Formation and Interfacial Behavior of Chitosan–Alginate Interpolyelectrolyte Complexes: From Bulk Dispersions to Layer-by-Layer Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Polysaccharide Solutions
2.3. Preparation of CS-ALG Mixtures
2.4. Experimental Techniques
3. Results and Discussion
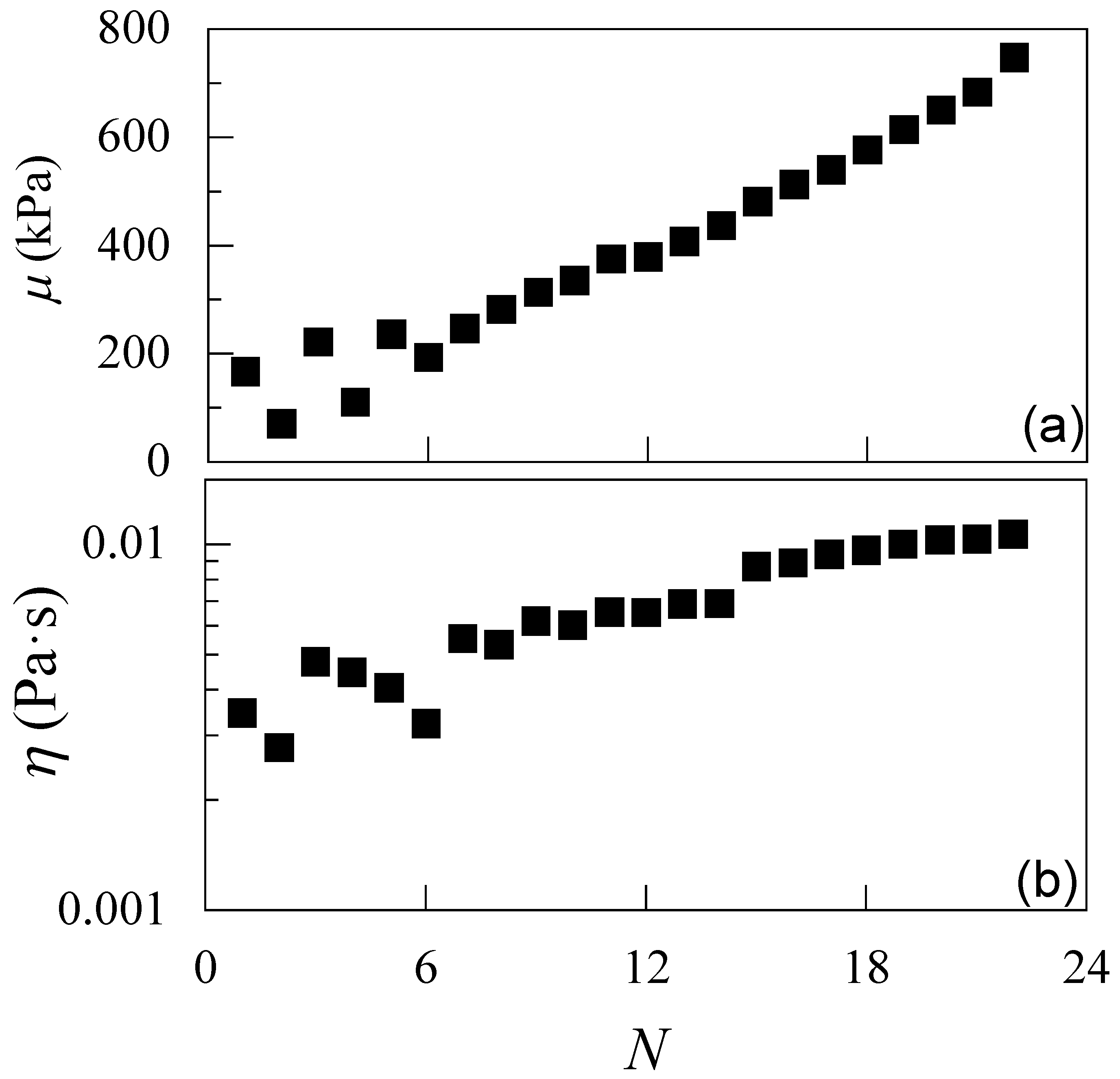
3.1. Understanding the Formation of IPECs in Solution
3.2. Influence of Mixing Procedure on the Formation of IPECs
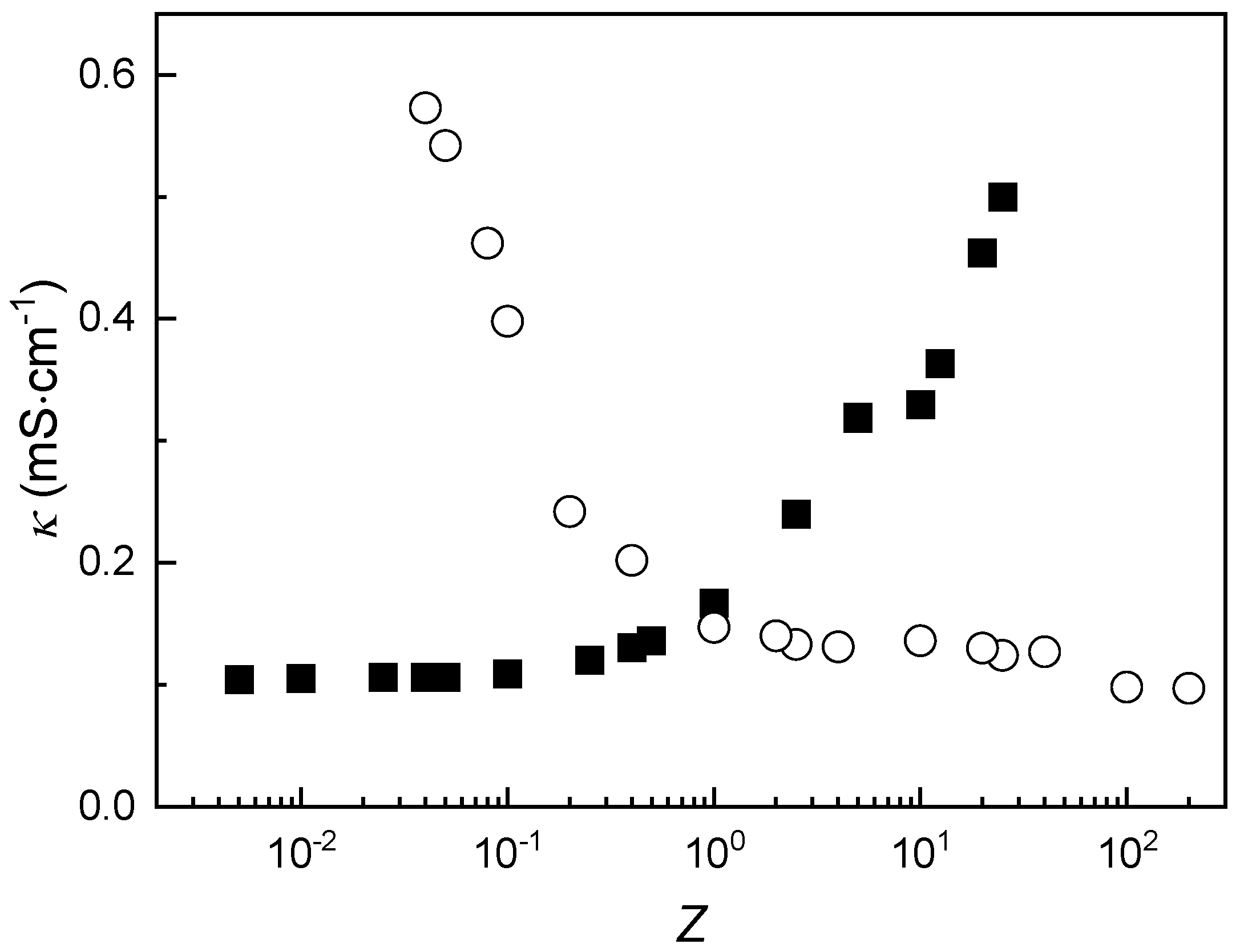
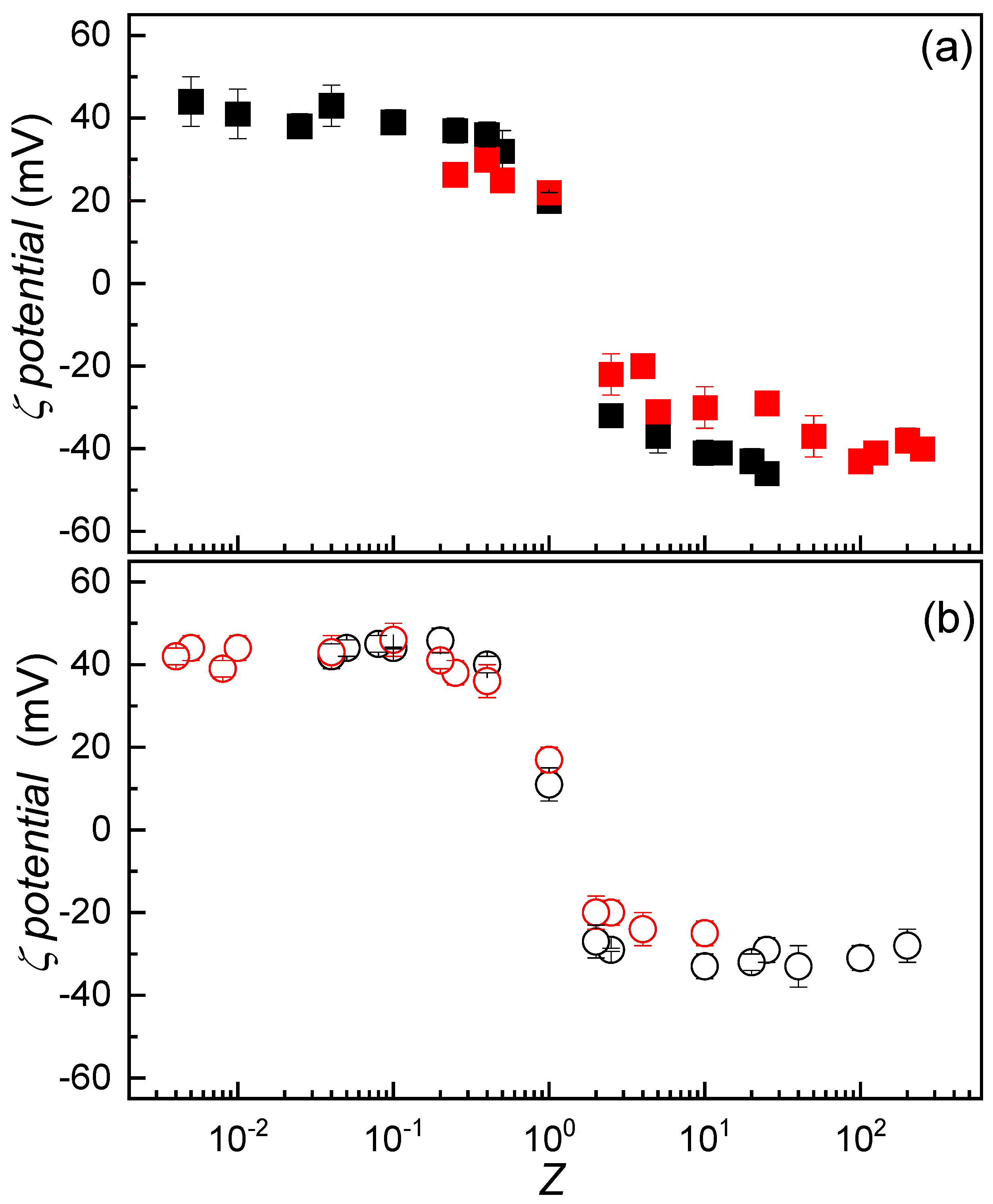
3.3. Influence of Solution Composition on the Formation of IPECs
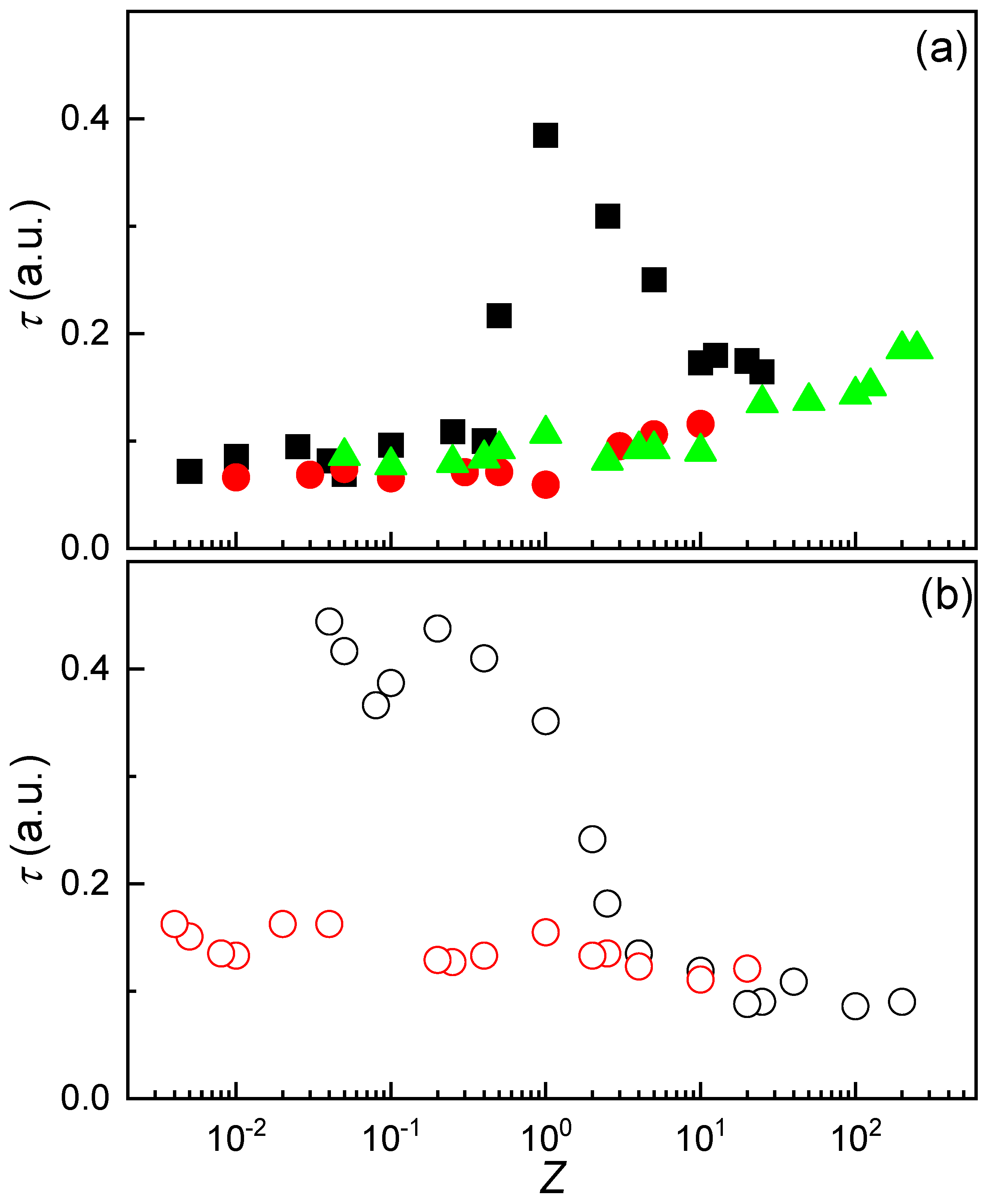
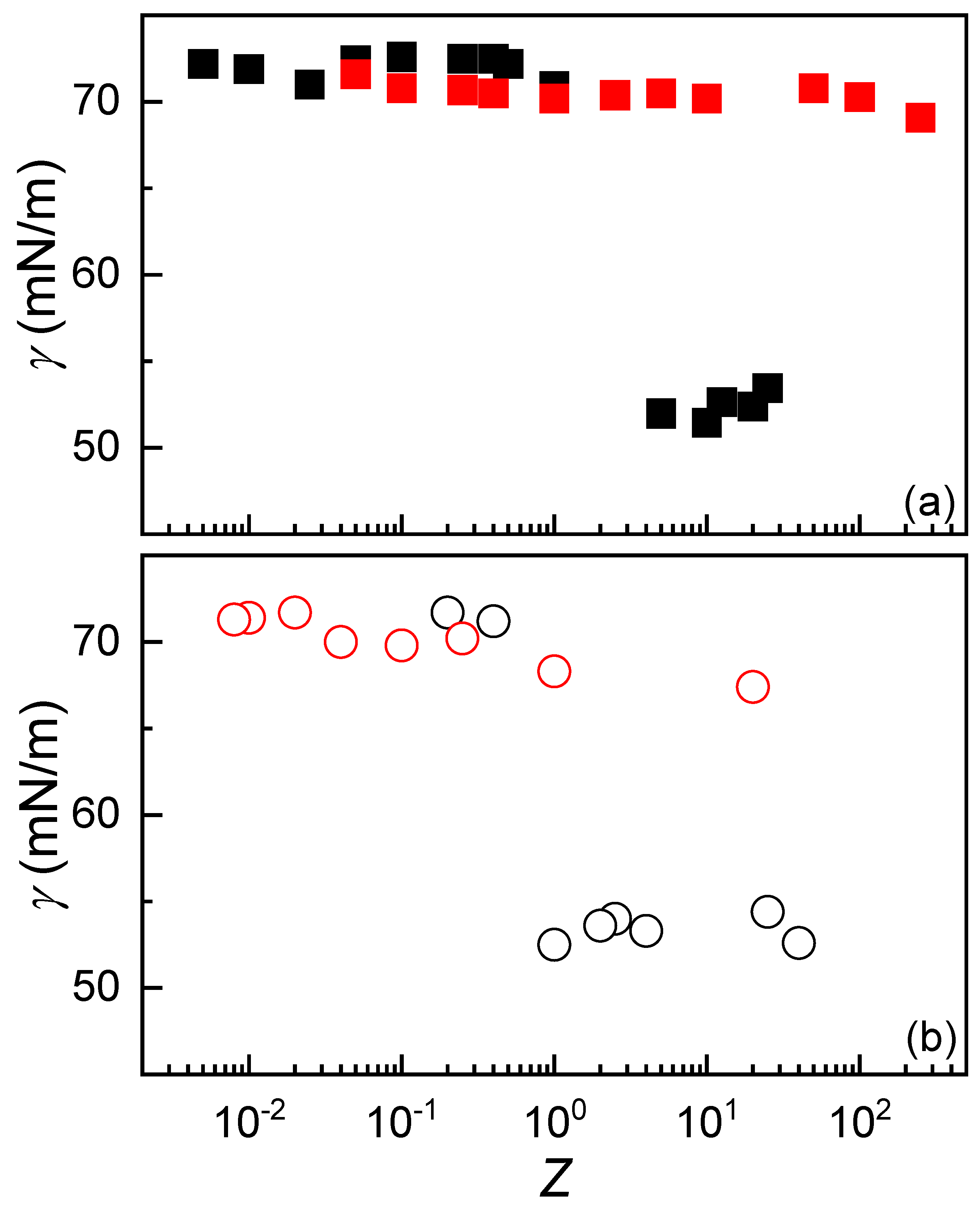
3.4. Adsorption of IPECs to the Air/Dispersion Interface
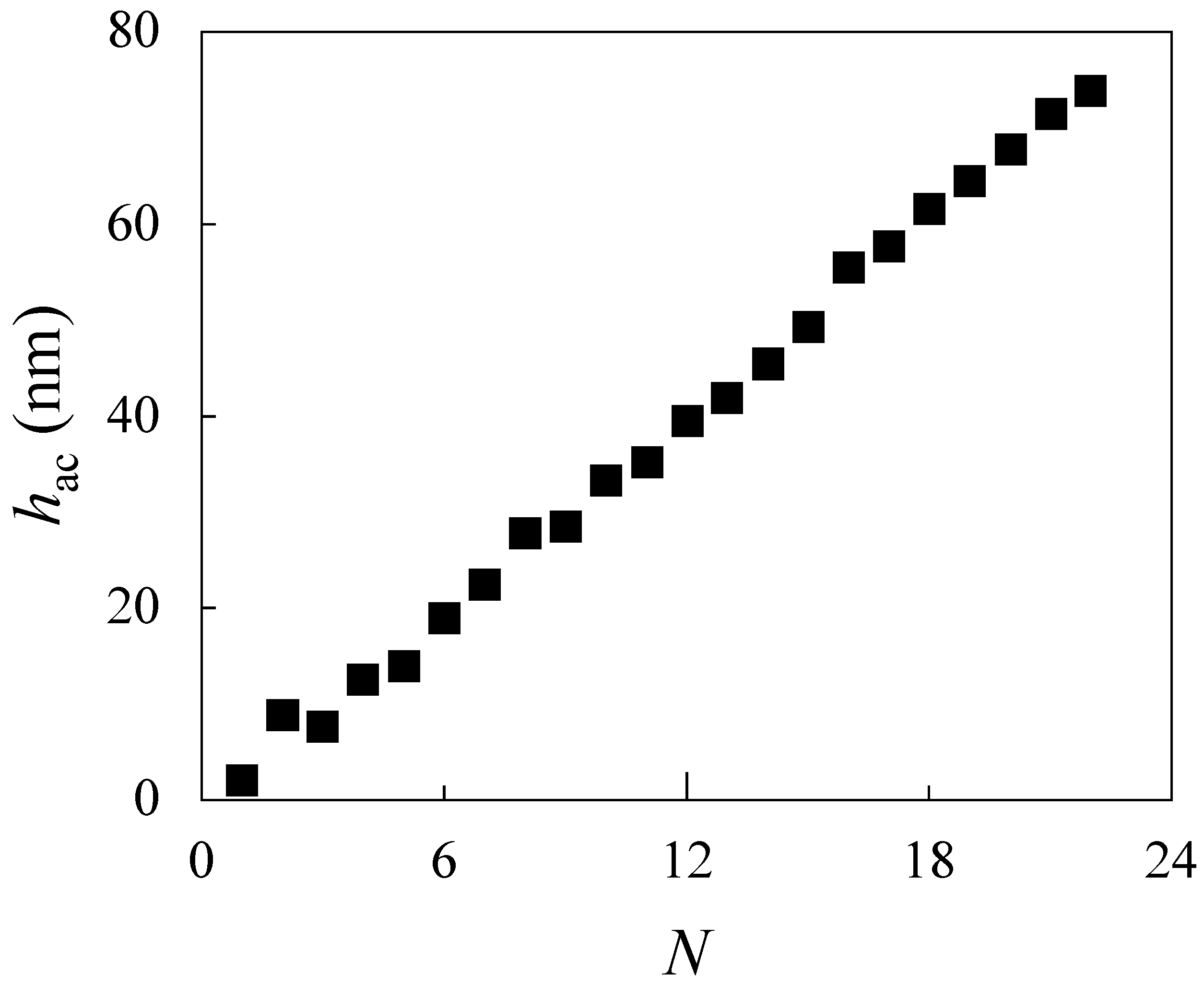
3.5. Assembly of LbL Films by Alternate Deposition of CS and ALG on Flat Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaroslavov, A.A.; Sybachin, A.V.; Efimova, A.A. Stabilization of Electrostatic Polymer-Colloid Complexes. Colloids Surf. A 2018, 558, 1–7. [Google Scholar] [CrossRef]
- Roy, P.S. Complex Coacervate-Based Materials for Biomedicine: Recent Advancements and Future Prospects. Ind. Eng. Chem. Res. 2024, 63, 5414–5487. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Mussabayeva, B.K.; Kassymova, Z.S.; Klivenko, A.N.; Orazzhanova, L.K. Interpolyelectrolyte Complexes: Advances and Prospects of Application. Russ. Chem. Rev. 2019, 88, 1046–1062. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A Comprehensive Review on Polyelectrolyte Complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Cristescu, R. Polysaccharide-Based Coatings as Drug Delivery Systems. Pharmaceutics 2023, 15, 2227. [Google Scholar] [CrossRef]
