PVA-Cellulose Fibers Composites Impregnated with Antimicrobial Particles: The Solvent Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Silver Particles (AgPs)
2.3. Preparation of Particle Mixtures and Design of Cellulose-Based Composites
2.4. Characterization Methods of Particles and Composites
2.4.1. Transmission Electron Microscopy (TEM)
2.4.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Spectroscopy
2.4.3. UV-Vis Spectroscopy
- In-plane dipole mode: This mode gives rise to a strong, dominant surface plasmon resonance (SPR) peak in the UV–Vis region;
2.4.4. Dynamic Light Scattering (DLS)
2.4.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4.6. Contact Angle Measurements
2.4.7. Antimicrobial Screening
2.4.8. Statistical Analysis
3. Results and Discussion
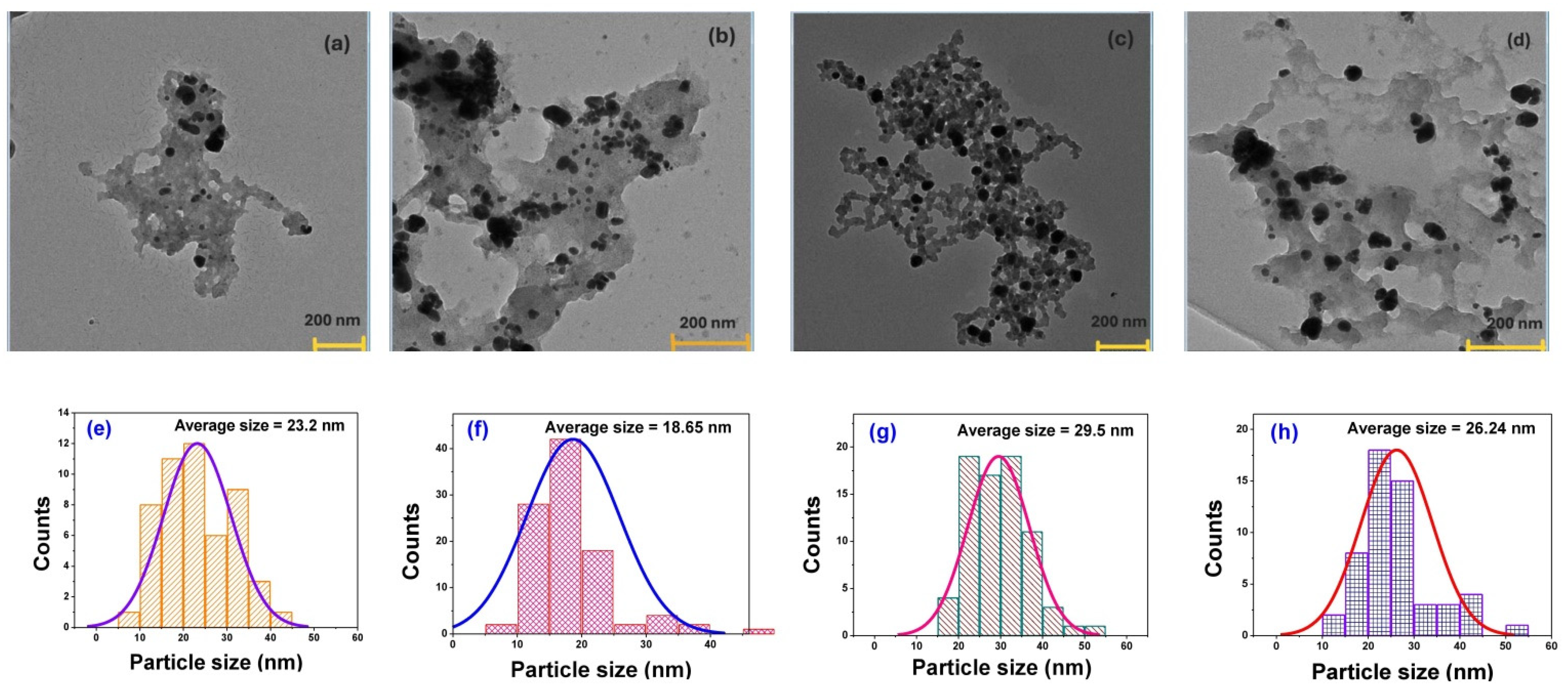
3.1. Morphology and Size of Silver Particles in Bulk
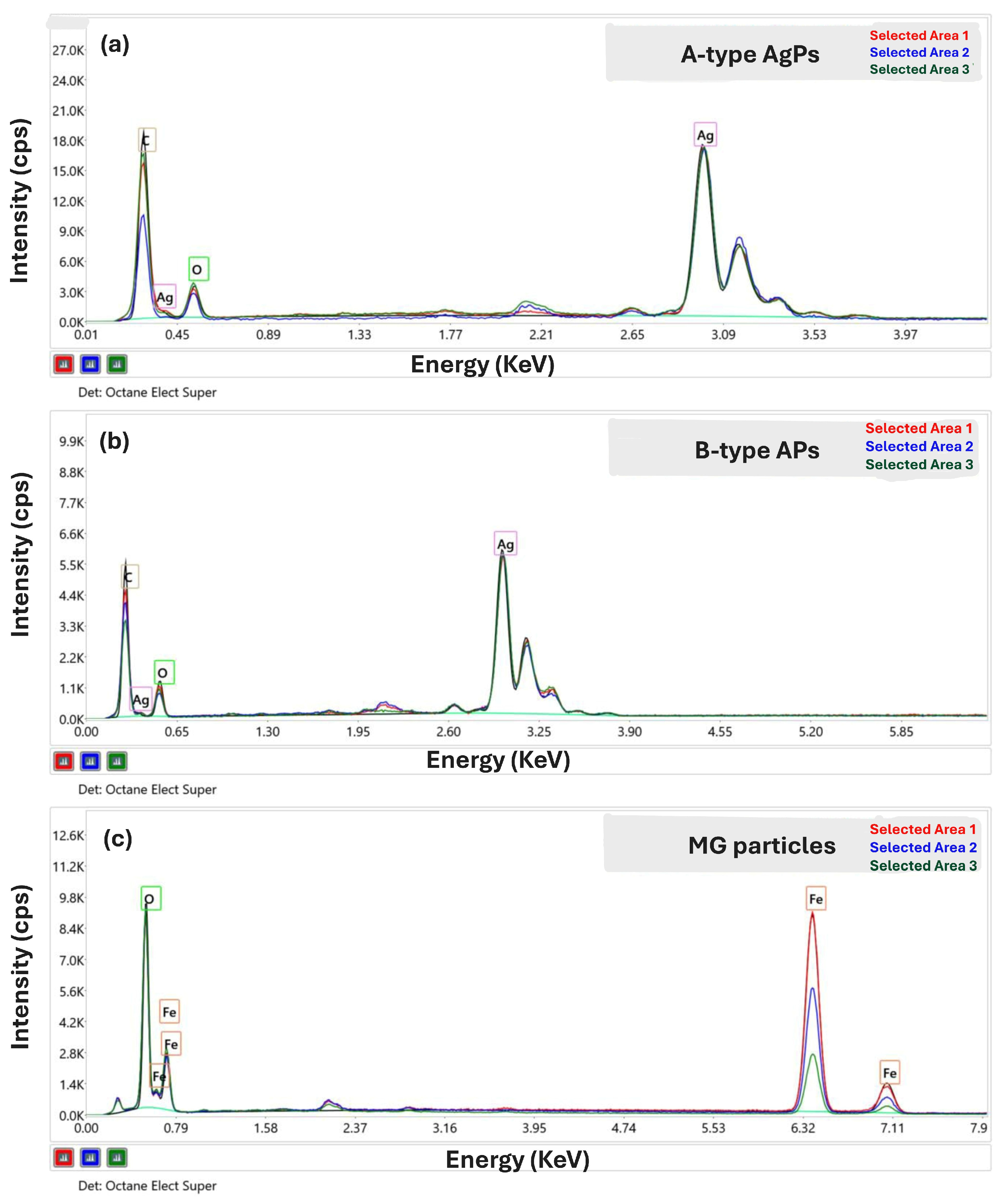
3.2. Chemical Composition of Inorganic Particles
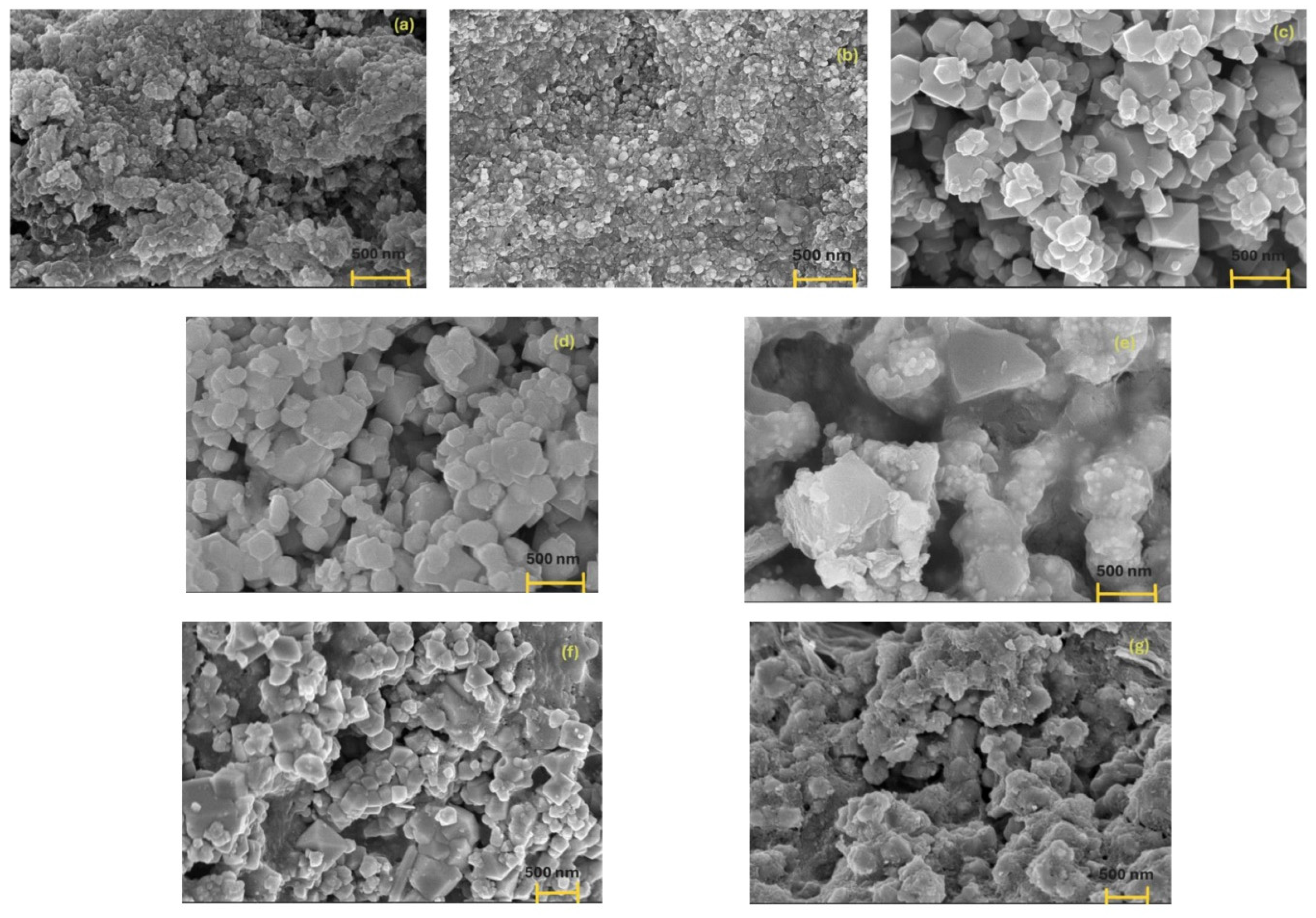
3.3. Surface Properties of Inorganic Particles and Their Mixture
- (a)
- MG aggregates formed due to dipole–dipole magnetic interactions characteristic of such materials,
- (b)
- A-type AgPs electrostatically adsorbed onto MG surfaces, and
- (c)
- agglomerates of A-type AgPs embedded in the more irregular and disrupted MG surface.
