Engineering Poly(L-Lactic Acid)/Hydroxyapatite Scaffolds via Melt-Electrowriting: Enhancement of Osteochondral Cell Response in Human Nasal Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Composite Materials
2.2. Melt-Electrowriting
2.3. Characterization Techniques
2.3.1. Confocal Imaging
2.3.2. Scanning Electron Microscope
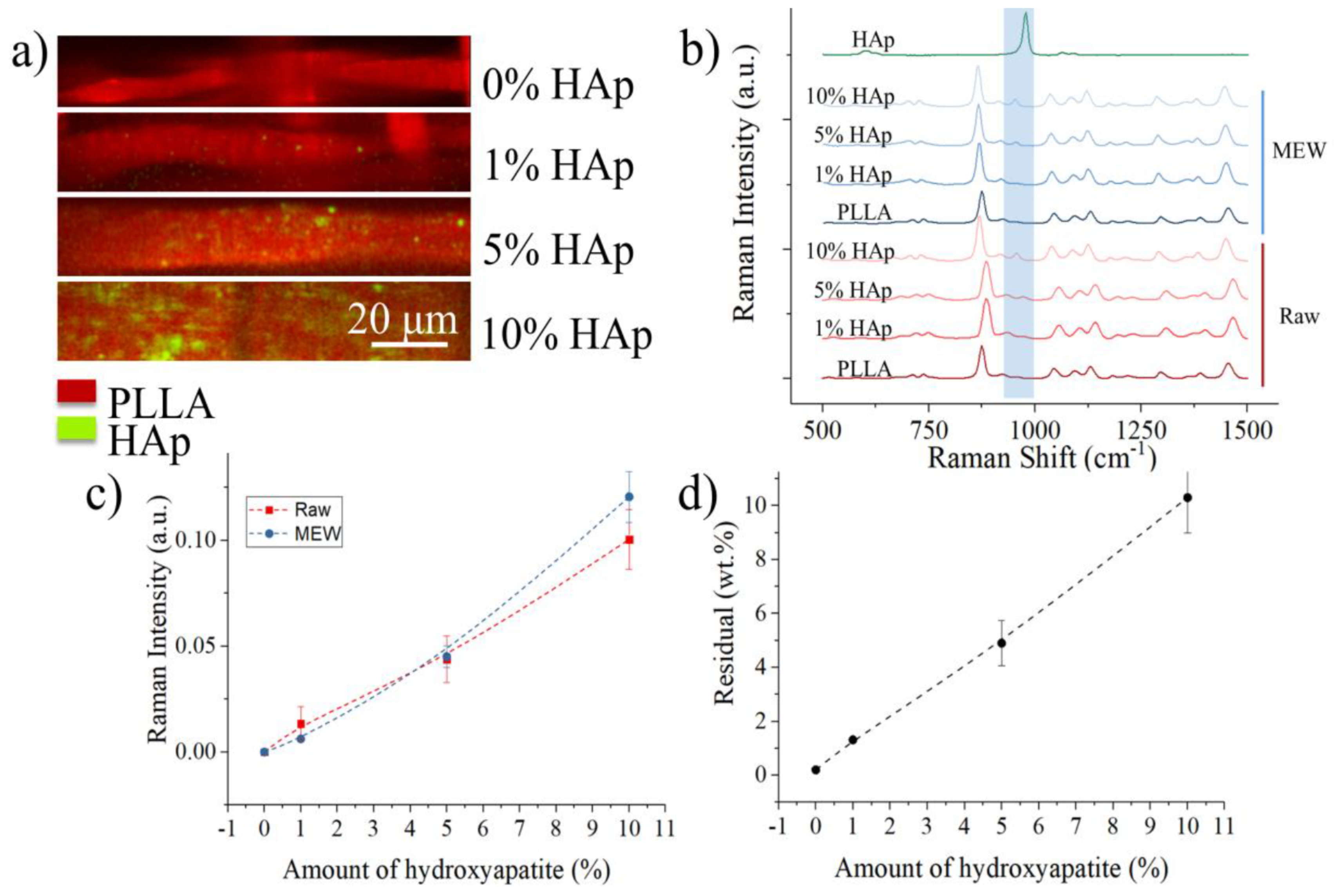
2.3.3. Raman Spectroscopy
2.3.4. X-ray Diffraction
2.3.5. Thermogravimetric Analysis
2.4. Biological Testing
2.4.1. Cell Culture for Toxicity Evaluation
2.4.2. Cell Culture for Osteochondral Interface Differentiation
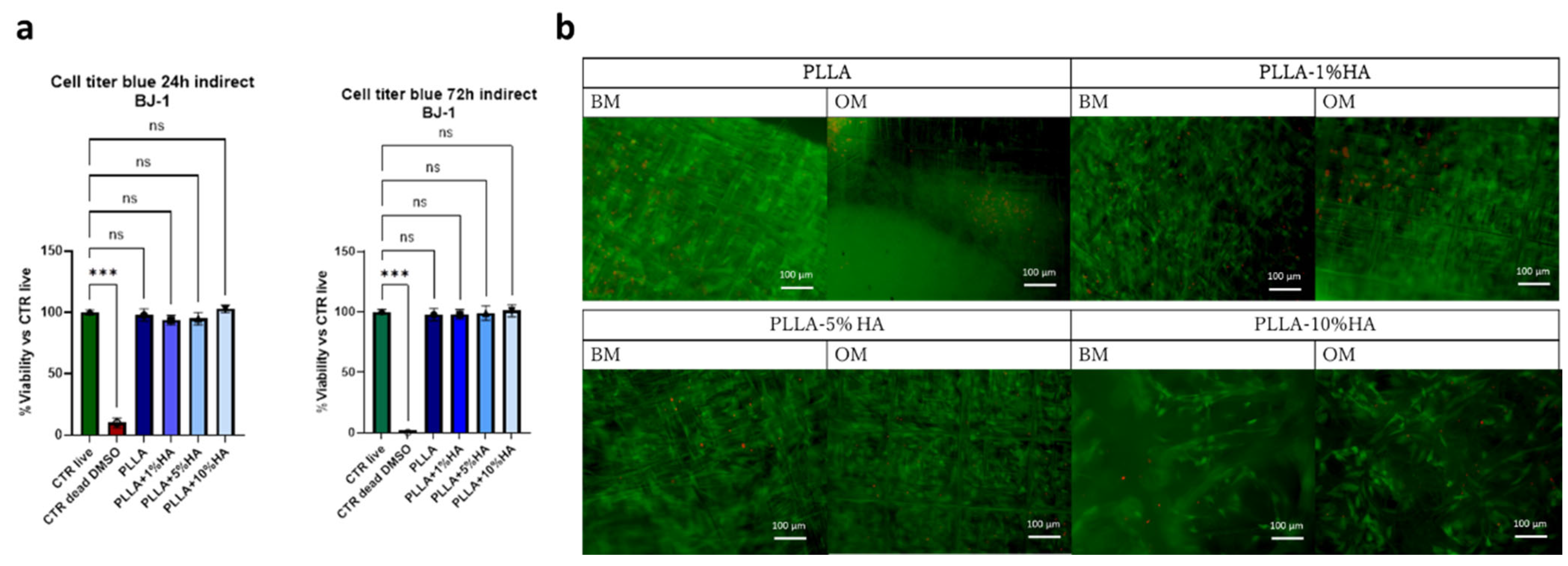
2.4.3. Metabolic Activity and Indirect Toxicity Test
2.4.4. Nasal Chondrocytes Cell Adhesion and Live and Dead
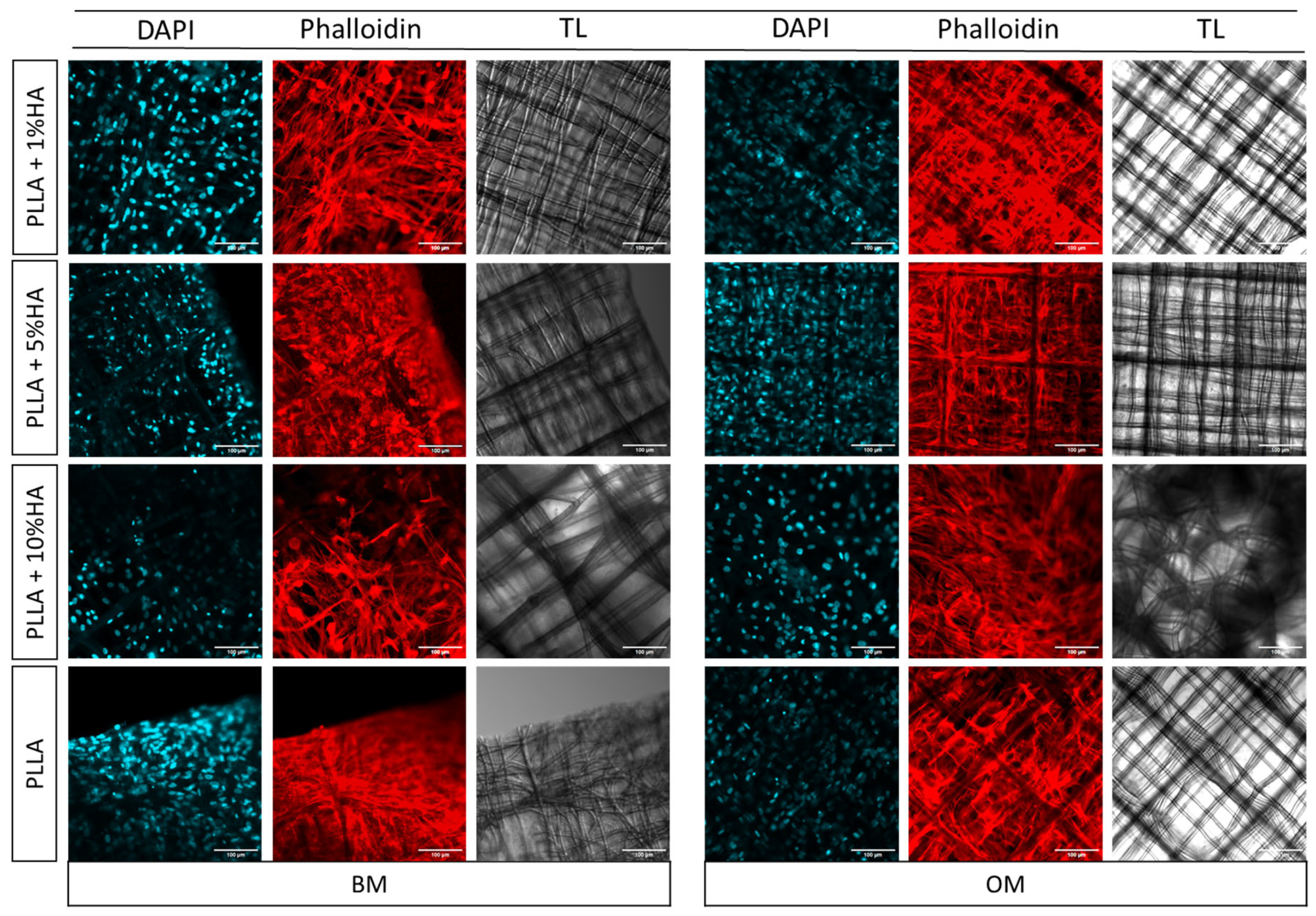
2.4.5. Nasal Chondrocytes Phalloidin Staining (Morphology)
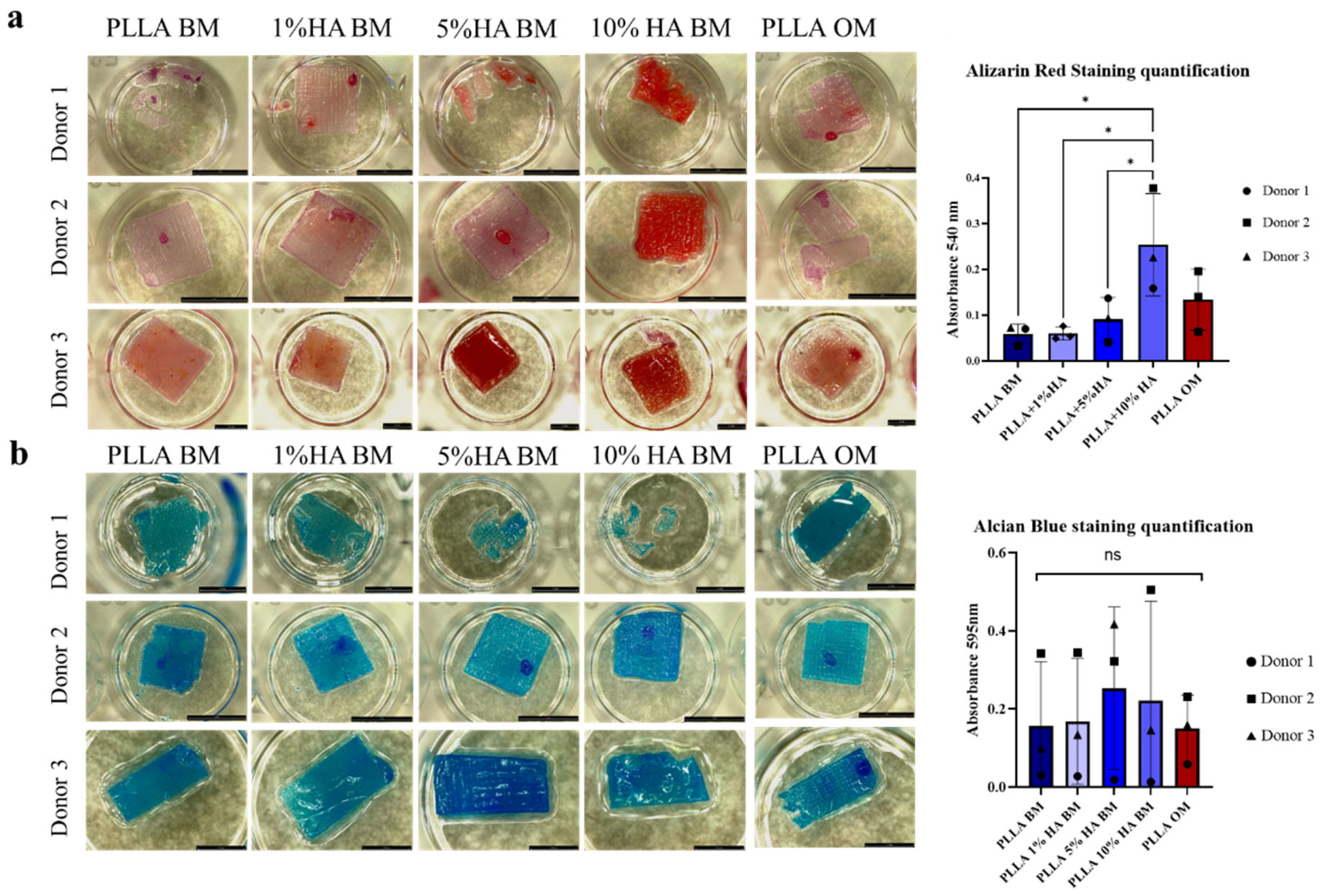
2.4.6. Alizarin Red Staining
2.4.7. Alcian Blue Staining
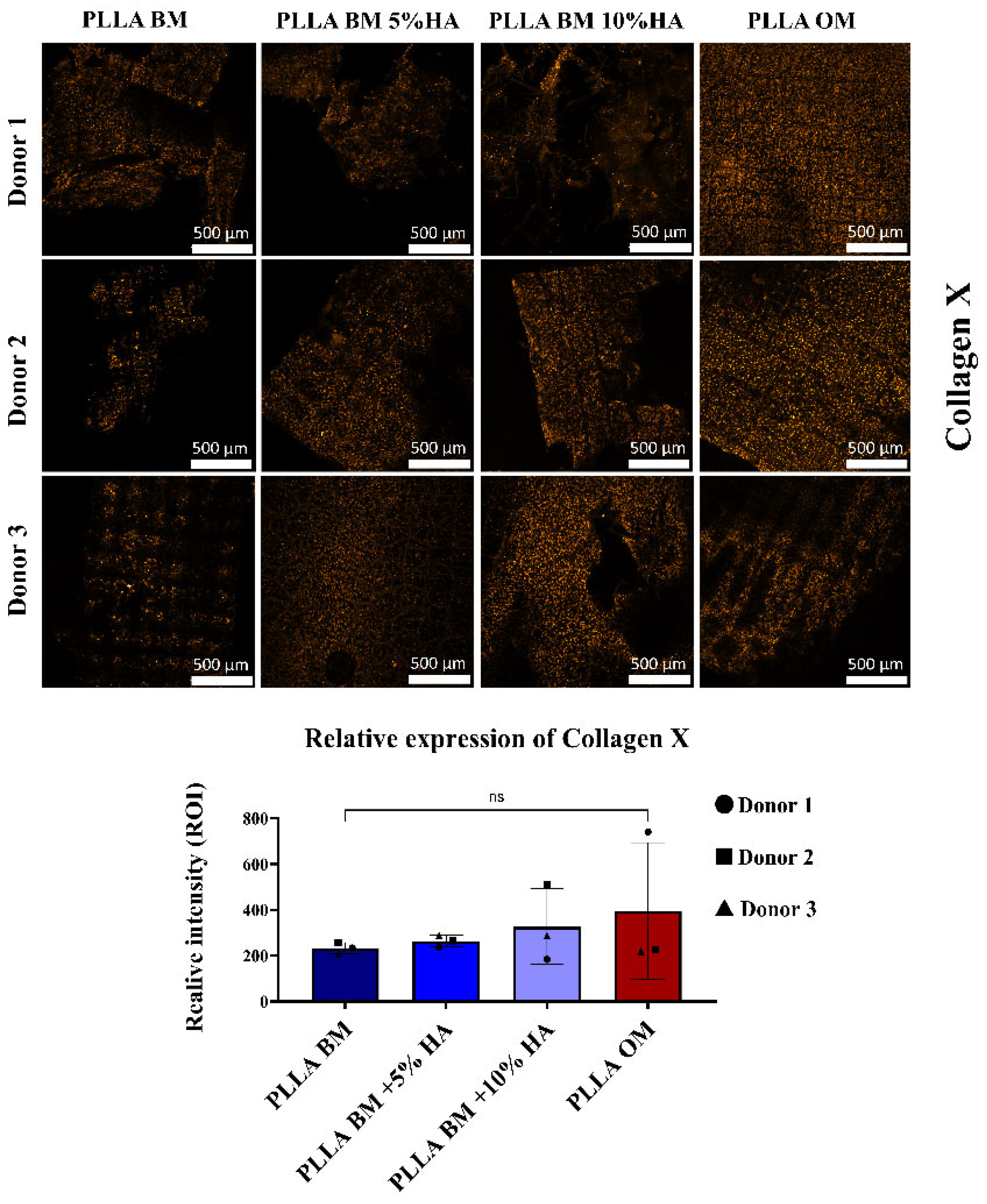
2.4.8. Immunofluorescence
2.5. Imaging Analysis
2.6. Statistical Analysis
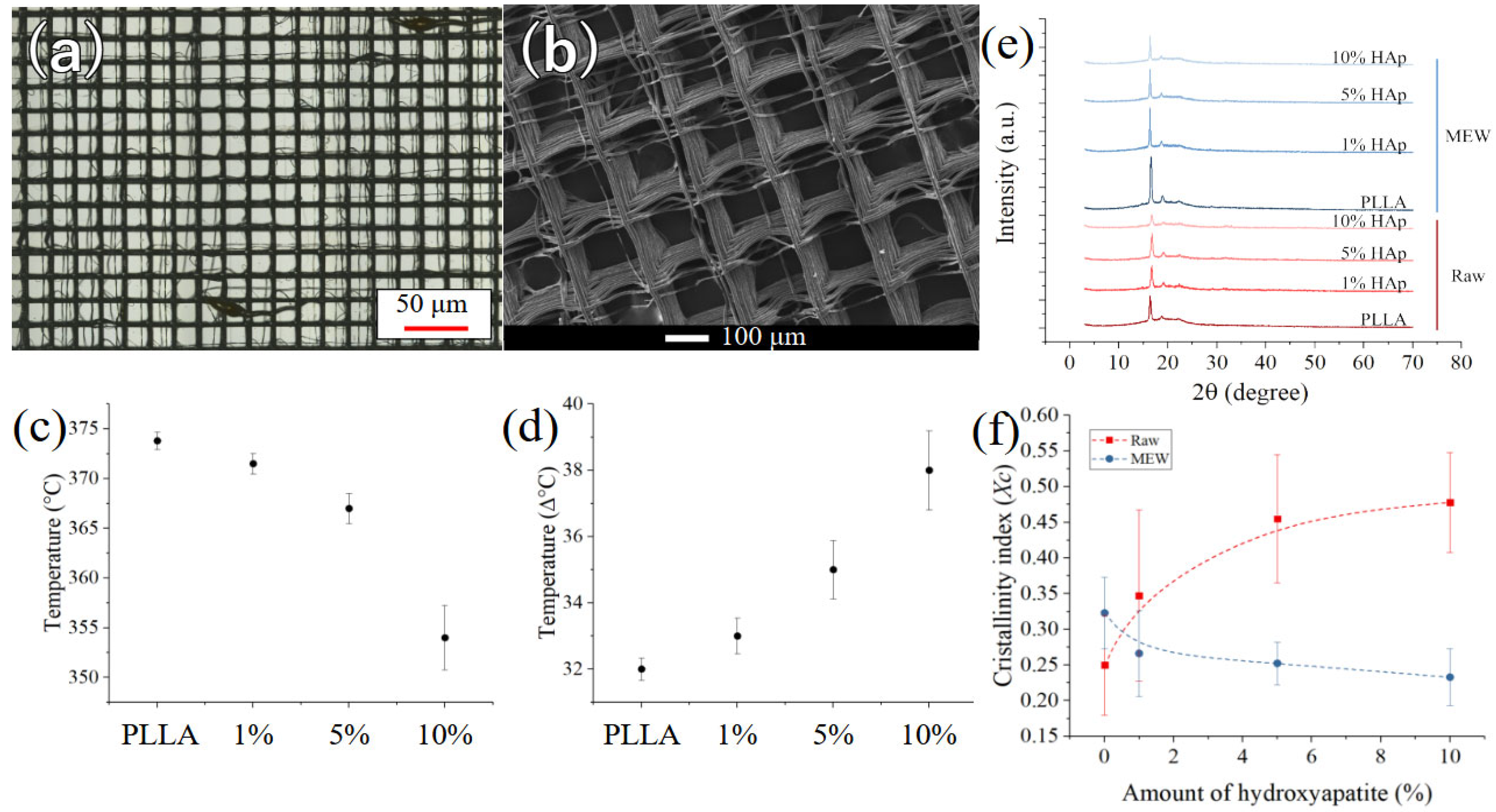
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Basal medium |
| BSA | Bovine serum albumin |
| CCD | Charge-coupled device |
| COL10 | Collagen X |
| CTR | Control |
| DMEM | Dulbecco’s modified Eagle medium |
| DSC | Differential scanning calorimetry |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| HAp | Hydroxyapatite |
| hNCs | Nasal condrocytes |
| MEW | Melt-electrowriting |
| OM | Osteogenic medium |
| PBS | Phosphate-buffer saline |
| PLLA | Poly-l-lactic acid |
| ROIs | Regions of interest |
| SEM | Scanning electron microscope |
| TGA | Thermogravimetric analysis |
| XRD | X-ray Diffraction |
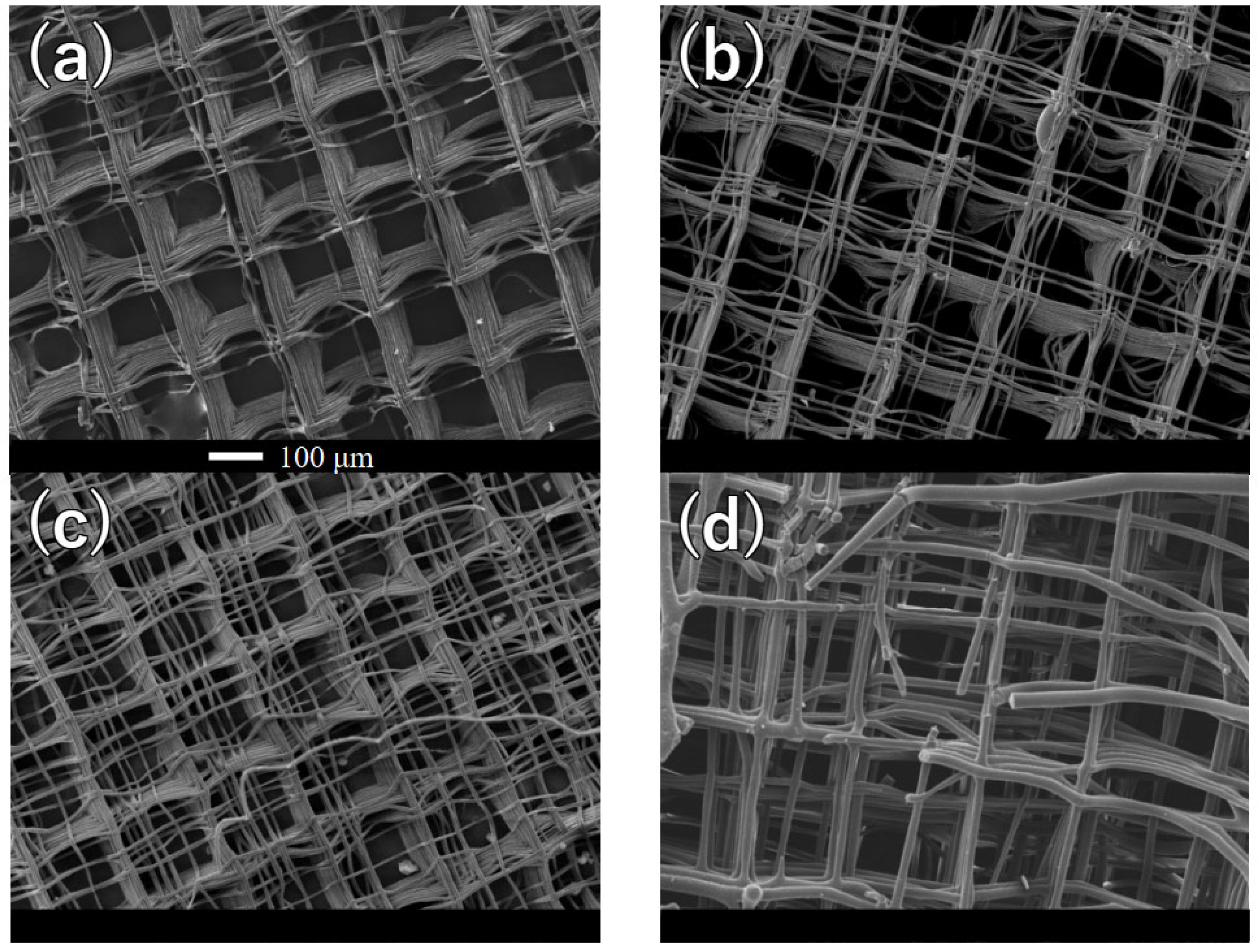
Appendix A. Morphology

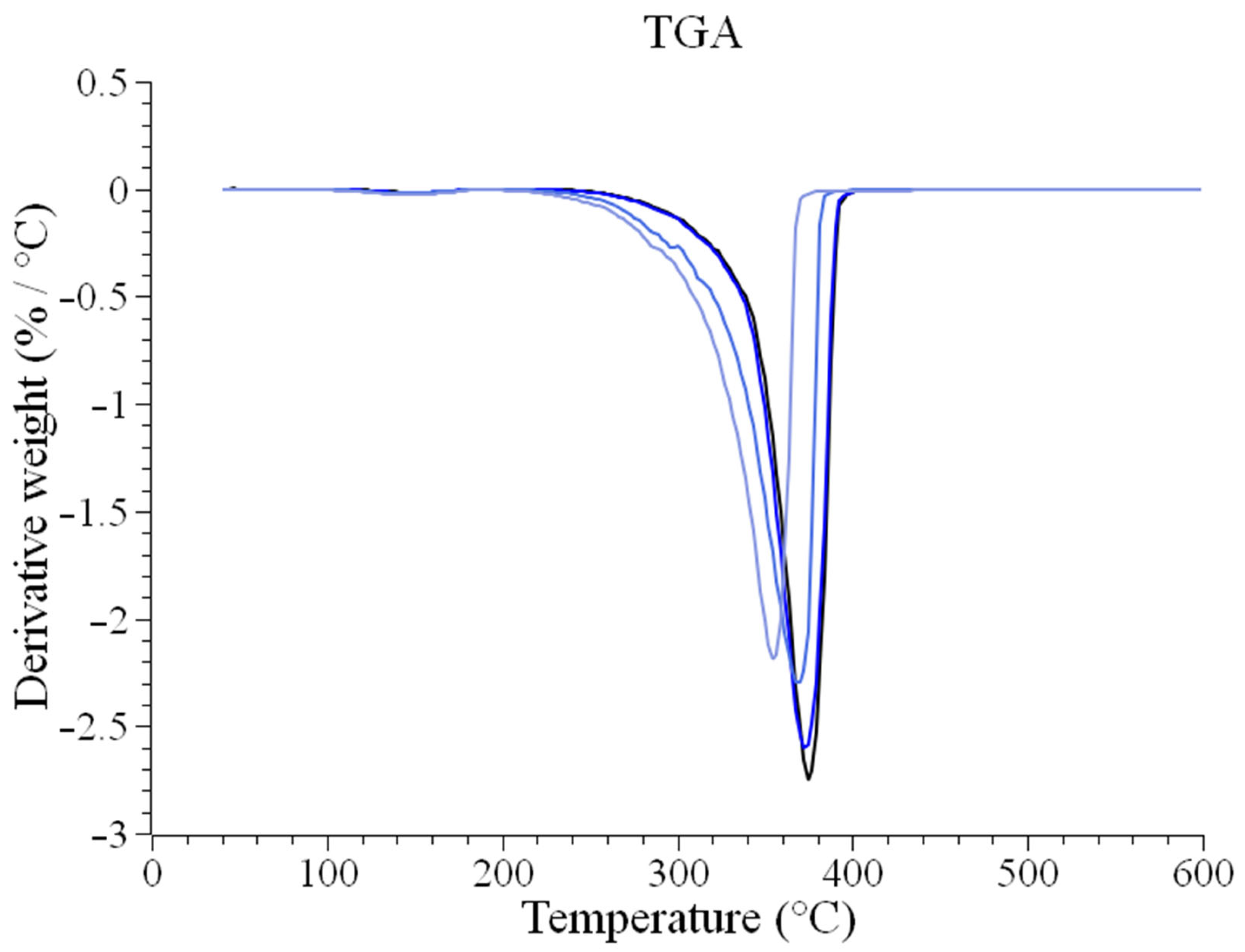
Appendix B. Thermogravimetric Analysis
Appendix C. Spectroscopic Characterization
| Band Position [cm−1] | Assignment | Material [51,52,53,54] |
|---|---|---|
| ~1450 | CH3 asymmetric stretch | PLLA |
| ~1388 | CH3 symmetric stretch | PLLA |
| ~1365 | CH deformation and CH3 symmetric deformation | PLLA |
| ~1295 | CH deformation | PLLA |
| ~1220 | COC asymmetric stretching | PLLA |
| ~1180 | COC asymmetric stretch | PLLA |
| ~1127 | CH3 asymmetric rocking | PLLA |
| ~1095 | COC symmetric stretch | PLLA |
| ~1076 | PO43− symmetric stretching mode | HAp |
| ~1049 | PO43− asymmetric stretching | HAp |
| ~1044 | C-CH3 stretch | PLLA |
| ~960 | PO43− symmetric stretching | HAp |
