The Effect of Bilayered Bioactive Coating on Polycaprolactone Electrospun Scaffold Biocompatibility, Bioabsorption and Cellular Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Scaffolds
2.2. Scaffolds Characterizations
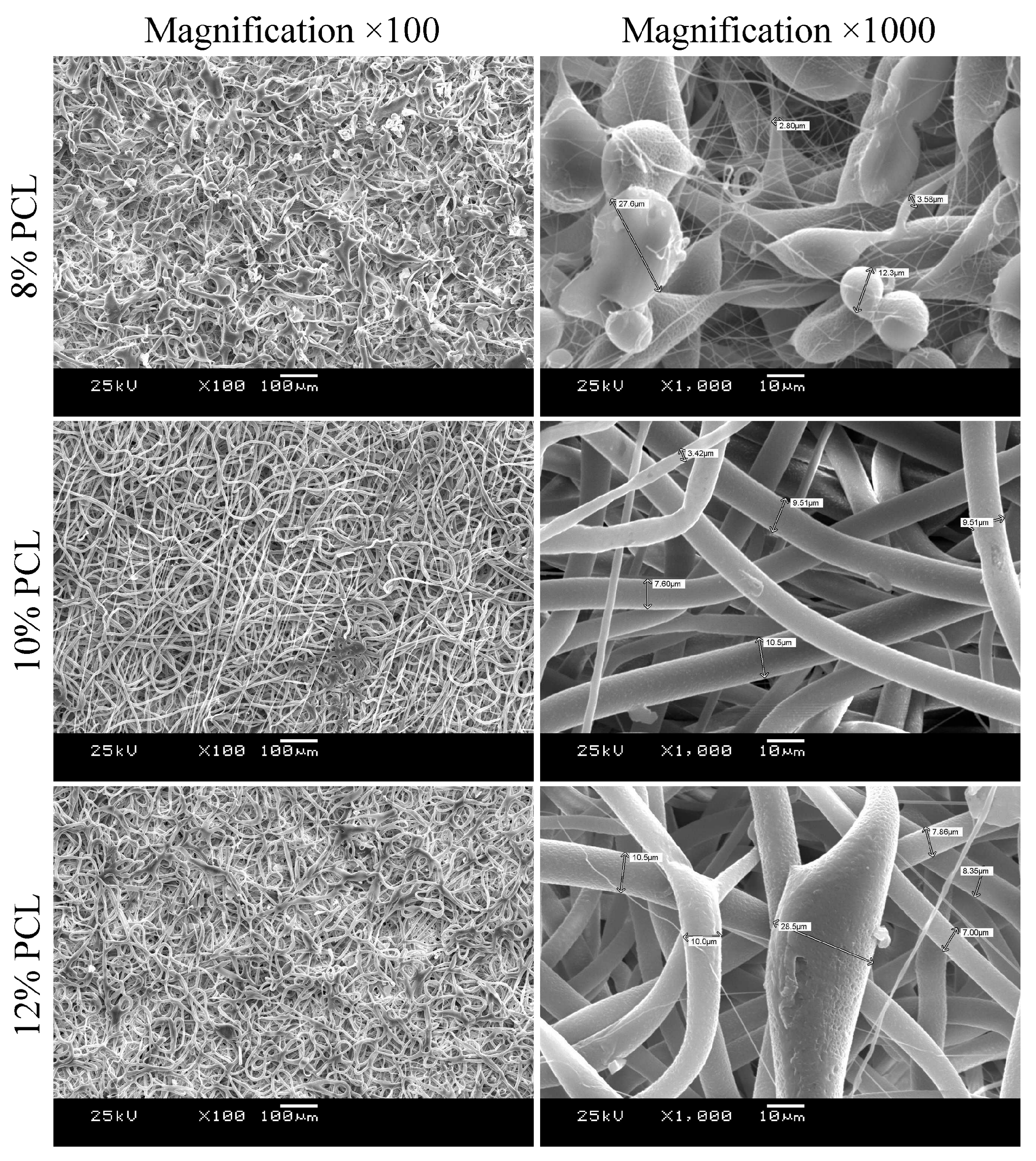
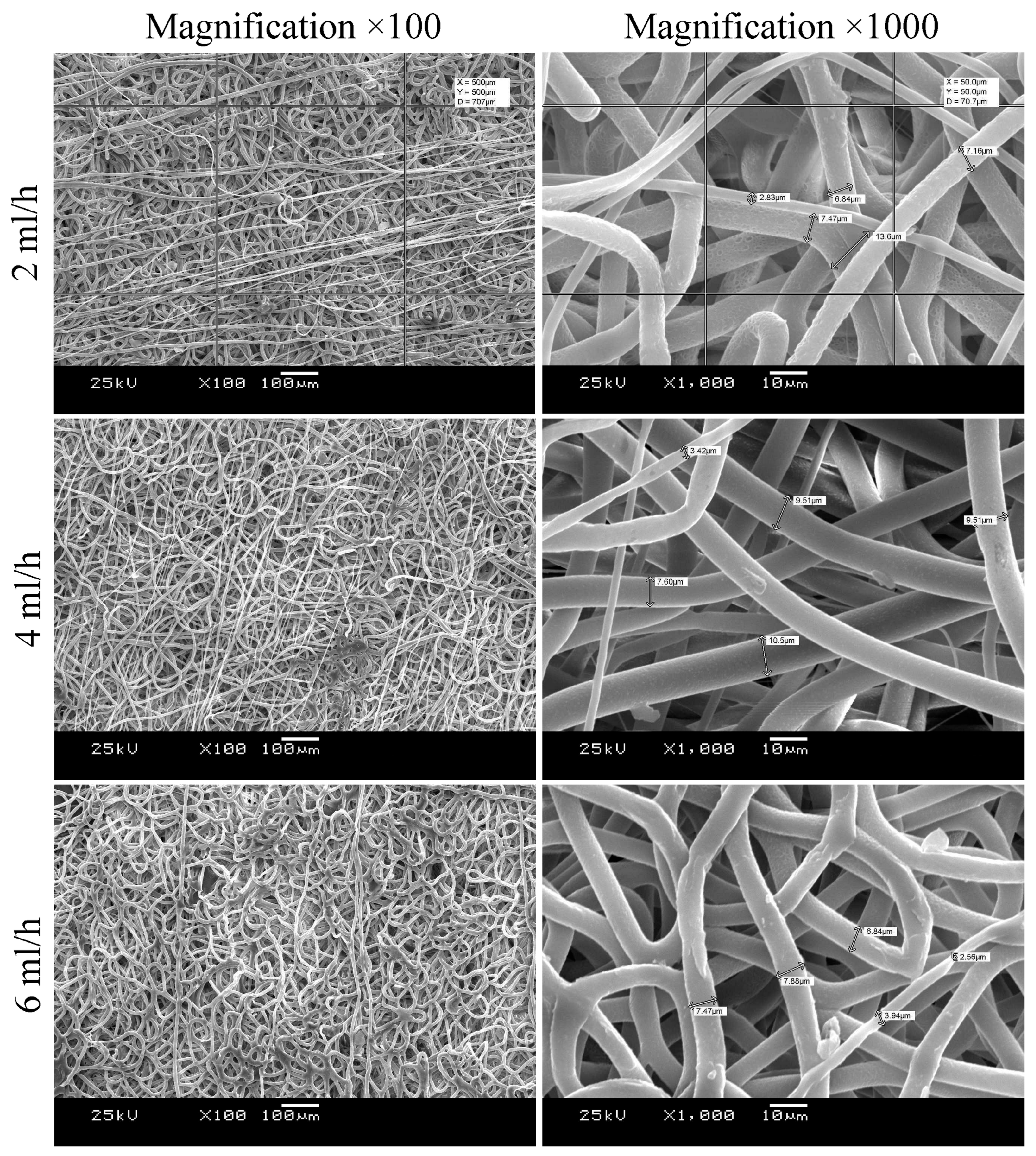
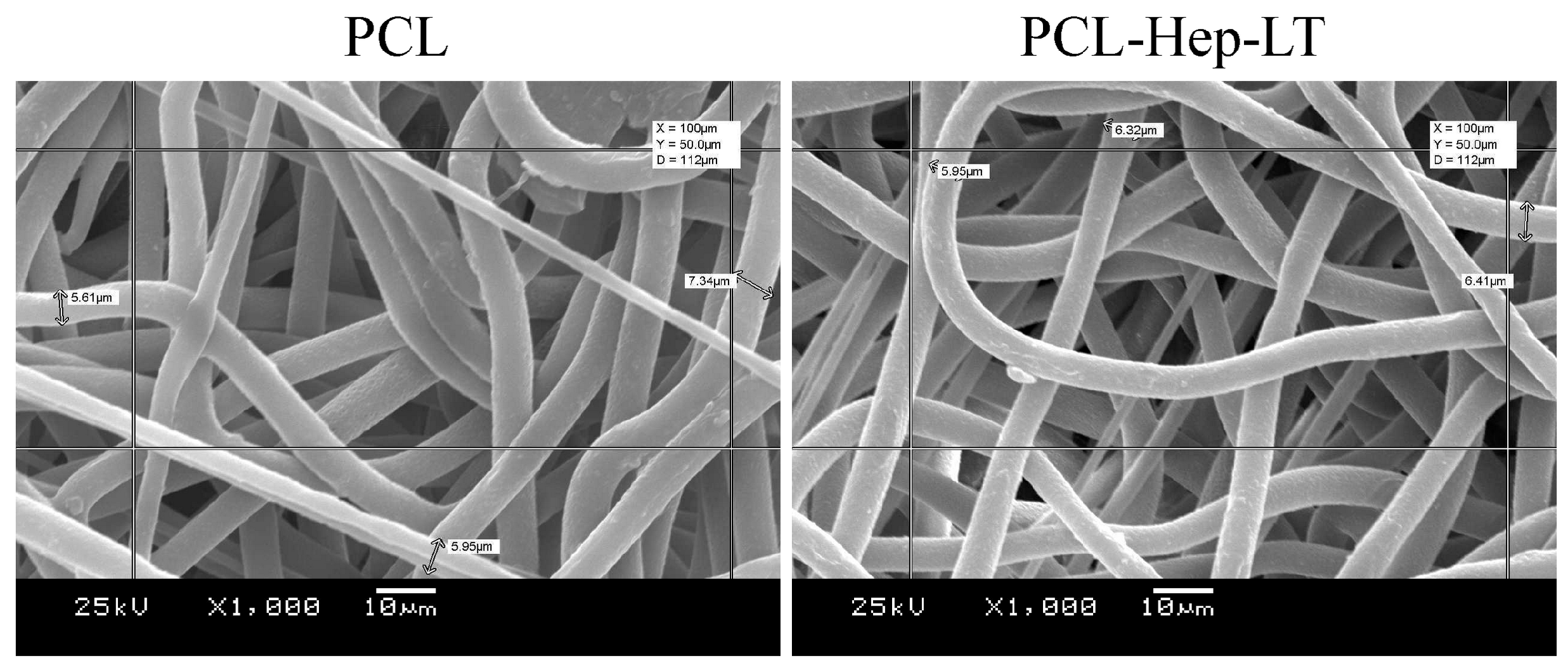
2.2.1. Scanning Electron Microscopy
2.2.2. Estimation of Biomechanical Properties of the Samples
2.3. Bioactive Agents Immobilization
2.4. Heparin Determination
2.4.1. Surface-Bound Heparin
2.4.2. Heparin Release
2.5. In Vitro Biocompatibility Testing
2.5.1. Surface Induced Hemolysis
2.5.2. Complement Activation
2.6. Cytotoxicity Assay
2.7. In Vitro Cell Cultures
2.7.1. Cells Source
2.7.2. Cell Seeding
2.7.3. Cell Staining
2.8. Scaffolds Local Effect and Absorption Testing In Vivo Study
2.8.1. Scaffold Implantation
2.8.2. Histological Investigation
2.9. VEGF Adsorption/Desorption Determination
2.10. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Mechanical Characterization
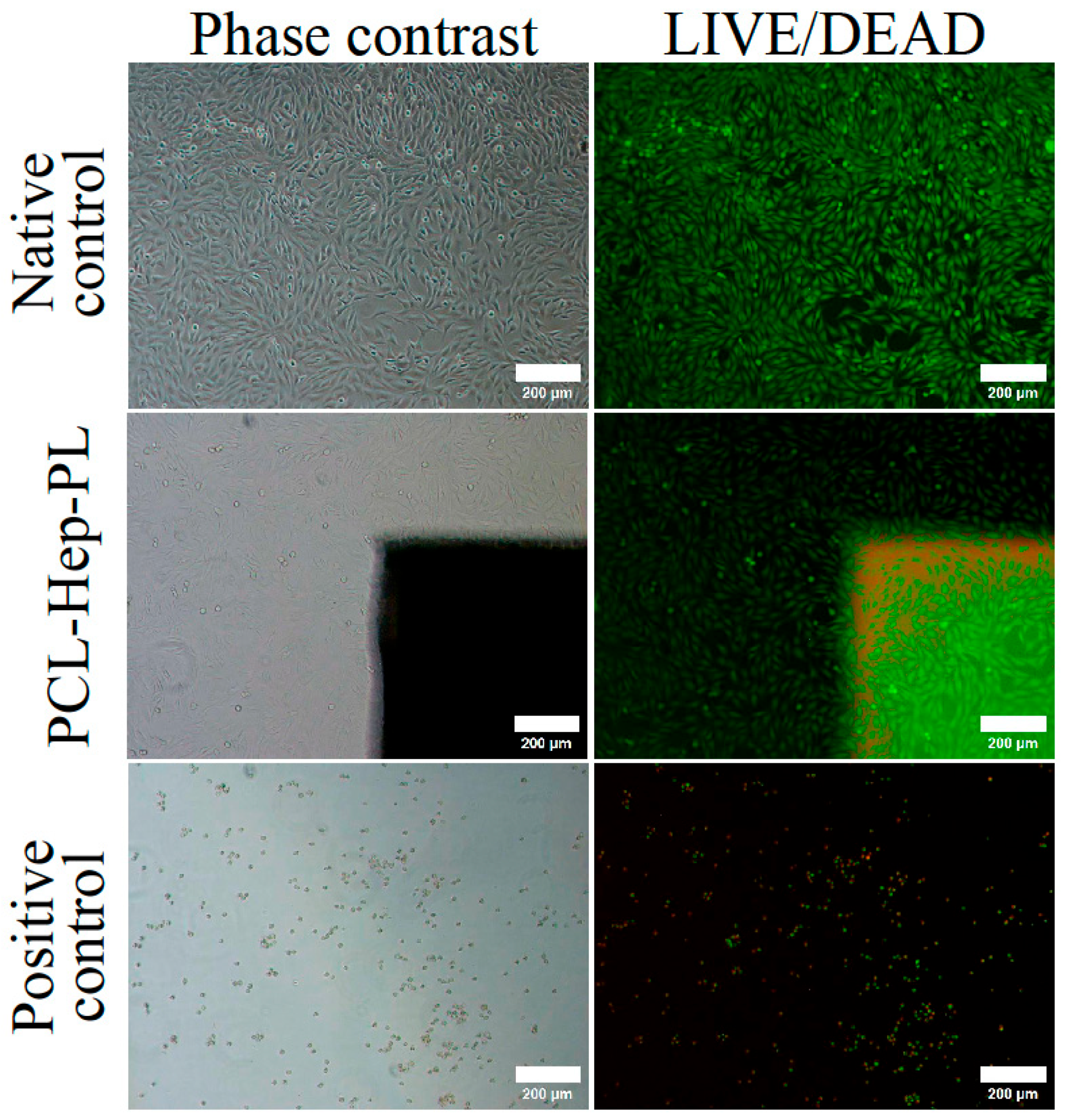
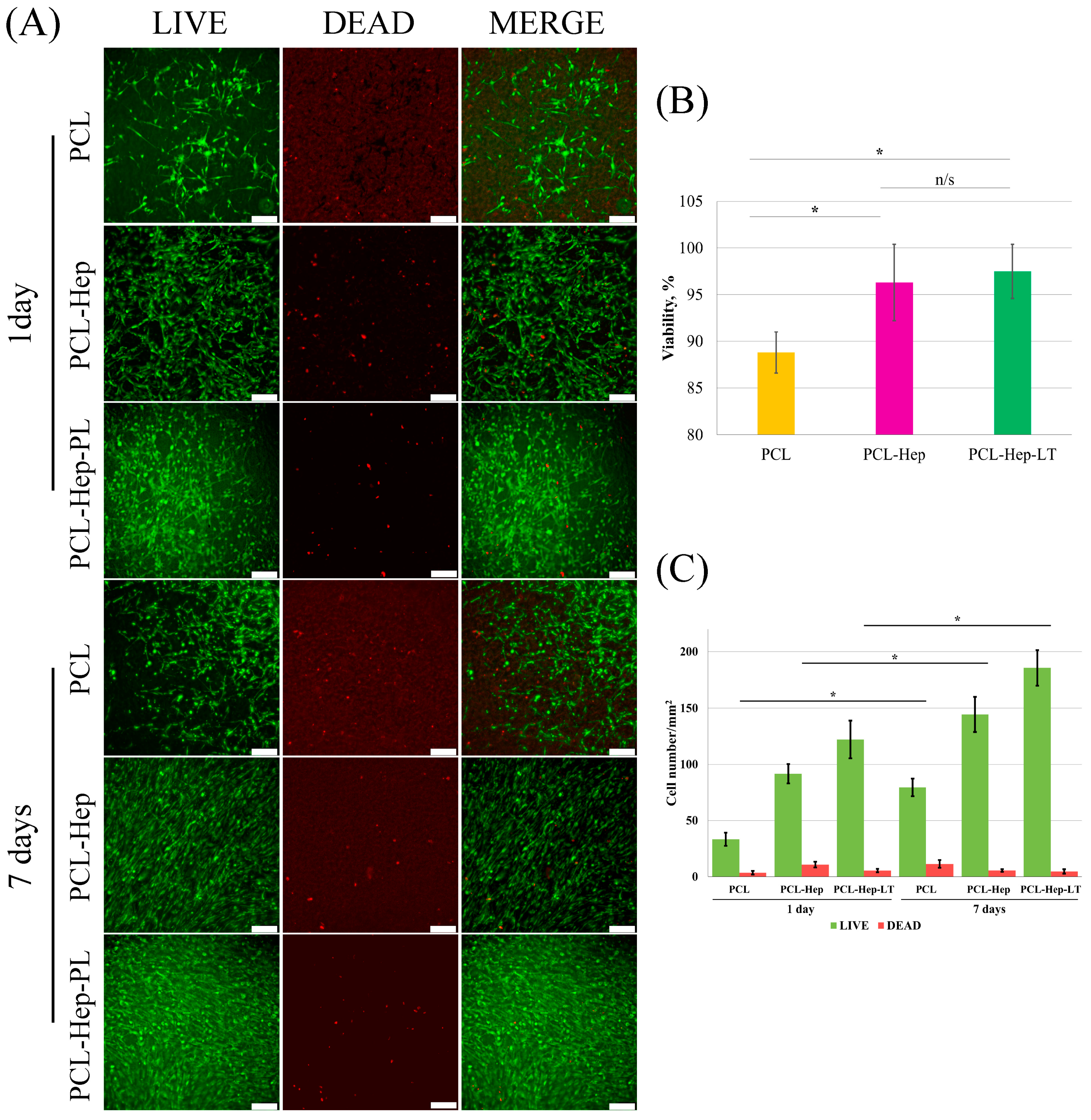
3.2. In Vitro Scaffolds Biocompatibility
3.3. Effect of the Treatment on the In Vitro Cells-Surface Interaction
3.3.1. Model Surface
3.3.2. Scaffold Surface
3.4. Scaffold In Vivo Biocompatibility and Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| TEC | Tissue-engineered constructs |
| PCL | Polycaprolactone |
| PLA | Polylactic acid |
| APTT | Activated partial thromboplastin time |
| DMEM | Dulbecco’s modified Eagle medium |
| MSC | Mesenchymal stromal cells |
| VEGF | Vascular endothelial growth factor |
| FBGC | Foreign body giant cells |
References
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 153–165. [Google Scholar] [CrossRef]
- Mao, J.; Duan, S.; Song, A.; Cai, Q.; Deng, X.; Yang, X. Macroporous and nanofibrous poly(lactide-co-glycolide) (50/50) scaffolds via phase separation combined with particle-leaching. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and application. Russian Chem. Reviews 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Meng, Q.; Xie, E.; Li, K.; Hu, J.; Chen, Q.; Li, J.; Han, F. Engineered biomimetic micro/nano-materials for tissue regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1205792. [Google Scholar] [CrossRef]
- Muthukrishnan, L. An overview on electrospinning and its advancement toward hard and soft tissue engineering applications. Colloid. Polym. Sci. 2022, 300, 875–901. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Li, Z.; Zhou, P.; Mao, Y. Electrospunnanofibrous membrane for biomedical application. SN Appl. Sci. 2022, 4, 172. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110716. [Google Scholar] [CrossRef]