- Alfinaikh, R.S.; Alamry, K.A.; Hussein, M.A. Sustainable and Biocompatible Hybrid Materials-Based Sulfated Polysaccharides for Biomedical Applications: A Review. RSC Adv. 2025, 15, 4708–4767. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Yermak, I.M.; Davydova, V.N.; Volod’ko, A.V. Mucoadhesive Marine Polysaccharides. Mar. Drugs 2022, 20, 522. [Google Scholar] [CrossRef]
- Etrych, T.; Leclercq, L.; Boustta, M.; Vert, M. Polyelectrolyte Complex Formation and Stability When Mixing Polyanions and Polycations in Salted Media: A Model Study Related to the Case of Body Fluids. Eur. J. Pharm. Sci. 2005, 25, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Klivenko, A.; Orazzhanova, L.; Mussabayeva, B.; Yelemessova, G.; Kassymova, Z. Soil Structuring Using Interpolyelectrolyte Complexes of Water-soluble Polysaccharides. Polym. Adv. Technol. 2020, 31, 3292–3301. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Gasilova, E.R.; Skorik, Y.A. Nano-Sized Fucoidan Interpolyelectrolyte Complexes: Recent Advances in Design and Prospects for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 2615. [Google Scholar] [CrossRef]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Ruiz-Caro, R.; Veiga, M.-D. Naturally Occurring Polyelectrolytes and Their Use for the Development of Complex-Based Mucoadhesive Drug Delivery Systems: An Overview. Polymers 2021, 13, 2241. [Google Scholar] [CrossRef]
- Nasibullin, S.F.; Dunaeva, J.V.; Akramova, L.A.; Timergalieva, V.R.; Moustafine, R.I. Characteristics of Interpolyelectrolyte Complexes Based on Different Types of Pectin with Eudragit® EPO as Novel Carriers for Colon-Specific Drug Delivery. Int. J. Mol. Sci. 2023, 24, 17622. [Google Scholar] [CrossRef]