| ~950 | CC stretching and CH3 rocking | PLLA |
| ~920 | CC stretching and CH3 rocking | PLLA |
| ~870 | C-COO stretch | PLLA |
| ~737 | C=O deformation (in-plane) | PLLA |
| ~708 | C=O deformation (out-of-plane) | PLLA |
| ~610 | PO43− asymmetric bending | HAp |
| ~592 | PO43− asymmetric bending | HAp |
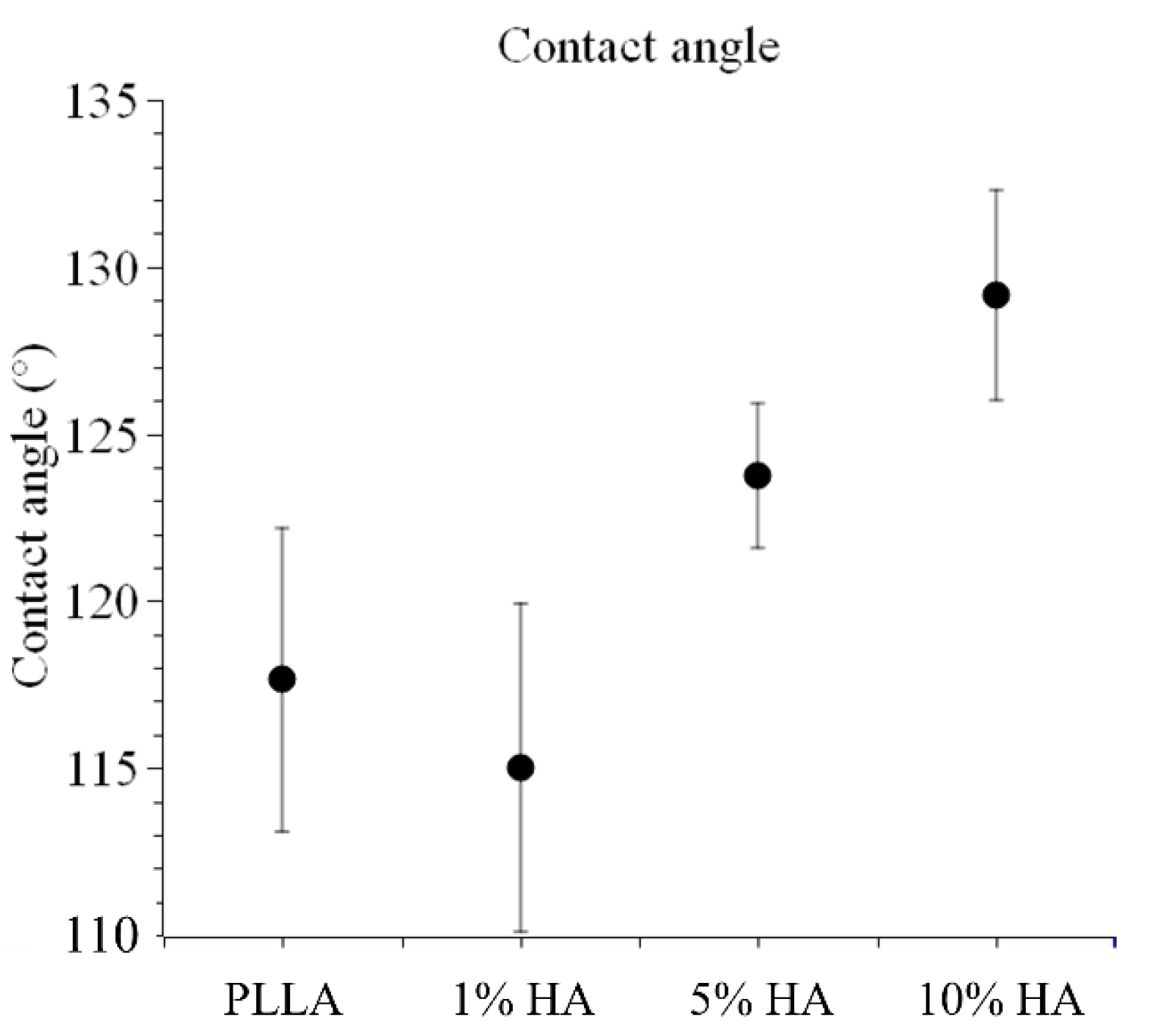
Appendix D. Contact Angle
Appendix E. Macro Script for Fijii ImageJ
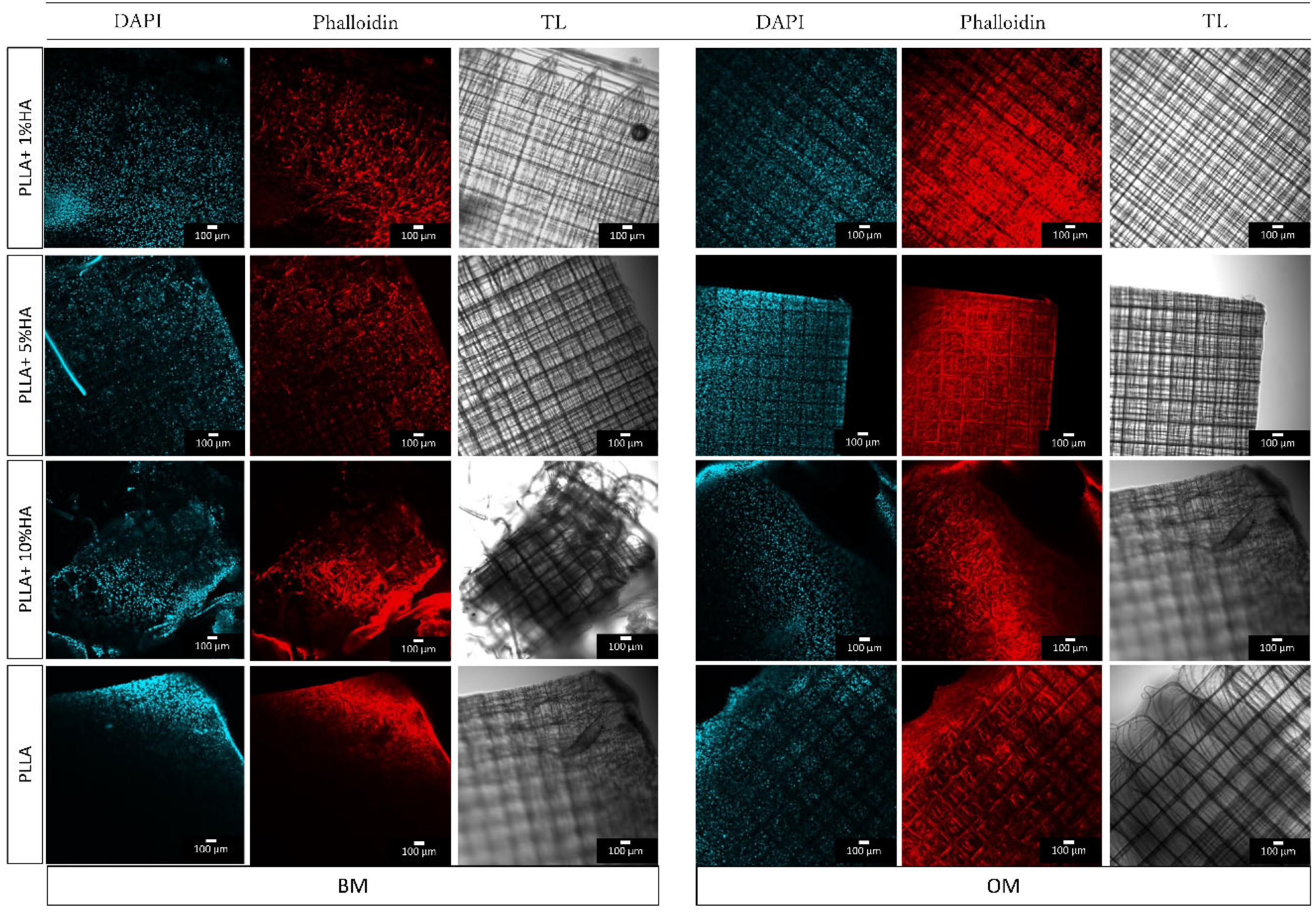
Appendix F
References
- Tuan, R.S.; Chen, A.F.; Klatt, B.A. Cartilage regeneration. JAAOS J. Am. Acad. Orthop. Surg. 2013, 21, 303–311. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Walter, S.G.; Ossendorff, R.; Schildberg, F.A. Articular cartilage regeneration and tissue engineering models: A systematic review. Arch. Orthop. Trauma Surg. 2019, 139, 305–316. [Google Scholar] [CrossRef] [PubMed]
- O’driscoll, S.W. Current concepts review-the healing and regeneration of articular cartilage. Jbjs 1998, 80, 1795–1812. [Google Scholar] [CrossRef]
- Guo, X.; Xi, L.; Yu, M.; Fan, Z.; Wang, W.; Ju, A.; Liang, Z.; Zhou, G.; Ren, W. Regeneration of articular cartilage defects: Therapeutic strategies and perspectives. J. Tissue Eng. 2023, 14, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Andrades, J.A.; Guerado, E.; Zamora-Navas, P.; López-Puertas, J.M.; Reddi, A.H. Articular Cartilage: Structure and Regeneration. Tissue Eng. Part B Rev. 2010, 16, 617–627. [Google Scholar] [CrossRef]
- Rosa, D.; Di Donato, S.; Balato, G.; D’Addona, A.; Smeraglia, F.; Correra, G.; Di Vico, G. How to Manage a Failed Cartilage Repair: A Systematic Literature Review. Joints 2017, 5, 093–106. [Google Scholar] [CrossRef]
- Khan, I.M.; Gilbert, S.J.; Singhrao, S.K.; Duance, V.C.; Archer, C.W. Cartilage integration: Evaluation of the reasons for failure of integration during cartilage repair. A review. Eur. Cells Mater. 2008, 16, 26–39. [Google Scholar] [CrossRef]
- Zuncheddu, D.; Della Bella, E.; Petta, D.; Bärtschi, C.; Häckel, S.; Deml, M.C.; Stoddart, M.J.; Grad, S.; Basoli, V. Effect of glucose depletion and fructose administration during chondrogenic commitment in human bone marrow-derived stem cells. Stem Cell Res. Ther. 2022, 13, 533. [Google Scholar] [CrossRef]
- Kovermann, N.; Basoli, V.; Della Bella, E.; Alini, M.; Lischer, C.; Schmal, H.; Kubosch, E.; Stoddart, M. BMP2 and TGF-β cooperate differently during synovial-derived stem-cell chondrogenesis in a dexamethasone-dependent manner. Cells 2019, 8, 636. [Google Scholar] [CrossRef]
