Bioinspired Nanoplatforms: Polydopamine and Exosomes for Targeted Antimicrobial Therapy
Abstract
1. Introduction
2. Overview of AMR
Mechanism of Antibiotic Resistance
3. Polydopamine (PDA)
3.1. Physicochemical Characteristics of PDA
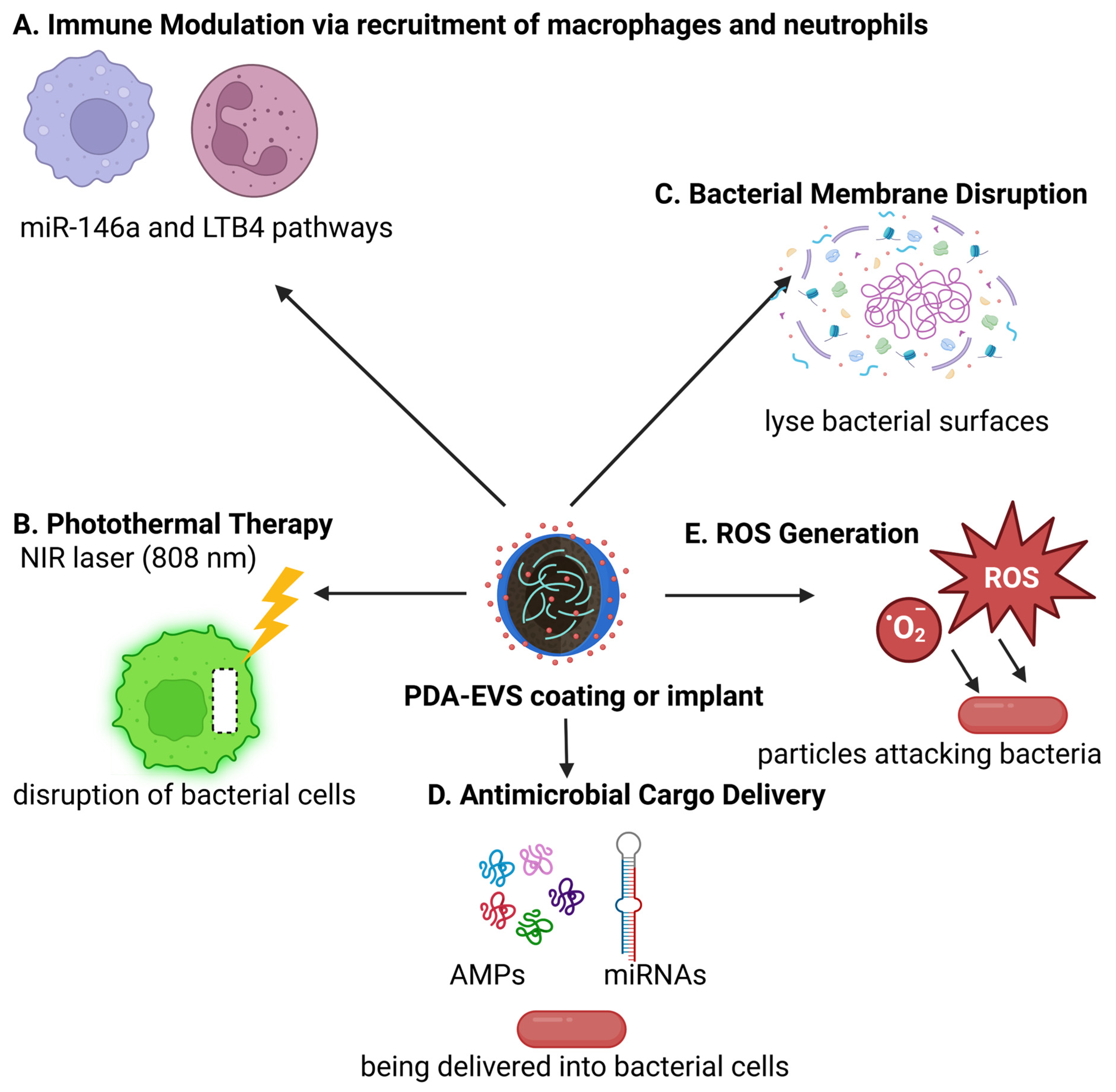
3.2. Antimicrobial Activity of Polydopamine
3.3. Synergistic Antimicrobial Systems Based on Polydopamine
4. Extracellular Vesicle-Based Therapeutics
4.1. Biology and Function of Extracellular Vesicles
4.2. EVs in Infectious Diseases
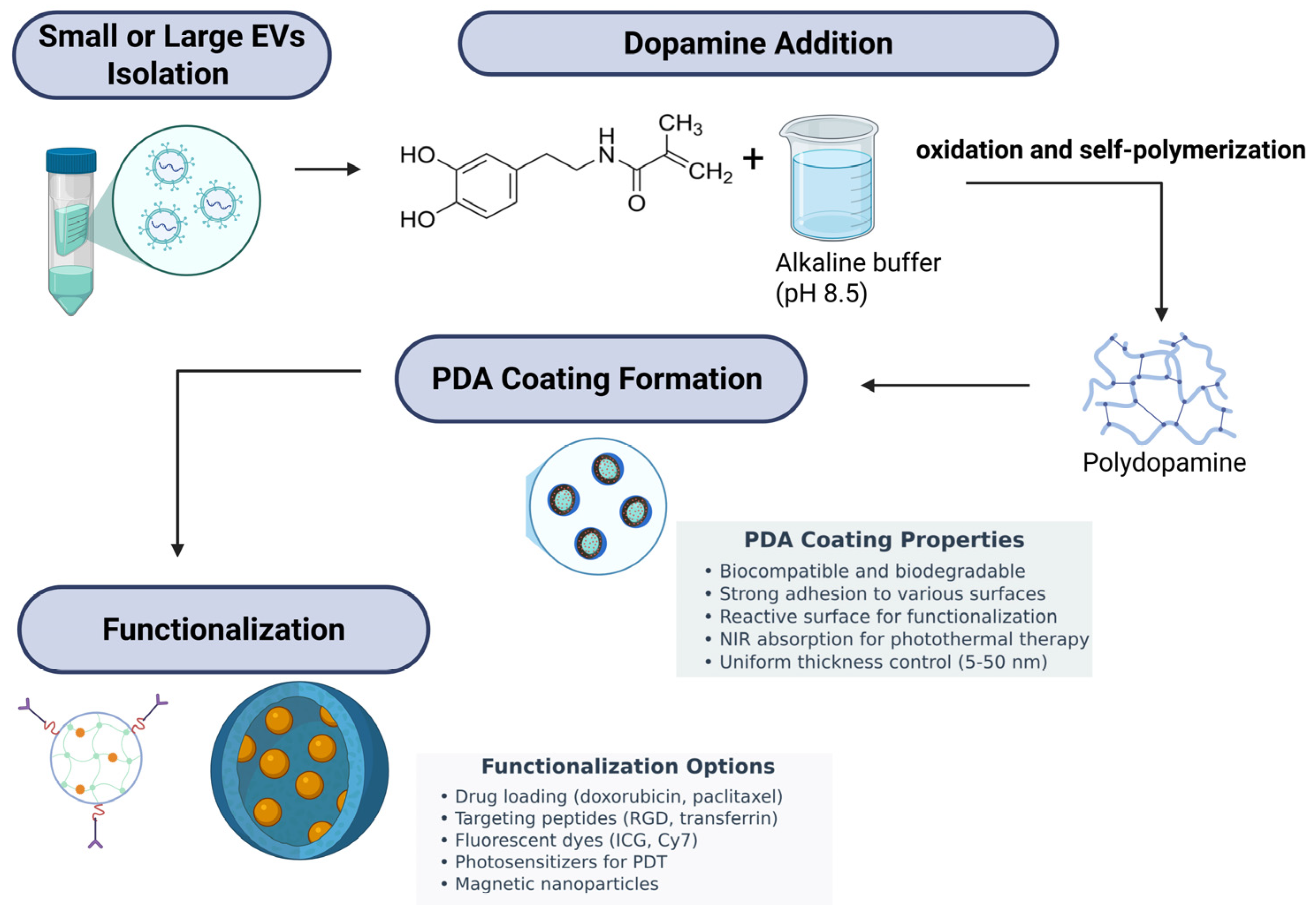
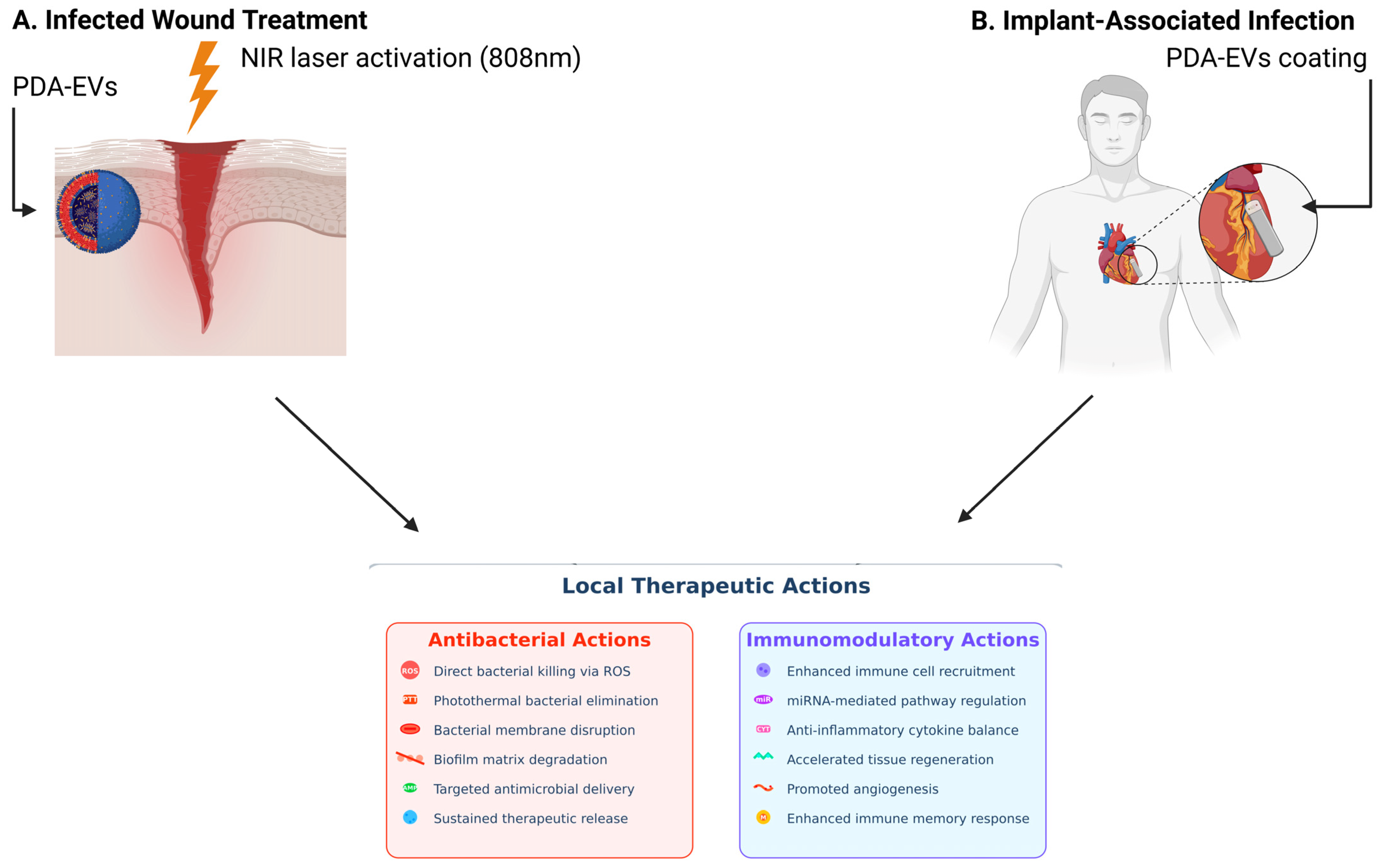
5. PDA Coatings and Exosomes in Antimicrobial Applications: A Synergistic Strategy
6. Advantages of Exosome-Encapsulated Antibiotics
7. Other Promising Polymers for Antimicrobial Exosome Coatings
8. Challenges in Applying Antimicrobial Coatings to Exosomes
9. Translational Challenges and Regulatory Hurdles of Exosome-Based Nanotherapies
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Chauhan, A.; Ranjan, A.; Mathkor, D.M.; Haque, S.; Ramniwas, S.; Tuli, H.S.; Jindal, T.; Yadav, V. Emerging challenges in antimicrobial resistance: Implications for pathogenic microorganisms, novel antibiotics, and their impact on sustainability. Front. Microbiol. 2024, 15, 1403168. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Ndaki, P.M.; Mwanga, J.R.; Mushi, M.F.; Konje, E.T.; Mwita, S.M.; Mshana, S.E. Drivers of inappropriate use of antibiotics among community members in low- and middle-income countries: A systematic review of qualitative studies. BMC Public Health 2025, 25, 705. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Dias da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Acter, S.; Moreau, M.; Ivkov, R.; Viswanathan, A.; Ngwa, W. Polydopamine Nanomaterials for Overcoming Current Challenges in Cancer Treatment. Nanomaterials 2023, 13, 1656. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, A.; Mordini, D.; Montalti, M. Polydopamine Nanosystems in Drug Delivery: Effect of Size, Morphology, and Surface Charge. Nanomaterials 2024, 14, 303. [Google Scholar] [CrossRef]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2021, 11, 607099. [Google Scholar] [CrossRef]
- Sall, I.M.; Flaviu, T.A. Plant and mammalian-derived extracellular vesicles: A new therapeutic approach for the future. Front. Bioeng. Biotechnol. 2023, 11, 1215650. [Google Scholar] [CrossRef]
- Aslam, B.; Asghar, R.; Muzammil, S.; Shafique, M.; Siddique, A.B.; Khurshid, M.; Ijaz, M.; Rasool, M.H.; Chaudhry, T.H.; Aamir, A.; et al. AMR and Sustainable Development Goals: At a crossroads. Glob. Health 2024, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.; Enciu, A.-M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds With Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, S.M.; Palmeiro-Silva, Y.K.; Llerena-Cayo, C.; Blanco-Villafuerte, L.; Escobar, L.E.; Diaz, A.; Sarmiento, J.H.; Lescano, A.G.; Melo, O.; Rojas-Rueda, D.; et al. The 2023 Latin America report of the Lancet Countdown on health and climate change: The imperative for health-centred climate-resilient development. Lancet regional health. Americas 2024, 33, 100746. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Egorov, A.M.; Ulyashova, M.M.; Rubtsova, M.Y. Bacterial Enzymes and Antibiotic Resistance. Acta Naturae 2018, 10, 33–48. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, M.C.; Maugeri, A.; Favara, G.; La Mastra, C.; Magnano San Lio, R.; Barchitta, M.; Agodi, A. The Impact of Wastewater on Antimicrobial Resistance: A Scoping Review of Transmission Pathways and Contributing Factors. Antibiotics 2025, 14, 131. [Google Scholar] [CrossRef]
- Bokhary, H.; Pangesti, K.N.A.; Rashid, H.; Abd El Ghany, M.; Hill-Cawthorne, G.A. Travel-Related Antimicrobial Resistance: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 11. [Google Scholar] [CrossRef]
- Sakalauskienė, G.V.; Malcienė, L.; Stankevičius, E.; Radzevičienė, A. Unseen Enemy: Mechanisms of Multidrug Antimicrobial Resistance in Gram-Negative ESKAPE Pathogens. Antibiotics 2025, 14, 63. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Mahey, N.; Tambat, R.; Kalia, R.; Ingavale, R.; Kodesia, A.; Chandal, N.; Kapoor, S.; Verma, D.K.; Thakur, K.G.; Jachak, S.; et al. Pyrrole-based inhibitors of RND-type efflux pumps reverse antibiotic resistance and display anti-virulence potential. PLoS Pathog. 2024, 20, e1012121. [Google Scholar] [CrossRef]
- Huang, L.; Wu, C.; Gao, H.; Xu, C.; Dai, M.; Huang, L.; Hao, H.; Wang, X.; Cheng, G. Bacterial Multidrug Efflux Pumps at the Frontline of Antimicrobial Resistance: An Overview. Antibiotics 2022, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial Multidrug Efflux Pumps: Much More Than Antibiotic Resistance Determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281–290. [Google Scholar] [CrossRef]
- Dashtbani-Roozbehani, A.; Brown, M.H. Efflux Pump Mediated Antimicrobial Resistance by Staphylococci in Health-Related Environments: Challenges and the Quest for Inhibition. Antibiotics 2021, 10, 1502. [Google Scholar] [CrossRef] [PubMed]
- Hajiagha, M.N.; Kafil, H.S. Efflux pumps and microbial biofilm formation. Infect. Genet. Evol. 2023, 112, 105459. [Google Scholar] [CrossRef]
- Jang, S. AcrAB-TolC, a major efflux pump in Gram negative bacteria: Toward understanding its operation mechanism. BMB Rep. 2023, 56, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Zack, K.M.; Sorenson, T.; Joshi, S.G. Types and Mechanisms of Efflux Pump Systems and the Potential of Efflux Pump Inhibitors in the Restoration of Antimicrobial Susceptibility, with a Special Reference to Acinetobacter baumannii. Pathogens 2024, 13, 197. [Google Scholar] [CrossRef]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Rahman, T.; Yarnall, B.; Doyle, D.A. Efflux drug transporters at the forefront of antimicrobial resistance. Eur. Biophys. J. EBJ 2017, 46, 647–653. [Google Scholar] [CrossRef]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G.; et al. Mechanism of antibacterial resistance, strategies and next-generation antimicrobials to contain antimicrobial resistance: A review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Thacharodi, A.; Lamont, I.L. Aminoglycoside-Modifying Enzymes Are Sufficient to Make Pseudomonas aeruginosa Clinically Resistant to Key Antibiotics. Antibiotics 2022, 11, 884. [Google Scholar] [CrossRef]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-Inactivating Enzymes. Front. Microbiol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Surette, M.D.; Spanogiannopoulos, P.; Wright, G.D. The Enzymes of the Rifamycin Antibiotic Resistome. Acc. Chem. Res. 2021, 54, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- van Duijkeren, E.; Schink, A.K.; Roberts, M.C.; Wang, Y.; Schwarz, S. Mechanisms of Bacterial Resistance to Antimicrobial Agents. Microbiol. Spectr. 2018, 6, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Badawy, N.K.; Ashraf, Y.; Zatioun, A.A.; Masriya, H.H.; Ammar, M.M.; Mohamed, N.A.; Mourad, S.; Assy, A.M. Combating Antibiotic Resistance: Mechanisms, Multidrug-Resistant Pathogens, and Novel Therapeutic Approaches: An Updated Review. Pharmaceuticals 2025, 18, 402. [Google Scholar] [CrossRef]
- Jagielski, T.; Bakuła, Z.; Brzostek, A.; Minias, A.; Stachowiak, R.; Kalita, J.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Żaczek, A.; Vasiliauskiene, E.; et al. Characterization of Mutations Conferring Resistance to Rifampin in Mycobacterium tuberculosis Clinical Strains. Antimicrob. Agents Chemother. 2018, 62, e01093-18. [Google Scholar] [CrossRef]
- Weigel, L.M.; Anderson, G.J.; Tenover, F.C. DNA gyrase and topoisomerase IV mutations associated with fluoroquinolone resistance in Proteus mirabilis. Antimicrob. Agents Chemother. 2002, 46, 2582–2587. [Google Scholar] [CrossRef] [PubMed]
