Solvent-Free Synthesis of P(MMA-AA) Copolymers and Their Application as Sustainable Primers for Concrete Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Copolymers of Methyl Methacrylate and Acrylic Acid
2.3. Primer Film Formation
2.4. Characterization of Materials and Analytical Methods
3. Results and Discussion
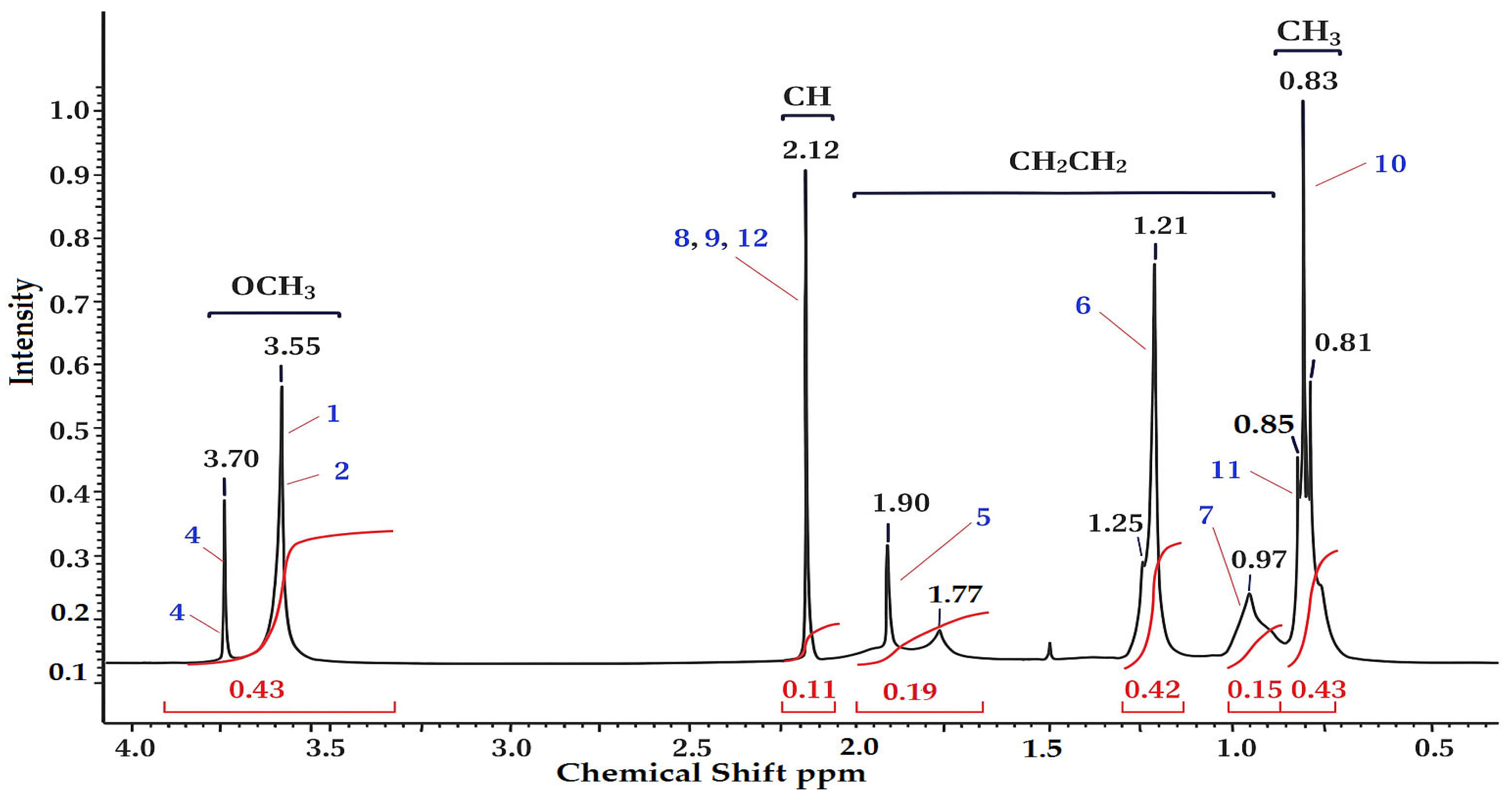
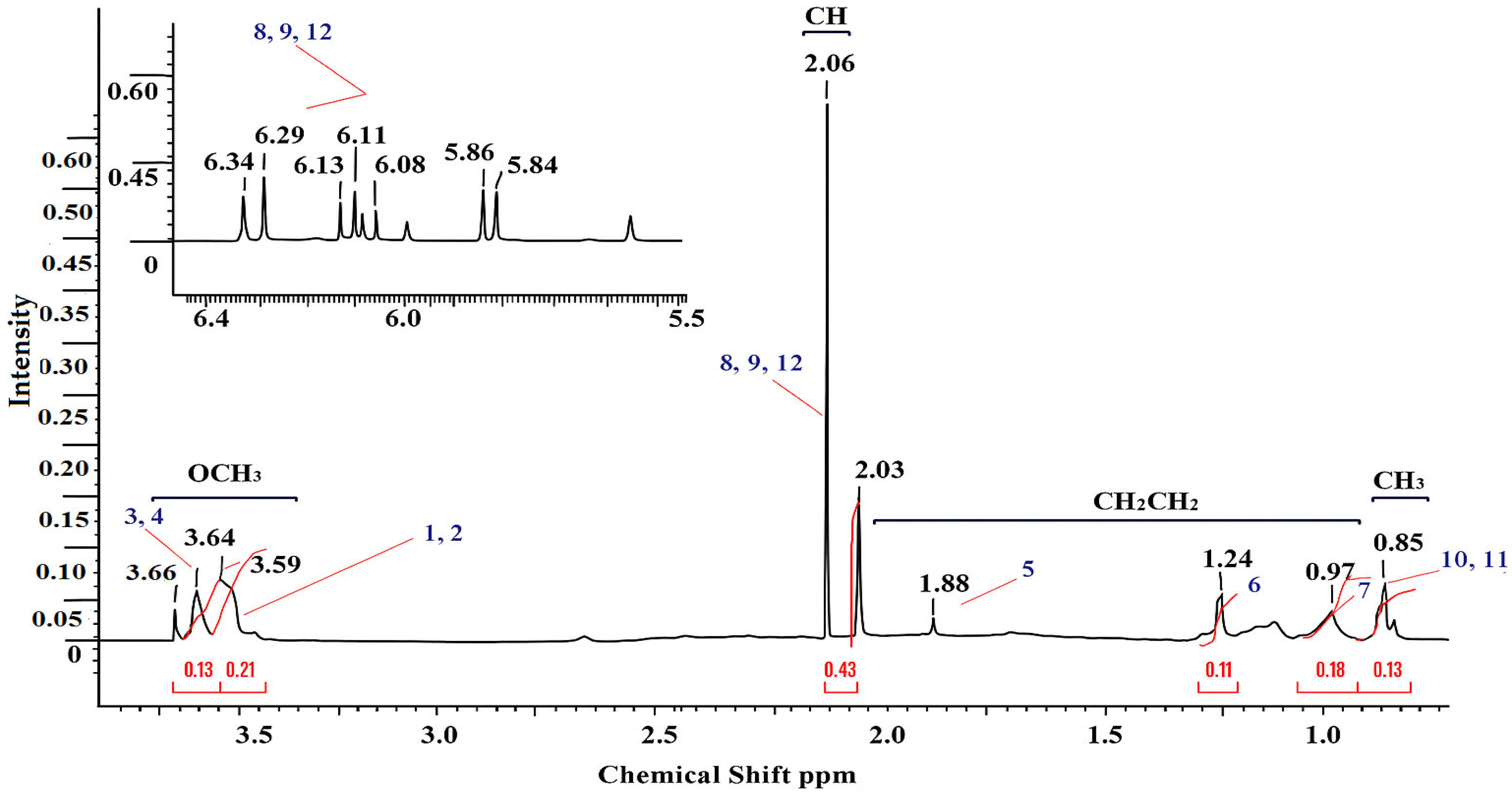
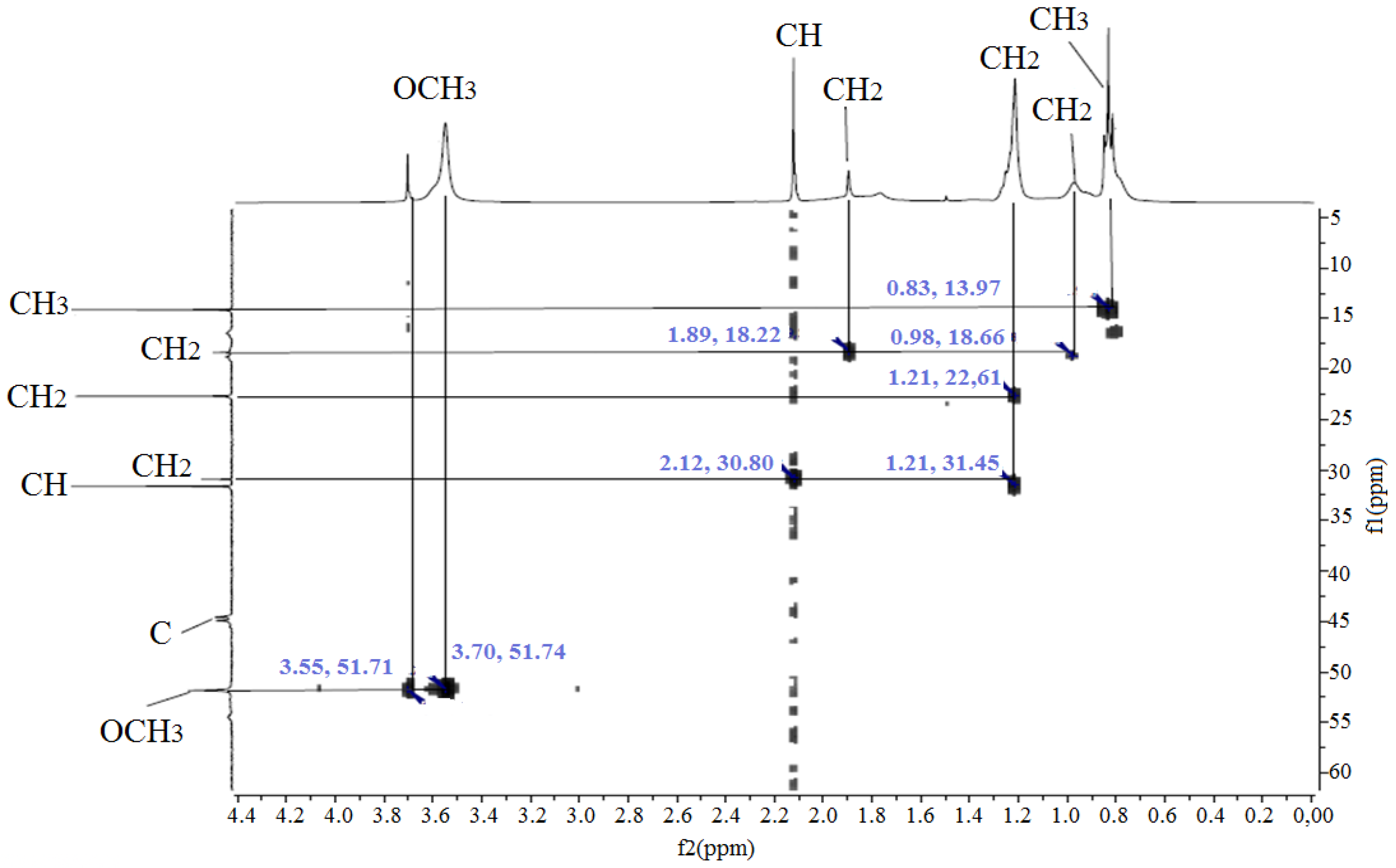
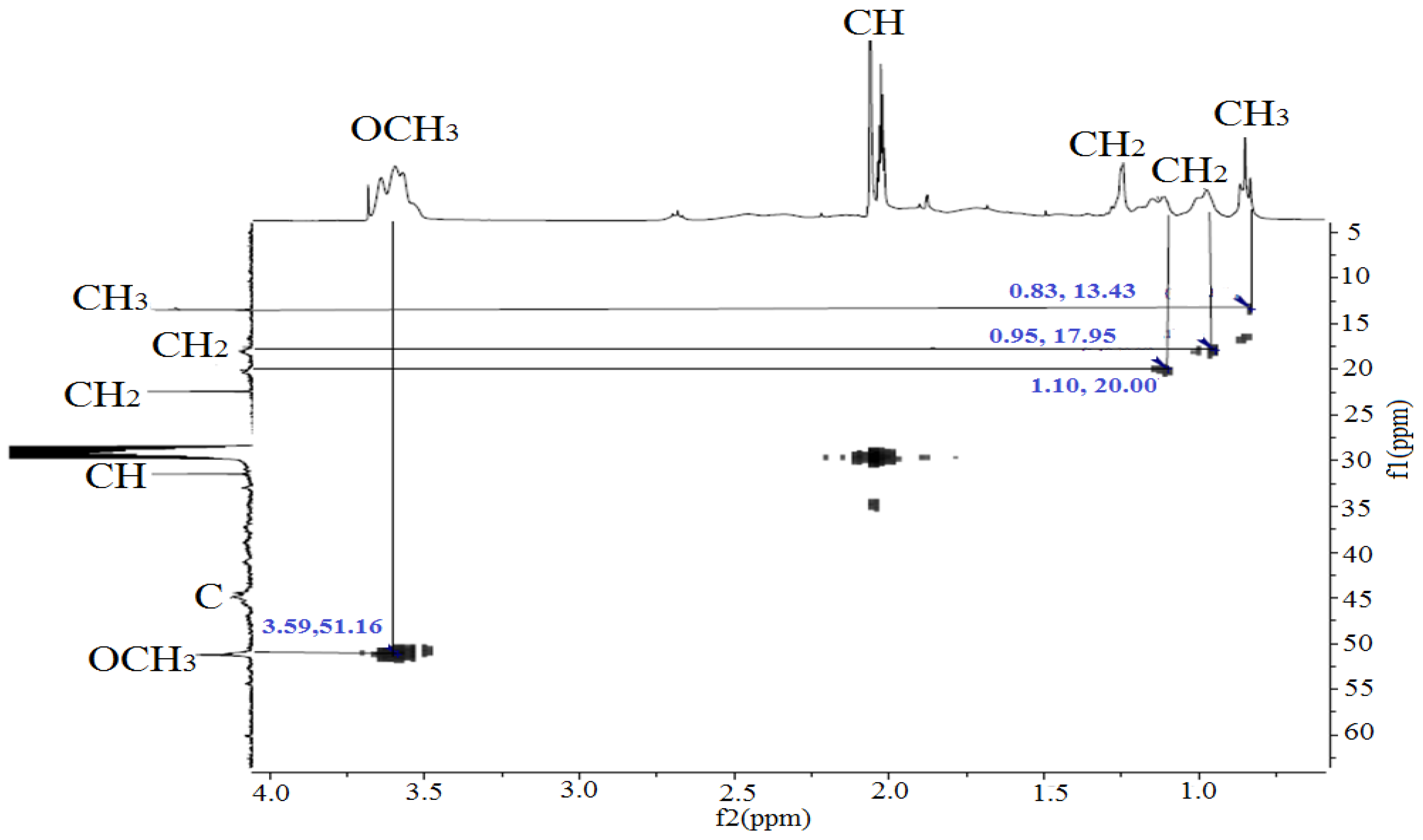
3.1. 1H and 13C NMR Spectroscopy
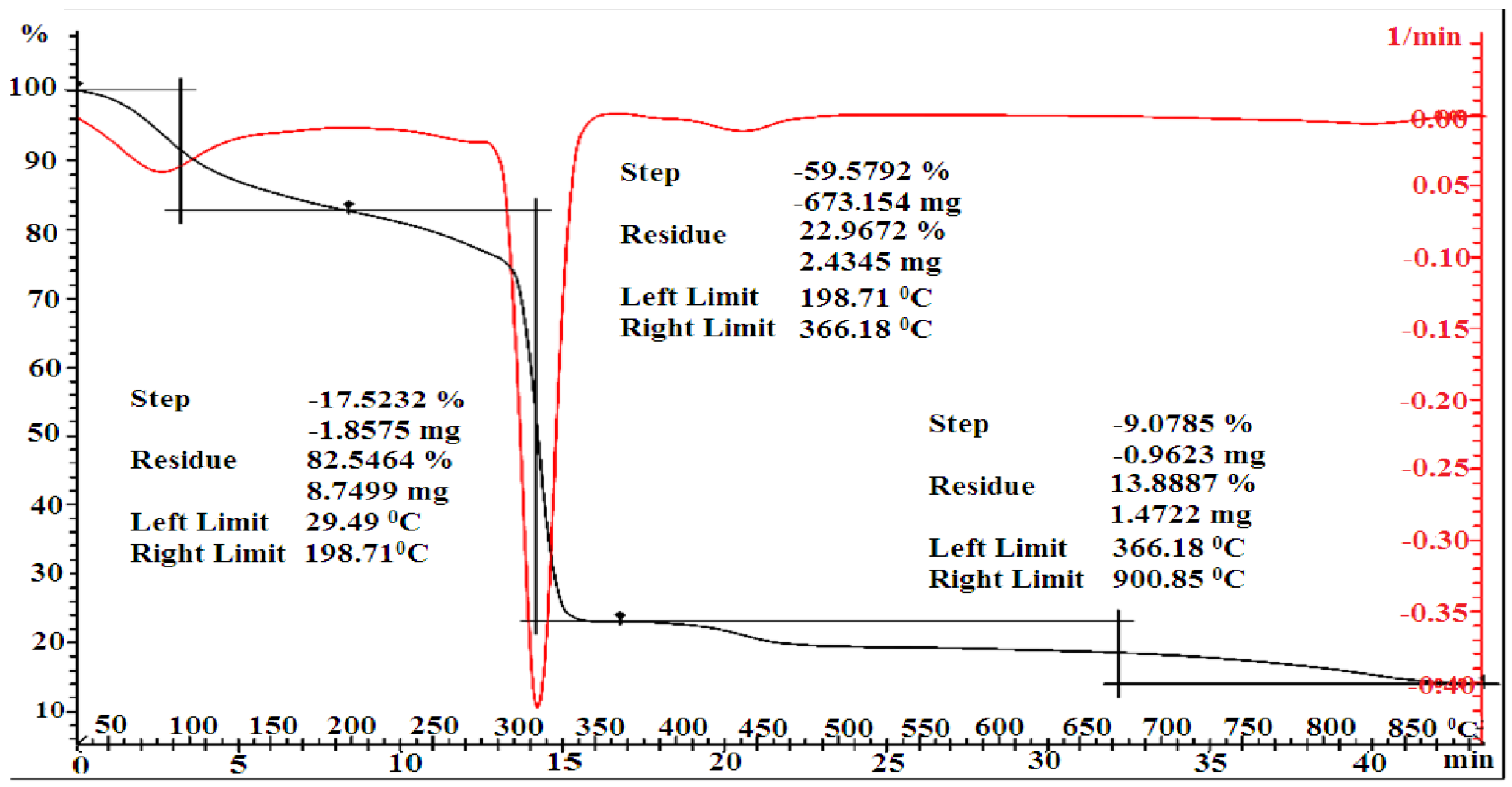
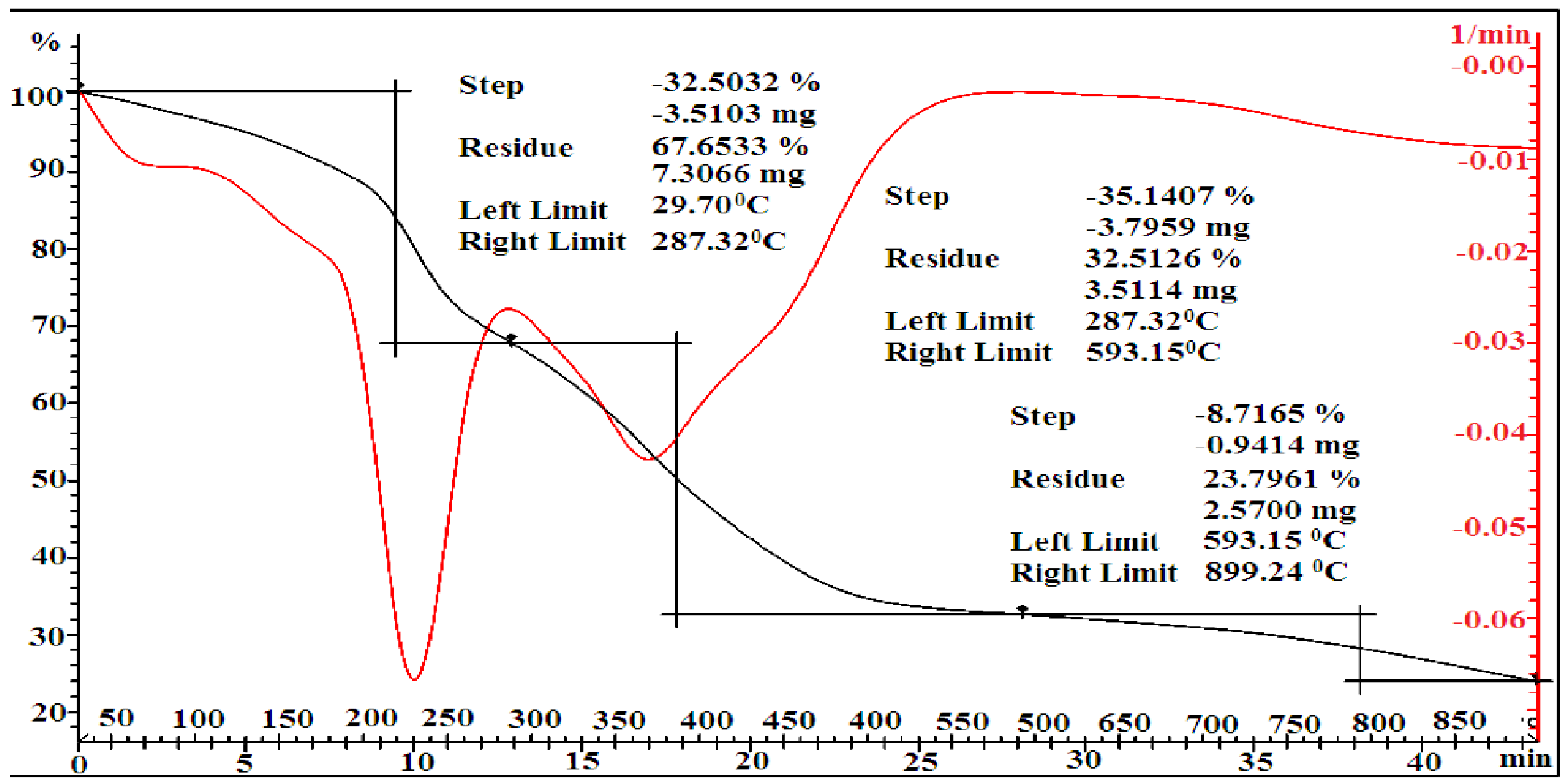
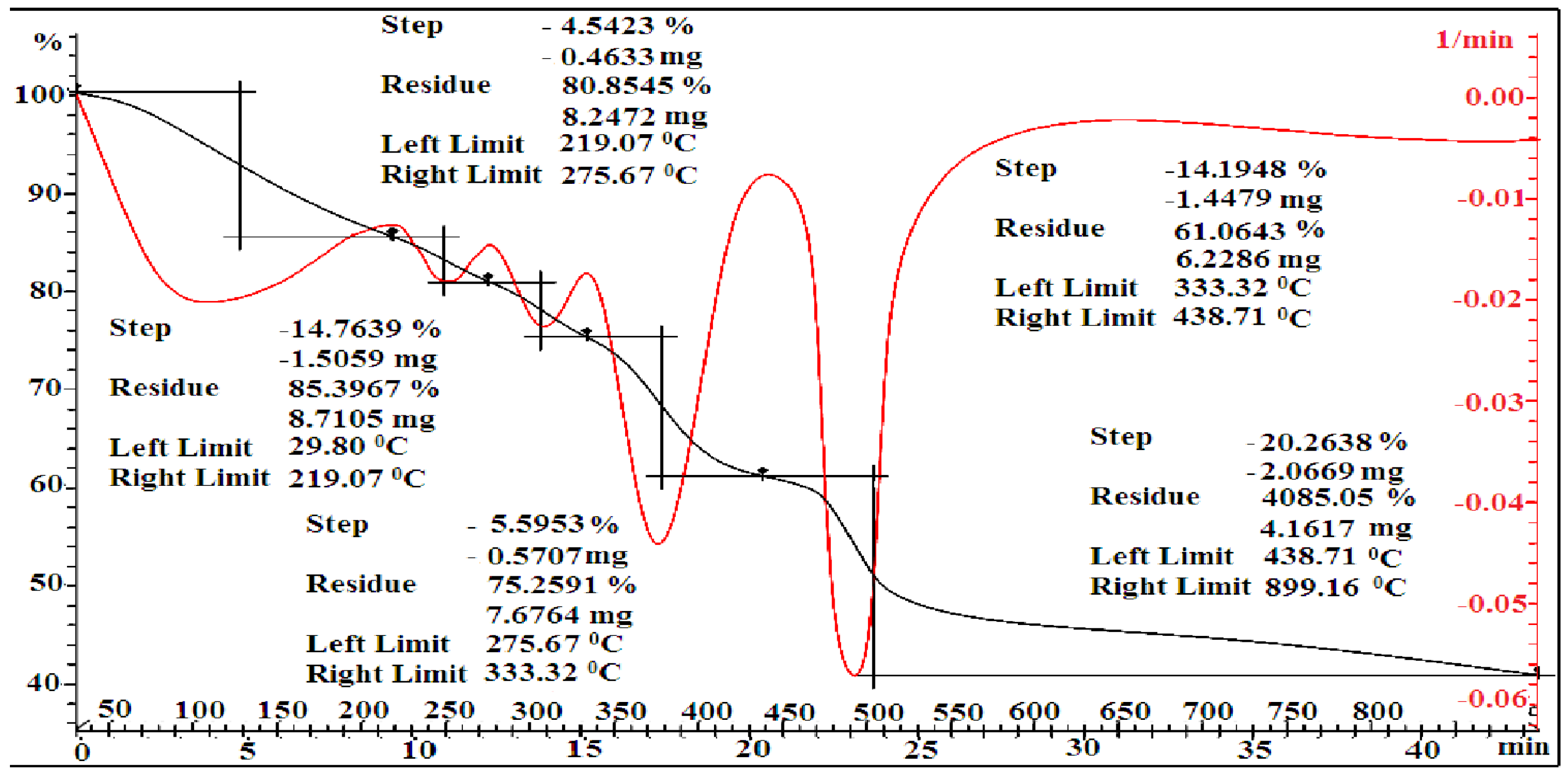
3.2. Thermal Analysis
3.3. Gel Permeation Chromatography
3.4. Characterization of Primer Films
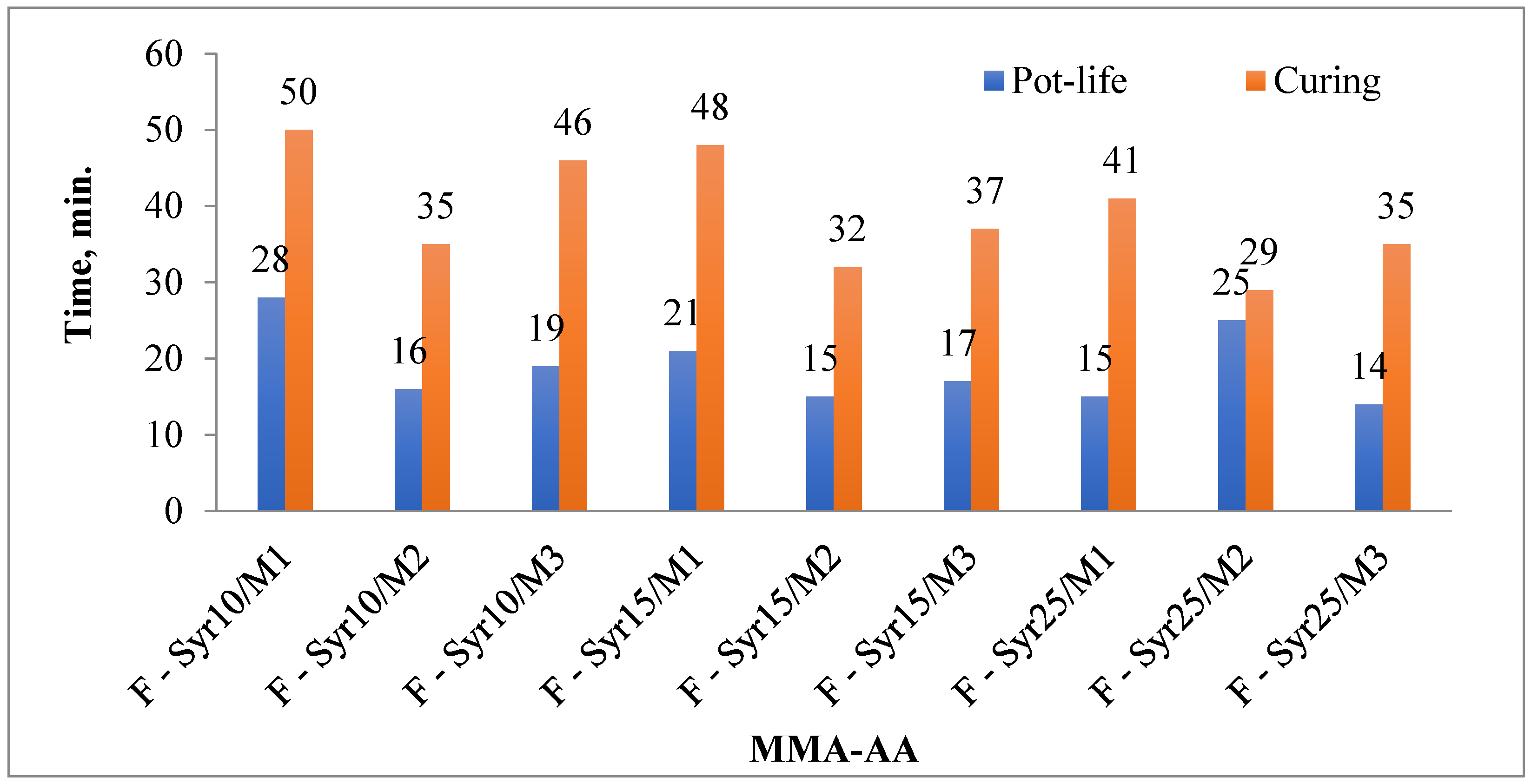
3.4.1. Pot-Life and Curing Test
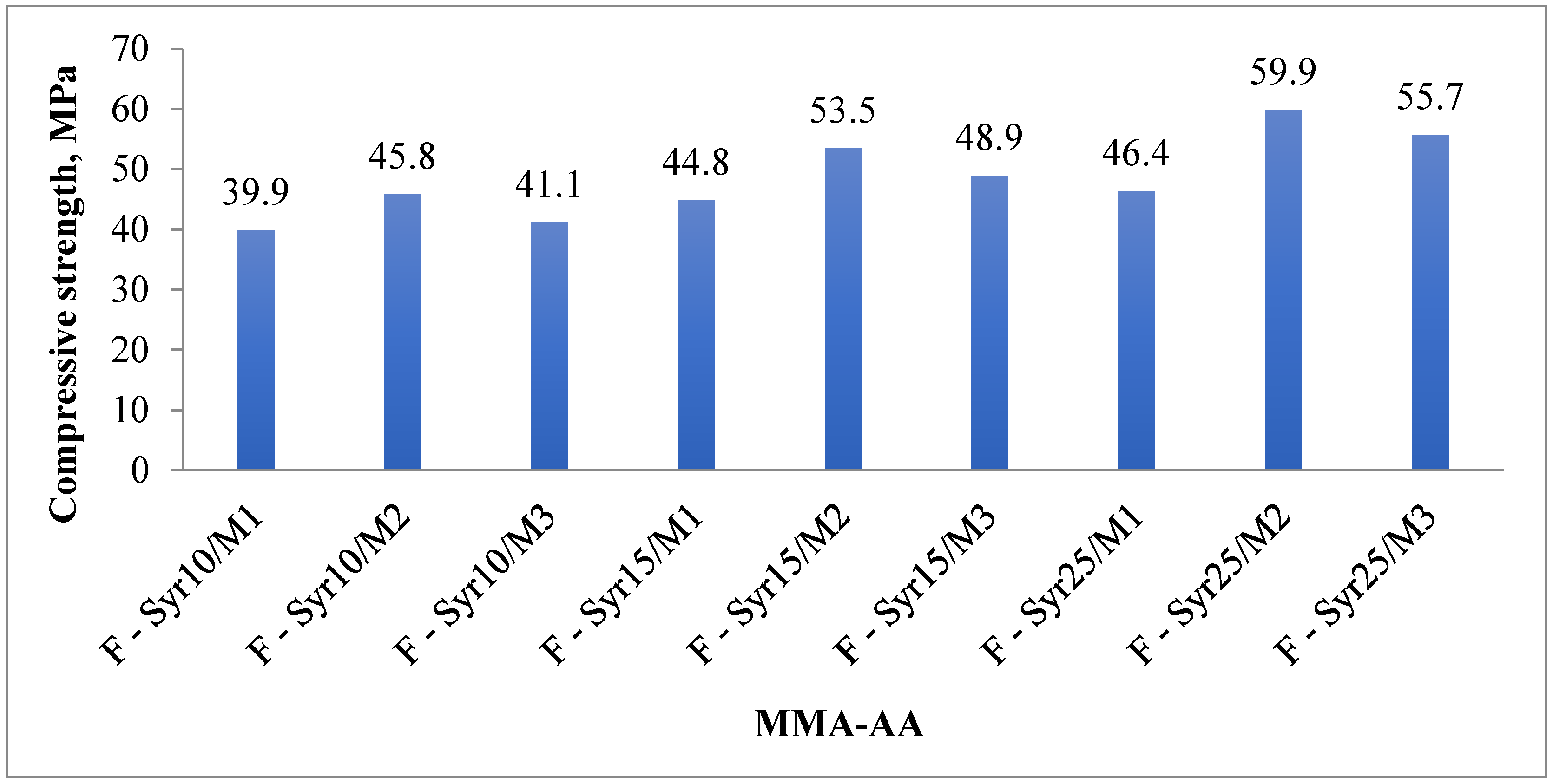
3.4.2. Compressive Strength
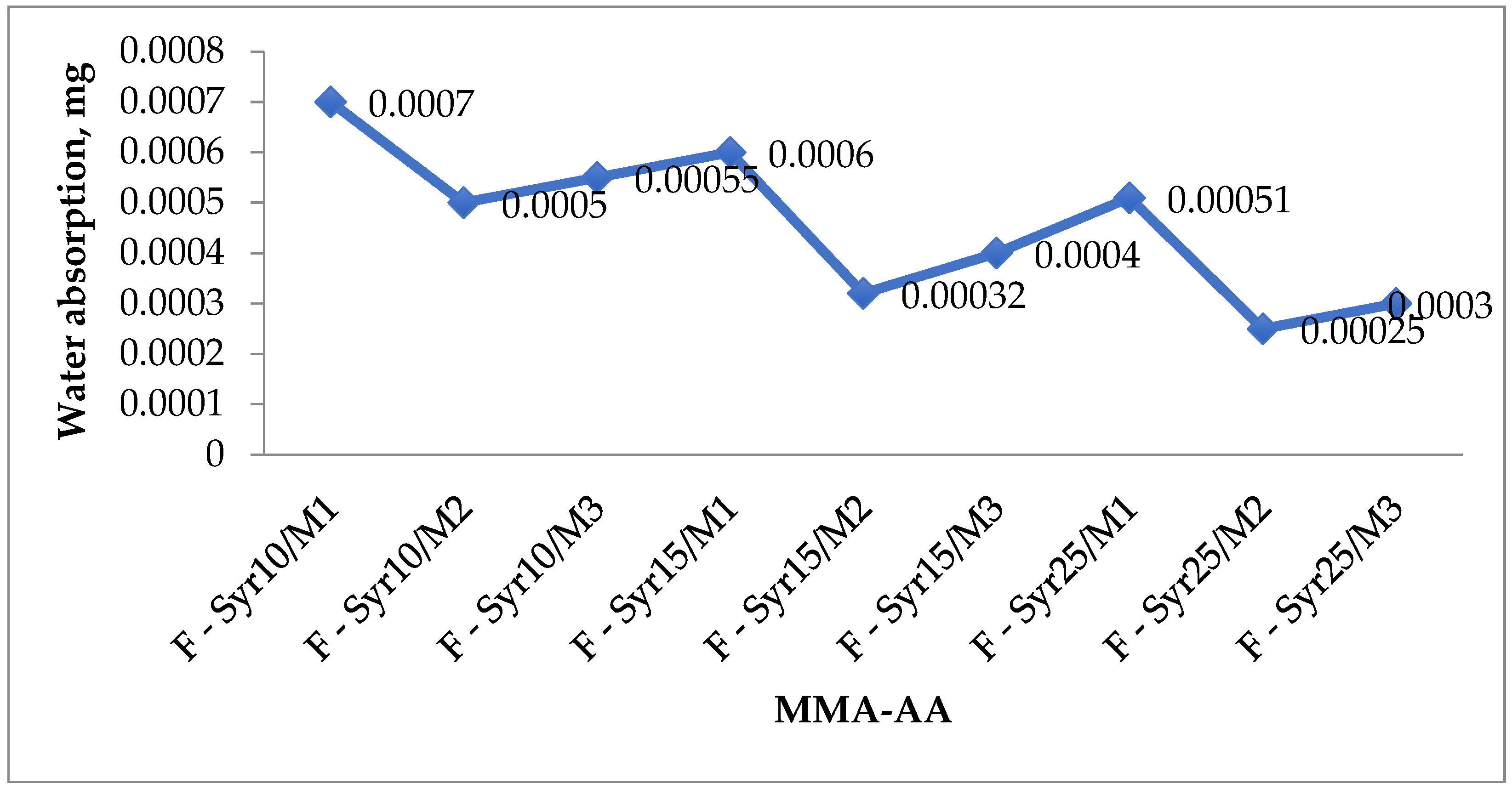
3.4.3. Water Absorption Properties of the Primer
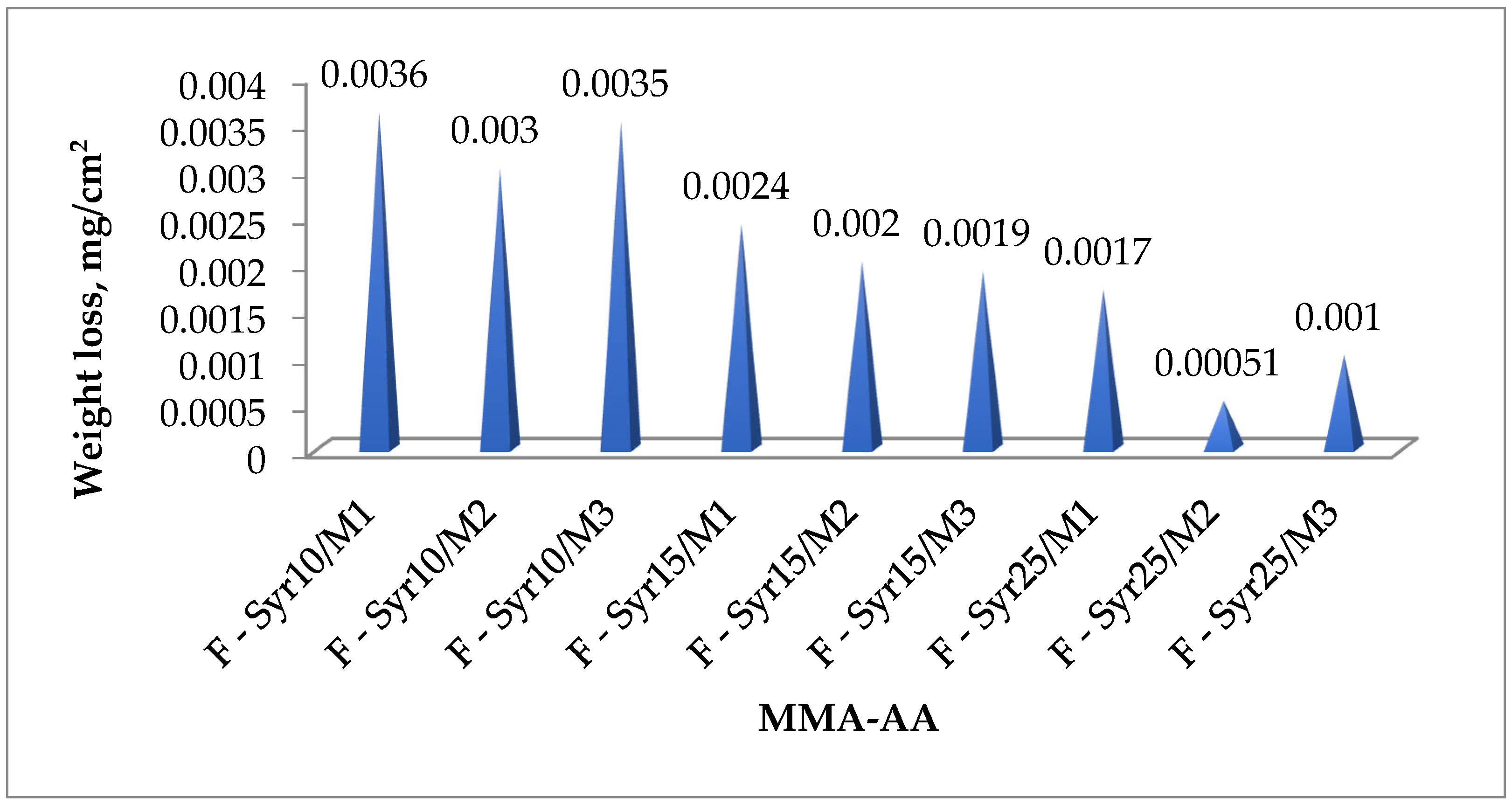
3.4.4. Weight Loss
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Su, F.; He, T.; He, Z.; Yu, Q.; Wang, H. Mechanism of Acrylate Emulsion-Modified Cement-Based Materials. Molecules 2024, 29, 1260. [Google Scholar] [CrossRef]
- Abbas, T.M.; Hussein, S.I. Improving the Mechanical Properties, Roughness, Thermal Stability, and Contact Angle of the Acrylic Polymer by Graphene and Carbon Fiber Doping for Waterproof Coatings. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3788–3796. [Google Scholar] [CrossRef]
