Anticancer Potential of Fisetin Against Glioblastoma: In Vitro Evaluation, Radiostability Assessment, and Preliminary PLGA Encapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.2.1. Cell Cycle Distribution
2.2.2. Apoptosis Analysis
2.3. Preparation Process of Fisetin-Loaded PLGA Nanoparticles
2.4. Characterization of Developed Fisetin-Loaded PLGA Nanoparticles
2.4.1. Mean Particle Size (MPS), Polydispersity Index (PDI), and Zeta Potential (ZP) Analysis
2.4.2. Drug Loading (DL%) and Entrapment Efficiency (EE%)
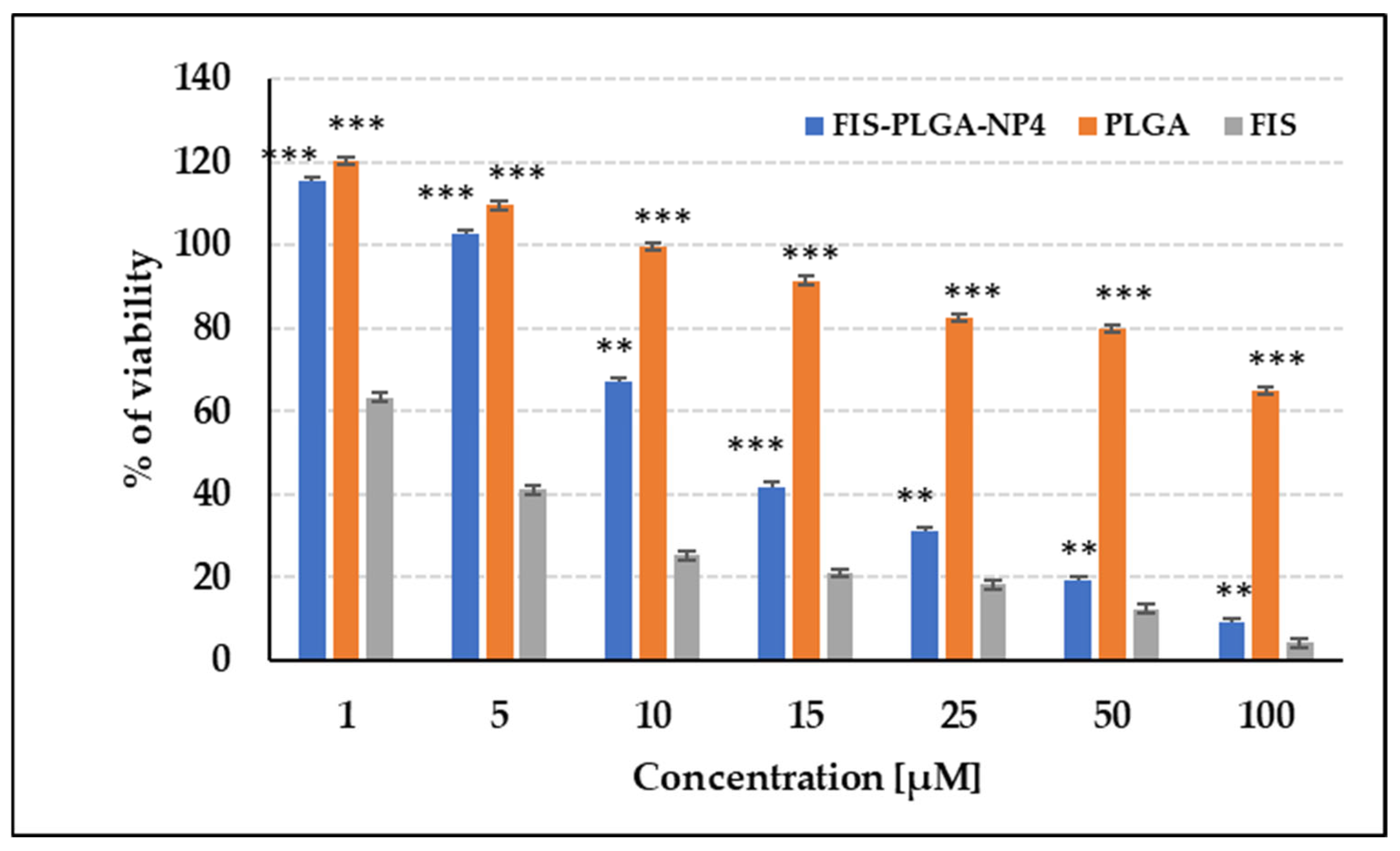
2.4.3. The Effect of Developed Fisetin-Loaded PLGA Nanoparticles on Cell Viability
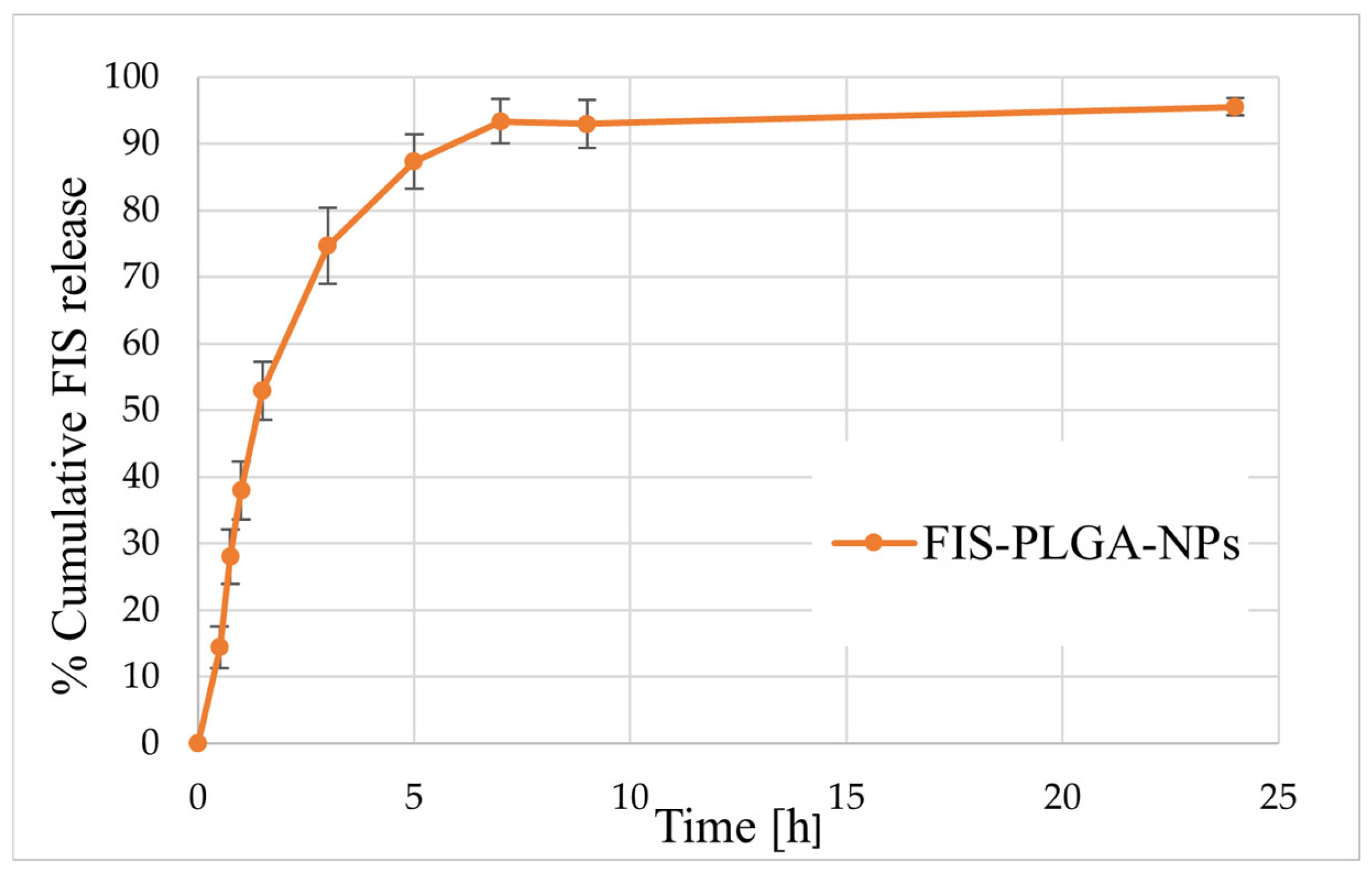
2.4.4. In Vitro Drug Release Studies
2.5. Development of the Fisetin Sterilization Method
2.5.1. Electron Paramagnetic Resonance (EPR) Spectroscopy
2.5.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5.3. Nuclear Magnetic Resonance (NMR) Analysis
2.5.4. High-Performance Liquid Chromatography (HPLC)
3. Results and Discussion
3.1. Biological Activity of Fisetin
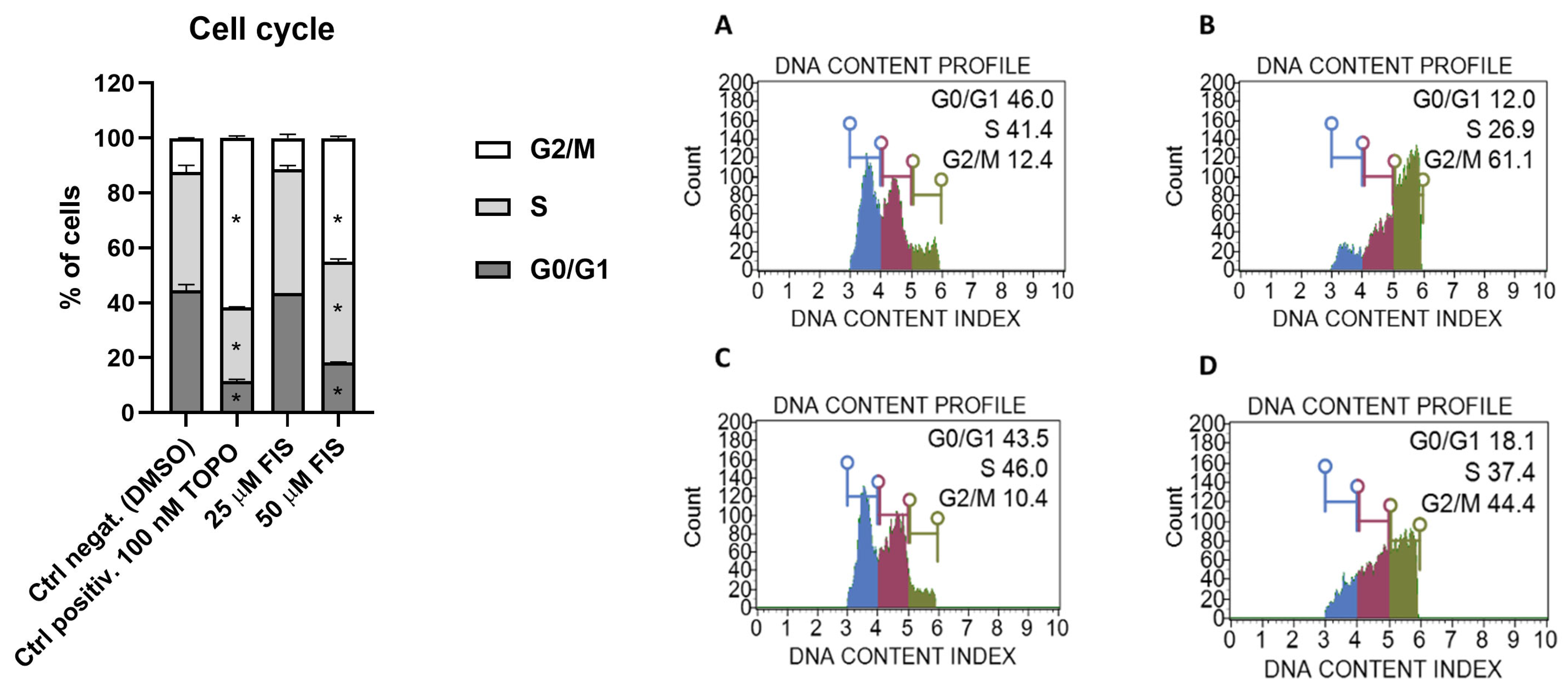
3.1.1. Effect of Fisetin on Cell Cycle Distribution in U-138 MG Glioblastoma Cells
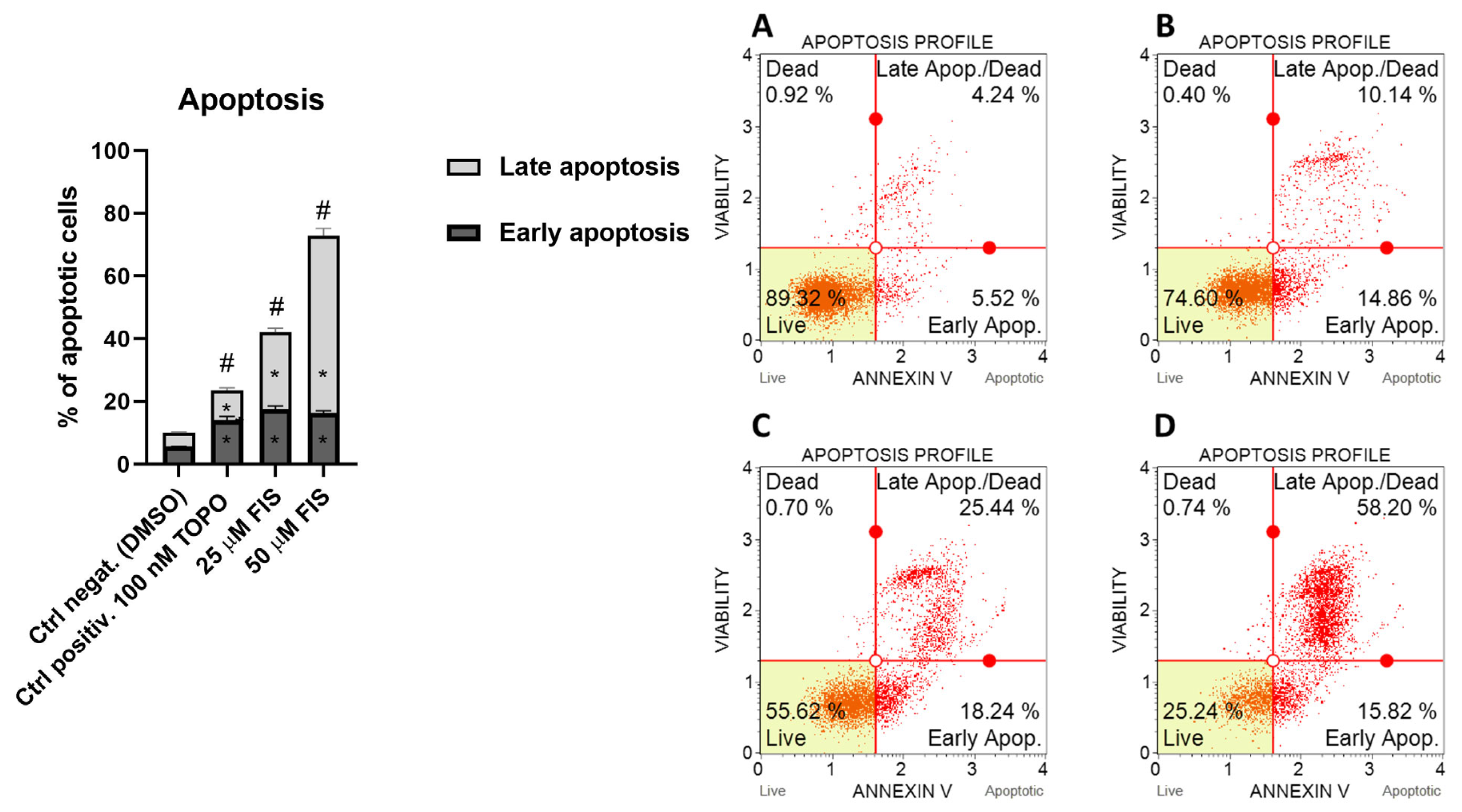
3.1.2. Effect of Fisetin on Apoptosis in U-138 MG Glioma Cells
3.2. Characterization of Fisetin-Loaded PLGA Nanoparticles
3.3. Biological Activity of FIS-Loaded PLGA NPs
3.4. Evaluation of the Impact of Radiation Sterilization on Fisetin Stability
3.4.1. Visual Examination
3.4.2. EPR Analysis
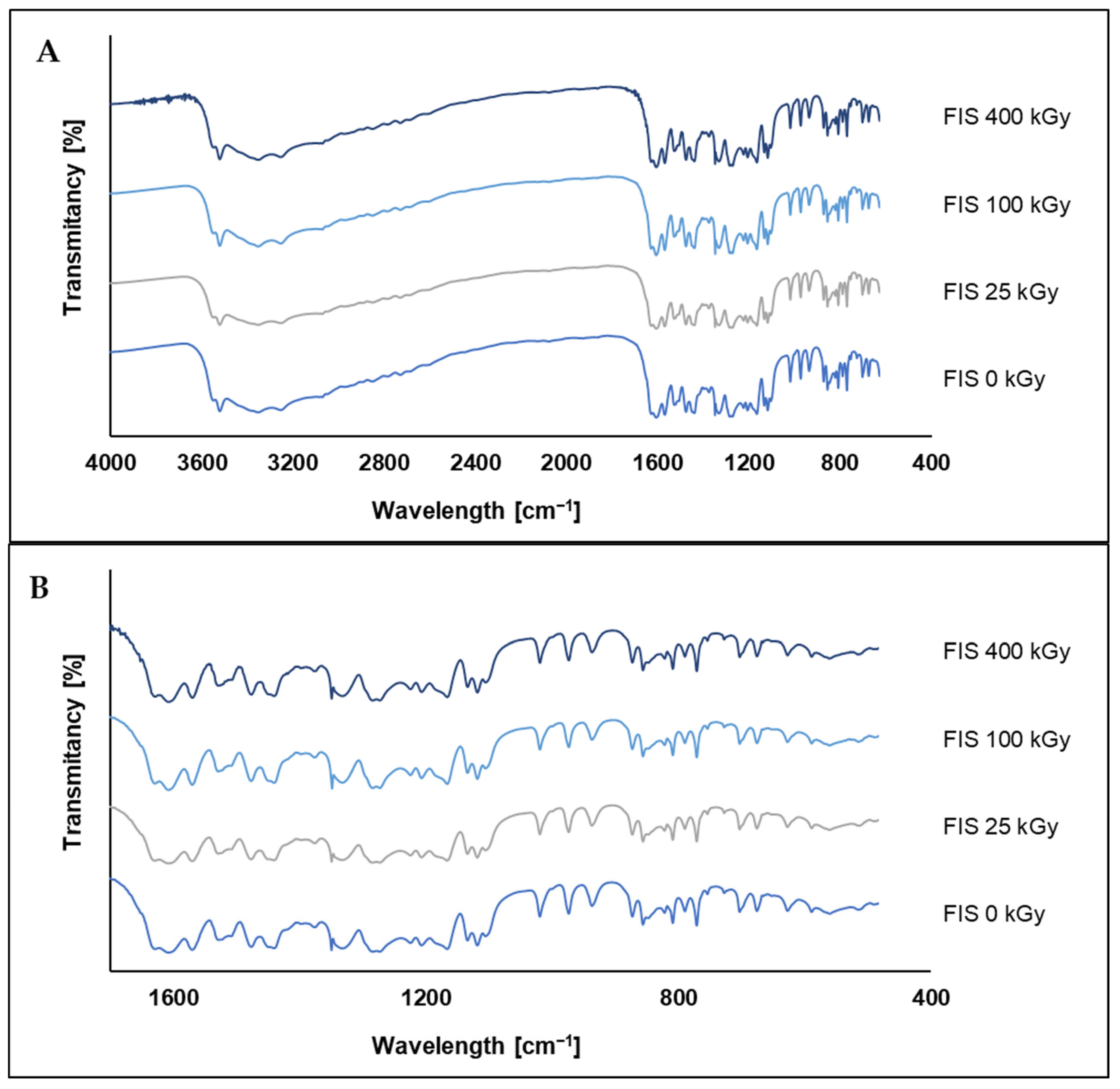
3.4.3. HPLC, FT-IR, and NMR Analysis
4. Limitations and Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.M.; Kumar, H.; Bhatt, T.; Jain, R.; Panchal, K.; Chaurasiya, A.; Jain, V. Fisetin in Cancer: Attributes, Developmental Aspects, and Nanotherapeutics. Pharmaceuticals 2023, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, Y.; Nie, S.; Zhou, S.; Gao, X.; Chen, G. Biological Effects and Mechanisms of Fisetin in Cancer: A Promising Anti-Cancer Agent. Eur. J. Med. Res. 2023, 28, 297. [Google Scholar] [CrossRef]