- Ercolani, E.; Del Gaudio, C.; Bianco, A. Vascular tissue engineering of small-diameter blood vessels: Reviewing the electrospinning approach. J. Tissue Eng. Regen. Med. 2015, 9, 861–888. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, C.; Chen, F.; Sun, Y.; Xia, Y.; Ji, A.; Wang, D.A. Progress in Articular Cartilage Tissue Engineering: A Review on Therapeutic Cells and Macromolecular Scaffolds. Macromol. Biosci. 2020, 20, e1900278. [Google Scholar] [CrossRef]
- Jacobs, T.; Declercq, H.; De Geyter, N.; Cornelissen, R.; Dubruel, P.; Leys, C.; Beaurain, A.; Payen, E.; Morent, R. Enhanced cell-material interactions on medium-pressure plasma-treated polyhydroxybutyrate/polyhydroxyvalerate. J. Biomed. Mater. Res. A 2013, 101, 1778–1786. [Google Scholar] [CrossRef]
- Yan, D.; Jones, J.; Yuan, X.Y.; Xu, X.H.; Sheng, J.; Lee, J.C.-M.; Ma, G.Q.; Yu, Q.S. Plasma treatment of electrospun PCL random nanofiber meshes (NFMs) for biological property improvement. J. Biomed. Mater. Res. 2013, 101A, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Dufay, M.; Jimenez, M.; Degoutin, S. Effect of Cold Plasma Treatment on Electrospun Nanofibers Properties: A Review. ACS Appl. Bio. Mater. 2020, 3, 4696–4716. [Google Scholar] [CrossRef] [PubMed]
- Yaseri, R.; Fadaie, M.; Mirzaei, E.; Samadian, H.; Ebrahiminezhad, A. Surface modification of polycaprolactone nanofibers through hydrolysis and aminolysis: A comparative study on structural characteristics, mechanical properties, and cellular performance. Sci. Rep. 2023, 13, 9434. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, J.; Zhang, Y.; Zhang, L.; Yang, Y.B.; Manor, O.; You, J. Crystallinity Dependence of PLLA Hydrophilic Modification during Alkali Hydrolysis. Polymers 2022, 15, 75. [Google Scholar] [CrossRef]
- Nemets, E.A.; Surguchenko, V.A.; Pankina, A.P.; Metelsky, S.T.; Sevastianov, V.I. Methods for Regulation of Physicochemical and Biological Properties of the Surface of Poly(hydroxybutyrate-co-hydroxyvalerate) Film Specimens. Inorg. Mater Appl. Res. 2019, 10, 628–633. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly(hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Antonova, L.V.; Seifalian, A.M.; Kutikhin, A.G.; Sevostyanova, V.V.; Matveeva, V.G.; Velikanova, E.A.; Mironov, A.V.; Shabaev, A.R.; Glushkova, T.V.; Senokosova, E.A.; et al. Conjugation with RGD Peptides and Incorporation of Vascular Endothelial Growth Factor Are Equally Efficient for Biofunctionalization of Tissue-Engineered Vascular Grafts. Int. J. Mol. Sci. 2016, 17, 1920. [Google Scholar] [CrossRef]