- Basoli, V.; Della Bella, E.; Kubosch, E.J.; Alini, M.; Stoddart, M.J. Effect of expansion media and fibronectin coating on growth and chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Sci. Rep. 2021, 11, 13089. [Google Scholar] [CrossRef] [PubMed]
- Erggelet, C.; Vavken, P. Anon Microfracture for the treatment of cartilage defects in the knee joint-A golden standard? J. Clin. Orthop. Trauma 2016, 7, 145–152. [Google Scholar] [CrossRef]
- Mumme, M.; Steinitz, A.; Nuss, K.M.; Klein, K.; Feliciano, S.; Kronen, P.; Jakob, M.; von Rechenberg, B.; Martin, I.; Barbero, A.; et al. Regenerative potential of tissue-engineered nasal chondrocytes in goat articular cartilage defects. Tissue Eng. Part A 2016, 22, 1286–1295. [Google Scholar] [CrossRef]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzschmar, M.; et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: An observational first-in-human trial. Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef]
- Mumme, M.; Wixmerten, A.; Ivkovic, A.; Peretti, G.M.; Yilmaz, T.; Reppenhagen, S.; Pullig, O.; Miot, S.; Izadpanah, K.; Jakob, M.; et al. Clinical relevance of engineered cartilage maturation in a randomized multicenter trial for articular cartilage repair. Sci. Transl. Med. 2025, 17, eads0848. [Google Scholar] [CrossRef]
- Wixmerten, A.; Miot, S.; Bittorf, P.; Wolf, F.; Feliciano, S.; Hackenberg, S.; Häusner, S.; Krenger, W.; Haug, M.; Martin, I.; et al. Good Manufacturing Practice-compliant change of raw material in the manufacturing process of a clinically used advanced therapy medicinal product-a comparability study. Cytotherapy 2023, 25, 548–558. [Google Scholar] [CrossRef]
- Pelttari, K.; Mumme, M.; Barbero, A.; Martin, I. Nasal chondrocytes as a neural crest-derived cell source for regenerative medicine. Curr. Opin. Biotechnol. 2017, 47, 1–6. [Google Scholar] [CrossRef]
- Cao, Z.; Dou, C.; Dong, S. Scaffolding biomaterials for cartilage regeneration. J. Nanomater. 2014, 2014, 489128. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liang, C.; Zheng, Y.; Wang, L.; Chen, L.; Huang, D.; Gu, Q. Establishing a tube foam scaffold for tracheal cartilage tissue engineering by using solvent casting/particulate leaching method. Chin. J. Tissue Eng. Res. 2007, 53, 6278–6281. [Google Scholar]
