Effects of Stoichiometric Variations in L-Arginine-Cured Epoxy Resins
Abstract
1. Introduction
2. Materials and Methods
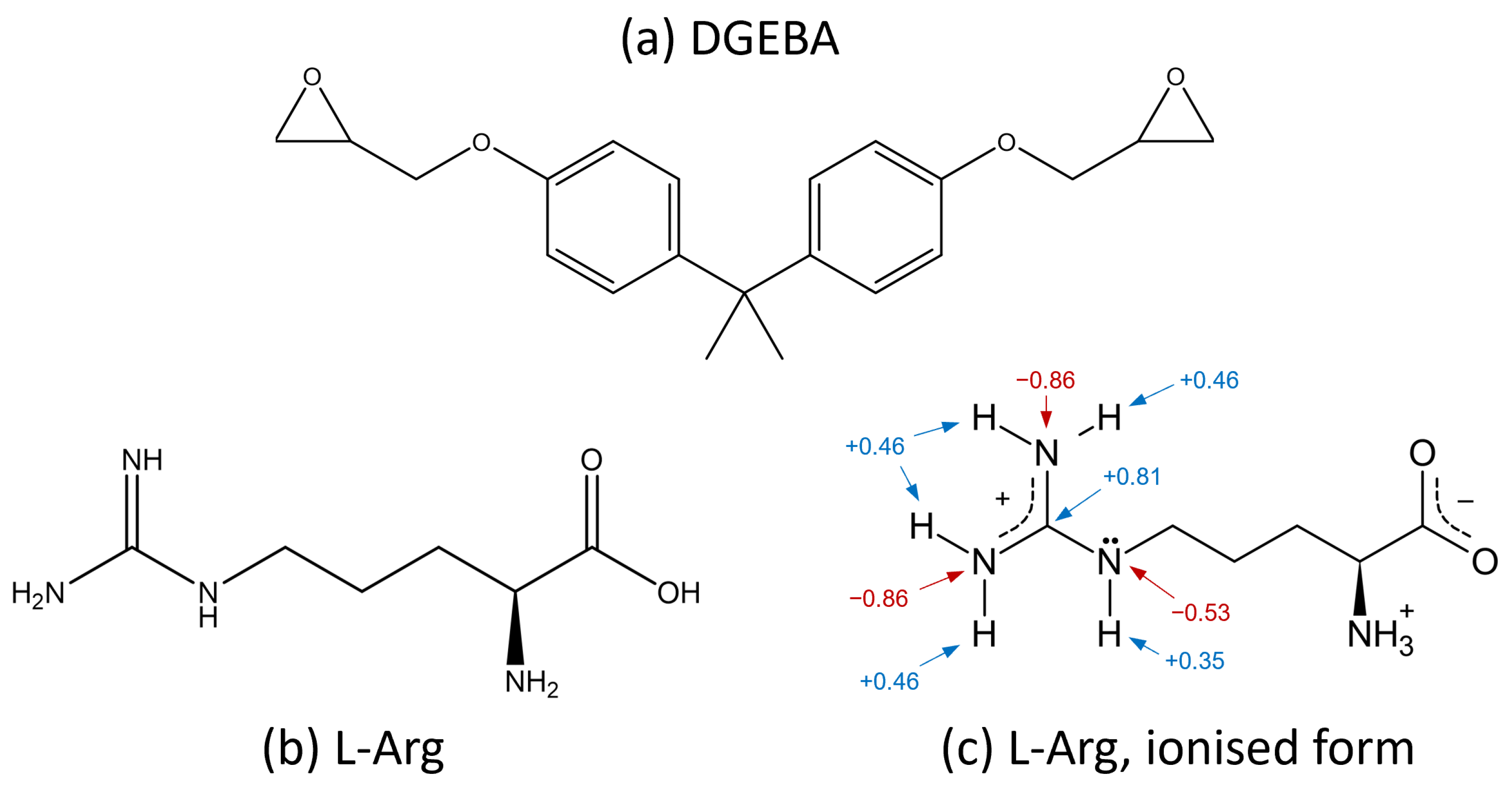
2.1. Materials and Stoichiometric Variations
2.2. Manufacturing and Testing
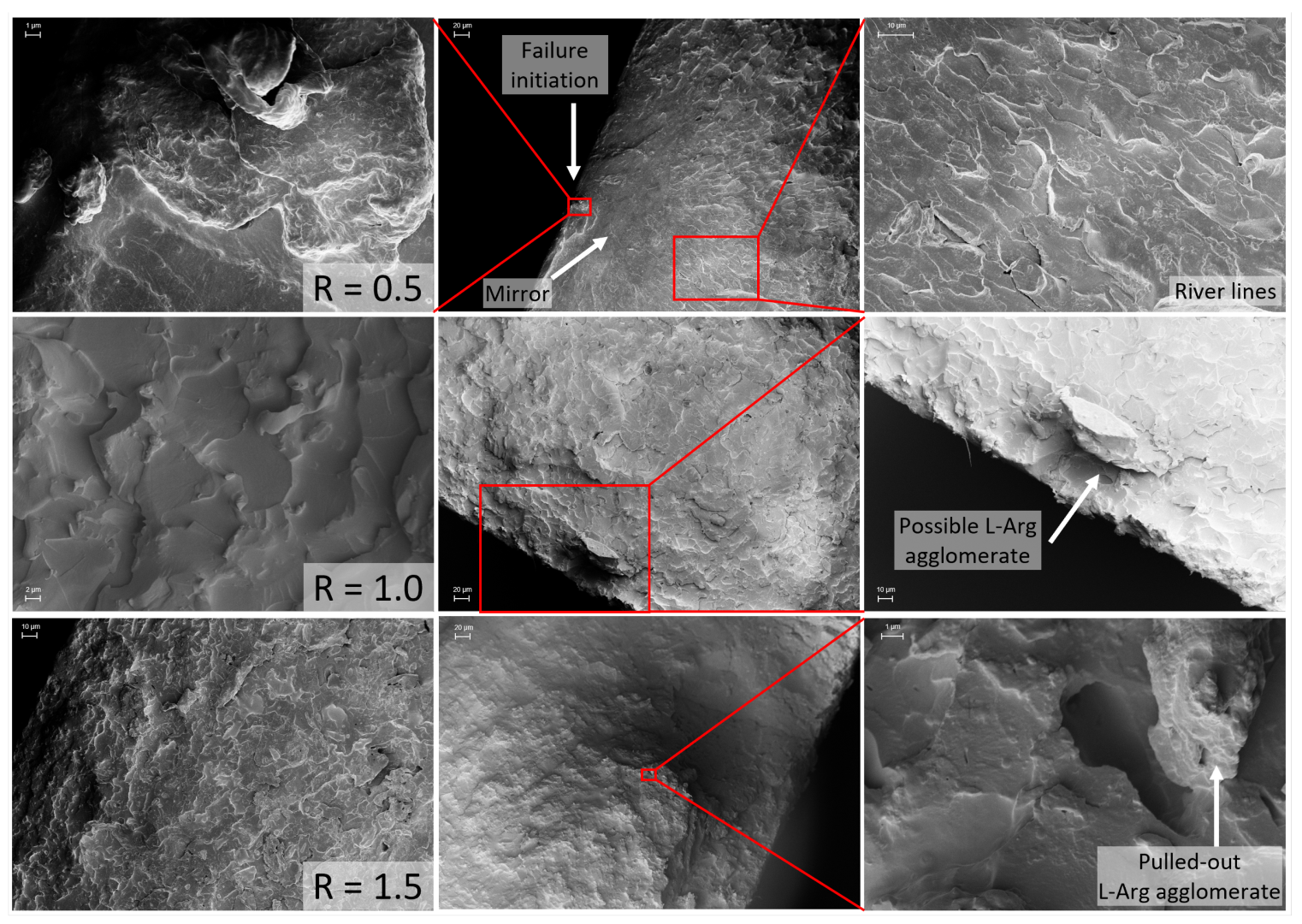
2.3. Fracture Surface Analysis
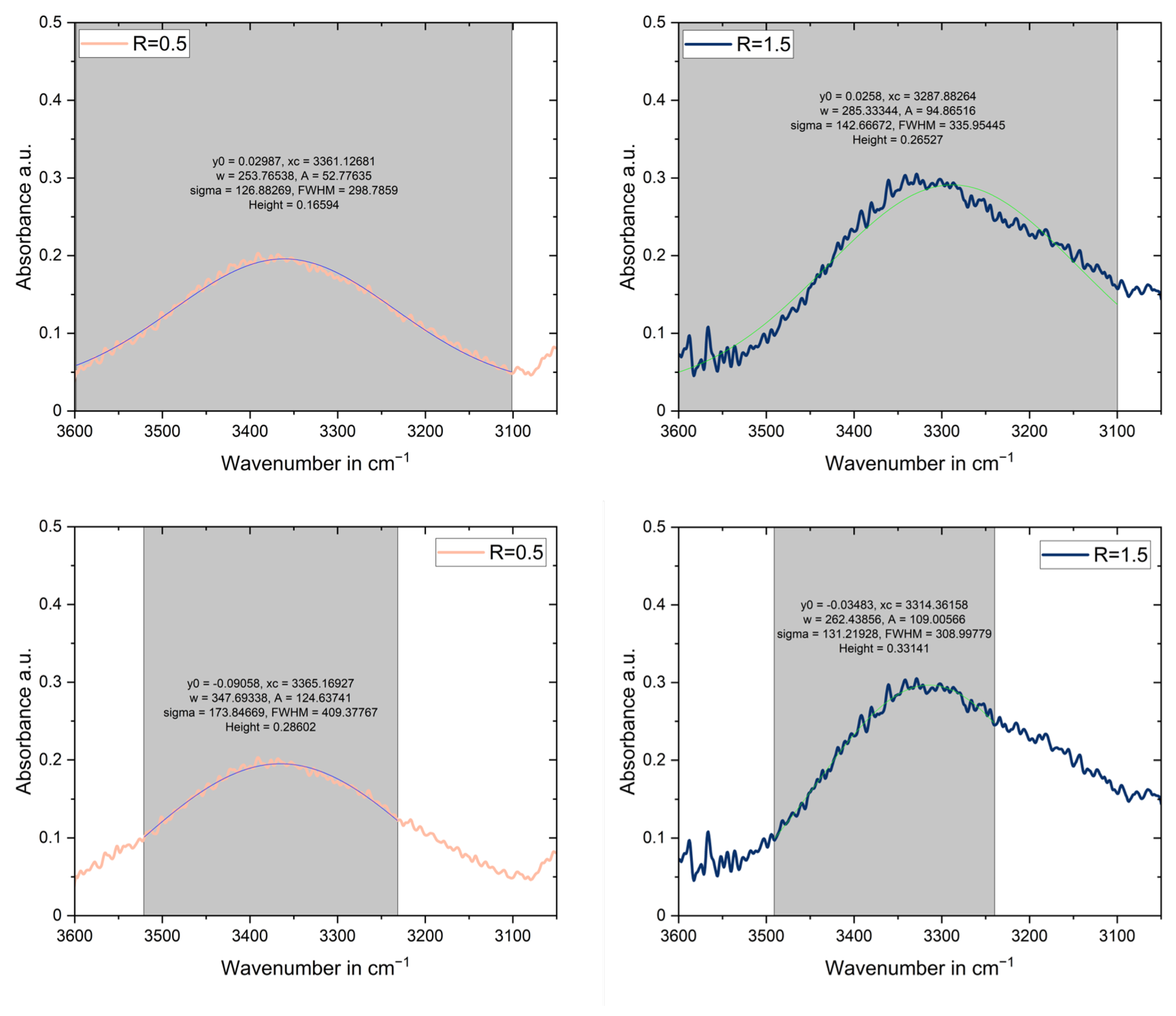
2.4. Fourier Transform Infrared Spectroscopy
3. Results and Discussion
3.1. Optical Examination
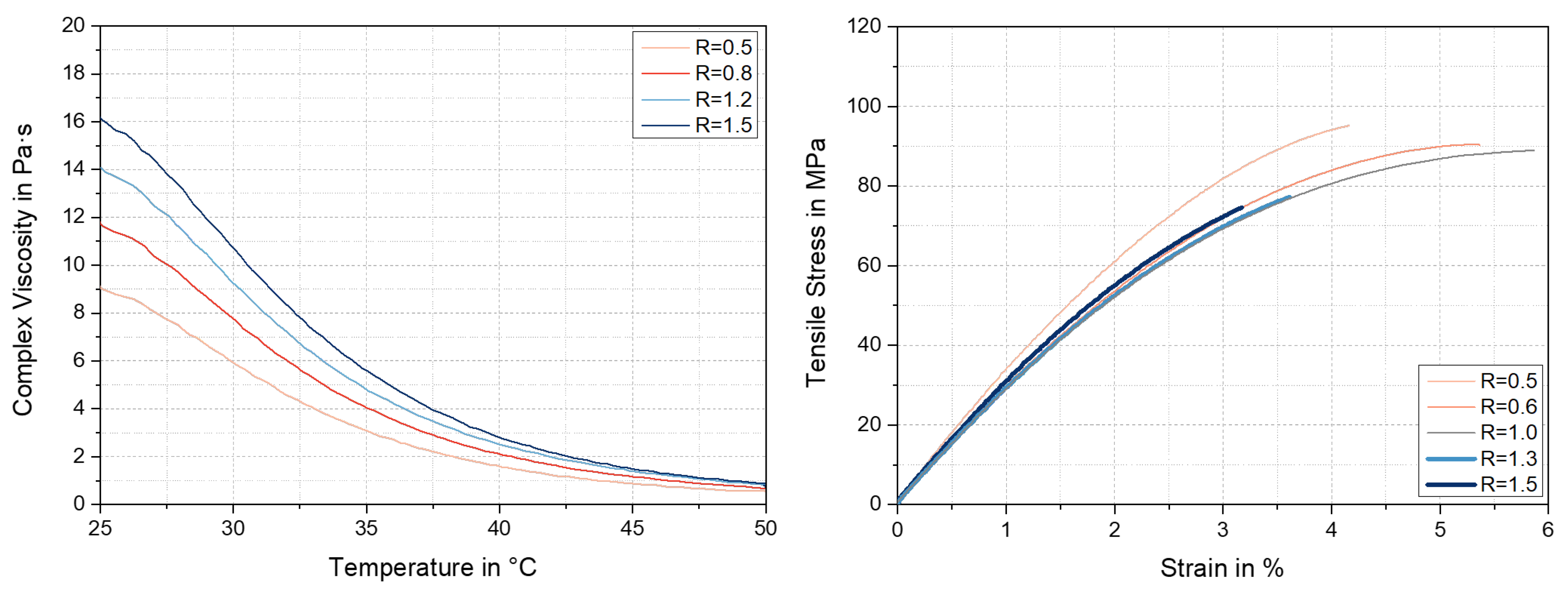
3.2. Rheological Characterisation
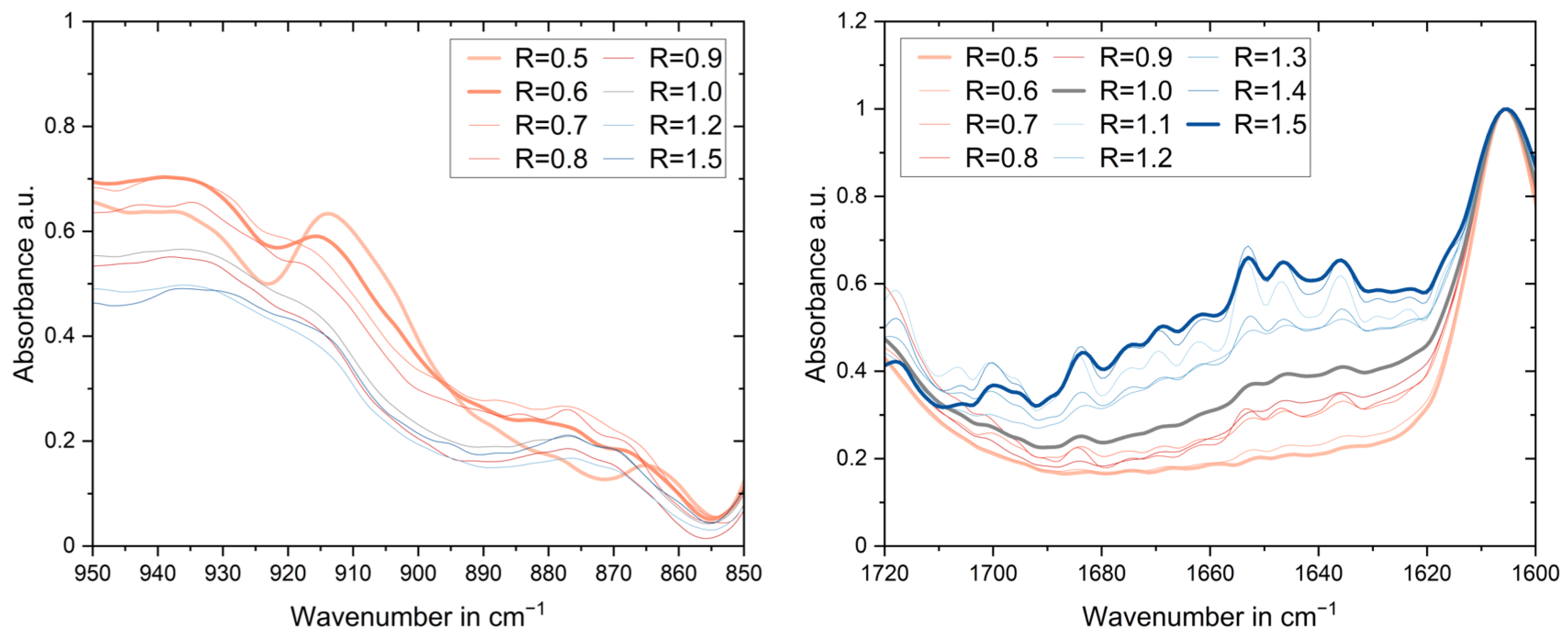
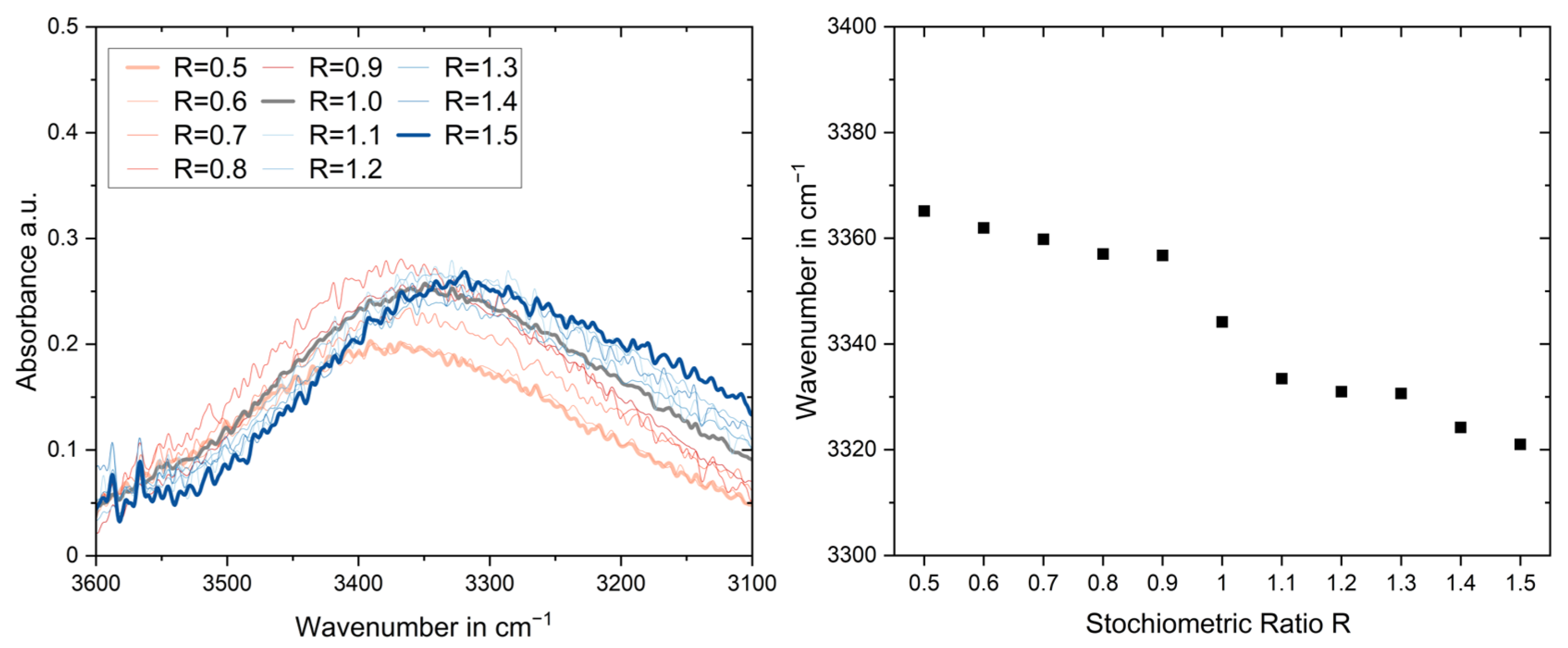
3.3. Fourier Transform Infrared Spectroscopy
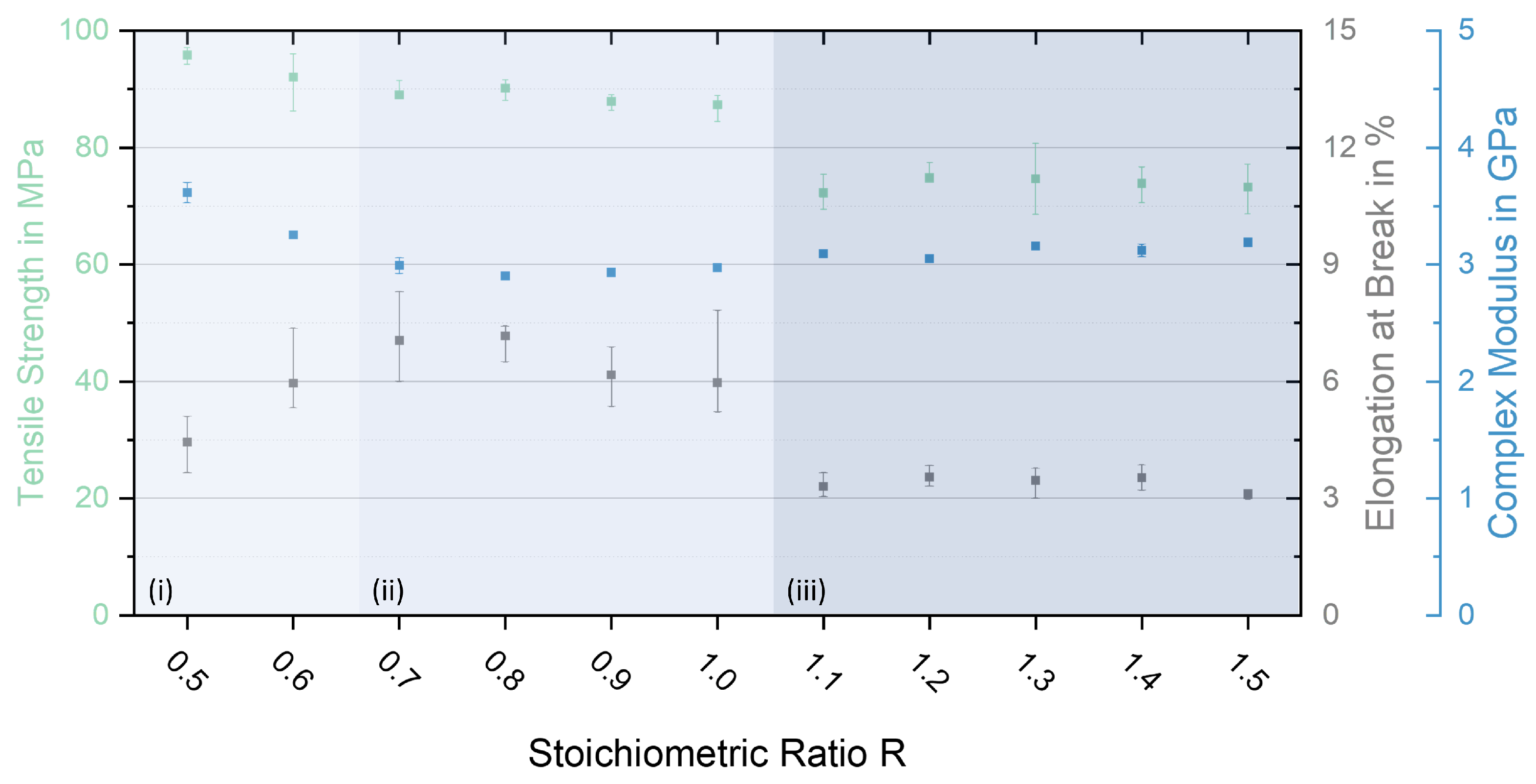
3.4. Mechanical Characterisation
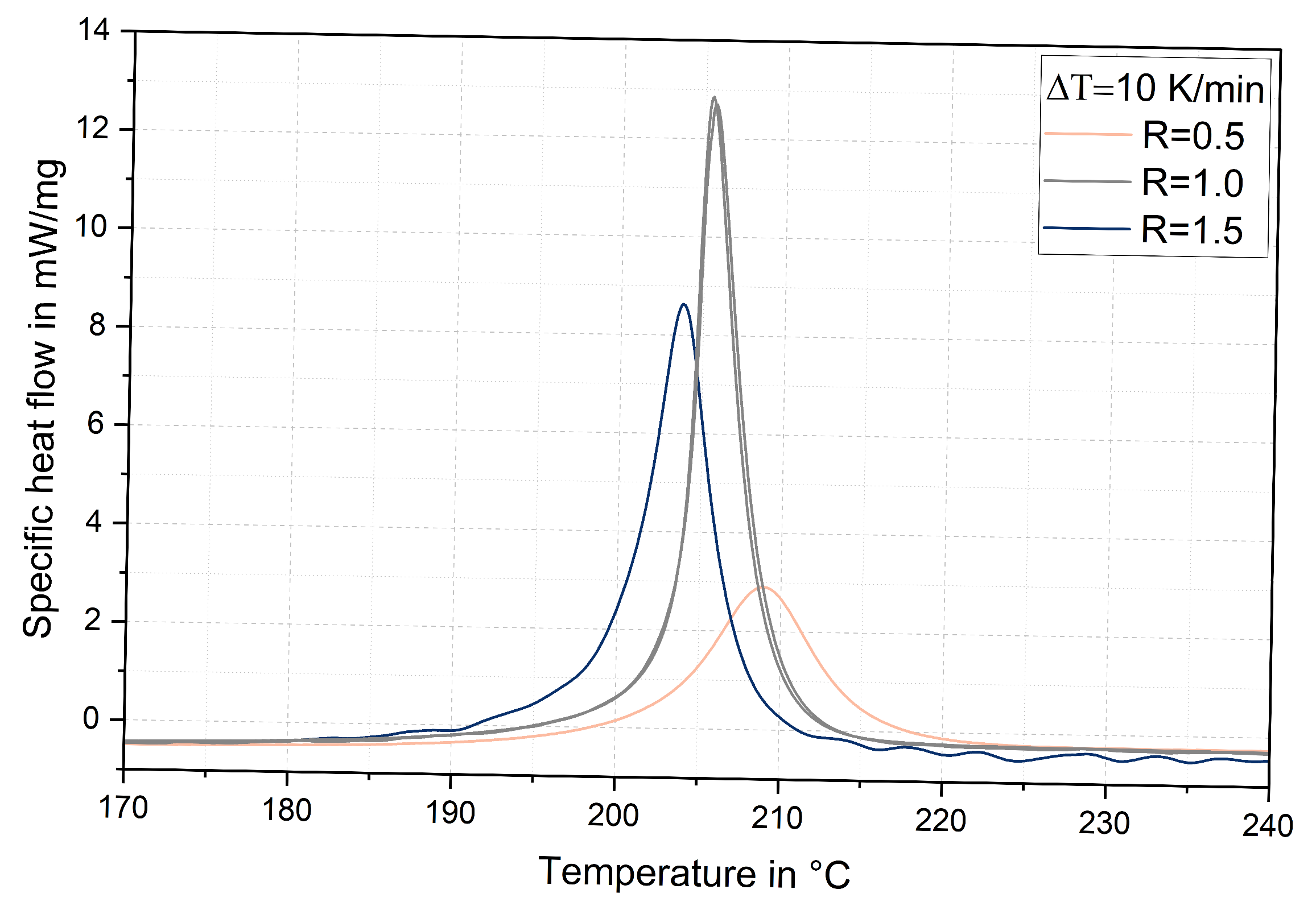
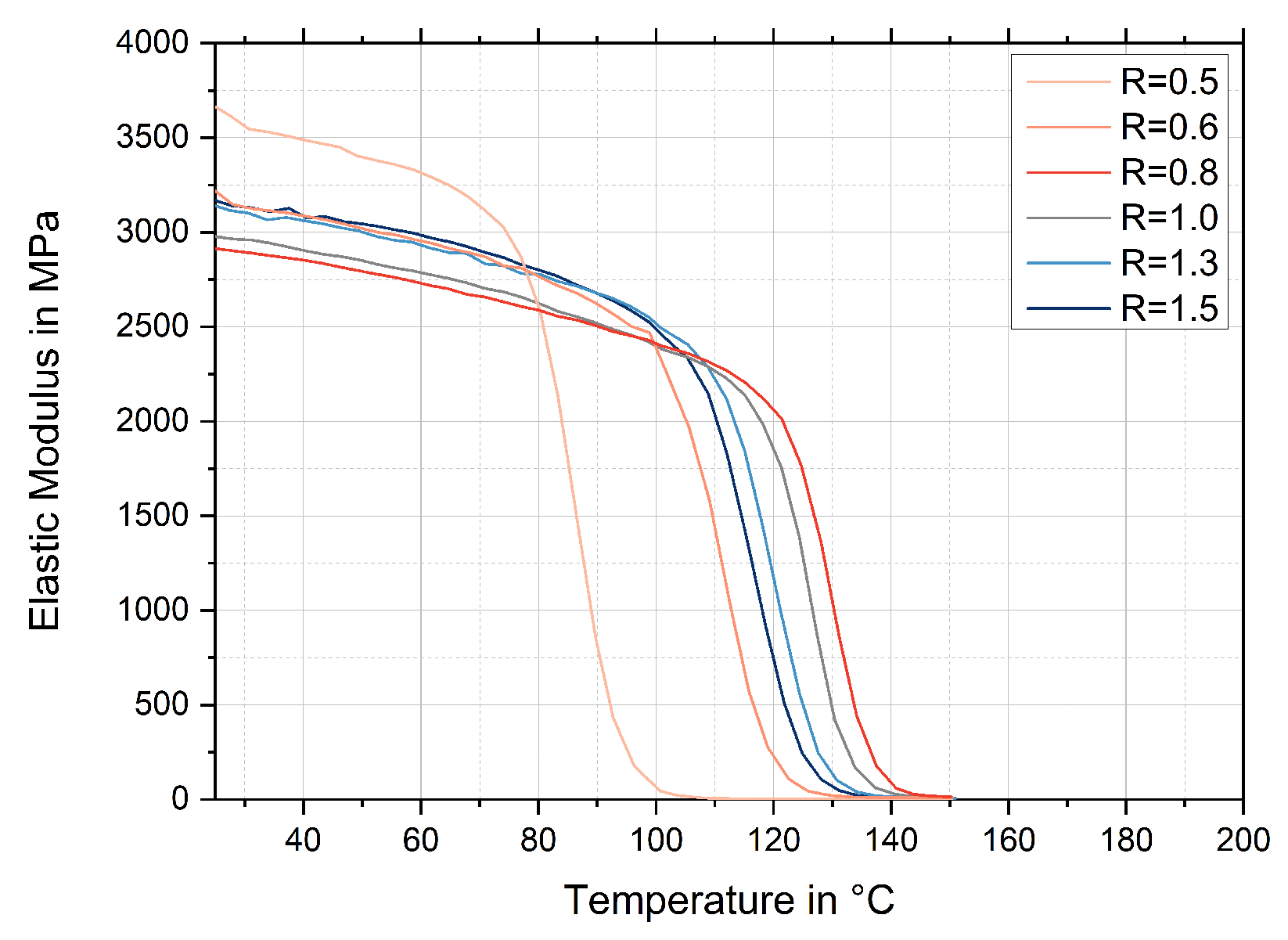
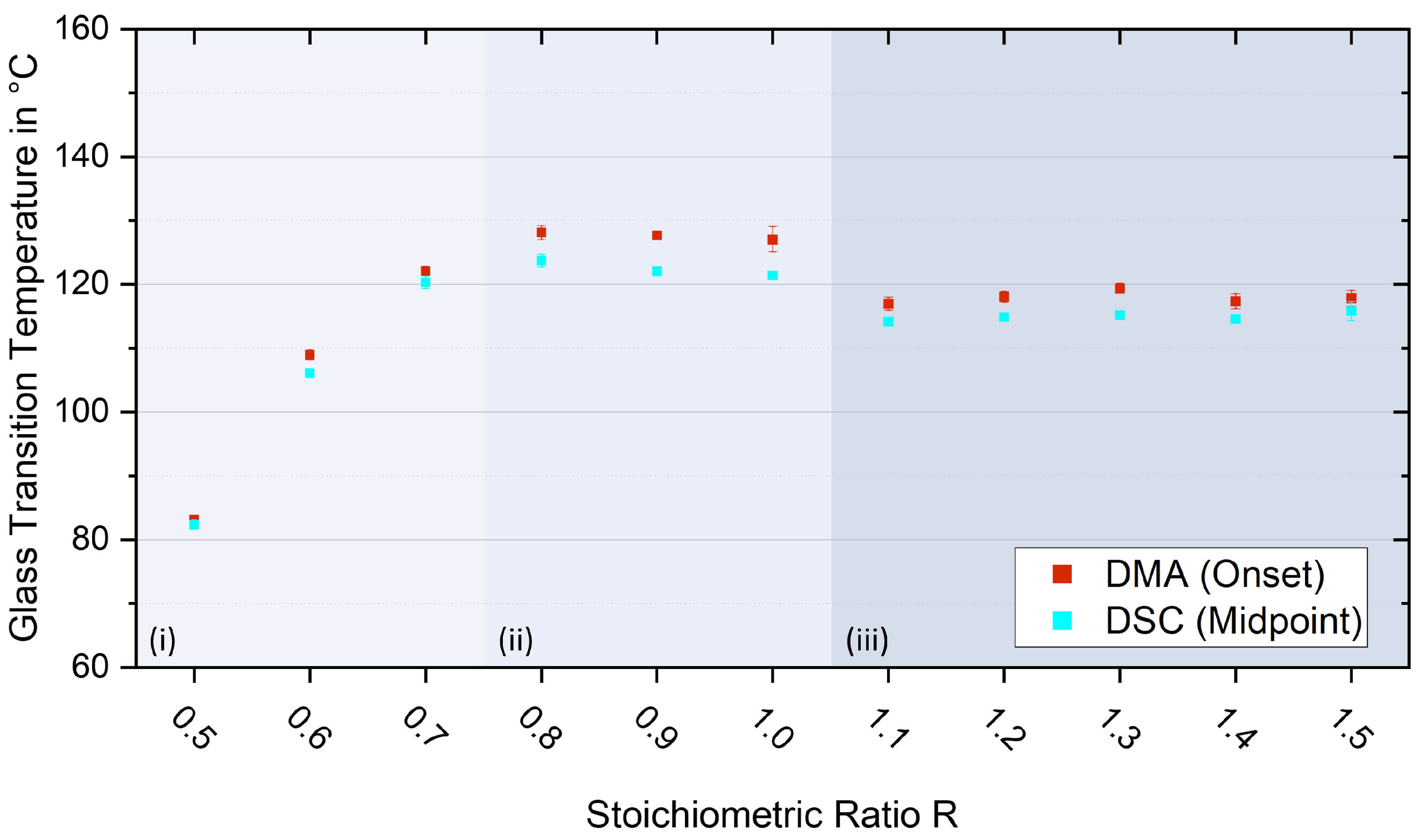
3.5. Glass Transition Temperatures
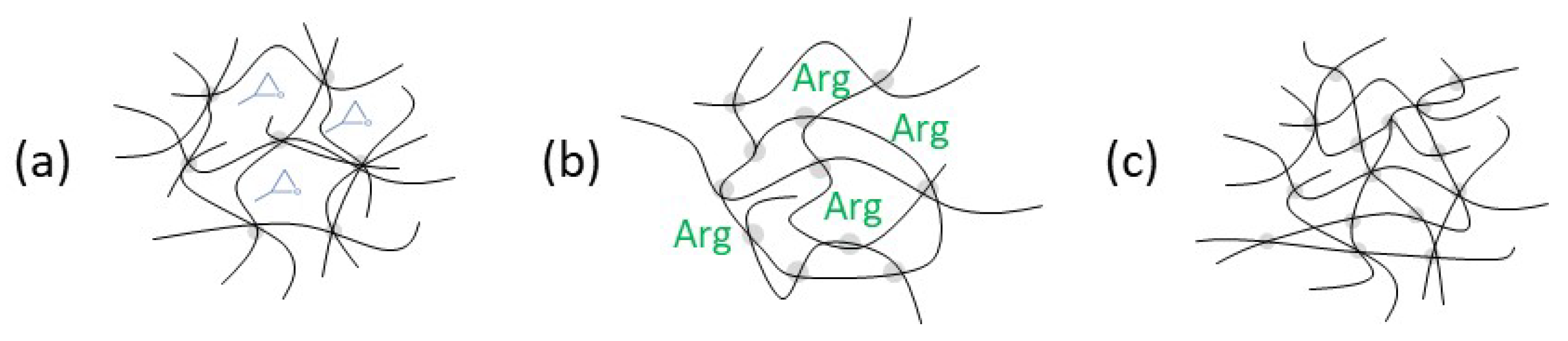
4. Postulation of Reaction Mechanism and Resulting Network Structures
- (1)
- The temperatures at which -NH3+ is deprotonated: Compared to other amino acids, L-Arg probably requires more energy to deprotonate -NH3+ due to the effects of the stable guanidium ion.
- (2)
- The density of the network, for example, due to steric hindrance.
- (3)
- Secondary/valence forces: It is conceivable that, e.g., the ionic bonds of the guanidium ions in L-Arg may improve thermo-mechanical properties.
- (4)
- Additional reactions of the side chains.
4.1. SeverelySub-Stoichiometric Configurations R = 0.5–R = 0.6
4.2. Over-Stoichiometric Configurations R > 1.0
4.3. Slightly Sub-Stoichiometric to Stoichiometric Configurations R = 0.8–R = 1.0
