Early Cell Adhesion of Periodontal Ligament Stem Cells on 3D Printed Polylactic Acid/Hydroxyapatite Scaffolds: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining the Material
2.2. Sample Preparation
2.3. Cell Culture
2.4. Cell Seeding
2.4.1. Seeding of Wells for the Study of Adhesion and Cell Morphology
2.4.2. Seeding of Wells for Viability and Apoptosis Studies
2.5. Sample Processing
2.5.1. Adhesion and Cell Morphology Test
2.5.2. Cell Viability Assay (MTT)
2.5.3. Apoptosis or Cell Death Assay
2.6. Image Analysis
2.7. Statistical Analysis
3. Results
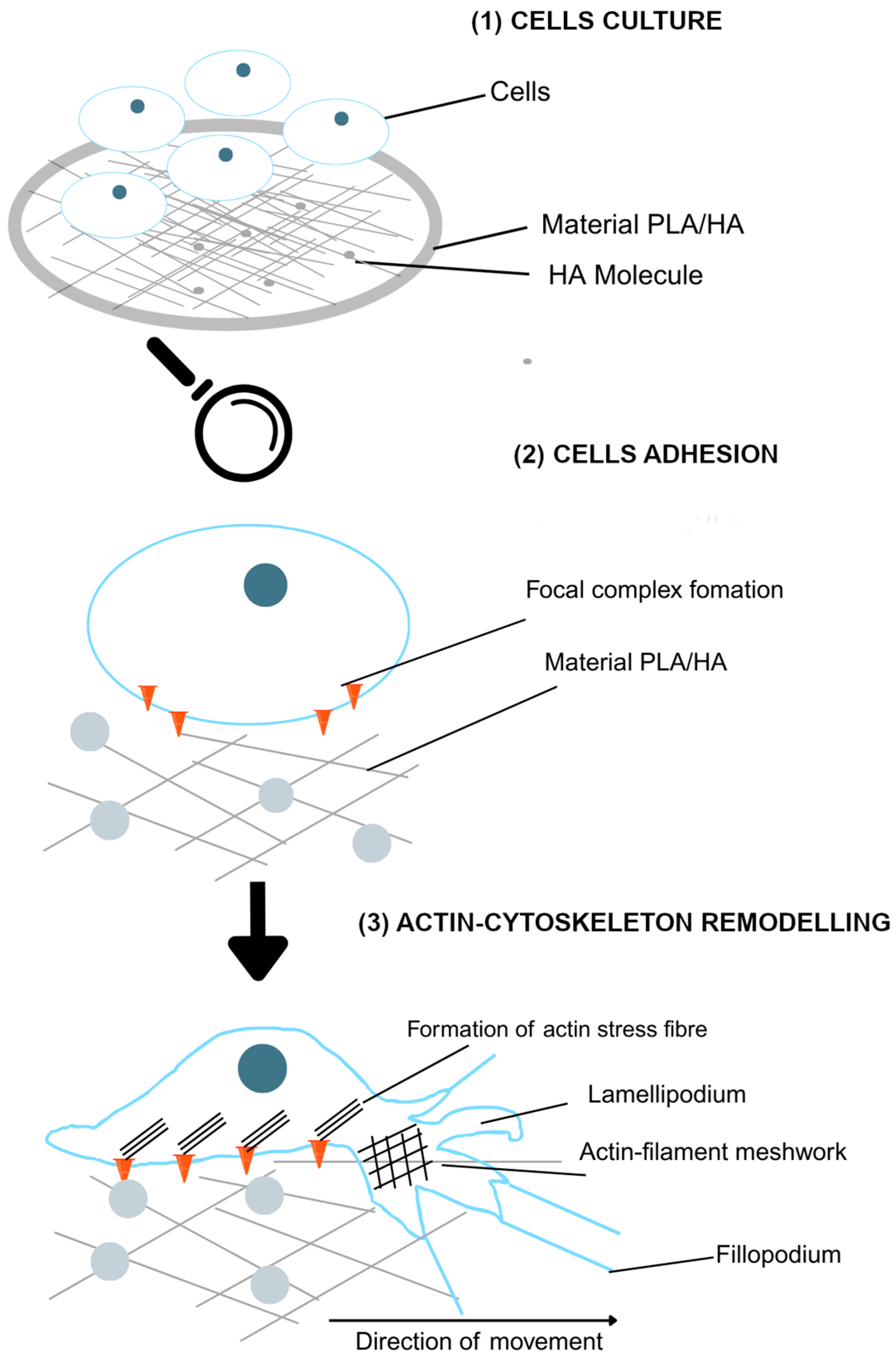
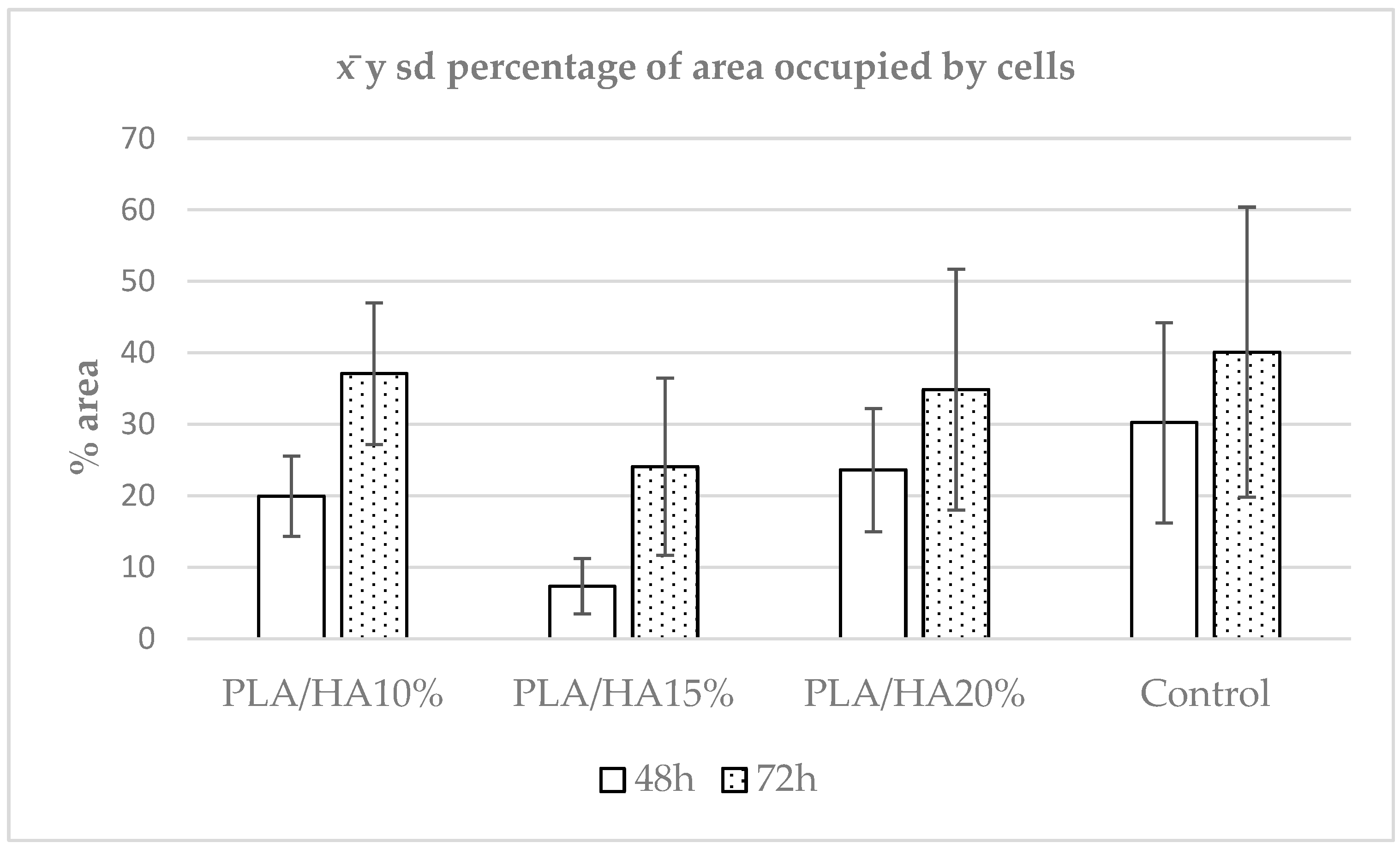
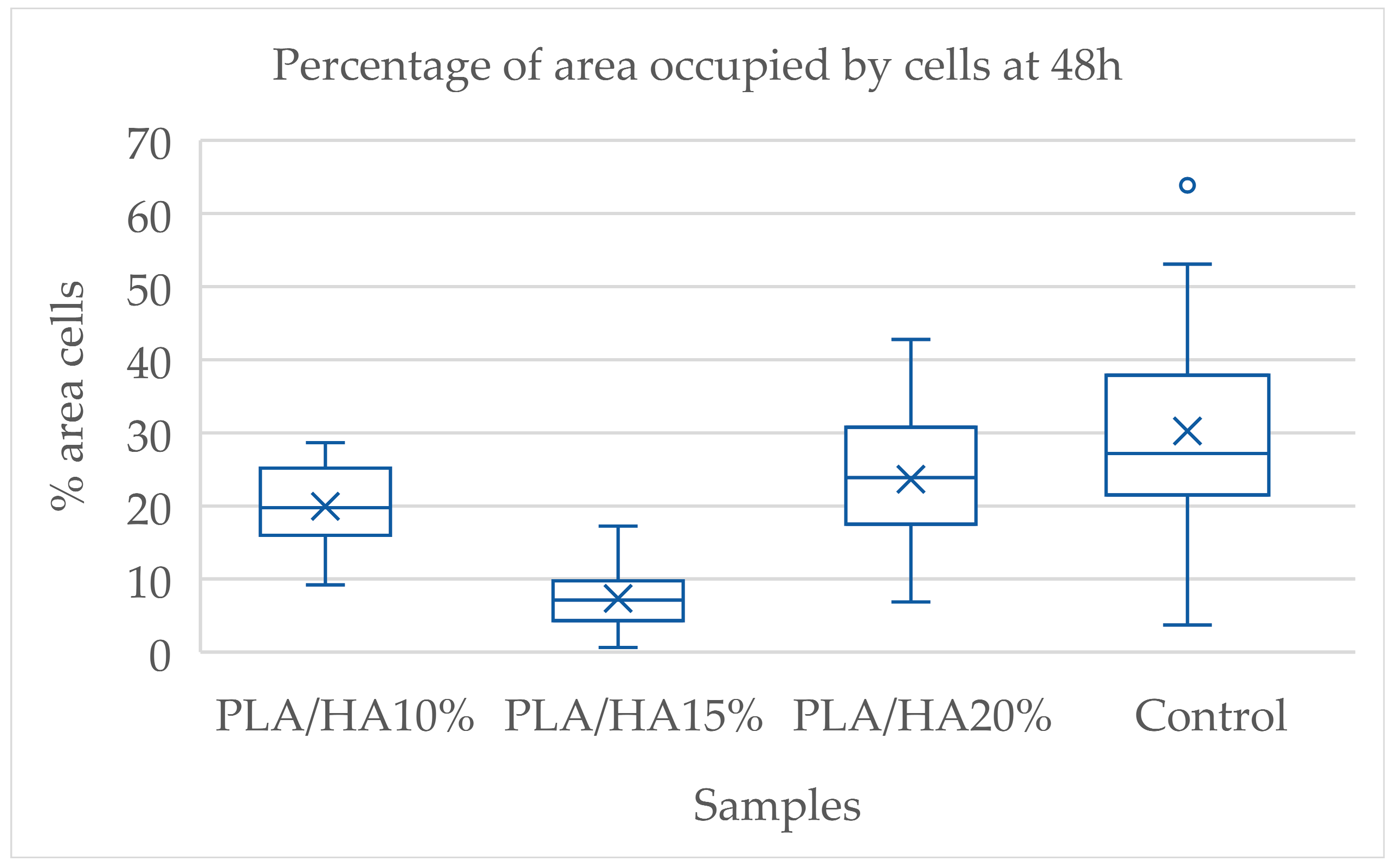
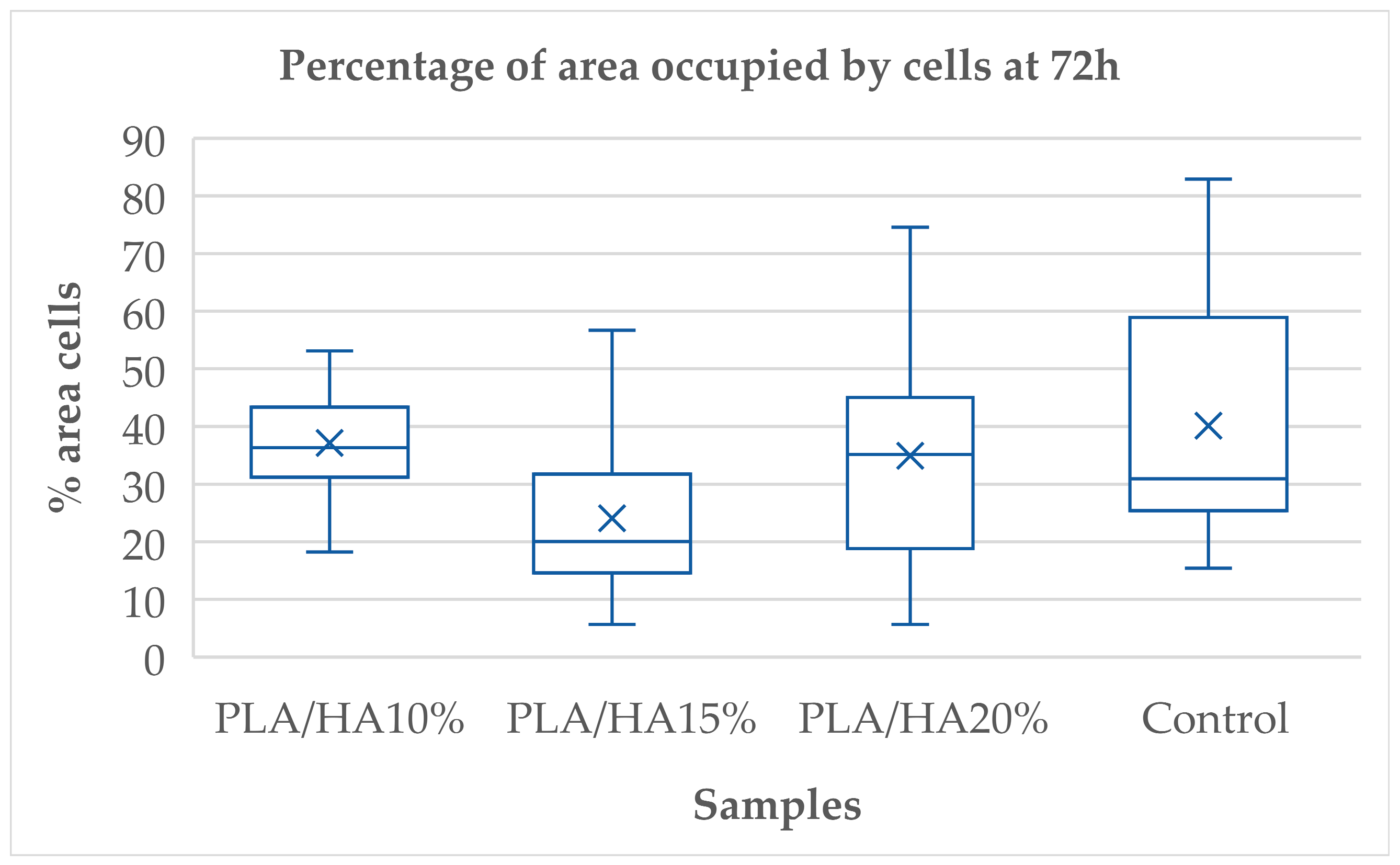
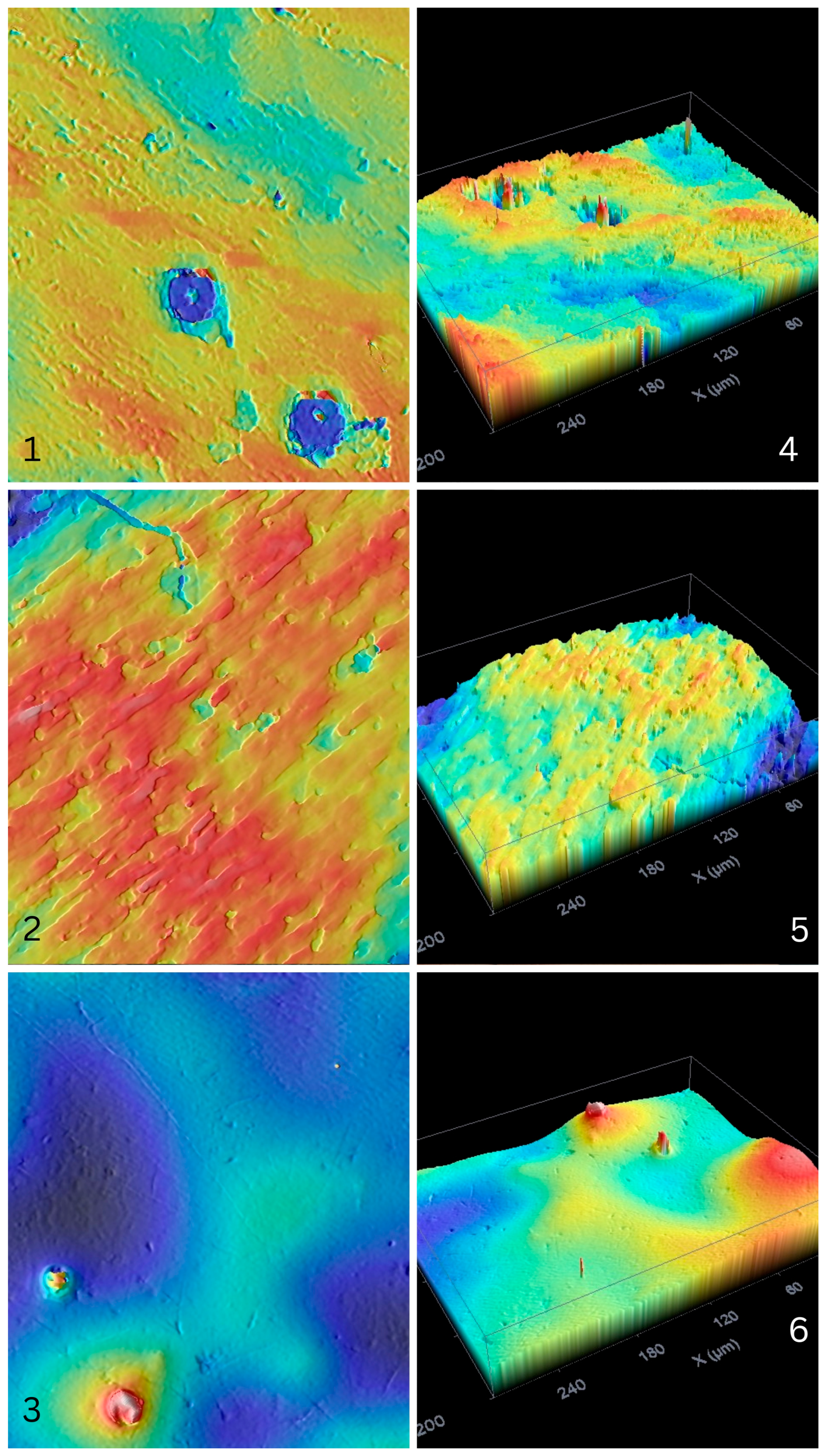
3.1. Cell Adhesion
3.2. Morphological Analysis
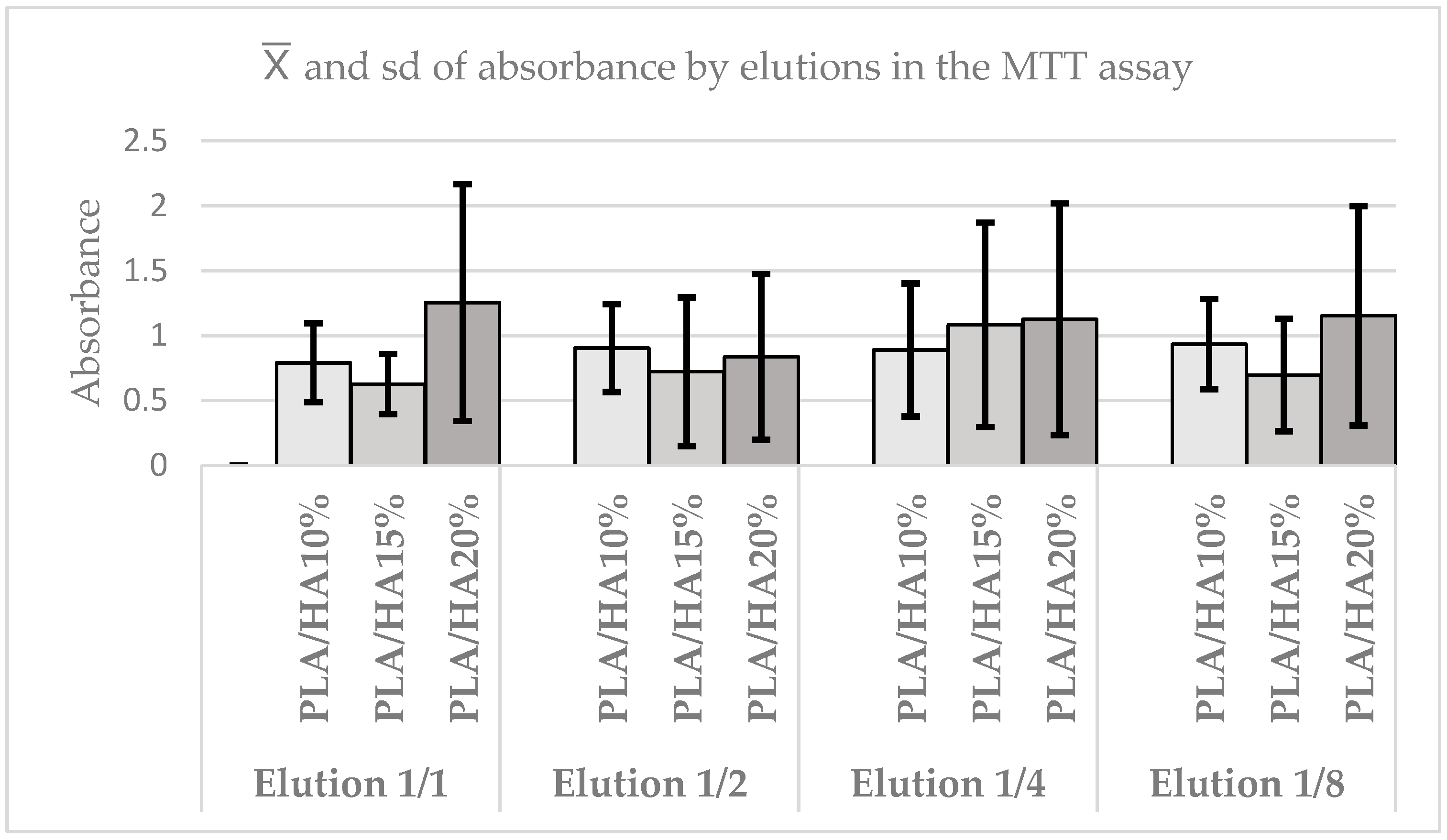
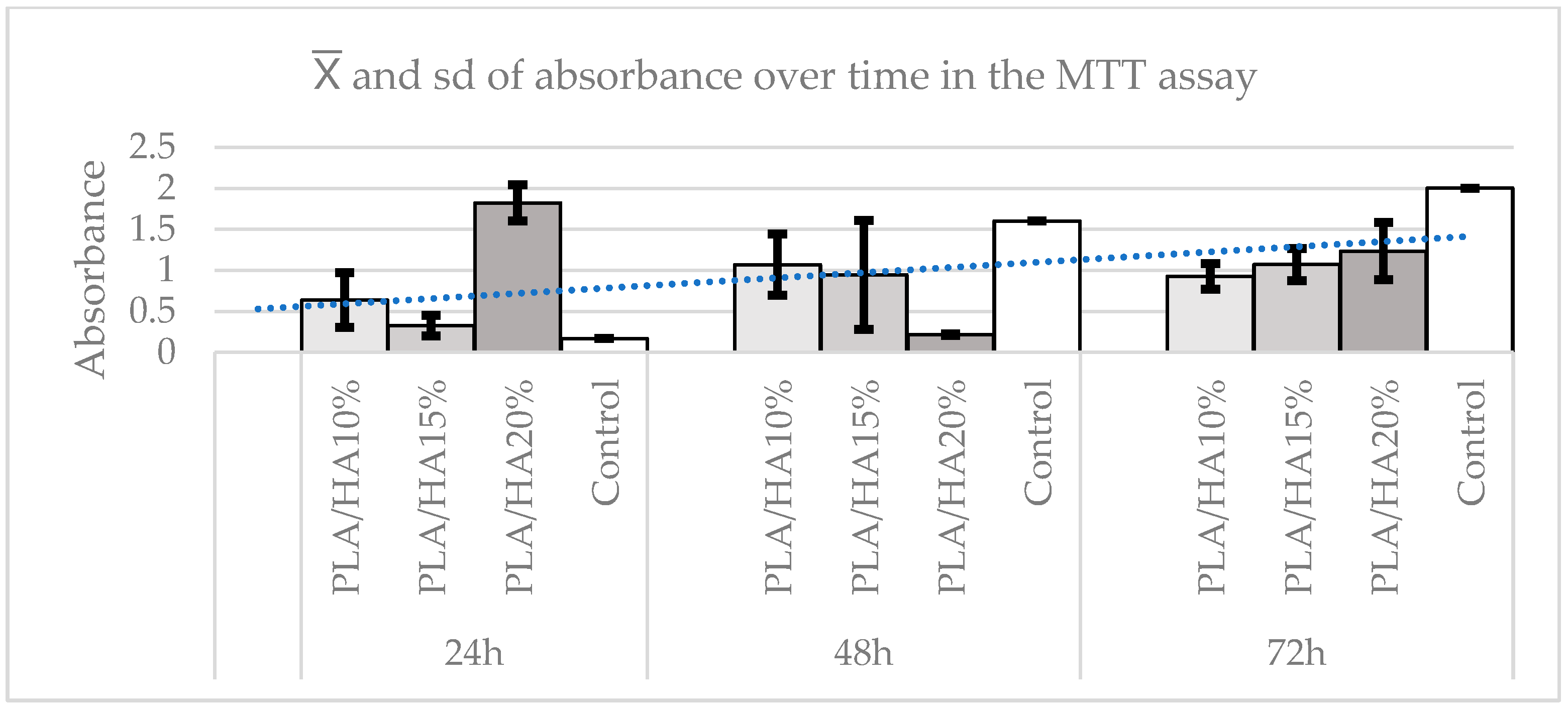
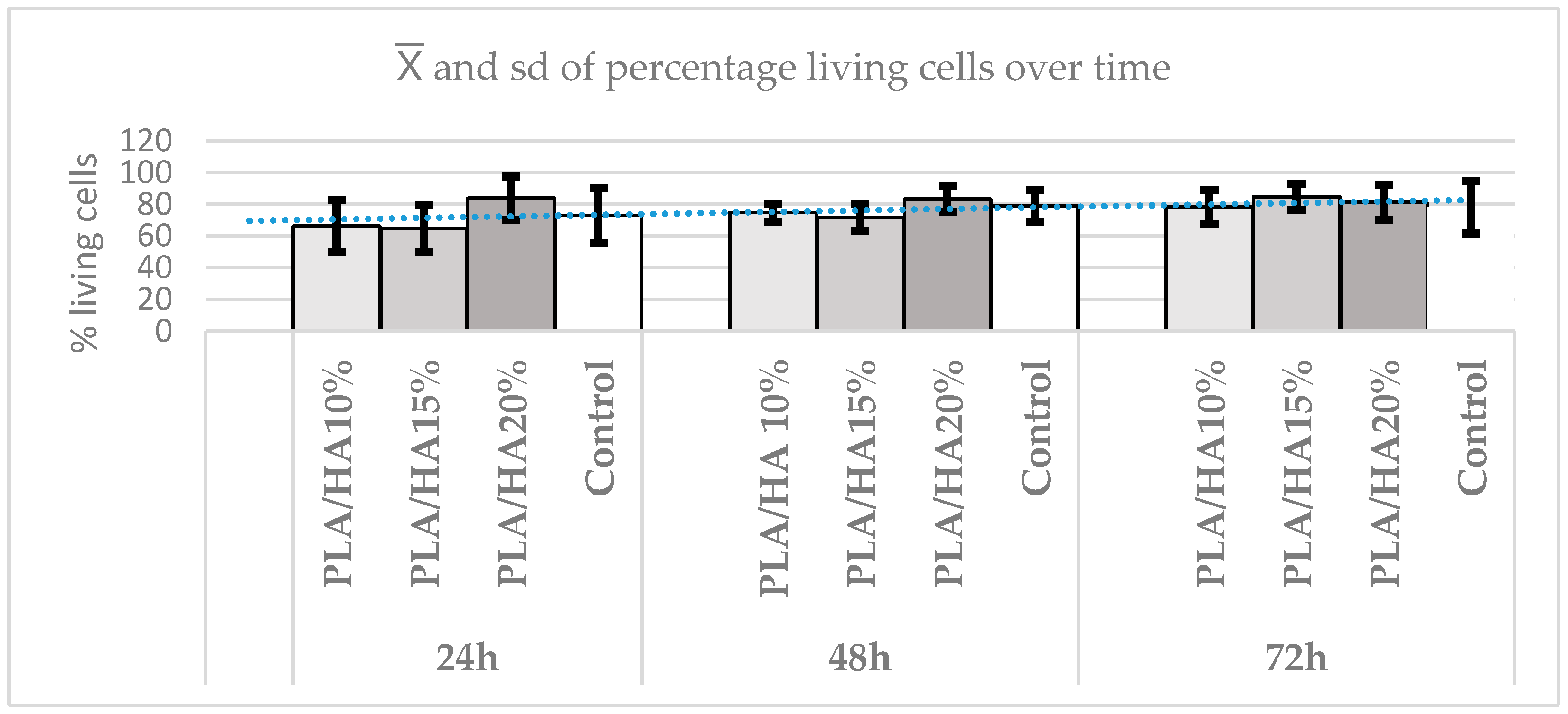
3.3. Cell Viability Assay Using MTT
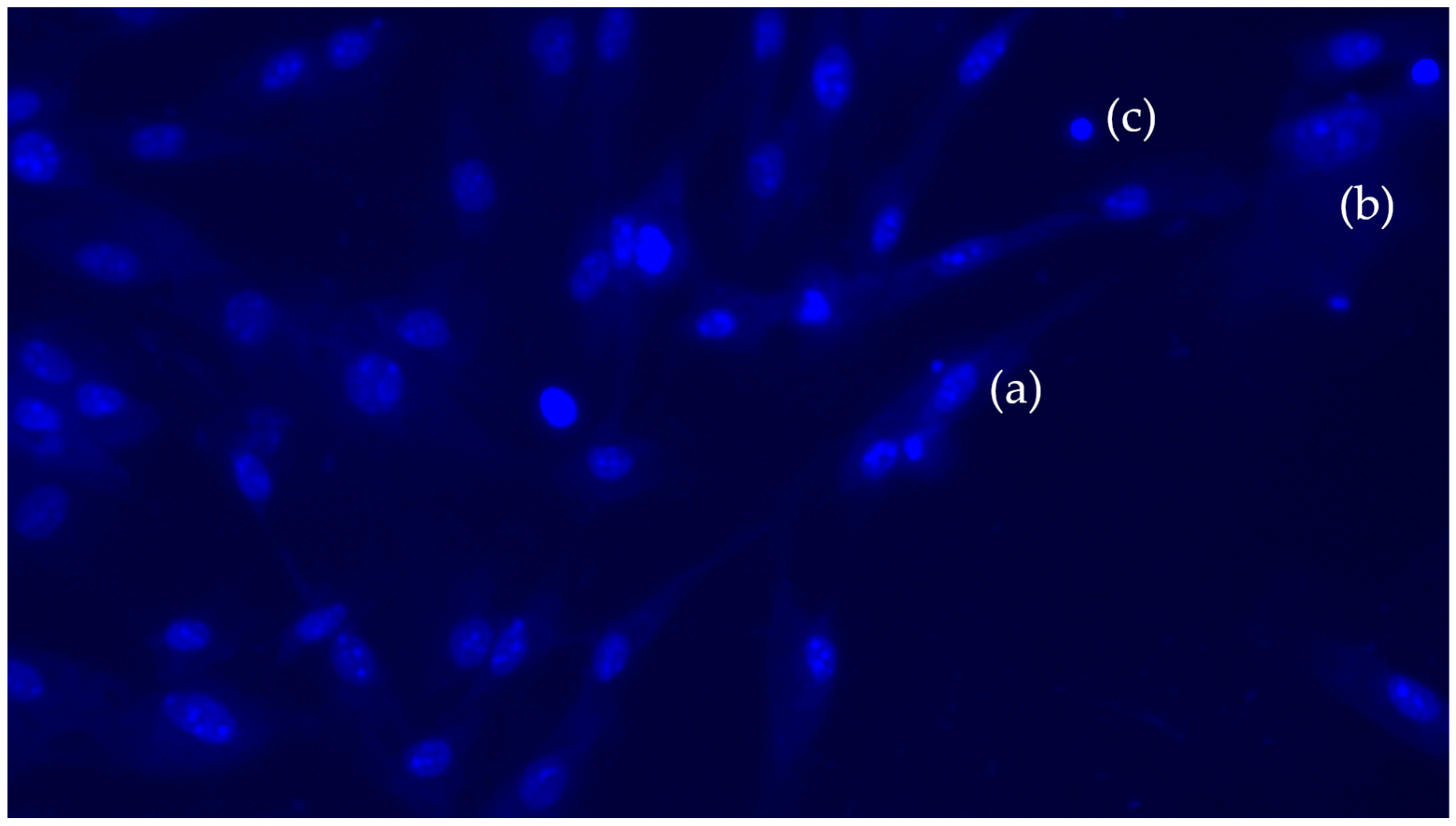
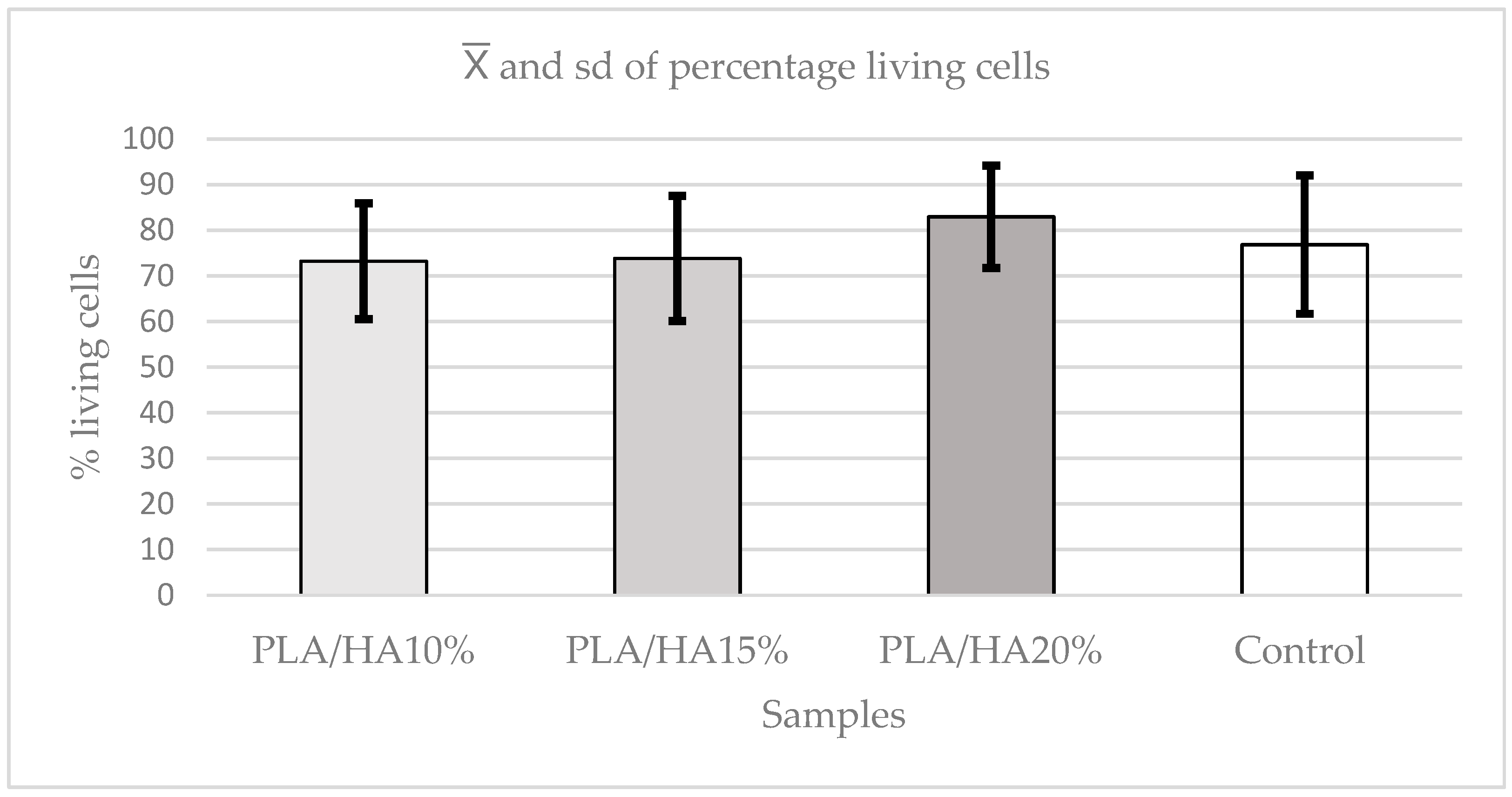
3.4. Apoptosis or Cell Death Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLDSC | Periodontal ligament stem cells |
