A Carboxylated Nitrile Butadiene Rubber Latex Film with Synergistically Enhanced Water-Based Lubricity and Tensile Strength: Fabrication and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Main Equipment and Instruments
2.3. Experimental Formulations
2.4. Sample Preparation
- (1)
- Preparation of Dispersions, Solutions, and Coagulant.
- (2)
- Preparation of Compounded Latex.
- (3)
- Film Preparation via Dip Molding.
- (4)
- Lubricant Incorporation Methods.
2.5. Characterization and Analysis
- (1)
- Scanning Electron Microscopy (SEM)
- (2)
- Contact Angle Measurement
- (3)
- Coefficient of Friction (COF)
- (4)
- Rheological Measurement
- (5)
- Mechanical Property Testing.
3. Results
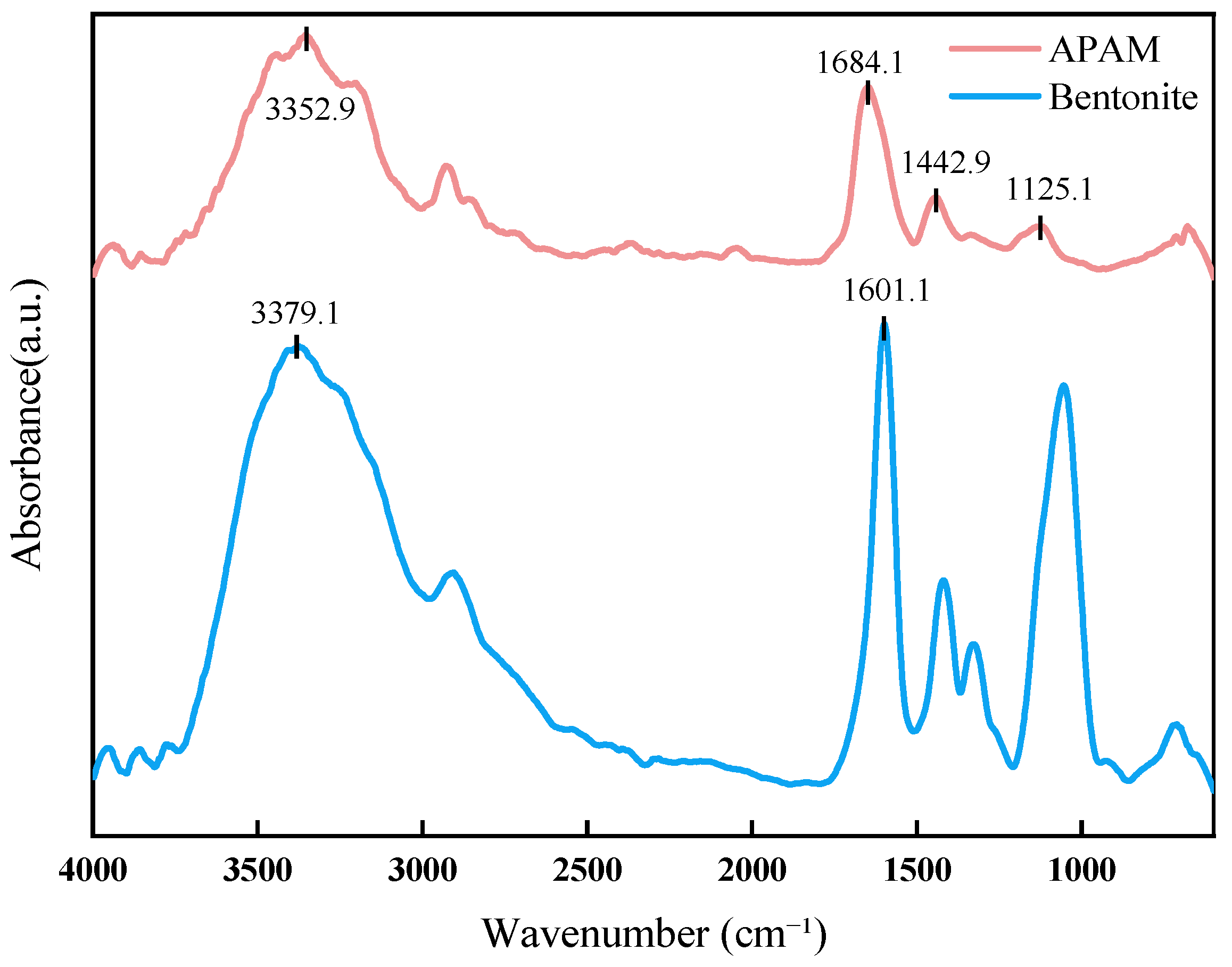
3.1. Enhancement of Lubricity and Mechanical Properties of XNBR Films by APAM
3.1.1. Lubricity of XNBR/APAM Films
3.1.2. Mechanical Properties of XNBRL/APAM Films
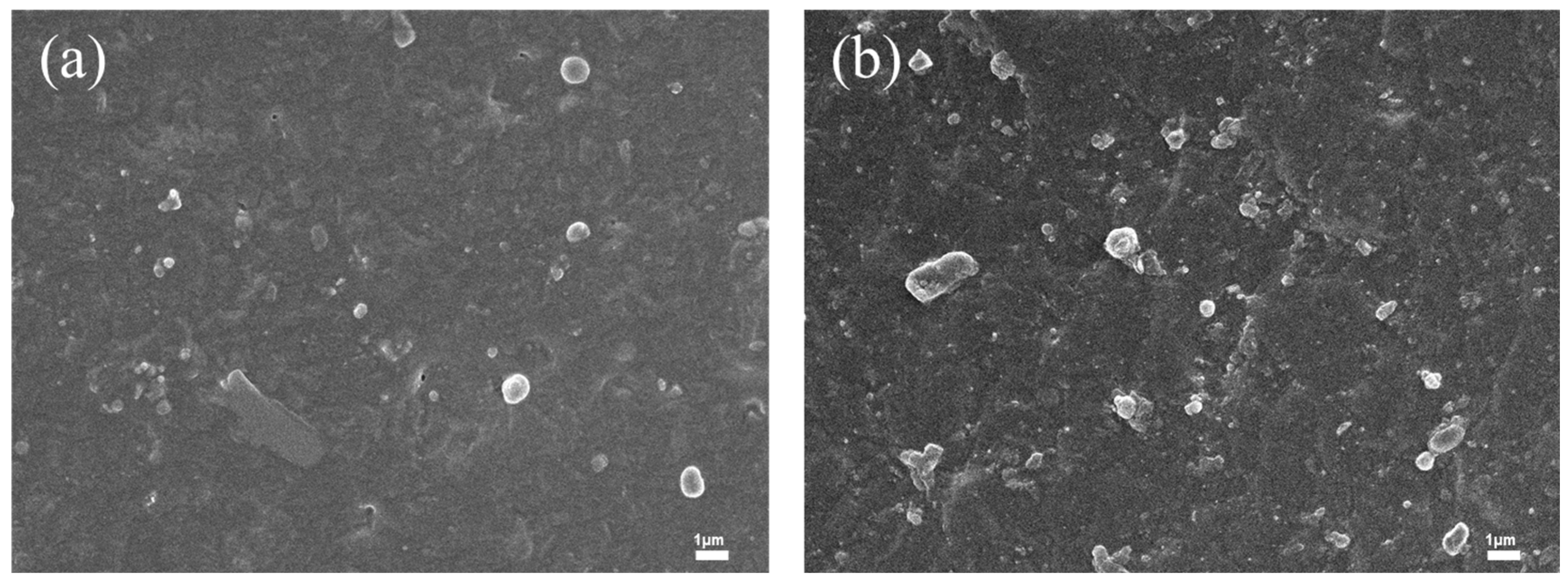
3.2. Lubrication and Tensile Properties of Internally Lubricated XNBR/Bentonite Films