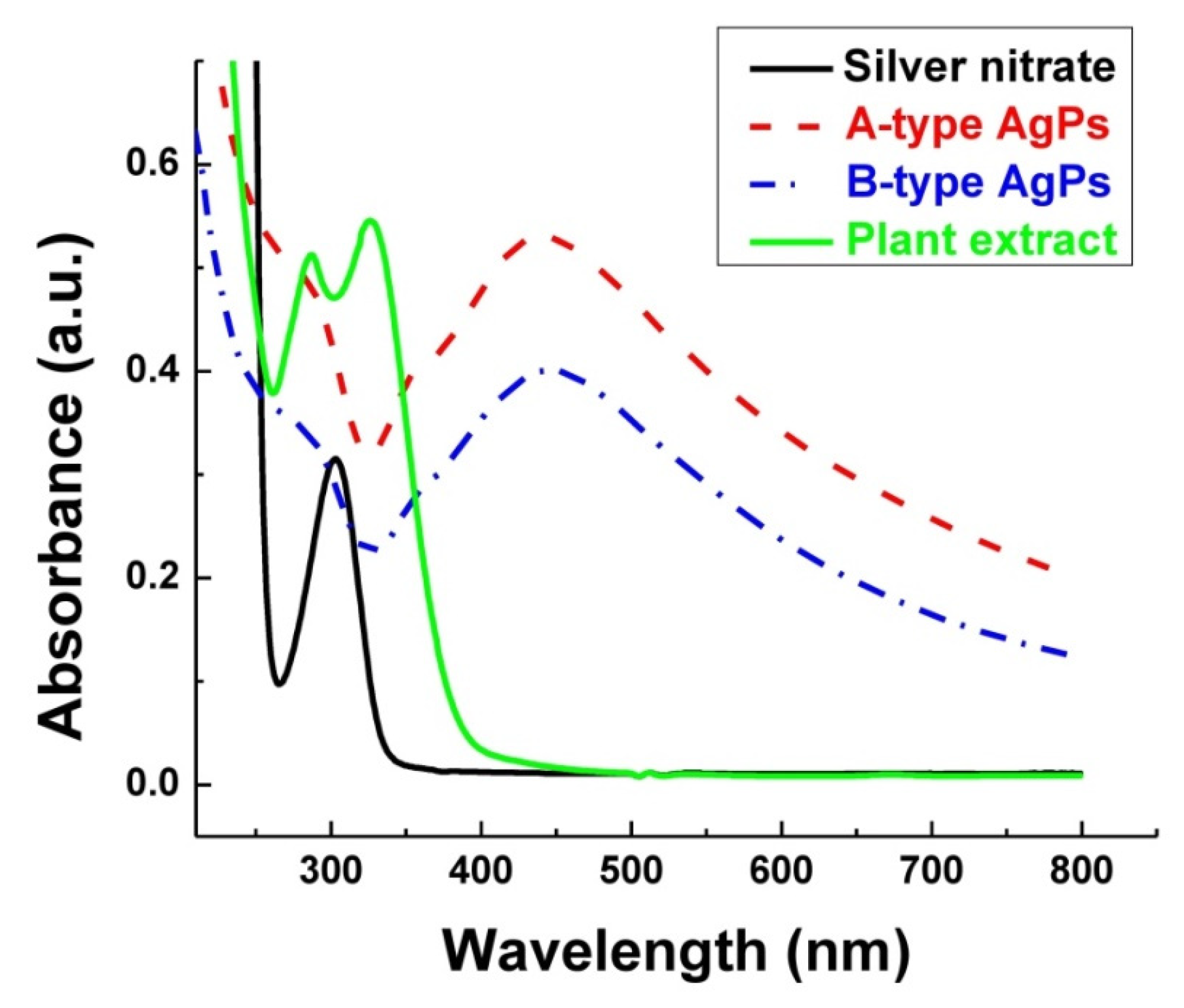
3.4. Optical Properties of Inorganic Particles in Solution
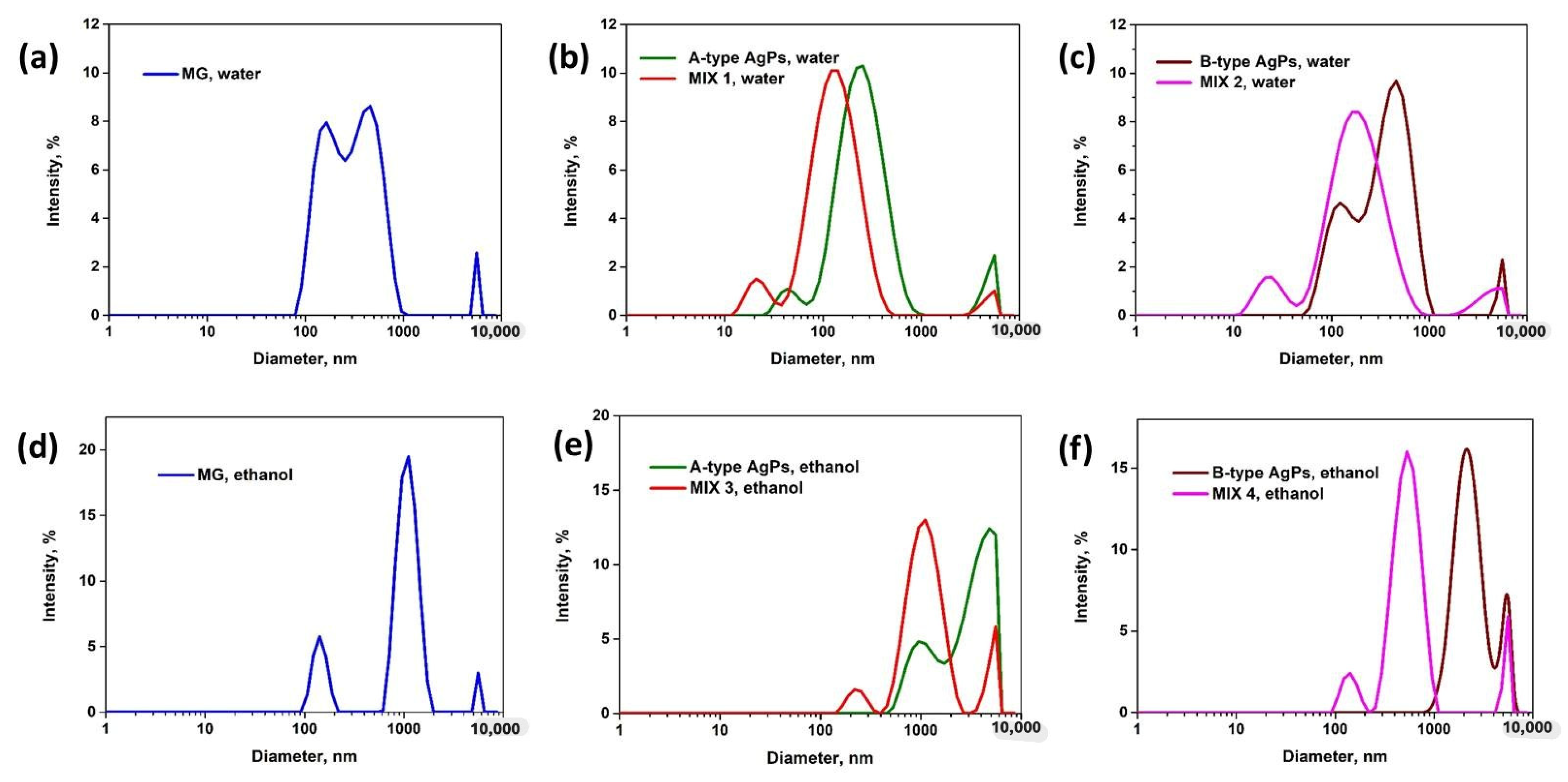
3.5. Size Distributions and Colloidal Stability of Inorganic Particle in Solution
3.6. Interactions and Functional Groups of Inorganic Particles
- (1)
- The signals centered at ~3435 cm−1, from 3670 to 3070 cm−1 broad strong region, are assigned to the stretching vibration of hydroxyl (−OH) or H−OH, and N−H, which could be arising from alcohols, phenols, carboxylic acids, amines, or amides. Remarkably, this band is broader in ethanol-dried samples—especially AgPs—due to enhanced hydrogen bonding.
- (2)
- The 3010–2800 cm−1 band with a maximum at ~2920 cm−1 is common to the alkanes possessing CH2 or CH3 groups and is associated with the aliphatic C-H stretching vibration of sp3 carbons.
- (3)
- The 1633 cm−1 peak in the 1760–1490 cm−1 region is due to the C=C stretching of alkenes, aromatics, or N–H bending (amide II) vibrations of proteins, peptides of primary amines. Combined 3435 cm−1 and 1633 cm−1 peaks may indicate moisture or surface oxidation.
- (4)
- The 1460 cm−1 peak in the 1490–1230 cm−1 region is likely assigned to the C–N stretching or COO− symmetric stretch from amine or carboxylate groups, and to the bending vibration of the C-H bond. The maximum of about 1380 cm−1 could be the signal of NO3− symmetric stretch if AgNO3 was used for AgPs synthesis, or the vibration of the CH2 groups. The peak of 1260/1270 cm−1 was generated by the stretching vibration of hydroxyl (−OH) groups.