- Shields, K.E.; Ranava, D.; Tan, Y.; Zhang, D.; Yap, M.F. Epitranscriptional m6A modification of rRNA negatively impacts translation and host colonization in Staphylococcus aureus. PLoS Pathog. 2024, 20, e1011968. [Google Scholar] [CrossRef] [PubMed]
- Lade, H.; Joo, H.S.; Kim, J.S. Molecular Basis of Non-β-Lactam Antibiotics Resistance in Staphylococcus aureus. Antibiotics 2022, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Rajput, P.; Nahar, K.S.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Positive Bacteria. Antibiotics 2024, 13, 1197. [Google Scholar] [CrossRef]
- Krawczyk, S.J.; Leśniczak-Staszak, M.; Gowin, E.; Szaflarski, W. Mechanistic Insights into Clinically Relevant Ribosome-Targeting Antibiotics. Biomolecules 2024, 14, 1263. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Wilson, A.; Ruiz, N. Transport of lipopolysaccharides and phospholipids to the outer membrane. Curr. Opin. Microbiol. 2021, 60, 51–57. [Google Scholar] [CrossRef]
- Prajapati, J.D.; Kleinekathöfer, U.; Winterhalter, M. How to Enter a Bacterium: Bacterial Porins and the Permeation of Antibiotics. Chem. Rev. 2021, 121, 5158–5192. [Google Scholar] [CrossRef]
- Mayse, L.A.; Movileanu, L. Gating of β-Barrel Protein Pores, Porins, and Channels: An Old Problem with New Facets. Int. J. Mol. Sci. 2023, 24, 12095. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.F.; Williams, B.J.; Blackwell, T.S.; Xie, C.M. Structure and function of OprD protein in Pseudomonas aeruginosa: From antibiotic resistance to novel therapies. Int. J. Med. Microbiol. IJMM 2012, 302, 63–68. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Wang, Y.; Wen, X.; Peng, H.; Peng, R.; Shi, Q.; Xie, X.; Li, L. Outer Membrane Porins Contribute to Antimicrobial Resistance in Gram-Negative Bacteria. Microorganisms 2023, 11, 1690. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Azeem, K.; Fatima, S.; Ali, A.; Ubaid, A.; Husain, F.M.; Abid, M. Biochemistry of Bacterial Biofilm: Insights into Antibiotic Resistance Mechanisms and Therapeutic Intervention. Life 2025, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Rohde, H.; Vilanova, M.; Cerca, N. Fighting Staphylococcus epidermidis Biofilm-Associated Infections: Can Iron Be the Key to Success? Front. Cell. Infect. Microbiol. 2021, 11, 798563. [Google Scholar] [CrossRef]
- Cigana, C.; Bianconi, I.; Baldan, R.; De Simone, M.; Riva, C.; Sipione, B.; Rossi, G.; Cirillo, D.M.; Bragonzi, A. Staphylococcus aureus Impacts Pseudomonas aeruginosa Chronic Respiratory Disease in Murine Models. J. Infect. Dis. 2018, 217, 933–942. [Google Scholar] [CrossRef]
- Worley, M.J. Immune evasion and persistence in enteric bacterial pathogens. Gut Microbes 2023, 15, 2163839. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Hassan, N.; Rafiq, M.; Hassan, F.; Rehman, M.; Iqbal, N.; Ali, H.; Hasan, F.; Kang, Y.-Q. Biofilms as Battlefield Armor for Bacteria against Antibiotics: Challenges and Combating Strategies. Microorganisms 2023, 11, 2595. [Google Scholar] [CrossRef]
- Haque, T.T.; Frischmeyer-Guerrerio, P.A. The Role of TGFβ and Other Cytokines in Regulating Mast Cell Functions in Allergic Inflammation. Int. J. Mol. Sci. 2022, 23, 10864. [Google Scholar] [CrossRef] [PubMed]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Antimicrobial Mechanisms of Macrophages and the Immune Evasion Strategies of Staphylococcus aureus. Pathogens 2015, 4, 826–868. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.R.; Menon, S.S.; Cortes, C.; Ferreira, V.P. Hijacking Factor H for Complement Immune Evasion. Front. Immunol. 2021, 12, 602277. [Google Scholar] [CrossRef] [PubMed]
- Doran, K.S.; Fulde, M.; Gratz, N.; Kim, B.J.; Nau, R.; Prasadarao, N.; Schubert-Unkmeir, A.; Tuomanen, E.I.; Valentin-Weigand, P. Host–pathogen interactions in bacterial meningitis. Acta Neuropathol. 2016, 131, 185–209. [Google Scholar] [CrossRef]
- Olorunmola, F.O.; Kolawole, D.O.; Lamikanra, A. Antibiotic resistance and virulence properties in Escherichia coli strains from cases of urinary tract infections. Afr. J. Infect. Dis. 2013, 7, 1–7. [Google Scholar] [CrossRef][Green Version]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
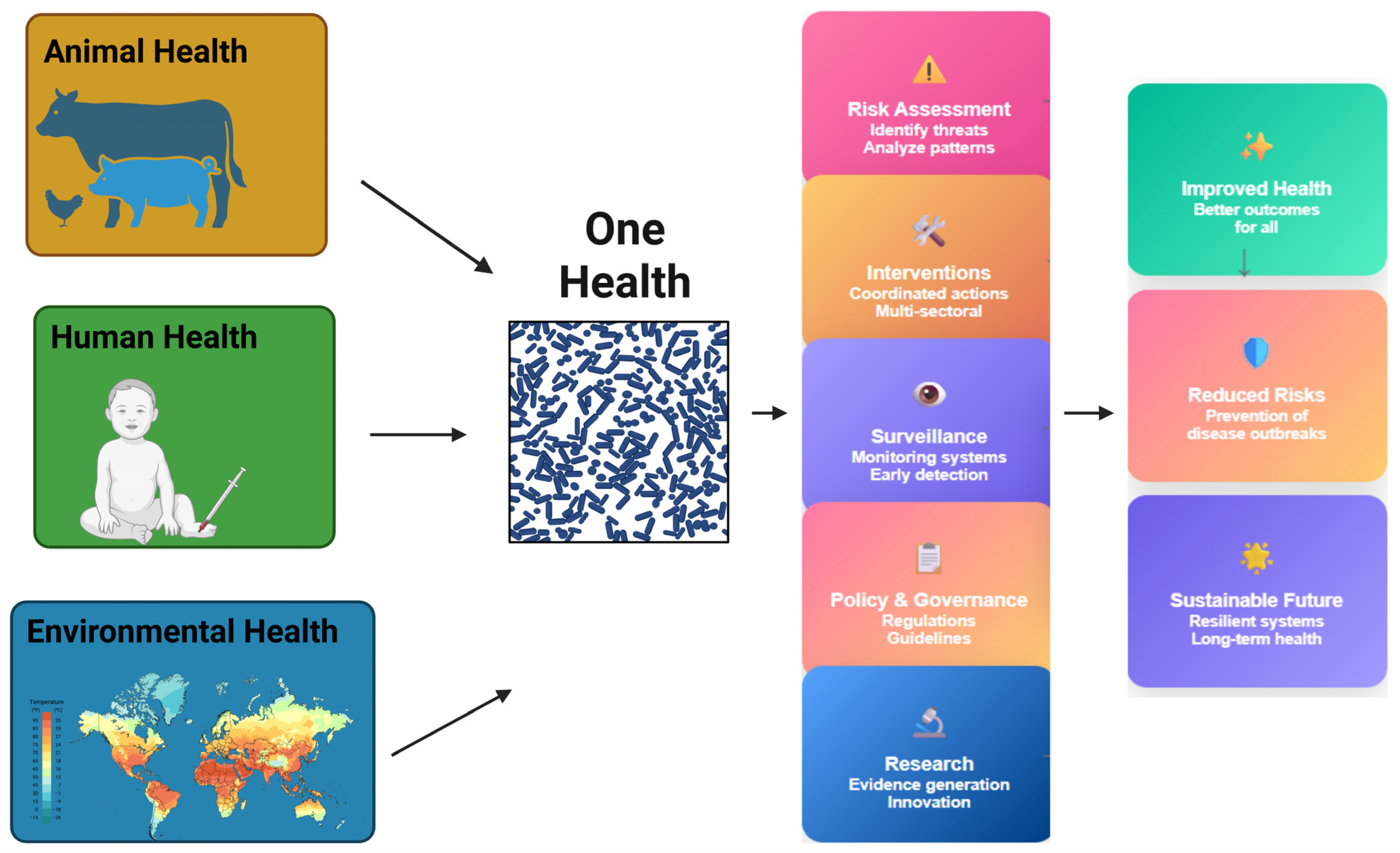
- Tesema, M.Y.; Birhanu, A.G. One health initiative to mitigate the challenge of antimicrobial resistance in the perspectives of developing countries. Bull Natl. Res. Cent. 2024, 48, 19. [Google Scholar] [CrossRef]
- Allué-Guardia, A.; García, J.I.; Torrelles, J.B. Evolution of Drug-Resistant Mycobacterium tuberculosis Strains and Their Adaptation to the Human Lung Environment. Front. Microbiol. 2021, 12, 612675. [Google Scholar] [CrossRef]
- Al-Hadidi, S.H.; Alhussain, H.; Hadi, H.A.; Johar, A.; Yassine, H.M.; Al Thani, A.A.; Eltai, N.O. The Spectrum of Antibiotic Prescribing During COVID-19 Pandemic: A Systematic Literature Review. Microb. Drug Resist. 2021, 27, 1705–1725. [Google Scholar] [CrossRef]
- Franklin, A.M.; Weller, D.L.; Durso, L.M.; Bagley, M.; Davis, B.C.; Frye, J.G.; Grim, C.J.; Ibekwe, A.M.; Jahne, M.A.; Keely, S.P.; et al. A one health approach for monitoring antimicrobial resistance: Developing a national freshwater pilot effort. Front. Water 2024, 6, 1359109. [Google Scholar] [CrossRef] [PubMed]
- Capuozzo, M.; Zovi, A.; Langella, R.; Ottaiano, A.; Cascella, M.; Scognamiglio, M.; Ferrara, F. Optimizing Antibiotic Use: Addressing Resistance Through Effective Strategies and Health Policies. Antibiotics 2024, 13, 1112. [Google Scholar] [CrossRef]
- Kapoor, A.; Mudaliar, S.B.; Bhat, V.G.; Chakraborty, I.; Prasad, A.S.B.; Mazumder, N. Phage therapy: A novel approach against multidrug-resistant pathogens. 3 Biotech 2024, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Buttaro, B.A.; Xue, H.Y.; Tran, N.T.; Wong, H.L. Lipid-polymer hybrid nanoparticles carrying linezolid improve treatment of methicillin-resistant Staphylococcus aureus (MRSA) harbored inside bone cells and biofilms. Eur. J. Pharm. Biopharm. 2020, 151, 189–198. [Google Scholar] [CrossRef]
- Lee, D.; Muir, P.; Lundberg, S.; Lundholm, A.; Sandegren, L.; Koskiniemi, S. A CRISPR-Cas9 system protecting E. coli against acquisition of antibiotic resistance genes. Sci. Rep. 2025, 15, 1545. [Google Scholar] [CrossRef]
- Bai, Y.-B.; Shi, M.-Y.; Wang, W.-W.; Wu, L.-Y.; Bai, Y.-T.; Li, B.; Zhou, X.-Z.; Zhang, J.-Y. Novel quorum sensing inhibitor Echinatin as an antibacterial synergist against Escherichia coli. Front. Microbiol. 2022, 13, 1003692. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.J.; O’BRien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.-Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Bober, J.R.; Beisel, C.L.; Nair, N.U. Synthetic Biology Approaches to Engineer Probiotics and Members of the Human Microbiota for Biomedical Applications. Annu. Rev. Biomed. Eng. 2018, 20, 277–300. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef]
- Shahid, A.; Aslam, B.; Muzammil, S.; Aslam, N.; Shahid, M.; Almatroudi, A.; Allemailem, K.S.; Saqalein, M.; Nisar, M.A.; Rasool, M.H.; et al. The prospects of antimicrobial coated medical implants. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211040304. [Google Scholar] [CrossRef]
- Babutan, I.; Lucaci, A.-D.; Botiz, I. Antimicrobial Polymeric Structures Assembled on Surfaces. Polymers 2021, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Al-Salmi, F.A.; Saleh, M.A.; Sabatier, J.-M.; Alatawi, F.A.; Alenezi, M.A.; Albalwe, F.M.; Albalawi, H.M.R.; Darwish, D.B.E.; Sharaf, E.M. Inhibition Mechanism of Methicillin-Resistant Staphylococcus aureus by Zinc Oxide Nanorods via Suppresses Penicillin-Binding Protein 2a. ACS Omega 2023, 8, 9969–9977. [Google Scholar] [CrossRef]
- Fatahian, R.; Erfani, R. Surrogate modeling of electrospun PVA/PLA nanofibers using artificial neural network for biomedical applications. Sci. Rep. 2025, 15, 12886. [Google Scholar] [CrossRef]