- Liao, Q.; Pang, L.; Li, J.-J.; Zhang, C.; Li, J.-X.; Zhang, X.; Mao, T.; Wu, D.-T.; Ma, X.-Y.; Geng, F.-N.; et al. Characterization and Diabetic Wound Healing Benefits of Protein-Polysaccharide Complexes Isolated from an Animal Ethno-Medicine Periplaneta americana L. Int. J. Biol. Macromol. 2022, 195, 466–474. [Google Scholar] [CrossRef]
- Ma, L.; Lu, L.; Zhao, X.; Zhou, Q.; Luo, L.; Ma, D.; Wang, W.; Zhang, W. Dual-Crosslinked Cirsiumsetosum Polysaccharide/Quaternary Chitosan Self-Healing Hydrogel Promotes Wound Healing. Carbohydr. Res. 2025, 556, 109616. [Google Scholar] [CrossRef]
- Li, N.; Huang, J.; Chen, C.; Yu, H.; Tian, H. Construction of Protein-Polysaccharide Complexes: Category, Organoleptic Impaction and Application in the Food Industry. Int. J. Biol. Macromol. 2025, 321, 146422. [Google Scholar] [CrossRef]
- Atheena, P.V.; Basawa, R.; Raval, R. Advancing Wastewater Treatment: Chitin and Derivatives for PPCP Contaminant Mitigation. Polym. Bull. 2024, 81, 14307–14336. [Google Scholar] [CrossRef]
- Yadav, D.; Dutta, J. A Systematic Review on Recent Development of Chitosan/Alginate-Based Polyelectrolyte Complexes for Wastewater Treatment. Int. J. Environ. Sci. Technol. 2024, 21, 3381–3406. [Google Scholar] [CrossRef]
- Das, A.; Prévost, S.; Gradzielski, M. Positional Correlation Length-Induced Morphological Transformation of Interpolyelectrolyte Complexes (IPECs) Made of Polysaccharides: The Role of Molar Charge Ratio. Macromolecules 2025, 58, 10763–10777. [Google Scholar] [CrossRef]