- (5)
- In 1230–900 cm−1 region the following peaks were remarked: 1086/1096 cm−1 for stretching vibration of (C−O−H); 1022/1028 cm−1 for vibration of etheric bond (−C−O−); 998/1000/1005 only for B-type AgPs and MG dried from water, and due to the C–O–C, C–N, or C–OH stretching from alcohols, ethers, or polysaccharides.
- (6)
- In the 900–650 cm−1 region, the C–H out-of-plane bending of alkenes or aromatics is recorded. This region is partially overlapped with the fingerprint region of 800–350 cm−1 specific to the metallic particles (Figures S1–S4 and Table 4).
- (1)
- The peak at ~840 cm−1, observed in the spectra of AgPs and MG particles dried from water, disappears in the MIX 1 and MIX 2 mixtures. Notably, this peak is absent in all samples dried from ethanol.
- (2)
- The peak observed at 803, 805, and 808 cm−1 in the spectra of MG, A-type AgPs, and B-type AgPs dried from water slightly shifts to 799 cm−1 in MIX 1 (indicative of a possible blueshift) and to 801 cm−1 in MIX 2. These signals are attributed to the stretching vibrations of Me–N bonds and appear more intense in both MIX 1 and MIX 2.
- (3)
- For the samples dried from ethanol, the signal observed at 804, 793, and 795 cm−1 for MG, A-type AgPs, and B-type AgPs, respectively, appears at 802 cm−1 in MIX 3 and MIX 4. These bands are also attributed to the stretching vibrations of Me–N bonds.
- (4)
- The fingerprint of nanoparticles or bulk MG includes a main peak ~570 cm−1, assigned to Fe-O stretching vibration of tetrahedral Fe3+, and a secondary peak near 450 cm−1, assigned to Fe-O bending/stretching of octahedral Fe2+/Fe3+. In our study, MG showed two peaks at 562 cm−1 and 452 cm−1 for the sample dried from ethanol, and distinct peaks at 573 cm−1 and 451 cm−1 for the sample dried from water. Similar results were observed by Khalkhali M. et al. [8] at 577 cm−1, by Yang R. et al. [58] at 582.47 cm−1, and by Gharehaghaji N. and Divband B. [13] at wavenumbers of 568 cm−1 (in the tetrahedral position) and 430 cm−1 (in the octahedral position). The vibrational absorption at 573 cm−1 or 562 cm−1, corresponding to the Fe-O bond, shifted in MIX 1 (at ~556 cm−1), MIX 2 (at ~562 cm−1) and MIX 3 (at ~575 cm−1), indicating the successful adsorption of AgPs onto the MG surface. Similar observations were reported by Yang R. et al. [58] for their magnetic Fe3O4-loaded silver nanocomposites. In MIX 4, the specific band of MG disappeared.
- (5)
- The AgPs spectra contain signals specific to Ag-Cl bonds: at ~569 cm−1, ~620 cm−1 and ~636 cm−1 for samples dried from water, and at ~623 cm−1 and at ~653 cm−1 for samples dried from ethanol. The origin of the FTIR peak attributed to AgCl compounds or Ag–Cl chemical bonds in the fingerprint spectra of AgPs synthesized using Salvia officinalis extracts is also discussed. Hydroalcoholic sage extracts are rich in phenolic compounds (e.g., rosmarinic acid, caffeic acid), flavonoids (e.g., luteolin, apigenin), essential oils (e.g., thujone, camphor), tannins, and terpenoids. Although detailed analyses of chloride content in these hydroalcoholic extracts are limited, Cl− ions likely originate from the plant’s absorption of inorganic salts such as NaCl, KCl, or CaCl2 present in the soil [73,74,75,76,77]. The presence of chlorine in the synthesized AgPs is further confirmed by EDX spectra, showing a peak near 2.6 keV (Section 3.2), which can be attributed solely to the phytochemical composition rather than residual water, as Millipore-filtered water was used throughout the experiments.
- (6)
- For the samples dried from water, the signals at 451, 457, and 457 cm−1 for MG, A-type AgPs, and B-type AgPs, respectively, shift to 453 cm−1 in the MIX 1 and MIX 2 mixtures. These peaks are attributed to the Fe-O stretching vibrations of octahedral Fe2+/Fe3+ ions, as well as to Ag–O stretching vibrations associated with AgO and AgOH.
- (7)
- MG dried from ethanol exhibits a characteristic peak at 452 cm−1, while A-type AgPs and B-type AgPs show a distinct signal around 432 cm−1. In the mixtures MIX 3 and MIX 4, a single peak centered at 450 cm−1 is observed, assigned to the stretching vibrations of Fe-O and Ag-O bonds, originating from iron oxides, AgO, and AgOH.
- (8)
- The signal at ~395 cm−1 appears predominantly in samples dried from ethanol and is attributed to the stretching vibrations of Fe-O and Ag-O bonds, assigned to MG and AgPs.
- (9)
- In conclusion, the weak, indirect signatures observed in the ~800–300 cm−1 region serve as a distinctive fingerprint of inorganic metal particles, arising primarily from Me–O bonds and interactions, with additional contributions from Me–N and Me–Cl bonding environments.
- (10)
- In mixtures, most of the bands observed in the deconvoluted signals come from AgPs and/or MG, but new bands were recorded at ~723 cm−1, ~690 cm−1 and ~584 cm−1 for MIX 1 or MIX 2, or at ~701 cm−1 for MIX 3, too. These new bands are due to electrostatic interactions between the two types of inorganic particles.