| HAS | Hydroxyapatite |
| PLA | Polylactic acid |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMEM | Dulbecco modified Eagle medium |
| DMSO | Dimethyl sulfoxide |
References
- Zhou, X.; Zhou, G.; Junka, R.; Chang, N.; Anwar, A.; Wang, H.; Yu, X. Fabrication of polylactic acid (PLA)-based porous scaffold through the combination of traditional bio-fabrication and 3D printing technology for bone regeneration. Colloids Surf. B Biointerfaces 2021, 197, 111420. [Google Scholar] [CrossRef]
- Castro, J.I.; Valencia Llano, C.H.; Tenorio, D.L.; Saavedra, M.; Zapata, P.; Navia-Porras, D.P.; Delgado-Ospina, J.; Chaur, M.N.; Hernández, J.H.M.; Grande-Tovar, C.D. Biocompatibility Assessment of Polylactic Acid (PLA) and Nanobioglass (n-BG) Nanocomposites for Biomedical Applications. Molecules 2022, 27, 3640. [Google Scholar] [CrossRef]
- Dinatale, E.; Guercio, E. Guided bone regeneration. Literature review. Acta Odontol. Venez. 2012, 46, 554–561. [Google Scholar]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Davila, S.; Garrido-Gulías, N.; González-Rodríguez, L.; López-Álvarez, M.; Serra, J.; López-Periago, J.E.; González, P. Physicochemical Properties of 3D-Printed Polylactic Acid/Hydroxyapatite Scaffolds. Polymers 2023, 15, 2849. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Z.; Cheng, L.; Gao, X.; Sun, J.; Chen, L. Improvement of the mechanical properties and osteogenic activity of 3D-printed polylactic acid porous scaffolds by nano-hydroxyapatite and nano-magnesium oxide. Heliyon 2022, 8, e09748. [Google Scholar] [CrossRef] [PubMed]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M. Effects of surface modification on dispersion, mechanical, thermal and dynamic mechanical properties of injection molded PLA-hydroxyapatite composites. Compos. Part A Appl. Sci. Manuf. 2017, 103, 96–105. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Hlushchenko, V.; Ivakhniuk, T.; Oleshko, T.; Berladir, K.; Smiyanov, V.; Oleshko, O. Modern approaches and possibilities of application of 3D modeling for tissue engineering and bone regeneration. Literature review. East. Ukr. Med. J. 2023, 11, 337–351. [Google Scholar] [CrossRef]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-Dimensional Printed Multiphase Scaffolds for Regeneration of Periodontium Complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhao, N.; Wang, L.; Yu, M.; Liu, H.; Song, A.; Huang, J.; Wang, G. Bone repair by periodontal ligament stem cellseeded nanohydroxyapatite-chitosan scaffold. Int. J. Nanomed. 2012, 7, 5405–5414. [Google Scholar] [CrossRef]
- Campos-Bijit, V.; Inostroza, N.C.; Orellana, R.; Rivera, A.; Von Marttens, A.; Cortez, C.; Covarrubias, C. Influence of Topography and Composition of Commercial Titanium Dental Implants on Cell Adhesion of Human Gingiva-Derived Mesenchymal Stem Cells: An In Vitro Study. Int. J. Mol. Sci. 2023, 4, 16686. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Cavalcanti, M.; Trubiani, O.; Diomede, F. MicroRNA 210 Mediates VEGF Upregulation in Human Periodontal Ligament Stem Cells Cultured on 3DHydroxyapatite Ceramic Scaffold. Int. J. Mol. Sci. 2018, 19, 3916. [Google Scholar] [CrossRef]
- Neshati, Z.; Bahrami, A.R.; Eshtiagh-Hosseini, H.; Matin, M.M.; Housaindokht, M.R.; Tabari, T.; Edalatmanesh, M.A. Evaluating the biodegradability of Gelatin/Siloxane/Hydroxyapatite (GS-Hyd) complex in vivo and its ability for adhesion and proliferation of rat bone marrow mesenchymal stem cells. Cytotechnology 2012, 64, 485–495. [Google Scholar] [CrossRef]