- Shash, Y.H. Cranial reconstruction utilizing polymeric implants in two different designs: Finite element investigation. BMC Musculoskelet. Disord. 2024, 25, 935. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef]
- Hemmatpour, H.; De Luca, O.; Crestani, D.; Stuart, M.C.A.; Lasorsa, A.; van der Wel, P.C.A.; Loos, K.; Giousis, T.; Haddadi-Asl, V.; Rudolf, P. New insights in polydopamine formation via surface adsorption. Nat. Commun. 2023, 14, 664. [Google Scholar] [CrossRef]
- Sadrearhami, Z.; Shafiee, F.N.; Ho, K.K.K.; Kumar, N.; Krasowska, M.; Blencowe, A.; Wong, E.H.H.; Boyer, C. Antibiofilm Nitric Oxide-Releasing Polydopamine Coatings. ACS Appl. Mater. Interfaces 2019, 11, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Hauser, D.; Septiadi, D.; Turner, J.; Petri-Fink, A.; Rothen-Rutishauser, B. From Bioinspired Glue to Medicine: Polydopamine as a Biomedical Material. Materials 2020, 13, 1730. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, G.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.; Wei, Y. Recent developments in polydopamine: An emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef]
- Kwon, I.S.; Bettinger, C.J. Polydopamine Nanostructures as Biomaterials for Medical Applications. J. Mater. Chem. B 2018, 6, 6895–6903. [Google Scholar] [CrossRef]
- Suneetha, M.; Rao, K.M.; Han, S.S. Mussel-Inspired Cell/Tissue-Adhesive, Hemostatic Hydrogels for Tissue Engineering Applications. ACS Omega 2019, 4, 12647–12656. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Soto-Garcia, L.F.; Liao, J.; Zimmern, P.; Nguyen, K.T.; Hong, Y. Mussel-inspired bioadhesives in healthcare: Design parameters, current trends, and future perspectives. Biomater. Sci. 2020, 8, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, J.; Guo, W.; Dong, X.; Cao, K.; Tang, F. Polydopamine Nanocomposite Hydrogel for Drug Slow-Release in Bone Defect Repair: A Review of Research Advances. Gels 2025, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Baral, A.; Basu, K. Supramolecular Adhesives Inspired by Nature: Concept and Applications. Biomimetics 2025, 10, 87. [Google Scholar] [CrossRef]
- Su, Z.; Xue, B.; Xu, C.; Dong, X. Mussel-Inspired Calcium Alginate/Polyacrylamide Dual Network Hydrogel: A Physical Barrier to Prevent Postoperative Re-Adhesion. Polymers 2023, 15, 4498. [Google Scholar] [CrossRef]
- Lo Presti, M.; Rizzo, G.; Farinola, G.M.; Omenetto, F.G. Bioinspired Biomaterial Composite for All-Water-Based High-Performance Adhesives. Adv. Sci. 2021, 8, 2004786. [Google Scholar] [CrossRef]
- Kaushik, N.; Nguyen, L.N.; Kim, J.H.; Choi, E.H.; Kaushik, N.K. Strategies for Using Polydopamine to Induce Biomineralization of Hydroxyapatite on Implant Materials for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 6544. [Google Scholar] [CrossRef]
- Zuppolini, S.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Optimization of Polydopamine Coatings onto Poly–ε–Caprolactone Electrospun Fibers for the Fabrication of Bio-Electroconductive Interfaces. J. Funct. Biomater. 2020, 11, 19. [Google Scholar] [CrossRef]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef]
- Zmerli, I.; Michel, J.P.; Makky, A. Bioinspired polydopamine nanoparticles: Synthesis, nanomechanical properties, and efficient PEGylation strategy. J. Mater. Chem. B 2020, 8, 4489–4504. [Google Scholar] [CrossRef]
- Maurelli, A.M.; De Leo, V.; Catucci, L. Polydopamine-Modified Liposomes: Preparation and Recent Applications in the Biomedical Field. ACS Omega 2024, 9, 24105–24120. [Google Scholar] [CrossRef]
- Quan, W.-Y.; Hu, Z.; Liu, H.-Z.; Ouyang, Q.-Q.; Zhang, D.-Y.; Li, S.-D.; Li, P.-W.; Yang, Z.-M. Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications. Molecules 2019, 24, 2586. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; De Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species--bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Huang, N.; Zhang, S.; Yang, L.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. Multifunctional Electrochemical Platforms Based on the Michael Addition/Schiff Base Reaction of Polydopamine Modified Reduced Graphene Oxide: Construction and Application. ACS Appl. Mater. Interfaces 2015, 7, 17935–17946. [Google Scholar] [CrossRef] [PubMed]
- Gopi, C.V.V.M.; Alzahmi, S.; Narayanaswamy, V.; Raghavendra, K.V.G.; Issa, B.; Obaidat, I.M. A review on electrode materials of supercapacitors used in wearable bioelectronics and implantable biomedical applications. Mater. Horiz. 2025. [Google Scholar] [CrossRef]
- Yi, X.; Duan, Q.Y.; Wu, F.G. Low-Temperature Photothermal Therapy: Strategies and Applications. Research 2021, 2021, 9816594. [Google Scholar] [CrossRef]
- Batul, R.; Bhave, M.; J Mahon, P.; Yu, A. Polydopamine Nanosphere with In-Situ Loaded Gentamicin and Its Antimicrobial Activity. Molecules 2020, 25, 2090. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, G.; Xia, T.; Li, Z.; Zhao, K.; Deng, Z.; Guo, D.; Peng, B. Bioinspired synthesis of polydopamine/Ag nanocomposite particles with antibacterial activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 155–165. [Google Scholar] [CrossRef]
- Pinnataip, R.; Lee, B.P. Oxidation Chemistry of Catechol Utilized in Designing Stimuli-Responsive Adhesives and Antipathogenic Biomaterials. ACS Omega 2021, 6, 5113–5118. [Google Scholar] [CrossRef]
- Mukherjee, I.; Ghosh, A.; Bhadury, P.; De, P. Side-chain amino acid-based cationic antibacterial polymers: Investigating the morphological switching of a polymer-treated bacterial cell. ACS Omega 2017, 2, 1633–1644. [Google Scholar] [CrossRef]
- Parcheta, M.; Sobiesiak, M. Preparation and Functionalization of Polymers with Antibacterial Properties-Review of the Recent Developments. Materials 2023, 16, 4411. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meng, F.; Cao, Z. Improving Surface Antimicrobial Performance by Coating Homogeneous PDA-Ag Micro–Nano Particles. Coatings 2024, 14, 887. [Google Scholar] [CrossRef]
- Lingamgunta, S.; Xiao, Y.; Choi, H.; Christie, G.; Fruk, L. Microwave-enhanced antibacterial activity of polydopamine-silver hybrid nanoparticles. RSC Adv. 2024, 14, 8331–8340. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Syed, A.; Kastrat, E.; Cheng, H.-P. Antibacterial Silver Nanoparticle Containing Polydopamine Hydrogels That Enhance Re-Epithelization. Gels 2024, 10, 363. [Google Scholar] [CrossRef]
- Liu, H.; Qu, X.; Tan, H.; Song, J.; Lei, M.; Kim, E.; Payne, G.F.; Liu, C. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Acta Biomater. 2019, 88, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.; Sarkhel, S.; Mukherjee, N.; Jaiswal, A. Nanomaterial-Based Antimicrobial Coating for Biomedical Implants: New Age Solution for Biofilm-Associated Infections. ACS Omega 2022, 7, 45962–45980. [Google Scholar] [CrossRef]
- Fan, S.; Lin, W.; Huang, Y.; Xia, J.; Xu, J.F.; Zhang, J.; Pi, J. Advances and Potentials of Polydopamine Nanosystem in Photothermal-Based Antibacterial Infection Therapies. Front. Pharmacol. 2022, 13, 829712. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Tian, Y.; Wang, B.; You, X.; Liao, Y. Mitigation of membrane biofouling via immobilizing Ag-MOFs on composite membrane surface for extractive membrane bioreactor. Water Res. 2022, 209, 117940. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, J.; Liu, E.; Gao, J.; Lv, Y.; Li, Z. Injectable hydrogels doped with PDA nanoparticles for photothermal bacterial inhibition and rapid wound healing in vitro. RSC Adv. 2024, 14, 2778–2791. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.S. Friends against the Foe: Synergistic Photothermal and Photodynamic Therapy against Bacterial Infections. Pharmaceutics 2023, 15, 1116. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Amorati, R.; Montalti, M. Recent Applications of Melanin-like Nanoparticles as Antioxidant Agents. Antioxidants 2023, 12, 863. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Rehman, K.; Jabri, T.; Kanwal, T.; Perveen, S.; Rashid, A.; Kazi, M.; Khan, S.A.; Saifullah, S.; Shah, M.R. Improving curcumin bactericidal potential against multi-drug resistant bacteria via its loading in polydopamine coated zinc-based metal-organic frameworks. Drug Deliv. 2023, 30, 2159587. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Hai, L.; Li, Y.; Li, H.; Gong, M.; Wang, Z.; Tang, Z.; Deng, L.; He, D. An Ultrasmall Fe3 O4 -Decorated Polydopamine Hybrid Nanozyme Enables Continuous Conversion of Oxygen into Toxic Hydroxyl Radical via GSH-Depleted Cascade Redox Reactions for Intensive Wound Disinfection. Small 2022, 18, e2105465. [Google Scholar] [CrossRef] [PubMed]