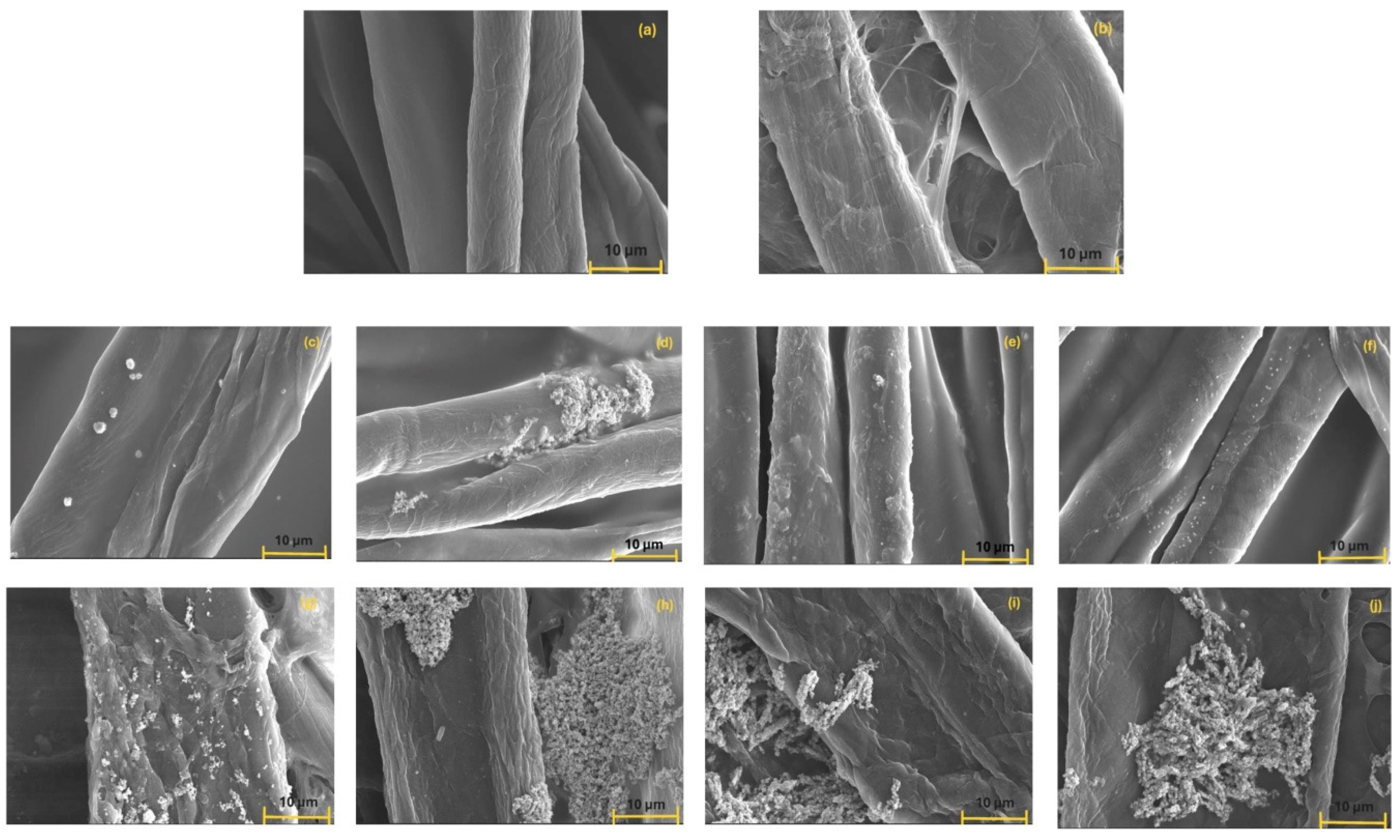
3.7. Surface Properties of Composites
3.8. Wettability of Composites
3.9. Preliminary Evaluation of the Antimicrobial Activity of Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeon, I.-Y.; Baek, J.-B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Damjanoviae-Vratnica, B.; Djakov, T.A.; Šukoviæ, D.; Damjanoviae, J. Chemical composition and antimicrobial activity of essential oil of wild-growing Salvia officinalis L. from Montenegro. J. Essent. Oil Bear. Plants 2008, 11, 79–89. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Liu, C.; He, F.; Song, Z.; Qiu, Q.; Yan, J.; Zhu, W. Interaction of polyphenols and Ag on the surface plasmon resonance absorption and resonance Rayleigh scattering spectra. Vib. Spectrosc. 2020, 107, 103037. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite nanoparticles for medical MR imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- Khalkhali, M.; Rostamizadeh, K.; Sadighian, S.; Khoeini, F.; Naghibi, M.; Hamidi, M. The impact of polymer coatings on magnetite nanoparticles performance as MRI contrast agents: A comparative study. J. Pharm. Sci. 2015, 23, 45. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Guadagnini, A.; Agnoli, S.; Badocco, D.; Pastore, P.; Fracasso, G.; Gerosa, M.; Vurro, F.; Busato, A.; Marzola, P. Polymer-coated silver-iron nanoparticles as efficient and biodegradable MRI contrast agents. J. Colloid Interface Sci. 2021, 596, 332–341. [Google Scholar] [CrossRef]
- Jain, N.; Jain, P.; Rajput, D.; Patil, U.K. Green synthesized plant-based silver nanoparticles: Therapeutic prospective for anticancer and antiviral activity. Micro Nano Syst. Lett. 2021, 9, 5. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Hoppe, H.; Okoh, A.I. Plant-based synthesis of silver nanoparticles using aqueous leaf extract of Salvia officinalis: Characterization and its antiplasmodial activity. J. Clust. Sci. 2021, 32, 101–109. [Google Scholar] [CrossRef]
- Gharehaghaji, N.; Divband, B. PEGylated magnetite/hydroxyapatite: A green nanocomposite for T2-weighted MRI and curcumin carrying. Evid. Based Complement. Alternat. Med. 2022, 2022, 1337588. [Google Scholar] [CrossRef]
- Ihsan, S.; Gul, H.; Jamila, N.; Khan, N.; Ullah, R.; Bari, A.; Nee, T.W.; Hwang, J.H.; Masood, R. Biogenic Salvia species synthesized silver nanoparticles with catalytic, sensing, antimicrobial, and antioxidant properties. Heliyon 2024, 10, e25814. [Google Scholar] [CrossRef]
- Shahzadi, S.; Fatima, S.; Ain, Q.U.; Shafiq, Z.; Janjua, M.R.S.A. A review on green synthesis of silver nanoparticles (SNPs) using plant extracts: A multifaceted approach in photocatalysis, environmental remediation, and biomedicine. RSC Adv. 2025, 15, 3858. [Google Scholar] [CrossRef]
- Errokh, A.; Magnin, A.; Putaux, J.-L.; Boufi, S. Hybrid nanocellulose decorated with silver nanoparticles as reinforcing filler with antibacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110044. [Google Scholar] [CrossRef]
- Bag, S.S.; Bora, A.; Golder, A.; Raina, K.; Haridhasapavalan, K.K.; Thummer, R.P. Gelatin-Pva-Agnps triad composite as wound healing hydrogel with wounded skin surface protective efficiency. Res. Sq. 2023; preprint research. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Zengjie, F. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mat. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef] [PubMed]
- Charti, I.; Sair, S.; Rafik, O.; Abboud, Y.; El Bouari, A. Ecofriendly synthesis of cellulose-silver nanocomposites and the evaluation of their antibacterial activity. Discov. Nano 2025, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Stauffer, S.R. Reinforced uncrosslinked poly(vinyl alcohol) gels produced by cyclic freezing–thawing processes: A structural and mechanical study. J. Control. Release 1991, 16, 305–310. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and applications. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Shaskolskii, B.L.; Babushkina, T.A.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems: 27. Physicochemical properties of poly(vinyl alcohol) cryogels and specific features of their macroporous morphology. Colloid J. 2007, 69, 747–764. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzyme Microb. Technol. 1998, 23, 227–242. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Kurochkin, I.N.; Kurochkin, I.I. Cryostructuring of polymeric systems. 36. Poly(vinyl alcohol) cryogels prepared from solutions of the polymer in water/low-molecular alcohol mixtures. Eur. Polym. J. 2014, 53, 189–205. [Google Scholar] [CrossRef]
- Fernandez-Lopez, J.; Zhi, N.; Aleson-Carbonell, L.; Pérez-Alvarez, J.A.; Kuri, V. Antioxidant and antibacterial activities of natural extracts: Application in beef meatballs. Meat Sci. 2005, 69, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, A.G.; Grigoras, V.C. Eco-friendly silver nanoparticles obtained by green synthesis from Salvia officinalis. Sustain. Chem. 2024, 5, 215–228. [Google Scholar] [CrossRef]
- Daoud, S.; Alkahtani, M.A.M.; Alkhalifah, D.H.M.; Elobeid, M.M.; Mohammed, A.E. Biosynthesis of silver nanoparticles using Salvia officinalis extract and assessment of their antibacterial activity. Int. J. Curr. Res. 2019, 7, 21548–21552. Available online: https://www.journalcra.com/sites/default/files/issue-pdf/11043.pdf (accessed on 1 June 2025).
