Synthesis of Polyfluorinated Aromatic Selenide-Modified Polysiloxanes: Enhanced Thermal Stability, Hydrophobicity, and Noncovalent Modification Potential
Abstract
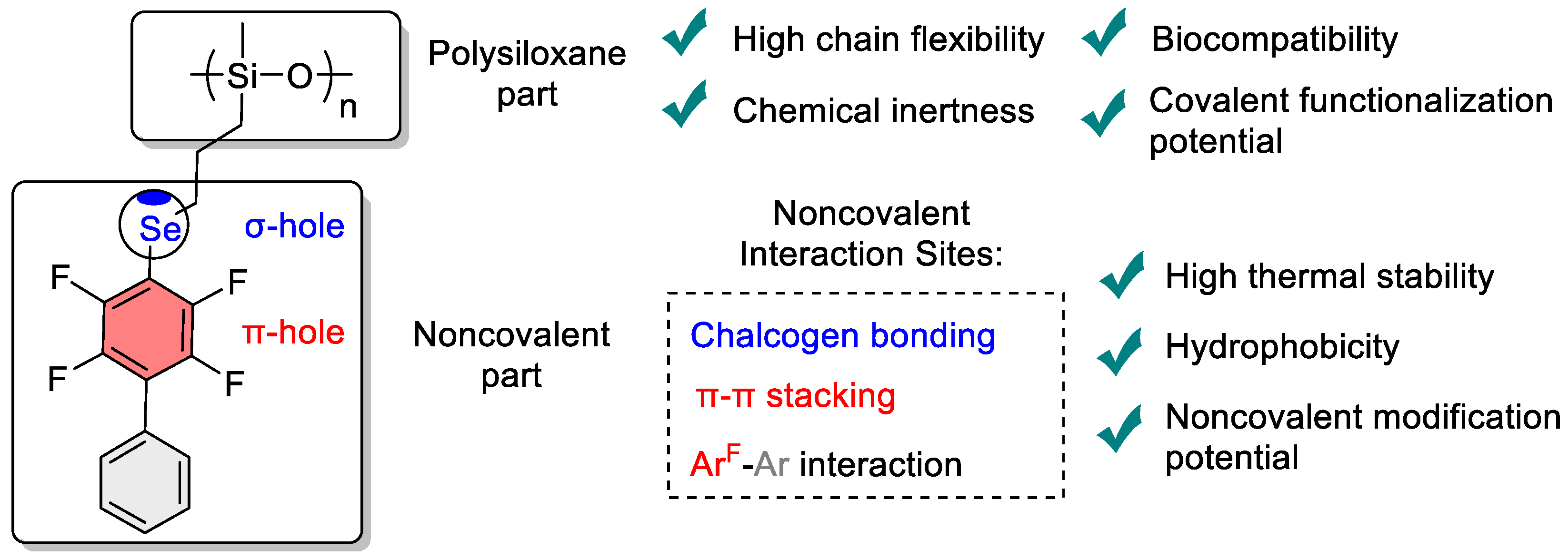
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
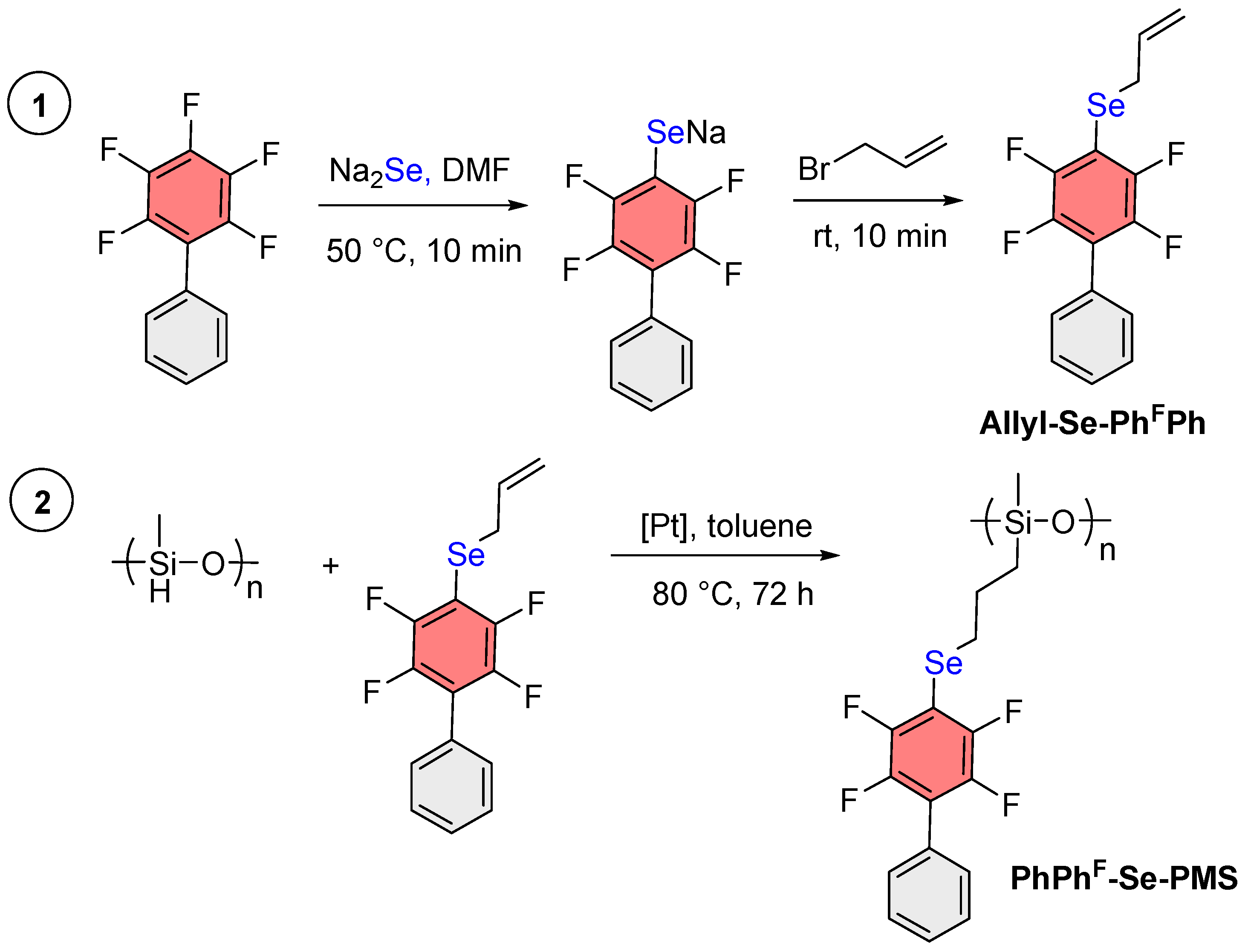
2.3. Synthesis of Allyl Selenide of PhFPh (Allyl-Se-PhFPh)
2.4. Synthesis of Allyl Sulfide of PhFPh (Allyl-S-PhFPh)
2.5. General Procedure of Hydrosilylation
2.6. General Procedure for Modification of Cl-PMS-1
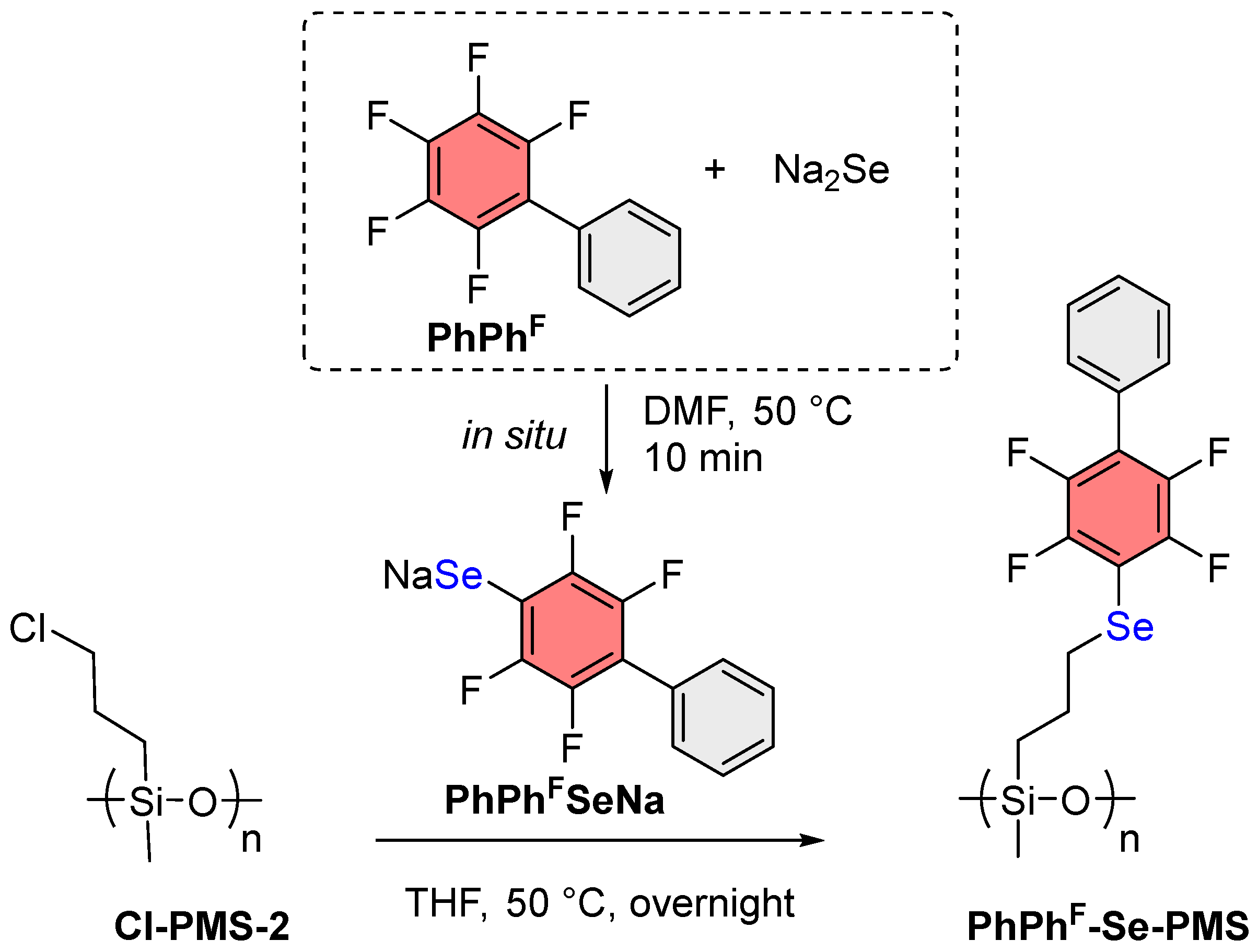
2.7. General Procedure of Cl-PMS-2 Modification
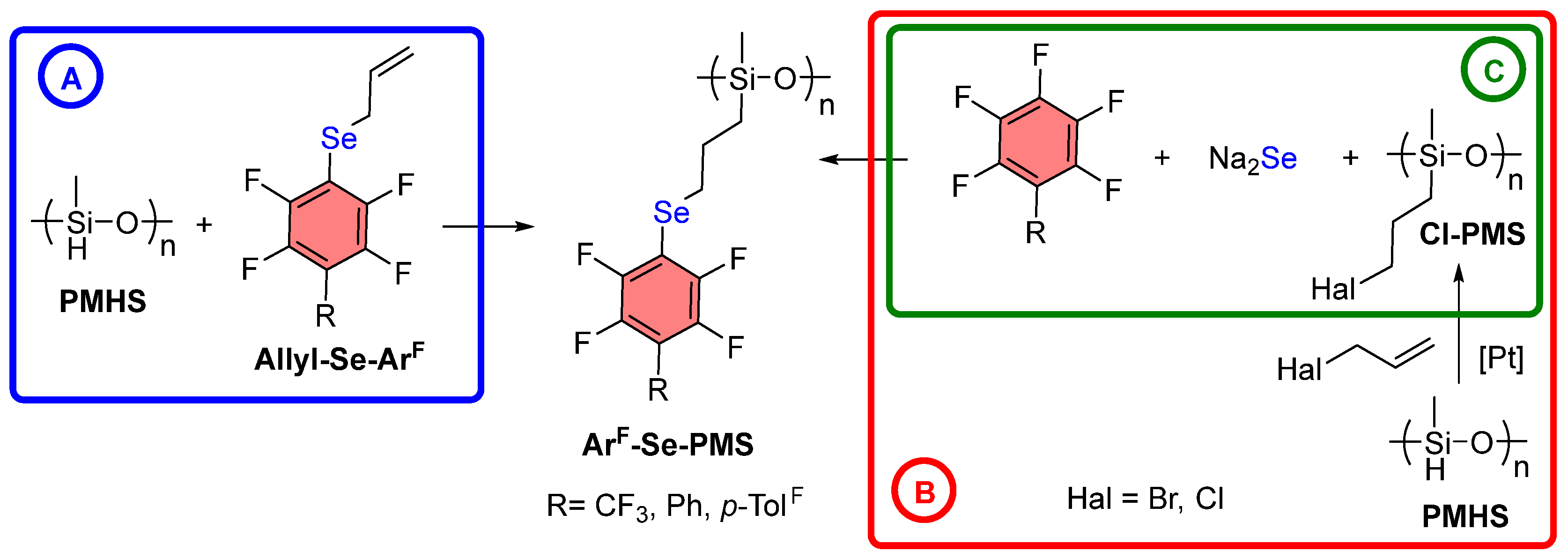
3. Results and Discussion
3.1. Synthesis of ArF-Se-PMS Through PMHS Modification
3.2. Synthesis of ArF-Se-PMS from Cl-PMS-2
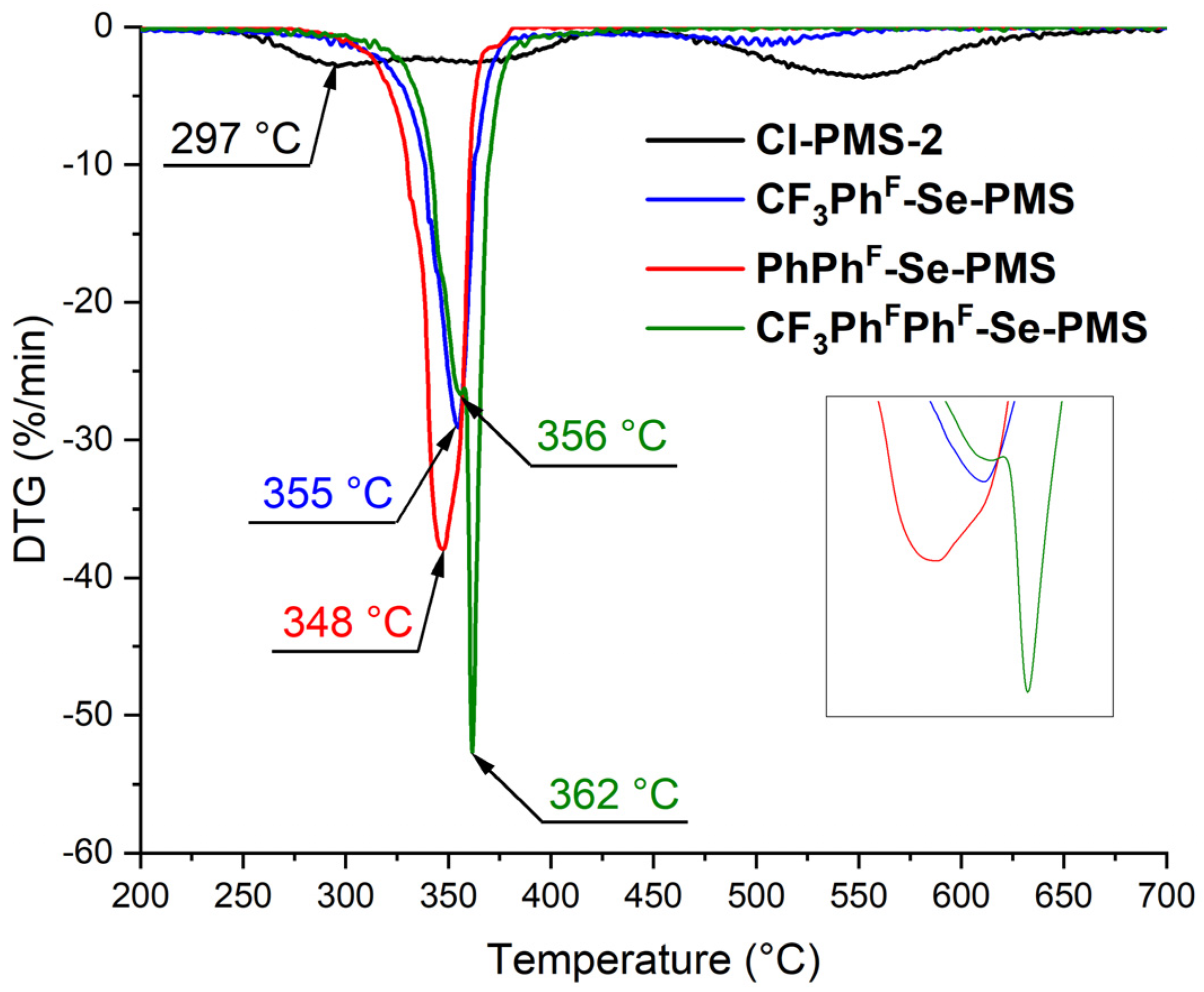
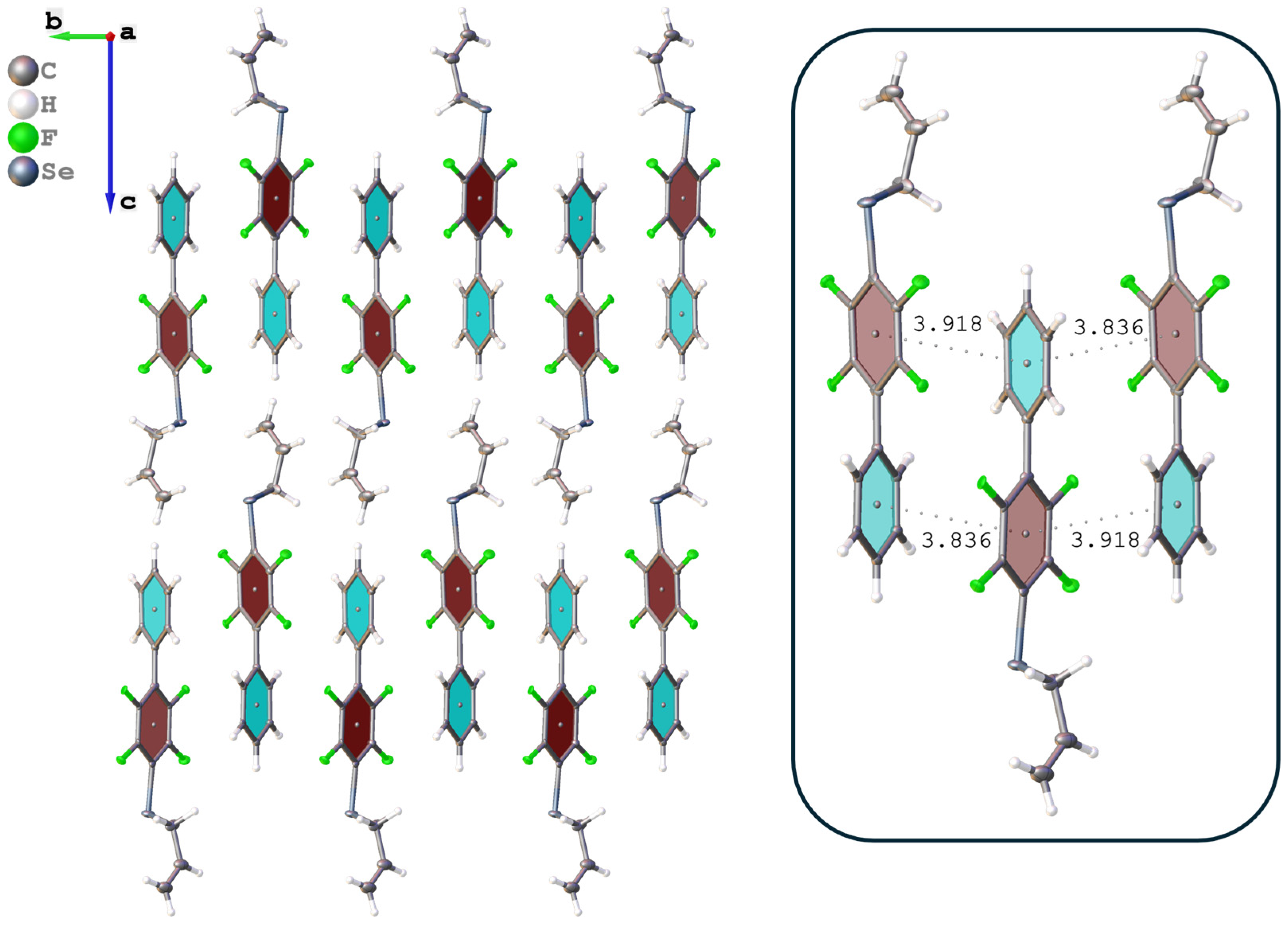
3.3. Polymer Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Köhler, T.; Gutacker, A.; Mejía, E. Industrial synthesis of reactive silicones: Reaction mechanisms and processes. Org. Chem. Front. 2020, 7, 4108–4120. [Google Scholar] [CrossRef]
- Vidal, F.; Jäkle, F. Functional Polymeric Materials Based on Main-Group Elements. Angew. Chem. Int. Ed. 2019, 58, 5846–5870. [Google Scholar] [CrossRef] [PubMed]
- Putzien, S.; Nuyken, O.; Kühn, F.E. Functionalized polysilalkylene siloxanes (polycarbosiloxanes) by hydrosilylation—Catalysis and synthesis. Prog. Polym. Sci. 2010, 35, 687–713. [Google Scholar] [CrossRef]
- Fröhlich, P.; Bertau, M. Polysiloxanes, Biocatalytic Functionalization. In Encyclopedia of Industrial Biotechnology; Flickinger, M.C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–15. [Google Scholar]
- Robeyns, C.; Picard, L.; Ganachaud, F. Synthesis, characterization and modification of silicone resins: An “Augmented Review”. Prog. Org. Coat. 2018, 125, 287–315. [Google Scholar] [CrossRef]
- Graffius, G.; Bernardoni, F.; Fadeev, A.Y. Covalent Functionalization of Silica Surface Using “Inert” Poly(dimethylsiloxanes). Langmuir 2014, 30, 14797–14807. [Google Scholar] [CrossRef]