- Boateng, S.Y.; Lateef, S.S.; Mosley, W.; Hartman, T.J.; Hanley, L.; Russell, B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am. J. Physiol. Cell Physiol. 2005, 288, 30–38. [Google Scholar] [CrossRef]
- Peng, G.; Yao, D.; Niu, Y.; Liu, H.; Fan, Y. Surface Modification of Multiple Bioactive Peptides to Improve Endothelialization of Vascular Grafts. Macromol. Biosci. 2019, 19, e1800368. [Google Scholar] [CrossRef]
- Wan, T.; Zhang, F.S.; Qin, M.Y.; Jiang, H.R.; Zhang, M.; Qu, Y.; Wang, Y.L.; Zhang, P.X. Growth factors: Bioactive macromolecular drugs for peripheral nerve injury treatment—Molecular mechanisms and delivery platforms. Biomed. Pharmacother. 2024, 170, 116024. [Google Scholar] [CrossRef] [PubMed]
- Táborská, J.; Blanquer, A.; Brynda, E.; Filová, E.; Stiborová, L.; Jenčová, V.; Havlíčková, K.; Riedelová, Z.; Riedel, T. PLCL/PCL Dressings with Platelet Lysate and Growth Factors Embedded in Fibrin for Chronic Wound Regeneration. Int. J. Nanomed. 2023, 18, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Elcin, A.E.; Elcin, Y.M. Localized angiogenesis induced by human vascular endothelial growth factor-activated PLGA sponge. Tissue Eng. 2006, 12, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Lee, Y.B.; Kim, S.J.; Kang, J.K.; Park, J.C.; Jang, W.; Shin, H. Mussel-inspired immobilization of vascular endothelial growth factor (VEGF) for enhanced endothelialization of vascular grafts. Biomacromolecules 2012, 13, 2020–2028. [Google Scholar] [CrossRef]
- De Visscher, G.; Mesure, L.; Meuris, B.; Ivanova, A.; Flameng, W. Improved endothelialization and reduced thrombosis by coating a synthetic vascular graft with fibronectin and stem cell homing factor SDF-1α. Acta Biomater. 2012, 8, 1330–1338. [Google Scholar] [CrossRef]
- Vijayan, A.; Nanditha, C.K.; Vinod Kumar, G.S. ECM-mimicking nanofibrous scaffold enriched with dual growth factor carrying nanoparticles for diabetic wound healing. Nanoscale Adv. 2021, 3, 3085–3092. [Google Scholar] [CrossRef]
- Ori, A.; Free, P.; Courty, J.; Wilkinson, M.C.; Fernig, D.G. Identification of heparin-binding sites in proteins by selective labeling. Mol. Cell Proteomics. 2009, 8, 2256–2265. [Google Scholar] [CrossRef]
- Aslani, S.; Kabiri, M.; Hossein Zadeh, S.; Hanaee-Ahvaz, H.; Taherzadeh, E.S.; Soleimani, M. The applications of heparin in vascular tissue engineering. Microvasc. Res. 2020, 131, 104027. [Google Scholar] [CrossRef]