- Pop, A.V.; Tofana, M.; Socaci, S.; Varban, D.; Fogarasi, M.; Muntean, M.-D.; Sfechis, S. Evaluation of antioxidant activity and phenolic content in different Salvia officinalis L. extracts. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2015, 72, 210–214. [Google Scholar] [CrossRef]
- Takci, D.; Ozdenefe, M.S.; Genc, S. Green synthesis of silver nanoparticles with an antibacterial activity using Salvia officinalis aqueous extract. J. Cryst. Growth 2023, 614, 127239. [Google Scholar] [CrossRef]
- Mihailović, V.; Srećković, N.; Nedić, Z.P.; Dimitrijević, S.; Matić, M.; Obradović, A.; Selaković, D.; Rosić, G.; Katanić Stanković, J. Green synthesis of silver nanoparticles using Salvia verticillata and Filipendula ulmaria extracts: Optimization of synthesis, biological activities, and catalytic properties. Molecules 2023, 28, 808. [Google Scholar] [CrossRef]
- Biswas, A.; Aktas, O.C.; Kanzow, J.; Saeed, U.; Strunskus, T.; Zaporojtchenko, V.; Faupel, F. Polymer-metal optical nanocomposites with tunable particle plasmon resonance prepared by vapor phase co-deposition. Mater. Lett. 2004, 58, 1530–1534. [Google Scholar] [CrossRef]
- Popov, A.K.; Brummer, J.; Tanke, R.S.; Taft, G.; Loth, M.; Langlois, R.; Wruck, A.; Schmitz, R. Synthesis of isolated silver nanoparticles and their aggregates manipulated by light. Laser Phys. Lett. 2006, 3, 546. [Google Scholar] [CrossRef][Green Version]
- Movsesyan, A. Plasmonic Properties of Metallic Nanoparticles: Beyond the Dipolar Resonance. Ph.D. Thesis, Universite de Technologie de Troyes, Troyes, France, 14 December 2018. Available online: https://theses.hal.science/tel-03610410v1 (accessed on 18 July 2025).
- Chhatre, A.; Solasa, P.; Sakle, S.; Thaokar, R.; Mehra, A. Color and surface plasmon effects in nanoparticle systems: Case of silver nanoparticles prepared by microemulsion route. Colloids Surf. A Physicochem. Eng. Asp. 2012, 404, 83–92. [Google Scholar] [CrossRef]
- Sorensen, L.K.; Khrennikov, D.E.; Gerasimov, V.S.; Ershov, A.E.; Polyutov, S.P.; Karpov, S.V.; Ågren, H. Nature of the anomalous size dependence of resonance red shifts in ultrafine plasmonic nanoparticles. J. Phys. Chem. C 2022, 126, 16804–16814. [Google Scholar] [CrossRef]
- Szanto, G.; Pritzke, P.; Kluitmann, J.J.; Köhler, J.M.; Csáki, A.; Fritzsche, W.; Csarnovics, I.; Bonyár, A. Optimization of the bulk refractive index sensitivity of silver nanoprisms. Adv. Opt. Mater. 2024, 12, 2302967. [Google Scholar] [CrossRef]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef]
- Hassellöv, M.; Readman, J.W.; Ranville, J.F.; Tiede, K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology 2008, 17, 344–361. [Google Scholar] [CrossRef]
- Powers, K.W.; Carpinone, P.L.; Siebein, K.N. Characterization of nanomaterials for toxicological studies. Methods Mol. Biol. 2012, 926, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Domingos, R.F.; Baalousha, M.A.; Ju-Nam, Y.; Reid, M.M.; Tufenkji, N.; Lead, J.R.; Leppard, G.G.; Wilkinson, K.J. Characterizing manufactured nanoparticles in the environment: Multimethod determination of particle sizes. Environ. Sci. Technol. 2009, 43, 7277–7284. [Google Scholar] [CrossRef] [PubMed]
- Mota, W.S.; Severino, P.; Kadian, V.; Rao, R.; Zielińska, A.; Silva, A.M.; Mahant, S.; Souto, E.B. Nanometrology: Particle sizing and influence on the toxicological profile. Front. Nanotechnol. 2025, 7, 1479464. [Google Scholar] [CrossRef]
- Pabisch, S.; Feichtenschlager, B.; Kickelbick, G.; Peterlik, H. Effect of interparticle interactions on size determination of zirconia and silica based systems—A comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 2012, 521, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, E.V.; Kuznetsov, N.M.; Kalinin, K.T.; Lebedev-Stepanov, P.V.; Novikov, A.A.; Chvalun, S.N. The role of integrated approach in the determination of nanoparticle sizes in dispersions. Colloid J. 2022, 84, 704–714. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. American Society for Microbiology. 2009. Available online: https://asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/kirby-bauer-disk-diffusion-susceptibility-test-protocol-pdf (accessed on 8 August 2025).
- Kapoor, S.; Sood, H.; Saxena, S.; Chaurasia, O.P. Green synthesis of silver nanoparticles using Rhodiola imbricata and Withania somnifera root extract and their potential catalytic, antioxidant, cytotoxic and growth-promoting activities. Bioprocess Biosyst. Eng. 2022, 45, 365–380. [Google Scholar] [CrossRef]
- Sreckovic, N.Z.; Nedic, Z.P.; Monti, D.M.; D’Elia, L.; Dimitrijevic, S.B.; Mihailovic, N.R.; Katanic Stankovic, J.S.; Mihailovic, V.B. Biosynthesis of silver nanoparticles using Salvia pratensis L. aerial part and root extracts: Bioactivity, biocompatibility, and catalytic potential. Molecules 2023, 28, 1387. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 318. [Google Scholar] [CrossRef]
- De Leersnyder, I.; Rijckaert, H.; De Gelder, L.; Van Driessche, I.; Vermeir, P. High variability in silver particle characteristics, silver concentrations, and production batches of commercially available products indicates the need for a more rigorous approach. Nanomaterials 2020, 10, 1394. [Google Scholar] [CrossRef]
- Prabhu, Y.T.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano. Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Xie, J.; Lee, J.Y.; Wang, D.I.C.; Ting, Y.P. Silver nanoplates: From biological to biomimetic synthesis. ACS Nano 2007, 1, 429–439. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Pannerselvam, B.; Durai, P.; Thiyagarajan, D.; Song, H.J.; Kim, K.J.; Jung, Y.S.; Kim, H.J.; Rangarajulu, S.K. Facile synthesis of silver nanoparticles using Asian Spider flower and its in vitro cytotoxic activity against human breast carcinoma cells. Processes 2020, 8, 430. [Google Scholar] [CrossRef]
