An Acid-Cleavable Lamellar Block Copolymer for Sub-30-nm Line Spacing Patterning via Graphoepitaxial Directed Self-Assembly and Direct Wet Etching
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses of Polymers
2.2.1. Synthesis of Bromide-Terminated Poly (Methyl Methacrylate) (PMMA28k-Br)
2.2.2. Synthesis of Polystyrene-Block-Poly (Methyl Methacrylate) (PS28k-b-PMMA28k)
2.2.3. Synthesis of Hydroxy-Terminated Poly (Methyl Methacrylate) (PMMA-OH)
2.2.4. Synthesis of Aromatic Aldehyde-Terminated Poly (Methyl Methacrylate) (PMMA-CHO)
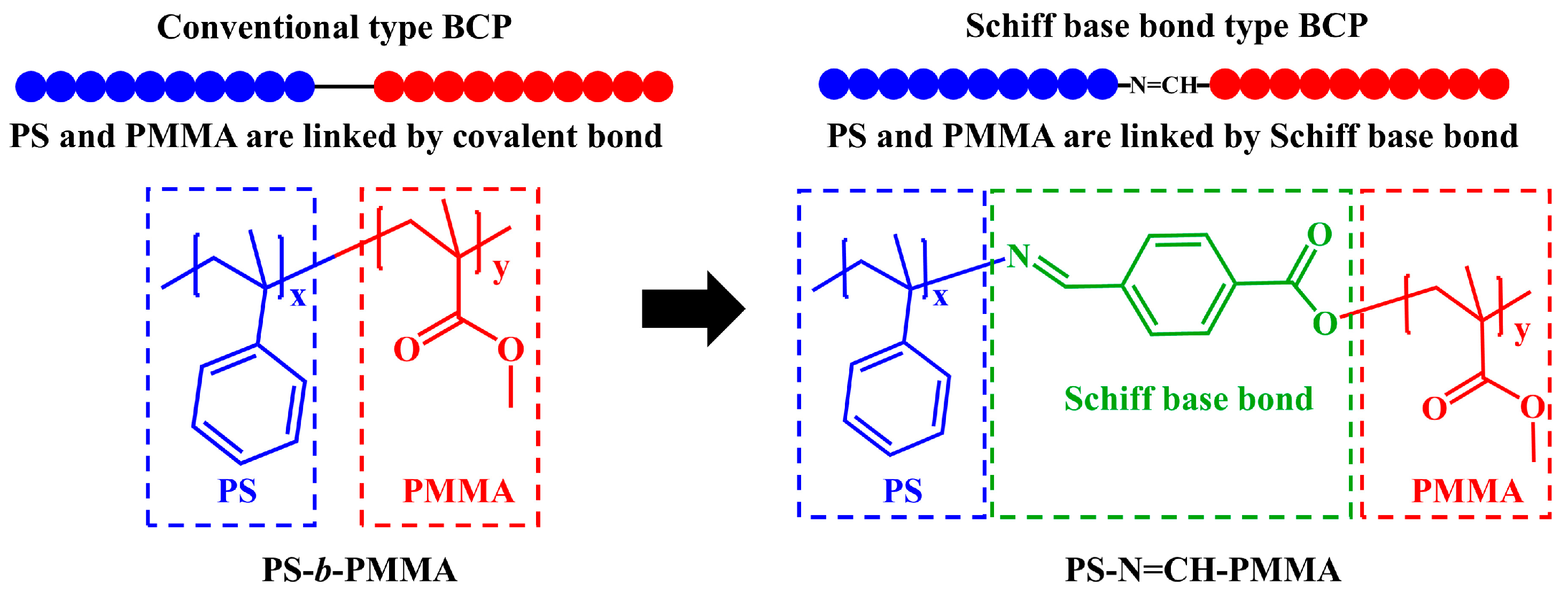
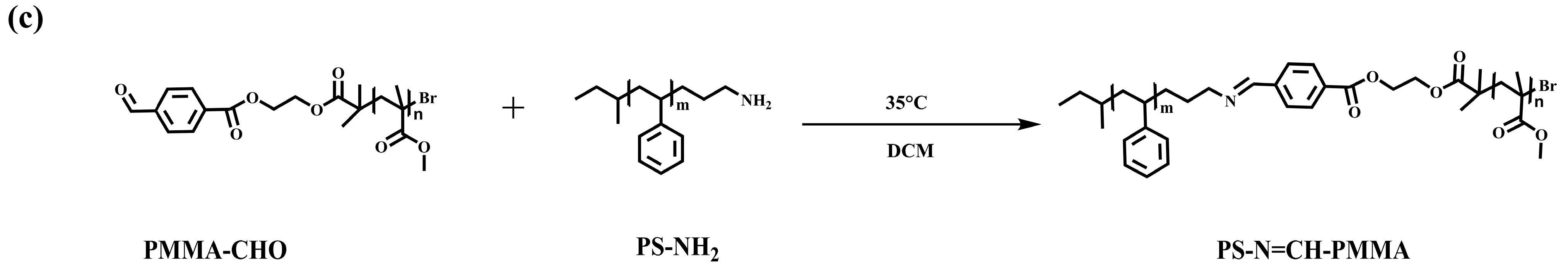
2.2.5. Synthesis of Acid-Cleavable Polystyrene-Block-Poly (Methyl Methacrylate) (PS-N=CH-PMMA)
2.2.6. Synthesis of the Random Copolymer PS-r-PMMA-r-PGMA
2.3. Thermal Annealing and Wet Etching Processes
2.3.1. Thin Film Self-Assembly of BCPs
2.3.2. Graphoepitaxial Directed Self-Assembly of BCPs
2.3.3. Wet Etching Process
2.4. Ohta–Kawasaki (OK) Model Simulation
2.5. Characterization
3. Results and Discussion
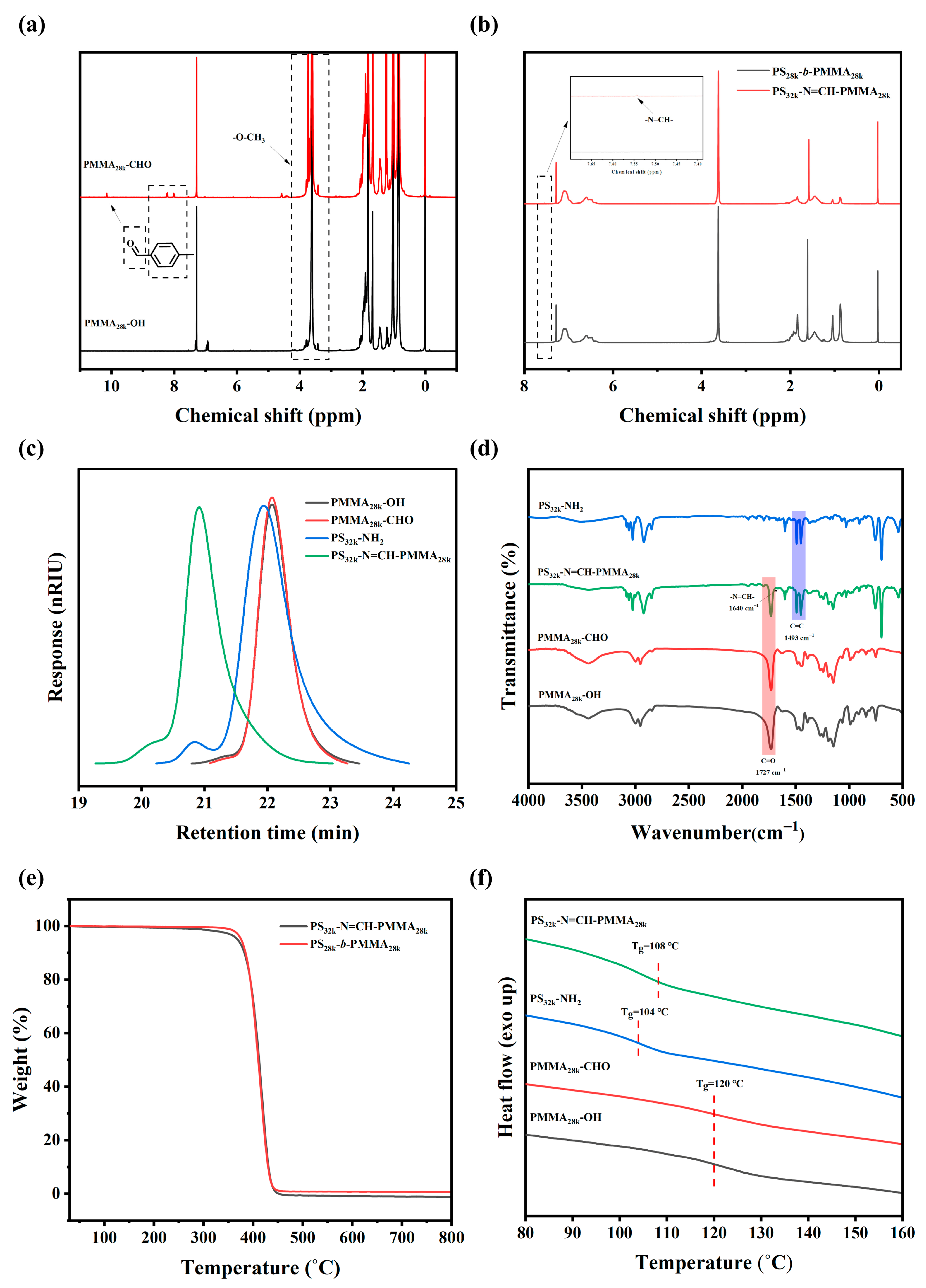
3.1. Synthesis and Characterization of Precursor Polymers and BCPs
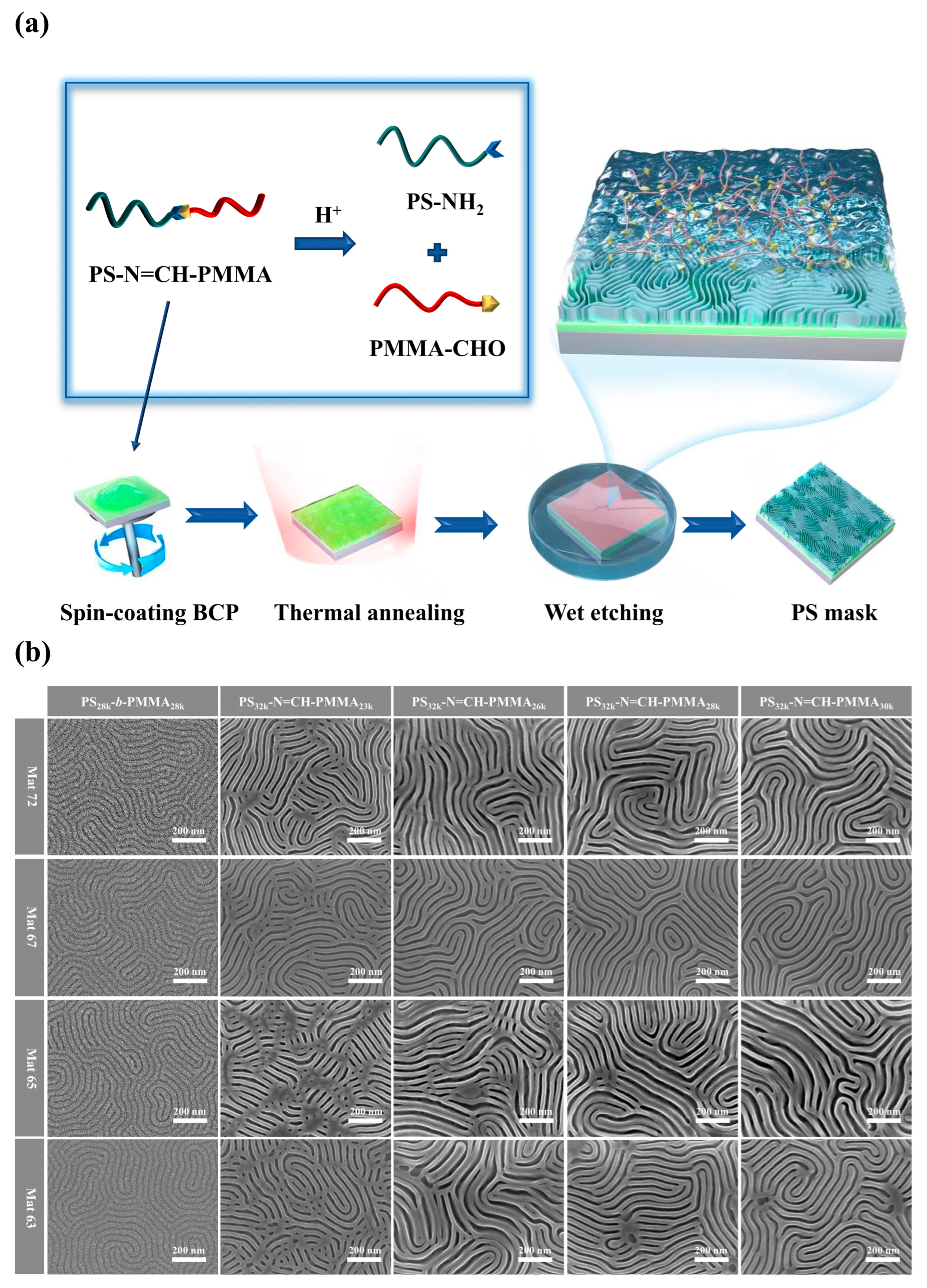
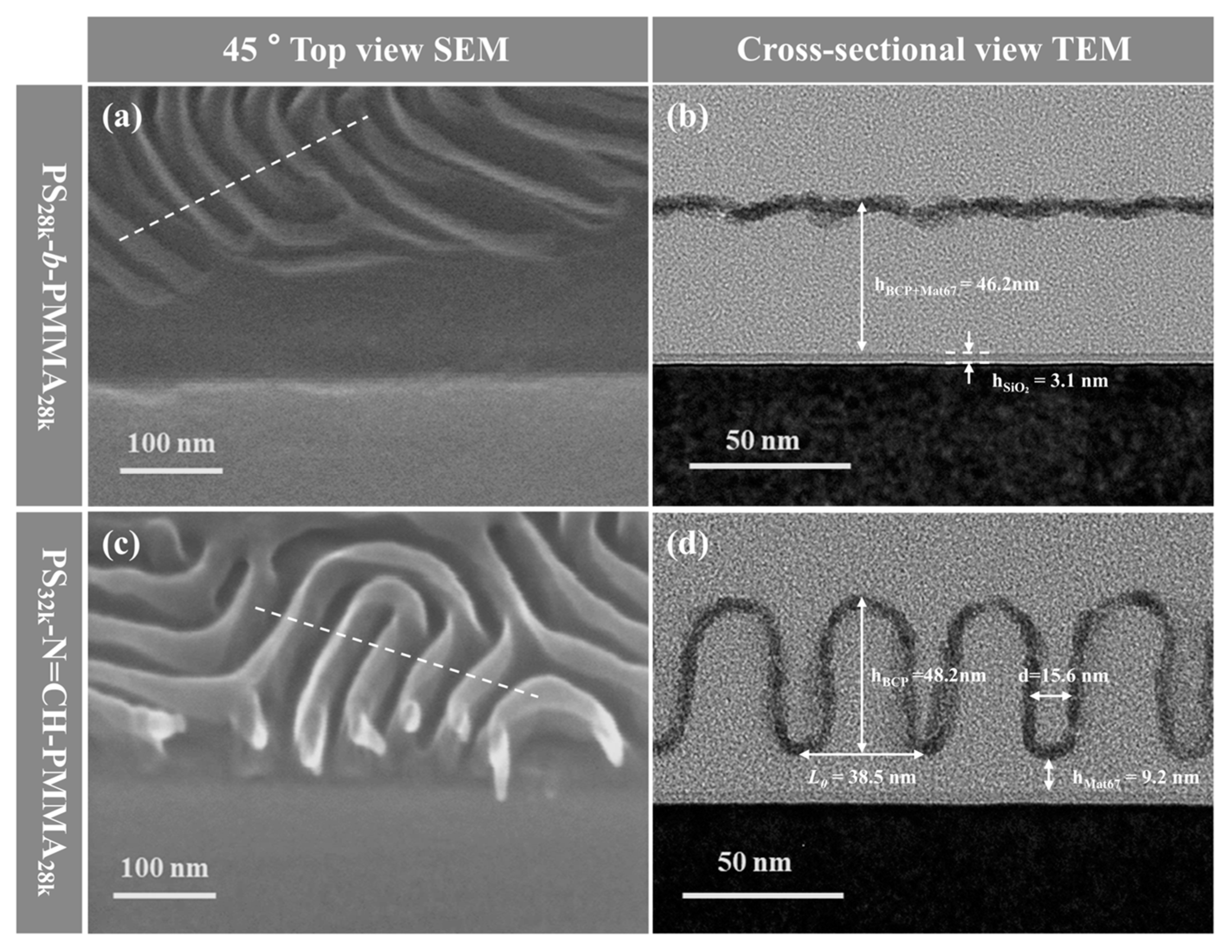
3.2. Morphological Analysis of BCP Self-Assembled Thin Films After Wet Etching
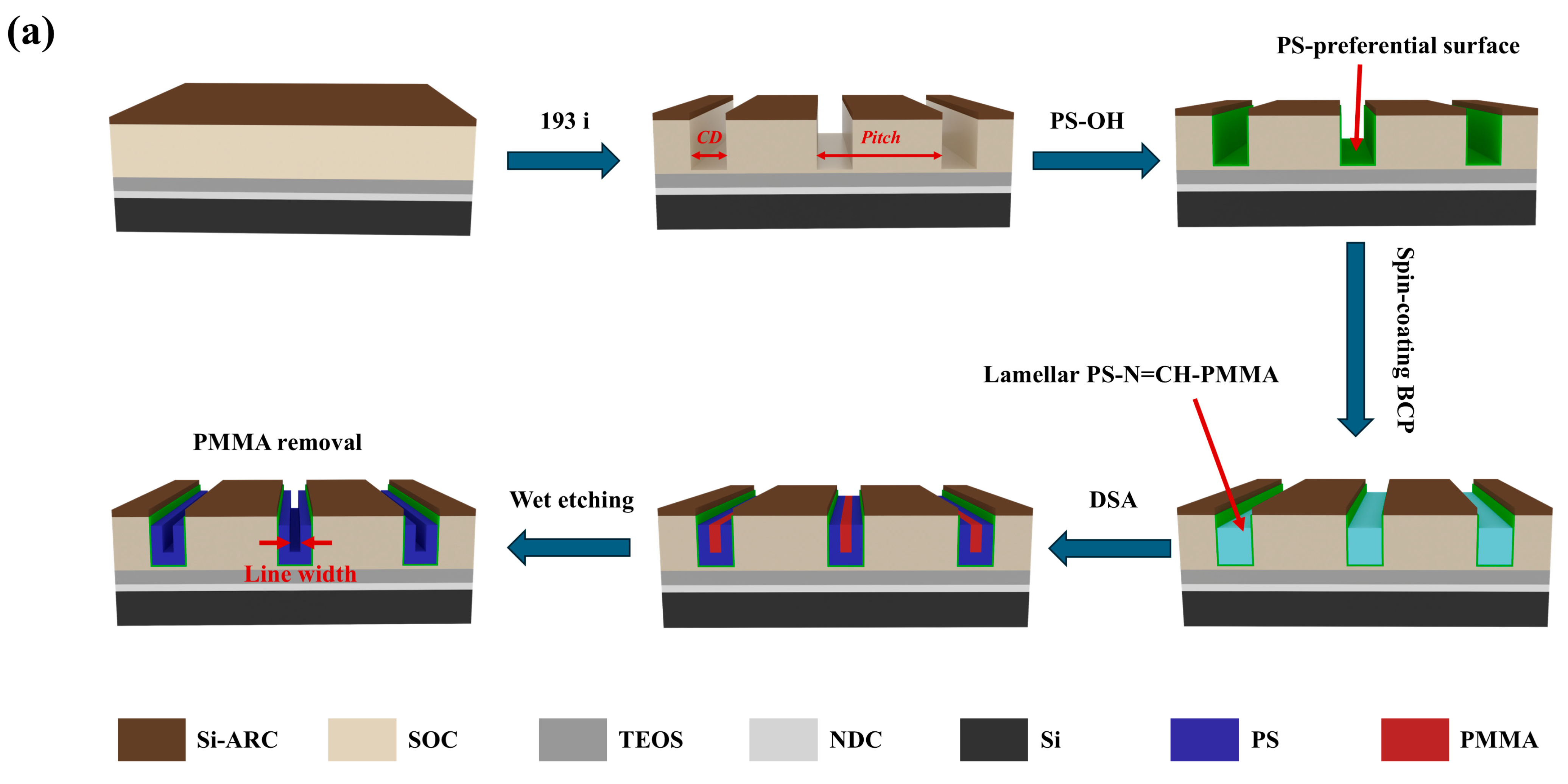
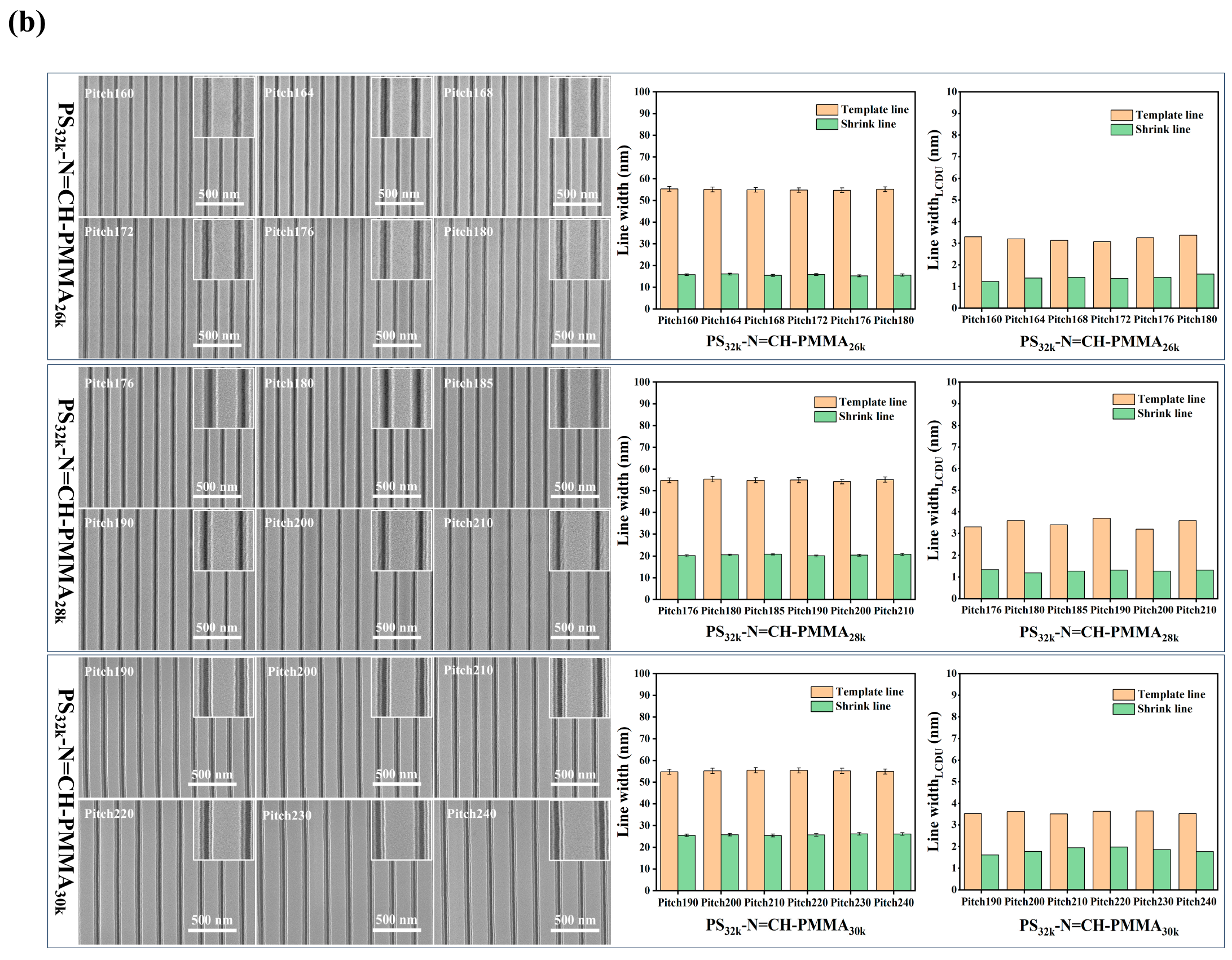
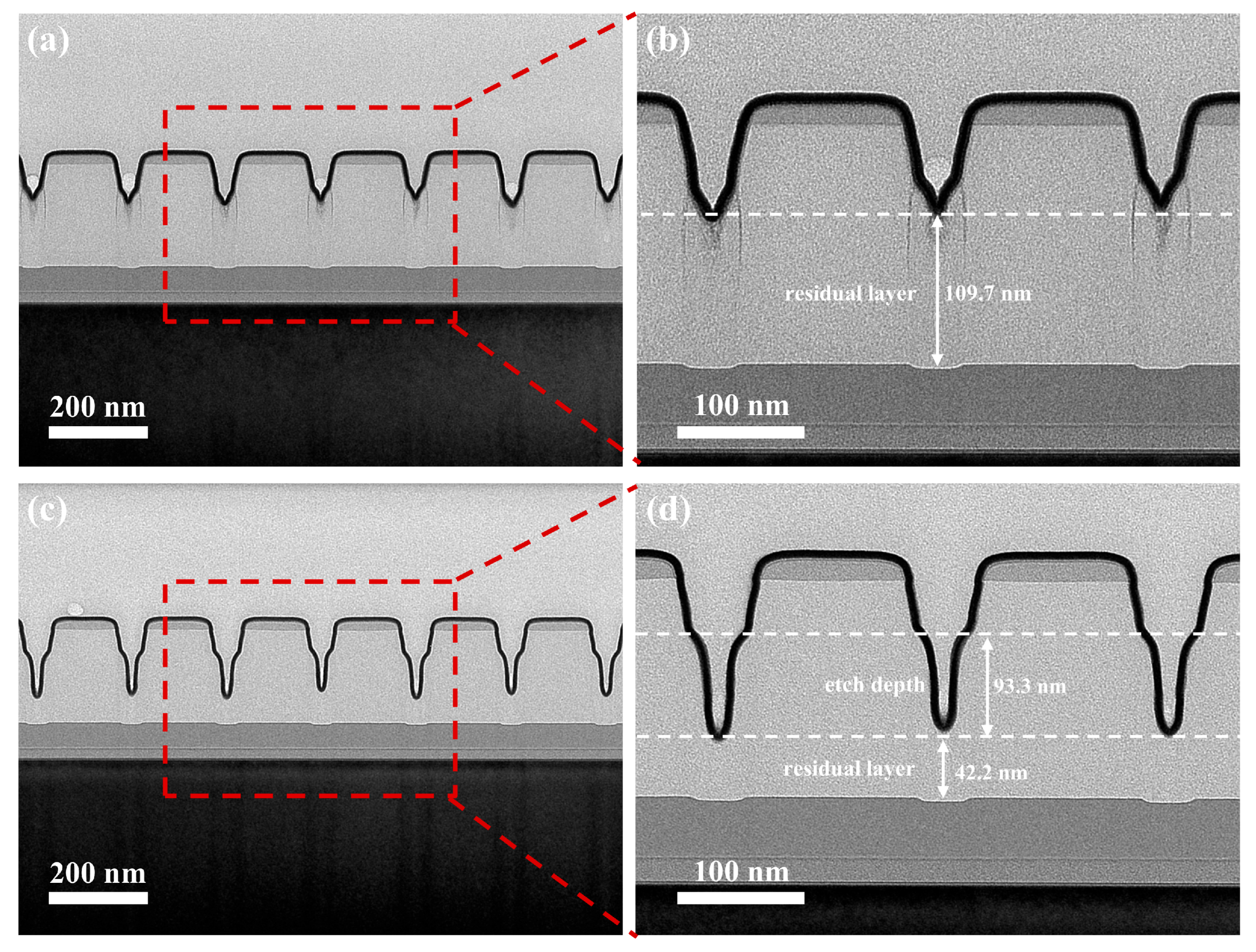
3.3. Graphoepitaxial DSA and Wet Etching Behavior of Lamella-Forming PS-N=CH-PMMA on Trench Templates
3.4. Ohta–Kawasaki Model Simulation of Trench Sidewall Angle Effects on the Graphoepitaxial DSA of Lamellar PS32k-N=CH-PMMA28k
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Shen, H.; Qiu, H.; Shi, Y.; Wang, X. Two-dimensional semiconductor transistors and integrated circuits for advanced technology nodes. Natl. Sci. Rev. 2024, 11, nwae001. [Google Scholar] [CrossRef]
- Basu, P.; Verma, J.; Abhinav, V.; Ratnesh, R.K.; Singla, Y.K.; Kumar, V. Advancements in Lithography Techniques and Emerging Molecular Strategies for Nanostructure Fabrication. Int. J. Mol. Sci. 2025, 26, 3027. [Google Scholar] [CrossRef]
- Morsy, M.; Znid, F.; Farraj, A. A critical review on improving and moving beyond the 2 nm horizon: Future directions and impacts in next-generation integrated circuit technologies. Mater. Sci. Semicond. Process. 2025, 190, 109376. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Z.; Zheng, W. Diamond immersion photodetector for 193 nm lithography. Adv. Opt. Mater. 2023, 11, 2301533. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kanaya, Y.; Saito, Y.; Sakamoto, T.; Masaki, K.; Owa, S.; Koo, T.; Tseng, D.; Sorensen, C.; Lin, B. Development of deep ultraviolet optical maskless exposure tool for advanced lithography. J. Micro/Nanopatterning Mater. Metrol. 2023, 22, 041403. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Xu, X.; Zhao, C.; Xu, X.; Weiss, P.S. Single-step dual-layer photolithography for tunable and scalable nanopatterning. ACS Nano 2021, 15, 12180–12188. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, S.; Miyaguchi, K.; Niroomand, A.; Verstraete, L.; Yang, K.; Gillijns, W.; Das, S.; Blanco, V.; Halder, S. EUV DRAM patterning historic overview and future assumption. In Proceedings of the DTCO and Computational Patterning IV, San Jose, CA, USA, 25–28 February 2025; Volume 13425, pp. 187–199. [Google Scholar]
- Kazazis, D.; Santaclara, J.G.; van Schoot, J.; Mochi, I.; Ekinci, Y. Extreme ultraviolet lithography. Nat. Rev. Methods Primers 2024, 4, 84. [Google Scholar] [CrossRef]
- Ashraf, N.S. High-NA EUV: Prospects and Challenges and Stochastic Defects Related Manufacturing Yield Loss. In Handbook of Emerging Materials for Semiconductor Industry; Springer: Berlin/Heidelberg, Germany, 2024; pp. 253–265. [Google Scholar]
- Li, M.; Aqad, E. Key Challenges and Opportunities for Advanced Extreme Ultraviolet Lithography Photoresist Materials. Adv. Funct. Mater. 2025, 35, 2420962. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, J.; Li, L.; Dong, Q.; Zhang, X.; Li, Z.; Liu, Y.; Ji, S.; Li, W.; Zhang, Y. Manipulating the processing window of directed self-assembly in contact hole shrinking with binary block copolymer/homopolymer blending. iScience 2024, 27, 109425. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiong, S. Directed self-assembly of block copolymers for sub-10 nm fabrication. Int. J. Extrem. Manuf. 2020, 2, 032006. [Google Scholar] [CrossRef]
- Pinto-Gómez, C.; Pérez-Murano, F.; Bausells, J.; Villanueva, L.G.; Fernández-Regúlez, M. Directed self-assembly of block copolymers for the fabrication of functional devices. Polymers 2020, 12, 2432. [Google Scholar] [CrossRef]
- Xiong, S.; Wan, L.; Ishida, Y.; Chapuis, Y.-A.; Craig, G.S.; Ruiz, R.; Nealey, P.F. Directed self-assembly of triblock copolymer on chemical patterns for sub-10-nm nanofabrication via solvent annealing. ACS Nano 2016, 10, 7855–7865. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, H.M.; Yang, G.G.; Han, K.H.; Yun, T.; Shin, J.Y.; Jeong, S.J.; Kim, S.O. Smart nanostructured materials based on self-assembly of block copolymers. Adv. Funct. Mater. 2020, 30, 1902049. [Google Scholar] [CrossRef]