- Racles, C.; Alexandru, M.; Bele, A.; Musteata, V.E.; Cazacu, M.; Opris, D.M. Chemical modification of polysiloxanes with polar pendant groups by co-hydrosilylation. RSC Adv. 2014, 4, 37620–37628. [Google Scholar] [CrossRef]
- Noro, A.; Matsushima, S.; He, X.; Hayashi, M.; Matsushita, Y. Thermoreversible Supramolecular Polymer Gels via Metal–Ligand Coordination in an Ionic Liquid. Macromolecules 2013, 46, 8304–8310. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; Mackay, M.E.; Hamley, I.W.; Rowan, S.J. A Healable Supramolecular Polymer Blend Based on Aromatic π–π Stacking and Hydrogen-Bonding Interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef]
- Vidal, F.; Gomezcoello, J.; Lalancette, R.A.; Jäkle, F. Lewis Pairs as Highly Tunable Dynamic Cross-Links in Transient Polymer Networks. J. Am. Chem. Soc. 2019, 141, 15963–15971. [Google Scholar] [CrossRef]
- Dodge, L.; Chen, Y.; Brook, M.A. Silicone Boronates Reversibly Crosslink Using Lewis Acid–Lewis Base Amine Complexes. Chem. Eur. J. 2014, 20, 9349–9356. [Google Scholar] [CrossRef]
- Hu, C.; Liao, H.; Li, F.; Xiang, J.; Li, W.; Duo, S.; Li, M. Noncovalent functionalization of multi-walled carbon nanotubes with siloxane polyether copolymer. Mater. Lett. 2008, 62, 2585–2588. [Google Scholar] [CrossRef]
- Vanderkooy, A.; Taylor, M.S. Solution-Phase Self-Assembly of Complementary Halogen Bonding Polymers. J. Am. Chem. Soc. 2015, 137, 5080–5086. [Google Scholar] [CrossRef]
- Zeng, R.; Gong, Z.; Chen, L.; Yan, Q. Solution Self-Assembly of Chalcogen-Bonding Polymer Partners. ACS Macro Lett. 2020, 9, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Ni, J.; Yue, Z.; Sun, T.; Chen, C.; Ma, X.; Wang, L. Polyoxometalate-Nanozyme-Integrated Nanomotors (POMotors) for Self-Propulsion-Promoted Synergistic Photothermal-Catalytic Tumor Therapy. Angew. Chem. Int. Ed. 2024, 63, e202315031. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wei, H.; Zhou, W.; Soto Rodriguez, P.E.D.; Lin, C.; Wang, L.; Zhang, Z. Advanced strategies for marine antifouling based on nanomaterial-enhanced functional PDMS coatings. Nano Mater. Sci. 2024, 6, 375–395. [Google Scholar] [CrossRef]