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Active chain end |
| AHEW | Amine hydrogen equivalent weight |
| AM | Activated monomer |
| ATR | Attenuated total reflectance |
| DGEBA | Bisphenol A diglycidyl ether |
| DMA | Dynamic mechanical analysis |
| DSC | Differential scanning calorimetry |
| EWW | Epoxy equivalent weight |
| FTIR | Fourier transform infrared spectroscopy |
| L-Arg | L-arginine |
| SEM | Scanning electron microscopy |
| Tg | Glass transition temperature |
| TMA | Thermo-mechanical analysis |
Appendix A
| Stoichiometric Ratio R | Tensile Strength in MPa | Elongation at Break in % | Young’s Modulus in GPa | Elastic Modulus (DMA) in GPa |
|---|---|---|---|---|
| 0.5 | 95.81 ± 4.05 | 4.44 ± 0.54 | 3.42 ± 0.11 | 3.61 ± 0.09 |
| 0.6 | 92.04 ± 3.81 | 5.95 ± 0.72 | 3.09 ± 0.12 | 3.25 ± 0.00 |
| 0.7 | 88.99 ± 3.05 | 7.05 ± 0.76 | 2.79 ± 0.09 | 2.99 ± 0.07 |
| 0.8 | 90.17 ± 1.27 | 7.16 ± 0.34 | 2.81 ± 0.15 | 2.90 ± 0.02 |
| 0.9 | 87.87 ± 3.78 | 6.17 ± 0.51 | 2.80 ± 0.13 | 2.93 ± 0.01 |
| 1.0 | 87.31 ± 1.40 | 5.97 ± 0.75 | 2.90 ± 0.11 | 2.97 ± 0.01 |
| 1.1 | 72.24 ± 2.02 | 3.30 ± 0.23 | 2.92 ± 0.08 | 3.09 ± 0.00 |
| 1.2 | 74.82 ± 1.89 | 3.55 ± 0.19 | 3.01 ± 0.10 | 3.05 ± 0.03 |
| 1.3 | 74.69 ± 4.53 | 3.45 ± 0.30 | 3.07 ± 0.08 | 3.16 ± 0.00 |
| 1.4 | 73.86 ± 2.42 | 3.54 ± 0.25 | 2.98 ± 0.05 | 3.12 ± 0.05 |
| 1.5 | 73.25 ± 2.83 | 3.11 ± 0.09 | 3.27 ± 0.10 | 3.19 ± 0.00 |
| Stoichiometric Ratio R | Tg DSC Midpoint in °C | Tg DMA Onset in °C |
|---|---|---|
| 0.5 | 82.35 ± 0.75 | 83.11 ± 0.29 |
| 0.6 | 106.10 ± 0.30 | 108.95 ± 0.76 |
| 0.7 | 120.30 ± 1.00 | 122.09 ± 0.72 |
| 0.8 | 123.70 ± 1.00 | 128.13 ± 1.10 |