- Kim, S.H.; Jung, Y.; Kim, S.H. A Biocompatible Tissue Scaffold Produced by Supercritical Fluid Processing for Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2013, 19, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsson, A.; Stenhamre, H.; Gatenholm, P.; Walkenström, P. Electrospinning of Highly Porous Scaffolds for Cartilage Regeneration. Biomacromolecules 2008, 9, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Wang, Z.; Zheng, G.; Chen, Q.; Luo, E. Application of electrospinning strategy on cartilage tissue engineering. Curr. Stem Cell Res. Ther. 2018, 13, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700298. [Google Scholar] [CrossRef]
- Daly, A.C.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef]
- Grande, D.A.; Breitbart, A.S.; Mason, J.; Paulino, C.; Laser, J.; Schwartz, R.E. Cartilage tissue engineering: Current limitations and solutions. Clin. Orthop. Relat. Res. 1999, 367, S176–S185. [Google Scholar] [CrossRef]
- Meng, J.; Boschetto, F.; Yagi, S.; Marin, E.; Adachi, T.; Chen, X.; Pezzotti, G.; Sakurai, S.; Yamane, H.; Xu, H. Design and manufacturing of 3D high-precision micro-fibrous poly (l-lactic acid) scaffold using melt electrowriting technique for bone tissue engineering. Mater. Des. 2021, 210, 110063. [Google Scholar] [CrossRef]
- Meng, J.; Boschetto, F.; Yagi, S.; Marin, E.; Adachi, T.; Chen, X.; Pezzotti, G.; Sakurai, S.; Yamane, H.; Xu, H. Melt-electrowritten poly (L-lactic acid)-and bioglass-reinforced biomimetic hydrogel for bone regeneration. Mater. Des. 2022, 219, 110781. [Google Scholar] [CrossRef]
- Meng, J.; Boschetto, F.; Yagi, S.; Marin, E.; Adachi, T.; Chen, X.; Pezzotti, G.; Sakurai, S.; Sasaki, S.; Aoki, T. Enhancing the bioactivity of melt electrowritten PLLA scaffold by convenient, green, and effective hydrophilic surface modification. Biomater. Adv. 2022, 135, 112686. [Google Scholar] [CrossRef]
- O’Neill, K.L.; Dalton, P.D. A Decade of Melt Electrowriting. Small Methods 2023, 7, 2201589. [Google Scholar] [CrossRef] [PubMed]
- Saiz, P.G.; Reizabal, A.; Vilas-Vilela, J.L.; Dalton, P.D.; Lanceros-Mendez, S. Materials and Strategies to Enhance Melt Electrowriting Potential. Adv. Mater. 2024, 36, 2312084. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zhang, F.; Zaeri, A.; Zhang, Y.; Zgeib, R.; Calzolaio, M.; Chang, R.C. Advances in design and quality of melt electrowritten scaffoldsK. Mater. Des. 2023, 226, 111618. [Google Scholar] [CrossRef]