- Schoeller, J.; Itel, F.; Wuertz-Kozak, K.; Gaiser, S.; Luisier, N.; Hegemann, D.; Ferguson, S.J.; Fortunato, G.; Rossi, R.M. PH-Responsive Chitosan/Alginate Polyelectrolyte Complexes on Electrospun PLGA Nanofibers for Controlled Drug Release. Nanomaterials 2021, 11, 1850. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. Thermosensitive Injectable Hydrogel Based on Chitosan-Polygalacturonic Acid Polyelectrolyte Complexes for Bone Tissue Engineering. Carbohydr. Polym. 2022, 294, 119769. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. Development of Chitosan-polygalacturonic Acid Polyelectrolyte Complex Fibrous Scaffolds Using the Hydrothermal Treatment for Bone Tissue Engineering. J. Biomed. Mater. Res. 2023, 111, 354–366. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; Ortega, F.; Rubio, R.G. Equilibrium and Kinetically Trapped Aggregates in Polyelectrolyte–Oppositely Charged Surfactant Mixtures. Curr. Opin. Colloid Interface Sci. 2020, 48, 91–108. [Google Scholar] [CrossRef]
- Varga, I.; Campbell, R.A. General Physical Description of the Behavior of Oppositely Charged Polyelectrolyte/Surfactant Mixtures at the Air/Water Interface. Langmuir 2017, 33, 5915–5924. [Google Scholar] [CrossRef]
- Naderi, A.; Claesson, P.M.; Bergström, M.; Dėdinaitė, A. Trapped Non-Equilibrium States in Aqueous Solutions of Oppositely Charged Polyelectrolytes and Surfactants: Effects of Mixing Protocol and Salt Concentration. Colloids Surf. A 2005, 253, 83–93. [Google Scholar] [CrossRef]
- Mészáros, R.; Thompson, L.; Bos, M.; Varga, I.; Gilányi, T. Interaction of Sodium Dodecyl Sulfate with Polyethyleneimine: Surfactant-Induced Polymer Solution Colloid Dispersion Transition. Langmuir 2003, 19, 609–615. [Google Scholar] [CrossRef]