- Maitz, M.F.; Martins, M.C.L.; Grabow, N.; Matschegewski, C.; Huang, N.; Chaikof, E.L.; Barbosa, M.A.; Werner, C.; Sperling, C. The blood compatibility challenge. Part 4: Surface modification for hemocompatible materials: Passive and active approaches to guide blood-material interactions. Acta Biomater. 2019, 94, 33–43. [Google Scholar] [CrossRef]
- Ebert, C.D.; Kim, S.W. Immobilized heparin: Spacer arm effects on biological interactions. Thromb. Res. 1982, 26, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Y.; Sheardown, H.; Brook, M.A. Immobilization of heparin on a silicone surface through a heterobifunctional PEG spacer. Biomaterials 2005, 26, 7418–7424. [Google Scholar] [CrossRef] [PubMed]
- Nojiri, C.; Kido, T.; Sugiyama, T.; Horiuchi, K.; Kijima, T.; Hagiwara, K.; Kuribayashi, E.; Nogawa, A.; Ogiwara, K.; Akutsu, T. Can heparin immobilized surfaces maintain nonthrombogenic activity during in vivo long-term implantation? ASAIO J. 1996, 42, M468–M475. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, M. Customized Ca-P/PHBV nanocomposite scaffolds for bone tissue engineering: Design, fabrication, surface modification and sustained release of growth factor. J. R. Soc. Interface 2010, 7 (Suppl. S5), S615–S629. [Google Scholar] [CrossRef]
- Nemets, E.A.; Sevastianov, V.I. The interaction of heparinized biomaterials with human serum, albumin, fibrinogen, antithrombin III, and platelets. Artif. Organs. 1991, 15, 381–385. [Google Scholar] [CrossRef]
- van Delden, C.J.; Engbers, G.H.; Feijen, J. Interaction of antithrombin III with surface-immobilized albumin-heparin conjugates. J. Biomed. Mater. Res. 1995, 29, 1317–1329. [Google Scholar] [CrossRef]
- Mirow, N.; Zimmermann, B.; Maleszka, A.; Knobl, H.; Tenderich, G.; Koerfer, R.; Herberg, F.W. Plasma protein binding properties to immobilized heparin and heparin-albumin conjugate. Artif. Organs. 2007, 31, 466–471. [Google Scholar] [CrossRef]
- Duysens, J.; Graide, H.; Niesten, A.; Mouithys-Mickalad, A.; Deby-Dupont, G.; Franck, T.; Ceusters, J.; Serteyn, D. Culture and Immunomodulation of Equine Muscle-Derived Mesenchymal Stromal Cells: A Comparative Study of Innovative 2D versus 3D Models Using Equine Platelet Lysate. Cells 2024, 13, 1290. [Google Scholar] [CrossRef]
- Mareschi, K.; Marini, E.; Niclot, A.G.S.B.; Barone, M.; Pinnetta, G.; Adamini, A.; Spadea, M.; Labanca, L.; Lucania, G.; Ferrero, I.; et al. A New Human Platelet Lysate for Mesenchymal Stem Cell Production Compliant with Good Manufacturing Practice Conditions. Int. J. Mol. Sci. 2022, 23, 3234. [Google Scholar] [CrossRef]