- Kubina, R.; Krzykawski, K.; Kabała-Dzik, A.; Wojtyczka, R.D.; Chodurek, E.; Dziedzic, A. Fisetin, a Potent Anticancer Flavonol Exhibiting Cytotoxic Activity Against Neoplastic Malignant Cells and Cancerous Conditions: A Scoping, Comprehensive Review. Nutrients 2022, 14, 2604. [Google Scholar] [CrossRef]
- Ferah, S.; Çamlıbel, M.; Erçelik, M.; Tekin, Ç.; Bekar, A.; Kocaeli, H.; Taşkapılıoğlu, Ö.M.; Tunca, B. Investigation of the Effects of Fisetin with the Combination of Chemotherapy Drug Te-609 Mozolomide to the Biological Behaviour of Glioblastoma Cells. In Proceedings of the 5th Inter-610 National Eurasian Conference on Biological and Chemical Sciences (EurasianBioChem 2022), Antalya, Turkey, 23–25 November 2022. [Google Scholar]
- Pak, F.; Oztopcu-Vatan, P. Fisetin Effects on Cell Proliferation and Apoptosis in Glioma Cells. Z. Für Naturforsch. C 2019, 74, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sundarraj, K.; Raghunath, A.; Perumal, E. A Review on the Chemotherapeutic Potential of Fisetin: In Vitro Evidences. Biomed. Pharmacother. 2018, 97, 928–940. [Google Scholar] [CrossRef]
- Chen, C.-M.; Hsieh, Y.-H.; Hwang, J.-M.; Jan, H.-J.; Hsieh, S.-C.; Lin, S.-H.; Lai, C.-Y. Fisetin Suppresses ADAM9 Expression and Inhibits Invasion of Glioma Cancer Cells through Increased Phosphorylation of ERK1/2. Tumor Biol. 2015, 36, 3407–3415. [Google Scholar] [CrossRef] [PubMed]
- Beltzig, L.; Christmann, M.; Dobreanu, M.; Kaina, B. Genotoxic and Cytotoxic Activity of Fisetin on Glioblastoma Cells. Anticancer Res. 2024, 44, 901–910. [Google Scholar] [CrossRef]
- Joma, N.; Zhang, I.; Righetto, G.L.; McKay, L.; Gran, E.R.; Kakkar, A.; Maysinger, D. Flavonoids Regulate Redox-Responsive Transcription Factors in Glioblastoma and Microglia. Cells 2023, 12, 2821. [Google Scholar] [CrossRef]
