Valorization of Agro-Industrial Lignin as a Functional Polymer for Sustainable Wastewater Treatment
Abstract
1. Introduction
- Hypothesis of Performance and Selectivity: We hypothesize that the unmodified Sarkanda grass lignin, owing to its distinct physicochemical properties (e.g., high density of carboxyl/phenolic groups), will exhibit significant but highly selective adsorption capacities towards a diverse suite of cationic, anionic, and neutral heavy metal(loid) pollutants.
- Hypothesis of Mechanistic Unification: We hypothesize that despite the varied chemical nature of the pollutants, a common, underlying rate-limiting mechanism—namely chemisorption, as evidenced by pseudo-second-order kinetics—will consistently govern the adsorption process across all systems, demonstrating a unified reactive behavior of the lignin’s surface.
2. Materials and Methods
2.1. Materials
2.1.1. Chemical Characteristics
- Acidic Functional Groups: The material possesses a notable content of carboxyl groups (COOH), quantified at 3.3 mmol/g [19,20]. Additionally, aromatic hydroxyl (phenolic OH) groups were reported at 1.7 mmol/g [19,20]. These acidic moieties are crucial for cation adsorption, primarily through ion exchange mechanisms where protons are released upon binding of metal cations (Mn+).
- Additional Oxygen-containing Groups: functional moieties inherent to the lignin structure include aliphatic hydroxyl groups (e.g., –CH2OH, –CHOH–), carbonyl groups (C=O), and methoxy groups (–OCH3). While aliphatic hydroxyls and carbonyls can participate in metal coordination and hydrogen bonding, methoxy groups—though less reactive—contribute to the overall polarity and hydrophilic/hydrophobic balance of the material.
- Chelating Capacity: Consistent with the presence of oxygen-containing functional groups amenable to coordination, the lignin exhibited a significant chelating capacity, reported as 67.14 meq/100 g [19,20]. This suggests a strong potential for forming stable chelate complexes with various metal ions, contributing significantly to the adsorption process, especially via mechanisms characterized by chemisorption.
- General Composition: Further compositional analysis indicated high purity, with acid-insoluble lignin content reported at 87% [20,21,22]. A low ash content of 2.2% signifies minimal inorganic contamination, while a nitrogen content of 1.2% [20,21,22] might suggest the presence of minor protein residues or inherent nitrogenous structures within the lignin framework, although specific nitrogen-containing functional groups like amines were not explicitly quantified or discussed in the context of adsorption mechanisms in these studies.
2.1.2. Physical Properties and Surface Morphology
- Particle Size: Particle size analysis indicated dimensions primarily in the micrometer range, with an average particle diameter reported as approximately 1–4 µm [19,20], although particle size distribution data suggests a broader range may exist. These dimensions have implications for diffusion pathways and settling characteristics in potential applications.
2.2. Experimental Procedure
2.2.1. Adsorption Methodology
2.2.2. Analytical Techniques for Metal Ion Quantification
2.2.3. Equilibrium and Kinetic Modeling
- Equilibrium Isotherms: The Langmuir and Freundlich isotherm models were universally applied to describe the equilibrium distribution of metal ions between the aqueous and solid phases [18,19,20,21,22,23,24,25,26,27,28]. The goodness-of-fit was typically evaluated based on the correlation coefficient (R2) of the linearized forms of these models.
2.3. Supporting Analyses
2.4. Methodology: Seed Germination and Seedling Growth Assays
- Filtrates: The aqueous phase remaining after the separation of lignin post-adsorption.
- Contaminated Lignin: The solid lignin adsorbent after it had been loaded with the target metal ion.
3. Results and Discussion
3.1. Optimization of Experimental Conditions
3.1.1. Influence of Adsorbent Dose
3.1.2. Critical Role of Solution pH and Contact Time
- pH 6.5 for Fe(II) [26].
3.2. Equilibrium Studies: Adsorption Isotherms and Capacity
- Freundlich Model Predominance: For many of the metal ions, including As(III) [18], Cd(II) [19,20], Co(II) [23], Fe(II) [26], and Ni(II) [27], the Freundlich isotherm generally provided a better fit to the experimental data (higher R2 values) compared to the Langmuir model. This suggests that the adsorption primarily occurs on a heterogeneous surface with a non-uniform distribution of binding energies, which is characteristic of complex biopolymers like lignin, and that the adsorption of these species onto the heterogeneous surface of Sarkanda grass lignin, possibly involving multilayer adsorption or sites with varying binding energies, is well described by this empirical model.
- Comparable Fits/Langmuir Applicability: For Cr(VI) [21,22], Cu(II) [24,25], Pb(II), and Zn(II) [28], both models often described the data well, with R2 values being high and comparable for both ((R2 > 0.98)). In some of these cases, the Langmuir model also showed good applicability, implying that monolayer adsorption on a finite number of energetically equivalent sites might also contribute significantly to the overall uptake.
3.3. Kinetic Studies: Adsorption Rate and Mechanism Pathway
3.3.1. Proposed Mechanisms for Divalent Cations (Cu(II), Ni(II), Co(II), Cd(II), Pb(II), Zn(II), and Fe(II))
- Ion Exchange: This is a prominent mechanism explaining the high uptake of divalent cations on the Sarkanda grass lignin studied by stoichiometric ion exchange. This process specifically involves the acidic carboxyl and phenolic hydroxyl groups characterized in Section 2.1. At the operational pH range of 5–6.5, these groups are sufficiently deprotonated to facilitate a direct exchange of surface protons (H+) for metal cations (M2+) from the solution, as represented by the following equilibrium reactions:
- Complexation/Chelation: Metal ion binding to lignin is largely governed by coordinate interactions with electron-donating functional groups, particularly oxygen atoms in hydroxyl, carboxyl, and carbonyl moieties. These interactions lead to the formation of surface complexes, often involving chelation—a process where a metal ion binds simultaneously to multiple donor atoms within the same lignin macromolecule. Such binding contributes to the chemisorptive nature of the process, which cannot be fully explained by simple ion exchange alone. Adsorption likely proceeds through an initial, non-specific electrostatic attraction between hydrated metal cations and the negatively charged lignin surface, forming outer-sphere complexes. This is followed by a slower, rate-determining transition to inner-sphere complexes, in which direct, specific coordination bonds are established. Chelation plays a central role in this step, with multidentate binding sites on lignin facilitating the formation of stable five- or six-membered rings. Notable examples include coordination between a carboxyl and adjacent hydroxyl group or between neighboring phenolic hydroxyl and methoxyl groups in guaiacyl units. Lignin’s high chelating capacity (67.14 meq/100 g) underscores its affinity for such metal–ligand interactions. This is particularly evident in the case of Cu(II), which demonstrates the highest adsorption capacity (qmax = 22.12 mg/g) among the studied divalent cations. Its superior uptake is likely due to a combination of its favorable ionic radius, higher electronegativity, and strong preference for forming stable chelates with oxygen-containing ligands—factors consistent with the Irving–Williams series [25].