- Corsaro, C.; Neri, G.; Santoro, A.; Fazio, E. Acrylate and Methacrylate Polymers’ Applications: Second Life with Inexpensive and Sustainable Recycling Approaches. Materials 2021, 15, 282. [Google Scholar] [CrossRef]
- Vijayanand, P.S.; Selvamalar, C.S.J.; Penlidis, A.; Nanjundan, S. Copolymers of 3,5-Dimethylphenyl Acrylate and Methyl Methacrylate: Synthesis, Characterization and Determination of Monomer Reactivity Ratios. Polym. Int. 2003, 52, 1856–1862. [Google Scholar] [CrossRef]
- Samad, H.A.; Jaafar, M. Effect of Polymethyl Methacrylate (PMMA) Powder to Liquid Monomer (P/L) Ratio and Powder Molecular Weight on the Properties of PMMA Cement. Polym.-Plast. Technol. Eng. 2009, 48, 554–560. [Google Scholar] [CrossRef]
- Patra, N.; Salerno, M.; Cozzoli, P.D.; Barone, A.C.; Ceseracciu, L.; Pignatelli, F.; Carzino, R.; Marini, L.; Athanassiou, A. Thermal and Mechanical Characterization of Poly(Methyl Methacrylate) Nanocomposites Filled with TiO2 Nanorods. Compos. Part B Eng. 2012, 43, 3114–3119. [Google Scholar] [CrossRef]
- Chang, P.P.; Hansen, N.A.; Phoenix, R.D.; Schneid, T.R. The Effects of Primers and Surface Bonding Characteristics on the Adhesion of Polyurethane to Two Commonly Used Silicone Elastomers. J. Prosthodont. 2009, 18, 23–31. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, X.; Yu, J.; Mou, D.; Li, H.; Kong, L.; Lang, T.; Peng, L.; Chen, W.; Xu, X.; et al. High-Quality Cs3Cu2I5@PMMA Scintillator Films Assisted by Multiprocessing for X-Ray Imaging. ACS Appl. Mater. Interfaces 2023, 15, 38741–38749. [Google Scholar] [CrossRef]