- Nazi, N.; Marguier, A.; Debiemme-Chouvy, C.; Humblot, V. Optimization and Antibacterial Response of N-Halamine Coatings Based on Polydopamine. Colloids Interfaces 2022, 6, 9. [Google Scholar] [CrossRef]
- Chien, H.-W.; Tsai, M.-Y.; Kuo, C.-J.; Lin, C.-L. Well-Dispersed Silver Nanoparticles on Cellulose Filter Paper for Bacterial Removal. Nanomaterials 2021, 11, 595. [Google Scholar] [CrossRef]
- Nazi, N.; Humblot, V.; Debiemme-Chouvy, C. A New Antibacterial N-Halamine Coating Based on Polydopamine. Langmuir ACS J. Surf. Colloids 2020, 36, 11005–11014. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L. Polydopamine Applications in Biomedicine and Environmental Science. Materials 2024, 17, 3916. [Google Scholar] [CrossRef]
- Cong, Y.; Xia, T.; Zou, M.; Li, Z.; Peng, B.; Guo, D.; Deng, Z. Mussel-inspired polydopamine coating as a versatile platform for synthesizing polystyrene/Ag nanocomposite particles with enhanced antibacterial activities. J. Mater. Chem. B 2014, 2, 3450–3461. [Google Scholar] [CrossRef]
- Fadaka, A.O.; Sibuyi, N.R.S.; Madiehe, A.M.; Meyer, M. Nanotechnology-Based Delivery Systems for Antimicrobial Peptides. Pharmaceutics 2021, 13, 1795. [Google Scholar] [CrossRef]
- Trzcińska, Z.; Bruggeman, M.; Ijakipour, H.; Hodges, N.J.; Bowen, J.; Stamboulis, A. Polydopamine Linking Substrate for AMPs: Characterisation and Stability on Ti6Al4V. Materials 2020, 13, 3714. [Google Scholar] [CrossRef]
- Lamba, S.; Wang, K.; Lu, J.; Phillips, A.R.J.; Swift, S.; Sarojini, V. Polydopamine-mediated antimicrobial lipopeptide surface coating for medical devices. ACS Appl. Bio Mater. 2024, 7, 7574–7584. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Chua, R.R.; Bow, H.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Kuppusamy, R.; Chen, R.; Willcox, M.D.P.; Walsh, W.R.; Black, D.S.; Kumar, N. Bioinspired Polydopamine Coatings Facilitate Attachment of Antimicrobial Peptidomimetics with Broad-Spectrum Antibacterial Activity. Int. J. Mol. Sci. 2022, 23, 2952. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Berber, E.; Lutzweiler, G.; Ersen, O.; Bahri, M.; Lavalle, P.; Ball, V.; Vrana, N.E.; Barthes, J. Polyarginine Decorated Polydopamine Nanoparticles With Antimicrobial Properties for Functionalization of Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 982. [Google Scholar] [CrossRef]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics are Potential Anticancer Drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, P.; Cao, Z.; Rahimi, S.; Pandit, S.; Mijakovic, I. Polydopamine/graphene oxide coatings loaded with tetracycline and green Ag nanoparticles for effective prevention of biofilms. Appl. Surf. Sci. 2023, 626, 157221. [Google Scholar] [CrossRef]
- Batul, R.; Bhave, M.; Yu, A. Investigation of Antimicrobial Effects of Polydopamine-Based Composite Coatings. Molecules 2023, 28, 4258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, H.; Xie, P.; Yi, R.; Zhou, J.; Huang, S.; Zhang, L.; Huang, X.; Zhao, J.; Wang, S.; Zhu, C. Polydopamine-polyethylenimine nanoparticles with photothermal-antimicrobial synergy for enhanced wound healing. J. Colloid Interface Sci. 2025, 694, 137713. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Yu, Y.; Zhao, Y.; Liang, F.; Zhang, X. Strong Antibacterial Polydopamine Coatings Prepared by a Shaking-assisted Method. Sci. Rep. 2016, 6, 24420. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, M.; Chen, Y. Minimal Information for Studies of Extracellular Vesicles (MISEV): Ten-Year Evolution (2014–2023). Pharmaceutics 2024, 16, 1394. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, C.; Xiong, X.; Yang, Y.; Zhang, J. Extracellular vesicles: New horizons in neurodegeneration. EBioMedicine 2025, 113, 105605. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, J.; Zhang, D.; Qian, Z.; Zuo, X.; Sun, Y. Small extracellular vesicles: The origins, current status, future prospects, and applications. Stem Cell Res. Ther. 2025, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’DRiscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Cullison, S.R.J.; Flemming, J.P.; Karagoz, K.; Wermuth, P.J.; Mahoney, M.G. Mechanisms of extracellular vesicle uptake and implications for the design of cancer therapeutics. J. Extracell. Biol. 2024, 3, e70017. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.P.; Fan, S.; Mason, J.; Wells, A.; Mendes, C.C.; Wainwright, S.M.; Scott, S.; Fischer, R.; Harris, A.L.; Wilson, C.; et al. Accessory ESCRT-III proteins are conserved and selective regulators of Rab11a-exosome formation. J. Extracell. Vesicles 2023, 12, e12311. [Google Scholar] [CrossRef]
- Horbay, R.; Hamraghani, A.; Ermini, L.; Holcik, S.; Beug, S.T.; Yeganeh, B. Role of Ceramides and Lysosomes in Extracellular Vesicle Biogenesis, Cargo Sorting and Release. Int. J. Mol. Sci. 2022, 23, 15317. [Google Scholar] [CrossRef]
- Lau, N.C.H.; Yam, J.W.P. From Exosome Biogenesis to Absorption: Key Takeaways for Cancer Research. Cancers 2023, 15, 1992. [Google Scholar] [CrossRef]
- Chimal-Vega, B.; Maldonado-Arvizu, J.E.; Avalos, A.D.H.; Díaz-Villanueva, J.F.; Avila-Barrientos, L.P.; González, V.G.G. Inter-Tissue Communication Mechanisms via Exosomes and Their Implications in Metabolic Diseases: Opportunities for Pharmacological Regulation. Future Pharmacol. 2025, 5, 11. [Google Scholar] [CrossRef]
- Li, Z.; Yan, J.; Li, X.; Chen, H.; Lin, C.; Zhang, Y.; Gao, T.; Zhang, Y.; Shu, Y.; Pan, S.; et al. Advancements in extracellular vesicles biomanufacturing: A comprehensive overview of large-scale production and clinical research. Front. Bioeng. Biotechnol. 2025, 13, 1487627. [Google Scholar] [CrossRef]
- Wijaya, W.; Phyu, S.M.; Jiang, S. Extracellular Vesicle (EV) Survivin for Cancer Diagnostics and Therapeutics: A Review. Front. Biosci. 2024, 29, 302. [Google Scholar] [CrossRef] [PubMed]
- Ovčar, A.; Kovačič, B. Biogenesis of Extracellular Vesicles (EVs) and the Potential Use of Embryo-Derived EVs in Medically Assisted Reproduction. Int. J. Mol. Sci. 2024, 26, 42. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.I.; Bernard, M.P.; Vocelle, D.; Zarea, A.A.; Saleh, N.A.; Gagea, M.A.; Schneider, D.; Bauzon, M.; Hermiston, T.; Kanada, M. Phosphatidylserine-Exposing Annexin A1-Positive Extracellular Vesicles: Potential Cancer Biomarkers. Vaccines 2023, 11, 639. [Google Scholar] [CrossRef]
- Tang, J.; Li, D.; Wang, R.; Li, S.; Xing, Y.; Yu, F. Engineered extracellular vesicles: An emerging nanomedicine therapeutic platform. Chem. Commun. 2025, 61, 4123–4146. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Bao, J.; Wang, Y.; Liu, H.; Tang, L. Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions. Front. Immunol. 2018, 9, 90. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, S.; Zhang, K.; Liu, Y.; Li, L.; Qu, S. Enhanced antibacterial activity of bovine milk exosome-based drug formulation against bacterial pathogens. Food Chem. 2024, 447, 139034. [Google Scholar] [CrossRef]
- Leiva-Sabadini, C.; Alvarez, S.; Barrera, N.P.; Schuh, C.M.; Aguayo, S. Antibacterial Effect of Honey-Derived Exosomes Containing Antimicrobial Peptides Against Oral Streptococci. Int. J. Nanomed. 2021, 16, 4891–4900. [Google Scholar] [CrossRef]