3.3.2. Proposed Mechanisms for Cr(VI)
- Electrostatic Attraction: At acidic pH (e.g., pH 5), lignin’s surface functional groups (like carboxyls and even some hydroxyls or trace amines, if present) can become protonated, leading to a net positive surface charge. This facilitates electrostatic attraction with the negatively charged chromate species.
- Ligand Exchange/Complexation: Chromate ions might directly complex with protonated functional groups or displace weaker ligands (like water) from these sites.
- Reduction to Cr(III) followed by Cationic Adsorption: Lignin, being a phenolic polymer, possesses reducing capabilities. It is plausible that some Cr(VI) is reduced to the less toxic Cr(III) by the lignin. The resulting Cr(III) cations could then be adsorbed via the ion exchange or complexation mechanisms described for other cations in the previous paragraph. The paper on biological testing of Cr(VI) loaded lignin [16] mentions “formation of free radicals during the reduction of Cr(VI) to Cr(III)” as a possible toxic action, indirectly supporting this redox pathway.
3.3.3. Proposed Mechanisms for Metalloid As(III)
- Hydrogen Bonding: The multiple hydroxyl groups of H3AsO3 can readily form hydrogen bonds with the abundant oxygen-containing functional groups (hydroxyl, carboxyl, carbonyl, ether linkages) on the lignin surface. This is a plausible primary interaction for a neutral species.
- Surface Complexation: Direct complexation of H3AsO3 with specific lignin sites, particularly phenolic hydroxyls, cannot be entirely ruled out, potentially involving ligand exchange.
- Oxidation to As(V) followed by Anionic Adsorption: While less commonly discussed for As(III) interaction with lignin compared to other reducing agents, there is a possibility of partial surface oxidation of As(III) to As(V) (which forms H2AsO4−/HAsO42− at pH 6). These arsenate anions could then adsorb via mechanisms similar to Cr(VI), i.e., electrostatic interaction or ligand exchange with protonated sites.
3.3.4. Correlation Between Metal Properties and Adsorption Affinity
- Ionic Radius and Charge Density: Smaller ionic radii and higher charge densities can lead to stronger electrostatic interactions and more stable complex formation. However, this is often counter-balanced by hydration effects [31].
- Hydrated Ionic Radius: Metal ions exist as hydrated species in aqueous solution. The energy required for desolvation (removal of the hydration shell) before adsorption can be significant and varies between ions. Ions with larger hydrated radii might face greater steric hindrance when accessing binding sites within the lignin’s porous structure [32].
- Electronegativity and Lewis Acidity: Higher electronegativity (Pauling scale) generally correlates with a greater tendency to accept electrons and form stronger covalent/coordinate bonds. Metal ions act as Lewis acids, and their strength influences their ability to interact with Lewis base sites on lignin (e.g., oxygen atoms in -OH, -COOH, C=O) [33].
- Copper(II) (qmax = 22.12 mg/g) exhibits a significantly higher adsorption capacity than the other M2+ ions (~13–14 mg/g). This is a common observation in biosorption and can be attributed to several factors:
- Irving–Williams Series: This empirical series often predicts the relative stability of complexes formed by divalent transition metal ions. It generally follows the order of Mn(II) < Fe(II) < Co(II) < Ni(II) < Cu(II) > Zn(II), meaning that copper (Cu) complexes are generally the most stable, while zinc (Zn) complexes are the least stable. The exceptionally high stability of Cu(II) complexes aligns with its superior adsorption capacity on lignin, where complexation is a key mechanism [36].
- “Hard–Soft” Acid–Base (HSAB) Principle: Lignin’s oxygen-containing functional groups (-OH, -COOH) are considered “hard” Lewis bases. According to the HSAB principle, hard bases prefer to bind with hard Lewis acids. Most of the divalent metal ions studied (e.g., Fe2+, Co2+, Ni2+, Cu2+, Zn2+, and Cd2+ to some extent) have borderline to hard acid characteristics, making them suitable partners for lignin’s functional groups. Pb2+, being a softer acid, might exhibit slightly different binding preferences or strengths, though its capacity was comparable to other hard/borderline cations in this system [37,38].
- Hydrolysis Constants (pKa values for M(H2O)x2+): The tendency of metal ions to hydrolyze and form hydroxo-complexes can influence their speciation and adsorption. The operational pH was generally chosen to minimize precipitation, but subtle differences in hydrolysis behavior near the optimal pH could play a role [39].
- Coordination Number and Geometry Preferences: Different metal ions have preferences for specific coordination numbers (e.g., 4, 6) and geometries (e.g., tetrahedral, octahedral, square planar). The ability of lignin to provide a suitable coordination environment will influence binding affinity [40].