- Kade, J.C.; Dalton, P.D. Polymers for Melt Electrowriting. Adv. Healthc. Mater. 2021, 10, 2001232. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-L-lactic acid (PLLA)-based biomaterials for regenerative medicine: A review on processing and applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. 2017, 105, 431–459. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, T.; Shao, M.; Lyu, F. Recent advances in PLLA-based biomaterial scaffolds for neural tissue engineering: Fabrication, modification, and applications. Front. Bioeng. Biotechnol. 2022, 10, 1011783. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly (lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Degradation of poly(L-lactide) films under ultraviolet-induced photografting and sterilization conditions. J. Appl. Polym. Sci. 2007, 106, 1042–1047. [Google Scholar] [CrossRef]
- Ho, M.; Hou, L.; Tu, C.; Hsieh, H.; Lai, J.; Chen, W.; Wang, D. Promotion of Cell Affinity of Porous PLLA Scaffolds by Immobilization of RGD Peptides via Plasma Treatment. Macromol. Biosci. 2006, 6, 90–98. [Google Scholar] [CrossRef]
- Pippenger, B.E.; Ventura, M.; Pelttari, K.; Feliciano, S.; Jaquiery, C.; Scherberich, A.; Walboomers, X.F.; Barbero, A.; Martin, I. Bone-forming capacity of adult human nasal chondrocytes. J. Cell. Mol. Med. 2015, 19, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, N.; Savić, V.; Najman, S.; Plavšić, M.; Uskoković, D. A study of HAp/PLLA composite as a substitute for bone powder, using FT-IR spectroscopy. Biomaterials 2001, 22, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yu, L.; Yang, W.; Peng, S.; Zhong, Y.; Feng, P. Phosphonic acid coupling agent modification of HAP nanoparticles: Interfacial effects in PLLA/HAP bone scaffold. Polymers 2020, 12, 199. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Ye, Z.; Ren, H.; Chen, N.; Zeng, Q.; Wu, X.; Lu, T. Bioactivity assessment of PLLA/PCL/HAP electrospun nanofibrous scaffolds for bone tissue engineering. Life Sci. 2016, 148, 139–144. [Google Scholar] [CrossRef]
- Sun, J.; Yu, H.; Zhuang, X.; Chen, X.; Jing, X. Crystallization Behavior of Asymmetric PLLA/PDLA Blends. J. Phys. Chem. B 2011, 115, 2864–2869. [Google Scholar] [CrossRef]
- Zhao, L.-S.; Cai, Y.-H. Studies of poly (L-lactic acid)/hydroxyapatite composites through DSC and XRD. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 439, p. 042049. [Google Scholar]
- Shaltout, A.A.; Allam, M.A.; Moharram, M.A. FTIR spectroscopic, thermal and XRD characterization of hydroxyapatite from new natural sources. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 56–60. [Google Scholar] [CrossRef]