- Puente-Santamaría, A.; Ortega, F.; Maestro, A.; Rubio, R.G.; Guzmán, E. Non-Equilibrium States in Polyelectrolyte-Surfactant Systems at Fluid Interfaces: A Critical Review. Curr. Opin. Colloid Interface Sci. 2024, 71, 101804. [Google Scholar] [CrossRef]
- Sæther, H.V.; Holme, H.K.; Maurstad, G.; Smidsrød, O.; Stokke, B.T. Polyelectrolyte Complex Formation Using Alginate and Chitosan. Carbohydr. Polym. 2008, 74, 813–821. [Google Scholar] [CrossRef]
- Bago Rodriguez, A.M.; Binks, B.P.; Sekine, T. Novel Stabilisation of Emulsions by Soft Particles: Polyelectrolyte Complexes. Faraday Discuss. 2016, 191, 255–285. [Google Scholar] [CrossRef] [PubMed]
- Bago Rodriguez, A.M.; Binks, B.P.; Sekine, T. Emulsion Stabilisation by Complexes of Oppositely Charged Synthetic Polyelectrolytes. Soft Matter 2018, 14, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Vasquez, F.J.; Ortega, F.; Rubio, R.G.; Guzmán, E. Bio-Based Interpolyelectrolyte Complexes for the Stabilization of Pickering-like Emulsions. Colloids Interfaces 2025, 9, 9. [Google Scholar] [CrossRef]
- Llamas, S.; Guzman, E.; Akanno, A.; Fernadez-Peña, L.; Ortega, F.; Campbell, R.A.; Miller, R.; Rubio, R.G. Study of the Liquid/Vapor Interfacial Properties of Concentrated Polyelectrolyte−Surfactant Mixtures Using Surface Tensiometry and Neutron Reflectometry: Equilibrium, Adsorption Kinetics, and Dilational Rheology. J. Phys. Chem. C 2018, 122, 4419–4427. [Google Scholar] [CrossRef]
- Llamas, S.; Fernández-Peña, L.; Akanno, A.; Guzmán, E.; Ortega, V.; Ortega, F.; Csaky, A.G.; Campbell, R.A.; Rubio, R.G. Towards Understanding the Behavior of Polyelectrolyte-Surfactant Mixtures at the Water/Vapor Interface Closer to Technologically-Relevant Conditions. Phys. Chem. Chem. Phys. 2018, 20, 1395–1407. [Google Scholar] [CrossRef]