- Hotton, C.; Lisuzzo, L.; Cavallaro, G.; Paineau, E.; Bizien, T.; Lazzara, G.; Hotton, C.; Paineau, E.; Lisuzzo, L.; Cavallaro, G.; et al. Supramolecular Nanoarchitectonics of PNIPAAM-Co-MA and Imogolite to Obtain Thermoresponsive Clay Nanotubes Through Complexation Mechanism. Adv. Mater. Interfaces 2025, 12, 2500211. [Google Scholar] [CrossRef]
- Lejnieks, J.; Mourran, A.; Tillmann, W.; Keul, H.; Möller, M. Thin Film of Poly(Acrylic Acid-Co-Allyl Acrylate) as a Sacrificial Protective Layer for Hydrophilic Self Cleaning Glass. Materials 2010, 3, 3369–3384. [Google Scholar] [CrossRef]
- Moghadam, N.; Liu, S.; Srinivasan, S.; Grady, M.C.; Soroush, M.; Rappe, A.M. Computational Study of Chain Transfer to Monomer Reactions in High-Temperature Polymerization of Alkyl Acrylates. J. Phys. Chem. A 2013, 117, 2605–2618. [Google Scholar] [CrossRef]
- Czarnecki, L. Polymer-Concrete Composites for the Repair of Concrete Structures. MATEC Web Conf. 2018, 199, 01006. [Google Scholar] [CrossRef]
- Rahman, R.W.; Syarif, A.R.; Prasiska, C.; Tohamba, P.; Pendidikan, J.; Inggris, B.; Keguruan, F.; Pendidikan, I. Enhancing Vocabulary Through English Songs: A Self-Directed Learning Experienced for Efl Students. Klasikal J. Educ. Lang. Teach. Sci. 2024, 6, 864–882. [Google Scholar] [CrossRef]
- Gyurova, A.Y.; Halacheva, S.; Mileva, E. Aqueous Solutions of Random Poly(Methyl Methacrylate-Co-Acrylic Acid): Effect of the Acrylic Acid Content. RSC Adv. 2017, 7, 13372–13382. [Google Scholar] [CrossRef]