- Mir, M.; Leite, F.L.; Herrmann, P.S.d.P., Jr.; Pissetti, F.L.; Rossi, A.M.; Moreira, E.L.; Mascarenhas, Y.P. XRD, AFM, IR and TGA study of nanostructured hydroxyapatite. Mater. Res. 2012, 15, 622–627. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Noda, I.; Ochiai, S.; Ozaki, Y. Raman microspectroscopy study of structure, dispersibility, and crystallinity of poly(hydroxybutyrate)/poly(l-lactic acid) blends. Polymer 2006, 47, 3132–3140. [Google Scholar] [CrossRef]
- Pezzotti, G.; Rondinella, A.; Marin, E.; Zhu, W.; Aldini, N.N.; Ulian, G.; Valdrè, G. Raman spectroscopic investigation on the molecular structure of apatite and collagen in osteoporotic cortical bone. J. Mech. Behav. Biomed. Mater. 2017, 65, 264–273. [Google Scholar] [CrossRef]
- Yamini, D.; Venkatasubbu, G.D.; Kumar, J.; Ramakrishnan, V. Raman scattering studies on PEG functionalized hydroxyapatite nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Wong, Y.S.; Park, J.O.; Venkatraman, S.S.; Srinivasarao, M. A simple method for obtaining the information of orientation distribution using polarized Raman spectroscopy: Orientation study of structural units in poly(lactic acid). Macromolecules 2011, 44, 2120–2131. [Google Scholar] [CrossRef]
- Fulco, I.; Miot, S.; Haug, M.D.; Barbero, A.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Jundt, G.; Marsano, A.; Farhadi, J.; et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: An observational first-in-human trial. Lancet 2014, 384, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Acevedo Rua, L.; Mumme, M.; Manferdini, C.; Darwiche, S.; Khalil, A.; Hilpert, M.; Buchner, D.A.; Lisignoli, G.; Occhetta, P.; von Rechenberg, B.; et al. Engineered nasal cartilage for the repair of osteoarthritic knee cartilage defects. Sci. Transl. Med. 2021, 13, eaaz4499. [Google Scholar] [CrossRef]
- Pelttari, K.; Pippenger, B.; Mumme, M.; Feliciano, S.; Scotti, C.; Mainil-Varlet, P.; Procino, A.; von Rechenberg, B.; Schwamborn, T.; Jakob, M.; et al. Adult human neural crest-derived cells for articular cartilage repair. Sci. Transl. Med. 2014, 6, 251ra119. [Google Scholar] [CrossRef]
- Yang, W.; Han, W.; He, W.; Li, J.; Wang, J.; Feng, H.; Qian, Y. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 45–53. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Cai, Y.; Ji, H.; Zhou, G.; Zhao, X.; Tang, R.; Zhang, M. In vitro effects of nanophase hydroxyapatite particles on proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. A 2009, 90, 1083–1091. [Google Scholar] [CrossRef]
