Biocompatibility and Safety of 3D Printing Resins for Orthodontic Aligners: A Critical Review of Current Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection, Data Collection, and Data Items
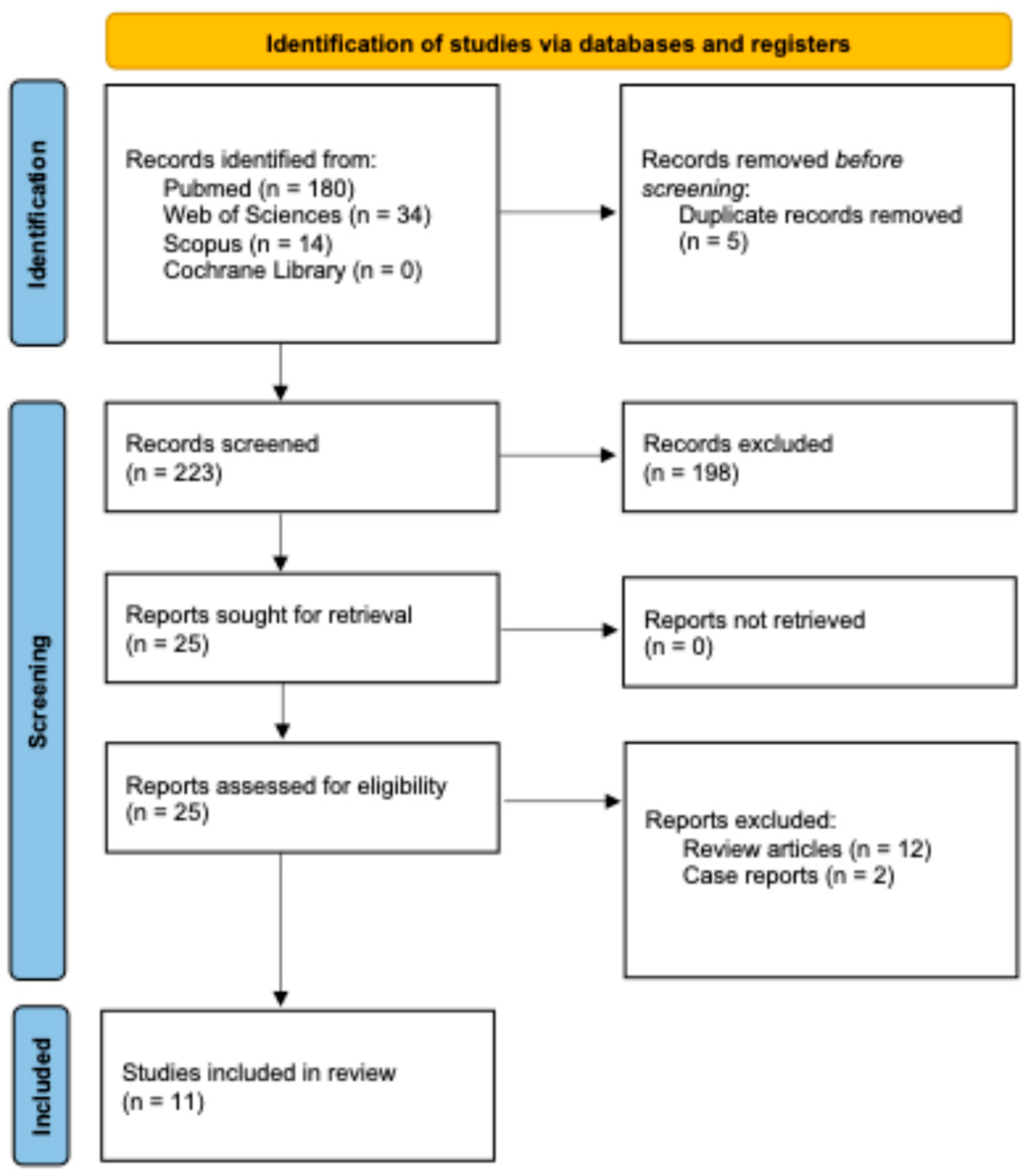
3. Results
3.1. In Vitro Tests of Leaching, Cytotoxicity, and Estrogenicity
3.2. Clinical Studies
3.3. Evaluation of Biofilm Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Torkomian, T.; de la Iglesia, F.; Puigdollers, A. 3D-Printed Clear Aligners: An Emerging Alternative to the Conventional Thermoformed Aligners?—A Systematic Review. J. Dent. 2025, 155, 105616. [Google Scholar] [CrossRef]
- Alkhamees, A. The New Additive Era of Orthodontics: 3D-Printed Aligners and Shape Memory Polymers—The Latest Trend—And Their Environmental Implications. J. Orthod. Sci. 2024, 13, 55. [Google Scholar] [CrossRef]
- Narongdej, P.; Hassanpour, M.; Alterman, N.; Rawlins-Buchanan, F.; Barjasteh, E. Advancements in Clear Aligner Fabrication: A Comprehensive Review of Direct-3D Printing Technologies. Polymers 2024, 16, 371. [Google Scholar] [CrossRef]
- Jungbauer, R.; Sabbagh, H.; Janjic Rankovic, M.; Becker, K. 3D Printed Orthodontic Aligners—A Scoping Review. Appl. Sci. 2024, 14, 10084. [Google Scholar] [CrossRef]
- Goracci, C.; Juloski, J.; D’Amico, C.; Balestra, D.; Volpe, A.; Juloski, J.; Vichi, A. Clinically Relevant Properties of 3D Printable Materials for Intraoral Use in Orthodontics: A Critical Review of the Literature. Materials 2023, 16, 2166. [Google Scholar] [CrossRef] [PubMed]
- Panayi, N.C. Directly Printed Aligner: Aligning with the Future. Turk. J. Orthod. 2023, 36, 62–69. [Google Scholar] [CrossRef]
- Kau, C.H.; Soh, J.; Christou, T.; Mangal, A. Orthodontic Aligners: Current Perspectives for the Modern Orthodontic Office. Medicina 2023, 59, 1773. [Google Scholar] [CrossRef] [PubMed]
- Campobasso, A.; Battista, G.; Lo Muzio, E.; Colombo, S.; Paglia, M.; Federici Canova, F.; Gianolio, A.; Beretta, M. New 3D Printed Polymers in Orthodontics: A Scoping Review. Eur. J. Paediatr. Dent. 2023, 24, 224–228. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Alwafi, A.; Liu, X.; Andrews, J.; Ludwig, B.; Bichu, A.Y.; Zou, B. Advances in Orthodontic Clear Aligner Materials. Bioact. Mater. 2023, 22, 384–403. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Mapelli, A.; Maspero, C.; Santaniello, T.; Serafin, M.; Farronato, M.; Caprioglio, A. Direct 3D Printing of Clear Orthodontic Aligners: Current State and Future Possibilities. Materials 2021, 14, 1799. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, H.; Kim, S.H.; Kim, S.J.; Cha, J.