- Yoon, J.-Y.; Vu, H.T.; Lee, J.H.; Shin, J.-S.; Kim, H.-W.; Lee, H.-H.; Kim, J.-B.; Lee, J.-H. Evaluation of Human Platelet Lysate as an Alternative to Fetal Bovine Serum for Potential Clinical Applications of Stem Cells from Human Exfoliated Deciduous Teeth. Cells 2024, 13, 847. [Google Scholar] [CrossRef]
- Grivet-Brancot, A.; Buscemi, M.; Ciardelli, G.; Bronco, S.; Sartori, S.; Cassino, C.; Al Kayal, T.; Losi, P.; Soldani, G.; Boffito, M. Cord Blood Platelet Lysate-Loaded Thermo-Sensitive Hydrogels for Potential Treatment of Chronic Skin Wounds. Pharmaceutics 2024, 16, 1438. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, C.; Foti, R.; Zeppieri, M.; Maniaci, A.; Lavalle, S.; Tancredi, G.; Gagliano, G.; Avitabile, A.; Cannizzaro, L.; Foti, R. Umbilical Cord Blood Platelet Lysate Eyedrops for the Treatment of Severe Ocular Surface Disorders in Graft vs. Host Disease Patients: Clinical Study. Life 2024, 14, 1268. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Kountouri, A.; Psahoulia, F.; Koliou, G.-A.; Lazaris, A.; Michalopoulos, E.; Mallis, P.; Korakas, E.; Eleftheriadou, I.; Balampanis, K.; et al. Treatment with Umbilical Cord Blood Platelet Lysate Gel Improves Healing of Diabetic Foot Ulcer. J. Clin. Med. 2024, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, A.; Hnydka, A.; Higuchi, J.; Michalak, A.; Tarczynska, M.; Gaweda, K.; Klimek, K. Recent Achievements in the Development of Biomaterials Improved with Platelet Concentrates for Soft and Hard Tissue Engineering Applications. Int. J. Mol. Sci. 2024, 25, 1525. [Google Scholar] [CrossRef]
- Nakamura, S.; Kubo, T.; Ijima, H. Heparin-conjugated gelatin as a growth factor immobilization scaffold. J. Biosci. Bioeng. 2013, 15, 562–567. [Google Scholar] [CrossRef]
- Nemets, E.A.; Belov, V.Y.; Ilina, T.S.; Surguchenko, V.A.; Pankina, A.P.; Sevastyanov, V.I. Composite porous tubular biopolymer matrix of small diameter. Perspekt. Materialy. 2018, 9, 49–59. [Google Scholar] [CrossRef]
- Surguchenko, V.A.; Nemets, E.A.; Belov, V.Y.; Sevastianov, V.I. Bioactive coating for tissue-engineered smalldiameter vascular grafts. Russ. J. Transplantol. Artif. Organs 2021, 23, 119–131. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Zhuravleva, I.Y.; Belomestnaya, Z.M.; Nemets, E.A.; Salomatina, L.A.; Krikovtsov, A.A.; Novikova, S.P.; Barbarash, L.S. The effect of conservation and heparinization on their mechanism interactions between small veselxenoprostheses and blood components. Biomater.-Living Syst. Interact. 1995, 3, 119–132. [Google Scholar]
- Byun, J.H.; Jang, I.S.; Kim, J.W.; Koh, E.H. Establishing the heparin therapeutic range using aPTT and anti-Xa measurements for monitoring unfractionated heparin therapy. Blood Res. 2016, 51, 171–174. [Google Scholar] [CrossRef]