- Feng, H.; Dolejsi, M.; Zhu, N.; Yim, S.; Loo, W.; Ma, P.; Zhou, C.; Craig, G.S.; Chen, W.; Wan, L. Optimized design of block copolymers with covarying properties for nanolithography. Nat. Mater. 2022, 21, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Agrawal, A.; Wu, W.; Masud, A.; Armijo, E.; Gonzalez, D.; Zhou, S.; Terlier, T.; Zhu, C.; Strzalka, J. Soft-shear-aligned vertically oriented lamellar block copolymers for template-free sub-10 nm patterning and hybrid nanostructures. ACS Appl. Mater. Interfaces 2022, 14, 12824–12835. [Google Scholar] [CrossRef]
- Huang, G.; Lai, H.; Song, J.; Jiang, Y.; Ji, S. A universal approach to fabricating 3D chemical patterns for directed self-assembly of block copolymers with density multiplication. Macromolecules 2023, 56, 5784–5791. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, H.U.; Yeom, H.I.; Han, K.H.; Yang, G.G.; Choi, H.J.; Kim, J.M.; Park, S.H.K.; Jin, H.M.; Kim, J.U. Atomically Flat, 2D Edge-Directed Self-Assembly of Block Copolymers. Adv. Mater. 2023, 35, 2207338. [Google Scholar] [CrossRef]
- Yang, G.G.; Choi, H.J.; Han, K.H.; Kim, J.H.; Lee, C.W.; Jung, E.I.; Jin, H.M.; Kim, S.O. Block copolymer nanopatterning for nonsemiconductor device applications. ACS Appl. Mater. Interfaces 2022, 14, 12011–12037. [Google Scholar] [CrossRef]
- Sparnacci, K.; Chiarcos, R.; Gianotti, V.; Laus, M.; Giammaria, T.J.; Perego, M.; Munaò, G.; Milano, G.; De Nicola, A.; Haese, M. Effect of Trapped Solvent on the Interface between PS-b-PMMA Thin Films and P (S-r-MMA) Brush Layers. ACS Appl. Mater. Interfaces 2020, 12, 7777–7787. [Google Scholar] [CrossRef]
- Yoshida, K.; Tanaka, S.; Yamamoto, T.; Tajima, K.; Borsali, R.; Isono, T.; Satoh, T. Chain-end functionalization with a saccharide for 10 nm microphase separation:“Classical” PS-b-PMMA versus PS-b-PMMA-saccharide. Macromolecules 2018, 51, 8870–8877. [Google Scholar] [CrossRef]
- Wan, L.; Ruiz, R.; Gao, H.; Patel, K.C.; Albrecht, T.R.; Yin, J.; Kim, J.; Cao, Y.; Lin, G. The limits of lamellae-forming PS-b-PMMA block copolymers for lithography. ACS Nano 2015, 9, 7506–7514. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.A.; Petkov, N.; Shaw, M.T.; Djara, V.; Holmes, J.D.; Morris, M.A. Monitoring PMMA elimination by reactive ion etching from a lamellar PS-b-PMMA thin film by ex situ TEM methods. Macromolecules 2010, 43, 8651–8655. [Google Scholar] [CrossRef]
- Bürger, J.; Venugopal, H.; Kool, D.; de los Arcos, T.; Gonzalez Orive, A.; Grundmeier, G.; Brassat, K.; Lindner, J.K. High-Resolution Study of Changes in Morphology and Chemistry of Cylindrical PS-b-PMMA Block Copolymer Nanomasks during Mask Development. Adv. Mater. Interfaces 2022, 9, 2200962. [Google Scholar] [CrossRef]
