Plant-Based Polysaccharide Gums as Sustainable Bio-Polymers: Focus on Tragacanth Gum and Its Emerging Applications
Abstract
1. Introduction
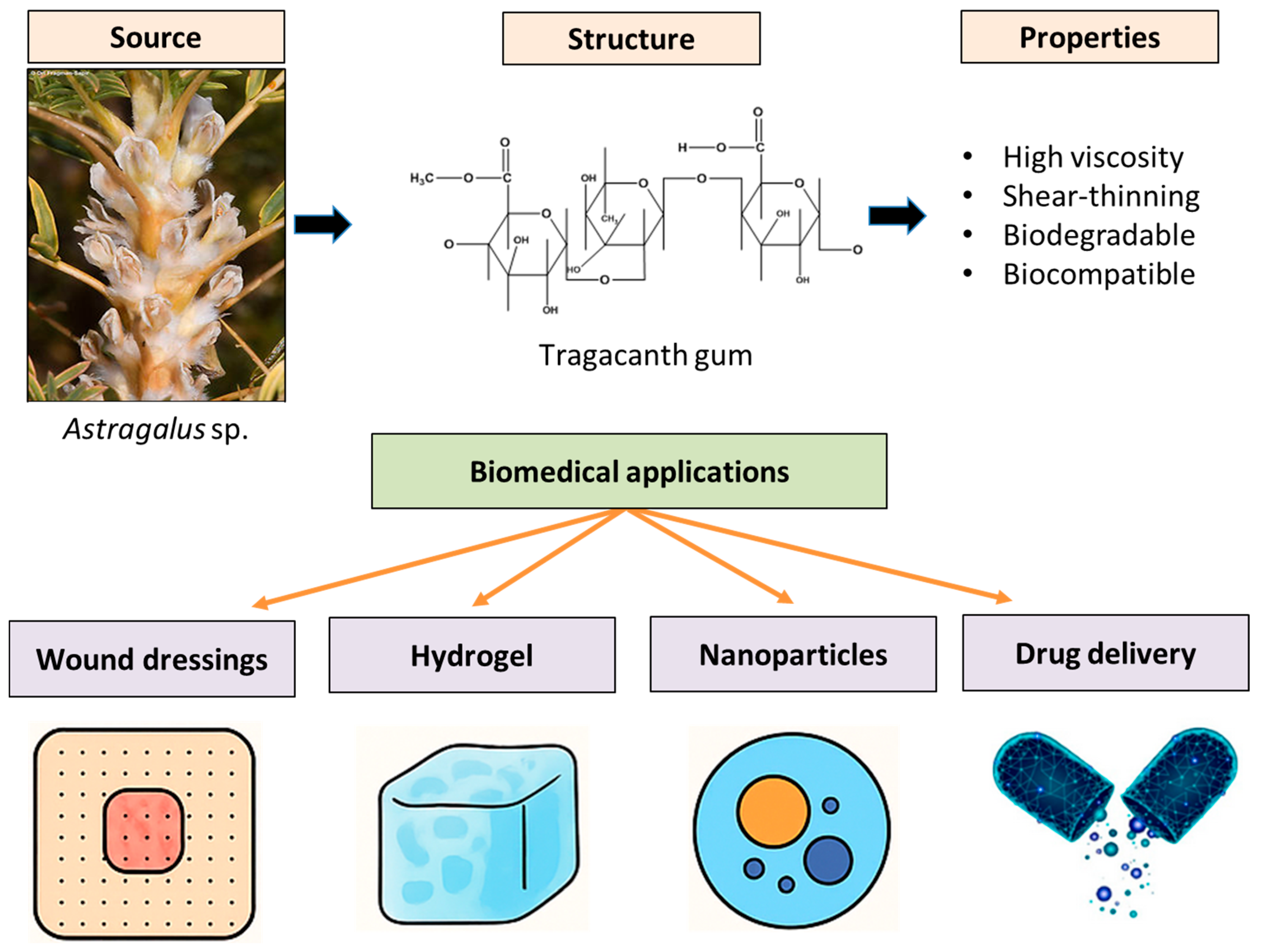
2. Tragacanth Gum as a Sustainable Biopolymer
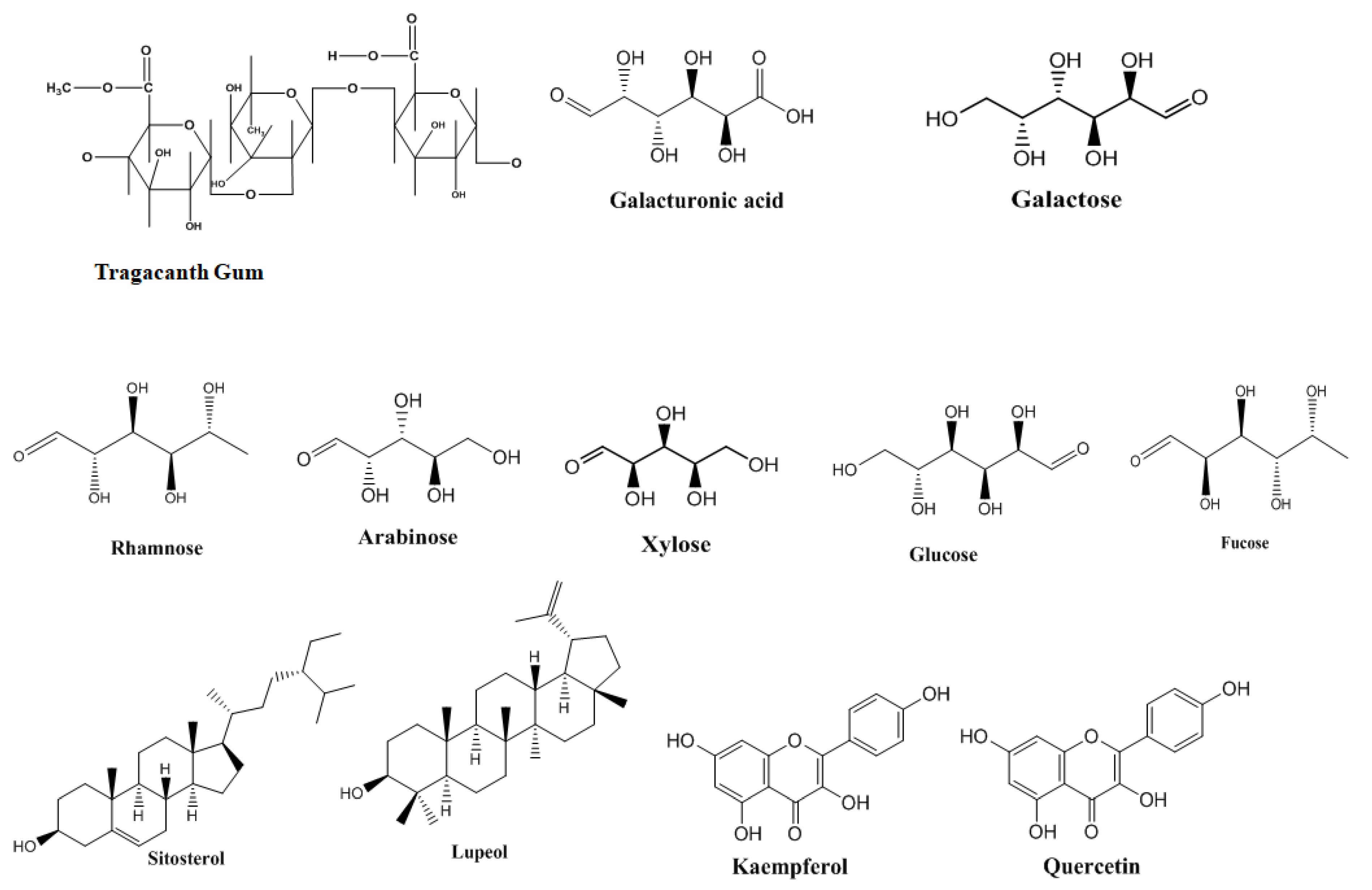
3. Structural and Physicochemical Features of Tragacanth Gum
Chemical Composition and Functional Properties
4. Advanced Materials Perspective
4.1. Nanocomposites: Enhancing Mechanical and Barrier Properties
4.2. Hydrogels: Applications in Drug Delivery and Tissue Engineering
4.3. Hybrid Systems: Synergistic Enhancements in Material Properties
5. Biomedical Innovations
5.1. Wound-Healing Applications
5.2. Drug-Delivery Systems
5.3. Tissue Engineering and Regenerative Medicine
| S. No. | Type of Polymer | Origin | Biodegradability % | Tensile Strength | O2 Barrier * | Cost * | Renewability | Toxicity Concerns | Applications | Green Economy Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | TG (Natural polysaccharide) | Astragalus sap; | 85–95% | 15–30 | 1000–1500 | 8–15 | Fully plant-based; minimal fossil-fuel use | Non-toxic; food-grade; pharma-safe when purified | Edible films, thickeners, and drug-delivery hydrogels | Renewable and low-toxicity | Hydrophilic and moisture-sensitive; | [27,73] |
| 2. | Polyethylene (Synthetic, petroleum based) PE | Petrochemical; ethylene-derived | <1% | 20–35 | 100–300 | 1–1.5 | Non-renewable; fossil-fuel based | Generally inert; additives may be hazardous; | Packaging films, bags, and containers; | Low cost; strong mechanical and barrier properties | Fossil fuel dependence; environmental persistence; plastic pollution; GHG emission | [74] |
| 3. | Polyethylene terephthalate (Synthetic) PET | Petrochemical; terephthalic acid + ethylene glycol | <5% | 50–75 | 10–20 | 1.2–1.8 | Non-renewable; bio-based PTA under research | Food-contact safe when processed correctly; microplastic and additive concerns | Bottles, fibers, packaging films | High strength, clarity, barrier properties; established recycling streams | Persistent if unrecycled; and limited replacement. | [75] |
| 4. | Polylactic acid (PLA); bio-based, industrial polymer | Fermentation of sugars → lactic acid → polymerization; corn/sugarcane feedstocks | 85–95% | 50–70 | 500–800 | 2–3 | Partly renewable; land use and fertilizer inputs matter | Low toxicity; | Compostable cups/packaging, fibers, 3D printing | Bio-based; good mechanical properties; familiar processing | Requires industrial composting; environmental fragmentation; agricultural footprint | [76,77] |
| 5. | Polyhydroxyalkanoates (PHA); microbial biopolymer | Microbial fermentation of organic feedstocks, including waste streams | 95–100% | 20–40 | 200–500 | 6–8 | Renewable; feedstock-flexible, can use waste streams | Low toxicity; biocompatible | Bioplastics, packaging, medical devices | Excellent biodegradability; strong green credentials; viable fossil–plastic replacement | Higher production cost; scale limitations; mechanical properties variable | [78,79] |
| 6. | Starch-based polymers; natural polysaccharide | Plant starch (corn, potato, cassava) | 90–100% | 5–30 | 1500–2500 | 2–5 | Renewable; abundant and low-cost | Low toxicity; food-grade | Disposable cutlery, films, fillers, blended bioplastics | Low-cost, renewable, compostable; blends with tragacanth improve film flexibility | Moisture sensitivity; lower mechanical strength; requires plasticizers or blending | [80] |
| 7. | Cellulose/nanocellulose; natural polysaccharide | Plant cellulose (wood, pulp); nanocellulose | >95% | 100–200 | 5–50 | 4–10 | Renewable; wood and agricultural residues | Low toxicity; biocompatible | Reinforcements, films, packaging, composites | Strong mechanical properties; green profile; blends with tragacanth enhance composites | Requires controlled processing; energy and chemical inputs needed | [81] |
| 8. | Chitosan; natural polysaccharide | Deacetylated chitin from crustacean shells or fungi | 80–95% | 25–50 | 800–1200 | 10–20 | Renewable; waste seafood shells or fungal biomass | Generally biocompatible; allergy risk minimized through processing | Wound dressings, films, active packaging, coatings | Biodegradable; antimicrobial; complements tragacanth for biomedical or packaging uses | Higher cost; variability in quality; limited solubility at neutral pH unless modified | [82] |
5.4. Material Properties and Biological Evaluation
6. Processing and Modification Techniques
6.1. Chemical Modifications Approaches
6.2. Physical and Green Processing Techniques
6.3. Enzymatic and Biological Functionalization
6.4. Composite and Blending Strategies
6.5. Development of Tragacanth-Based Composites and Their Functional Roles
7. Comparative Analysis with Other Plant Gums
| Properties | Tragacanth Gum | Guar Gum | Xanthan Gum | Gum Arabic | References |
|---|---|---|---|---|---|
| Microbial/botanical source | Astragalus spp. Exudates (A. gummifer, A. microcephalus) | Cyamopsis tetragonoloba (seed endosperm) | Xanthomonas campestris (bacterial fermentation) | Exudate of Acacia Senegal and A. seyal trees | [90,91] |
| Main composition | Galacturonic acid, fucose, arabinogalactans and rhamnose | Galactomannan (Mannose: Galactose: 2:1) | Beta-D-glucose backbone with glucuronic acid and mannose side chains | Arabinogalactan with Ca, Mg, K salts of glucuronic acid | [33,90,91] |