-Y.; Lee, S.Y.; Lee, J.; Min, J.; Jang, S.; Khan, T.A.; et al. Mechanical and Viscoelastic Properties of a Temperature-Responsive Photocurable Resin for 3D Printed Orthodontic Clear Aligners. Sci. Rep. 2025, 15, 23530. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, H.; Kim, H.-J.; Chung, C.J.; Choi, Y.J.; Kim, S.-J.; Cha, J.-Y. Thermo-Mechanical Properties of 3D Printed Photocurable Shape Memory Resin for Clear Aligners. Sci. Rep. 2022, 12, 6246. [Google Scholar] [CrossRef]
- Atta, I.; Bourauel, C.; Alkabani, Y.; Mohamed, N.; Kim, H.; Alhotan, A.; Ghoneima, A.; Elshazly, T. Physiochemical and Mechanical Characterisation of Orthodontic 3D Printed Aligner Material Made of Shape Memory Polymers (4D Aligner Material). J. Mech. Behav. Biomed. Mater. 2024, 150, 106337. [Google Scholar] [CrossRef] [PubMed]
- Bleilöb, M.; Welte-Jzyk, C.; Knode, V.; Ludwig, B.; Erbe, C. Biocompatibility of Variable Thicknesses of a Novel Directly Printed Aligner in Orthodontics. Sci. Rep. 2025, 15, 3279. [Google Scholar] [CrossRef]
- Bor, S.; Kaya, Y.; Demiral, A.; Güngörmüş, M. Post-Process Cytotoxicity of Resins in Clear Aligner Fabrication. Polymers 2025, 17, 1776. [Google Scholar] [CrossRef] [PubMed]
- Jahng, K.J.; Lee, M.M.; McCray, J.F.; Adel, S.M.; Miranda, G.; Kim, K.B. Forces and Moments on a Maxillary Lateral Incisor Using 3D-Printed Aligners with Pressure Points: An In Vitro Study. Eur. J. Orthod. 2025, 47, cjaf061. [Google Scholar] [CrossRef]
- Kim, D.-W.; Lee, H.-J.; Kim, K.B.; Kim, S.-H.; Kim, S.-S.; Park, S.-B.; Choi, Y.-K.; Kim, Y.-I. Force and Moment Analysis of Clear Aligners: Impact of Material Properties and Design on Premolar Rotation. Korean J. Orthod. 2025, 55, 212–223. [Google Scholar] [CrossRef]
- Šimunović, L.; Miličević, A.M.; Brenko, L.; Haramina, T.; Meštrović, S. Absorption and Desorption Dynamics of Thermoformed and 3-Dimensional-Printed Orthodontic Aligners. Am. J. Orthod. Dentofac. Orthop. 2025, 168, 466–476. [Google Scholar] [CrossRef]
- Zecca, P.A.; Borgese, M.; Raspanti, M.; Zara, F.; Fastuca, R.; Serafin, M.; Caprioglio, A. Comparative Microscopic Analysis of Plastic Dispersion from 3D-Printed and Thermoformed Orthodontic Aligners. Eur. J. Orthod. 2025, 47, cjaf014. [Google Scholar] [CrossRef]
- Wendl, T.; Wendl, B.; Proff, P. An Analysis of Initial Force and Moment Delivery of Different Aligner Materials. Biomed. Eng./Biomed. Tech. 2025, 70, 259–267. [Google Scholar] [CrossRef]
- Goracci, C.; Bosoni, C.; Marti, P.; Scotti, N.; Franchi, L.; Vichi, A. Influence of Printing Orientation on Surface Roughness and Gloss of 3D Printed Resins for Orthodontic Devices. Materials 2025, 18, 523. [Google Scholar] [CrossRef]