- Sarrazin, A.; Posseme, N.; Pimenta-Barros, P.; Barnola, S.; Gharbi, A.; Argoud, M.; Tiron, R.; Cardinaud, C. PMMA removal selectivity to polystyrene using dry etch approach. J. Vac. Sci. Technol. B 2016, 34, 061802. [Google Scholar] [CrossRef]
- Wu, H.-C.; Liao, M.-C.; Hirahara, E.; Iwaki, T. Wet etch process for high-resolution DSA patterning for advanced node DRAM. In Advances in Patterning Materials and Processes XLI; SPIE: Bellingham, WA, USA, 2024; Volume 12957, pp. 374–383. [Google Scholar]
- Gu, X.; Liu, Z.; Gunkel, I.; Chourou, S.; Hong, S.W.; Olynick, D.L.; Russell, T.P. High aspect ratio sub-15 nm silicon trenches from block copolymer templates. Adv. Mater. 2012, 24, 5688–5694. [Google Scholar] [CrossRef]
- Borah, D.; Senthamaraikannan, R.; Rasappa, S.; Kosmala, B.; Holmes, J.D.; Morris, M.A. Swift nanopattern formation of PS-b-PMMA and PS-b-PDMS block copolymer films using a microwave assisted technique. ACS Nano 2013, 7, 6583–6596. [Google Scholar] [CrossRef]
- Delalande, M.; Cunge, G.; Chevolleau, T.; Bézard, P.; Archambault, S.; Joubert, O.; Chevalier, X.; Tiron, R. Development of plasma etching processes to pattern sub-15 nm features with PS-b-PMMA block copolymer masks: Application to advanced CMOS technology. J. Vac. Sci. Technol. B 2014, 32, 051806. [Google Scholar] [CrossRef]
- Imamura, T.; Yamamoto, H.; Omura, M.; Sakai, I.; Hayashi, H. Highly selective removal of poly (methyl methacrylate) from polystyrene-block-poly (methyl methacrylate) by CO/H2 plasma etching. J. Vac. Sci. Technol. B 2015, 33, 061601. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface modification of polymers: Methods and applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Zajadacz, J.; Lorenz, P.; Frost, F.; Fechner, R.; Steinberg, C.; Scheer, H.-C.; Zimmer, K. Reactive ion beam etching of fused silica using vertical lamellar patterns of PS-b-PMMA diblock copolymer masks. Microelectron. Eng. 2015, 141, 289–293. [Google Scholar] [CrossRef]
- Gharbi, A.; Tiron, R.; Pimenta Barros, P.; Argoud, M.; Servin, I.; Chevalier, X.; Nicolet, C.; Navarro, C. PMMA removal options by wet development in PS-b-PMMA block copolymer for nanolithographic mask fabrication. J. Vac. Sci. Technol. B 2015, 33, 051602. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic covalent polymer networks: A molecular platform for designing functions beyond chemical recycling and self-healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J. Molecular assembly of Schiff base interactions: Construction and application. Chem. Rev. 2015, 115, 1597–1621. [Google Scholar] [CrossRef]