3.2.1. Lubrication Performance of Internally Lubricated XNBR/Bentonite Films
3.2.2. Mechanical Properties of Internally Lubricated XNBR/Bentonite Films
3.3. Shear-Thinning Behavior of the Bentonite Aqueous Dispersion
3.4. Tribological and Mechanical Properties of Externally Lubricated XNBRL/Bentonite Films
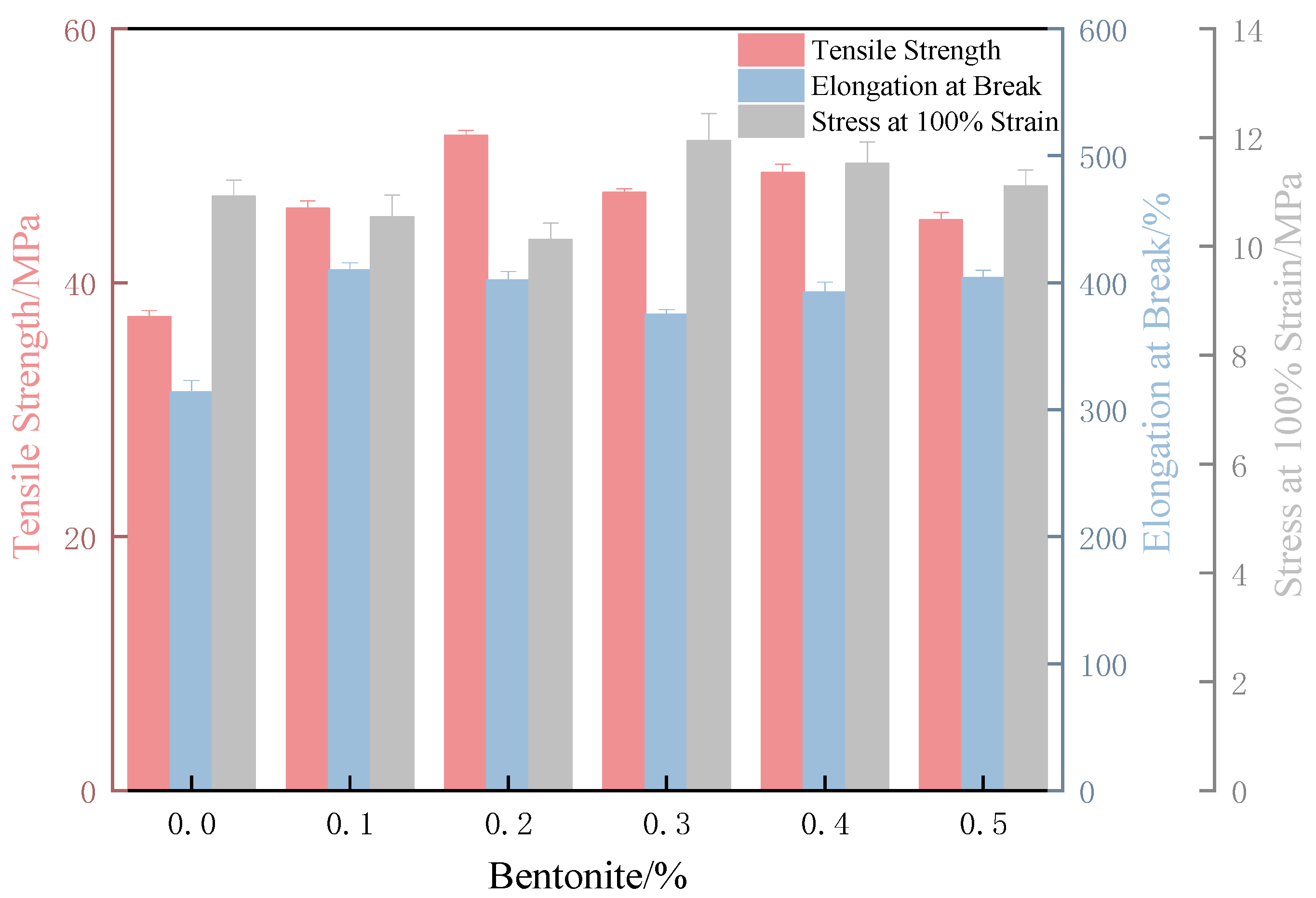
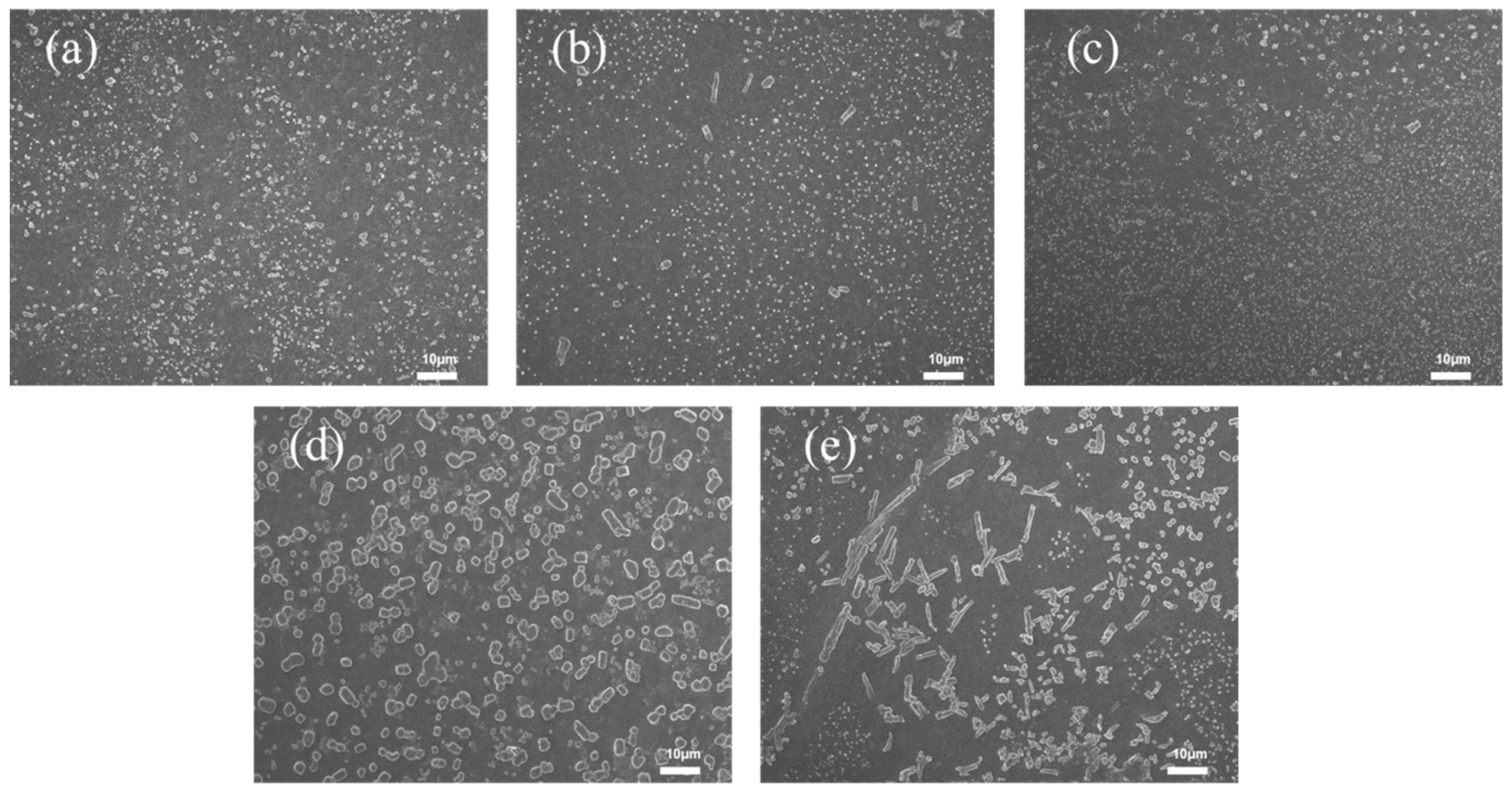
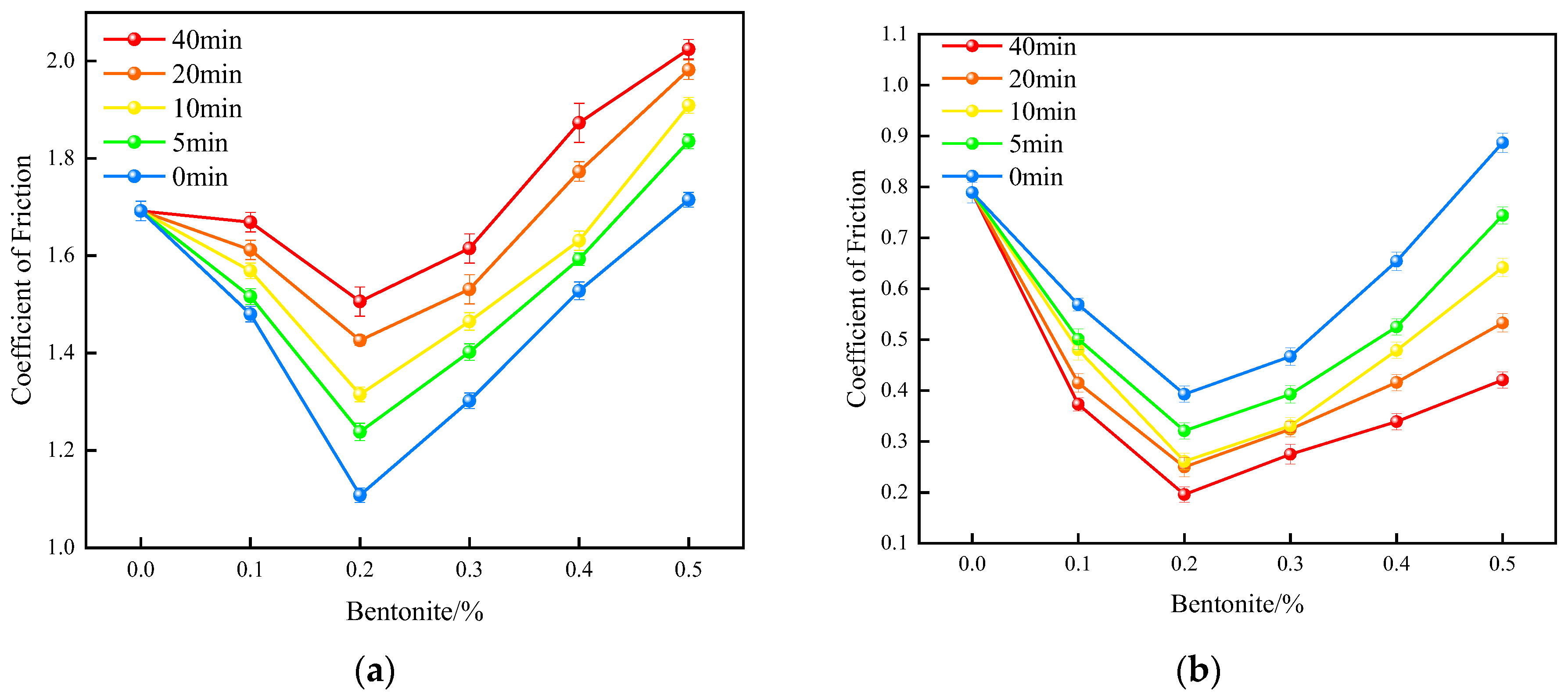
3.4.1. Effect of Bentonite Content on the Lubrication Performance of XNBRL/Bentonite Films
3.4.2. Effect of Bentonite Content on the Mechanical Properties of XNBR/Bentonite Composite Films
4. Conclusions
- (1)
- The incorporation of APAM into the XNBR matrix via blending yielded only modest gains in mechanical strength and tribological performance. This limited efficacy is attributed to the inherent tendency of APAM’s long polymer chains to entangle and form agglomerates, which compromises its reinforcing and lubricating functions.
- (2)
- The bentonite/XNBR films prepared via internal lubrication showed improved tensile and lubricating properties compared to APAM/XNBR, with a 48% reduction in friction coefficient and a 38% increase in tensile strength. However, due to the shear-thinning behavior of the bentonite aqueous solution, external lubrication further enhanced the surface lubricity, resulting in a 57% reduction in the wet friction coefficient and a 29% increase in tensile strength.