- You, Y.; Zheng, A.; Wei, D.; Xu, X.; Guan, Y.; Chen, J. Improving the thermal stability of poly[methyl(trifluoropropyl)siloxane] by introducing diphenylsiloxane units. RSC Adv. 2023, 13, 11424–11431. [Google Scholar] [CrossRef]
- Meier, D.; Huch, V.; Kickelbick, G. Aryl-group substituted polysiloxanes with high-optical transmission, thermal stability, and refractive index. J. Polym. Sci. 2021, 59, 2265–2283. [Google Scholar] [CrossRef]
- Briesenick, M.; Gallei, M.; Kickelbick, G. High-Refractive-Index Polysiloxanes Containing Naphthyl and Phenanthrenyl Groups and Their Thermally Cross-Linked Resins. Macromolecules 2022, 55, 4675–4691. [Google Scholar] [CrossRef]
- Nobuoka, H.; Ajiro, H. Development of Ester Free Type Poly(trimethylene carbonate) Derivatives with Pendant Fluoroaromatic Groups. Macromol. Chem. Phys. 2019, 220, 1900051. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Yang, H.; Bao, Y.; Liu, C.; Yu, S.; Guo, R.; Zhang, W.; Jiao, Z. Tailored Architecture for Enhanced Self-Healing: Visible Light-Responsive Polyacrylates with Dynamic Se–Se Bonds and Fluorinated Surfaces. ACS Appl. Polym. Mater. 2025, 7, 4944–4954. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, Y.; Meng, L.; Wang, Y.; Pang, A.; Guo, X.; Xiao, J.; Li, W. A review of poly[(3,3,3-trifluoropropyl)methylsiloxane]: Synthesis, properties and applications. Eur. Polym. J. 2022, 163, 110903. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Jin, K.; Wang, L.; Sun, J.; Fang, Q. A New Fluorinated Polysiloxane with Good Optical Properties and Low Dielectric Constant at High Frequency Based on Easily Available Tetraethoxysilane (TEOS). Macromolecules 2017, 50, 9394–9402. [Google Scholar] [CrossRef]
- Cai, L.; Lv, C.; Kang, J.; Wang, L.; He, X.; Zhou, T. Fabrication and investigation of multifunctional fluorinated polysiloxane coatings with phenyl as bridging group. J. Appl. Polym. Sci. 2022, 139, 51672. [Google Scholar] [CrossRef]