- Bae, B.G.; Kim, Y.H.; Lee, G.H.; Lee, J.; Min, J.; Kim, H.; Shin, J.W.; Chae, H.S. A Study on the Compressive Strength of Three-Dimensional Direct Printing Aligner Material for Specific Designing of Clear Aligners. Sci. Rep. 2025, 15, 2489. [Google Scholar] [CrossRef]
- Kim, J.-E.; Mangal, U.; Yu, J.-H.; Kim, G.-T.; Kim, H.; Seo, J.-Y.; Cha, J.-Y.; Lee, K.-J.; Kwon, J.-S.; Choi, S.-H. Evaluation of the Effects of Temperature and Centrifugation Time on Elimination of Uncured Resin from 3D-Printed Dental Aligners. Sci. Rep. 2024, 14, 15206. [Google Scholar] [CrossRef]
- Sayahpour, B.; Eslami, S.; Stuhlfelder, J.; Bühling, S.; Dahmer, I.; Goteni, M.; Kopp, S.; Nucci, L. Evaluation of Thickness of 3D Printed versus Thermoformed Aligners: A Prospective In Vivo Ageing Experiment. Orthod. Craniofac. Res. 2024, 27, 831–838. [Google Scholar] [CrossRef]
- Iodice, G.; Ludwig, B.; Polishchuk, E.; Petruzzelli, R.; Di Cunto, R.; Husam, S.; Farella, M. Effect of Post-printing Curing Time on Cytotoxicity of Direct Printed Aligners: A Pilot Study. Orthod. Craniofac. Res. 2024, 27, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, L.; Jurela, A.; Sudarević, K.; Bačić, I.; Haramina, T.; Meštrović, S. Influence of Post-Processing on the Degree of Conversion and Mechanical Properties of 3D-Printed Polyurethane Aligners. Polymers 2023, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Sayahpour, B.; Zinelis, S.; Polychronis, G.; Eliades, T.; Goteni, M.; Kopp, S.; Eslami, S. Effects of Intraoral Aging on Mechanical Properties of Directly Printed Aligners vs. Thermoformed Aligners: An In Vivo Prospective Investigation. Eur. J. Orthod. 2024, 46, cjad063. [Google Scholar] [CrossRef] [PubMed]
- Eslami, S.; Kopp, S.; Goteni, M.; Dahmer, I.; Sayahpour, B. Alterations in the Surface Roughness and Porosity Parameters of Directly Printed and Invisalign Aligners after 1 Week of Intraoral Usage: An in Vivo Prospective Investigation. Am. J. Orthod. Dentofac. Orthop. 2024, 165, 73–79. [Google Scholar] [CrossRef]
- Migliorati, M.; Drago, S.; Castroflorio, T.; Pesce, P.; Battista, G.; Campobasso, A.; Gastaldi, G.; Valvecchi, F.F.; Mari, A. De Accuracy of Orthodontic Movements with 3D Printed Aligners: A Prospective Observational Pilot Study. Korean J. Orthod. 2024, 54, 160–170. [Google Scholar] [CrossRef]
- Šimunović, L.; Čekalović Agović, S.; Marić, A.J.; Bačić, I.; Klarić, E.; Uribe, F.; Meštrović, S. Color and Chemical Stability of 3D-Printed and Thermoformed Polyurethane-Based Aligners. Polymers 2024, 16, 1067. [Google Scholar] [CrossRef]
- Manoukakis, T.; Nikolaidis, A.K.; Koulaouzidou, E.A. Polymerization Kinetics of 3D-Printed Orthodontic Aligners under Different UV Post-Curing Conditions. Prog. Orthod. 2024, 25, 42. [Google Scholar] [CrossRef]