- Sapugahawatte, D.N.; Godakumara, K.; Mäesaar, M.; Ekanayake, G.; Midekessa, G.B.; Prasadani, M.; Kodithuwakku, S.; Roasto, M.; Andronowska, A.; Fazeli, A. Harnessing Nature’s Defence: The Antimicrobial Efficacy of Pasteurised Cattle Milk-Derived Extracellular Vesicles on Staphylococcus aureus ATCC 25923. Int. J. Mol. Sci. 2024, 25, 4759. [Google Scholar] [CrossRef] [PubMed]
- Shaban, A.M.; Raslan, M.; Sharawi, Z.W.; Abdelhameed, M.S.; Hammouda, O.; El-Masry, H.M.; Elsayed, K.N.M.; El-Magd, M.A. Antibacterial, Antifungal, and Anticancer Effects of Camel Milk Exosomes: An In Vitro Study. Vet. Sci. 2023, 10, 124. [Google Scholar] [CrossRef]
- Ahn, G.; Shin, W.-R.; Lee, S.; Yoon, H.-W.; Choi, J.-W.; Kim, Y.-H.; Ahn, J.-Y. Bovine Colostrum Exosomes Are a Promising Natural Bacteriostatic Agent against Staphylococcus aureus. ACS Infect. Dis. 2023, 9, 993–1003. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Fakouri, A.; Razavi, Z.-S.; Mohammed, A.T.; Hussein, A.H.A.; Afkhami, H.; Hooshiar, M.H. Applications of mesenchymal stem cell-exosome components in wound infection healing: New insights. Burn. Trauma 2024, 12, tkae021. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Feng, X.-M.; Abbott, J.; Fang, X.-H.; Hao, Q.; Monsel, A.; Qu, J.-M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Hiemstra, T.F.; Charles, P.D.; Gracia, T.; Hester, S.S.; Gatto, L.; Al-Lamki, R.; Floto, R.A.; Su, Y.; Skepper, J.N.; Lilley, K.S.; et al. Human urinary exosomes as innate immune effectors. J. Am. Soc. Nephrol. JASN 2014, 25, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Gong, A.-Y.; Roth, A.L.; Huang, B.Q.; Ward, H.D.; Zhu, G.; LaRusso, N.F.; Hanson, N.D.; Chen, X.-M.; Petri, W.A. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013, 9, e1003261. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, J.; Xue, K.; Chen, J.; Zhang, Y.; Zhang, G.; Wang, K.; Yao, Z.; Hu, Q.; Lin, C.; et al. Highly Bioactive MXene-M2-Exosome Nanocomposites Promote Angiogenic Diabetic Wound Repair through Reconstructing High Glucose-Derived Immune Inhibition. ACS Nano 2024, 18, 4269–4286. [Google Scholar] [CrossRef]
- Hui, W.W.; Emerson, L.E.; Clapp, B.; Sheppe, A.E.; Sharma, J.; del Castillo, J.; Ou, M.; Maegawa, G.H.B.; Hoffman, C.; Larkin, I.J.; et al. Antigen-encapsulating host extracellular vesicles derived from Salmonella-infected cells stimulate pathogen-specific Th1-type responses in vivo. PLoS Pathog. 2021, 17, e1009465. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, Y.; Liu, H.; Wu, Y.; Zhou, X. Extracellular Vesicles: Recent Insights Into the Interaction Between Host and Pathogenic Bacteria. Front. Immunol. 2022, 13, 840550. [Google Scholar] [CrossRef]
- Wang, Y.; Hoffmann, J.P.; Baker, S.M.; zu Bentrup, K.H.; Wimley, W.C.; Fuselier, J.A.; Bitoun, J.P.; Morici, L.A. Inhibition of Streptococcus mutans biofilms with bacterial-derived outer membrane vesicles. BMC Microbiol. 2021, 21, 234. [Google Scholar] [CrossRef]
- Cheng, Y.; Schorey, J.S. Extracellular vesicles deliver Mycobacterium RNA to promote host immunity and bacterial killing. EMBO Rep. 2019, 20, e46613. [Google Scholar] [CrossRef]
- Li, M.; Lee, K.; Hsu, M.; Nau, G.; Mylonakis, E.; Ramratnam, B. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Semeradtova, A.; Liegertova, M.; Herma, R.; Capkova, M.; Brignole, C.; Del Zotto, G. Extracellular vesicles in cancer´s communication: Messages we can read and how to answer. Mol. Cancer 2025, 24, 86. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, P.K.; Kashyap, V.; Kumar, S. Proteomics of Extracellular Vesicles: Recent Updates, Challenges and Limitations. Proteomes 2025, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef]
- Dou, W.; Zeng, X.; Zhu, S.; Zhu, Y.; Liu, H.; Li, S. Mussel-Inspired Injectable Adhesive Hydrogels for Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 9100. [Google Scholar] [CrossRef]
- Lupu, A.; Gradinaru, L.M.; Gradinaru, V.R.; Bercea, M. Diversity of Bioinspired Hydrogels: From Structure to Applications. Gels 2023, 9, 376. [Google Scholar] [CrossRef]
- Forooshani, P.K.; Polega, E.; Thomson, K.; Bhuiyan, S.A.; Pinnaratip, R.; Trought, M.; Kendrick, C.; Gao, Y.; Perrine, K.A.; Pan, L.; et al. Antibacterial Properties of Mussel-Inspired Polydopamine Coatings Prepared by a Simple Two-Step Shaking-Assisted Method. Front. Chem. 2019, 7, 631. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef]
- Liscano, Y.; Salamanca, C.H.; Vargas, L.; Cantor, S.; Laverde-Rojas, V.; Oñate-Garzón, J. Increases in Hydrophilicity and Charge on the Polar Face of Alyteserin 1c Helix Change its Selectivity towards Gram-Positive Bacteria. Antibiotics 2019, 8, 238. [Google Scholar] [CrossRef]
- Yang, X.; Shi, G.; Guo, J.; Wang, C.; He, Y. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2018, 13, 8095–8104. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; D’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Brunet, M.Y.; Jones, M.-C.; Cox, S.C. Engineered Extracellular Vesicles: Tailored-Made Nanomaterials for Medical Applications. Nanomaterials 2020, 10, 1838. [Google Scholar] [CrossRef]
- Ghadami, S.; Dellinger, K. The lipid composition of extracellular vesicles: Applications in diagnostics and therapeutic delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef] [PubMed]
- Benyamini, P. Beyond Antibiotics: What the Future Holds. Antibiotics 2024, 13, 919. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Huemer, M.; Shambat, S.M.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Chen, J.; Hu, S.; Liu, J.; Jiang, H.; Wang, S.; Yang, Z. Exosomes: A double-edged sword in cancer immunotherapy. MedComm 2025, 6, e70095. [Google Scholar] [CrossRef]
- Abbasi, H.; Kouchak, M.; Mirveis, Z.; Hajipour, F.; Khodarahmi, M.; Rahbar, N.; Handali, S. What We Need to Know about Liposomes as Drug Nanocarriers: An Updated Review. Adv. Pharm. Bull. 2023, 13, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, L.; Liu, T.; Zhao, H.; Liu, Y.; Shu, F.; Huang, H.; Liu, W.; Zhang, W.; Jiang, L.; et al. Mannose-modified exosomes loaded with MiR-23b-3p target alveolar macrophages to alleviate acute lung injury in Sepsis. J. Control. Release 2025, 379, 832–847. [Google Scholar] [CrossRef]
- Elliott, R.O.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef]
- Alikhani, M.S.; Nazari, M.; Hatamkhani, S. Enhancing antibiotic therapy through comprehensive pharmacokinetic/pharmacodynamic principles. Front. Cell. Infect. Microbiol. 2025, 15, 1521091. [Google Scholar] [CrossRef]
- Lopes, D.; Lopes, J.; Pereira-Silva, M.; Peixoto, D.; Rabiee, N.; Veiga, F.; Moradi, O.; Guo, Z.-H.; Wang, X.-D.; Conde, J.; et al. Bioengineered exosomal-membrane-camouflaged abiotic nanocarriers: Neurodegenerative diseases, tissue engineering and regenerative medicine. Military Med. Res. 2023, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Han, Z.; Song, D.; Peng, Y.; Xiong, M.; Chen, Z.; Duan, S.; Zhang, L. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int. J. Nanomed. 2023, 18, 7923–7940. [Google Scholar] [CrossRef] [PubMed]
- Mosselhy, D.A.; Assad, M.; Sironen, T.; Elbahri, M. Nanotheranostics: A Possible Solution for Drug-Resistant Staphylococcus aureus and their Biofilms? Nanomaterials 2021, 11, 82. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, H.; Wang, X.; Dong, S.; Guo, L.; Zhang, S.; Yang, X.; Liu, C.; Jiang, X.; Kan, M.; et al. Advances in preparation and application of antibacterial hydrogels. J. Nanobiotechnology 2023, 21, 300. [Google Scholar] [CrossRef]
- Wu, X.; Jin, S.; Ding, C.; Wang, Y.; He, D.; Liu, Y. Mesenchymal Stem Cell-Derived Exosome Therapy of Microbial Diseases: From Bench to Bed. Front. Microbiol. 2022, 12, 804813. [Google Scholar] [CrossRef]
- Olteanu, G.; Neacșu, S.M.; Joița, F.A.; Musuc, A.M.; Lupu, E.C.; Ioniță-Mîndrican, C.-B.; Lupuliasa, D.; Mititelu, M. Advancements in Regenerative Hydrogels in Skin Wound Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 3849. [Google Scholar] [CrossRef]