| 0.9 | 122.05 ± 0.05 | 127.65 ± 0.41 |
| 1.0 | 121.35 ± 0.35 | 127.09 ± 1.97 |
| 1.1 | 114.15 ± 0.05 | 116.96 ± 1.03 |
| 1.2 | 114.85 ± 0.25 | 118.05 ± 0.85 |
| 1.3 | 115.15 ± 0.15 | 119.38 ± 0.78 |
| 1.4 | 114.55 ± 0.15 | 117.34 ± 1.17 |
| 1.5 | 115.80 ± 1.50 | 117.81 ± 1.22 |
References
- Li, Y.; Xiao, F.; Wong, C.P. Novel, environmentally friendly crosslinking system of an epoxy using an amino acid: Tryptophan–cured diglycidyl ether of bisphenol A epoxy. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 181–190. [Google Scholar] [CrossRef]
- Merighi, S.; Mazzocchetti, L.; Benelli, T.; Giorgini, L. Evaluation of Novel Bio-Based Amino Curing Agent Systems for Epoxy Resins: Effect of Tryptophan and Guanine. Processes 2021, 9, 42. [Google Scholar] [CrossRef]
- Walter, M.; Neubacher, M.; Fiedler, B. Using thermokinetic methods to enhance properties of epoxy resins with amino acids as biobased curing agents by achieving full crosslinking. Sci. Rep. 2024, 14, 4367. [Google Scholar] [CrossRef] [PubMed]
- Hristova, K.; Wimley, W.C. A look at arginine in membranes. J. Membr. Biol. 2011, 239, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, F.; Elvers, B. (Eds.) Ullmann’s Polymers and Plastics: Products and Processes; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Fang, M.; Zhang, Z.; Li, J.; Zhang, H.; Lu, H.; Yang, Y. Constructing hierarchically structured interphases for strong and tough epoxy nanocomposites by amine-rich graphene surfaces. J. Mater. Chem. 2010, 20, 9635. [Google Scholar] [CrossRef]
- Morsch, S.; Kefallinou, Z.; Liu, Y.; Lyon, S.B.; Gibbon, S.R. Controlling the nanostructure of epoxy resins: Reaction selectivity and stoichiometry. Polymer 2018, 143, 10–18. [Google Scholar] [CrossRef]