- Razack, S.A.; Suresh, A.; Sriram, S.; Ramakrishnan, G.; Sadanandham, S.; Veerasamy, M.; Nagalamadaka, R.B.; ·Sahadevan, R. Green synthesis of iron oxide nanoparticles using Hibiscus rosa-sinensis for fortifying wheat biscuits. SN Appl. Sci. 2020, 2, 898. [Google Scholar] [CrossRef]
- Cloutis, E.A.; McCormack, K.A.; Bell, J.F., III; Hendrix, A.R.; Bailey, D.T.; Craig, M.A.; Mertzman, S.A.; Robinson, M.S.; Riner, M.A. Ultraviolet spectral reflectance properties of common planetary minerals. Icarus 2008, 197, 321–347. [Google Scholar] [CrossRef]
- Rajendran, K.; Balakrishnan, G.S.; Kalirajan, J. Synthesis of magnetite nanoparticles for arsenic removal from ground water pond. Int. J. PharmTech Res. 2015, 8, 670–677. [Google Scholar]
- Ma, S.; Zhan, S.; Jia, Y.; Zhou, Q. Superior antibacterial activity of Fe3O4-TiO2 nanosheets under solar light. ACS Appl. Mater. Interfaces 2015, 7, 21875–21883. [Google Scholar] [CrossRef]
- Yang, R.; Liang, B.; Han, D.; Guo, Z.; Yang, C.; Yang, J.; Qiu, Y.; Li, Q.; Guo, S.; Shi, J.; et al. Synthesis and antibacterial activity of magnetic Fe3O4-loaded silver nanocomposites. J. Alloys Compd. 2024, 973, 172849. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzan, L.M. Formation of PVP-protected metal nanoparticles in DMF. Langmuir 2002, 18, 2888–2894. [Google Scholar] [CrossRef]
- Haes, A.J.; Stuart, D.A.; Nie, S.; Van Duyne, R.P. Using solution-phase nanoparticles, surface-confined nanoparticle arrays and single nanoparticles as biological sensing platforms. J. Fluoresc. 2004, 14, 355–367. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Qian, X.; Yin, J.; Zhu, Z. Preparation of polychrome silver nanoparticles in different solvents. J. Mater. Chem. 2002, 12, 3783–3786. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef]
- Antropova, I.G.; Revina, A.A.; Oo, P.M.; Kurakina, E.S.; Butorova, I.A.; Magomedbekov, E.P. Synthesis of silver nanoparticles using reactive water−ethanol extracts from Murraya paniculata. ACS Omega 2021, 6, 8313–8321. [Google Scholar] [CrossRef]
- Lee, J.-M.; Lim, D.-S.; Jeon, S.-H.; Hur, D.H. Zeta potentials of magnetite particles and alloy 690 surfaces in alkaline solutions. Materials 2020, 13, 3999. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Arias, J.L.; Reddy, L.H.; Couvreur, P. Magnetoresponsive squalenoyl gemcitabine composite nanoparticles for cancer active targeting. Langmuir 2008, 24, 7512. [Google Scholar] [CrossRef]
- Jorge, M.J.; Nilson, M.C.; Aracely, H.R.; Machuca-Martínez, F. Data on the removal of metals (Cr3+, Cr6+, Cd2+, Cu2+, Ni2+, Zn2+) from aqueous solution by adsorption using magnetite particles from electrochemical synthesis. Data Brief 2019, 24, 103956. [Google Scholar] [CrossRef]
- Singh, S.; Arya, H.; Sahu, W.; Reddy, K.S.; Nimesh, S.; Alotaibi, B.S.; Kumar Bhatt, T. Green synthesized silver nanoparticles of Terminalia bellirica leaves extract: Synthesis, characterization, in-silico studies, and antimalarial activity. Artif. Cells Nanomed. Biotechnol. 2024, 52, 238–249. [Google Scholar] [CrossRef]
- Khane, Y.; Benouis, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Al Ali, A.; Aouf, D.; Fenniche, F.; Khane, S.; Chaibi, W.; et al. Green synthesis of silver nanoparticles using aqueous Citrus limon zest extract: Characterization and evaluation of their antioxidant and antimicrobial properties. Nanomaterials 2022, 12, 2013. [Google Scholar] [CrossRef]
- Shanwaz, M.M.; Shyam, P. Synthesis of Silver nanoparticles from Vitex negundo plant by green method and their bactericidal effects. Lett. Appl. NanoBioSci. 2023, 12, 59. [Google Scholar] [CrossRef]
- Dabagh, S.; Haris, S.A.; Isfahani, B.K.; Ertas, Y.N. Silver-decorated and silica-capped magnetite nanoparticles with effective antibacterial activity and reusability. ACS Appl. Bio Mater. 2023, 6, 2266–2276. [Google Scholar] [CrossRef] [PubMed]
- Shameli, K.; Ahmad, M.B.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef] [PubMed]
- Bandoniene, D.; Venskutonis, P.; Gruzdiene, D.; Murkovic, M. Antioxidative activity of Sage (Salvia officinalis L.), Savory (Satureja hortensis L.) and Borage (Borago officinalis L.) extracts in rapeseed oil. Eur. J. Lipid Sci. Technol. 2002, 104, 286–292. [Google Scholar] [CrossRef]
- Zaguła, G.; Fabisiak, A.; Bajcar, M.; Czernicka, M.; Saletnik, B.; Puchalski, C. Mineral components analysis of selected dried herbs. EconTechMod—Int. Q. J. 2016, 5, 127–132. [Google Scholar]
- Mot, M.-D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.-M.; Copolovici, L.; Copolovici, D.M.; et al. Salvia officinalis L. essential oil: Characterization, antioxidant properties, and the effects of aromatherapy in adult patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef]
- Koçak, M.Z.; Karadağ, M.; Çelikcan, F. Essential oil composition of Salvia officinalis and Rosmarinus officinalis. J. Agric. 2021, 4, 39–47. [Google Scholar] [CrossRef]
- Gogoasa, I.; Jurca, V.; Alda, L.M.; Velciov, A.; Rada, M.; Alda, S.; Sirbulescu, C.; Bordean, D.M.; Gergen, I. Mineral content of some medicinal herbs. J. Hort. Forest. Biotechnol. 2013, 17, 65–67. Available online: https://www.usab-tm.ro/Journal-HFB/2013/Lista%20Lucrari%20PDF/Lucrari%20Vol%2017(4)%20PDF/14.pdf (accessed on 5 August 2025).