- ISO 10993-4:2017; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. ISO: Geneva, Switzerland, 2009.
- GOST 33215-2014; Guidelines for Accommodation and Care of Animals. Environment, Housing and Management. The Federal Agency for Technical Regulation and Metrology (Rosstandart): Khabarovsk, Russia, 2014.
- GOST 33216-2014; Guidelines for Accommodation and Care of Animals. Species-Specific Provisions for Laboratory Rodents and Rabbits. The Federal Agency for Technical Regulation and Metrology (Rosstandart): Khabarovsk, Russia, 2014.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Vakhrushev, I.V.; Basok, Y.B.; Baskaev, K.K.; Novikova, V.D.; Leonov, G.E.; Grigoriev, A.M.; Belova, A.D.; Kirsanova, L.A.; Lupatov, A.Y.; Burunova, V.V.; et al. Cartilage-Specific Gene Expression and Extracellular Matrix Deposition in the Course of Mesenchymal Stromal Cell Chondrogenic Differentiation in 3D Spheroid Culture. Int. J. Mol. Sci. 2024, 25, 5695. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bünger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- John, J.V.; Sharma, N.S.; Tang, G.; Luo, Z.; Su, Y.; Weihs, S.; Shahriar, S.M.S.; Wang, G.; McCarthy, A.; Dyke, J.; et al. Nanofiber Aerogels with Precision Macrochannels and LL-37-Mimic Peptides Synergistically Promote Diabetic Wound Healing. Adv. Funct. Mater. 2023, 33, 2206936. [Google Scholar] [CrossRef]
- Karan, A.; Sharma, N.S.; Darder, M.; Su, Y.; Andrabi, S.M.; Shahriar, S.M.S.; John, J.V.; Luo, Z.; DeCoster, M.A.; Zhang, Y.S.; et al. Copper-Cystine Biohybrid-Embedded Nanofiber Aerogels Show Antibacterial and Angiogenic Properties. ACS Omega 2024, 9, 9765–9781. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre Diameter Fibres of Polymer, Produced by Electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.Y.; Wu, L.L.; Han, Y.; Sheng, J. Study on the Morphology of Electrospun Poly(Vinyl Alcohol) Fiber Mat. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of Electrospinning Parameters on the Nanofiber Diameter and Length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y.N. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Casanova, M.R.; Reis, R.L.; Martins, A.; Neves, N.M. The Use of Electrospinning Technique on Osteochondral Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1058, 247–263. [Google Scholar] [CrossRef]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef] [PubMed]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, G. Heparin Binding Proteins as Therapeutic Target: An Historical Account and Current Trends. Medicines 2019, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, Y.; Mizumachi, H.; Yoshida, K.; Ijima, H. Heparin-conjugated collagen as a potent growth factor-localizing and stabilizing scaffold for regenerative medicine. Regen. Ther. 2020, 15, 236–242. [Google Scholar] [CrossRef]