- McKay, A.; McCray, J.; Bankhead, B.; Lee, M.M.; Miranda, G.; Adel, S.M.; Kim, K.B. Forces and Moments Generated during Extrusion of a Maxillary Central Incisor with Clear Aligners: An In Vitro Study. BMC Oral. Health 2023, 23, 495. [Google Scholar] [CrossRef]
- Grant, J.; Foley, P.; Bankhead, B.; Miranda, G.; Adel, S.M.; Kim, K.B. Forces and Moments Generated by 3D Direct Printed Clear Aligners of Varying Labial and Lingual Thicknesses during Lingual Movement of Maxillary Central Incisor: An In Vitro Study. Prog. Orthod. 2023, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, S.-H.; Yu, H.-S.; Kim, S.-J.; Kim, H.; Kim, K.B.; Cha, J.-Y. Comparison of Translucency, Thickness, and Gap Width of Thermoformed and 3D-Printed Clear Aligners Using Micro-CT and Spectrophotometer. Sci. Rep. 2023, 13, 10921. [Google Scholar] [CrossRef]
- Willi, A.; Patcas, R.; Zervou, S.-K.; Panayi, N.; Schätzle, M.; Eliades, G.; Hiskia, A.; Eliades, T. Leaching from a 3D-Printed Aligner Resin. Eur. J. Orthod. 2023, 45, 244–249. [Google Scholar] [CrossRef]
- Alessandra, C.; Anastasia, A.; Giovanni, B.; Francesca, P.; Marco, M.; Sara, D.; Eleonora, L.M.; Giorgio, M. Comparison of the Cytotoxicity of 3D-Printed Aligners Using Different Post-Curing Procedures: An In Vitro Study. Australas. Orthod. J. 2023, 39, 49–56. [Google Scholar] [CrossRef]
- Koenig, N.; Choi, J.-Y.; McCray, J.; Hayes, A.; Schneider, P.; Kim, K.B. Comparison of Dimensional Accuracy between Direct-Printed and Thermoformed Aligners. Korean J. Orthod. 2022, 52, 249–257. [Google Scholar] [CrossRef]
- Zinelis, S.; Panayi, N.; Polychronis, G.; Papageorgiou, S.N.; Eliades, T. Comparative Analysis of Mechanical Properties of Orthodontic Aligners Produced by Different Contemporary 3D Printers. Orthod. Craniofac Res. 2022, 25, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, H.; Papageorgiou, S.N.; Panayi, N.; Iliadi, A.; Eliades, T.; Kletsas, D. Cytotoxicity and Estrogenicity of a Novel 3-Dimensional Printed Orthodontic Aligner. Am. J. Orthod. Dentofac. Orthop. 2022, 162, e116–e122. [Google Scholar] [CrossRef]
- Knode, V.; Ludwig, B.; Retrouvey, J.-M.; Pandis, N.; Schmid, J.Q.; Erbe, C.; Fleming, P.S. Directly Printed Aligner Therapy: A 12-Month Evaluation of Application and Effectiveness. Am. J. Orthod. Dentofac. Orthop. 2025, 167, 73–79. [Google Scholar] [CrossRef]
- Erbe, C.; Ludwig, B.; Bleilöb, M. Unlocking the Biological Insights of 3D Printed Aligners: A Look at Current Findings. Semin. Orthod. 2025, 31, 139–143. [Google Scholar] [CrossRef]