- Bell, C.G.; Breward, C.J.W.; Howell, P.D.; Penfold, J.; Thomas, R.K. A Theoretical Analysis of the Surface Tension Profiles of Strongly Interacting Polymer–Surfactant Systems. J. Colloid Interface Sci. 2010, 350, 486–493. [Google Scholar] [CrossRef]
- Jing, Z.; Li, Y.; Zhang, Y.; Chen, K.; Sun, Y.; Wang, M.; Chen, B.; Zhao, S.; Jin, Y.; Du, Q.; et al. Simple Synthesis of Chitosan/Alginate/Graphene Oxide/UiO-67 Amphoteric Aerogels: Characterization, Adsorption Mechanism and Application for Removal of Cationic and Anionic Dyes from Complex Dye Media. Int. J. Biol. Macrmol. 2023, 242, 124683. [Google Scholar] [CrossRef]
- Braccini, S.; Pecorini, G.; Biagini, S.; Tacchini, C.; Battisti, A.; Puppi, D. Chitosan/Alginate Polyelectrolyte Complex Hydrogels by Additive Manufacturing for in Vitro 3D Ovarian Cancer Modeling. Int. J. Biol. Macromol. 2025, 296, 139795. [Google Scholar] [CrossRef]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; G Rubio, R.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Ormeño-Martínez, M.; Guzmán, E.; Fernández-Peña, L.; Greaves, A.J.; Bureau, L.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Roles of Polymer Concentration and Ionic Strength in the Deposition of Chitosan of Fungal Origin onto Negatively Charged Surfaces. Biomimetics 2024, 9, 534. [Google Scholar] [CrossRef]
- Ravera, F.; Santini, E.; Loglio, G.; Ferrari, M.; Liggieri, L. Effect of Nanoparticles on the Interfacial Properties of Liquid/Liquid and Liquid/Air Surface Layers. J. Phys. Chem. B 2006, 110, 19543–19551. [Google Scholar] [CrossRef]
- Guzmán, E.; Maestro, A.; Ortega, F.; Rubio, R.G. Association of Oppositely Charged Polyelectrolyte and Surfactant in Solution: Equilibrium and Nonequilibrium Features. J. Phys. Cond. Matter 2023, 35, 323001. [Google Scholar] [CrossRef]