- (3)
- In terms of overall performance, the XNBR film with 0.2% bentonite added by internal lubrication (denoted as Film 1) exhibited the highest tensile strength (51.59 MPa), high elongation at break (401%), and intermediate stress at 100% elongation. Meanwhile, the film impregnated with 0.4% bentonite at 20 min of curing via external lubrication (denoted as Film 2) demonstrated the maximum elongation at break (480%), high tensile strength (47.21 MPa), and also intermediate stress at 100% elongation. Both films achieved optimal comprehensive mechanical properties. Although Film 1 showed higher dry and wet friction coefficients than Film 2, the latter maintained a continuously low-friction interface due to its high surface bentonite content and shear-thinning-enabled external lubrication. Thus, both Film 1 and Film 2 are preferable choices for high-performance, low-friction carboxylated nitrile rubber films.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zainal, H.; Hee, S.Q. Folpet permeation through nitrile gloves. Appl. Occup. Environ. Hyg. 2003, 18, 658–668. [Google Scholar] [CrossRef]
- Miller, J.M.; Collier, C.; Griffith, N. Permeability of surgical rubber gloves. Am. J. Surg. 1972, 124, 57–59. [Google Scholar] [CrossRef]
- Creta, M.; Savory, L.D.; Duca, R.; Chu, W.K.; Poels, K.; Pan, J.; Zheng, J.; Godderis, L.; Draper, M.; Vanoirbeek, J.A. An alternative method to assess permeation through disposable gloves. J. Hazard. Mater. 2021, 411, 125045. [Google Scholar] [CrossRef]
- Mahaling, R.N.; Jana, G.K.; Das, C.K.; Jeong, H.; Ha, C.S. Carboxylated nitrile elastomer/filler nanocomposite: Effect of silica nanofiller in thermal, dynamic mechanical behavior, and interfacial adhesion. Macromol. Res. 2005, 13, 306–313. [Google Scholar] [CrossRef]
- Daud, S.; You, Y.S.; Azura, A.R. The effect of acid hydrolyzed sago starch on mechanical properties of natural rubber and carboxylated nitrile butadiene rubber latex. Mater. Today Proc. 2019, 17, 1047–1055. [Google Scholar] [CrossRef]
- Bian, X.; Tuo, R.; Yang, W.; Zhang, Y.; Xie, Q.; Zha, J.; Lin, J.; He, S. Mechanical, thermal, and electrical properties of bn–epoxy composites modified with carboxyl-terminated butadiene nitrile liquid rubber. Polymers 2019, 11, 1548. [Google Scholar]
- Das, M.; Bhattacharya, A.B.; Parathodika, A.R.; Naskar, K. Room temperature self-healable and extremely stretchable elastomer with improved mechanical properties: Exploring a simplistic metal-ligand interaction. Eur. Polym. J. 2022, 174, 111341. [Google Scholar] [CrossRef]
- Paran, S.M.R.; Naderi, G.; Mosallanezhad, H.; Movahedifar, E.; Formela, K.; Saeb, M.R. Microstructure and mechanical properties of carboxylated nitrile butadiene rubber/epoxy/XNBR-grafted halloysite nanotubes nanocomposites. Polymers 2020, 12, 1192. [Google Scholar] [PubMed]
- Azizli, M.J.; Iranpoury, A.; Barghamadi, M.; Rezaeeparto, K.; Parham, S.; Jahankhah, Z.; Mokhtary, M.; Hashemi, M. Compatibilization of novel GO-XNBR-g-GMAC/XNBR/XSBR nanocomposites: The relationship between structure and properties. Compos. Interfaces 2023, 30, 803–826. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A. Effect of water adsorption on lubricating film stability in slippery coatings. Langmuir 2024, 40, 1633–1645. [Google Scholar] [CrossRef]
- Zuo, X.; Yu, H.; Zhou, F.; Zhang, X. Synergistic lubrication achieved by proton-ionic liquids and MoS2 as high-performance water-based lubricant additives. Langmuir 2025, 41, 5534–5545. [Google Scholar]