- Renault-Mahieux, M.; Vieillard, V.; Seguin, J.; Espeau, P.; Le, D.T.; Lai-Kuen, R.; Mignet, N.; Paul, M.; Andrieux, K. Co-Encapsulation of Fisetin and Cisplatin into Liposomes for Glioma Therapy: From Formulation to Cell Evaluation. Pharmaceutics 2021, 13, 970. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Jian, Y.; He, S.; Liu, J.; Cheng, Y.; Zheng, S.; Wang, X.; Qian, Z.; Gao, X. Sensitizing Chemotherapy for Glioma with Fisetin Mediated by Microenvironment-Responsive Nano-Drug Delivery System. Nanoscale 2023, 16, 97–109. [Google Scholar] [CrossRef]
- Lin, K.; Ramesh, S.; Agarwal, S.; Kuo, W.; Kuo, C.; Chen, M.Y.; Lin, Y.; Ho, T.; Huang, P.; Huang, C. Fisetin Attenuates Doxorubicin-induced Cardiotoxicity by Inhibiting the Insulin-like Growth Factor. Phytother. Res. 2023, 37, 3964–3981. [Google Scholar] [CrossRef]
- Grzegorzewski, J.; Michalak, M.; Wołoszczuk, M.; Bulicz, M.; Majchrzak-Celińska, A. Nanotherapy of Glioblastoma—Where Hope Grows. Int. J. Mol. Sci. 2025, 26, 1814. [Google Scholar] [CrossRef]
- Seguin, J.; Brullé, L.; Boyer, R.; Lu, Y.M.; Ramos Romano, M.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Mignet, N.; Chabot, G.G. Liposomal Encapsulation of the Natural Flavonoid Fisetin Improves Bioavailability and Antitumor Efficacy. Int. J. Pharm. 2013, 444, 146–154. [Google Scholar] [CrossRef]
- Kuźmińska, J.; Sobczak, A.; Majchrzak-Celińska, A.; Żółnowska, I.; Gostyńska, A.; Jadach, B.; Krajka-Kuźniak, V.; Jelińska, A.; Stawny, M. Etoricoxib-Cannabidiol Combo: Potential Role in Glioblastoma Treatment and Development of PLGA-Based Nanoparticles. Pharmaceutics 2023, 15, 2104. [Google Scholar] [CrossRef] [PubMed]