- Liu, W.; Huang, W.; Song, N.; Wu, Y.; Zhao, X.; Chen, K. Effect of stoichiometry on chemical structure, dielectric and mechanical properties of epoxy resin under gamma irradiation. Radiat. Phys. Chem. 2023, 202, 110551. [Google Scholar] [CrossRef]
- Li, H.; Chen, G.; Su, H.; Li, D.; Sun, L.; Yang, J. Effect of the stoichiometric ratio on the crosslinked network structure and cryogenic properties of epoxy resins cured at low temperature. Eur. Polym. J. 2019, 112, 792–798. [Google Scholar] [CrossRef]
- D’Almeida, J.; Monteiro, S.N. The effect of the resin/hardener ratio on the compressive behavior of an epoxy system. Polym. Test. 1996, 15, 329–339. [Google Scholar] [CrossRef]
- Tavares, M.I.B.; D’Almeida, J.R.M.; Monteiro, S.N. 13C solid-state NMR analysis of the DGEBA/TETA epoxy system. J. Appl. Polym. Sci. 2000, 78, 2358–2362. [Google Scholar] [CrossRef]
- D’Almeida, J.R.M.; de Menezes, G.W.; Monteiro, S.N. Ageing of the DGEBA/TETA epoxy system with off-stoichiometric compositions. Mater. Res. 2003, 6, 415–420. [Google Scholar] [CrossRef]
- Rothenhäusler, F.; Ruckdaeschel, H. Influence of the stoichiometric ratio on the in–situ formation of crystals and the mechanical properties of epoxy resin cured with L–tyrosine. Polym. Eng. Sci. 2023, 63, 4007–4018. [Google Scholar] [CrossRef]
- DIN EN ISO 3219:1994-10; Kunststoffe_-Polymere/Harze in Flüssigem, Emulgiertem oder Dispergiertem Zustand_-Bestimmung der Viskosität mit Einem Rotationsviskosimeter bei Definiertem Geschwindigkeitsgefälle (ISO_3219:1993). Beuth Verlag GmbH: Berlin, Germany, 1994.