- Lovato, M.J.; del Valle, L.J.; Puiggalí, J.; Franco, L. Performance-enhancing materials in medical gloves. J. Funct. Biomater. 2023, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Esmizadeh, E.; Chang, B.P.; Jubinville, D.; Ojogbo, E.; Seto, C.; Tzoganakis, C.; Mekonnen, T.H. Can medical-grade gloves provide protection after repeated disinfection? ACS Appl. Polym. Mater. 2021, 3, 445–454. [Google Scholar] [CrossRef]
- Luo, M.; Chen, Z.; He, X.; Xie, T.; Bo, W.; Fan, P.; Bi, Q.; Xia, Q.; Li, C. Evaluation of the effects on the tensile properties of medical gloves after repeated disinfection. Sci. Rep. 2025, 15, 5266. [Google Scholar] [CrossRef]
- Gao, P.; Horvatin, M.; Niezgoda, G.; Weible, R. Effect of multiple alcohol-based hand rub applications on the tensile properties of thirteen brands of medical exam nitrile and latex gloves. J. Occup. Environ. Hyg. 2016, 13, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chang, C.; Lin, Y.; Chu, W. Evaluating the protective effectiveness of rubber glove materials against organic solvents upon repeated exposure and decontamination. Saf. Health Work 2024, 15, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Holtan, J.R.; Lua, M.J.; Belvedere, P.C.; Lambert, D.L. Evaluating the effect of glove coating on the shear bond strength of porcelain laminate veneers. J. Am. Dent. Assoc. 1995, 126, 611–616. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Li, N.; Jiang, Z. Preparation, characterization, and lubrication performances of water-based nanolubricant for micro rolling strips. Materials 2024, 17, 516. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, L.; Cheng, G.; Yuan, N.; Ding, J.; Pesika, N.S. Water-based lubrication of hard carbon microspheres as lubricating additives. Tribol. Lett. 2018, 66, 148. [Google Scholar] [CrossRef]
- Miao, K.; Wang, J.; Zhao, Q.; Wang, K.; Wen, M.; Zhang, K. Water-based lubrication of niobium nitride. Friction 2022, 10, 842–853. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Sun, F.; Shiu, B.; Ren, H.; Lou, C.; Lin, J. pH-responsive nonwoven fabric with reversibly wettability for controllable oil-water separation and heavy metal removal. Environ. Res. 2022, 215, 114355. [Google Scholar] [CrossRef]
- Baumli, P.; D’Acunzi, M.; Hegner, K.I.; Naga, A.; Wong, W.S.; Butt, H.; Vollmer, D. The challenge of lubricant-replenishment on lubricant-impregnated surfaces. Adv. Colloid Interface Sci. 2021, 287, 102329. [Google Scholar] [CrossRef] [PubMed]
- Najafi, P.; Zabihi, M.; Faghihi, M. Remarkable adsorption of anionic dye on the supported magnetic and non-magnetic polymeric nanocomposites including chitosan/polyacrylamide and chitosan/polylactic acid. Water Air Soil Pollut. 2024, 235, 366. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Di, H.; Liu, D.; Wang, X.; Zhao, Y. Influence of anionic polyacrylamide on the freeze–thaw resistance of silty clay. Cold Reg. Sci. Technol. 2024, 219, 104111. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. Optimal synthesis, characterization and antifouling performance of Pluronic F127/bentonite-based super-hydrophilic polyvinyl chloride ultrafiltration membrane for enhanced oilfield produced water treatment. J. Ind. Eng. Chem. 2020, 90, 58–75. [Google Scholar] [CrossRef]
- Koşarsoy Ağçeli, G.; Hammamchi, H.; Cihangir, N. Novel levan/bentonite/essential oil films: Characterization and antimicrobial activity. J. Food Sci. Technol. 2022, 59, 249–256. [Google Scholar] [CrossRef]
- Zewudie, A.G.; Zereffa, E.A.; Segne, T.A.; Murthy, H.C.A.; Ravikumar, C.R.; Muniswamy, D.; Binagdie, B.B. Biosynthesis of Ag/bentonite, ZnO/bentonite, and Ag/ZnO/bentonite nanocomposites by aqueous leaf extract of Hagenia abyssinica for antibacterial activities. Rev. Adv. Mater. Sci. 2023, 62, 20220307. [Google Scholar] [CrossRef]