- Bilski, P.; Drużbicki, K.; Jenczyk, J.; Mielcarek, J.; Wąsicki, J. Molecular and Vibrational Dynamics in the Cholesterol-Lowering Agent Lovastatin: Solid-State NMR, Inelastic Neutron Scattering, and Periodic DFT Study. J. Phys. Chem. B 2017, 121, 2776–2787. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation. ICH Q2(R2) Guideline on Validation of Analytical Procedures—Step 5; International Council for Harmonisation: Geneva, Switzerland, 2023. [Google Scholar]
- Mazzio, E.A.; Bauer, D.; Mendonca, P.; Taka, E.; Soliman, K.F.A. Natural Product HTP Screening for Attenuation of Cytokine-Induced Neutrophil Chemo Attractants (CINCs) and NO2—In LPS/IFNγ Activated Glioma Cells. J. Neuroimmunol. 2017, 302, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, I.; Pandit, J.; Emad, N.A.; Bano, S.; Dar, K.I.; Rizvi, M.M.A.; Ansari, M.D.; Aqil, M.; Sultana, Y. Brain Targeted Delivery of Carmustine Using Chitosan Coated Nanoparticles via Nasal Route for Glioblastoma Treatment. Int. J. Biol. Macromol. 2022, 221, 435–445. [Google Scholar] [CrossRef]
- Sousa De Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Messina, S.; Zuchegna, C.; Bruzzi, M. Chemotherapeutic Nanoparticles for Glioblastoma. Front. Oncol. 2025, 15, 1641752. [Google Scholar] [CrossRef]
- Gao, K.; Jiang, X. Influence of Particle Size on Transport of Methotrexate across Blood Brain Barrier by Polysorbate 80-Coated Polybutylcyanoacrylate Nanoparticles. Int. J. Pharm. 2006, 310, 213–219. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Bertacco, J.; Gaglio, S.C.; Minoia, A.; Cominacini, M.; Cheri, S.; Deiana, M.; Marchetto, G.; Bisognin, A.; Gandini, A.; et al. Fisetin: An Integrated Approach to Identify a Strategy Promoting Osteogenesis. Front. Pharmacol. 2022, 13, 890693. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Lin, C.-C.; Hsieh, Y.-S.; Wu, Y.-T. Nanoformulation Development to Improve the Biopharmaceutical Properties of Fisetin Using Design of Experiment Approach. Molecules 2021, 26, 3031. [Google Scholar] [CrossRef]