- Gibhardt, D.; Buggisch, C.; Meyer, D.; Fiedler, B. Hygrothermal Aging History of Amine-Epoxy Resins: Effects on Thermo-Mechanical Properties. Front. Mater. 2022, 9, 826076. [Google Scholar] [CrossRef]
- Zheng, J.; Png, Z.M.; Ng, S.H.; Tham, G.X.; Ye, E.; Goh, S.S.; Loh, X.J.; Li, Z. Vitrimers: Current research trends and their emerging applications. Mater. Today 2021, 51, 586–625. [Google Scholar] [CrossRef]
- DIN EN ISO 6721-1; Kunststoffe_- Bestimmung Dynamisch-Mechanischer Eigenschaften_- Teil_1: Allgemeine Grundlagen (ISO_6721-1:2019). DIN Media GmbH: Berlin, Germany, 2019.
- DIN EN ISO 11357-2; Kunststoffe_- Dynamische Differenz-Thermoanalyse_(DSC)_- Teil_2: Bestimmung der Glasübergangstemperatur und der Glasübergangsstufenhöhe (ISO_11357-2:2013). Beuth Verlag GmbH: Berlin, Germany, 2014.
- Williams, J.G. The beta relaxation in epoxy resin–based networks. J. Appl. Polym. Sci. 1979, 23, 3433–3444. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast Fourier transform IR characterization of epoxy GY systems crosslinked with aliphatic and cycloaliphatic EH polyamine adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef]
- Mora, A.S.; Tayouo, R.; Boutevin, B.; David, G.; Caillol, S. A perspective approach on the amine reactivity and the hydrogen bonds effect on epoxy-amine systems. Eur. Polym. J. 2020, 123, 109460. [Google Scholar] [CrossRef]
- Kolev, T. Solid-state IR-LD spectroscopic and theoretical analysis of arginine-containing peptides. Biopolymers 2006, 83, 39–45. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared Spectrum Characteristics and Quantification of OH Groups in Coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Antoniou, A.; Rosemeier, M.; Tazefidan, K.; Krimmer, A.; Wolken-Möhlmann, G. Impact of Site-Specific Thermal Residual Stress on the Fatigue of Wind-Turbine Blades. AIAA J. 2020, 58, 4781–4793. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Zhang, J.; Li, T.; Hu, Z.; Yu, Y. Effect of curing conversion on the water sorption, corrosion resistance and thermo-mechanical properties of epoxy resin. RSC Adv. 2015, 5, 11358–11370. [Google Scholar] [CrossRef]
- Gupta, V.B.; Brahatheeswaran, C. Molecular packing and free volume in crosslinked epoxy networks. Polymer 1991, 32, 1875–1884. [Google Scholar] [CrossRef]
- Ogata, M.; Kinjo, N.; Kawata, T. Effects of crosslinking on physical properties of phenol–formaldehyde novolac cured epoxy resins. J. Appl. Polym. Sci. 1993, 48, 583–601. [Google Scholar] [CrossRef]
- Li, S.; He, J.; Han, S.; Chen, F.; Zhang, Y.; Chen, Y.; Zou, H. Hydrogen bond-driven rigid filling strategy: Regulation of epoxy resin network structure and properties by anchored groups. Polymer 2025, 329, 128511. [Google Scholar] [CrossRef]
- Altaweel, A.M.A.M.; Ravikumar, H.B.; Ranganathaiah, C. Influence of free volume on the mechanical properties of epoxy based composites: A correlation study. Phys. Status Solidi C 2009, 6, 2401–2403. [Google Scholar] [CrossRef]
- Karunakaran, K.; Singh, S.S.; Kitey, R. Investigating the role of filler shape on the dynamic mechanical properties of glass–filled epoxy composites. Polym. Compos. 2022, 43, 6912–6925. [Google Scholar] [CrossRef]
- Cantwell, W.J.; Kausch, H.H. Fracture behaviour of epoxy resins. In Chemistry and Technology of Epoxy Resins; Springer: Dordrecht, The Netherlands, 1993; pp. 144–174. [Google Scholar]