- Shi, Z.; Reddy, N.; Shen, L.; Hou, X.; Yang, Y. Effects of Monomers and Homopolymer Contents on the Dry and Wet Tensile Properties of Starch Films Grafted with Various Methacrylates. J. Agric. Food Chem. 2014, 62, 4668–4676. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xu, L.; Feng, T.; Shi, X.; Zhang, P. Effect of PCE on Properties of MMA-Based Repair Material for Concrete. Materials 2021, 14, 859. [Google Scholar] [CrossRef] [PubMed]
- Sarcinella, A.; Frigione, M. Sustainable and Bio-Based Coatings as Actual or Potential Protective Treatments for Concrete. Coatings 2022, 13, 44. [Google Scholar] [CrossRef]
- Moharram, M.A.; Allam, M.A. Study of the Interaction of Poly(Acrylic Acid) and Poly(Acrylic Acid-Polyacrylamide) Complex with Bone Powders and Hydroxyapatite Using TGA and DSC. J. Appl. Polym. Sci. 2007, 105, 3220–3227. [Google Scholar] [CrossRef]
- Geng, P.; Gade, S.V.; Van De Mark, M.R. DSC and TGA Characterization of Free and Surface Water of Colloidal Unimolecular Polymer (CUP) Particles for Coatings Applications. J. Coat. Technol. Res. 2021, 18, 143–154. [Google Scholar] [CrossRef]
- Geng, P.; Zore, A.; Van De Mark, M.R. Thermodynamic Characterization of Free and Surface Water of Colloidal Unimolecular Polymer (CUP) Particles Utilizing DSC. Polymers 2020, 12, 1417. [Google Scholar] [CrossRef]
- Yanar, N.; Park, S.; Yang, E.; Choi, H. Surface Fouling Characterization Methods for Polymeric Membranes Using a Short Experimental Study. Polymers 2024, 16, 2124. [Google Scholar] [CrossRef]
- Murali, G.; Karthikeyan, K.; Senthilpandian, M.; Wong, L.S.; Abid, S.R.; Hemanth Kumar, A. Synergistic Effects of Graphene Oxide, Steel Wire Mesh and Fibers on the Impact Resistance of Preplaced Aggregate Concrete. J. Build. Eng. 2024, 95, 110363. [Google Scholar] [CrossRef]