- Kadari, A.; Gudem, S.; Kulhari, H.; Bhandi, M.M.; Borkar, R.M.; Kolapalli, V.R.M.; Sistla, R. Enhanced Oral Bioavailability and Anticancer Efficacy of Fisetin by Encapsulating as Inclusion Complex with HPβCD in Polymeric Nanoparticles. Drug Deliv. 2017, 24, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of Dietary Flavonoid Fisetin: Formulation and In Vitro Antioxidant and α-Glucosidase Inhibition Activities. Mater. Sci. Eng. C 2016, 68, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Burgess, D.J. Accelerated in-vitro release testing methods for extended-release parenteral dosage forms. J. Pharm. Pharmacol. 2012, 64, 986–996. [Google Scholar] [CrossRef]
- Goel, M.; Leung, D.; Famili, A.; Chang, D.; Nayak, P.; Al-Sayah, M. Accelerated in vitro release testing method for a long-acting peptide-PLGA formulation. Eur. J. Pharm. Biopharm. 2021, 165, 185–192. [Google Scholar] [CrossRef]
- Marciniec, B.; Stawny, M.; Kozak, M.; Naskrent, M. Theeffect of ionizing radiation on chloramphenicol. J. Therm. Anal. Calorim. 2006, 84, 741–746. [Google Scholar] [CrossRef]
- Sakiroff, L.-M.; Chennell, P.; Yessaad, M.; Pereira, B.; Bouattour, Y.; Sautou, V. Evaluation of Color Changes During Stability Studies Using Spectrophotometric Chromaticity Measurements Versus Visual Examination. Sci. Rep. 2022, 12, 8959. [Google Scholar] [CrossRef] [PubMed]
- Gonet, M.; Baranowski, M.; Czechowski, T.; Kucinska, M.; Plewinski, A.; Szczepanik, P.; Jurga, S.; Murias, M. Multiharmonic Electron Paramagnetic Resonance Imaging as an Innovative Approach for In Vivo Studies. Free Radic. Biol. Med. 2020, 152, 271–279. [Google Scholar] [CrossRef]
- Kubo, R.; Tomita, K. A General Theory of Magnetic Resonance Absorption. J. Phys. Soc. Jpn. 1954, 9, 888–919. [Google Scholar] [CrossRef]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 73, 679–712. [Google Scholar] [CrossRef]