| Solubility behavior | Partially soluble as tragacanthin soluble and bassorin swellable | Highly soluble in cold water | Completely soluble and forms pseudoplastic solutions | Highly soluble in cold water and generally forms low viscosity solutions | [92,93] |
| Rheology | High viscosity, stable at temperature variations and pH 1–10 | Very high viscosity but pH sensitive | High viscosity, shear thinning and stable under moderate pH | Mild pseudoplasticity at high concentration | [92,93] |
| Gel forming ability | Strong due to tragacanthin and bassorin fractions | Moderate, limited gelation and forms viscous solutions | Viscosity control but poor gelation | Poor gel formation but primarily acts as emulsifier | [90,91] |
| pH and Thermal stability | Excellent pH and thermal tolerance (pH 2–10 and thermal stability: 180–220 °C) | Moderate tolerance and viscosity decrease with acids pH 5–8; thermal stability: 180–200 °C | Moderate to good. (pH 4–9; thermal stability: 200–230 °C | pH 3–9; thermal stability: 160–180 °C | [90,91] |
| Emulsifying capacity | Excellent emulsifying, stable emulsions and cohesive films | Good thickener and moderate emulsifying | Excellent stabilizer but limited film strength | High surface activity, film forming ability and excellent emulsifier | [90,91,92] |
| Biocompatibility | Biodegradable and non-toxic | Food grade and non-toxic | Generally safe but salt sensitivity limits its uses | Generally same, excellent in food and pharma | [33,90,91,92] |
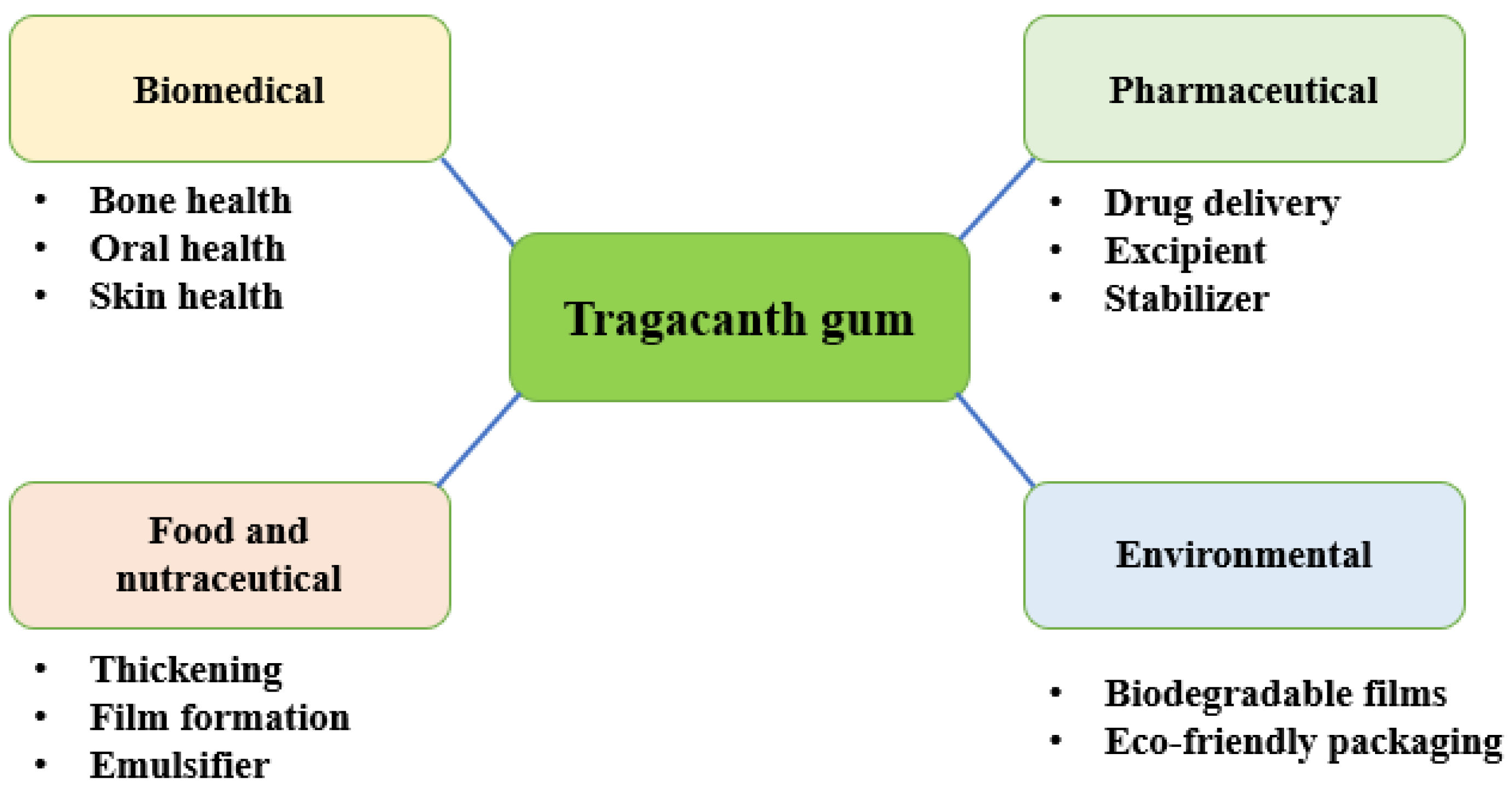
8. Applications of TG
8.1. Biomedical Applications
| S. No. | Synthesis and Characterization | Evaluation Methods | Main Findings | Limitations | Application | Ref. |
|---|---|---|---|---|---|---|
| 1. | CMGT synthesis, poly sodium acrylate crosslink, FTIR, XRD, NMR, SEM, TGA | Aceclofenac release, Higuchi, Korsmeyer–Peppas | CMGT- based hydrogel exhibited favorable rheological properties and facilitated drug release predominantly | Potential aspects, such as long-term mechanical stability, in vivo or in situ effectiveness, and issues related to scale-up and reproducibility | Controlled drug delivery | [98] |
| 2. | TG hydrogel, A. vestita extract, viscosity, spreadability | In vitro drug release, scratch assay (HaCaT cells), antimicrobial testing. | The hydrogel demonstrated effective wound closure (~95% within 24 h), notable antimicrobial activity, and exhibited pH-responsive behavior | Need animal or in vivo models to assess long-term stability and safety | A. vestita leaf extract for wound healing, topical drug delivery | [62] |
| 3. | Thermo-responsive magnetic hydrogel, TG, anticancer drug loading, magnetic and polymeric characterization | Temperature and magnetic responsiveness, drug release | The incorporation of magnetic components with TG enhanced control over drug release | Further investigation is needed to assess in vivo anticancer efficacy, systemic toxicity, and biodegradation | Targeted cancer therapy, controlled drug delivery | [95] |
| 4. | Hydrogel: TG + collagen + polyurethane, varying gum concentrations, swelling, viscoelasticity, pH-dependent degradation | Antibacterial activity, cytotoxicity, and immune-modulatory testing | High swelling (>2600%), strong antibacterial, biocompatible, immune-modulatory properties | Need to evaluate long-term in vivo wound healing, mechanical stability under stress, and scalability | Wound dressing, tissue engineering, and synthesis of immuno-modulatory biomaterial | [54] |
| 5. | Murine excisional wound model; planimetry, histology (AA + castor oil + TG formulation) | Enhanced wound healing (~87% compared to~70% in controls). | Clarifying the underlying mechanisms, such as identifying active polysaccharide fractions, comparison with standard treatments, and dosage optimization | Topical wound healing formulation, and skin repair | [55] | |
| 6. | Nanohydrogel: TG + cellulose + g-C3N4; lornoxicam loading; drug loading characterization | pH and temperature-dependent release, cell viability | Biocompatible, pH and temperature-responsive release, controlled drug delivery potential. | In vivo studies, stability and biodegradability, immune response, industrial-scale formulation | Controlled drug delivery, and smart nanohydrogel system | [35] |
| 7. | Systematic literature review, TG composition, gelation behavior | Biomedical applications, and drug delivery, cell therapy | TG is under-exploited biomaterial, potential in drug delivery and cell therapy highlighted | Lack of raw material standardization, and absence of direct comparisons with other gums | Drug delivery, cell therapy, and biomedical biomaterial | [26] |
| 8. | ZnO-tragacanth composite hydrogel, characterization | Murine burn wound model. | Accelerated burn wound healing, and enhanced tissue regeneration | Lack of mechanistic studies (immune modulation), systemic toxicity, and dose optimization | Burn wound healing, and tissue regeneration hydrogel | [86] |
| 9. | TG–CNF films, free/encapsulated cumin oil (CEO/CNE), ± oxygen absorber (OA) | Applied to turkey burgers (food application outcomes) | Ternary system (TG–CNF + CNE + OA) most effective, lowest microbial counts, reduced spoilage (TVN, TBARS), minimal pH rise/cooking loss, shelf-life ≥20 days | Industrial scalability, cost–benefit, consumer acceptance, and validation in other foods remain unexplored. | Turkey burger packaging, food preservation | [99] |