3.4. Direct Evidence of Adsorption from Surface Analysis
3.5. Thermodynamic Insights
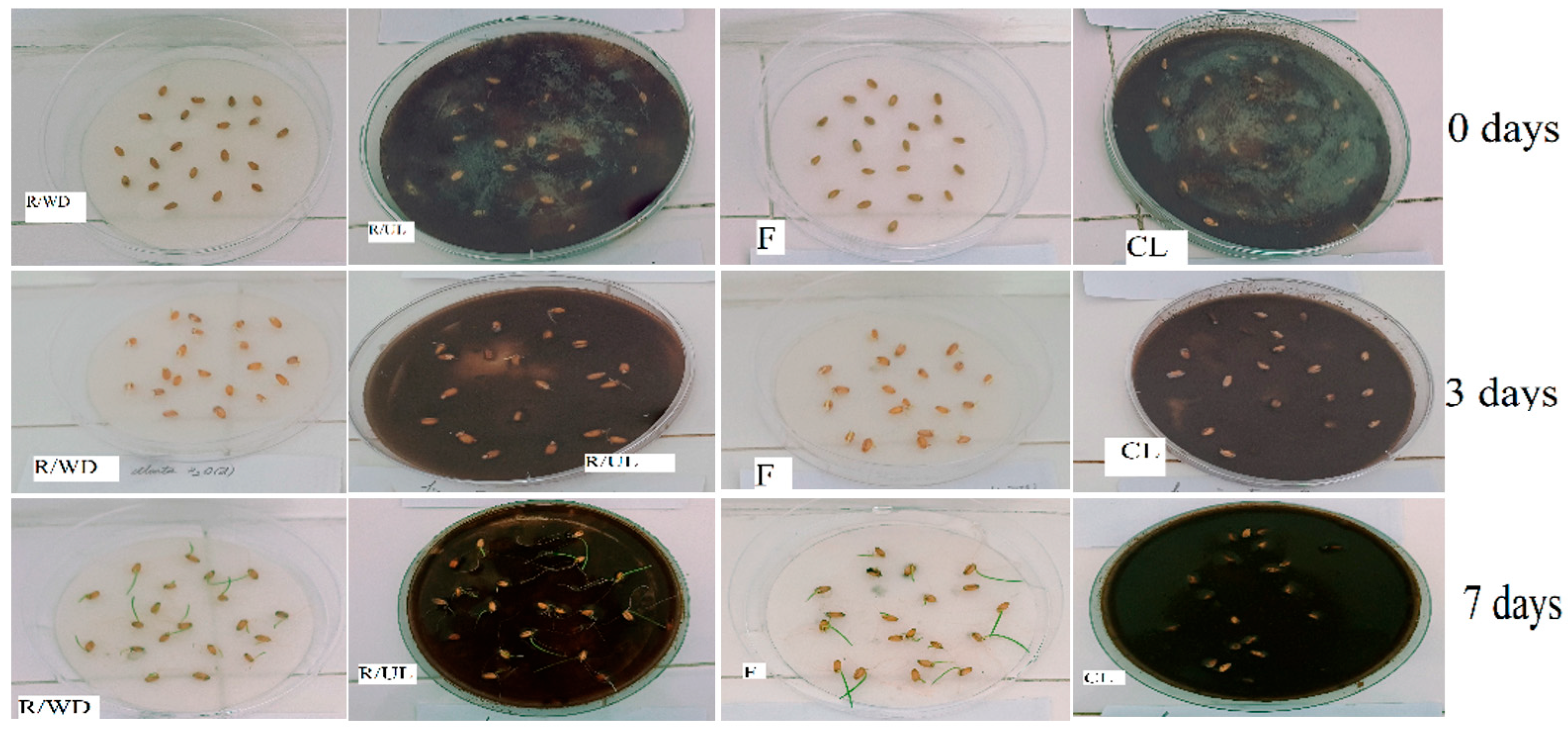
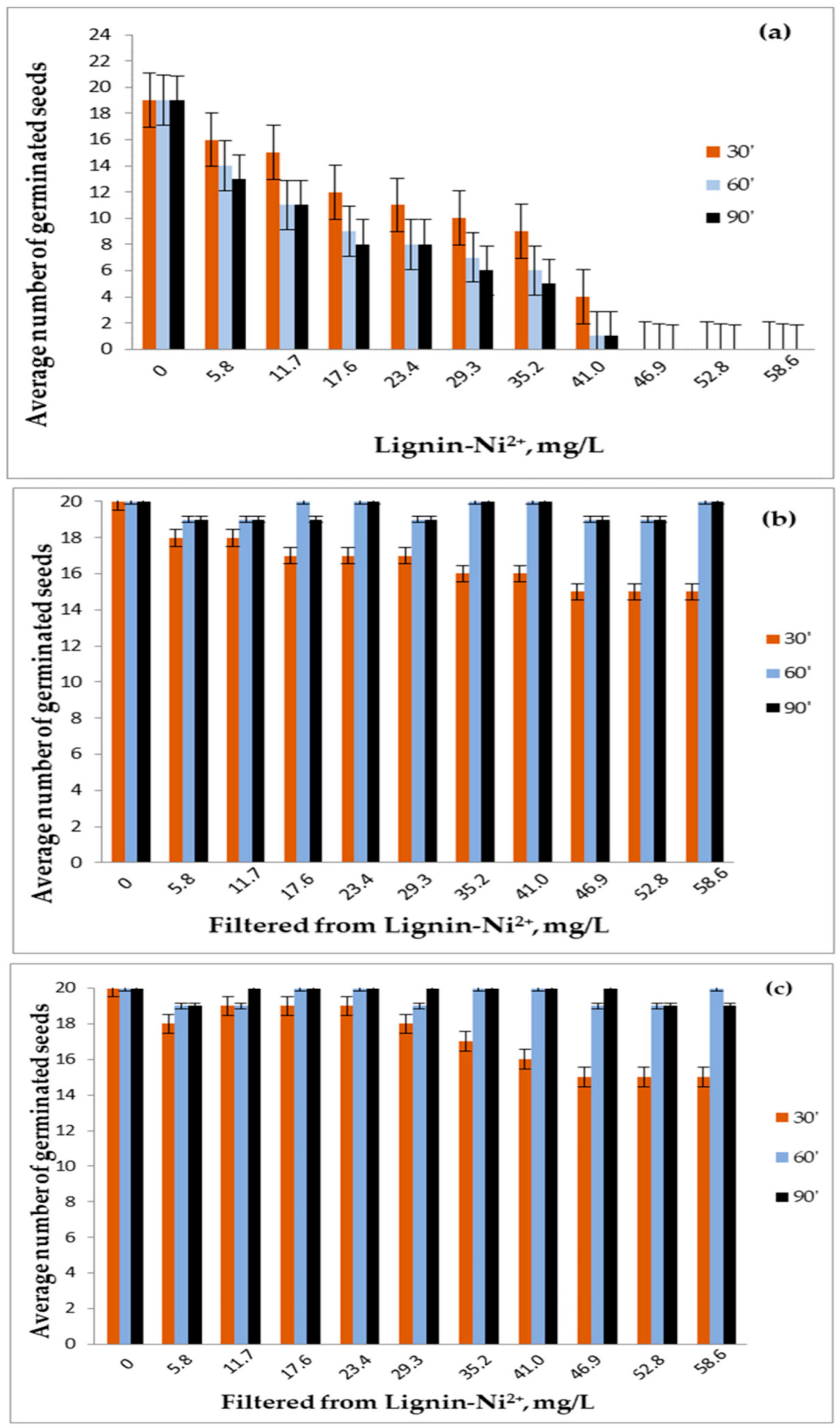
3.6. Biological Assessment of Adsorption Efficiency and Ecotoxicity
3.6.1. Evidence for Effective Pollutant Removal from Aqueous Phase (Filtrate Bioassays)
3.6.2. Evidence for Metal Sequestration and Immobilization (Contaminated Lignin Bioassays)
3.6.3. Ecological Implications of Bioassay Findings
3.7. Integration with Physicochemical Adsorption Data
3.8. Comparative Context: Performance of Sarkanda Grass Lignin in Relation to Other Lignin-Based Adsorbents
4. Conclusions and Future Perspectives
4.1. Overall Significance
4.2. Concluding Remarks
- Advanced Mechanistic Elucidation: While chemisorption is evident, employing advanced spectroscopic techniques (e.g., FTIR, XPS, NMR) pre- and post-adsorption for each metal could provide more definitive evidence of the specific functional groups involved in binding, their coordination environments, and the nature of the metal–lignin bonds. This could further explain the observed affinity differences (e.g., the Cu(II) preference).