- Dominiak, K.; Gostyńska-Stawna, A.; Sobczak, A.; Paluszczak, J.; Woźniak-Braszak, A.; Baranowski, M.; Bilski, P.; Wicher, B.; Tykarska, E.; Jelińska, A.; et al. Formulation of Honokiol- and Magnolol-Loaded Nanoemulsions for Head and Neck Cancer Adjuvant Therapy: Evaluation of Radiation Sterilization Effects on Active Substance Properties. Int. J. Mol. Sci. 2025, 26, 8032. [Google Scholar] [CrossRef]
- Moya, E.L.J.; Lombardo, S.M.; Vandenhaute, E.; Schneider, M.; Mysiorek, C.; Türeli, A.E.; Kanda, T.; Shimizu, F.; Sano, Y.; Maubon, N.; et al. Interaction of Surfactant Coated PLGA Nanoparticles with In Vitro Human Brain-Like Endothelial Cells. Int. J. Pharm. 2022, 621, 121780. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Barcia, E.; Negro, S.; Paccione, N.; Rahmani, M.; Montejo, C.; García-García, L.; Fernández-Carballido, A. Regional Brain Distribution of PLGA Nanoparticles Functionalized with Glutathione or Phenylalanine Dipeptide. J. Drug Deliv. Sci. Technol. 2025, 105, 106680. [Google Scholar] [CrossRef]

| Sample | FIS:PLGA Ratio | PVA [%] | Sonication Time [min] |
|---|---|---|---|
| FIS-PLGA-NP1 | 1:20 | 1 | 2 |
| FIS-PLGA-NP2 | 1:5 | ||
| FIS-PLGA-NP3 | 1:20 | 2 | 4 |
| FIS-PLGA-NP4 | 1:5 | ||
| FIS-PLGA-NP5 | 1:20 | 3 | 6 |
| FIS-PLGA-NP6 | 1:5 |
| Parameter | Value |
|---|---|
| Modulation frequency [kHz] | 100,000 |
| Center field [mT] | 338 |
| Sweep width [mT] | 15 |
| Sweep time [s] | 60 |
| Time constant [s] | 0.008 |
| Second modulation amplitude [G] | 1 |
| Radio Frequency power [mW] | 0.291 |
| Radio frequency [GHz] | 9.460048 |
| Temperature [K] | 296.15 |
| Precision/Recovery | ||||
|---|---|---|---|---|
| Intra-Day | Inter-Day | |||
| Series I n = 9 | Series II n = 9 | Series I-II n = 18 | ||
| Concentration [μg/mL] | RSD [%] Recovery [%] | Concentration [μg/mL] | RSD [%] Recovery [%] | RSD [%] Recovery [%] |