| 10. | TG-g-PAMPS hydrogel; microwave-assisted copolymerization; AgNP synthesis; FTIR, TGA, XRD, SEM, EDS, TEM; swelling, biodegradation | Antibacterial, diclofenac release, kinetic modeling | SN improved swelling and antibacterial activity, gels biodegradable, drug release non-Fickian, fitted Korsmeyer–Peppas model | Lack of in vivo validation, cytocompatibility/toxicity, SN concentration effect on mechanics/degradation, scale-up, biomedical application | Antibacterial hydrogel, and controlled drug delivery | [100] |
| 11. | TG hydrogel + Ca2+ crosslink; silk fibroin + in situ Fe3O4; biocompatibility characterization | Hemolysis, cytotoxicity, hyperthermia (SAR) | Good dispersibility, biocompatible, low hemolysis, supports cell growth, SAR up to 41.2 W/g | The study is limited by unclear long-term stability and biodegradation, limited assessment of heating performance, and absence of tests in real physiological or targeted therapeutic conditions | Cancer hyperthermia therapy, biocompatible nanocomposite | [101] |
| 12. | Double-layer hydrogel: TG + PVA + GrF + PANI; XRD, conductivity, contact angle, FESEM, tensile | MTT, biodegradation | PANI reduced crystallinity, enhanced conductivity (TP: 20,481×; TPG: 1804×), improved tensile strength (4.59×), good hydrophilicity (61.4°), uniform GrF, biocompatible (>90% viability), full biodegradation in 2 months | In vivo TENS validation, long-term stability, large-scale fabrication, optimize conductivity–biodegradability balance | TENS devices, conductive biodegradable hydrogel | [102] |
| 13. | GT coating (0–2%) on bell peppers | Weight loss, firmness, biochemical traits, antioxidant enzymes, marketability | 1% GT most effective: reduced weight loss (10.46% vs. 18.92%), maintained firmness, antioxidants, enzyme activity (SOD 97.02 U g−1, CAT 24.38 U g−1), improved antioxidant capacity (81.74%), marketability (~75% vs. 40%) | Scalability, consumer acceptance, cost analysis, combining GT with other natural additives for extended preservation across different fruits/vegetables | Bell pepper preservation, natural edible coating, shelf-life extension | [103] |
| 14. | Alginate/aloe vera films with TG (2–14%), evaluated mechanical, barrier, optical, biodegradability properties. | TG improved strength and flexibility, tensile strength ↑ (max 67.64 N/mm2 at 12%), WVP/swelling/thickness ↑, solubility ↓, films darker with higher TG. | Optimization for packaging, impact on active compounds (antimicrobials/antioxidants), real-food storage testing | Edible films, food packaging, biodegradable composites | [104] | |
| 15. | Oxidized TG (up to 57%), characterized by FTIR, 1H NMR, SEM, TGA, DSC, rheology | Antibacterial activity, hemolysis, cell viability tested | Oxidation ↓ pore size (23 ± 0.8 µm), rheology, thermal stability; new functional groups (hemiacetal, NMR 5.38 ppm); gained antibacterial activity; biocompatible (≤1% hemolysis, >85% viability) | Structure–function relationship, long-term stability, in vivo performance, scalable OTG synthesis | Antibacterial biomaterial, biomedical applications, tissue engineering | [105] |
| 16. | ST–GT hydrogels (varied ratios), PVA crosslink; FTIR, FESEM, TGA-DSC | Dye adsorption (MB, CR), kinetics, biodegradability | ST/GT 0.5:1.5 most efficient: MB 97.6% (pH 10), CR 93.7% (pH 2) in 90 min; adsorption pseudo-second-order; fully biodegradable in soil | Real wastewater testing, regeneration/reuse, large-scale production optimization | Biodegradable hydrogels for dye removal, wastewater treatment | [106] |
| 17. | Films with cherry wine pomace extract | WVTR, OTR, opacity | Improved barrier properties: WVTR 7.96–10.95 g/m2·d, OTR 0.50–0.94 cm3/m2·d·0.1 MPa; increased opacity (~19%); high antioxidant potential; eco-friendly | Mechanical strength, real food shelf-life testing, scalability, cost-effectiveness | Eco-friendly bioactive food packaging, antioxidant films | [47] |
| 18. | Eutectogels: TG bassorin + ChCl–malic acid; solubility, tensile, rheology, adhesion, texture | DXM transdermal permeability, anti-inflammatory (rat RA model) | DXM solubility ↑>1700×, gel stable up to 80 °C, strong mechanical and adhesive properties; 20% water: highest skin flux (332.7 mg/cm2·h); reduced paw swelling and TNF-α, normal knee histology | Long-term stability, large-scale production, human transdermal efficacy, testing other therapeutics | Transdermal DXM delivery, anti-inflammatory eutectogel | [107] |
| 19. | TG hydrogel encapsulation: beetroot juice + basil | Nano-nutraceutical formation outcomes | Retained nutrients (iron, folic acid, vitamins C, niacin, chlorophyll, sugars); preserved TG encapsulation ability at nanoscale; no chemical additives | Bioavailability, gastrointestinal absorption, long-term stability, in vivo human efficacy | TG-based nano-nutraceuticals, functional foods, nutrient delivery | [108] |
| 20. | TG + chitosan + MnFe2O4 hydrogels; hydrophilicity, biodegradation, adhesion | Biocompatibility (hemolysis, MTT), antibacterial, biofilm inhibition, tooth de/re-mineralization | Biocompatible, strong tooth adhesion, 100% S. mutans inhibition, high activity vs. S. aureus and E. coli, biofilm inhibition ≥85.94%, promoted remineralization, prevented demineralization | In vivo validation, long-term oral stability, scalability for dental use, impact on oral microbiome | Dental caries prevention, remineralization, antibacterial dental hydrogel | [109] |