- Ghayor, C.; Bhattacharya, I.; Guerrero, J.; Özcan, M.; Weber, F.E. 3D-Printed HA-Based Scaffolds for Bone Regeneration: Microporosity, Osteoconduction and Osteoclastic Resorption. Materials 2022, 15, 1433. [Google Scholar] [CrossRef]
- Kang, J.; Zheng, J.; Hui, Y.; Li, D. Mechanical Properties of 3D-Printed PEEK/HA Composite Filaments. Polymers 2022, 14, 4293. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yu, J.; Cao, J.; E, L.; Wang, D.; Zhang, H.; Liu, H. Biocompatibility and Osteogenic Capacity of Periodontal Ligament Stem Cells on nHAC/PLA and HA/TCP Scaffolds. J. Biomater. Sci. Polym. Ed. 2011, 22, 179–194. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef]
- Al Rahraway, M. Hydroxyapatite as a Biomaterial to Improve StemCell-Related Tissue Engineering. Biomed. J. Sci. Technol. Res. 2020, 25, 19209–19226. [Google Scholar] [CrossRef]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef] [PubMed]
- Marrella, A.; Tedeschi, G.; Giannoni, P.; Lagazzo, A.; Sbrana, F.; Barberis, F.; Quarto, R.; Puglisi, F.; Scaglione, S. “Green-reduced” graphene oxide induces in vitro an enhanced biomimetic mineralization of polycaprolactone electrospun meshes. Mater. Sci. Eng. C 2018, 93, 1044–1053. [Google Scholar] [CrossRef]
- Balaji Raghavendran, H.R.; Puvaneswary, S.; Talebian, S.; Raman Murali, M.; Vasudevaraj Naveen, S.; Krishnamurithy, G.; McKean, R.; Kamarul, T. A Comparative Study on In Vitro Osteogenic Priming Potential of Electron Spun Scaffold PLLA/HA/Col, PLLA/HA, and PLLA/Col for Tissue Engineering Application. PLoS ONE 2014, 9, e104389. [Google Scholar] [CrossRef] [PubMed]
- Frone, A.N.; Batalu, D.; Chiulan, I.; Oprea, M.; Gabor, A.R.; Nicolae, C.A.; Raditoiu, V.; Trusca, R.; Panaitescu, D.M. Morpho-Structural, Thermal and Mechanical Properties of PLA/PHB/Cellulose Biodegradable Nanocomposites Obtained by Compression Molding, Extrusion, and 3D Printing. Nanomaterials 2019, 10, 51. [Google Scholar] [CrossRef]
- He, J.; Hu, X.; Cao, J.; Zhang, Y.; Xiao, J.; Peng, L.; Chen, D.; Xiong, C.; Zhang, L. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Carbohydr. Polym. 2021, 253, 117198. [Google Scholar] [CrossRef]