- Mirzaei, R.; Babakhani, S.; Ajorloo, P.; Ahmadi, R.H.; Hosseini-Fard, S.R.; Keyvani, H.; Ahmadyousefi, Y.; Teimoori, A.; Zamani, F.; Karampoor, S.; et al. The emerging role of exosomal miRNAs as a diagnostic and therapeutic biomarker in Mycobacterium tuberculosis infection. Mol. Med. 2021, 27, 34. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, Y.; Hu, J.; Peng, X.; Liu, Z. CRISPR/Cas9 systems: Delivery technologies and biomedical applications. Asian J. Pharm. Sci. 2023, 18, 100854. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Madhyastha, H.; Madhyastha, R.; Rajendran, R.L.; Nakajima, Y.; Watanabe, N.; Velikkakath, A.K.G.; Hong, C.M.; Gopi, R.V.; Muthukalianan, G.K.; et al. The emerging role of exosomes in innate immunity, diagnosis and therapy. Front. Immunol. 2023, 13, 1085057. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ding, X.; Zhang, H.; Liu, Z.; Han, Y.; Wei, Q.; Okoro, O.V.; Shavandi, A.; Nie, L. Recent Advances of Chitosan-Based Hydrogels for Skin-Wound Dressings. Gels 2024, 10, 175. [Google Scholar] [CrossRef]
- Pachla, J.; Kopiasz, R.J.; Marek, G.; Tomaszewski, W.; Głogowska, A.; Drężek, K.; Kowalczyk, S.; Podgórski, R.; Butruk-Raszeja, B.; Ciach, T.; et al. Polytrimethylenimines: Highly Potent Antibacterial Agents with Activity and Toxicity Modulated by the Polymer Molecular Weight. Biomacromolecules 2023, 24, 2237–2249. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir ACS J. Surf. Colloids 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Ghazzy, A.; Naik, R.R.; Shakya, A.K. Metal-Polymer Nanocomposites: A Promising Approach to Antibacterial Materials. Polymers 2023, 15, 2167. [Google Scholar] [CrossRef]
- Olmos, D.; González-Benito, J. Polymeric Materials with Antibacterial Activity: A Review. Polymers 2021, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hong, X.; Zhao, M.; Liu, N.; Liu, H.; Zhao, J.; Shao, L.; Xue, W.; Zhang, H.; Zhu, P.; et al. Nanocomposite hydrogels for biomedical applications. Bioeng. Transl. Med. 2022, 7, e10315. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Villarreal-Leal, R.A.; Cooke, J.P.; Corradetti, B. Biomimetic and immunomodulatory therapeutics as an alternative to natural exosomes for vascular and cardiac applications. Nanomed. Nanotechnol. Biol. Med. 2021, 35, 102385. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s frontier in combatting infectious and inflammatory diseases: Prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [CrossRef]
- Smola-Dmochowska, A.; Lewicka, K.; Macyk, A.; Rychter, P.; Pamuła, E.; Dobrzyński, P. Biodegradable Polymers and Polymer Composites with Antibacterial Properties. Int. J. Mol. Sci. 2023, 24, 7473. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, A.; Nandy, A.; Ghatak, S. Insight into the Functional Dynamics and Challenges of Exosomes in Pharmaceutical Innovation and Precision Medicine. Pharmaceutics 2024, 16, 709. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Gao, H.; Zhang, X.; Sun, L.; Huang, Y.; Zhang, J.; Ding, B. Cell Membrane-Coated Biomimetic Nanoparticles in Cancer Treatment. Pharmaceutics 2024, 16, 531. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Polymeric Micellar Systems-A Special Emphasis on "Smart" Drug Delivery. Pharmaceutics 2023, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Dilsiz, N. A comprehensive review on recent advances in exosome isolation and characterization: Toward clinical applications. Transl. Oncol. 2024, 50, 102121. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Li, H.; Xu, G.; Hu, Y.; Zhang, W.; Tian, K. Current Knowledge and Future Perspectives of Exosomes as Nanocarriers in Diagnosis and Treatment of Diseases. Int. J. Nanomed. 2023, 18, 4751–4778. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, A.; Hu, J.; Feng, L.; Liu, L.; Shen, Z. Recent developments in isolating methods for exosomes. Front. Bioeng. Biotechnol. 2023, 10, 1100892. [Google Scholar] [CrossRef]
- Schwarz, G.; Ren, X.; Xie, W.; Guo, H.; Jiang, Y.; Zhang, J. Engineered exosomes: A promising drug delivery platform with therapeutic potential. Front. Mol. Biosci. 2025, 12, 1583992. [Google Scholar] [CrossRef]
- Kawai-Harada, Y.; Nimmagadda, V.; Harada, M. Scalable isolation of surface-engineered extracellular vesicles and separation of free proteins via tangential flow filtration and size exclusion chromatography (TFF-SEC). BMC Methods 2024, 1, 9. [Google Scholar] [CrossRef]
- Neupane, Y.R.; Handral, H.K.; Alkaff, S.A.; Chng, W.H.; Venkatesan, G.; Huang, C.; Lee, C.K.; Wang, J.-W.; Sriram, G.; Dienzo, R.A.; et al. Cell-derived nanovesicles from mesenchymal stem cells as extracellular vesicle-mimetics in wound healing. Acta Pharm. Sinica B 2023, 13, 1887–1902. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, J.; Gui, X.; Zheng, Y.; Schaar, E.; Liu, G.; Shi, J. Bioengineered nanotechnology for nucleic acid delivery. J. Control. Release 2023, 364, 124–141. [Google Scholar] [CrossRef]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Arif, T.; Mahmood, R.; Harris, D.T. Stem Cell-Based Acellular Therapy: Insight into Biogenesis, Bioengineering and Therapeutic Applications of Exosomes. Biomolecules 2024, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.M.; Mahapatra, D.K.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Tsai, T.H.; Lee, C.H. Regulation of exosomes as biologic medicines: Regulatory challenges faced in exosome development and manufacturing processes. Clin. Transl. Sci. 2024, 17, e13904. [Google Scholar] [CrossRef] [PubMed]
- Gabaran, S.G.; Ghasemzadeh, N.; Rahnama, M.; Karatas, E.; Akbari, A.; Rezaie, J. Functionalized exosomes for targeted therapy in cancer and regenerative medicine: Genetic, chemical, and physical modifications. Cell Commun. Signal. CCS 2025, 23, 265. [Google Scholar] [CrossRef]
- Kropp, M.; Harmening, N.; Bascuas, T.; Johnen, S.; De Clerck, E.; Fernández, V.; Ronchetti, M.; Cadossi, R.; Zanini, C.; Scherman, D.; et al. GMP-Grade Manufacturing and Quality Control of a Non-Virally Engineered Advanced Therapy Medicinal Product for Personalized Treatment of Age-Related Macular Degeneration. Biomedicines 2022, 10, 2777. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Lu, Y.; Luo, X.; Huang, Y.; Xie, T.; Pilarsky, C.; Dang, Y.; Zhang, J. Microfluidic Technology for the Isolation and Analysis of Exosomes. Micromachines 2022, 13, 1571. [Google Scholar] [CrossRef]
| Mechanism | Description | Examples | Clinical Relevance |
|---|---|---|---|
| Efflux Pumps | Active transport proteins that expel antibiotics out of bacterial cells. | AcrAB-TolC (E. coli), MexAB-OprM (P. aeruginosa) | Multidrug resistance; overexpression in UPEC and P. aeruginosa leads to resistance to β-lactams, fluoroquinolones, etc. |
| Enzymatic Inactivation | Production of enzymes that degrade or modify antibiotics, rendering them inactive. | β-lactamases (classes A–D), aminoglycoside-modifying enzymes [AAC(6′)-Ib], macrolide esterases, TetX, ADP-ribosyltransferases | Key mechanism in resistance to β-lactams, aminoglycosides, macrolides, rifamycins, etc. |
| Target Site Alteration | Modification of antibiotic targets to reduce or abolish drug binding. | rpoB (rifamycin resistance), gyrA/parC (fluoroquinolones), mecA (MRSA), PBP modifications (S. pneumoniae), 23S/16S rRNA methylation | Resistance in M. tuberculosis, S. aureus, S. pneumoniae; major contributor to therapeutic failure. |
| Reduced Membrane Permeability | Altered outer membrane composition or porin expression, limiting drug entry. | OprD (P. aeruginosa), OmpK36 (K. pneumoniae), OmpF (E. coli) | Reduces susceptibility to β-lactams, quinolones, and other hydrophilic antibiotics in Gram-negative bacteria. |