- ISO 11358-1:2014; Plastics—Thermogravimetry (TG) of Polymers—Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2014.
- DIN EN ISO 9514:2006; Paints and Varnishes—Determination of the Pot-Life of Multicomponent Coating Materials—Preparation and Conditioning of Samples and Testing of the Properties of the Mixed Material. German Institute for Standardization (DIN): Berlin, Germany, 2006.
- ASTM D5895-03(2018); Standard Test Method for Evaluating Drying or Curing During Film Formation of Organic Coatings Using Mechanical Recorders. ASTM International: West Conshohocken, PA, USA, 2018.
- KS F 2419:2016; Testing Method for Compressive Strength of Cement Mortar and Concrete. Korean Industrial Standards (KATS): Seoul, Korea, 2016.
- KS F 2405:2020; Method of Test for Compressive Strength of Concrete. Korean Industrial Standards (KATS): Seoul, Korea, 2020.
- BS 1881-122:2011; Testing Concrete—Method for Determination of Water Absorption. British Standards Institution (BSI): London, UK, 2011.
- Sato, H.; Kawaguchi, M.; Yamamoto, H. Water Sorption and Dynamic Mechanical Properties of PMMA-Based Copolymers. Polymer Journal 2019, 51, 1103–1112. [Google Scholar]
- Chen, X.; Li, Z.; Wu, J. Hydrophobic Behavior of Acrylic Copolymers during Long-Term Water Immersion. Progress in Organic Coatings 2020, 147, 105–114. [Google Scholar]
- ASTM D2688-94(2016); Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser. ASTM International: West Conshohocken, PA, USA, 2016.