- Fiedler, B.; Hojo, M.; Ochiai, S.; Schulte, K.; Ando, M. Failure behavior of an epoxy matrix under different kinds of static loading. Compos. Sci. Technol. 2001, 61, 1615–1624. [Google Scholar] [CrossRef]
- Startsev, O.V.; Vapirov, Y.M.; Lebedev, M.P.; Kychkin, A.K. Comparison of Glass-Transition Temperatures for Epoxy Polymers Obtained by Methods of Thermal Analysis. Mech. Compos. Mater. 2020, 56, 227–240. [Google Scholar] [CrossRef]
- Yong, A.X.H.; Sims, G.D.; Gnaniah, S.J.P.; Ogin, S.L.; Smith, P.A. Heating rate effects on thermal analysis measurement of Tg in composite materials. Adv. Manuf. Polym. Compos. Sci. 2017, 3, 43–51. [Google Scholar] [CrossRef]
- Courvoisier, E.; Williams, P.A.; Lim, G.K.; Hughes, C.E.; Harris, K.D.M. The crystal structure of L-arginine. Chem. Commun. 2012, 48, 2761–2763. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Arakawa, T.; Shiraki, K. Effect of counter ions of arginine as an additive for the solubilization of protein and aromatic compounds. Int. J. Biol. Macromol. 2016, 91, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Ramsdale-Capper, R.; Foreman, J.P. Internal antiplasticisation in highly crosslinked amine cured multifunctional epoxy resins. Polymer 2018, 146, 321–330. [Google Scholar] [CrossRef]
- Morancho, J.M.; Ramis, X.; Fernández-Francos, X.; Salla, J.M.; Konuray, A.O.; Serra, À. Curing of off-stoichiometric amine–epoxy thermosets. J. Therm. Anal. Calorim. 2018, 133, 519–527. [Google Scholar] [CrossRef]
- Ranoux, G.; Tataru, G.; Coqueret, X. Cationic Curing of Epoxy–Aromatic Matrices for Advanced Composites: The Assets of Radiation Processing. Appl. Sci. 2022, 12, 2355. [Google Scholar] [CrossRef]