- Regeneration, Reusability, and Circularity: To fully realize the sustainability potential of Sarkanda grass lignin, comprehensive regeneration and reusability studies are important. The current work establishes its efficacy for a single use; future research must focus on developing effective and environmentally benign methods for desorbing the bound metal ions to regenerate the active sites. Potential strategies could involve washing with dilute acidic solutions (e.g., HCl, HNO3) to displace the metal cations via ion exchange with protons, or using chelating agents (e.g., EDTA) to strip the metals. The success of regeneration, the number of cycles the adsorbent can withstand without significant loss of capacity, and the management of the concentrated metal-rich eluate are all critical factors that will determine the material’s economic viability and its true place in a circular economy. The toxicity of the metal-loaded lignin, as demonstrated in our bioassays, underscores the importance of either safe disposal or, preferably, effective regeneration for sustainable application.
- Performance in Complex Systems: Real-world wastewaters are complex matrices containing multiple competing ions, organic matter, and varying ionic strengths. Future studies should focus on:
- Competitive Adsorption: Evaluating the selectivity of Sarkanda grass lignin in multi-metal systems.
- Real Wastewater Testing: Assessing performance using actual industrial or contaminated water samples.
- Optimization of Adsorption Conditions and Influence of Ionic Strength: Further exploration of the effect of temperature (to validate the endothermic nature across all metals and find optimal ranges) is warranted. Critically, investigating the impact of ionic strength by adding a background electrolyte (e.g., NaCl) would provide deeper mechanistic insight. A significant decrease in cation adsorption with increasing ionic strength would confirm the major role of non-specific electrostatic interactions (outer-sphere complexation), while processes less affected by ionic strength would point towards strong, specific inner-sphere complexation. This is essential for assessing the adsorbent’s performance in real, high-salinity industrial wastewaters.
- Process Scale-Up and Engineering: Transitioning from batch studies to continuous-flow column experiments is essential for evaluating dynamic adsorption behavior, breakthrough characteristics, and for designing practical treatment systems.
- Targeted Lignin Modification: Based on the baseline performance of this unmodified lignin, targeted chemical modifications could be strategically designed to improve capacity or selectivity for specific pollutants, particularly those for which the unmodified form shows lower affinity (like Cr(VI) and As(III)). Such modifications could include amination to introduce positive charges for anion binding, phosphorylation, or grafting of specific chelating ligands. Critically, any such modification study must be accompanied by a thorough characterization of the resulting material, including a clear summary of morphological changes to assess alterations in particle size, porosity, and surface texture, and how these physical changes correlate with the enhanced adsorption performance.
- Life Cycle and Economic Assessment: A thorough life cycle assessment (LCA) and techno-economic analysis would be important to evaluate the overall environmental benefits and financial viability of using Sarkanda grass lignin for wastewater treatment in comparison to conventional methods and other biosorbents.
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Iordache, A.M.; Nechita, C.; Pluhacek, T.; Iordache, M.; Zgavarogea, R.; Ionete, R.E. Past and present anthropic environmental stress reflect high susceptibility of natural freshwater ecosystems in Romania. Environ. Pollut. 2020, 267, 115505. [Google Scholar] [CrossRef] [PubMed]
- Vintila, T.; Negrea, A.; Barbu, H.; Sumalan, R.; Kovacs, K. Metal distribution in the process of lignocellulosic ethanol production from heavy metal contaminated sorghum biomass. J. Chem. Technol. Biotechnol. 2016, 91, 1607–1614. [Google Scholar] [CrossRef]
- Rahoui, S.; Chaoui, A.; El Ferjani, E. Membrane damage and solute leakage from germinating pea seed under cadmium stress. J. Hazard. Mater. 2010, 178, 1128–1131. [Google Scholar] [CrossRef]
- Tanase, C.; Volf, I.; Popa, V.I. Enhancing Copper and Lead Bioaccumulation in Rapeseed by Adding Hemp Shives as Soil Natural Amendments. J. Environ. Eng. Landsc. Manag. 2014, 22, 245–253. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Das, S. Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: A comprehensive review. J. Environ. Chem. Eng. 2020, 9, 104686. [Google Scholar] [CrossRef]
- Macena, M.; Pereira, H.; Cruz-Lopes, L.; Grosche, L.; Esteves, B. Competitive Adsorption of Metal Ions by Lignocellulosic Materials: A Review of Applications, Mechanisms and Influencing Factors. Separations 2025, 12, 70. [Google Scholar] [CrossRef]
- Shree, B.; Kumari, S.; Singh, S.; Rani, I.; Dhanda, A.; Chauhan, R. Exploring various types of biomass as adsorbents for heavy metal remediation: A review. Environ. Monit. Assess. 2025, 197, 406. [Google Scholar] [CrossRef]