- Xu, J.; Xu, P.; Li, Z.; Huang, J.; Yang, Z. Oxidative stress and apoptosis induced by hydroxyapatite nanoparticles in C6 cells. J. Biomed. Mater. Res. A 2012, 100, 738–745. [Google Scholar] [CrossRef]


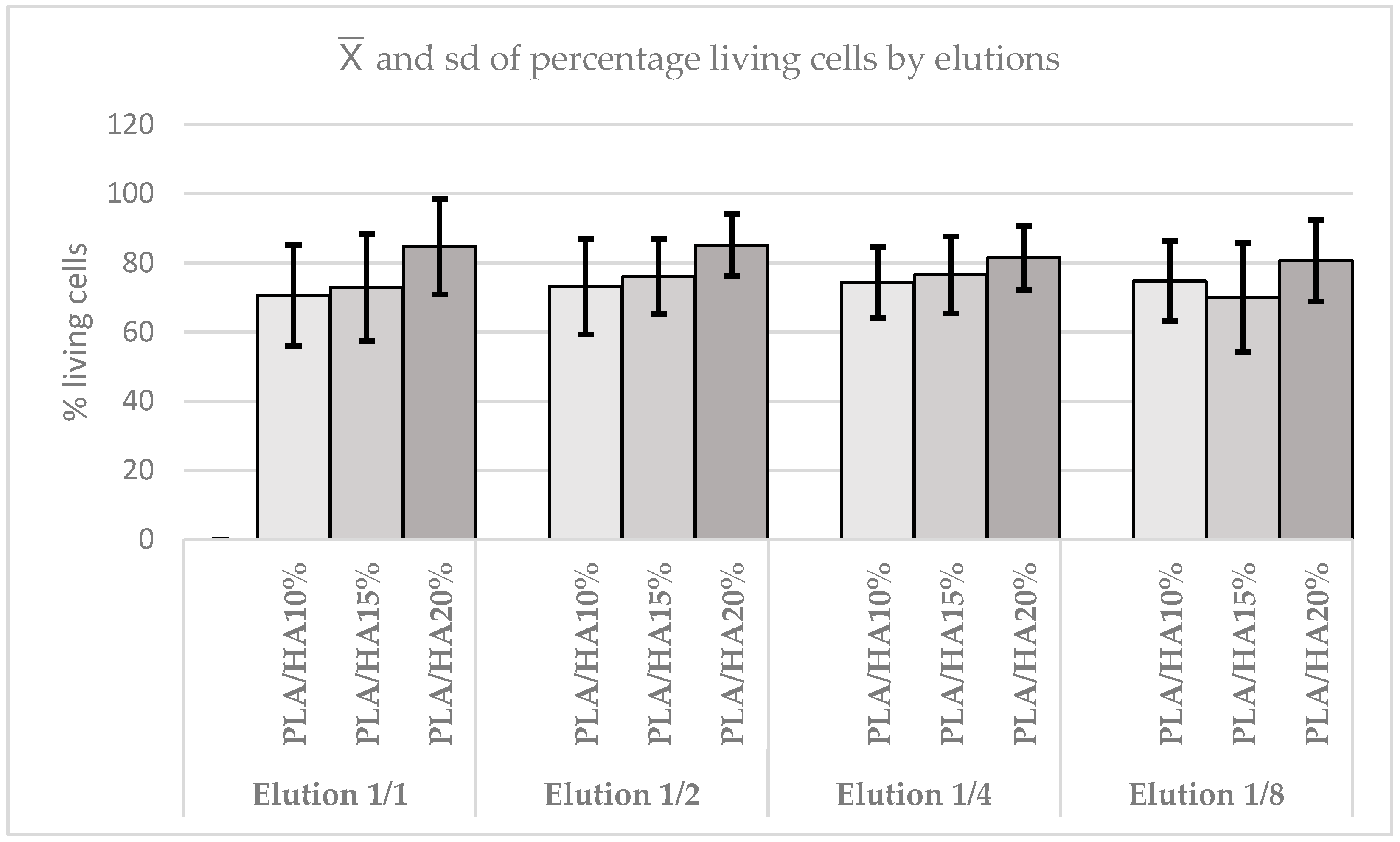
| p Value | PLA/HA 10% | PLA/HA 15% | PLA/HA 20% |
| PLA/HA 15% | 0.0001 | ||
| PLA/HA 20% | 0.6130 | 0.0001 | |
| Control group | 0.0287 | 0.0001 | 0.5593 |
| p Value | PLA/HA 10% | PLA/HA 15% | PLA/HA 20% |
| PLA/HA 15% | 0.0012 | ||
| PLA/HA 20% | 0.9691 | 0.0133 | |
| Control group | 1.0000 | 0.0017 | 1.0000 |
| Group | Topography | Wetting | |||
|---|---|---|---|---|---|
| Ra (µm) | Rq (µm) | Rsk (-) | Kurtosis (-) | Contact Angle (°) | |
| PLA/HA 10 PLA/HA 15 PLA/HA 20 | 2.1 (0.6) a 1.7 (0.1) c 1.1 (0.2) b | 2.6 (0.8) a 2.1 (0.3) a 1.4 (0.3) b | −0.2 (1) a −0.1 (0.9) a 0.4 (0.9) a | 6 (2) a 3 (1) b 7 (3) a | 40 (2) a 48 (3) c 30 (2) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Belmonte, I.; Montero, J.; Guerrero-González, E.; Munteanu, A.; Pérez-Fernández, V.; Pérez-Silva, A.; Martínez-Cánovas, A. Early Cell Adhesion of Periodontal Ligament Stem Cells on 3D Printed Polylactic Acid/Hydroxyapatite Scaffolds: An In Vitro Study. Polymers 2025, 17, 3088. https://doi.org/10.3390/polym17223088