- Xin, J.; Lu, X.; Cao, J.; Wu, W.; Liu, Q.; Wang, D.; Zhou, X.; Ding, D. Fluorinated Organic Polymers for Cancer Drug Delivery. Adv. Mater. 2024, 36, 2404645. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Seo, E.W.; Hong, S.; Jung, K.O.; Kim, D. Pentafluorobenzene: Promising Applications in Diagnostics and Therapeutics. ACS Appl. Bio Mater. 2023, 6, 4081–4099. [Google Scholar] [CrossRef] [PubMed]
- Yampolskii, Y.P.; Belov, N.A.; Alentiev, A.Y. Fluorine in the structure of polymers: Influence on the gas separation properties. Russ. Chem. Rev. 2019, 88, 387–405. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Z.; Zhang, W.; Wang, L.; Yu, G. Recent Progress of Fluorinated Conjugated Polymers. Adv. Mater. 2024, 36, 2403961. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Krykova, M.A.; Ivanov, D.M.; Novikov, A.S.; Sinelshchikova, A.A.; Volostnykh, M.V.; Konovalov, M.A.; Grigoriev, M.S.; Gorbunova, Y.G.; Kukushkin, V.Y. Reverse Arene Sandwich Structures Based upon π-Hole·[MII] (d8 M=Pt, Pd) Interactions, where Positively Charged Metal Centers Play the Role of a Nucleophile. Angew. Chem. Int. Ed. 2019, 58, 4164–4168. [Google Scholar] [CrossRef]
- Wang, W.; Wu, W.X.; Zhang, Y.; Jin, W.J. Perfluoroaryl⋯aryl interaction: The most important subset of π-hole·π bonding. Chem. Phys. Rev. 2024, 5, 031303. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, A.; An, Y.; Li, Q. Arene-perfluoroarene interaction: Properties, constructions, and applications in materials science. Matter 2024, 7, 3317–3350. [Google Scholar] [CrossRef]
- Cheng, Q.; Hao, A.; Xing, P. Selective chiral dimerization and folding driven by arene–perfluoroarene force. Chem. Sci. 2024, 15, 618–628. [Google Scholar] [CrossRef]
- Lee, L.M.; Tsemperouli, M.; Poblador-Bahamonde, A.I.; Benz, S.; Sakai, N.; Sugihara, K.; Matile, S. Anion Transport with Pnictogen Bonds in Direct Comparison with Chalcogen and Halogen Bonds. J. Am. Chem. Soc. 2019, 141, 810–814. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Eliseeva, A.A.; Baykov, S.V.