- Wang, B.; Lan, J.; Bo, C.; Gong, B.; Ou, J. Adsorption of heavy metal onto biomass-derived activated carbon: Review. RSC Adv. 2023, 13, 4275–4302. [Google Scholar] [CrossRef] [PubMed]
- Ben Amar, M.; Mallek, M.; Valverde, A.; Monclús, H.; Myers, T.G.; Salvadó, V.; Cabrera-Codony, A. Competitive Heavy Metal Adsorption on Pinecone Shells: Mathematical Modelling of Fixed-Bed Column and Surface Interaction Insights. Sci. Total Environ. 2024, 917, 170398. [Google Scholar] [CrossRef] [PubMed]
- González-Feijoo, R.; Santás-Miguel, V.; Arenas-Lago, D.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Arias-Estévez, M.; Pérez-Rodríguez, P. Effectiveness of Cork and Pine Bark Powders as Biosorbents for Potentially Toxic Elements Present in Aqueous Solution. Environ. Res. 2024, 250, 118455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Wang, D.; Yu, D.; Wu, C. Lignin-based adsorbents for heavy metals. Ind. Crops Prod. 2023, 193, 116119. [Google Scholar] [CrossRef]
- Singh, A.K.; Bilal, M.; Iqbal, H.M.N.; Meyer, A.S.; Raj, A. Bioremediation of lignin derivatives and phenolics in wastewater with lignin modifying enzymes: Status, opportunities and challenges. Sci. Total Environ. 2021, 777, 145988. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, B.; Chen, J.; Yang, G.; Zhang, L.; Liu, K.; Jiang, Q.; Fatehi, P. Lignin-based Hydrogel: Mechanism, Properties, and Applications. In Lignin Chemistry; Liao, Y., Sels, B.F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New opportunities in the valorization of technical lignins. ChemSusChem 2020, 14, 1016–1036. [Google Scholar] [CrossRef]
- Capraru, A.M.; Ungureanu, E.; Trinca, L.C.; Malutan, T.; Popa, V.I. Chemical and spectral characteristics of annual plant lignins modified by hydroxymethylation reaction. Cellul. Chem. Technol. 2012, 46, 589. [Google Scholar]
- Ungureanu, E.; Ungureanu, O.; Capraru, A.M.; Popa, V.I. Chemical Modification and Characterization of Straw Lignin. Cellul. Chem. Technol. 2009, 43, 261–267. [Google Scholar]
- Ungureanu, E.; Jităreanu, D.C.; Trofin, A.E.; Fortună, M.E.; Ungureanu, O.; Ariton, A.M.; Trincă, L.C.; Brezuleanu, S.; Popa, V.I. Use of Sarkanda Grass lignin as a possible adsorbent for As(III) from aqueous solutions—Kinetic and equilibrium studies. Cellul. Chem. Technol. 2022, 56, 681–689. [Google Scholar] [CrossRef]
- Ungureanu, E.; Fortună, M.E.; Țopa, D.C.; Brezuleanu, C.O.; Ungureanu, V.I.; Chiruță, C.; Rotaru, R.; Tofanica, B.M.; Popa, V.I.; Jităreanu, D.C. Comparison Adsorption of Cd (II) onto Lignin and Polysaccharide-Based Polymers. Polymers 2023, 15, 3794. [Google Scholar] [CrossRef]
- Ungureanu, E.; Chelariu, L.E.; Ungureanu, O.C.; Fortună, M.E.; Popa, V.I.; Jităreanu, D.C. Thermodynamic studies on Cd (II) retention from aqueous medium on Sarkanda grass lignin nanoparticles. Sci. Pap. Ser. B Hortic. 2023, 66, 7–10. [Google Scholar]
- Ungureanu, E.; Chelariu, L.E.; Tofănică, B.M.; Popa, V.I.; Jităreanu, D.C. Optimization and modeling of Cr (VI) biosorption from aqueous solution on biosorbent—Sarkanda grass lignin. Sci. Pap. Ser. B Hortic. 2022, 65, 13–18. [Google Scholar]
- Ungureanu, E.; Tofănică, B.M.; Ungureanu, O.C.; Fortună, M.E.; Volf, I.; Popa, V.I. Lignin-based biomass fractions for Cr(VI) adsorption from aqueous media—Thermodynamic, spectral and biological analysis. Bul. Inst. Polit. Iasi 2024, 70, 57–64. [Google Scholar]
- Ungureanu, E.; Ulea, E.; Samuil, C.; Ungureanu, O.C.; Fortună, M.E.; Rotaru, R.; Tofanica, B.M.; Popa, V.I. Renewable Lignin-Based Biomaterials for the Adsorption of Co(II) Ions from Wastewaters. Cellul. Chem. Technol. 2024, 58, 917–928. [Google Scholar] [CrossRef]
- Ungureanu, E.; Jităreanu Doina, C.; Trofin Alina, E.; Ungureanu, O.C.; Fortună Maria, E.; Ariton Adina, M.; Trincă Lucia, C.; Popa, V.I. Adsorption of Cu (II) from aqueous solution on Sarkanda Grass lignin: Equilibrium and kinetic studies. Sci. Pap. Ser. B Hortic. 2022, 65, 15–20. [Google Scholar]
- Ungureanu, E.; Tofănică, B.M.; Fortună, M.E.; Ungureanu, O.C.; Volf, I. Bioremediation in circular economy: Case study of Cu(II) removal via lignin-based biomass. Bul. Inst. Polit. Iasi 2025, 71, 75–84. [Google Scholar]
- Ungureanu, E.; Tofanică, B.M.; Ungureanu, O.C.; Fortună, M.E.; Rotaru, R.; Brezuleanu, C.O.; Frunză, G.; Popa, V.I. Lignin-based biowaste and its use for adsorbing Fe(II) ions from aqueous environments. Cellul. Chem. Technol. 2025, 59, 451–462. [Google Scholar] [CrossRef]
- Ungureanu, E.; Samuil, C.; Țopa, D.C.; Ungureanu, O.C.; Tofanica, B.-M.; Fortună, M.E.; Brezuleanu, C.O. Adsorption of Ni(II) from Aqueous Media on Biodegradable Natural Polymers—Sarkanda Grass Lignin. Crystals 2024, 14, 381. [Google Scholar] [CrossRef]
- Ungureanu, E.; Trofin, A.E.; Trincă, L.C.; Ariton, A.M.; Ungureanu, O.C.; Fortună, M.E.; Jităreanu, C.D.; Popa, V.I. Studies on kinetics and adsorption equilibrium of lead and zinc ions from aqueous solutions on Sarkanda Grass lignin. Cellul. Chem. Technol. 2021, 55, 939–948. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 6 August 2025).