| 20.06 | 1.15 96.87 ± 0.86 | 20.02 | 1.15 102.57 ± 0.91 | 3.14 99.72 ± 1.56 |
| Sample | MPS ± SD [nm] | PDI ± SD | ZP ± SD [mV] | DL% ± SD [%] | EE% ± SD [%] |
|---|---|---|---|---|---|
| Formulation conditions I: 1% PVA; 2 min. of emulsification | |||||
| FIS-PLGA-NP1 * | 660 ± 80 | 0.45 ± 0.03 | −13.60 ± 0.44 | 0.89 ± 0.20 | 18.69 ± 4.16 |
| FIS-PLGA-NP2 ** | 560 ± 21 | 0.49 ± 0.03 | −11.37 ± 0.15 | 13.05 ± 1.80 | 78.30 ± 10.78 |
| Formulation conditions II: 2% PVA; 4 min. of emulsification | |||||
| FIS-PLGA-NP3 * | 323 ± 2 | 0.20 ± 0.01 | −12.27 ± 0.81 | 0.61 ± 0.01 | 12.71 ± 0.15 |
| FIS-PLGA-NP4 ** | 330 ± 6 | 0.25 ± 0.03 | −7.18 ± 1.19 | 13.93 ± 0.07 | 83.58 ± 0.42 |
| Formulation conditions II: 3% PVA; 6 min. of emulsification | |||||
| FIS-PLGA-NP5 * | 380 ± 7 | 0.23 ± 0.01 | −10.00 ± 0.09 | 0.57 ± 0.01 | 11.87 ± 0.15 |
| FIS-PLGA-NP6 ** | 300 ± 4 | 0.18 ± 0.01 | −6.92 ± 0.90 | 11.81 ± 0.28 | 70.86 ± 1.70 |
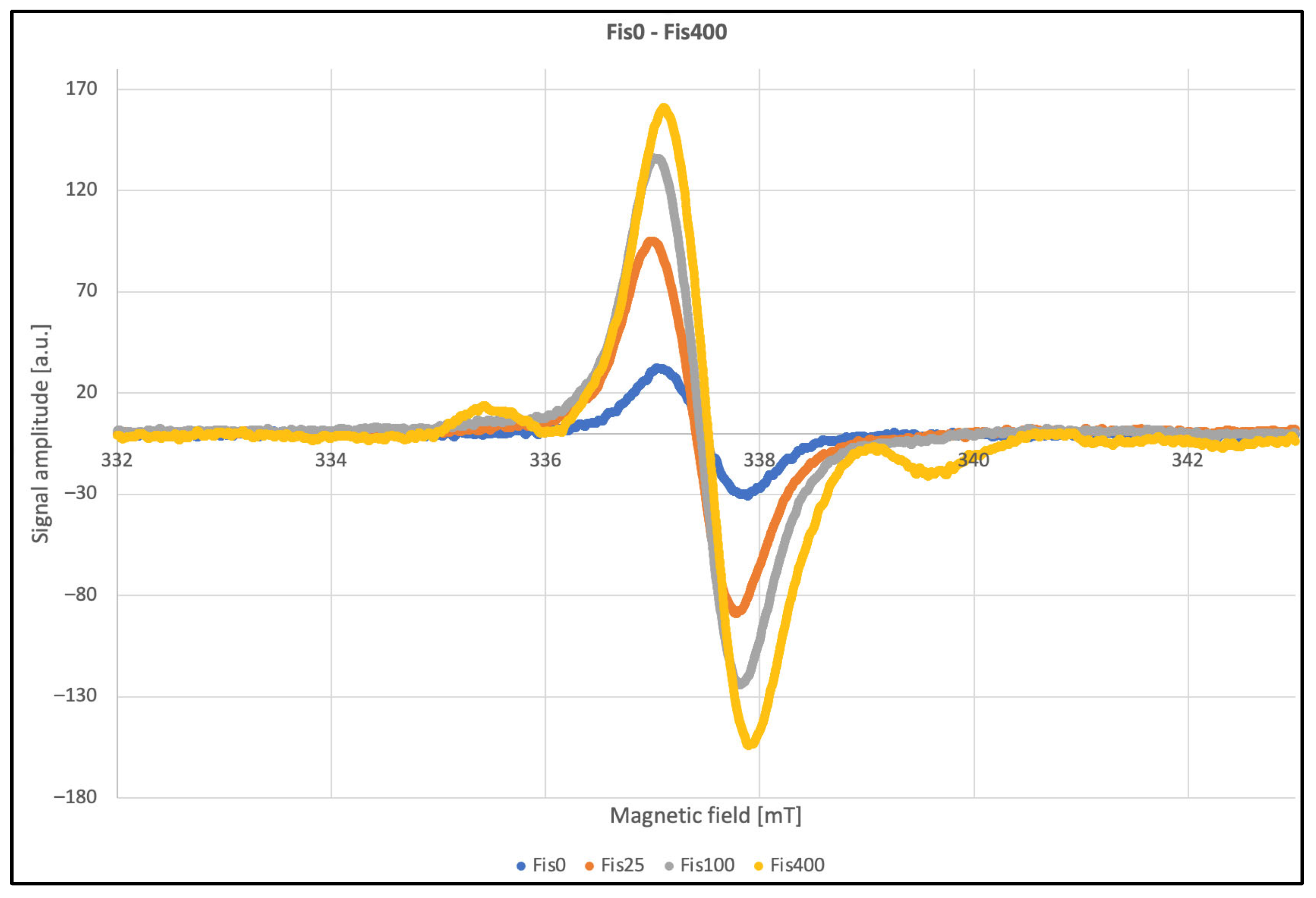
| Parameter | 0 kGy | 25 kGy | 100 kGy | 400 kGy |
|---|---|---|---|---|
| g-factor | 2.0028 | 2.0031 | 2.0032 | 2.0031 |
| dH [mT] | 0.82 | 0.8 | 0.83 | 0.82 |
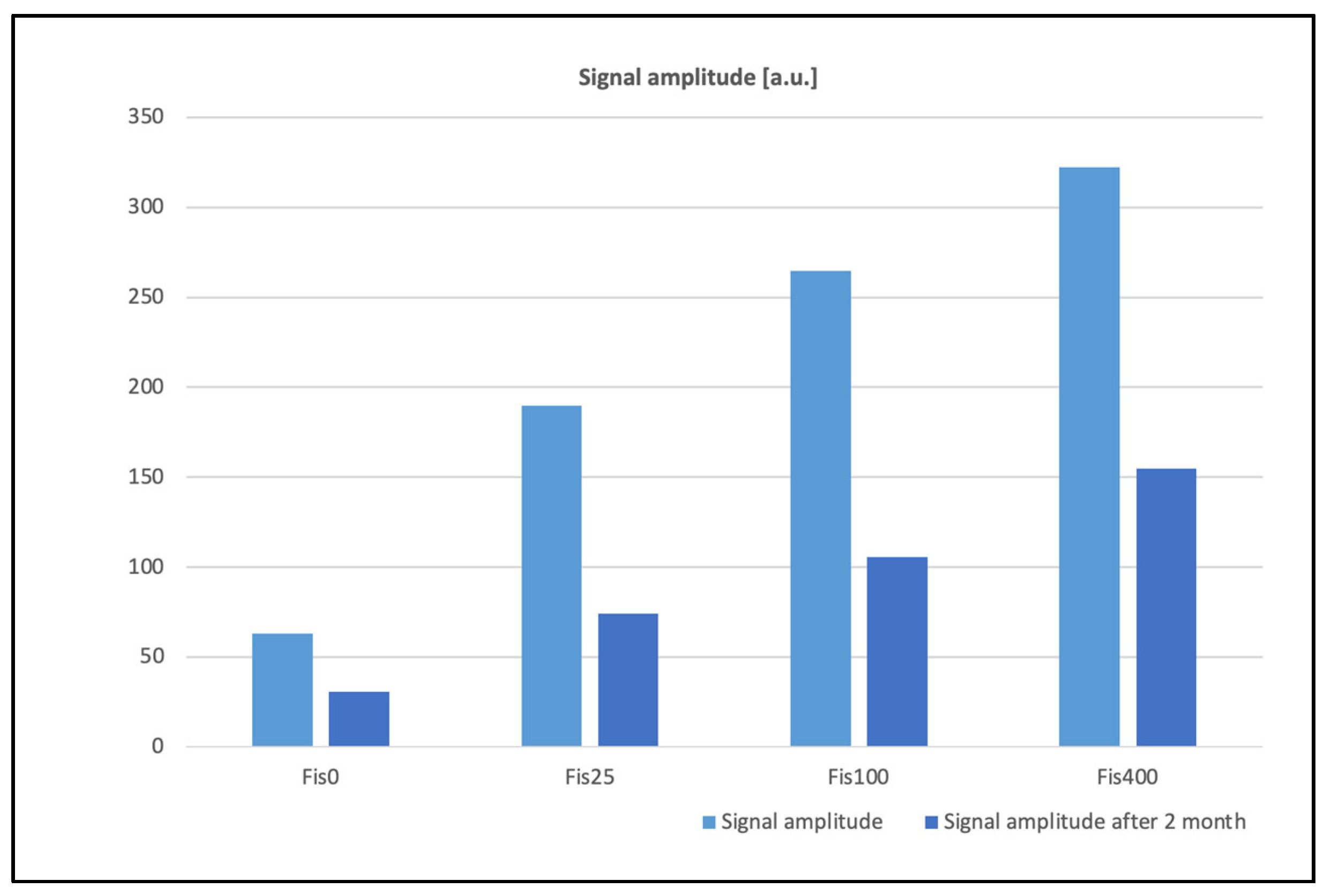
| Signal amplitude | 63.1 | 189.6 | 264.6 | 322.0 |
| Signal amplitude after 2 months | 30.5 | 74.2 | 105.6 | 154.8 |
| Line center [mT] | 337.46 | 337.38 | 337.41 | 337.52 |
| Dose of Radiation | Average Amount [%] |
|---|---|
| 0 kGy | 99.72 ± 1.56 * |
| 25 kGy | 98.20 ± 2.38 ** |
| 100 kGy | 97.70 ± 1.14 ** |
| 400 kGy | 97.13 ± 1.37 ** |
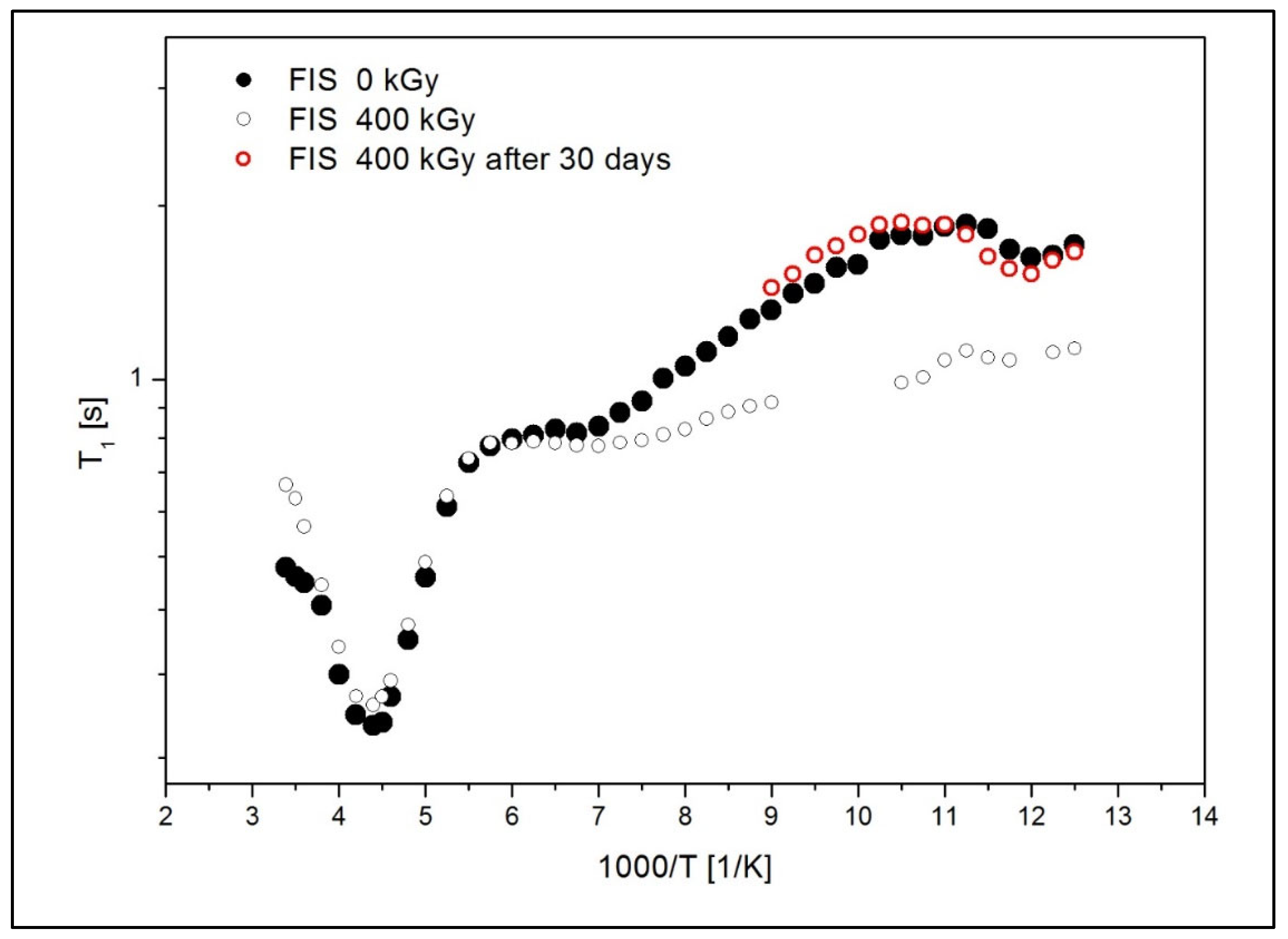
| Sample | Internal Motion | ||
|---|---|---|---|
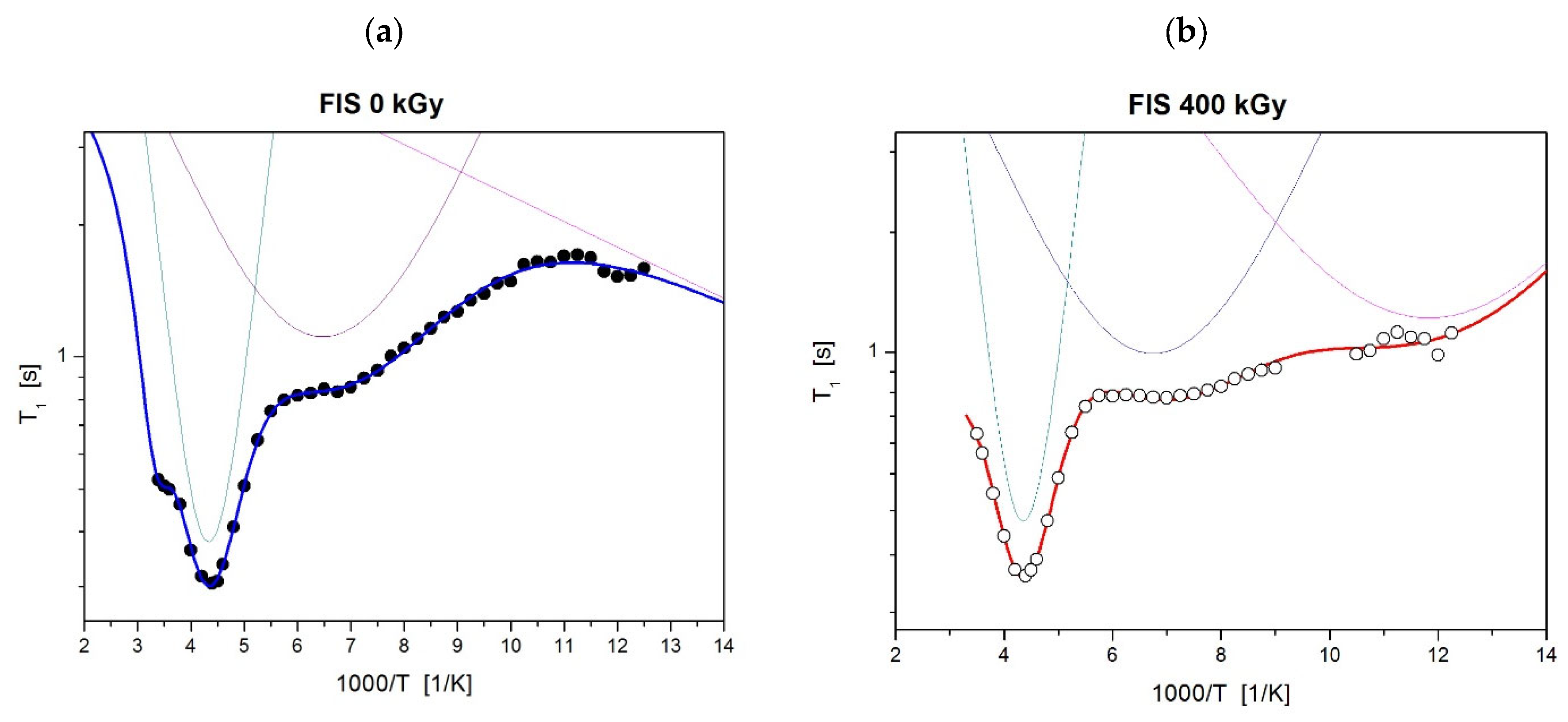
| FIS 0 kGy | oscillations of the ring | motion of local group (C=O) | proton jump in hydrogen bonds |
| τ02 = 0.9 Ea2 = 20.4 (kJ/mol) = 0.6 (10−8T2) | τ03 = 6.7 Ea3 = 5.2 (kJ/mol) = 0.2 (10−8T2) | τ03 = 2.8 Ea3 = 1.1 (kJ/mol) = 1.6 (10−8T2) | |