- Zhang, M.; Chu, L.; Chen, J.; Qi, F.; Li, X.; Chen, X.; Yu, D.-G. Asymmetric wettability fibrous membranes: Preparation and biologic applications. Compos. Part B 2024, 269, 111095. [Google Scholar] [CrossRef]
- Sun, L.; Guo, J.; Chen, H.; Zhang, D.; Shang, L.; Zhang, B.; Zhao, Y. Tailoring materials with specific wettability in biomedical engineering. Adv. Sci. 2021, 8, 2100126. [Google Scholar] [CrossRef] [PubMed]
- Erbil, Y. Contact angle of liquid drops on solids. In Surface Chemistry of Solid and Liquid Interfaces; Erbil, Y., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 308–334. [Google Scholar]
- Kumar, R.; Sahani, A.K. Role of superhydrophobic coatings in biomedical applications. Mater. Today Proc. 2021, 45, 5655–5659. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, Q.; Yu, H.; Shao, L.; Cui, X.; Pang, Q.; Zhu, Y.; Hou, R. Construction methods and biomedical applications of PVA-based hydrogels. Front. Chem. 2024, 12, 1376799. [Google Scholar] [CrossRef]
- Ahmad, D.; van den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I. Modification of the wettability of polymer surfaces using nanoparticles. Prog. Org. Coat. 2014, 77, 331–338. [Google Scholar] [CrossRef]
- Dubey, P.; Matai, I.; Kumar, S.U.; Sachdev, A.; Bhushan, B.; Gopinath, P. Perturbation of cellular mechanistic system by silver nanoparticle toxicity: Cytotoxic, genotoxic and epigenetic potentials. Adv. Colloid Interface Sci. 2015, 221, 4–21. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Girma, A.; Abera, B.; Mekuye, B.; Mebratie, G. Antibacterial activity and mechanisms of action of inorganic nanoparticles against foodborne bacterial pathogens: A systematic review. IET Nanobiotechnol. 2024, 2024, 5417924. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Webster, T.J. The use of superparamagnetic nanoparticles for prosthetic biofilm. Int. J. Nanomed. 2009, 4, 145–152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Bahari, A.; Roeinfard, M.; Ramzannezhad, A.; Khodabakhshi, M.; Mohseni, M. Nanostructured features and antimicrobial properties of Fe3O4/ZnO nanocomposites. Natl. Acad. Sci. Lett. 2019, 42, 9–12. [Google Scholar] [CrossRef]
- Maher, C.; Hassan, K.A. The Gram-negative permeability barrier: Tipping the balance of the in and the out. mBio 2023, 14, e01205-23. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]


| Composite | Cellulose Support | Dispersant for Inorganic Particles | A-Type AgPs | B-Type AgPs | MG | PVA aq. sol. |
|---|---|---|---|---|---|---|
| CPZ-1 | medical gauze | H2O | x | x | x | |
| CPZ-2 | H2O | x | x | x | ||
| CPZ-3 | EtOH | x | x | x | ||
| CPZ-4 | EtOH | x | x | x | ||
| CZH-1 | filter paper | H2O | x | x | x | |
| CZH-2 | H2O | x | x | x | ||
| CZH-3 | EtOH | x | x | x | ||
| CZH-4 | EtOH | x | x | x |
| Sample | A-Type AgPs | B-Type AgPs | MG | |
| Elements | ||||
| C (K) | 22.03 | 19.28 | - | |
| O (K) | 13.27 | 13.63 | 18.82 | |
| Ag (L) | 64.69 | 67.08 | - | |
| Fe (K) | - | - | 81.17 | |
| Sample | Solvent | Z-Average Diameter (nm) | PdI | ZP (mV) |
|---|---|---|---|---|
| MG | H2O | 450 ± 26 | 0.55 ± 0.05 | −21.8 ± 0.3 |
| A-type AgPs | 224 ± 4 | 0.37 ± 0.01 | −25.3 ± 0.7 | |
| B-type AgPs | 287 ± 19 | 0.50 ± 0.08 | −20.7 ± 0.9 | |
| MIX 1 (A-type AgPs+MG) | 105 ± 0.87 | 0.37 ± 0.01 | −28.3 ± 1.1 | |
| MIX 2 (B-type AgPs+MG) | 132 ± 1.77 | 0.48 ± 0.30 | −26.8 ± 2.3 | |
| MG | EtOH | 1451 ± 110 | 0.93 ± 0.07 | +17.8 ± 0.4 |
| A-type AgPs | 2513 ± 130 | 0.31 ± 0.04 | −2.0 ± 0.4 | |
| B-type AgPs | 3051 ± 175 | 0.40 ± 0.06 | −1.9 ± 0.3 | |
| MIX 3 (A-type AgPs+MG) | 1116 ± 107 | 0.40 ± 0.05 | −17.2 ± 2.2 | |
| MIX 4 (B-type AgPs+MG) | 782 ± 78 | 0.65 ± 0.01 | −21.1 ± 0.4 |
| MG (lit.) | AgNPs (lit.) | MG * | A-Type AgPs * | B-Type AgPs * | MIX 1 * | MIX2 * | MG ** | A-Type AgPs ** | B-Type AgPs ** | MIX 3 ** | MIX 4 ** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 839 | 838 | 841 | |||||||||
| 800–700 Ag-N st.v. | 803 | 805 | 808 | 799 | 801 | 804 | 793 | 795 | 802 | 802 | |
| 723 | 723 | 720 | 723 | 724 723 | |||||||
| 692 | 690 | 678 | 701 | ||||||||
| 650–550 Ag-Cl st.v. | 658 | 656 | 654 | 654 | 667 | ||||||
| 639 | 635 | ||||||||||
| 621 | 619 | 621 | 617 | 620 | 623 | 625 | 624 | 623 | |||
| 604 | |||||||||||
| 540–500 Fe-O st.v. | 573 | 584 | 590 | 575 | 586 | ||||||
| 569 | 569 | 556 | 562 | 562 | |||||||
| 500–480 Fe-O st.v. | 500–400 Ag-O st.v. | 451 | 457 | 457 | 453 | 453 | 452 | 450 | 450 | ||
| 432 | 432 (low) | ||||||||||
| 417 | 419 | 420 (low) | |||||||||
| 400 | 400 | ||||||||||
| 396 | 392 | 396 (low) | 396 | 395 | |||||||
| 381 |
| Sample | Contact Angle (θ°) |
|---|---|
| CPZ-M | 111.86 ± 1.26 |
| CPZ-1 | 104.75 ± 0.36 |
| CPZ-2 | 112.56 ± 0.36 |
| CPZ-3 | 111.40 ± 0.68 |
| CPZ-4 | 115.88 ± 0.28 |
| CZH-M | 91.33 ± 1.09 |
| CZH-1 | 98.42 ± 1.03 |
| CZH-2 | 106.36 ± 0.99 |
| CZH-3 | 116.14 ± 0.40 |
| CZH-4 | 117.51 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoras, A.G.; Popescu, I.; Gradinaru, L.M.; Mihalache, G.; Lipsa, F.D.; Nica, S.L.; Grigoras, V.C. PVA-Cellulose Fibers Composites Impregnated with Antimicrobial Particles: The Solvent Effect. Polymers 2025, 17, 2456. https://doi.org/10.3390/polym17182456
Grigoras AG, Popescu I, Gradinaru LM, Mihalache G, Lipsa FD, Nica SL, Grigoras VC. PVA-Cellulose Fibers Composites Impregnated with Antimicrobial Particles: The Solvent Effect. Polymers. 2025; 17(18):2456. https://doi.org/10.3390/polym17182456
Chicago/Turabian StyleGrigoras, Anca Giorgiana, Irina Popescu, Luiza Madalina Gradinaru, Gabriela Mihalache, Florin Daniel Lipsa, Simona Luminita Nica, and Vasile Cristian Grigoras. 2025. "PVA-Cellulose Fibers Composites Impregnated with Antimicrobial Particles: The Solvent Effect" Polymers 17, no. 18: 2456. https://doi.org/10.3390/polym17182456
APA StyleGrigoras, A. G., Popescu, I., Gradinaru, L. M., Mihalache, G., Lipsa, F. D., Nica, S. L., & Grigoras, V. C. (2025). PVA-Cellulose Fibers Composites Impregnated with Antimicrobial Particles: The Solvent Effect. Polymers, 17(18), 2456. https://doi.org/10.3390/polym17182456