- Kim, S.E.; Jeong, S.I.; Shim, K.M.; Jang, K.; Park, J.S.; Lim, Y.M.; Kang, S.S. In Vivo Evaluation of Gamma-Irradiated and Heparin-Immobilized Small-Diameter Polycaprolactone Vascular Grafts with VEGF in Aged Rats. Polymers 2022, 14, 1265. [Google Scholar] [CrossRef]
- Cabric, S.; Sanchez, J.; Johansson, U.; Larsson, R.; Nilsson, B.; Korsgren, O.; Magnusson, P.U. Anchoring of vascular endothelial growth factor to surface-immobilized heparin on pancreatic islets: Implications for stimulating islet angiogenesis. Tissue Eng. Part. A 2010, 16, 961–970. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Poomani, M.S.; Mariappan, I.; Perumal, R.; Regurajan, R.; Muthan, K.; Subramanian, V. Mesenchymal stem cell (MSCs) therapy for ischemic heart disease: A promising frontier. Glob. Heart. 2022, 17, 19. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Shi, G.; Lei, X.; Huang, Y.; Bai, L.; Qin, C. Effectiveness and mechanisms of adipose-derived stem cell therapy in animal models of Parkinson’s disease: A systematic review and meta-analysis. Transl. Neurodegener. 2021, 10, 14. [Google Scholar] [CrossRef]
- Zaripova, L.N.; Midgley, A.; Christmas, S.E.; Beresford, M.W.; Pain, C.; Baildam, E.M.; Oldershaw, R.A. Mesenchymal stem cells in the pathogenesis and therapy of autoimmune and autoinflammatory diseases. Int. J. Mol. Sci. 2023, 24, 16040. [Google Scholar] [CrossRef]
- Cruz, F.F.; Rocco, P.R.M. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev. Respir. Med. 2020, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Han, H.T.; Jin, W.L.; Li, X. Mesenchymal stem cells-based therapy in liver diseases. Mol. Biomed. 2022, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, N.; Xia, C.; Wang, H.; Shao, L.; Zhou, C.; Wang, J. Mesenchymal stem cell therapy in kidney diseases: Potential and challenges. Cell Transplant. 2023, 32, 9636897231164251. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Luo, F.; Lin, Y.; Luo, J.; Ke, D.; Song, C.; Xu, J. Research trends of mesenchymal stem cells application in orthopedics: A bibliometric analysis of the past 2 decades. Front. Public Health 2022, 10, 1021818. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Vasilets, V.N.; Surguchenko, V.A.; Ponomareva, A.S.; Nemetz, E.A.; Sevastianov, V.I.; Bae, J.W.; Park, K.D. Effects of surface properties of bacterial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) on adhesion and proliferation of mouse fibroblasts. Macromol. Res. 2015, 23, 205–213. [Google Scholar] [CrossRef]