| Biofilm Formation | Structured communities embedded in an extracellular matrix that restricts antibiotic penetration and enhances tolerance. | S. epidermidis, K. pneumoniae, P. aeruginosa (CF patients), S. aureus, CoNS | Biofilms increase resistance 10–1000×; associated with chronic infections and medical device colonization. |
| Immune Evasion | Strategies to avoid host immune responses and prolong infection, allowing more time for resistance to develop. | Induction of IL-10, TGF-β; intracellular survival; LPS/capsule modification; biofilm-mediated immune evasion | Enables chronic infection and persistence; enhances time for resistance trait selection and maintenance. |
| Strategy | Mechanism/Description | Examples | Potential Benefits |
|---|---|---|---|
| Exosome-Based Drug Delivery | Natural vesicles deliver antimicrobial agents directly to target cells. | MSC-derived exosomes carrying antibiotics or siRNA. | Targeted delivery, reduced toxicity, immune evasion. |
| CRISPR-Cas Antimicrobials | Gene editing tools used to disrupt resistance genes in bacteria. | CRISPR-Cas9 targeting blaNDM, mecA genes. | Specific targeting of resistance genes; prevents horizontal gene transfer. |
| Quorum Sensing Inhibitors (QSIs) | Block bacterial communication to prevent biofilm formation and virulence. | Furanones, AHL analogs. | Disarm pathogens without killing, reducing selective pressure. |
| Phage Therapy | Use of bacteriophages to infect and lyse resistant bacteria. | Listeria-specific phages in food safety; Pseudomonas phages in lung infections. | Host-specific, can co-evolve with bacteria, minimal dysbiosis. |
| Nanoparticles | Nanomaterials with inherent antimicrobial properties or used as drug carriers. | Silver nanoparticles, liposomes, polymeric NPs. | Enhanced penetration, controlled release, membrane disruption. |
| Synthetic Polymers | Engineered molecules for targeted delivery or direct antimicrobial action. | Cationic polymers, dendrimers, polymer-drug conjugates. | Broad-spectrum activity, biofilm penetration, reduced resistance development. |
| Engineered Probiotics | Genetically modified microbes that detect and kill resistant pathogens. | Lactobacillus strains producing bacteriocins or CRISPR systems. | Gut microbiome protection, pathogen-specific killing. |
| Microbiota-Based Interventions | Use of beneficial microbes to outcompete or modulate pathogens. | Fecal microbiota transplantation (FMT), synbiotics. | Restore healthy microbiota, indirect suppression of resistance. |
| Combination Therapy | Use of multiple agents to target different resistance mechanisms. | Colistin + rifampin for MDR Acinetobacter; β-lactam + β-lactamase inhibitor. | Synergistic effects, delayed resistance emergence. |
| Category | Specific Property | Functional Implications/Applications |
|---|---|---|
| Adhesion | Strong, universal adhesion to diverse substrates | Surface coating, implant modification, wet-interface applications |
| Adhesion in aqueous environments | Biomedical use (e.g., tissue contact, wound dressings) | |
| Chemical Reactivity | Catechol, amine, imine groups | Covalent bonding, surface engineering, biomolecule immobilization |
| Quinone formation via catechol oxidation | Metal chelation, redox activity, cross-linking, catalytic functionality | |
| Michael addition/Schiff base reactivity | Conjugation with thiols, amines, hydroxyls | |
| Biocompatibility | Non-toxic, supports cell adhesion and proliferation | Tissue engineering, drug delivery, biosensors |
| Morphology & Structure | Tunable to nanoparticles, films, capsules, core–shell | Nanoplatforms for imaging, targeting, catalysis |
| Solubility | Water-insoluble (bulk), organic-solvent soluble (nano) | Organic-phase processing; solubility enhanced by PEGylation |
| Enhanced colloidal stability via surface modification | Long-term physiological stability | |
| Bioactivity | ROS generation (e.g., H2O2, superoxide) | Antibacterial, anticancer therapy (requires control for safety) |
| Membrane disruption | Antimicrobial coatings | |
| Photothermal Properties | NIR light-to-heat conversion | Photothermal therapy (PTT) for cancer and infections |
| Electrochemical Behavior | Redox-active; electron donor under stimuli | Biosensors, energy devices, electrocatalysis |
| Catalysis | Intrinsic and synergistic with nanoparticles (e.g., Ag) | Environmental cleanup, antibacterial agents, smart nanomaterials |
| Strategy/Mechanism | Description | Examples | Antimicrobial Benefits |
|---|---|---|---|
| ROS Generation | Catechol oxidation generates H2O2 and superoxide radicals | PDA thin films, PDA-metal hybrids (e.g., PDA-Ag) | Oxidative damage to membranes, proteins, and DNA |
| Electrostatic Membrane Disruption | Protonated amine groups interact with bacterial membranes | PDA nanoparticles with polyarginine | Membrane lysis and bacterial death |
| Surface Roughness Enhancement | rPDA coatings improve contact and mechanical disruption | Rough PDA coatings on surfaces | Higher bacterial adhesion and enhanced killing compared to smooth PDA |
| Metal Chelation and Hybridization | Catechol groups chelate metals for synergistic killing | PDA-Ag NPs, PDA-Au NPs, PDA–metal–organic frameworks | Sustained release of ions, increased ROS, prolonged antimicrobial action |
| Photothermal Therapy (PTT) | PDA absorbs NIR light and converts it into localized heat | PDA-ZIF-8 nanostructures, PDA-based microneedles, PDA-Polymyxin B | Thermal denaturation of bacterial proteins and biofilm disruption |
| Photodynamic Therapy (PDT) | Light-activated ROS generation using PDA’s redox properties | PDA-MOFs, PDA-curcumin, PDA/Fe3O4 nanozymes | Light-induced oxidative stress leads to bacterial death |
| N-Halamine Functionalization | Chlorinated amine groups release halide species | PDA/PEI chlorinated films, halamine-modified PDA coatings | Oxidative damage to bacterial enzymes and proteins, durable and repeatable killing |
| Antimicrobial Peptide (AMP) Immobilization | Covalent or non-covalent attachment of AMPs | PDA-KR-12, PDA-CWR11, PDA-lipopeptide (SL1.15), PDA-Mel4 | Localized, sustained antimicrobial action, minimal toxicity, biofilm inhibition |
| Peptidomimetic Conjugation | PDA facilitates binding of synthetic antimicrobial peptides | PDA-coated surfaces with RK758, Melimine, etc. | Broad-spectrum antimicrobial action, biofilm prevention |
| Antibiotic Loading and Controlled Release | PDA enables sustained antibiotic delivery | PDA-GO-tetracycline–Ag composites, PDA-Ag@Gen vs. Ag/Gen@PDA | Prolonged antimicrobial action, reduced burst release, biofilm suppression |
| Enzyme Immobilization | PDA coatings stabilize antimicrobial enzymes | PDA-lysostaphin functionalized surfaces | Enzyme-mediated bacterial lysis, preserved enzymatic activity |
| Synergistic Hybrid Nanoplatforms | Combined strategies for enhanced effects | PDA-TiO2 (photocatalytic), PDA-AuNPs (delivery + dispersion), PDA-PEI–Polymyxin B + PTT | Multifunctional action: targeting, penetration, ROS, PTT, and sustained delivery |
| Feature | Small EVs (Exosomes) | Large EVs (Microvesicles) |
|---|---|---|
| Size Range | ~30–150 nm | ~100 nm to >1 µm |
| Biogenesis Origin | Endosomal pathway (MVBs/ILVs) | Plasma membrane budding and fission |
| Key Formation Process | Endocytosis → Early/late endosomes → ILVs → MVBs → Exocytosis | Direct outward budding from plasma membrane |
| Mechanisms Involved | ESCRT-dependent and ESCRT-independent (e.g., ceramide, tetraspanins) | Cytoskeletal rearrangement, calcium signaling, ARF6, RhoA, calpain |
| Surface Markers | CD9, CD63, CD81, ALIX, TSG101 | Phosphatidylserine (detected by annexin A5/A1), integrins, CD40, ARF6 |
| Cargo Composition | Proteins, lipids, miRNAs, mRNAs, lncRNAs, metabolites | Cytosolic proteins, membrane proteins, organelle fragments |
| Release Mechanism | Fusion of MVB with plasma membrane (Rab GTPases, SNAREs) | Budding and shedding from cell surface |
| Interaction with Target Cells | Endocytosis, membrane fusion, or ligand–receptor interaction | Same as exosomes, depending on content and surface molecules |