- Carbone, C.; Navarro-Arrebola, I.; Liggieri, L.; Ortega, F.; Rubio, R.G.; Guzmán, E. Interfacial Dilational Rheology of Chitosan-Silica Nanocomposite Films at the Aqueous Dispersion/Air Interface. J. Mol. Liquids 2025, 425, 127273. [Google Scholar] [CrossRef]
- Smoluchowski, M. Handbuch Der Elektrizität und Des Magnetismus; Barth-Verlag: Leipzig, Germany, 1921. [Google Scholar]
- Voinova, M.V.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic Acoustic Response of Layered Polymer Films at Fluid-Solid Interfaces: Continuum Mechanics Approach. Phys. Script. 1999, 59, 391–396. [Google Scholar] [CrossRef]
- De Stefano, C.; Gianguzza, A.; Piazzese, D.; Sammartano, S. Modelling of Proton and Metal Exchange in the Alginate Biopolymer. Anal. Bioanal. Chem. 2005, 383, 587–596. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.; Cabrera, G.; Peniche, C.; Rinaudo, M. Conductimetric Study of the Interpolyelectrolyte Reaction between Chitosan and Polygalacturonic Acid. Polymer 2000, 41, 2373–2378. [Google Scholar] [CrossRef]
- Nivard, M.; Ortega, F.; Rubio, R.G.; Guzmán, E. Adsorption and Bulk Assembly of Quaternized Hydroxyethylcellulose–Anionic Surfactant Complexes on Negatively Charged Substrates. Polymers 2025, 17, 207. [Google Scholar] [CrossRef]
- Akanno, A.; Guzmán, E.; Ortega, F.; Rubio, R.G. Behavior of the Water/Vapor Interface of Chitosan Solutions with an Anionic Surfactant: Effect of Polymer-Surfactant Interactions. Phys. Chem. Chem. Phys. 2020, 22, 23360–23373. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Llamas, S.; Maestro, A.; Fernández-Peña, L.; Akanno, A.; Miller, R.; Ortega, F.; Rubio, R.G. Polymer-Surfactant Systems in Bulk and at Fluid Interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Rubio, R.G.; Ortega, F. A Closer Physico-Chemical Look to the Layer-by-Layer Electrostatic Self-Assembly of Polyelectrolyte Multilayers. Adv. Colloid Interface Sci. 2020, 282, 102197. [Google Scholar] [CrossRef]
- Subbotin, A.V.; Semenov, A.N. The Structure of Polyelectrolyte Complex Coacervates and Multilayers. Macromolecules 2021, 54, 1314–1328. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-López, R.; Puente-Santamaría, A.; Rubio, R.G.; Ortega, F.; Guzmán, E. Formation and Interfacial Behavior of Chitosan–Alginate Interpolyelectrolyte Complexes: From Bulk Dispersions to Layer-by-Layer Films. Polymers 2025, 17, 3073. https://doi.org/10.3390/polym17223073
Martín-López R, Puente-Santamaría A, Rubio RG, Ortega F, Guzmán E. Formation and Interfacial Behavior of Chitosan–Alginate Interpolyelectrolyte Complexes: From Bulk Dispersions to Layer-by-Layer Films. Polymers. 2025; 17(22):3073. https://doi.org/10.3390/polym17223073
Chicago/Turabian StyleMartín-López, Rafael, Ana Puente-Santamaría, Ramón G. Rubio, Francisco Ortega, and Eduardo Guzmán. 2025. "Formation and Interfacial Behavior of Chitosan–Alginate Interpolyelectrolyte Complexes: From Bulk Dispersions to Layer-by-Layer Films" Polymers 17, no. 22: 3073. https://doi.org/10.3390/polym17223073
APA StyleMartín-López, R., Puente-Santamaría, A., Rubio, R. G., Ortega, F., & Guzmán, E. (2025). Formation and Interfacial Behavior of Chitosan–Alginate Interpolyelectrolyte Complexes: From Bulk Dispersions to Layer-by-Layer Films. Polymers, 17(22), 3073. https://doi.org/10.3390/polym17223073