- Nurlybayeva, A.N.; Sahy, M.S.; Rustem, E.I. Study of New Methacrylic Monomers Using 1H NMR Spectroscopy. Vestn. L. N. Gumilyov Eurasian Natl. Univ. 2015, 6, 337. [Google Scholar]
- Park, S.E.; Chao, M.; Raj, P.A. Mechanical Properties of Surface-Charged Poly(Methyl Methacrylate) as Denture Resins. Int. J. Dent. 2009, 2009, 841431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zielecka, M.; Rabajczyk, A.; Cygańczuk, K.; Pastuszka, Ł.; Jurecki, L. Silicone Resin-Based Intumescent Paints. Materials 2020, 13, 4785. [Google Scholar] [CrossRef]
- Negim, E.S.; Aisha, N.; Mun, G.A.; Iskakov, R.; Irmukhametova, G.S.; Sakhy, M. Preparation and Characterization of Acrylic Primer for Concrete Substrate Application. Int. J. Polym. Sci. 2016, 2016, 1754168. [Google Scholar] [CrossRef]
- Talha Junaid, M.; Saboor Karzad, A.; Altoubat, S. Stress-Strain Behaviour and Strength Properties of Ambient Cured Geo-Polymer Concrete. IOP Conf. Ser. Mater. Sci. Eng. 2020, 839, 012005. [Google Scholar] [CrossRef]
- Nurlybayeva, A.N.; Rustem, E.I.; Sadiyeva, H.R.; Seitbekova, G.A.; Darmenbayeva, A.S.; Kalmakhanova, M.S.; Egisinova, A.M.; Otinshieva, U.T. Synthesis and Application of Acrylic Films in Paint and Varnish Materials. Ser. Chem. Technol. 2020, 2, 14–22. [Google Scholar] [CrossRef]
- Schantz, S.; Carlsson, H.T.; Andersson, T.; Erkselius, S.; Larsson, A.; Karlsson, O.J. Poly(Methyl Methacrylate-Co-Ethyl Acrylate) Latex Particles with Poly(Ethylene Glycol) Grafts: Structure and Film Formation. Langmuir 2007, 23, 3590–3602. [Google Scholar] [CrossRef]
- Celentano, W.; Neri, G.; Distante, F.; Li, M.; Messa, P.; Chirizzi, C.; Chaabane, L.; De Campo, F.; Metrangolo, P.; Baldelli Bombelli, F.; et al. Design of Fluorinated Hyperbranched Polyether Copolymers for F-19 MRI Nanotheranostics. Polym. Chem. 2025, 11, 3951–3963. [Google Scholar] [CrossRef]
- Momber, A.W.; Irmer, M.; Marquardt, T. Effects of Polymer Hardness on the Abrasive Wear Resistance of Thick Organic Offshore Coatings. Prog. Org. Coat. 2020, 146, 105720. [Google Scholar] [CrossRef]
- Hussein, S.I.; Kadhem, S.J.; Ali, N.A.; Alraih, A.M.; Abd-Elnaiem, A.M. Improving the Mechanical, Thermoelectric Insulations, and Wettability Properties of Acrylic Polymers: Effect of Silica or Cement Nanoparticles Loading and Plasma Treatment. Polymers 2024, 16, 2965. [Google Scholar] [CrossRef] [PubMed]
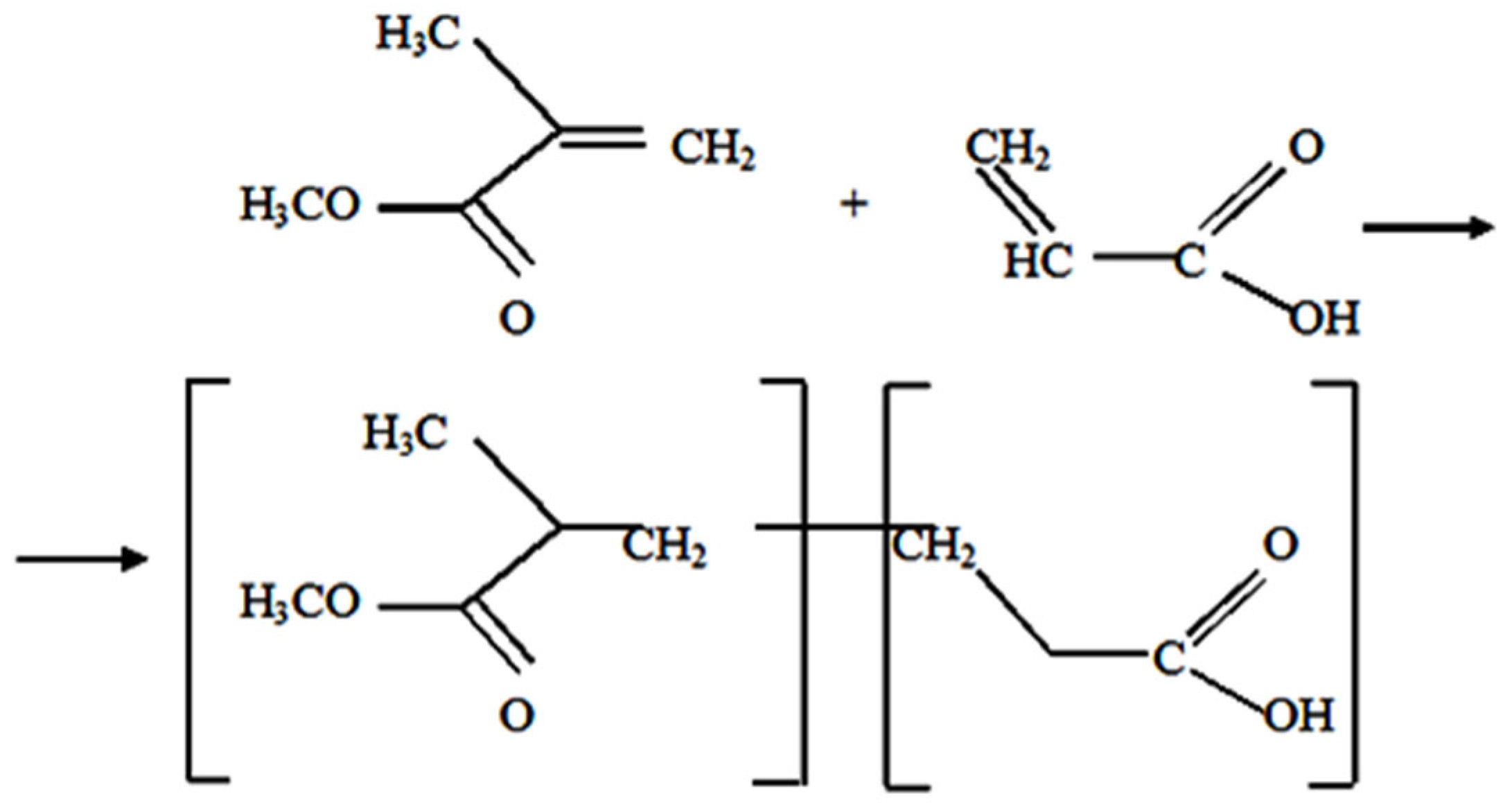
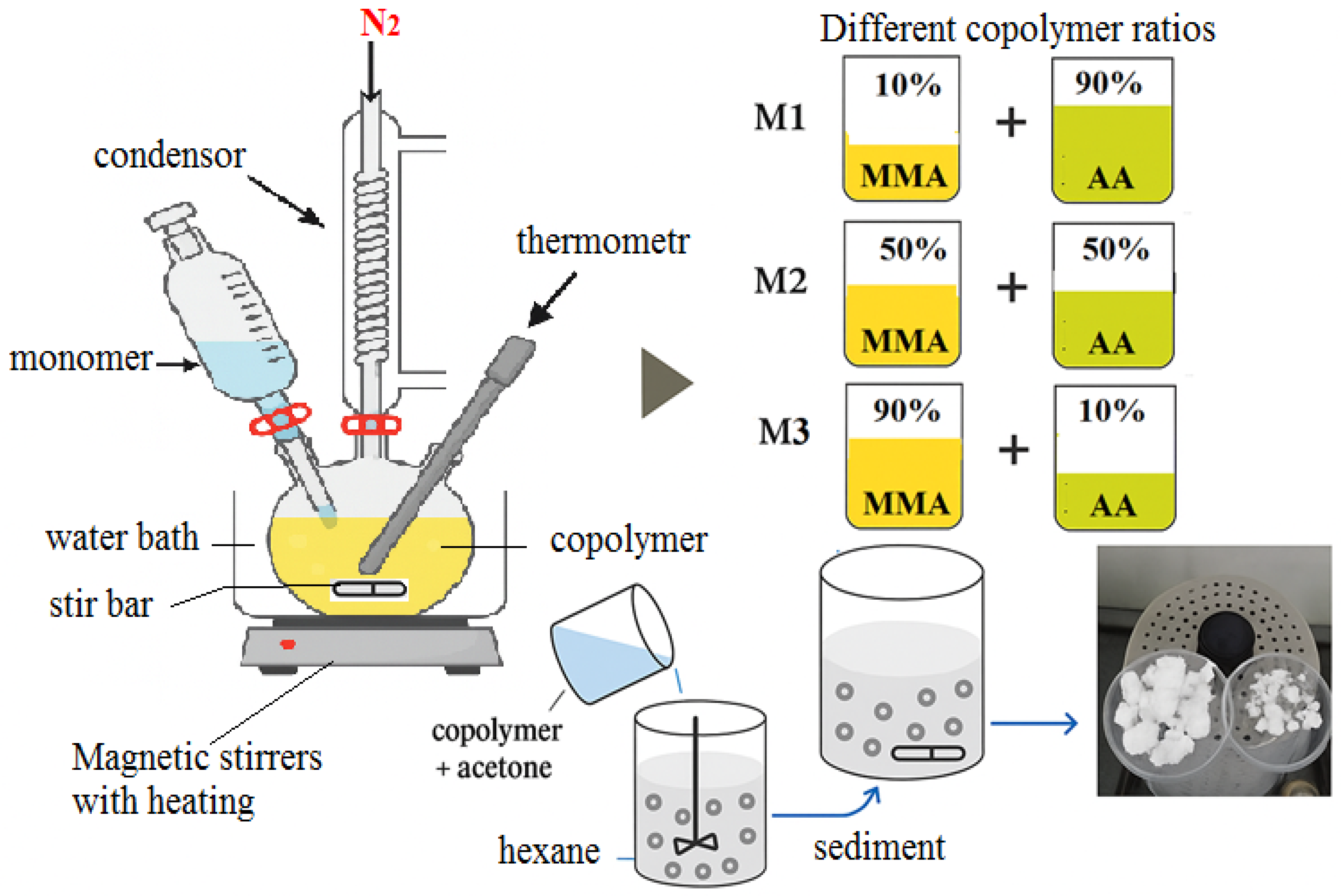

| Group | Syrups | Copolymer (Powder, gm) | MMA (Liquid, gm) |
|---|---|---|---|