| 21. | 3D scaffolds TG + PVP (DIW printing); printability, shear-thinning, SEM porosity, FTIR | Hydrophilicity, degradation, cell proliferation (WST-8), antibacterial | Good printability and shear-thinning, interconnected porous structure, hydrophilic, controlled degradation (168 h), sustained lawsone release, prolonged antibacterial activity (E. coli, S. aureus), supported cell attachment/proliferation | In vivo wound healing, long-term stability, mechanical optimization, combined bioactive molecules evaluation | 3D wound healing scaffold, antibacterial, controlled drug delivery | [110] |
| 22. | TG-based films and coatings; polymer blending, chemical properties | Food preservation applications | Hydrophilic, biodegradable, renewable, edible; composite films improve mechanical, barrier, functional properties; coatings extend shelf-life, maintain food quality | Large-scale industrial processing, cost-effectiveness, mechanical/barrier optimization, diverse food storage evaluation | Food packaging, edible coatings, shelf-life extension, functional food preservation | [66] |
| 23. | TG extraction, hydrogel formulation; physicochemical characterization | Drug delivery and tissue engineering | Biocompatible, biodegradable, high water absorption, controlled drug release, effective for bone, skin, cartilage, periodontal regeneration | Limited in vivo studies, physiological stability, long-term biodegradability, immune response, scalable industrial formulation | Drug delivery, tissue engineering, regenerative medicine | [27] |
| 24. | PVA/GT/CNC nanocomposite films, CNC from sugarcane bagasse (steam explosion + acid hydrolysis), solution casting, TEM, SEM, FTIR, mechanical andswelling tests | Cytotoxicity, antibacterial assays, betel leaf extract loading | Transparent, mechanically strong (elastic modulus 1526 MPa, tensile 80.39 MPa), stable 7 days, swelling ↓ with CNC, non-toxic (95% L929 viability), strong antibacterial activity (S. aureus, P. aeruginosa) | In vivo wound healing, long-term stability and biodegradability, CNC dispersion optimization, large-scale production feasibility | Wound dressing, antibacterial films, regenerative biomaterials | [110] |
| 25. | Self-healing hydrogel: Tragacanth + PVA + borax, one-pot synthesis; characterized by rheology, self-healing tests | Cytotoxicity (L929), scratch assay, real-time PCR (TGFβ1, TGFβ2, VEGF-A) | Good mechanical strength, high self-healing, non-toxic (>100% viability), enhanced cell migration and scratch healing, increased growth factor expression, higher borax ↑ storage modulus | In vivo wound healing validation, long-term biodegradability andstability, large-scale production optimization, clinical application | Wound healing, tissue engineering, regenerative hydrogel | [87] |
| 26. | Literature review: cellulose derivatives and nanocellulose, physicochemical properties, bio-ink design, cross-linking | 3D bioprinting, cell–matrix interactions | Biocompatible, biodegradable, cost-effective, printable; cross-linking tunes mechanics; functional groups/surface charge influence cell adhesion, survival, proliferation | Limited tissue-specific bio-inks, long-term stability, in vivo performance, optimized multi-component formulations | 3D bioprinting, tissue engineering, bio-ink development | [111] |
8.2. Food Industry
8.3. Environmental and Water Treatment
9. Research Gaps and Future Perspectives
9.1. Reviews, Overviews, and Comparative Analyses
9.2. Processing, Functionalization, and Material Science
9.3. Standardization, Quality Control, and Supply-Chain Considerations
9.4. Scalability and Industrial Limitations
9.5. Clinical Status and Translational Prospects
10. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TG | Tragacanth Gum |
| TG–CNF | Tragacanth Gum–Cellulose Nanofiber |
| CNF | Cellulose Nanofiber |
| CNC | Cellulose Nanocrystals |
| CEO | Cumin Essential Oil |
| CNE | Encapsulated Cumin Essential Oil |
| OA | Oxygen Absorber |
| PVA | Polyvinyl Alcohol |
| GrF | Carboxylated Graphene |
| PANI | Polyaniline |
| ST | Starch |
| MB | Methylene Blue |
| CR | Congo Red |
| DXM | Dexamethasone |
| MNP | Magnetic Nanoparticles |
| PVP | Polyvinylpyrrolidone |
| DIW | Direct Ink Writing |
| RTC | Ready to Cook |
| FTIR | Fourier Transform Infrared Spectroscopy |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| FESEM | Field Emission Scanning Electron Microscopy |
| XRD | X-ray Diffraction |
| TGA | Thermogravimetric Analysis |
| DSC | Differential Scanning Calorimetry |
| NMR | Nuclear Magnetic Resonance |
| PCR | Polymerase Chain Reaction |
| TGFβ1 | Transforming Growth Factor Beta-1 |
| TGFβ2 | Transforming Growth Factor Beta-2 |
| VEGF-A | Vascular Endothelial Growth Factor-A |
| SAR | Specific Absorption Rate |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| WVTR | Water Vapor Transmission Rate |
| OTR | Oxygen Transmission Rate |
References
- Shiam, M.A.H.; Islam, M.S.; Ahmad, I.; Haque, S.S. A review of plant-derived gums and mucilages: Structural chemistry, film forming properties and application. J. Plast. Film Sheet. 2025, 41, 195–237. [Google Scholar] [CrossRef]
- Kaur, R.; Pathak, L.; Vyas, P. Biobased polymers of plant and microbial origin and their applications—A review. Biotech. Sustain. Mater. 2024, 1, 13. [Google Scholar] [CrossRef]
- Bilal, M.; Munir, H.; Khan, M.I.; Khurshid, M.; Rasheed, T.; Rizwan, K.; Franco, M.; Iqbal, H.M. Gums-based engineered bio-nanostructures for greening the 21st-century biotechnological settings. Crit. Rev. Food Sci. Nutr. 2022, 62, 3913–3929. [Google Scholar] [CrossRef]
- WFO. World Flora Online. Version 2025.06.2025. Available online: http://www.worldfloraonline.org (accessed on 15 August 2025).