| FIS 400 kGy | oscillations of the ring | motion of local group (C=O) | additional local motion in the low temperature |
| τ02 = 0.9 Ea2 = 20.3 (kJ/mol) = 0.5 (10−8T2) | τ03 = 6.4 Ea3 = 5.1 (kJ/mol) = 0.2 (10−8T2) | τ03 = 3.6 Ea3 = 3.3 (kJ/mol) = 0.2 (10−8T2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak, A.; Dominiak, K.; Sztenc, B.; Jadach, B.; Woźniak-Braszak, A.; Baranowski, M.; Bilski, P.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V.; Jelińska, A.; et al. Anticancer Potential of Fisetin Against Glioblastoma: In Vitro Evaluation, Radiostability Assessment, and Preliminary PLGA Encapsulation. Polymers 2025, 17, 3074. https://doi.org/10.3390/polym17223074
Sobczak A, Dominiak K, Sztenc B, Jadach B, Woźniak-Braszak A, Baranowski M, Bilski P, Majchrzak-Celińska A, Krajka-Kuźniak V, Jelińska A, et al. Anticancer Potential of Fisetin Against Glioblastoma: In Vitro Evaluation, Radiostability Assessment, and Preliminary PLGA Encapsulation. Polymers. 2025; 17(22):3074. https://doi.org/10.3390/polym17223074
Chicago/Turabian StyleSobczak, Agnieszka, Katarzyna Dominiak, Bartłomiej Sztenc, Barbara Jadach, Aneta Woźniak-Braszak, Mikołaj Baranowski, Paweł Bilski, Aleksandra Majchrzak-Celińska, Violetta Krajka-Kuźniak, Anna Jelińska, and et al. 2025. "Anticancer Potential of Fisetin Against Glioblastoma: In Vitro Evaluation, Radiostability Assessment, and Preliminary PLGA Encapsulation" Polymers 17, no. 22: 3074. https://doi.org/10.3390/polym17223074
APA StyleSobczak, A., Dominiak, K., Sztenc, B., Jadach, B., Woźniak-Braszak, A., Baranowski, M., Bilski, P., Majchrzak-Celińska, A., Krajka-Kuźniak, V., Jelińska, A., Stawny, M., & Gostyńska-Stawna, A. (2025). Anticancer Potential of Fisetin Against Glioblastoma: In Vitro Evaluation, Radiostability Assessment, and Preliminary PLGA Encapsulation. Polymers, 17(22), 3074. https://doi.org/10.3390/polym17223074