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. One-Pot Route to X-perfluoroarenes (X = Br, I) Based on FeIII-Assisted C–F Functionalization and Utilization of These Arenes as Building Blocks for Crystal Engineering Involving Halogen Bonding. Cryst. Growth Des. 2020, 20, 5908–5921. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Novikov, A.S.; Ivanov, D.M.; Bolotin, D.S.; Bokach, N.A.; Kukushkin, V.Y. Structure-Directing Weak Interactions with 1,4-Diiodotetrafluorobenzene Convert One-Dimensional Arrays of [MII(acac)2] Species into Three-Dimensional Networks. Cryst. Growth Des. 2018, 18, 3626–3636. [Google Scholar] [CrossRef]
- Mehrparvar, S.; Klinksiek, M.; Gauld, R.M.; Wolf, J.; Poliyodath Mohanan, M.; Vonnemann, C.; Haase, J.; Papagna, R.; Völkel, M.H.H.; Gessner, V.H.; et al. Halogen Bonding in Solution: Under Pressure. J. Am. Chem. Soc. 2025, 147, 29659–29665. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Zhmykhova, M.V.; Torubaev, Y.V.; Katlenok, E.A.; Kryukov, D.M.; Kukushkin, V.Y. Bis(perfluoroaryl)chalcolanes ArF2Ch (Ch = S, Se, Te) as σ/π-Hole Donors for Supramolecular Applications Based on Noncovalent Bonding. Cryst. Growth Des. 2023, 23, 2593–2601. [Google Scholar] [CrossRef]
- Dhaka, A.; Jeannin, O.; Jeon, I.-R.; Aubert, E.; Espinosa, E.; Fourmigué, M. Activating Chalcogen Bonding (ChB) in Alkylseleno/Alkyltelluroacetylenes toward Chalcogen Bonding Directionality Control. Angew. Chem. Int. Ed. 2020, 59, 23583–23587. [Google Scholar] [CrossRef]
- Kryukov, D.M.; Rozhkov, A.V.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y. Perfluoroaromatic Bisselanes: From Molecular Design to Supramolecular Architecture Through Tandem σ/π-hole Interactions. Chem. Asian, J. 2025, 20, e202500247. [Google Scholar] [CrossRef]
- Deriabin, K.V.; Vereshchagin, A.A.; Kirichenko, S.O.; Rashevskii, A.A.; Levin, O.V.; Islamova, R.M. Self-cross-linkable ferrocenyl-containing polysiloxanes as flexible electrochromic materials. Mater. Today Chem. 2023, 29, 101399. [Google Scholar] [CrossRef]