- Mayes, J.M. Gum tragacanth and karaya. In Food Stabilisers, Thickeners and Gelling Agents; Blackwell Publishing Ltd.: Oxford, UK, 2009; pp. 167–179. [Google Scholar]
- Emam-Djomeh, Z.; Fathi, M.; Askari, G. Gum tragacanth (Astragalus gummifer Labillardiere). In Emerging Natural Hydrocolloids: Rheology and Functions; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 299–326. [Google Scholar]
- Adnan, M.; Oh, K.K.; Cho, D.H.; Alle, M. Nutritional, pharmaceutical, and industrial potential of forest-based plant gum. In Non-Timber Forest Products: Food, Healthcare and Industrial Applications. Springer International Publishing: Cham, Switzerland, 2021; pp. 105–128. [Google Scholar]
- Askari, V.R.; Fathani, M.; Roudi, H.S.; Fadaei, M.S.; Nejad, S.S.M.; Keshik, S.D.; Rahimi, V.B.; Hasnain, M.S.; Nayak, A.K. Gum tragacanth in drug delivery. In Natural Biopolymers for Drug Delivery; Woodhead Publishing: Cambridge, UK, 2025; pp. 51–71. [Google Scholar]
- Lacomis, D.; Giuliani, M.J.; Van Cott, A.; Kramer, D.J. Acute myopathy of intensive care: Clinical, electromyographic, and pathological aspects. Ann. Neurol. 1996, 40, 645–654. [Google Scholar] [CrossRef]
- Mahboubi, M. The beneficial effects of Ferulaasafoetida oleo-gum resin in gastrointestinal disorders. Bull. Fac. Pharm. Cairo Univ. 2021, 59, 50–63. [Google Scholar]
- Malabadi, R.B.; Kolkar, K.P.; Chalannavar, R.K. Natural plant gum exudates and mucilage: Pharmaceutical updates. Int. J.Innov. Sci. Res. Rev. 2021, 3, 1897–1912. [Google Scholar]
- Koyyada, A.; Orsu, P. Natural gum polysaccharides as efficient tissue engineering and drug delivery biopolymers. J. Drug Deliv. Sci. Technol. 2021, 63, 102431. [Google Scholar] [CrossRef]
- Ahmed, S.; Ali, A. (Eds.) Natural Gums: Extraction, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Rohilla, S.; Chawla, G.; Bhagwat, D.P.; Rohilla, A. Natural gum: An option to prepare nanocarriers. Med. Theor. Hypothesis 2023, 6, 15. [Google Scholar] [CrossRef]
- Thakur, S. Gum based green nanocomposites and their applications. In Green-Based Nanocomposite Materials and Applications; Springer International Publishing: Cham, Switzerland, 2023; pp. 295–315. [Google Scholar]
- Ebrahimian, E.; Seyyedi, S.M. Important of katira gum (gondkatira) or TG as a herbal medicine. In Proceedings of the1st International Conference on Natural Food Hydrocolloids, Mashhad, Iran, 22 October 2014. [Google Scholar]
- Kundalia, A.; Mishra, A.; Rani, C.; Gupta, D.; Gautam, K.; Sushma, K.M.; Meghwal, M. Nutritional profile, functional characteristics, health benefits, and potential application of edible gum (Gond). In Nutritional Science and Technology: Concept to Application; Scrivener Publishing LLC: Newport Beach, CA, USA, 2023; pp. 83–102. [Google Scholar]
- Pansare, K.; Sonawane, G.; Patil, C.; Sonawane, D.; Mahajan, S.; Somavanshi, D.; Ahire, Y.; Bairagi, V.; Sharma, S. Gond Katira: A Natural Remedy for Summer Heat and Hydration. Res. J. Pharmacol. Pharmacodyn. 2025, 17, 95–101. [Google Scholar] [CrossRef]
- Yadav, H.; Gupta, L.; Dubey, S.; Maiti, S.; Yadav, N. Economical importance, structural diversity, and properties of natural gums. In Natural Gums; Elsevier: Amsterdam, The Netherlands, 2023; pp. 23–53. [Google Scholar]
- Aspinall, G.O.; Baillie, J. Gum tragacanth. Part I. Fractionation of the gum and the structure of tragacanthic acid. J. Chem. Soc. 1963, 1702–1714. [Google Scholar] [CrossRef]
- James, S.P.; Smith, F. The chemistry of gum tragacanth; derivatives of d-and l-fucose. J. Chem. Soc. 1945, 746–748. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Musavi, S.M.; Kiumarsi, A.; Williams, P.A. Solution properties of targacanthin (water-soluble part of gum tragacanth exudate from Astragalus gossypinus). Int. J. Biol. Macromol. 2006, 38, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A. Physicochemical, rheological and structural characteristics of alcohol precipitated fraction of gum tragacanth. Food Health 2018, 4, 183–193. [Google Scholar] [CrossRef]
- Fattahi, A.; Petrini, P.; Munarin, F.; Shokoohinia, Y.; Golozar, M.A.; Varshosaz, J.; Tanzi, M.C. Polysaccharides derived from tragacanth as biocompatible polymers and gels. J. Appl. Polym. Sci. 2013, 129, 2092–2102. [Google Scholar] [CrossRef]
- Boamah, P.O.; Afoakwah, N.A.; Onumah, J.; Osei, E.D.; Mahunu, G.K. Physicochemical properties, biological properties and applications of gum tragacanth—A review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100288. [Google Scholar] [CrossRef]
- Nazemi, Z.; Sahraro, M.; Janmohammadi, M.; Nourbakhsh, M.S.; Savoji, H. A review on tragacanth gum: A promising natural polysaccharide in drug delivery and cell therapy. Int. J. Biol. Macromol. 2023, 241, 124343. [Google Scholar] [CrossRef]
- Abdi, G.; Jain, M.; Patil, N.; Tariq, M.; Choudhary, S.; Kumar, P.; Raj, N.S.; Mohsen Ali, S.S.; Uthappa, U.T. Tragacanth gum-based hydrogels for drug delivery and tissue engineering applications. Front. Mater. 2024, 11, 1296399. [Google Scholar] [CrossRef]
- Balaghi, S.; Mohammadifar, M.A.; Zargaraan, A.; Gavlighi, H.A.; Mohammadi, M. Compositional analysis and rheological characterization of gum tragacanth exudates from six species of Iranian Astragalus. Food Hydrocoll. 2011, 25, 1775–1784. [Google Scholar] [CrossRef]
- Tavakol, M.; Mohammadifar, M.A. A review on gum tragacanth and its biomedical applications. Nashr. ShimiMohand. Shimi Iran 2017, 36, 1–20. [Google Scholar]
- Farzi, M.; Emam-Djomeh, Z.; Mohammadifar, M.A. A comparative study on the emulsifying properties of various species of gum tragacanth. Int. J. Biol. Macromol. 2013, 57, 76–82. [Google Scholar] [CrossRef]
- Dhupal, M.; Gupta, M.K.; Tripathy, D.R.; Kumar, M.; Yi, D.K.; Nanda, S.S.; Chowdhury, D. Recent advances in pharmaceutical applications of natural carbohydrate polymer gum tragacanth. Nat. Polym. Pharm. Appl. 2019, 1, 49–86. [Google Scholar]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef]
- TaghavizadehYazdi, M.E.; Nazarnezhad, S.; Mousavi, S.H.; Sadegh Amiri, M.; Darroudi, M.; Baino, F.; Kargozar, S. Gum tragacanth (GT): A versatile biocompatible material beyond borders. Molecules 2021, 26, 1510. [Google Scholar] [CrossRef]
- Ahmed, S.; Singh, N.; Annu. Biomedical applications of gums. In Biopolymers for Biomedical Applications; Scrivener Publishing LLC: Newport Beach, CA, USA, 2024; pp. 175–197. [Google Scholar]
- Nawaz, A.; Alghamdi, S.; Taj, M.B.; Carabineiro, S.A.C.; Raheel, A.; Ahmad, F.; Rao, K.A.; Khan, M.I.; Shananbleh, A. Essential insights on advancing biomedical innovations with TG nanocomposites: A review. Inorg. Chem. Commun. 2025, 173, 113807. [Google Scholar] [CrossRef]
- Thombare, N.; Lohot, V.D.; Kanani, M.R.; Nadjafi, F.; Prasad, N.; Schulz, H. Taxonomy and distribution of botanical resources providing most important gums and resins. In Natural Gums and Resins: Botany and Sustainable Uses in Medicine, Nutrition, Perfumery and Cosmetics; CABI: London, UK, 2025; pp. 38–106. [Google Scholar]
- Dogra, S.; Koul, B.; Singh, J.; Mishra, M.; Yadav, D. Phytochemical analysis, antimicrobial screening and in vitro pharmacological activity of Artemisia vestita leaf extract. Molecules 2024, 29, 1829. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Bahramsoltani, R. (Eds.) Therapeutic Medicinal Plants in Traditional Persian Medicine; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Nur, M. Tragacanth as a Novel Excipient in Oral Insulin Delivery. Doctoral Dissertation, Victoria University, Melbourne, VIC, Australia, 2019. [Google Scholar]
- Nayak, A.K.; Hasnain, M.S.; Pal, D. (Eds.) Natural Polymers for Pharmaceutical Applications: Volume 1: Plant-Derived Polymers; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Glicksman, M. Gum tragacanth. In Food Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2019; pp. 49–60. [Google Scholar]