- Zhan, J.; Wu, Y.; Wang, H.; Liu, J.; Ma, Q.; Xiao, K.; Li, Z.; Li, J.; Luo, F.; Tan, H. An injectable hydrogel with pH-sensitive and self-healing properties based on 4armPEGDA and N-carboxyethyl chitosan for local treatment of hepatocellular carcinoma. Int. J. Biol. Macromol. 2020, 163, 1208–1222. [Google Scholar] [CrossRef]
- Xu, X.; Ma, S.; Wu, J.; Yang, J.; Wang, B.; Wang, S.; Li, Q.; Feng, J.; You, S.; Zhu, J. High-performance, command-degradable, antibacterial Schiff base epoxy thermosets: Synthesis and properties. J. Mater. Chem. A 2019, 7, 15420–15431. [Google Scholar] [CrossRef]
- Wang, W.; Shi, D.; Zhang, Y.; Li, W.; Li, F.; Feng, H.; Ma, L.; Yang, C.; Peng, Z.; Song, G. An injectable hydrogel based on hyaluronic acid prepared by Schiff base for long-term controlled drug release. Int. J. Biol. Macromol. 2023, 245, 125341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, P.; Peng, X.; Chen, S.; Zhang, K. Interfacial synthesis of cellulose-derived solvent-responsive nanoparticles via Schiff base reaction. ACS Sustain. Chem. Eng. 2019, 7, 16595–16603. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Li, C.; Gao, L. Degradable block copolymer-derived nanoporous membranes and their applications. Giant 2023, 16, 100183. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, J. Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- He, Q.; Huang, J.; Liang, H.; Lu, J. Light-responsive fluorescent cross-linked polymeric micelles based on a salicylidene Schiff base pendant-functionalized block copolymer. Polym. Chem. 2014, 5, 4348–4357. [Google Scholar] [CrossRef]
- Miksa, B. Recent progress in designing shell cross-linked polymer capsules for drug delivery. RSC Adv. 2015, 5, 87781–87805. [Google Scholar] [CrossRef]
- Williams, V.A.; Matyjaszewski, K. Expanding the ATRP toolbox: Methacrylate polymerization with an elemental silver reducing agent. Macromolecules 2015, 48, 6457–6464. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, C.; Lee, K.S.; Kim, E.; Ahn, S.; Lee, J.; Kim, J.K. Microdomain orientation of star-shaped block copolymer thin film depending on molecular weight. Macromolecules 2020, 53, 3611–3618. [Google Scholar] [CrossRef]
- Perego, M.; Kuschlan, S.; Seguini, G.; Chiarcos, R.; Gianotti, V.; Antonioli, D.; Sparnacci, K.; Laus, M. Silicon doping by polymer grafting: Size distribution matters. ACS Appl. Polym. Mater. 2021, 3, 6383–6393. [Google Scholar] [CrossRef]