- McGowen, J.A.; Mathias, L.J. Cationic polymerization of diglycidly ether of bisphenol a resins initiated by benzylsulfonium salts. Polym. Compos. 1997, 18, 348–367. [Google Scholar] [CrossRef]
- Ekbrant, B.E.F.; Skov, A.L.; Daugaard, A.E. Epoxy-Rich Systems with Preference for Etherification over Amine-Epoxy Reactions for Tertiary Amine Accelerators. Macromolecules 2021, 54, 4280–4287. [Google Scholar] [CrossRef]


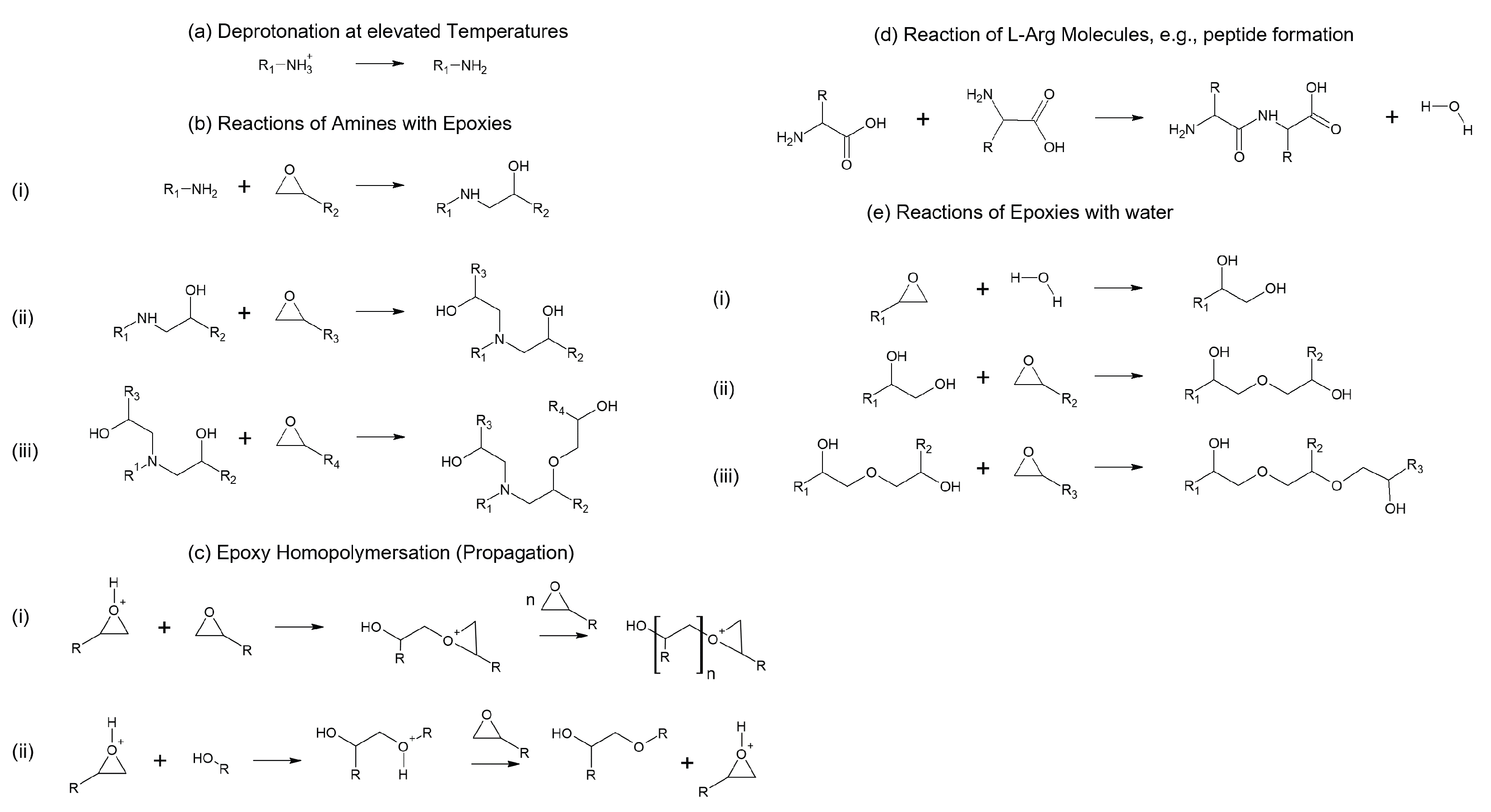
| Stoichiometric Ratio R | 827 in g | : | L-Arg in g |
|---|---|---|---|
| 0.5 | 100 | : | 6.87 |
| 0.6 | 100 | : | 8.24 |
| 0.7 | 100 | : | 9.62 |
| 0.8 | 100 | : | 10.99 |
| 0.9 | 100 | : | 12.36 |
| 1.0 | 100 | : | 13.74 |
| 1.1 | 100 | : | 15.11 |
| 1.2 | 100 | : | 16.49 |
| 1.3 | 100 | : | 17.86 |
| 1.4 | 100 | : | 19.24 |
| 1.5 | 100 | : | 20.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, M.; Gibhardt, D.; Fiedler, B. Effects of Stoichiometric Variations in L-Arginine-Cured Epoxy Resins. Polymers 2025, 17, 3089. https://doi.org/10.3390/polym17223089
Walter M, Gibhardt D, Fiedler B. Effects of Stoichiometric Variations in L-Arginine-Cured Epoxy Resins. Polymers. 2025; 17(22):3089. https://doi.org/10.3390/polym17223089
Chicago/Turabian StyleWalter, Melissa, Dennis Gibhardt, and Bodo Fiedler. 2025. "Effects of Stoichiometric Variations in L-Arginine-Cured Epoxy Resins" Polymers 17, no. 22: 3089. https://doi.org/10.3390/polym17223089
APA StyleWalter, M., Gibhardt, D., & Fiedler, B. (2025). Effects of Stoichiometric Variations in L-Arginine-Cured Epoxy Resins. Polymers, 17(22), 3089. https://doi.org/10.3390/polym17223089