- Pegg, A.M. The application of natural hydrocolloids to foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Woodhead Publishing: Cambridge, UK, 2012; pp. 175–196. [Google Scholar]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural polymer-based hydrogels: From polymer to biomedical applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef] [PubMed]
- Nejatian, M.; Abbasi, S.; Azarikia, F. Gum tragacanth: Structure, characteristics and applications in foods. Int. J. Biol. Macromol. 2020, 160, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Makvandi, P.; Tay, F.R. Recent progress in the industrial and biomedical applications of tragacanth gum: A review. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef]
- Iravani, S. Plant gums for sustainable and eco-friendly synthesis of nanoparticles: Recent advances. Inorg. Nano-Met. Chem. 2020, 50, 469–488. [Google Scholar] [CrossRef]
- Dobrucka, R.; Vapenka, L.; Szymański, M.; Pawlik, M.; Lasik-Kurdyś, M.; Gumienna, M. Bio-packaging based on pectin/TG with added extracts of cherry waste from the wine industry as a new generation of active films for the food industry. Foods 2025, 14, 2203. [Google Scholar] [CrossRef]
- Tabassum, B.A.; Ahmed, H.S.; Naik, T.; KP, B.; Jeevan Prasad Reddy, D.; Prabhakar, P.R.; Vivek Babu, C.S.; Varaprasad, K.; Keshava Murthy, P.S. Development of antibacterial edible films for food packaging using TG, carrageenan, and clove essential oil. J. Appl. Polym. Sci. 2024, 141, e55495. [Google Scholar] [CrossRef]
- Tabarsa, A.; Latifi, N.; Zangeneh, S.; Phunpeng, V.; Horpibulsuk, S.; Hoy, M. Evaluation of sandy soil stabilized with TG biopolymer for geotechnical applications. Results Eng. 2024, 24, 103532. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Dhoble, S.; Tobin, D. Natural gum (xanthan, gellan, arabic, guar, ghatti gum, etc.)-based bio-scaffold and their application in tissue engineering. In Natural Product Inspired Scaffolds: Applications in Tissue Engineering; Springer Nature: Singapore, 2024; pp. 61–91. [Google Scholar]
- Shahvalizadeh, R.; Ahmadi, R.; Davandeh, I.; Pezeshki, A.; Moslemi, S.A.S.; Karimi, S.; Rahimi, M.; Hamishehkar, H.; Mohammadi, M. Antimicrobial bio-nanocomposite films based on gelatin, tragacanth, and zinc oxide nanoparticles–Microstructural, mechanical, thermo-physical, and barrier properties. Food Chem. 2021, 354, 129492. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, J.A.A.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Biopolymer-based films reinforced with green synthesized zinc oxide nanoparticles. Polymers 2022, 14, 5202. [Google Scholar] [CrossRef] [PubMed]
- León-Campos, M.I.; Claudio-Rizo, J.A.; Becerra-Rodriguez, J.J.; González-Díaz, M.O.; Flores-Guía, T.E.; Soriano-Corral, F.; Herrera-Guerrero, A. Novel TG-collagen-polyurethane hydrogels: Super-swelling, antibacterial, and fibrillogenesis-enhancing properties for efficient wound healing. Int. J. Biol. Macromol. 2025, 310, 143281. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J. Application of TG and alginate in hydrogel wound dressing’s formation using gamma radiation. Carbohydr. Polym. Tech. 2021, 2, 100058. [Google Scholar]
- Polez, R.T.; Kimiaei, E.; Madani, Z.; Österberg, M.; Baniasadi, H. TG hydrogels with cellulose nanocrystals: A study on optimizing properties and printability. Int. J. Biol. Macromol. 2024, 280, 136182. [Google Scholar] [CrossRef]
- Das, D.; Roy, A.; Pal, S. A polysaccharide-based pH-sensitive hybrid hydrogel as a sustained release matrix for antimicrobial drugs. ACSAppl. Polym. Mater. 2023, 5, 3348–3358. [Google Scholar] [CrossRef]
- Khoshnood, N.; Shahrezaee, M.H.; Shahrezaee, M.; Zamanian, A. Three-dimensional bioprinting of tragacanth/hydroxyapaptite modified alginate bioinks for bone tissue engineering with tunableprintability and bioactivity. J. Appl. Polym. Sci. 2022, 139, e52833. [Google Scholar] [CrossRef]
- Kuo, C.C.; Qin, H.; Cheng, Y.; Jiang, X.; Shi, X. An integrated manufacturing strategy to fabricate delivery system using gelatin/alginate hybrid hydrogels: 3D printing and freeze-drying. Food Hydrocoll. 2021, 111, 106262. [Google Scholar] [CrossRef]
- Cleymand, F.; Poerio, A.; Mamanov, A.; Elkhoury, K.; Ikhelf, L.; Jehl, J.P.; Kahn, C.J.; Poncot, M.; Arab-Tehrany, E.; Mano, J.F. Development of novel chitosan/guar gum inks for extrusion-based 3D bioprinting: Process, printability and properties. Bioprinting 2021, 21, e00122. [Google Scholar] [CrossRef]
- Foroughi, P.; Koupaei, N. Physically crosslinked polyvinyl alcohol/chitosan/gum tragacanth hydrogels loaded with vitamin E for wound healing applications. J. Vinyl Addit. Technol. 2023, 29, 268–282. [Google Scholar] [CrossRef]
- Azadi, A.; Rafieian, F.; Sami, M.; Rezaei, A. Investigating the effects of chitosan/TG/polyvinyl alcohol composite coating incorporated with cinnamon essential oil nanoemulsion on safety and quality features of chicken breast fillets during storage in the refrigerator. Int. J. Biol. Macromol. 2023, 253, 126481. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Koul, B.; Singh, J.; Mishra, M.; Rabbee, M.F. Formulation and in vitro assessment of TG-based hydrogel loaded with Artemisia vestita leaf extract for wound healing. Processes 2024, 12, 2750. [Google Scholar] [CrossRef]
- Iqbal, Y.; Ameer, M.E.; Ahmed, I.; Ullah, A.; Irfan, M.F.; Chatha, S.A.S. Green Biopolymers in Food Packaging. In Green Biopolymers for Packaging Applications; CRC Press: Boca Raton, FL, USA, 2025; pp. 93–148. [Google Scholar]
- Mallakpour, S.; Ramezanzade, V. Green fabrication of chitosan/TG bionanocomposite films having TiO2@ Ag hybrid for bioactivity and antibacterial applications. Int. J. Biol. Macromol. 2020, 162, 512–522. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F.; Hussain, C.M. Potential of TG in the industries: A short journey from past to the future. Polym. Bull. 2023, 80, 4643–4662. [Google Scholar] [CrossRef]
- Ma, K.; Yang, J.; Goksen, G.; Alamri, A.S.; Alhomrani, M.; Xia, G.; Zhang, W. Application of TG as a potential food packaging film and its performance enhancement strategy. Food Hydrocoll. 2025, 162, 110894. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, P.; Gupta, K.; Singh, M.; Tomer, V. Recent advances in extraction, techno-functional properties, food and therapeutic applications as well as safety aspects of natural and modified stabilizers. Food Rev. Int. 2023, 39, 2233–2276. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Santos, A.C.P.; Varma, R.S.; Berrada, M. Smart stimuli-responsive polysaccharide nanohydrogels for drug delivery: A review. J. Mater. Chem. B 2023, 11, 10538–10565. [Google Scholar] [CrossRef]
- Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Hydrogels as potential nano-, micro-and macro-scale systems for controlled drug delivery. Materials 2020, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S. Gum tragacanth-based nanosystems for therapeutic applications. In Polymeric Nanosystems; Academic Press: Cambridge, MA, USA, 2023; pp. 367–404. [Google Scholar]
- Aghajani, M.; Garshasbi, H.R.; Naghib, S.M.; Mozafari, M.R. 3D printing of hydrogel polysaccharides for biomedical applications: A Review. Biomedicines 2025, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Avinash, A.; Kumar, B.; Kulshrestha, R.; Kapur, D.; Prabhat, S.K. Efficacy of astragalus extract against S. mutans and C. albicans an in vitro Study. Int. J. Recent Sci. Res. 2019, 10, 32417–32420. [Google Scholar]
- Kumar, P.; Lata, K.; Gacem, A.; Tariq, M.; Singh, S.; Sharma, A.; Yadav, V.K.; Bhutto, J.K.; Kumar, M.; Alreshidi, M.A.; et al. A review on the environmental fate, toxicological risks, and cutting-edge degradation methods of microplastics contamination. Environ. Sci. Eur. 2025, 37, 114. [Google Scholar] [CrossRef]
- Joseph, T.M.; Azat, S.; Ahmadi, Z.; Jazani, O.M.; Esmaeili, A.; Kianfar, E.; Haponiuk, J.; Thomas, S. Polyethylene terephthalate (PET) recycling: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100673. [Google Scholar] [CrossRef]
- Rajendran, D.S.; Venkataraman, S.; Jha, S.K.; Chakrabarty, D.; Kumar, V.V. A review on bio-based polymer polylactic acid potential on sustainable food packaging. Food Sci. Biotechnol. 2024, 33, 1759–1788. [Google Scholar] [CrossRef]