- Jiang, X.; Zhong, Y.; Zheng, L.; Zhao, J. Nano-hydroxyapatite/collagen film as a favorable substrate to maintain the phenotype and promote the growth of chondrocytes cultured in vitro. Int. J. Mol. Med. 2018, 41, 2150–2158. [Google Scholar] [CrossRef]
- Yin, H.; Yue, H.; Wang, M.; Zhang, T.; Zhao, Y.-T.; Liu, H.; Wang, J.; Zheng, H.; Xue, C. Preparation of novel sea cucumber intestinal peptides to promote tibial fracture healing in mice by inducing differentiation of hypertrophic chondrocytes to the osteoblast lineage. Mol. Nutr. Food Res. 2024, 68, 2300344. [Google Scholar] [CrossRef]
- Zhao, D.; Xing, S.; Qi, J.; Wei, Z.; Huang, J.; Sun, J.; Wen, X.; Wang, Y. Preparation of silk fibroin/chitosan/nano-Hydroxyapatite and its effect on the repair of cartilage damage in rats. Sci. Adv. Mater. 2022, 14, 1458–1465. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Lafantaisie-Favreau, C.H.; Lascau-Coman, V.; Chen, G.; Guzmán-Morales, J. The cartilage-bone interface. J. Knee Surg. 2012, 25, 085–098. [Google Scholar] [CrossRef]
- Khotib, J.; Gani, M.A.; Budiatin, A.S.; Lestari ML, A.D.; Rahadiansyah, E.; Ardianto, C. Signaling pathway and transcriptional regulation in osteoblasts during bone healing: Direct involvement of hydroxyapatite as a biomaterial. Pharmaceuticals 2021, 14, 615. [Google Scholar] [CrossRef]
- Xia, L.; Lin, K.; Jiang, X.; Xu, Y.; Zhang, M.; Chang, J.; Zhang, Z. Enhanced osteogenesis through nano-structured surface design of macroporous hydroxyapatite bioceramic scaffolds via activation of ERK and p38 MAPK signaling pathways. J. Mater. Chem. B 2013, 1, 5403–5416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, S.; Kuss, M.A.; Streubel, P.N.; Duan, B. Effects of hydroxyapatite and hypoxia on chondrogenesis and hypertrophy in 3D bioprinted ADMSC laden constructs. ACS Biomater. Sci. Eng. 2017, 3, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, H.; Hao, G.; Zhang, Y.; Ding, H.; Fan, Z.; Sun, L. 3D printing hydrogel scaffolds with nanohydroxyapatite gradient to effectively repair osteochondral defects in rats. Adv. Funct. Mater. 2021, 31, 2006697. [Google Scholar] [CrossRef]
- Ege, D.; Hasirci, V. Is 3D printing promising for osteochondral tissue regeneration? ACS Appl. Bio Mater. 2023, 6, 1431–1444. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhu, Z.; Liu, J.; Bao, X.; Wang, Q.; Shi, C.; Zhao, C.; Xu, G.; Li, D. 3D-printed gradient scaffolds for osteochondral defects: Current status and perspectives. Int. J. Bioprinting 2023, 9, 724. [Google Scholar] [CrossRef]