- Allegretti, C.; Boumezgane, O.; Rossato, L.; Strini, A.; Troquet, J.; Turri, S.; Griffini, G.; D’Arrigo, P. Tuning lignin characteristics by fractionation: A versatile approach based on solvent extraction and Membrane-Assisted ultrafiltration. Molecules 2020, 25, 2893. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Thermodynamics of solvation of ions. Part 5—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Huheey, J.E.; Keiter, E.A.; Keiter, R.L. Inorganic Chemistry: Principles of Structure and Reactivity; HarperCollins: New York, NY, USA, 1993. [Google Scholar]
- Halcrow, M.A. Jahn–Teller distortions in transition metal compounds, and their importance in functional molecular and inorganic materials. Chem. Soc. Rev. 2012, 42, 1784–1795. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. 637. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Burgot, G.; Burgot, J.L. General Analytical Chemistry, 1st ed.; Taylor & Francis Ltd.: Milton Park, UK, 2023. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1996. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2009, 156, 2–10. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater 2008, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A.; Hasan, S.H.; Ducoste, J.J. Cellulosic substrates for removal of pollutants from aqueous systems: A review. 1. Metals. BioResources 2011, 6, 2161–2287. [Google Scholar] [CrossRef]
- Tkachenko, O.; Diment, D.; Rigo, D.; StrøMme, M.; Budnyak, T.M. Unveiling the Nature of lignin’s Interaction with Molecules: A Mechanistic Understanding of Adsorption of Methylene Blue Dye. Biomacromolecules 2024, 25, 4292–4304. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, Y.-C.; Sun, R.-C. Fractionational and structural characterization of lignin and its modification as biosorbents for efficient removal of chromium from wastewater: A review. J. Leather Sci. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Reddad, Z.; Gerente, C.; Andres, Y.; Cloirec, P.L. Adsorption of Several Metal Ions onto a Low-Cost Biosorbent: Kinetic and Equilibrium Studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef]
- McKay, G.; Porter, J.F. Equilibrium Parameters for the Sorption of Copper, Cadmium and Zinc Ions onto Peat. J. Chem. Technol. Biotechnol. 1997, 69, 309–320. [Google Scholar] [CrossRef]
- Sun, G.; Shi, W. Sunflower Stalks as Adsorbents for the Removal of Metal Ions from Wastewater. Ind. Eng. Chem. Res. 1998, 37, 1324–1328. [Google Scholar] [CrossRef]
- Lourenço, A.; Kukić, D.; Vasić, V.; Costa, R.A.; Antov, M.; Šćiban, M.; Gominho, J. Valorisation of Lignocellulosic Wastes, the Case Study of Eucalypt Stumps Lignin as Bioadsorbent for the Removal of Cr(VI). Molecules 2022, 27, 6246. [Google Scholar] [CrossRef]
- Demirbas, A. Adsorption of lead and cadmium ions in aqueous solutions onto modified lignin from alkali glycerol delignication. J. Hazard. Mater. B. 2004, 109, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gloaguen, V.; Morvan, H. Removal of heavy metal ions from aqueous solution by modified barks. J. Environ. Sci. Health Part A 1997, 32, 901–912. [Google Scholar] [CrossRef]
- Wu, P.; Cui, P.; Fang, G.; Gao, J.; Zhou, D.; Wang, Y. Sorption mechanism of zinc on reed, lignin, and reed- and lignin-derived biochars: Kinetics, equilibrium, and spectroscopic studies. J. Soils Sediments 2018, 18, 2535–2543. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin—A biosorbent. J. Colloid Interface Sci. 2005, 297, 489–504. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Al-Muhtaseb, A.H.; Al-Laqtah, N.A.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20–30. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.-H.; Lee, S.-L.; Hwang, S.-W.; Seo, D.-C. Adsorption behavior of arsenic onto lignin-based biochar decorated with zinc. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127095. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of lignin and its derivatives in adsorption of heavy metal ions in water: A review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Zambrano, G.B.; De Almeida, O.N.; Duarte, D.S.; Velasco, F.G.; Luzardo, F.H.M.; Nieto-González, L. Adsorption of arsenic anions in water using modified lignocellulosic adsorbents. Results Eng. 2022, 13, 100340. [Google Scholar] [CrossRef]
- Dang, J.; Wang, H.; Wang, C. Adsorption of Toxic Zinc by Functionalized Lignocellulose Derived from Waste Biomass: Kinetics, Isotherms and Thermodynamics. Sustainability 2021, 13, 10673. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Guo, X.; Huang, H. Adsorption of chromium(III) on lignin. Bioresour. Technol. 2008, 99, 7709–7715. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, M.; Melegari, M.; Gazzurelli, C.; Maffini, M.; Mucchino, C.; Mazzeo, P.P.; Carcelli, M.; Perego, J.; Migliori, A.; Leonardi, G.; et al. Industrial lignins as efficient biosorbents for Cr(VI) water remediation: Transforming a waste into an added value material. RSC Sustain. 2023, 1, 1423–1435. [Google Scholar] [CrossRef]