- Hansen-Felby, M.; Henriksen, M.L.; Pedersen, S.U.; Daasbjerg, K. Postfunctionalization of self-immolative poly (dithiothreitol) using Steglich esterification. Macromolecules 2022, 55, 5788–5794. [Google Scholar] [CrossRef]
- Liu, J.; Guo, H.; Pang, X.; Tan, X.; Akinc, M.; Lin, Z.; Bowler, N. Dynamics of polystyrene-block-poly(methylmethacrylate) (PS-b-PMMA) diblock copolymers and PS/PMMA blends: A dielectric study. J. Non-Cryst. Solids 2013, 359, 27–32. [Google Scholar] [CrossRef]
- Ham, S.; Shin, C.; Kim, E.; Ryu, D.Y.; Jeong, U.; Russell, T.P.; Hawker, C.J. Microdomain Orientation of PS-b-PMMA by Controlled Interfacial Interactions. Macromolecules 2008, 41, 6431–6437. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Taniguchi, T. Large-scale simulations of directed self-assembly with simplified model. J. Photopolym. Sci. Technol. 2013, 26, 809–816. [Google Scholar] [CrossRef]
- Tsakos, M.; Schaffert, E.S.; Clement, L.L.; Villadsen, N.L.; Poulsen, T.B. Ester coupling reactions–an enduring challenge in the chemical synthesis of bioactive natural products. Nat. Prod. Rep. 2015, 32, 605–632. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, X.; Fan, J.; Wang, G.; Liu, S. Aldehyde surface-functionalized shell cross-linked micelles with pH-tunable core swellability and their bioconjugation with lysozyme. Macromolecules 2007, 40, 9074–9083. [Google Scholar] [CrossRef]
- Jung, S.; Yoon, H.J. Mechanical Force for the Transformation of Aziridine into Imine. Angew. Chem. Int. Ed. 2021, 60, 23564–23568. [Google Scholar] [CrossRef]
- Ferrarese Lupi, F.; Giammaria, T.J.; Seguini, G.; Vita, F.; Francescangeli, O.; Sparnacci, K.; Antonioli, D.; Gianotti, V.; Laus, M.; Perego, M. Fine tuning of lithographic masks through thin films of PS-b-PMMA with different molar mass by rapid thermal processing. ACS Appl. Mater. Interfaces 2014, 6, 7180–7188. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Lee, Y.; Lee, H.; Park, S.; Kim, Y.; Cho, J.; Ryu, D.Y. Microdomain expansion and transition behavior of PS-b-PMMA/PS homopolymers by SAXS analysis. Polymer 2012, 53, 5163–5169. [Google Scholar] [CrossRef]
- Dolejsi, M.; Nealey, P. Utilization of metal–polymer interactions for self-aligned directed self-assembly of device relevant features. J. Micro/Nanolithogr. MEMS MOEMS 2018, 17, 031204. [Google Scholar] [CrossRef]
- Suh, H.S.; Dudash, V.; Lorusso, G.; Mack, C. Roughness study on line and space patterning with chemo-epitaxy directed self-assembly. In Advances in Patterning Materials and Processes XXXVII; SPIE: Bellingham, WA, USA, 2020; Volume 11326, pp. 146–154. [Google Scholar]
