Localized Combination Therapy Using Collagen–Hydroxyapatite Bone Grafts for Simultaneous Bone Cancer Inhibition and Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Materials
2.3. Characterization Techniques
2.3.1. Water Absorption
2.3.2. Enzymatic Degradation
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. FTIR Spectroscopy
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. X-Ray Diffraction (XRD)
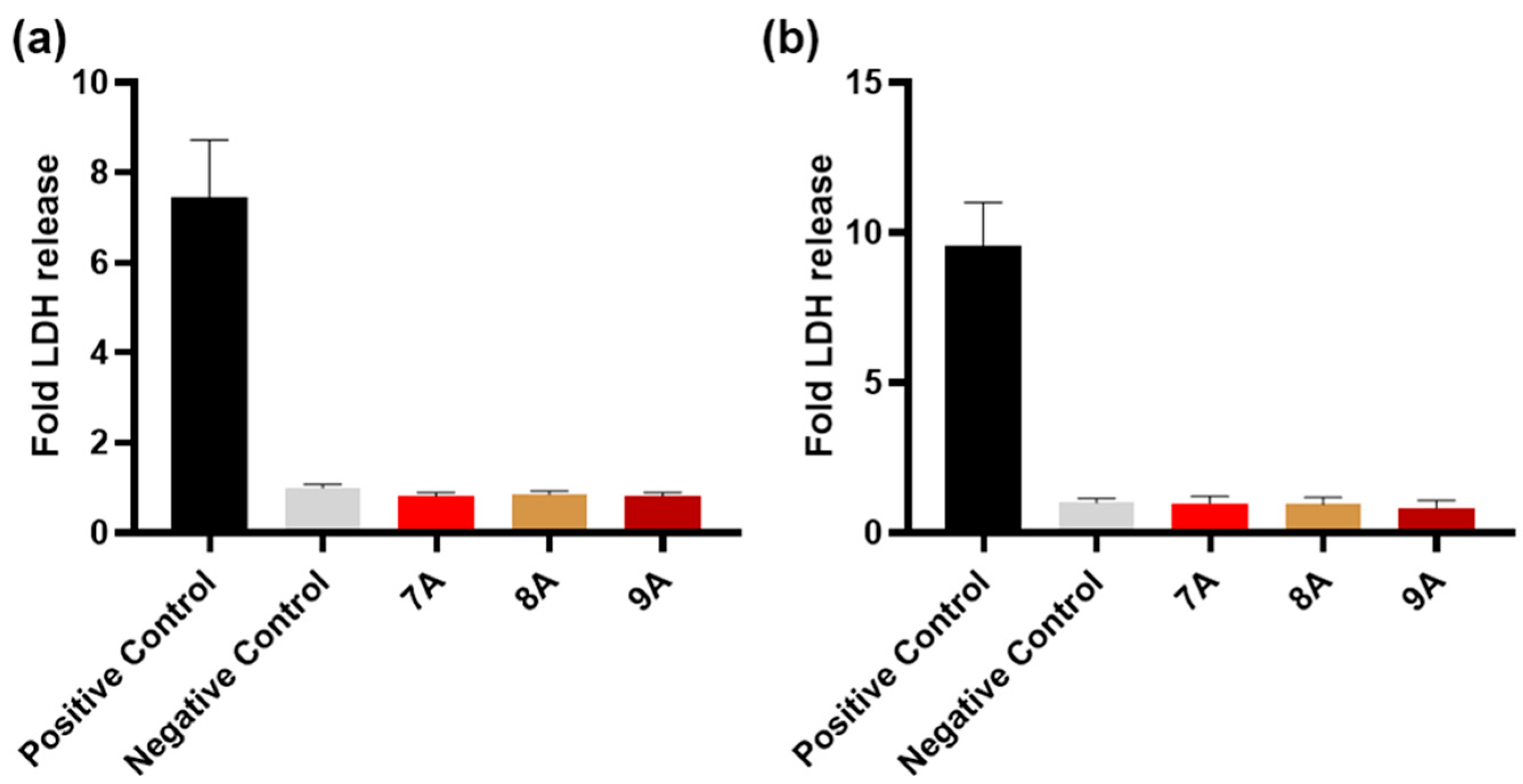
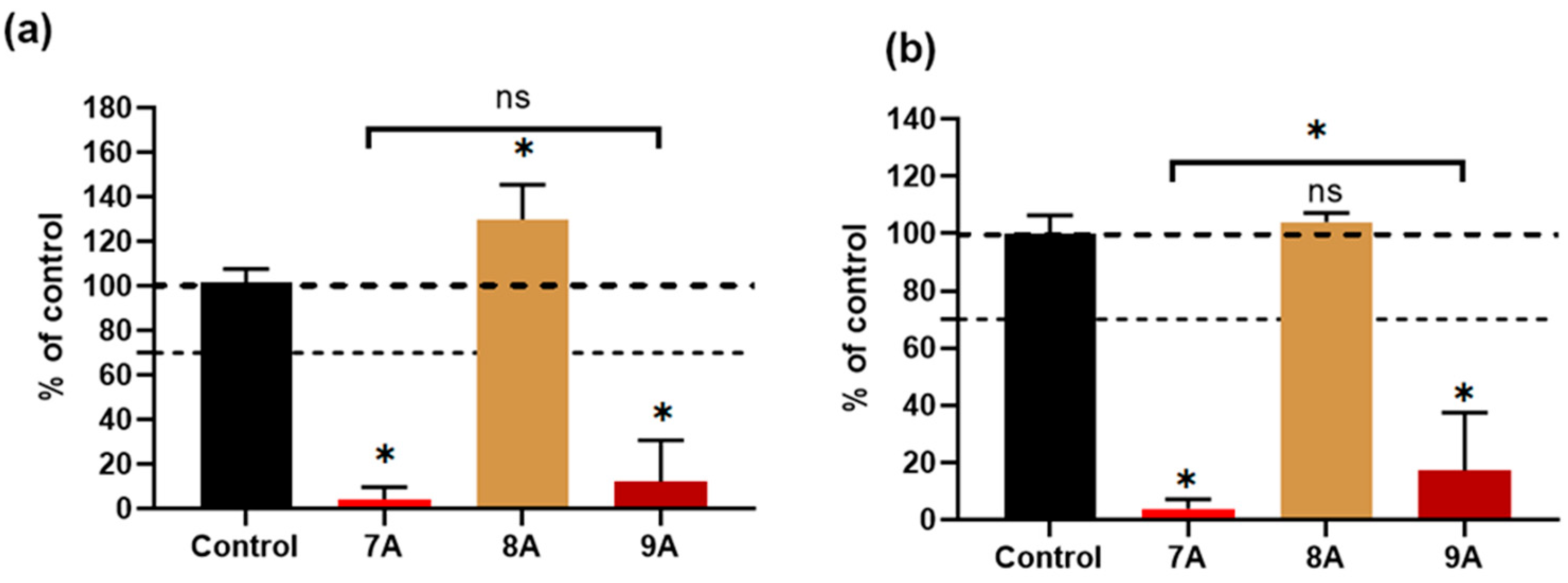
2.3.7. Lactase Dehydrogenase Enzyme (LDH) and XTT Assays
2.3.8. Statistical Analysis
3. Results and Discussion
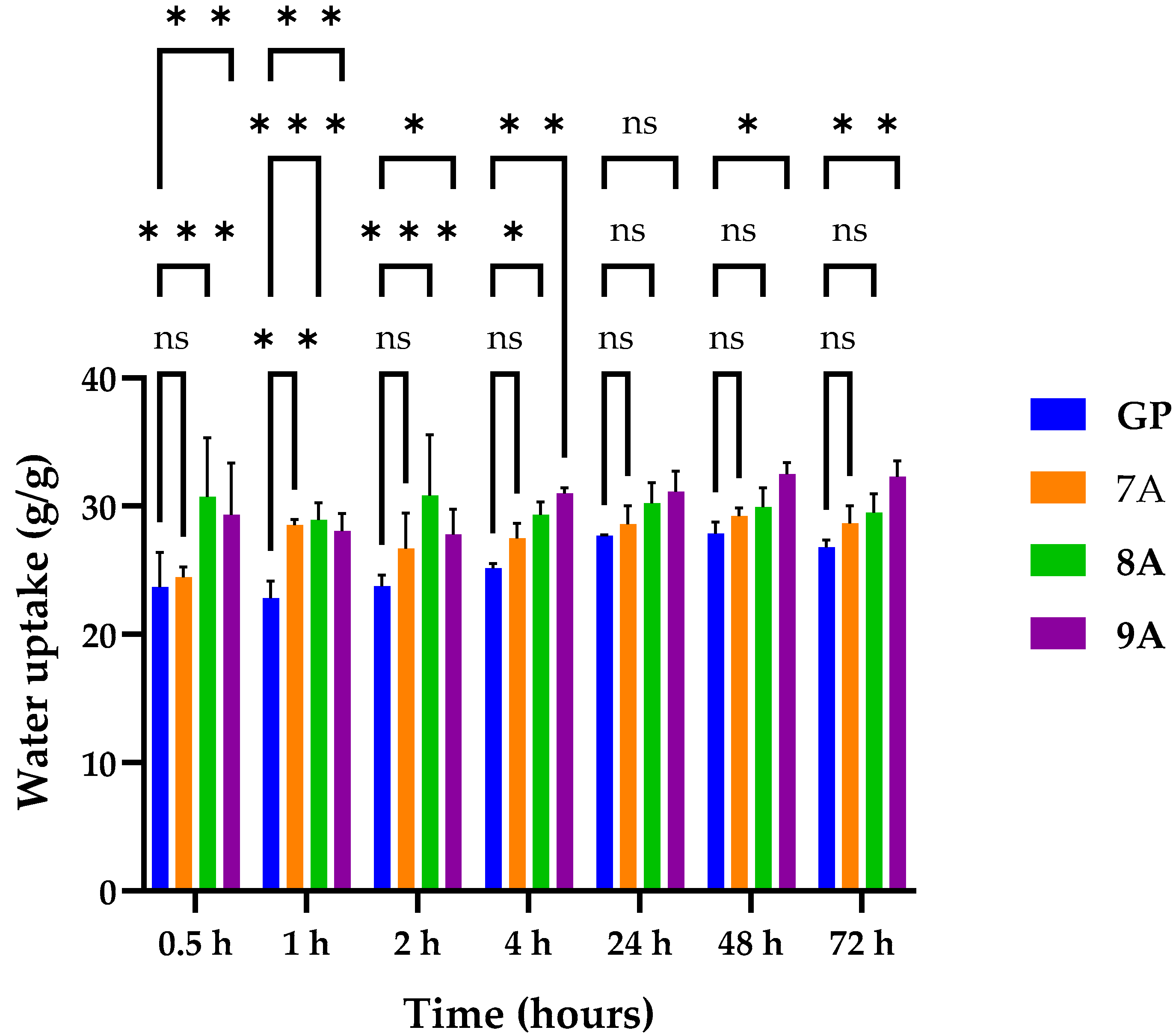