- Low, K.S.; Lee, C.K.; Mak, S.M. Sorption of copper and lead by citric acid modified wood. Wood Sci. Technol. 2004, 38, 629–640. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Shan, X.-Q. Adsorption of metal ions on lignin. J. Hazard. Mater. 2008, 151, 134–142. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P. Biosorption of heavy metals by lignocellulosic biomass and chemical analysis. BioRes. 2019, 14, 4952–4995. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Tsang, D.C.W.; Hunt, A.J. Lignin materials for adsorption: Current trend, perspectives and opportunities. Bioresour. Technol. 2018, 272, 570–581. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Ragnar, M.; Lindgren, C.T.; Nilvebrant, N.-O. PKA-Values of guaiacyl and syringyl phenols related to lignin. J. Wood Chem. Technol. 2000, 20, 277–305. [Google Scholar] [CrossRef]
- Suksabye, P.; Thiravetyan, P.; Nakbanpote, W. Column study of chromium(VI) adsorption from electroplating industry by coconut coir pith. J. Hazard. Mater. 2008, 160, 56–62. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Wang, Q.; Liu, S.; Ji, X. Lignin Nanoparticles Produced from Wheat Straw Black Liquor Using γ-Valerolactone. Polymers 2024, 16, 49. [Google Scholar] [CrossRef]
- Lara-Flores, A.A.; Araújo, R.G.; Rodríguez-Jasso, R.M.; Aguedo, M.; Aguilar, C.N.; Trajano, H.L.; Ruiz, H.A. Bioeconomy and Biorefinery: Valorization of Hemicellulose from Lignocellulosic Biomass and Potential Use of Avocado Residues as a Promising Resource of Bioproducts. In Waste to Wealth. Energy, Environment, and Sustainability; Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R., Eds.; Springer: Singapore, 2018; pp. 141–170. [Google Scholar]
- Rusu, G. Studies on the use of Cellulosic Wastes in Reducing Environmental Pollution. Ph.D. Thesis, Polytechnic University of Iasi, Iasi, Romania, 2015; pp. 29–48. [Google Scholar]
- Shi, X.; Qiao, Y.; An, X.; Tian, Y.; Zhou, H. High-capacity adsorption of Cr(VI) by lignin-based composite: Characterization, performance and mechanism. Int. J. Biol. Macromol. 2020, 159, 839–849. [Google Scholar] [CrossRef]



| Metal/Metalloid | Limited Concentration * (mg L−1) | Common Sources | Effects |
|---|---|---|---|
| Arsenic | 0.01 | Pesticides, mining, natural groundwater contamination, treated wood, glass, and electronics production wastes | Skin lesions, cancers (skin/lung/bladder), cardiovascular disease, neurotoxicity |
| Cadmium | 0.003 | Batteries, pigments, electroplating, phosphate fertilizers, smoking | Kidney damage, osteoporosis, lung cancer, hypertension, bone demineralization |
| Chromium | 0.05 | Leather tanning, industrial effluents, stainless steel production, wood preservatives | Lung cancer, dermatitis, liver/kidney damage, neurotoxicity |
| Cobalt | NS ** | Alloys, batteries (Li-ion), mining, orthopedic implants | Cardiomyopathy, thyroid dysfunction, dermatitis, respiratory irritation |
| Copper | 2.0 | Plumbing pipes, mining, agricultural fungicides | Gastrointestinal distress, liver/kidney damage |
| Iron | 2.0 | Corroded pipes, natural deposits, industrial waste | Liver damage, diabetes, oxidative stress (hemochromatosis) |
| Nickel | 0.07 | Batteries, electroplating, stainless steel, volcanic emissions | Dermatitis, respiratory carcinogenicity, lung cancer |
| Lead | 0.01 | Leaded paints, old plumbing, recycling batteries, contaminated soil | Neurodevelopmental deficits (children), anemia, hypertension, renal failure |
| Zinc | 3.0 | Galvanized pipes, mining runoff, fertilizers | Gastrointestinal distress, vomiting, immune dysfunction, deficiency growth |
| Metal/ Metalloid | Concentration (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arsenic | 7.49 | 14.98 | 22.48 | 29.97 | 34.46 | 44.95 | 52.44 | 59.94 | 67.43 | 74.92 |
| Cadmium | 11.24 | 22.48 | 33.72 | 44.96 | 56.21 | 67.45 | 78.69 | 89.93 | 101.17 | 112.41 |
| Chromium | 5.20 | 10.40 | 15.60 | 20.08 | 25.00 | 31.50 | 36.40 | 41.60 | 46.80 | 52.00 |
| Cobalt | 5.89 | 11.39 | 17.68 | 23.60 | 29.50 | 35.40 | 41.30 | 47.19 | 53.09 | 58.99 |
| Copper | 6.36 | 12.71 | 19.07 | 25.42 | 32.67 | 38.13 | 44.49 | 50.84 | 57.20 | 63.55 |
| Iron | 5.58 | 11.17 | 16.75 | 22.34 | 27.92 | 33.50 | 39.09 | 44.67 | 50.25 | 55.84 |
| Nickel | 5.87 | 11.74 | 17.61 | 23.48 | 29.35 | 35.22 | 41.09 | 46.95 | 52.82 | 58.69 |
| Lead | 20.72 | 41.44 | 62.16 | 82.88 | 103.60 | 124.32 | 145.04 | 165.76 | 186.48 | 207.20 |
| Zinc | 6.54 | 13.08 | 19.61 | 26.15 | 32.69 | 39.23 | 45.77 | 52.30 | 58.84 | 65.38 |
| Ionic Specie | Symbol | pH | Contact Time (min) | Reference | |||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | ||||
| Arsenic | As(III) | 6 | 30 | 60 | - | 120 | [18] |
| Cadmium | Cd(II) | 6.2 | 30 | 60 | - | 120 | [19,20] |
| Chromium | Cr(VI) | 5 | 30 | 60 | - | 120 | [21,22] |
| Cobalt | Co(II) | 5 | 30 | 60 | 90 | [23] | |
| Copper | Cu(II) | 5 | 30 | 60 | - | 120 | [24,25] |
| Iron | Fe(II) | 6.5 | 30 | 60 | 90 | [26] | |
| Nickel | Ni(II) | 5 | 30 | 60 | 90 | [27] | |
| Lead | Pb(II) | 6 | 30 | 60 | 90 | [28] | |
| Zinc | Zn(II) | 6 | 30 | 60 | 90 | [28] | |
| Ionic Specie | Symbol | Method | Indicator | λ, nm | Reference |