| Group 1 | Syp10/M1 | 10 | 90 |
| Syp10/M2 | 10 | 90 | |
| Syp10/M3 | 10 | 90 | |
| Group 2 | Syp15/M1 | 15 | 85 |
| Syp15/M2 | 15 | 85 | |
| Syp15/M3 | 15 | 85 | |
| Group 3 | Syp25/M1 | 25 | 75 |
| Syp25/M2 | 25 | 75 | |
| Syp25/M3 | 25 | 75 |
| Group | Name of Mix | Syrups (gm) | Sand (gm) | Calcium Carbonate (gm) | BPO (gm) | DMPT (gm) | Pigment (gm) |
|---|---|---|---|---|---|---|---|
| Group 1 | F-Syp10/M1 | 100 | 150 | 100 | 1 | 0.75 | 5 |
| F-Syp10/M2 | |||||||
| F-Syp10/M3 | |||||||
| Group 2 | F-Syp15/M1 | ||||||
| F-Syp15/M2 | |||||||
| F-Syp15/M3 | |||||||
| Group 3 | F-Syp25/M1 | ||||||
| F-Syp25/M2 | |||||||
| F-Syp25/M3 |
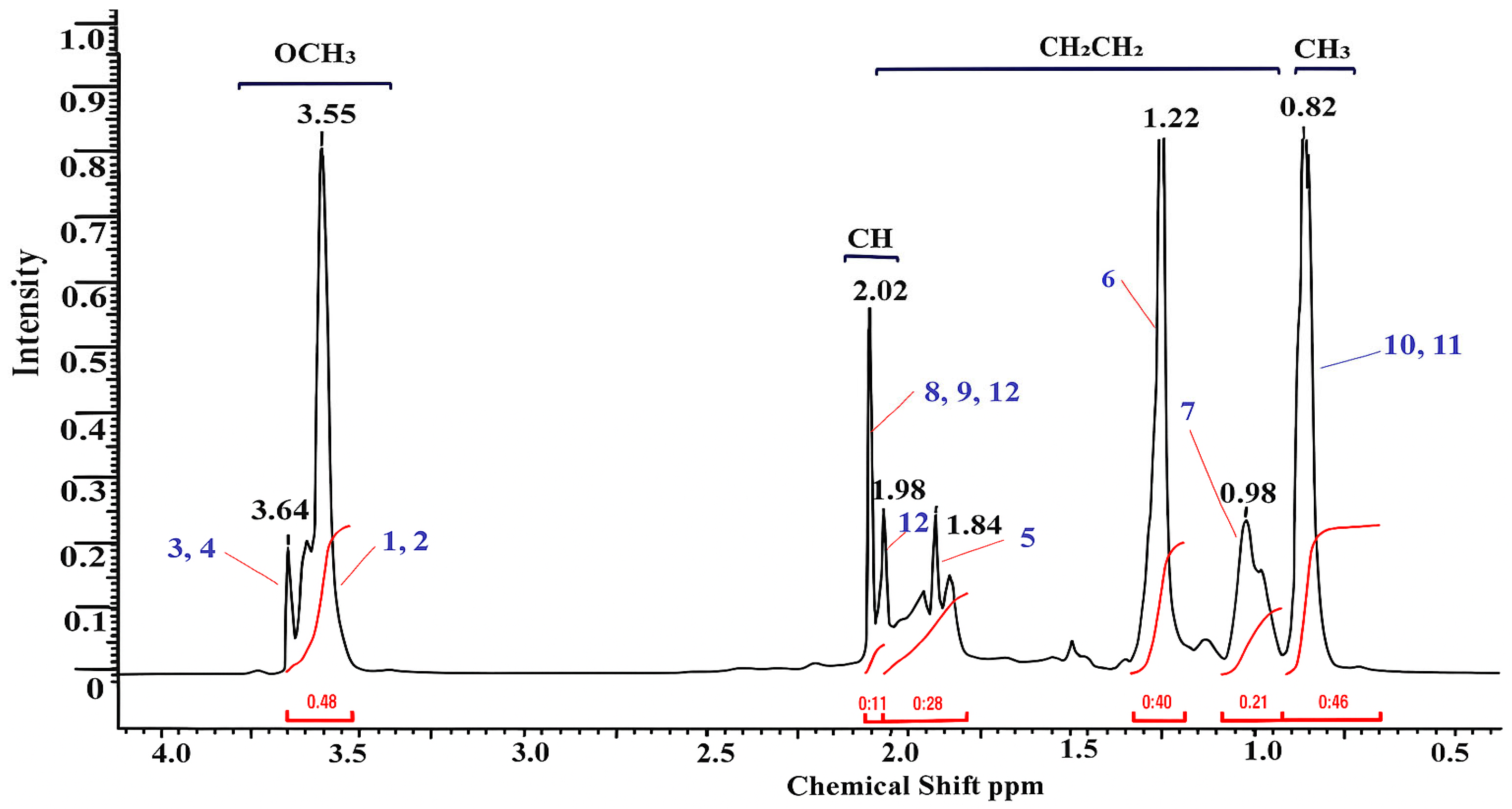
| Functional Group | Signals (ppm) | Description |
|---|---|---|
| –OCH3 (1, 2, 3, 4) | 3.55–3.64 | Methoxyl protons of the methyl methacrylate unit (–COOCH3) |
| –CH– (8, 9, 12) | 2.02–2.12 | The methine protons in the main unit of the polymer (–CH–C–) |
| –CH2– and COO–(5) | 0.97–1.90 | Methylene protons of the side chains and carboxyl groups of the basic skeleton |
| –CH2–, –CH3 (6, 7) | 1.22–0.98 | Alkyl protons (end groups or initiator residues) |
| –CH3 (10, 11) | 0.83–0.85 | Methyl protons of the side groups MMA (–C(CH3)–) |
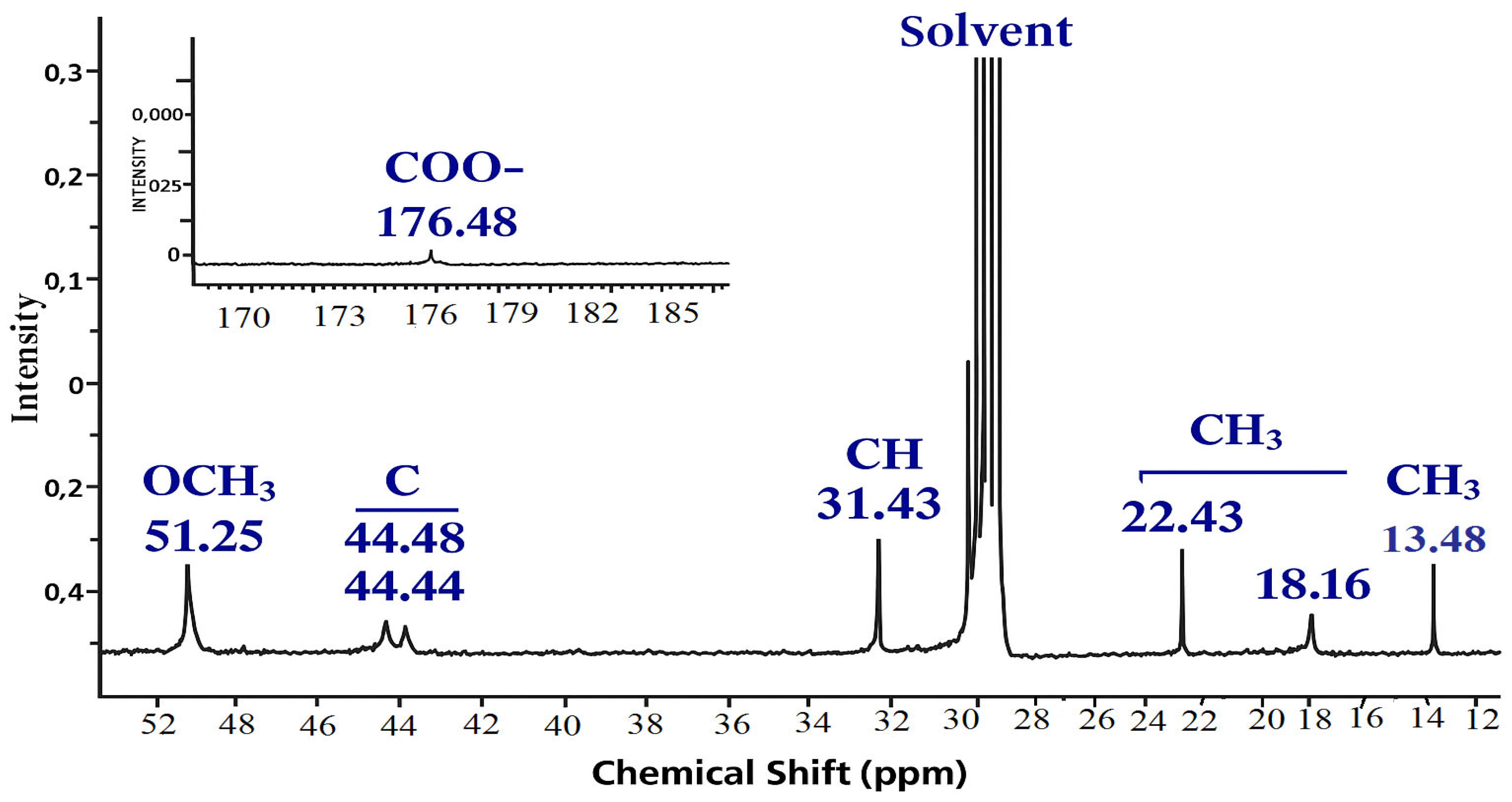
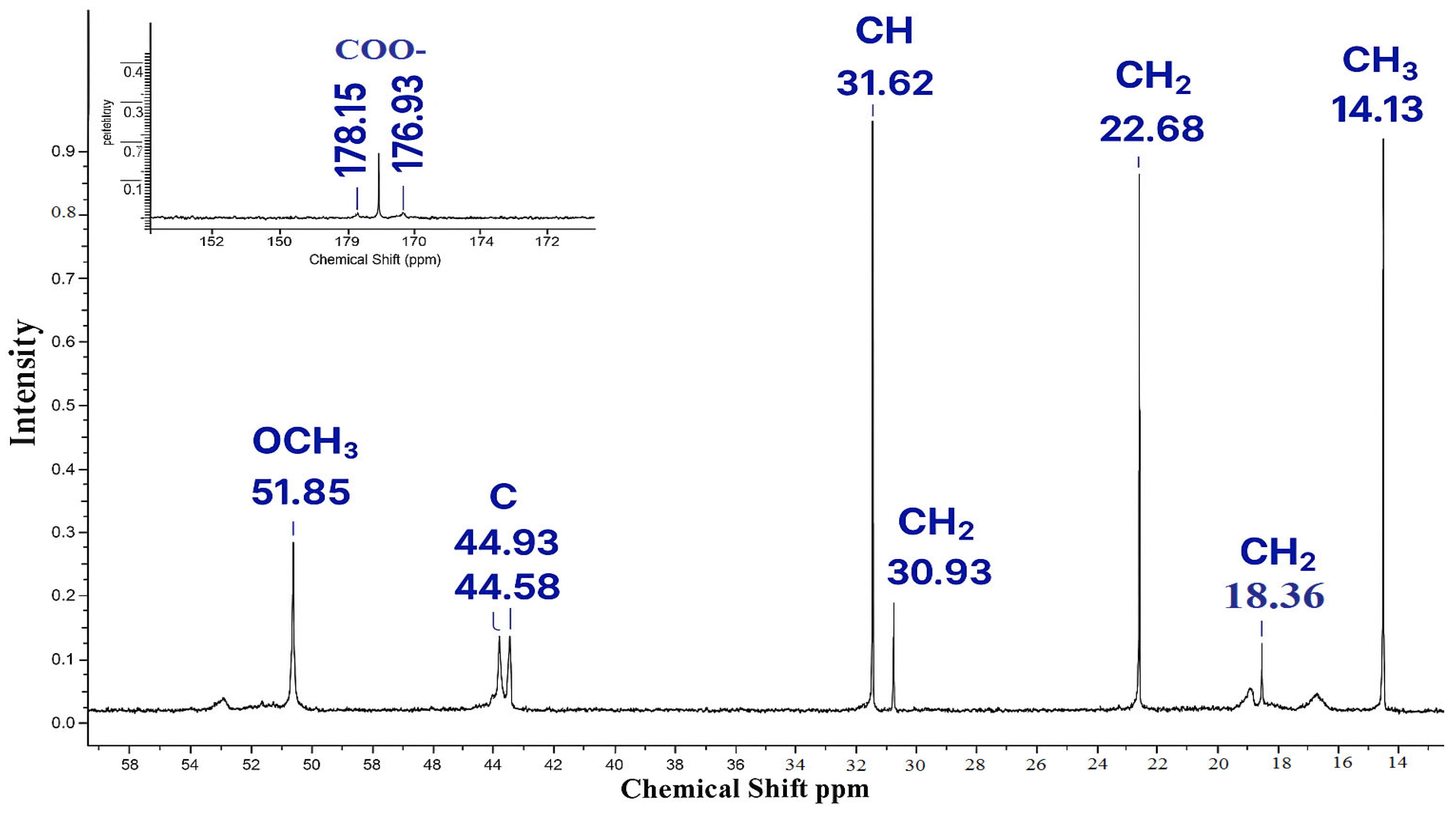
| Functional Group | Signals (ppm) | Description |
|---|---|---|
| –COO– (carbonyl carbon) | 176.4–178.2 | Signals of carboxyl groups of acrylic acid and methyl methacrylate esters. |
| –OCH3 | 51.2–51.8 | Methoxyl carbons of the MMA unit. |
| –C– (Quaternary carbon at C=O) | 44.4–44.9 | It is characteristic of the methyl methacrylate unit after polymerization. |
| –CH– | 31.4–31.6 | The methyl carbon bound to C=CH2 in the AA monomer |
| –CH2– | 22.4–30.9 18.1–18.3 | Additional methylene fragments, probably from impurities or a chain transporter |
| –CH3 | 13.4–14.1 | Methyl carbons of the methyl methacrylate unit. |
| [MMA-AA] mol. % | Tg, °C a | Tg, °C b | Temperature Range, °C b | Lost Mass b % | Residual Weight a % | PDTmax °C b |
|---|---|---|---|---|---|---|
| 10-90 | 97.24 | 100 | 29.49–198.71 198.71–366.18 366.18–900.85 | 17.53 59.57 9.075 | 82.47 22.96 13.89 | 320 |
| 50-50 | 102.28 | 100 | 29.70–287.32 287.32–593.15 593.15–899.24 | 32.50 35.14 8.7165 | 67.65 32.51 23.76 | 380 |
| 90-10 | 103.01 | 100 | 29.80–219.07 219.07–275.67 275.67–333.32 333.32–438.71 438.71–899.16 | 14.76 4.54 5.59 14.19 20.26 | 85.24 75.15 40.80 | 500 |
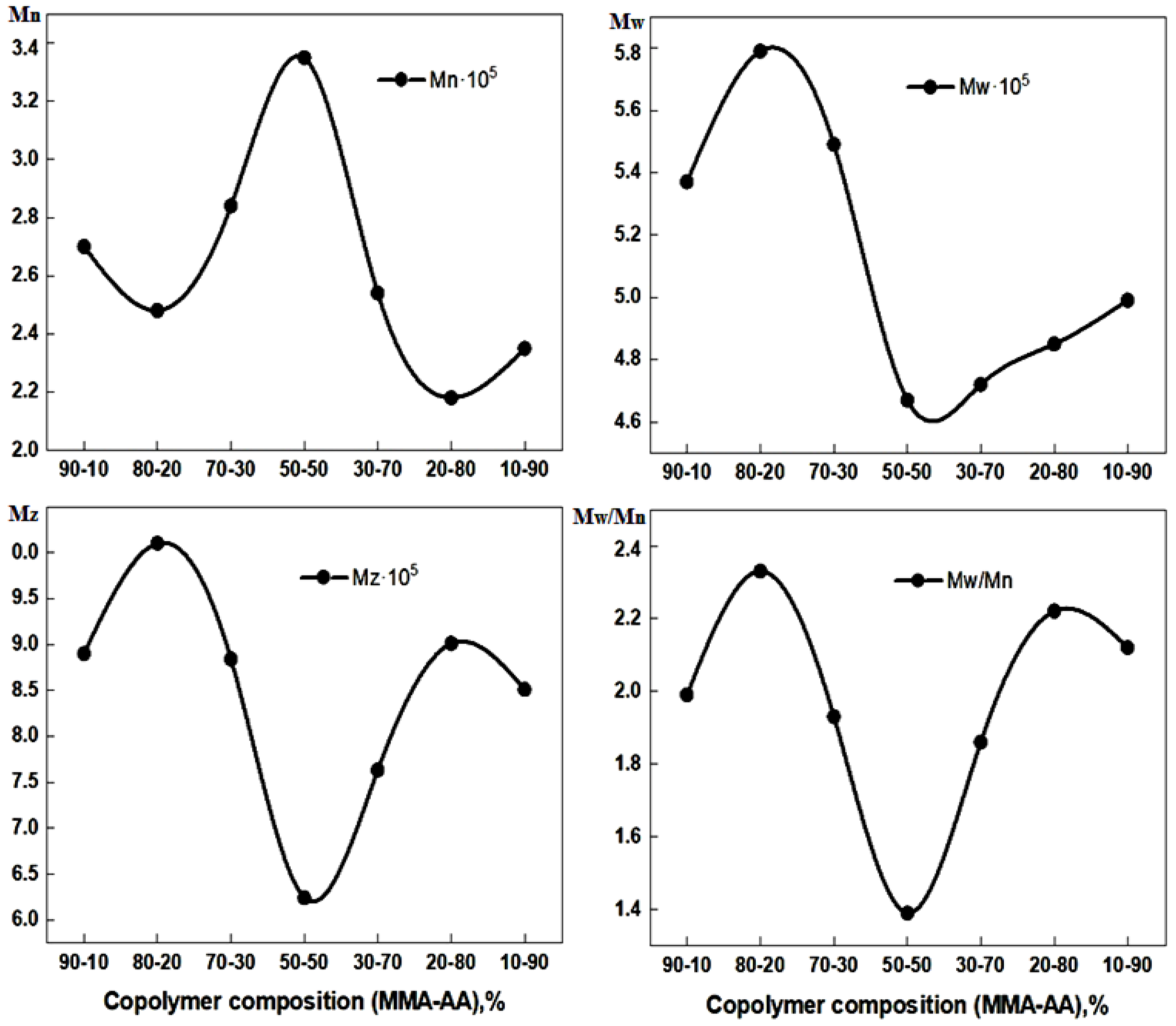