- Martsouka, F.; Papagiannopoulos, K.; Hatziantoniou, S.; Barlog, M.; Lagiopoulos, G.; Tatoulis, T.; Tekerlekopoulou, A.G.; Lampropoulou, P.; Papoulis, D. The antimicrobial properties of modified pharmaceutical bentonite with zinc and copper. Pharmaceutics 2021, 13, 1190. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Qin, L.; Liang, W.; Chen, K.; Li, K.; Yan, R. Adsorption properties of cesium by natural Na-bentonite and Ca-bentonite. J. Radioanal. Nucl. Chem. 2024, 333, 5347–5361. [Google Scholar] [CrossRef]
- Oguntade, T.; Rotimi, O.; Mojisole, A.; Solomon, A.; Angye, G. Experimental dataset of enhanced rheological properties and lubricity of Nigerian bentonite mud using kelzan® xcd polymer and identifying it optimal combination. Data Brief 2018, 19, 1804–1809. [Google Scholar] [CrossRef]
- Makhoukhi, B.; Didi, M.A.; Villemin, D. Modification of bentonite with diphosphonium salts: Synthesis and characterization. Mater. Lett. 2008, 62, 2493–2496. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; De Oliveira, V.R.L.; Santos, F.K.G.D.; De Barros Neto, E.L.; De Lima Leite, R.H.; Aroucha, E.M.M.; De Oliveira Silva, K.N. Synergistic effect of the sequential intercalation of three types of surfactants in the exfoliation degree of bentonite clay in films of cassava. J. Mol. Liq. 2018, 266, 770–780. [Google Scholar] [CrossRef]
- Yu, L.; Wang, D.; Tan, Y.; Du, J.; Xiao, Z.; Wu, R.; Xu, S.; Huang, J. Super tough bentonite/SiO2-based dual nanocomposite hydrogels using silane as both an intercalator and a crosslinker. Appl. Clay Sci. 2018, 156, 53–60. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, Z.; Tong, L. On promoting intercalation and exfoliation of bentonite in high-density polyethylene by grafting acrylic acid. J. Appl. Polym. Sci. 2005, 96, 2429–2434. [Google Scholar] [CrossRef]
- Mohanta, S.; Hussain, S.; Sarangi, P.L.; Mohanty, S.P. Exfoliation of bentonite clay by ultrasonication and evaluation of the polypropylene-based composites manufactured thereof. Bull. Mater. Sci. 2025, 48, 57. [Google Scholar] [CrossRef]
- Sarkar, M.; Dana, K.; Ghatak, S.; Banerjee, A. Polypropylene-clay composite prepared from Indian bentonite. Bull. Mater. Sci. 2008, 31, 23–28. [Google Scholar] [CrossRef]
- Alekseeva, O.V.; Rodionova, A.N.; Bagrovskaya, N.A.; Noskov, A.V.; Agafonov, A.V. Bentonite filler effect on structure and properties of polystyrene-based composites. Iran. Polym. J. 2019, 28, 123–133. [Google Scholar]











| Raw Material Name | Dry Weight Formulation/phr |
|---|---|
| XNBRL | 100 |
| S | 1.25 |
| ZDEC | 0.5 |
| TMTD | 0.5 |
| Titanium dioxide | 1 |
| ZnO | 3 |
| KOH | 1.2 |
| Casein | 0.4 |
| Antioxidant445 | 0.5 |
| ZDMA | 5 |
| APAM | Variable |
| Bentonite | Variable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, J.; Wu, M. A Carboxylated Nitrile Butadiene Rubber Latex Film with Synergistically Enhanced Water-Based Lubricity and Tensile Strength: Fabrication and Characterization. Polymers 2025, 17, 2436. https://doi.org/10.3390/polym17182436
Zhai J, Wu M. A Carboxylated Nitrile Butadiene Rubber Latex Film with Synergistically Enhanced Water-Based Lubricity and Tensile Strength: Fabrication and Characterization. Polymers. 2025; 17(18):2436. https://doi.org/10.3390/polym17182436
Chicago/Turabian StyleZhai, Jinting, and Mingsheng Wu. 2025. "A Carboxylated Nitrile Butadiene Rubber Latex Film with Synergistically Enhanced Water-Based Lubricity and Tensile Strength: Fabrication and Characterization" Polymers 17, no. 18: 2436. https://doi.org/10.3390/polym17182436
APA StyleZhai, J., & Wu, M. (2025). A Carboxylated Nitrile Butadiene Rubber Latex Film with Synergistically Enhanced Water-Based Lubricity and Tensile Strength: Fabrication and Characterization. Polymers, 17(18), 2436. https://doi.org/10.3390/polym17182436