|---|---|---|---|---|---|
| Arsenic | As(III) | Inductively Coupled Plasma Optical Emission Spectrometry with Hydride Generation (HG-ICP-OES) | - | 189.042 | [18] |
| Cadmium | Cd(II) | Spectrophotometry | Xylenol orange | 575 | [19,20] |
| Chromium | Cr(VI) | Spectrophotometry | Diphenylcarbazide | 545 | [21,22] |
| Cobalt | Co(II) | Spectrophotometry | Rubeanic acid | 580 | [23] |
| Copper | Cu(II) | Spectrophotometry | Rubeanic acid | 390 | [24,25] |
| Iron | Fe(II) | Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) | - | 238.204 | [26] |
| Nickel | Ni(II) | Spectrophotometry | Rubeanic acid | 590 | [27] |
| Lead | Pb(II) | Spectrophotometry | 4-(2-pyridylazo)-resorcinol | 530 | [28] |
| Zinc | Zn(II) | Spectrophotometry | Xylenol orange | 570 | [28] |
| Metal/Metalloid | Maximum Adsorption Capacity qmax, mg·g−1 | Adsorption Capacity qe, mg·g−1 | Reference |
|---|---|---|---|
| Arsenic | 1.16 | 1.07 (±0.08) | [18] |
| Cadmium | ~13.4 * | 13.43 (±0.21) | [19,20] |
| Chromium | 0.841 | 0.84 (±0.16) | [21,22] |
| Cobalt | 13.67 | 11.56 (±0.07) | [23] |
| Copper | 22.12 | 11.44 (±0.11) | [24,25] |
| Iron | 13.06 | 12.38 (±0.19) | [26] |
| Nickel | 13.74 | 12.56 (±0.23) | [27] |
| Lead | 13.21 | 12.07 (±0.09) | [28] |
| Zinc | 13.19 | 11.91 (±0.14) | [28] |
| Pollutant | Times | Freundlich Model | Langmuir Model | ||||
|---|---|---|---|---|---|---|---|
| (min) | R2 | 1/n | kF | R2 | qm (mg/g) | KL | |
| As(III) | 30 | 0.9930 | 0.3387 | 0.8645 | 0.8951 | 2.7070 | 0.2296 |
| 60 | 0.9875 | 0.2323 | 1.0949 | 0.8251 | 2.8042 | 0.3145 | |
| 120 | 0.9888 | 0.2320 | 1.0960 | 0.8287 | 2.8145 | 0.3134 | |
| Cd(II) | 30 | 0.9810 | 0.9125 | 0.8321 | 0.8863 | 2.4224 | 0.2457 |
| 60 | 0.9726 | 0.9312 | 1.0432 | 0.8276 | 2.5562 | 0.3411 | |
| 120 | 0.9637 | 0.9041 | 1.0359 | 0.8219 | 2.7593 | 0.3528 | |
| Cr(VI) | 30 | 0.9930 | 0.9387 | 0.8645 | 0.8951 | 2.7070 | 0.2296 |
| 60 | 0.9875 | 0.9423 | 1.0949 | 0.8251 | 2.8042 | 0.3145 | |
| 120 | 0.9888 | 0.9112 | 1.0960 | 0.8287 | 2.8450 | 0.3134 | |
| Co(II) | 30 | 0.9743 | 0.9014 | 2.1732 | 0.9061 | 13.2187 | 0.0703 |
| 60 | 0.9826 | 0.9124 | 1.9342 | 0.8217 | 14.0061 | 0.0690 | |
| 90 | 0.9632 | 0.9281 | 1.9382 | 0.7324 | 14.1398 | 0.0651 | |
| Cu(II) | 30 | 0.9969 | 0.9020 | 2.1421 | 0.9820 | 13.0204 | 0.0643 |
| 60 | 0.9971 | 0.9394 | 2.0001 | 0.9854 | 13.1122 | 0.0638 | |
| 90 | 0.9982 | 0.9500 | 1.9643 | 0.9882 | 13.1872 | 0.0631 | |
| Fe(II) | 30 | 0.9656 | 0.9142 | 2.0327 | 0.7852 | 12.8821 | 0.0693 |
| 60 | 0.9789 | 0.9242 | 1.9856 | 0.8712 | 13.6117 | 0.0687 | |
| 90 | 0.9684 | 0.9411 | 1.9902 | 0.7931 | 13.7002 | 0.0641 | |
| Ni(II) | 30 | 0.9822 | 0.9028 | 2.1586 | 0.9179 | 12.4073 | 0.0801 |
| 60 | 0.9942 | 0.9205 | 2.0075 | 0.8920 | 12.5206 | 0.0795 | |
| 90 | 0.9844 | 0.9994 | 1.9776 | 0.7766 | 12.5398 | 0.0794 | |
| Pb(II) | 30 | 0.9971 | 0.9028 | 2.1586 | 0.9822 | 13.0208 | 0.0651 |
| 60 | 0.9987 | 0.9595 | 2.0070 | 0.9868 | 13.1233 | 0.0759 | |
| 90 | 0.9990 | 0.9694 | 1.9776 | 0.9899 | 13.2100 | 0.0754 | |
| Zn(II) | 30 | 0.9982 | 0.5506 | 1.9286 | 0.9926 | 12.5470 | 0.0786 |
| 60 | 0.9966 | 0.6061 | 1.9466 | 0.9917 | 13.1061 | 0.0754 | |
| 90 | 0.9952 | 0.6341 | 1.9460 | 0.9910 | 13.1926 | 0.0750 | |
| Pollutant | pH | Time (min) | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/mol K) |
|---|---|---|---|---|---|
| Cr(VI) | 2.09 5 | 30 60 90 30 60 90 | −26.08 −27.94 −28.82 −30.76 −35.41 −38.14 | 12.96 11.37 11.82 15.34 14.25 15.01 | 98.17 88.72 93.14 111.02 132.24 124.38 |
| Cd(II) | 1.02 6.03 | 30 60 120 30 60 120 | −25.29 −27.02 −27.98 −30.67 −36.28 −37.05 | 12.01 11.21 11.43 15.18 14.36 14.81 | 99.21 86.24 87.31 112.32 137.92 126.83 |
| Cu(II) | 2.11 5.02 | 30 60 90 30 60 90 | −26.17 −28.04 −29.13 −31.31 −36.18 −37.99 | 13.07 11.98 12.92 15.02 13.95 14.93 | 97.76 88.06 92.89 110.28 131.47 12.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, E.; Tofanica, B.-M.; Ulea, E.; Ungureanu, O.C.; Fortună, M.E.; Rotaru, R.; Volf, I.; Popa, V.I. Valorization of Agro-Industrial Lignin as a Functional Polymer for Sustainable Wastewater Treatment. Polymers 2025, 17, 2263. https://doi.org/10.3390/polym17162263
Ungureanu E, Tofanica B-M, Ulea E, Ungureanu OC, Fortună ME, Rotaru R, Volf I, Popa VI. Valorization of Agro-Industrial Lignin as a Functional Polymer for Sustainable Wastewater Treatment. Polymers. 2025; 17(16):2263. https://doi.org/10.3390/polym17162263
Chicago/Turabian StyleUngureanu, Elena, Bogdan-Marian Tofanica, Eugen Ulea, Ovidiu C. Ungureanu, Maria E. Fortună, Răzvan Rotaru, Irina Volf, and Valentin I. Popa. 2025. "Valorization of Agro-Industrial Lignin as a Functional Polymer for Sustainable Wastewater Treatment" Polymers 17, no. 16: 2263. https://doi.org/10.3390/polym17162263
APA StyleUngureanu, E., Tofanica, B.-M., Ulea, E., Ungureanu, O. C., Fortună, M. E., Rotaru, R., Volf, I., & Popa, V. I. (2025). Valorization of Agro-Industrial Lignin as a Functional Polymer for Sustainable Wastewater Treatment. Polymers, 17(16), 2263. https://doi.org/10.3390/polym17162263