| Copolymer Composition. % | Mn·105 | Mw·105 | Mz 105 | Mw/Mn | |
|---|---|---|---|---|---|
| MMA | AA | ||||
| 90 | 10 | 2.70 | 5.37 | 8.90 | 1.99 |
| 80 | 20 | 2.48 | 5.79 | 10.1 | 2.33 |
| 70 | 30 | 2.84 | 5.49 | 8.84 | 1.93 |
| 50 | 50 | 3.35 | 4.67 | 6.24 | 1.39 |
| 30 | 70 | 2.54 | 4.72 | 7.63 | 1.86 |
| 20 | 80 | 2.18 | 4.85 | 9.01 | 2.22 |
| 10 | 90 | 2.35 | 4.99 | 8.51 | 2.12 |
| Copolymer Composition MMA-AA % | Compressive Strength, MPa | Weight Loss (mg/cm2) | Water Absorption (mg) | Pot-Life (min) | Curing Time (min) |
|---|---|---|---|---|---|
| F-Syr10/M1 | 39.9 | 0.0038 | 0.0007 | 28 | 50 |
| F-Syr10/M2 | 45.8 | 0.0028 | 0.0005 | 16 | 35 |
| F-Syr10/M3 | 41.1 | 0.0035 | 0.00055 | 19 | 46 |
| F-Syr15/M1 | 44.8 | 0.0023 | 0.0006 | 21 | 48 |
| F-Syr15/M2 | 53.5 | 0.0016 | 0.00032 | 15 | 32 |
| F-Syr15/M3 | 48.9 | 0.0018 | 0.0004 | 17 | 37 |
| F-Syr25/M1 | 46.4 | 0.0015 | 0.0005 | 15 | 41 |
| F-Syr25/M2 | 59.9 | 0.0005 | 0.00051 | 25 | 29 |
| F-Syr25/M3 | 55.7 | 0.001 | 0.00025–0.0003 | 14 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurlybayeva, A.; Urkimbayeva, Z.; Rakhmetullayeva, R.; Taubayeva, R.; Sarova, N.; Seitkan, A.; Seitbekova, G.; Bulekbayeva, K.; Kussainova, B.; Shinibekova, A.; et al. Solvent-Free Synthesis of P(MMA-AA) Copolymers and Their Application as Sustainable Primers for Concrete Substrates. Polymers 2025, 17, 3039. https://doi.org/10.3390/polym17223039
Nurlybayeva A, Urkimbayeva Z, Rakhmetullayeva R, Taubayeva R, Sarova N, Seitkan A, Seitbekova G, Bulekbayeva K, Kussainova B, Shinibekova A, et al. Solvent-Free Synthesis of P(MMA-AA) Copolymers and Their Application as Sustainable Primers for Concrete Substrates. Polymers. 2025; 17(22):3039. https://doi.org/10.3390/polym17223039
Chicago/Turabian StyleNurlybayeva, Aisha, Zhansaya Urkimbayeva, Raikhan Rakhmetullayeva, Raushan Taubayeva, Nurbanu Sarova, Ainur Seitkan, Gulnaziya Seitbekova, Kamila Bulekbayeva, Bakytgul Kussainova, Assem Shinibekova, and et al. 2025. "Solvent-Free Synthesis of P(MMA-AA) Copolymers and Their Application as Sustainable Primers for Concrete Substrates" Polymers 17, no. 22: 3039. https://doi.org/10.3390/polym17223039
APA StyleNurlybayeva, A., Urkimbayeva, Z., Rakhmetullayeva, R., Taubayeva, R., Sarova, N., Seitkan, A., Seitbekova, G., Bulekbayeva, K., Kussainova, B., Shinibekova, A., & Ergali, R. (2025). Solvent-Free Synthesis of P(MMA-AA) Copolymers and Their Application as Sustainable Primers for Concrete Substrates. Polymers, 17(22), 3039. https://doi.org/10.3390/polym17223039