- Lors, C.; Leleux, P.; Park, C.H. State of the art on biodegradability of bio-based plastics containing polylactic acid. Front. Mater. 2025, 11, 1476484. [Google Scholar] [CrossRef]
- Acharjee, S.A.; Bharali, P.; Gogoi, B.; Sorhie, V.; Walling, B.; Alemtoshi, R. PHA-based bioplastic: A potential alternative to address microplastic pollution. Water Air Soil Pollut. 2023, 234, 21. [Google Scholar] [CrossRef]
- Hong, S.J.; Ahn, K.; Kim, J.T.; Kim, Y.T. Polyhydroxyalkanoates for sustainable food packaging: A comprehensive review on production, applications, and end-of-life scenarios for food packaging. Trends Food Sci. Technol. 2025, 165, 105294. [Google Scholar] [CrossRef]
- Fatima, S.; Khan, M.R.; Ahmad, I.; Sadiq, M.B. Recent advances in modified starch based biodegradable food packaging: A review. Heliyon 2024, 10, e27453. [Google Scholar] [CrossRef]
- Antony Jose, S.; Cowan, N.; Davidson, M.; Godina, G.; Smith, I.; Xin, J.; Menezes, P.L. A comprehensive review on cellulose nanofibers, nanomaterials, and composites: Manufacturing, properties, and applications. Nanomaterials 2025, 15, 356. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A. A review of chitosan-based materials for biomedical, food, and water treatment applications. Materials 2024, 17, 5770. [Google Scholar] [CrossRef] [PubMed]
- Kaith, B.S.; Jindal, R.; Kumar, V.; Bhatti, M.S. Optimal response surface design of gum tragacanth-based poly[(acrylic acid)-co-acrylamide] IPN hydrogel for the controlled release of the antihypertensive drug losartan potassium. RSC Adv. 2014, 4, 39822–39829. [Google Scholar]
- Ahmad, S.; Imran, S. Synthesis of gum tragacanth–starch hydrogels for water purification. Mater. Adv. 2024, 5, 8812–8825. [Google Scholar] [CrossRef]
- Iijima, M.; Hatakeyama, T.; Hatakeyama, H. DSC and TMA studies of polysaccharide physical hydrogels. Anal. Sci. 2021, 37, 211–219. [Google Scholar] [CrossRef]
- Shaw, P.; Sharma, A.K.; Kalonia, A.; Kumar, R.; Yashavarddhan, M.H.; Surya, P.; Singh, S.; Shukla, S.K. Zinc oxide and gum tragacanth-based composite hydrogel heals partial-thickness burn wound by attenuating pro-inflammatory genes and enhancing regenerating growth factors. Int. J. Biol. Macromol. 2025, 286, 138679. [Google Scholar] [CrossRef]
- Mohamadi-Sodkouieh, S.; Kalantari, M.; Askari, N. A bioactive self-healing hydrogel wound-dressing based on TG: Structural and invitro biomedical investigations. Int. J. Biol. Macromol. 2024, 278, 134980. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-based hydrogels as versatile materials with interesting functional properties for tissue engineering applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Jain, R.; Jain, B.; Al-Khateeb, L.A.; Alharthi, S.; Ghoneim, M.M.; AbdElrahman, M.; Alanazi, A.S. Advances in green sample preparation methods for bioanalytical laboratories focusing on drug analysis. Bioanalysis 2025, 17, 489–508. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef]
- Omidbakhsh Amiri, E.; Nayebzadeh, K.; Mohammadifar, M.A. Comparative studies of xanthan, guar and tragacanth gums on stability and rheological properties of fresh and stored ketchup. J. Food Sci. Technol. 2015, 52, 7123–7132. [Google Scholar] [CrossRef]
- Chenlo, F.; Moreira, R.; Silva, C. Rheological behaviour of aqueous systems of tragacanth and guar gums with storage time. J. Food Eng. 2010, 96, 107–113. [Google Scholar] [CrossRef]
- Padil, V.V.; Černík, M. Graphene oxide-plant gum nanocomposites for sustainable applications. In Graphene Based Biopolymer Nanocomposites; Springer: Singapore, 2020; pp. 149–171. [Google Scholar]
- Barhoum, A.; Meftahi, A.; Kashef Sabery, M.S.; Momeni Heravi, M.E.; Alem, F. A review on carbon dots as innovative materials for advancing biomedical applications: Synthesis, opportunities, and challenges. J. Mater. Sci. 2023, 58, 13531–13579. [Google Scholar] [CrossRef]
- Sayadnia, S.; Arkan, E.; Jahanban-Esfahlan, R.; Sayadnia, S.; Jaymand, M. Thermal-responsive magnetic hydrogels based on TG for delivery of anticancer drugs. J. Polym.Res. 2021, 28, 90. [Google Scholar] [CrossRef]
- Khani, A.; Eskandani, M.; Derakhshankhah, H.; Soleimani, K.; Nakhjavani, S.A.; Massoumi, B.; Jahanban-Esfahlan, R.; Moloudi, K.; Jaymand, M. A novel stimuli-responsive magnetic hydrogel based on nature-inspired TG for chemo/hyperthermia treatment of cancerous cells. J. Polym.Res. 2022, 29, 149. [Google Scholar] [CrossRef]
- Tokasi, S.; Mehrnia, M.R.; Roudsari, F.P. Antibacterial gelatin/TG films containing galbanum essential oil for in vitro scratch-healing. Int.J. Biol.Macromol. 2024, 281, 136284. [Google Scholar] [CrossRef]
- Tanwar, M.; Gupta, R.K.; Rani, A. Carboxymethylated gum tragacanth crosslinked poly (sodium acrylate) hydrogel: Fabrication, characterization, rheology and drug-delivery application. Indian J. Chem. Technol. 2023, 30, 308–319. [Google Scholar] [CrossRef]
- Shahabi, N.; Fallah, A.A.; Sami, M.; HabibianDehkordi, S. Effect of TG–chitin nanofiber film containing free or nano-encapsulated cumin essential oil on the quality of chilled turkey burgers packed withoxygen absorber. Food Sci. Nutr. 2024, 12, 5605–5618. [Google Scholar] [CrossRef]
- Chapi, S.; Babaladimath, G.; Murugendrappa, M.V.; Raghu, A.V. Microwave-Assisted TG–based grafted silver nanocomposite hydrogel for sustained release formulations of diclofenac sodium and antibacterial assay. Nano Select. 2024, 5, e202300200. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Mohammadi, A.; AghamirzaMoghimAliabadi, H.; Kashtiaray, A.; Bani, M.S.; Karimi, A.H.; Maleki, A.; Mahdavi, M. A novel ternary magnetic nanobiocomposite based on tragacanth-silk fibroin hydrogel for hyperthermia and biological properties. Sci. Rep. 2024, 14, 8166. [Google Scholar] [CrossRef]
- Vafa, Z.J.; Zare, E.N.; Eslam, M.R.F. Double-layer biodegradable hydrogel based on TG as an electrically conductive nanoplatform for TENS device application. Carbohydr. Polym. 2025, 358, 123540. [Google Scholar] [CrossRef]
- Zare-Bavani, M.R.; Rahmati-Joneidabad, M.; Jooyandeh, H. Gum tragacanth, a novel edible coating, maintains biochemical quality, antioxidant capacity, and storage life in bell pepper fruits. Food Sci. Nutr. 2024, 12, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Nawab, A.; Alam, F.; Zehra, K. Alginate/aloe vera films reinforced with TG. Food Chem.-Mol. Sci. 2022, 4, 100105. [Google Scholar]
- Shabannejad, M.; Nourbakhsh, M.S.; Nezafati, N.; Nazemi, Z.; Nemati, F. Periodate oxidation of TG and evaluation of physicochemical and biological properties of oxidized TG. Sci. Rep. 2025, 15, 30214. [Google Scholar] [CrossRef] [PubMed]
- Sringeri Ganesh, P.; Kadri Jagadish, D.; Badalamoole, V. Development of Gum Tragacanth-Based Graft Copolymer Hydrogel for the Adsorptive Removal of Dyes from Wastewater. Macromol. Chem. Phys. 2025, 226, e00225. [Google Scholar] [CrossRef]
- Fatahi, A.; Varshosaz, J.; Hajhashemi, V. TG-deep eutectic solvent-based eutectogels for dextromethorphan transdermal delivery and induced rheumatoid arthritis treatment in rat. Int. J. Biol. Macromol. 2025, 321, 146381. [Google Scholar] [CrossRef]
- Singh, D.P.; Gopinath, P. TG-based nano-nutraceuticals synthesis by encapsulation of beetroot juice and Ocimumbasilicum leaves for micronutrient deficient population. Int.J. Biol.Macromol. 2023, 253, 127502. [Google Scholar] [CrossRef]
- Yadav, A.A.; Mujawar, S.; Shinde, N.; Phalake, S.S.; Khot, V.M.; Kashte, S.B. A multifunctional TG-chitosan hydrogel loaded with manganese ferrite nanoparticles for dental caries prevention and remineralization. Int. J. Biol. Macromol. 2025, 322, 146886. [Google Scholar] [CrossRef]
- Diem, L.N.; Torgbo, S.; Banerjee, I.; Pal, K.; Sukatta, U.; Rugthaworn, P.; Sukyai, P. Sugarcane bagasse-derived cellulose nanocrystal/polyvinyl alcohol/gum tragacanth composite film incorporated with betel leaf extract as a versatile biomaterial for wound dressing. Int. J. Biomater. 2023, 2023, 9630168. [Google Scholar] [CrossRef]