- Dantagnan, C.-A.; Babajko, S.; Nassif, A.; Porporatti, A.; Attal, J.-P.; Dursun, E.; Nguyen, J.-F.; Bosco, J. Biocompatibility of Direct Printed Clear Aligners: A Systematic Review of In Vitro Studies. Int. Orthod. 2025, 23, 101028. [Google Scholar] [CrossRef]
- Eliades, T.; Panayi, N.; Papageorgiou, S.N. From Biomimetics to Smart Materials and 3D Technology: Applications in Orthodontic Bonding, Debonding, and Appliance Design or Fabrication. Jpn. Dent. Sci. Rev. 2023, 59, 403–411. [Google Scholar] [CrossRef]
- Wu, C.; Mangal, U.; Seo, J.-Y.; Kim, H.; Bai, N.; Cha, J.-Y.; Lee, K.-J.; Kwon, J.-S.; Choi, S.-H. Enhancing Biofilm Resistance and Preserving Optical Translucency of 3D Printed Clear Aligners through Carboxybetaine-Copolymer Surface Treatment. Dent. Mater. 2024, 40, 1575–1583. [Google Scholar] [CrossRef]
- Pasaoglu Bozkurt, A.; Demirci, M.; Erdogan, P.; Kayalar, E. Comparison of Microbial Adhesion and Biofilm Formation on Different Orthodontic Aligners. Am. J. Orthod. Dentofac. Orthop. 2025, 167, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating Guidance for Reporting Systematic Reviews: Development of the PRISMA 2020 Statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Turkalj, M.; Pully, D.; Li, Q.; Sun, W.; Ghosh, M.; Čokić, S.M.; Vanoirbeek, J.; Van Meerbeek, B.; Van Landuyt, K.L. Monomer Elution and Cytotoxicity of 3D-Printed Resin-Based Composites. Dent. Mater. 2025, 41, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Cokic, S.M.; Duca, R.C.; De Munck, J.; Hoet, P.; Van Meerbeek, B.; Smet, M.; Godderis, L.; Van Landuyt, K.L. Saturation Reduces In-Vitro Leakage of Monomers from Composites. Dent. Mater. 2018, 34, 579–586. [Google Scholar] [CrossRef]
- Josic, U.; Teti, G.; Ionescu, A.; Maravic, T.; Mazzitelli, C.; Cokic, S.; Van Meerbeek, B.; Falconi, M.; Brambilla, E.; Mazzoni, A.; et al. Cytotoxicity and Microbiological Behavior of Universal Resin Composite Cements. Dent. Mater. 2024, 40, 1515–1523. [Google Scholar] [CrossRef]
- Finer, Y.; Santerre, J.P. Biodegradation of a Dental Composite by Esterases: Dependence on Enzyme Concentration and Specificity. J. Biomater. Sci. Polym. Ed. 2003, 14, 837–849. [Google Scholar] [CrossRef]
- Tsitrou, E.; Kelogrigoris, S.; Koulaouzidou, E.; Antoniades-Halvatjoglou, M.; Koliniotou-Koumpia, E.; Noort, R. Effect of Extraction Media and Storage Time on the Elution of Monomers from Four Contemporary Resin Composite Materials. Toxicol. Int. 2014, 21, 90. [Google Scholar] [CrossRef]
- ISO 10993-13:2010; Biological Evaluation of Medical Devices. Part 13, Identification and Quantification of Degradation Products from Polymeric Medical Devices. BSI: Kolkata, India, 2010; ISBN 9780580577192.