- Kocheva, A.N.; Deriabin, K.V.; Volkov, A.I.; Levin, O.V.; Islamova, R.M. Cobaltocenium-Containing Polysiloxanes: Catalytic Synthesis, Structure, and Properties. ACS Appl. Polym. Mater. 2024, 6, 12112–12122. [Google Scholar] [CrossRef]
- Golovenko, E.A.; Kocheva, A.N.; Semenov, A.V.; Baykova, S.O.; Deriabin, K.V.; Baykov, S.V.; Boyarskiy, V.P.; Islamova, R.M. Palladium-Functionalized Polysiloxane Drop-Casted on Carbon Paper as a Heterogeneous Catalyst for the Suzuki–Miyaura Reaction. Polymers 2024, 16, 2826. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, M.V.; Kukushkin, V.Y.; Islamova, R.M. Cellulose-based hybrid glycosilicones via grafted-to metal-catalyzed hydrosilylation: “When opposites unite”. Carbohydr. Polym. 2020, 241, 116327. [Google Scholar] [CrossRef] [PubMed]
- Filippova, S.S.; Deriabin, K.V.; Perevyazko, I.; Shamova, O.V.; Orlov, D.S.; Islamova, R.M. Metal- and Peroxide-Free Silicone Rubbers with Antibacterial Properties Obtained at Room Temperature. ACS Appl. Polym. Mater. 2023, 5, 5286–5296. [Google Scholar] [CrossRef]
- Vinogradov, A.S.; Platonov, V.E. Synthesis of perfluorinated biaryls by reaction of perfluoroarylzinc compounds with perfluoroarenes. Russ. J. Org. Chem. 2015, 51, 1388–1394. [Google Scholar] [CrossRef]
- Thompson, D.P.; Boudjouk, P. A convenient synthesis of alkali metal selenides and diselenides in tetrahydrofuran and the reactivity differences exhibited by these salts toward organic bromides. Effect of ultrasound. J. Org. Chem. 1988, 53, 2109–2112. [Google Scholar] [CrossRef]
- CrysAlisPro, A.; Agilent Technologies Inc. Yarnton, Oxfordshire, England; 2019. Available online: https://rigaku.com/products/crystallography/x-ray-diffraction/crysalispro (accessed on 9 October 2025).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Katlenok, E.A.; Zhmykhova, M.V.; Ivanov, A.Y.; Kuznetsov, M.L.; Bokach, N.A.; Kukushkin, V.Y. Metal-Involving Chalcogen Bond. The Case of Platinum(II) Interaction with Se/Te-Based σ-Hole Donors. J. Am. Chem. Soc. 2021, 143, 15701–15710. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Han, X.; Zhuang, H.; Nie, G.; Xu, H. Nanomedicine Assembled by Coordinated Selenium–Platinum Complexes Can Selectively Induce Cytotoxicity in Cancer Cells by Targeting the Glutathione Antioxidant Defense System. ACS Biomater. Sci. Eng. 2017, 4, 1954–1962. [Google Scholar] [CrossRef]