| Terminology (MISEV2023) | Preferred functional classification; biogenesis-based terms used if justified | Same; shift toward functional/operational definitions |
| Function | Intercellular communication, immune modulation, angiogenesis, metastasis | Cell signaling, coagulation, inflammation, immune response |
| EV Source | Antibacterial Target | Mechanism of Action | Applications |
|---|---|---|---|
| Bovine milk-derived exosomes | MDR bacteria | Deliver isobavachalcone and polymyxin B; 99% bacterial elimination | Wound healing, food preservation |
| Honey-derived EVs (HEc-EVs) | Streptococcus mutans | Contain MRJP1, defensin-1, jellein-3; disrupt biofilm | Oral health, natural antimicrobial agent |
| Pasteurized cow’s milk exosomes | Staphylococcus aureus | Dose-dependent growth inhibition; delayed lag/generation time | Food safety |
| Camel milk-derived exosomes (CM-EXOs) | Escherichia coli | Bacteriostatic; Gram-negative specificity | Animal health |
| Bovine colostrum-derived exosomes | Staphylococcus aureus | Disrupt oxidative phosphorylation; reduce ATP | Infection control |
| MSC-derived exosomes | Gram+ and Gram− bacteria | AMPs (e.g., beta-defensin-2), activate TLR-4, enhance macrophage/neutrophil activity | Tissue repair, infection therapy |
| BMSC-derived EVs | E. coli-induced ALI | Deliver KGF mRNA for tissue regeneration | Acute lung injury therapy |
| Urinary exosomes | E. coli (pathogenic and commensal) | Lysozyme C and myeloperoxidase; pH-dependent lysis | Urogenital infection defense |
| Biliary/Intestinal epithelial EVs | Broad spectrum | Contain LL-37, hBD-2; TLR-4 activation | Gastrointestinal immunity |
| MXene-M2-Exo (FM-Exo) | Broad spectrum (diabetic wound) | Sustained exosome release; antibacterial and immunosuppressive | Diabetic wound healing |
| Infection-derived host EVs | Salmonella, Gram-negative bacteria | Expand CD4+ T cells; induce Th1-biased response | Vaccine development |
| Host EVs (with ADAM10) | Staphylococcus aureus alpha-toxin | Bind/neutralize bacterial toxins | Antitoxin strategy |
| OMVs (Burkholderia thailandensis) | S. mutans | Antibiofilm; synergize with gentamicin | Biofilm-targeted therapy |
| EVs from M. tuberculosis-infected macrophages | M. tuberculosis | Deliver bacterial RNA via SecA2; trigger RIG-I/MAVS and LC3-associated phagosome maturation | TB adjunct therapy |
| Lactobacillus-derived EVs | VRE faecium | Host gene modulation; protection against infection | Probiotic therapy, resistance control |
| EV Source | Stability and Processing | Targeting and Therapeutic Potential | Immune Interaction and Safety | Key Applications |
|---|---|---|---|---|
| Bovine Milk-Derived Exosomes | Stable; affected by industrial processing | Drug delivery, tumor targeting | Cross-species tolerance; safe oral use | Cancer therapy, oral drug delivery |
| Honey-Derived EVs (HEc-EVs) | Stable; <150 nm size | Antibacterial, antibiofilm | Antimicrobial peptides; biofilm modulation | Dental caries prevention |
| Pasteurized Cow’s Milk Exosomes | Partial preservation of bioactive cargo | Similar to milk exosomes | Some immune proteins preserved | Nutritional and therapeutic supplements |
| Camel Milk-Derived Exosomes | Stable; unique cargo | Anticancer, antioxidant, anti-inflammatory | Immunomodulatory via lactoferrin and casein | Cancer, inflammation, oxidative stress |
| Bovine Colostrum-Derived Exosomes | Stable; rich in growth factors | Hair regeneration, tissue repair | Safe with minimal adverse effects | Hair loss, wound healing |
| MSC-Derived Exosomes | Stable; modifiable | Immunomodulation, anti-inflammatory, tissue repair | Low immunogenicity; promote immune tolerance | Autoimmune diseases, inflammation, cancer |
| BMSC-Derived EVs | Stable; miRNA-rich | Anti-fibrotic, anti-inflammatory | Modulate inflammatory cytokines | Fibrotic skin diseases |
| Urinary Exosomes | Stable with protease inhibitors | Diagnostic biomarkers | Low immune activation | Kidney/urinary diseases biomarkers |
| Biliary/Intestinal Epithelial EVs | Limited data | Gut immunity and homeostasis | Likely immune-modulatory | Gut health |
| MXene-M2-Exo (FM-Exo) | Emerging technology | Enhanced delivery and imaging | Under investigation | Nanomedicine |
| Infection-Derived Host EVs | Variable | Modulate infection and immunity | Influence pathogen-host interactions | Infectious disease research |
| OMVs (Burkholderia thailandensis) | Bacterial vesicles | Immune modulation, vaccine potential | Can trigger immune responses | Vaccine development, pathogenesis |
| EVs from M. tuberculosis-Infected Macrophages | Host-pathogen interaction vesicles | Biomarkers, immune modulation | Influence tuberculosis immunity | TB diagnosis and therapy |
| Lactobacillus-Derived EVs | Stable; probiotic origin | Immune modulation, gut homeostasis | Promote mucosal immunity | IBD, gut health |
| Aspect | Conventional Antibiotics | Standard Exosome Therapy | Exosome-Based Nanotherapies |
|---|---|---|---|
| Intracellular Delivery | Limited penetration into intracellular pathogens | Natural biodistribution, moderate targeting | Enhanced intracellular delivery via engineering |
| Efficacy | Variable, often limited by resistance and bioavailability | Effective in immune modulation and cargo delivery | Superior efficacy in intracellular infections and targeted therapy |
| Immunogenicity | Potentially high with systemic toxicity | Low immunogenicity | Very low immunogenicity, reduced toxicity |
| Cargo Versatility | Mostly small molecules | Proteins, nucleic acids, small molecules | Broad cargo loading (hydrophilic/lipophilic drugs, proteins, RNA) |
| Stability | Variable, often requires formulation | Stable, biocompatible | High stability, modifiable for enhanced targeting |
| Polymer/Hybrid | Antimicrobial Mechanism | ROS Generation | Coating Stability | Anti-Biofilm Effect | Functionalization Capacity |
|---|---|---|---|---|---|
| PDA (Polydopamine) | Sustained ROS (H2O2), surface charge repulsion | High (continuous) | Strong (catechol adhesion) | Strong | High (binds AMPs, metals, drugs) |
| PEG (Polyethylene Glycol) | Stealth coating; not inherently antimicrobial | None | Moderate (steric repulsion) | Weak | Moderate (limited active binding) |
| Quaternized Chitosan | Electrostatic bacterial membrane disruption | Low | Moderate (pH-sensitive) | Moderate | High (polyamine-rich structure) |
| PEI (Polyethyleneimine) | Strong membrane interaction; electrostatic killing | Moderate | Strong (polymeric film) | Moderate | High (modular chemical backbone) |
| Zwitterionic Polymers | Antifouling via hydration layer; passive microbial inhibition | None | Strong (hydrated layer) | Strong | Low–moderate |
| Metal NP Composites | Ion release, ROS, membrane damage | High | Variable (matrix-dependent) | Strong | Moderate–high |
| 2D Material Hybrids (e.g., MoS2) | Photothermal killing, ROS, high surface area | High | High | Strong | High (multifunctional surface) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muttiah, B.; Hanafiah, A. Bioinspired Nanoplatforms: Polydopamine and Exosomes for Targeted Antimicrobial Therapy. Polymers 2025, 17, 1670. https://doi.org/10.3390/polym17121670
Muttiah B, Hanafiah A. Bioinspired Nanoplatforms: Polydopamine and Exosomes for Targeted Antimicrobial Therapy. Polymers. 2025; 17(12):1670. https://doi.org/10.3390/polym17121670
Chicago/Turabian StyleMuttiah, Barathan, and Alfizah Hanafiah. 2025. "Bioinspired Nanoplatforms: Polydopamine and Exosomes for Targeted Antimicrobial Therapy" Polymers 17, no. 12: 1670. https://doi.org/10.3390/polym17121670
APA StyleMuttiah, B., & Hanafiah, A. (2025). Bioinspired Nanoplatforms: Polydopamine and Exosomes for Targeted Antimicrobial Therapy. Polymers, 17(12), 1670. https://doi.org/10.3390/polym17121670