3.1. Water Uptake Capacity of Loaded Coll/HAp Sponges
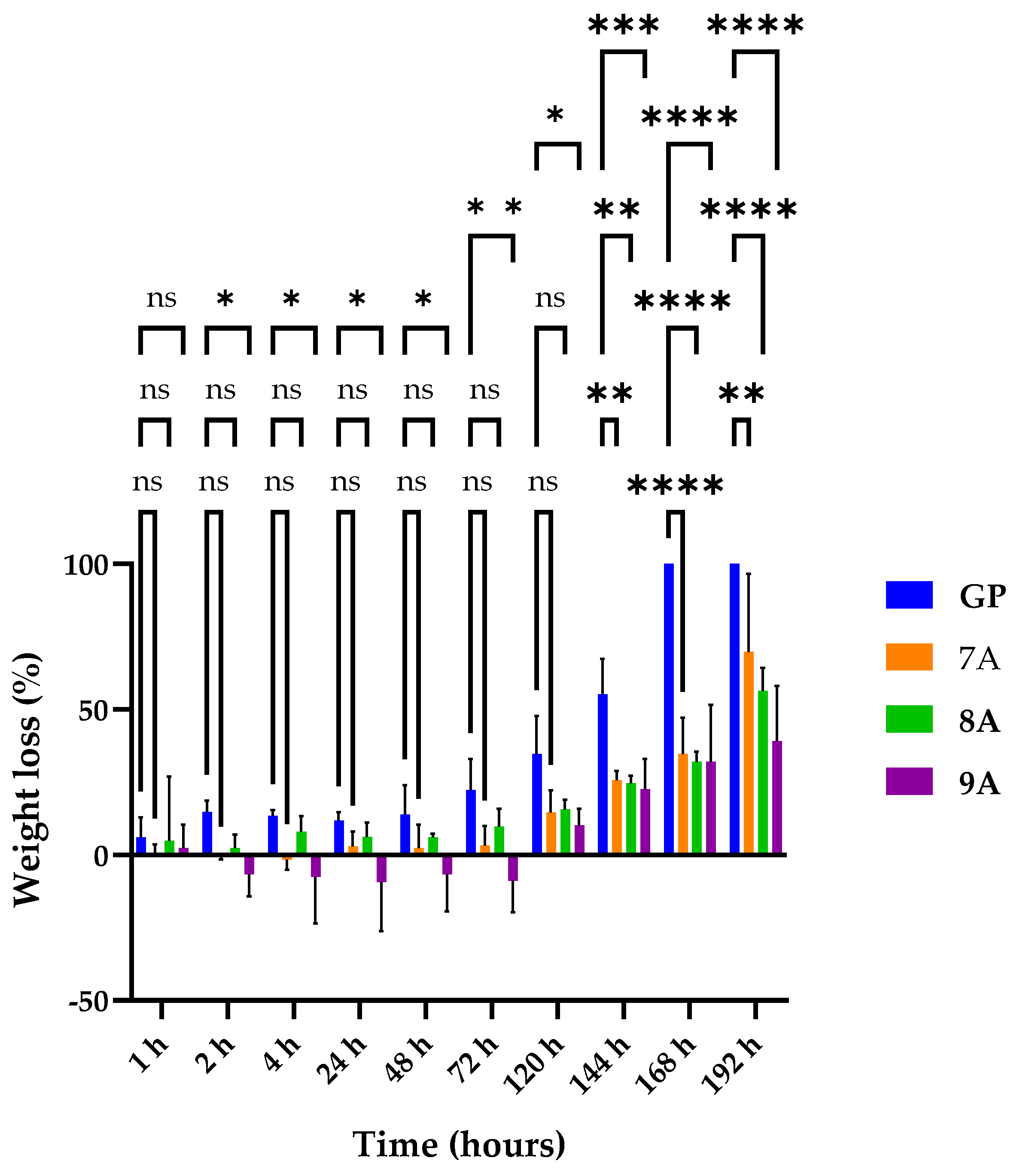
3.2. Enzymatic Degradation of Loaded Coll/HAp Sponges
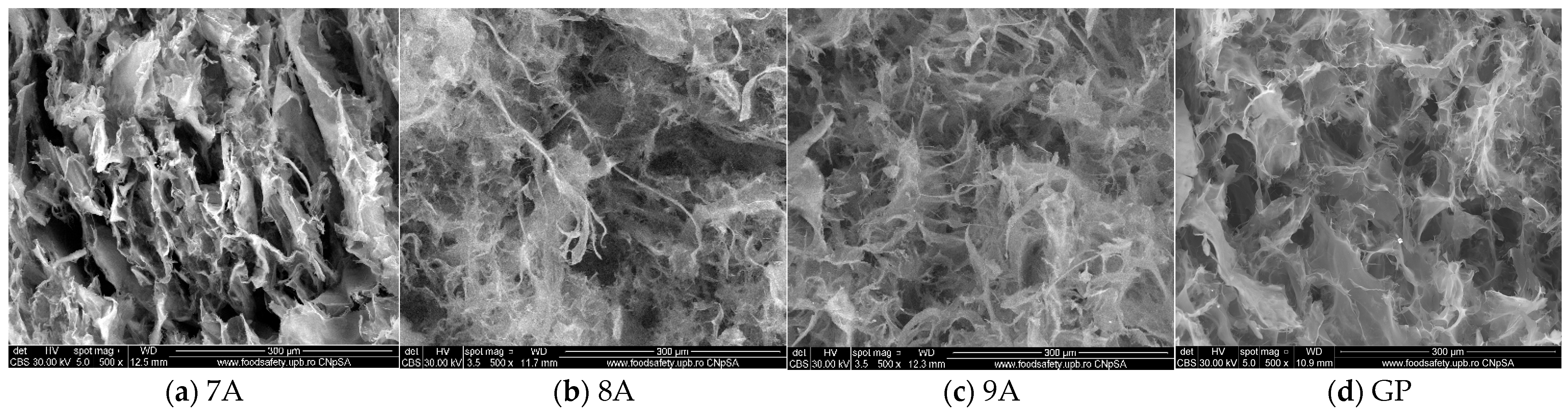
3.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDX) Characterization
3.4. FTIR Spectroscopy and Microscopy
3.5. TG-DSC Analysis
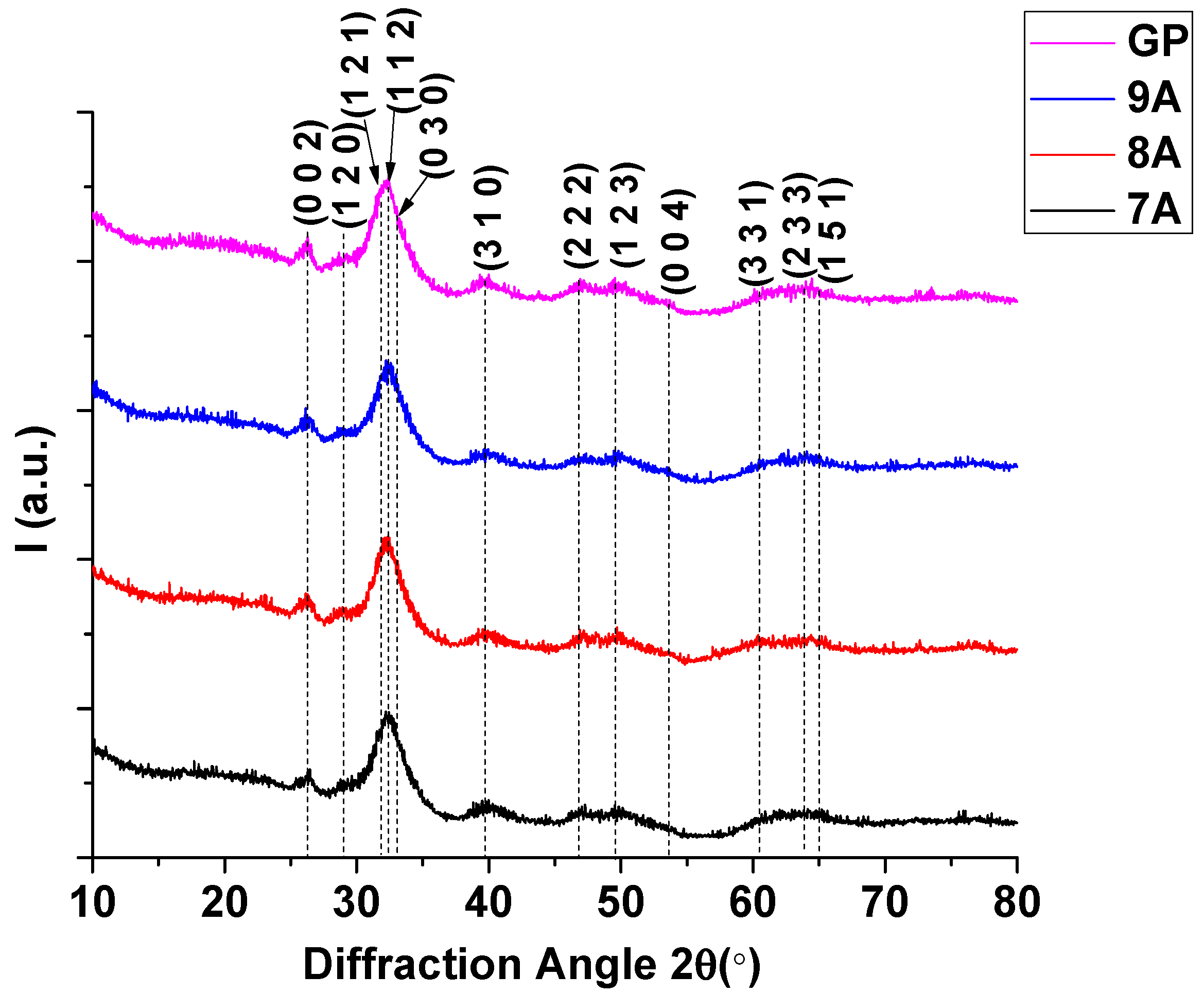
3.6. X-Ray Diffraction (XRD)
3.7. Biological Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The American Cancer Society. American Cancer Society Releases Latest Global Cancer Statistics; Cancer Cases Expected to Rise to 35 Million Worldwide by 2050. News Release, 4 April 2024. Available online: https://pressroom.cancer.org/GlobalCancerStatistics2024 (accessed on 8 April 2024).