- Kryukov, D.M.; Khrustaleva, A.A.; Baykov, S.V.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y.; Rozhkov, A.V.; Bokach, N.A. Synergy of chalcogen and halogen bonding in cocrystals of PdII and PtII acetylacetonates with perfluoroarene selanes: Impact of the arene iodination on supramolecular architecture. Inorg. Chem. Commun. 2025, 182, 115288. [Google Scholar] [CrossRef]
- Pikies, J.; Wojnowski, W. The reaction of isosteric isobutyl(isopropoxy)silanes iBun(iPrO)3–nSiH (n = 0–3) with allyl bromide in the presence of platinum compounds. J. Organomet. Chem. 1990, 393, 187–193. [Google Scholar] [CrossRef]
- Deriabin, K.V.; Lobanovskaia, E.K.; Novikov, A.S.; Islamova, R.M. Platinum-catalyzed reactions between Si–H groups as a new method for cross-linking of silicones. Org. Biomol. Chem. 2019, 17, 5545–5549. [Google Scholar] [CrossRef]
- Kihara, Y.; Ichikawa, T.; Abe, S.; Nemoto, N.; Ishihara, T.; Hirano, N.; Haruki, M. Synthesis of alkyne-functionalized amphiphilic polysiloxane polymers and formation of nanoemulsions conjugated with bioactive molecules by click reactions. Polym. J. 2014, 46, 175–183. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Yu, L.; Skov, A.L. Revisiting the Thermal Transitions of Polydimethylsiloxane (PDMS) Elastomers: Addressing Common Misconceptions with Comprehensive Data. Macromol. Mater. Eng. 2025, 310, 2500075. [Google Scholar] [CrossRef]
- Kryukov, D.M.; Rozhkov, A.V.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y.; Bokach, N.A. Positional Control of σ- and π-Hole Directionality in Iodoselane Cocrystals: Structural Impact of the Iodine Position on the Supramolecular Assembly with 1,4-Diazabicyclo[2.2.2]octane (DABCO). Organometallics 2025. [Google Scholar] [CrossRef]



| № | Cl-PMS-2: PhPhFSeNa Ratio | Cl Substitution Degree, a mol% | Byproduct, b mol% | |