- Sharma, C.; Raza, M.A.; Purohit, S.D.; Pathak, P.; Gautam, S.; Corridon, P.R.; Han, S.S. Cellulose-based 3D printing bio-inks for biomedical applications: A review. Int. J. Biol. Macromol. 2025, 305, 141174. [Google Scholar] [CrossRef]
- Amin, L.; Jesmeen, T.; BishwajitSutradhar, K.; Mannan, A. Development and in vitro evaluation of diclofenac sodium loaded mucoadhesive microsphere with natural gum for sustained delivery. Curr. Drug Deliv. 2013, 10, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Baus, R.A.; Zahir-Jouzdani, F.; Dünnhaupt, S.; Atyabi, F.; Bernkop-Schnürch, A. Mucoadhesive hydrogels for buccal drug delivery: In vitro-in vivo correlation study. Eur. J. Pharm. Biopharm. 2019, 142, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Taj, M.B.; Khan, M.I.; Beagan, A.M.; Maria, A.; Shanableh, A. A TG-infused graphitic carbon nitride/cellulose nanohydrogel for controlled lornoxicam release kinetics. New J. Chem. 2025, 49, 3370–3386. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M.; Rad, M.M. TG biopolymer as reducing and stabilizing agent in biosonosynthesis of urchin-like ZnO nanorod arrays: A low cytotoxic photocatalyst with antibacterial and antifungal properties. Carbohydr. Polym. 2016, 136, 232–241. [Google Scholar] [CrossRef]
- Singh, B.; Dhiman, A.; Kumar, S. Designing silver nanoparticles impregnated acacia and TG based copolymeric hydrogels for drug delivery applications. Results Surf. Interfaces 2024, 16, 100256. [Google Scholar] [CrossRef]
- Szymańska, E.; Potaś, J.; Baranowski, M.; Czarnomysy, R.; Sulewska, M.E.; Basa, A.; Pietruska, M.; Bielawski, K.; Winnicka, K. Evaluation of Oromucosal Natural Gum-Based Emulgels as Novel Strategy for Photodynamic Therapy of Oral Premalignant Lesions. Pharmaceutics 2023, 15, 2512. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, L.; Han, W.; Gu, L.; Cui, X.; Wang, C. Novel deep eutectic solvent–hydrogel systems for synergistic transdermal delivery of Chinese herb medicine and local treatments for rheumatoid arthritis. Pharm. Res. 2022, 39, 2431–2446. [Google Scholar] [CrossRef]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Janani, N.; Zare, E.N.; Salimi, F.; Makvandi, P. Antibacterial TG-based nanocomposite films carrying ascorbic acid antioxidant for bioactive food packaging. Carbohydr. Polym. 2020, 247, 116678. [Google Scholar] [CrossRef]
- Nehra, A.; Biswas, D.; Siracusa, V.; Roy, S. Natural gum-based functional bioactive films and coatings: A Review. Int. J. Mol. Sci. 2022, 24, 485. [Google Scholar] [CrossRef]
- Pulatsu, E.; Xie, J.; Wang, Q.; Udenigwe, C.C. Edible films based on gum tragacanth and gelatin. Phys. Fluids 2025, 37, 047101. [Google Scholar] [CrossRef]
- Amani, F.; Rezaei, A.; Akbari, H.; Dima, C.; Jafari, S.M. Active packaging films made by complex coacervation of TG and gelatin loaded with curcumin; characterization and antioxidant activity. Foods 2022, 11, 3168. [Google Scholar] [CrossRef]
- Goudar, N.; Vanjeri, V.N.; Hiremani, V.D.; Gasti, T.; Khanapure, S.; Masti, S.P.; Chougale, R.B. Ionically crosslinked chitosan/TG-based polyelectrolyte complexes for antimicrobial biopackaging applications. J. Polym. Environ. 2022, 30, 2419–2434. [Google Scholar] [CrossRef]
- Bhathiwal, A.S.; Sharma, H.; Yadav, G.; Raghav, N.; Bendi, A. Biopolymers in active food packaging: Current Landscape and Future Directions. In Green Materials for Active Food Packaging; Springer Nature Singapore: Singapore, 2025; pp. 35–55. [Google Scholar]
- Tanwar, M.; Gupta, R.K.; Rani, A. Natural gums and their derivatives-based hydrogels: In biomedical, environment, agriculture, and food industry. Crit. Rev. Biotechnol. 2024, 44, 275–301. [Google Scholar] [CrossRef]
- Kumar, V.; Mittal, H.; Alhassan, S.M. Biodegradable hydrogels of TG polysaccharide to improve water retention capacity of soil and environment-friendly controlled release of agrochemicals. Int. J. Biol. Macromol. 2019, 132, 1252–1261. [Google Scholar]
- Fardood, S.T.; Atrak, K.; Ramazani, A. Green synthesis using TG and characterization of Ni–Cu–Zn ferrite nanoparticles as a magnetically separable photocatalyst for organic dyes degradation from aqueous solution under visible light. J. Mater.Sci. Mater.Electron. 2017, 28, 10739–10746. [Google Scholar] [CrossRef]
- Ramos, P.; Broncel, M. Influence of storage conditions on the stability of gum arabic and tragacanth. Molecules 2022, 27, 1510. [Google Scholar] [CrossRef] [PubMed]
- Shiroodi, S.G.; Mohammadifar, M.A.; Gorji, E.G.; Ezzatpanah, H.; Zohouri, N. Influence of gum tragacanth on the physicochemical and rheological properties of kashk. J. Dairy Res. 2012, 79, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Matini, A.; Naghib, S.M. Microwave-assisted natural gums for drug delivery systems: Recent progresses and advances over emerging biopolymers and technologies. Curr. Med. Chem. 2025, 32, 2547–2571. [Google Scholar] [CrossRef]
- Najafian, S.; Eskandani, M.; Derakhshankhah, H.; Jaymand, M.; Massoumi, B. Extracellular matrix-mimetic electrically conductive nanofibrous scaffolds based on polyaniline-grafted TG and poly (vinyl alcohol) for skin tissue engineering application. Int. J. Biol. Macromol. 2023, 249, 126041. [Google Scholar] [CrossRef]
- Patel, R.; Trivedi, R.; Raj, M.; Raj, L. A brief review of polymeric blends based on natural polymers and synthetic thermoplastics polymers. Chem. Pap. 2024, 78, 665–697. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A review of natural polysaccharides: Sources, characteristics, properties, food, and pharmaceutical applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Dey, N.; Bhardwaj, S.; Maji, P.K. Recent breakthroughs in the valorization of lignocellulosic biomass for advancements in the construction industry: A Review. RSC Sustain. 2025, 3, 3307–3357. [Google Scholar] [CrossRef]
- Kaushik, R.; Upadhyaya, K.; Sharma, P. A comprehensive review of Ayurveda Aahar: Regulations, challenges, and future prospects. J. Drug Res. Ayurvedic Sci. 2025, 10, 181–188. [Google Scholar] [CrossRef]
- Eldigair, H. Quality Control Systems of Gum Arabic in Sudan. Doctoral Dissertation, University of Chester, Chester, UK, 2018. [Google Scholar]
- Yele, S.; Deshpande, A.; Salgar, K.; Kalaskar, M. Pharmaceutical aids of natural origin. In Pharmacognosy and Phytochemistry: Principles, Techniques, and Clinical Applications; Wiley: Hoboken, NJ, USA, 2025; pp. 273–298. [Google Scholar]
- Javadpour, M.; Hosseini, E.; Nateghi, L.; Bazrafshan, S. Enhancing margarine oxidative stability, antioxidant retention, and sensory quality via tragacanth-chitosan hydrogel microencapsulation of supercritical CO2-extracted green coffee. Food Chem. 2025, 28, 102580. [Google Scholar] [CrossRef] [PubMed]
- Golshirazi, A.; Labbaf, S.; Varshosaz, J. Optimised GelMA/tragacanth gum hydrogel loaded with vanillic acid for biomedical applications. Int. J. Biol. Macromol. 2025, 311, 143535. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogra, S.; Yadav, D.; Koul, B.; Rabbee, M.F. Plant-Based Polysaccharide Gums as Sustainable Bio-Polymers: Focus on Tragacanth Gum and Its Emerging Applications. Polymers 2025, 17, 3163. https://doi.org/10.3390/polym17233163
Dogra S, Yadav D, Koul B, Rabbee MF. Plant-Based Polysaccharide Gums as Sustainable Bio-Polymers: Focus on Tragacanth Gum and Its Emerging Applications. Polymers. 2025; 17(23):3163. https://doi.org/10.3390/polym17233163
Chicago/Turabian StyleDogra, Shivani, Dhananjay Yadav, Bhupendra Koul, and Muhammad Fazle Rabbee. 2025. "Plant-Based Polysaccharide Gums as Sustainable Bio-Polymers: Focus on Tragacanth Gum and Its Emerging Applications" Polymers 17, no. 23: 3163. https://doi.org/10.3390/polym17233163
APA StyleDogra, S., Yadav, D., Koul, B., & Rabbee, M. F. (2025). Plant-Based Polysaccharide Gums as Sustainable Bio-Polymers: Focus on Tragacanth Gum and Its Emerging Applications. Polymers, 17(23), 3163. https://doi.org/10.3390/polym17233163