- Rosa, V.; Silikas, N.; Yu, B.; Dubey, N.; Sriram, G.; Zinelis, S.; Lima, A.F.; Bottino, M.C.; Ferreira, J.N.; Schmalz, G.; et al. Guidance on the Assessment of Biocompatibility of Biomaterials: Fundamentals and Testing Considerations. Dent. Mater. 2024, 40, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, C.; Rahimi, B.; Padova, D.; Rooholghodos, S.A.; Bienek, D.R.; Luo, X.; Kaufman, G.; Raub, C.B. Oral mucosa-on-a-chip to assess layer-specific responses to bacteria and dental materials. Biomicrofluidics 2018, 12, 054106. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, M.; Słowik-Rylska, M.; Cyran-Stemplewska, S.; Gieroń, M.; Nowak-Starz, G.; Kręcisz, B. Acrylates as a Significant Causes of Allergic Contact Dermatitis—New Sources of Exposure. Adv. Dermatol. Allergol. 2021, 38, 555–560. [Google Scholar] [CrossRef]
- Freitas, M.P.M. Aligners, Environmental Contamination, and The Role of Orthodontics. Angle Orthod. 2022, 92, 148–149. [Google Scholar] [CrossRef]
- Raghavan, A.S.; Pottipalli Sathyanarayana, H.; Kailasam, V.; Padmanabhan, S. Comparative Evaluation of Salivary Bisphenol A Levels in Patients Wearing Vacuum-Formed and Hawley Retainers: An In-Vivo Study. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.K.; Miettinen, V.; Alakuijala, P. Residual Monomer Content and Its Release into Water from Denture Base Materials. Dent. Mater. 1995, 11, 338–342. [Google Scholar] [CrossRef]
- Martim, G.C.; Pfeifer, C.S.; Girotto, E.M. Novel Urethane-Based Polymer for Dental Applications with Decreased Monomer Leaching. Mater. Sci. Eng. C 2017, 72, 192–201. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Domarecka, M.; Kopacz, K.; Sokolowski, J.; Bociong, K. An Evaluation of the Properties of Urethane Dimethacrylate-Based Dental Resins. Materials 2021, 14, 2727. [Google Scholar] [CrossRef]
| Electronic Database (Number of Hits on 20 July 2025) | Search Strategy |
|---|---|
| Medline via PubMed (n = 180) https://pubmed.ncbi.nlm.nih.gov | ((3d AND printed AND aligner) OR (3d- AND printed AND aligner) OR (direct AND printed AND aligner)) AND (biocompatibility OR cytotoxicity OR estrogenicity OR genotoxicity OR allergy OR hypersensitivity OR biofilm) |
| Web of Science (n = 34) https://www.webofscience.com | TS = ((“3D printed aligner” OR “clear aligner” OR “orthodontic aligner”) AND (material * OR resin * OR polymer *)) AND TS = (biocompatibility OR toxicity OR degradation OR biofilm) |
| Scopus (n = 14) https://www.scopus.com | (TITLE-ABS-KEY (“3D printed aligner”) OR TITLE-ABS-KEY (“3D-printed aligner”) OR TITLE-ABS-KEY (“direct printed aligner”) AND TITLE-ABS-KEY (biocompatibility OR cytotoxicity OR estrogenicity OR genotoxicity OR allergy OR hypersensitivity OR biofilm)) |
| Cochrane Library (n = 0) https://www.cochranelibrary.com | (“3D printed aligner” OR “3D-printed aligner” OR “direct printed aligner”) AND (biocompatibility OR cytotoxicity OR estrogenicity OR genotoxicity OR allergy OR hypersensitivity OR biofilm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goracci, C.; Mangal, U.; Čokić, S.M.; Mazzoni, A.; Vichi, A.; Josic, U. Biocompatibility and Safety of 3D Printing Resins for Orthodontic Aligners: A Critical Review of Current Evidence. Polymers 2025, 17, 3060. https://doi.org/10.3390/polym17223060
Goracci C, Mangal U, Čokić SM, Mazzoni A, Vichi A, Josic U. Biocompatibility and Safety of 3D Printing Resins for Orthodontic Aligners: A Critical Review of Current Evidence. Polymers. 2025; 17(22):3060. https://doi.org/10.3390/polym17223060
Chicago/Turabian StyleGoracci, Cecilia, Utkarsh Mangal, Stevan M. Čokić, Annalisa Mazzoni, Alessandro Vichi, and Uros Josic. 2025. "Biocompatibility and Safety of 3D Printing Resins for Orthodontic Aligners: A Critical Review of Current Evidence" Polymers 17, no. 22: 3060. https://doi.org/10.3390/polym17223060
APA StyleGoracci, C., Mangal, U., Čokić, S. M., Mazzoni, A., Vichi, A., & Josic, U. (2025). Biocompatibility and Safety of 3D Printing Resins for Orthodontic Aligners: A Critical Review of Current Evidence. Polymers, 17(22), 3060. https://doi.org/10.3390/polym17223060