Serrano-Belmonte I, Montero J, Guerrero-González E, Munteanu A, Pérez-Fernández V, Pérez-Silva A, Martínez-Cánovas A. Early Cell Adhesion of Periodontal Ligament Stem Cells on 3D Printed Polylactic Acid/Hydroxyapatite Scaffolds: An In Vitro Study. Polymers. 2025; 17(22):3088. https://doi.org/10.3390/polym17223088
Chicago/Turabian StyleSerrano-Belmonte, Ildefonso, Javier Montero, Elena Guerrero-González, Alexandra Munteanu, Virginia Pérez-Fernández, Amparo Pérez-Silva, and Ascensión Martínez-Cánovas. 2025. "Early Cell Adhesion of Periodontal Ligament Stem Cells on 3D Printed Polylactic Acid/Hydroxyapatite Scaffolds: An In Vitro Study" Polymers 17, no. 22: 3088. https://doi.org/10.3390/polym17223088
APA StyleSerrano-Belmonte, I., Montero, J., Guerrero-González, E., Munteanu, A., Pérez-Fernández, V., Pérez-Silva, A., & Martínez-Cánovas, A. (2025). Early Cell Adhesion of Periodontal Ligament Stem Cells on 3D Printed Polylactic Acid/Hydroxyapatite Scaffolds: An In Vitro Study. Polymers, 17(22), 3088. https://doi.org/10.3390/polym17223088