- Claveau, G.; Quemere, P.; Argoud, M.; Hazart, J.; Barros, P.P.; Sarrazin, A.; Posseme, N.; Tiron, R.; Chevalier, X.; Nicolet, C. Surface affinity role in graphoepitaxy of lamellar block copolymers. J. Micro/Nanolithogr. MEMS MOEMS 2016, 15, 031604. [Google Scholar] [CrossRef]
- Zhang, B.; Meng, L.; Li, Z. Study of the ordered assembly morphologies of diblock copolymers on the same substrate. RSC Adv. 2022, 12, 28376–28387. [Google Scholar] [CrossRef]
- Barros, P.P.; Gharbi, A.; Fouquet, A.; Bos, S.; Hazart, J.; Delachat, F.; Chevalier, X.; Cayrefourcq, I.; Pain, L.; Tiron, R. Balancing Block Copolymer Thickness over Template Density in Graphoepitaxy Approach. Macromol. Mater. Eng. 2017, 302, 1700285. [Google Scholar] [CrossRef]
- Seino, Y.; Yonemitsu, H.; Sato, H.; Kanno, M.; Kato, H.; Kobayashi, K.; Kawanishi, A.; Azuma, T.; Muramatsu, M.; Nagahara, S. Contact hole shrink process using graphoepitaxial directed self-assembly lithography. J. Micro/Nanolithogr. MEMS MOEMS 2013, 12, 033011. [Google Scholar] [CrossRef][Green Version]
- Navarro, C.; Nicolet, C.; Ariura, F.; Chevalier, X.; Xu, K.; Hockey, M.A.; Mumtaz, M.; Fleury, G.; Hadziioannou, G.; Legrain, A. Recent Achievements in sub-10 nm DSA lithography for Line/Space patterning. J. Photopolym. Sci. Technol. 2017, 30, 69–75. [Google Scholar] [CrossRef]
- Bruinink, C.M.; Péter, M.; Maury, P.A.; De Boer, M.; Kuipers, L.; Huskens, J.; Reinhoudt, D.N. Capillary force lithography: Fabrication of functional polymer templates as versatile tools for nanolithography. Adv. Funct. Mater. 2006, 16, 1555–1565. [Google Scholar] [CrossRef]
- Wang, S.; Deng, W.; Seow, Y.A.; Chen, B.; Lin, Q.Y. Study of line-space pitch multiplication using graphoepitaxy directed self-assembly for semiconductor applications. J. Electron. Mater. 2017, 46, 4405–4413. [Google Scholar] [CrossRef]
- Tawfick, S.; De Volder, M.; Copic, D.; Park, S.J.; Oliver, C.R.; Polsen, E.S.; Roberts, M.J.; Hart, A.J. Engineering of micro-and nanostructured surfaces with anisotropic geometries and properties. Adv. Mater. 2012, 24, 1628–1674. [Google Scholar] [CrossRef] [PubMed]


| Samples | Mp (kg/mol) | Mn (kg/mol) | Mw (kg/mol) | PDI | DPn |
|---|---|---|---|---|---|
| PMMA23k-OH | 25.3 | 23.0 | 25.1 | 1.09 | 230 |
| PMMA26k-OH | 27.2 | 25.9 | 27.5 | 1.09 | 259 |
| PMMA28k-OH | 31.3 | 28.2 | 30.7 | 1.09 | 282 |
| PMMA30k-OH | 32.6 | 30.1 | 32.8 | 1.09 | 301 |
| Samples | Mp (kg/mol) | Mn (kg/mol) | Mw (kg/mol) | PDI | GE (%) |
|---|---|---|---|---|---|
| PMMA23k-CHO | 25.5 | 23.1 | 25.2 | 1.09 | 87.3 |