|---|---|---|---|---|
 PhPhFSe-SePhFPh | 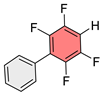 PhPhFH | |||
| 1 | 1:1 | 73 | 0 | 23 |
| 2 | 1:1.3 | 92 | 3 | 16 |
| 3 | 1:1.5 | 90 | 11 | 29 |
| 4 | 1:1.7 | 88 | 15 | 31 |
| 5 | 1:1.2 | 92 | 0 | 23 |
| Polymer | Atmosphere | Td,2%, a °C | Td,5%, b °C | Tmax%, c °C | Residue Mass, d % | Ref. |
|---|---|---|---|---|---|---|
| Cl-PMS-2 | Ar | 260 | 280 | 297 | 22 | This study |
| CF3PhF-Se-PMS | Ar | 237 | 293 | 355 | 10 | This study |
| CF3PhFPhF-Se-PMS | Ar | 271 | 314 | 356 | 2 | This study |
| PhPhF-Se-PMS | Ar | 301 | 317 | 348 | 1 | This study |
| Sylgard 186 | N2 | 248 | – | 400 | ≈80 | [62] |
| HM40_PHM20_PP40 | N2 | 220 | 282 | – | ≈30 | [20] |
| PMTFPS | N2 | 250 | 345 | 404 | ≈2 | [19] |
| P(MTFPS-co-DPS)-1 | N2 | 270 | 366 | 412 | ≈2 | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotsman, K.A.; Filippova, S.S.; Kukushkin, V.Y.; Islamova, R.M. Synthesis of Polyfluorinated Aromatic Selenide-Modified Polysiloxanes: Enhanced Thermal Stability, Hydrophobicity, and Noncovalent Modification Potential. Polymers 2025, 17, 2729. https://doi.org/10.3390/polym17202729
Lotsman KA, Filippova SS, Kukushkin VY, Islamova RM. Synthesis of Polyfluorinated Aromatic Selenide-Modified Polysiloxanes: Enhanced Thermal Stability, Hydrophobicity, and Noncovalent Modification Potential. Polymers. 2025; 17(20):2729. https://doi.org/10.3390/polym17202729
Chicago/Turabian StyleLotsman, Kristina A., Sofia S. Filippova, Vadim Yu. Kukushkin, and Regina M. Islamova. 2025. "Synthesis of Polyfluorinated Aromatic Selenide-Modified Polysiloxanes: Enhanced Thermal Stability, Hydrophobicity, and Noncovalent Modification Potential" Polymers 17, no. 20: 2729. https://doi.org/10.3390/polym17202729
APA StyleLotsman, K. A., Filippova, S. S., Kukushkin, V. Y., & Islamova, R. M. (2025). Synthesis of Polyfluorinated Aromatic Selenide-Modified Polysiloxanes: Enhanced Thermal Stability, Hydrophobicity, and Noncovalent Modification Potential. Polymers, 17(20), 2729. https://doi.org/10.3390/polym17202729