- Brennan, C.M.; Eichholz, K.F.; Hoey, D.A. The effect of pore size within fibrous scaffolds fabricated using melt electrowriting on human bone marrow stem cell osteogenesis. Biomed. Mater. 2019, 14, 065016. [Google Scholar] [CrossRef]
- Lai, X.; Huang, J.; Huang, S.; Wang, J.; Zheng, Y.; Luo, Y.; Tang, L.; Gao, B.; Tang, Y. Antibacterial and osteogenic dual-functional micronano composite scaffold fabricated via melt electrowriting and solution electrospinning for bone tissue engineering. ACS Appl. Mater. Interfaces 2024, 16, 37707–37721. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Kocak-Oztug, N.A.; Sheikh, F.A.; Han, P.; Anwar, S.; Fournier, B.P.; Ivanovski, S. Fabrication of 3D bioactive melt electrowriting composite scaffold with high osteogenic potential. Colloids Surf. B: Biointerfaces 2025, 245, 114270. [Google Scholar] [CrossRef]
- Bernardo, M.P.; da Silva, B.C.; Hamouda, A.E.; de Toledo, M.A.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso LH, C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basoli, V.; Barbano, V.; Bärtschi, C.; Loffreda, C.; Zanocco, M.; Rondinella, A.; Lanzutti, A.; Zhu, W.; Specchia, S.; Barbero, A.; et al. Engineering Poly(L-Lactic Acid)/Hydroxyapatite Scaffolds via Melt-Electrowriting: Enhancement of Osteochondral Cell Response in Human Nasal Chondrocytes. Polymers 2025, 17, 2455. https://doi.org/10.3390/polym17182455
Basoli V, Barbano V, Bärtschi C, Loffreda C, Zanocco M, Rondinella A, Lanzutti A, Zhu W, Specchia S, Barbero A, et al. Engineering Poly(L-Lactic Acid)/Hydroxyapatite Scaffolds via Melt-Electrowriting: Enhancement of Osteochondral Cell Response in Human Nasal Chondrocytes. Polymers. 2025; 17(18):2455. https://doi.org/10.3390/polym17182455
Chicago/Turabian StyleBasoli, Valentina, Vittorio Barbano, Cecilia Bärtschi, Cosimo Loffreda, Matteo Zanocco, Alfredo Rondinella, Alex Lanzutti, Wenliang Zhu, Stefania Specchia, Andrea Barbero, and et al. 2025. "Engineering Poly(L-Lactic Acid)/Hydroxyapatite Scaffolds via Melt-Electrowriting: Enhancement of Osteochondral Cell Response in Human Nasal Chondrocytes" Polymers 17, no. 18: 2455. https://doi.org/10.3390/polym17182455
APA StyleBasoli, V., Barbano, V., Bärtschi, C., Loffreda, C., Zanocco, M., Rondinella, A., Lanzutti, A., Zhu, W., Specchia, S., Barbero, A., Thieringer, F. M., Xu, H., & Marin, E. (2025). Engineering Poly(L-Lactic Acid)/Hydroxyapatite Scaffolds via Melt-Electrowriting: Enhancement of Osteochondral Cell Response in Human Nasal Chondrocytes. Polymers, 17(18), 2455. https://doi.org/10.3390/polym17182455