- Chernonosova, V.S.; Laktionov, P.P. Structural Aspects of Electrospun Scaffolds Intended for Prosthetics of Blood Vessels. Polymers 2022, 14, 1698. [Google Scholar] [CrossRef]


| Feedrate, mL/h | Young’s Modulus, MPa | Tensile Strength, MPa | Elongation at Break, % |
|---|---|---|---|
| 2 | 3.1 ± 0.3 | 1.3 ± 0.2 | 134 ± 9 |
| 4 | 5.5 ± 1.1 | 2.5 ± 0.4 | 321 ± 29 |
| 6 | 4.7 ± 0.3 | 1.9 ± 0.2 | 298 ± 18 |
| Rat aorta * | 1.6 ± 0.4 | 2.9 ± 0.5 | 327 ± 38 |
| Feedrate, mL/h | Young’s Modulus, Pa | Tensile Strength, MPa | Maximal Elongation, % |
|---|---|---|---|
| PCL | 5.5 ± 1.1 | 2.0 ± 0.4 | 321 ± 29 |
| PCL-Hep-PL | 4.7 ± 0.4 | 1.3 ± 0.3 | 257 ± 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevastianov, V.I.; Nemets, E.A.; Grigoriev, A.M.; Belova, A.D.; Belov, V.Y.; Kirsanova, L.A.; Ponomareva, A.S.; Grudinin, N.V.; Bogdanov, V.K.; Nikolskaya, A.O.; et al. The Effect of Bilayered Bioactive Coating on Polycaprolactone Electrospun Scaffold Biocompatibility, Bioabsorption and Cellular Properties. Polymers 2025, 17, 2813. https://doi.org/10.3390/polym17212813
Sevastianov VI, Nemets EA, Grigoriev AM, Belova AD, Belov VY, Kirsanova LA, Ponomareva AS, Grudinin NV, Bogdanov VK, Nikolskaya AO, et al. The Effect of Bilayered Bioactive Coating on Polycaprolactone Electrospun Scaffold Biocompatibility, Bioabsorption and Cellular Properties. Polymers. 2025; 17(21):2813. https://doi.org/10.3390/polym17212813
Chicago/Turabian StyleSevastianov, Victor I., Evgeniy A. Nemets, Alexey M. Grigoriev, Aleksandra D. Belova, Vyacheslav Yu. Belov, Lyudmila A. Kirsanova, Anna S. Ponomareva, Nikita V. Grudinin, Vladimir K. Bogdanov, Alla O. Nikolskaya, and et al. 2025. "The Effect of Bilayered Bioactive Coating on Polycaprolactone Electrospun Scaffold Biocompatibility, Bioabsorption and Cellular Properties" Polymers 17, no. 21: 2813. https://doi.org/10.3390/polym17212813
APA StyleSevastianov, V. I., Nemets, E. A., Grigoriev, A. M., Belova, A. D., Belov, V. Y., Kirsanova, L. A., Ponomareva, A. S., Grudinin, N. V., Bogdanov, V. K., Nikolskaya, A. O., Kuznetsova, E. G., Guseva, E. A., Basok, Y. B., & Gautier, S. V. (2025). The Effect of Bilayered Bioactive Coating on Polycaprolactone Electrospun Scaffold Biocompatibility, Bioabsorption and Cellular Properties. Polymers, 17(21), 2813. https://doi.org/10.3390/polym17212813