| PMMA26k-CHO | 27.7 | 26.1 | 28.4 | 1.09 | 86.1 |
| PMMA28k-CHO | 31.9 | 28.3 | 31.2 | 1.09 | 85.9 |
| PMMA30k-CHO | 33.6 | 30.3 | 33.1 | 1.09 | 84.2 |
| Samples | Mp (kg/mol) | Mn (kg/mol) | Mw (kg/mol) | PDI |
|---|---|---|---|---|
| PS28k-b-PMMA28k | 64.8 | 56.3 | 61.9 | 1.10 |
| PS32k-N=CH-PMMA23k | 65.2 | 55.3 | 62.5 | 1.13 |
| PS32k-N=CH-PMMA26k | 68.0 | 58.0 | 65.6 | 1.13 |
| PS32k-N=CH-PMMA28k | 70.2 | 60.1 | 68.0 | 1.13 |
| PS32k-N=CH-PMMA30k | 71.9 | 61.7 | 69.7 | 1.13 |
| Samples | Mp (kg/mol) | Mn (kg/mol) | Mw (kg/mol) | PDI | FSt (%) | Film Thickness (nm) |
|---|---|---|---|---|---|---|
| Mat63 | 39.2 | 24.1 | 37.4 | 1.55 | 63.2 | 7.8 |
| Mat65 | 41.3 | 25.1 | 39.4 | 1.57 | 65.4 | 8.5 |
| Mat67 | 44.1 | 28.1 | 43.8 | 1.56 | 67.1 | 9.7 |
| Mat72 | 45.6 | 28.5 | 44.2 | 1.55 | 72.3 | 10.2 |
| Samples | Mn of PMMA a (kg/mol) | ƒSt b (%) | Domain Width of PMMA c (nm) | L0 d (nm) | Film Thickness e (nm) |
|---|---|---|---|---|---|
| PS28k-b-PMMA28k | 28.1 | 51.2 | 12.9 | 29.8 | 38.3 |
| PS32k-N=CH-PMMA23k | 23.1 | 61.3 | - | - | - |
| PS32k-N=CH-PMMA26k | 26.1 | 57.2 | 13.3 | 36.1 | 45.1 |
| PS32k-N=CH-PMMA28k | 28.3 | 54.8 | 15.4 | 38.3 | 47.9 |
| PS32k-N=CH-PMMA30k | 30.3 | 52.4 | 18.2 | 40.2 | 50.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; Shang, C.; Niu, M.; Luo, J.; Gao, S.; Wu, Z.; Niu, S.; Xu, Y.; Zhang, X.; Li, Z.; et al. An Acid-Cleavable Lamellar Block Copolymer for Sub-30-nm Line Spacing Patterning via Graphoepitaxial Directed Self-Assembly and Direct Wet Etching. Polymers 2025, 17, 2435. https://doi.org/10.3390/polym17182435
Zhan J, Shang C, Niu M, Luo J, Gao S, Wu Z, Niu S, Xu Y, Zhang X, Li Z, et al. An Acid-Cleavable Lamellar Block Copolymer for Sub-30-nm Line Spacing Patterning via Graphoepitaxial Directed Self-Assembly and Direct Wet Etching. Polymers. 2025; 17(18):2435. https://doi.org/10.3390/polym17182435
Chicago/Turabian StyleZhan, Jianghao, Caiwei Shang, Muqiao Niu, Jiacheng Luo, Shengguang Gao, Zhiyong Wu, Shengru Niu, Yiming Xu, Xingmiao Zhang, Zili Li, and et al. 2025. "An Acid-Cleavable Lamellar Block Copolymer for Sub-30-nm Line Spacing Patterning via Graphoepitaxial Directed Self-Assembly and Direct Wet Etching" Polymers 17, no. 18: 2435. https://doi.org/10.3390/polym17182435
APA StyleZhan, J., Shang, C., Niu, M., Luo, J., Gao, S., Wu, Z., Niu, S., Xu, Y., Zhang, X., Li, Z., & Xiong, S. (2025). An Acid-Cleavable Lamellar Block Copolymer for Sub-30-nm Line Spacing Patterning via Graphoepitaxial Directed Self-Assembly and Direct Wet Etching. Polymers, 17(18), 2435. https://doi.org/10.3390/polym17182435