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Pullan, J.E.; Lotfollahzadeh, S. Primary Bone Cancer. [Updated 2024 Mar 20]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560830/ (accessed on 11 June 2025).
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. SICOT-J. 2018, 4, 12. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alonzo, M.; Primo, F.A.; Kumar, S.A.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Barajas-Pedroza, M.A.; Rodríguez-Rodríguez, R. Development of 3D-printed biocompatible materials for bone substitution. In Cartilage Tissue and Knee Joint Biomechanics; Nochehdehi, A.R., Nemavhola, F., Thomas, S., Maria, H.J., Eds.; Academic Press: Cambridge, UK, 2024; pp. 507–524. [Google Scholar]
- Jaisankar, S.N.; Haridharan, N.; Murali, A.; Sergii, P.; Špírková, M.; Mandal, A.B.; Matějka, L. Single-electron transfer living radical copolymerization of SWCNT-g-PMMA via graft from approach. Polymer 2014, 55, 2959–2966. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Yuan, J.; Ye, Z.; Zeng, Y.; Pan, Z.; Feng, Z.; Bao, Y.; Li, Y.; Liu, X.; He, Y.; Feng, Q. Bifunctional scaffolds for tumor therapy and bone regeneration: Synergistic effect and interplay between therapeutic agents and scaffold materials. Mater. Today Bio. 2022, 15, 100318. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Fu, Y.; Li, X.; Huang, J.; Guan, X.; Zhou, Y. A bifunctional bortezomib-loaded porous nano-hydroxyapatite/alginate scaffold for simultaneous tumor inhibition and bone regeneration. J. Nanobiotechnol. 2023, 21, 174. [Google Scholar] [CrossRef]
- Yan, Z.; Deng, Y.; Huang, L.; Zeng, J.; Wang, D.; Tong, Z.; Fan, Q.; Tan, W.; Yan, J.; Zang, X.; et al. Biopolymer-based bone scaffold for controlled Pt (IV) prodrug release and synergistic photothermal-chemotherapy and immunotherapy in osteosarcoma. J. Nanobiotechnol. 2025, 23, 286. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Wang, J.; Tang, F.; Tong, D.; Wang, Z.; Zhou, Z.; Ruan, R.; Zhang, J.; Song, J.; Yang, H. Bionic Bilayer Scaffold for Synchronous Hyperthermia Therapy of Orthotopic Osteosarcoma and Osteochondral Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 8538–8553. [Google Scholar] [CrossRef]
- Malla, S.; Niraula, N.P.; Singh, B.; Liou, K.K.; Sohng, J.K. Limitations in doxorubicin production from Streptomyces peucetius. Microbiol. Res. 2010, 165, 427–435. [Google Scholar] [CrossRef]
- Park, H.-J.; Yoon, S.-Y.; Park, J.-N.; Suh, J.-H.; Choi, H.-S. Doxorubicin Induces Bone Loss by Increasing Autophagy through a Mitochondrial ROS/TRPML1/TFEB Axis in Osteoclasts. Antioxidants 2022, 11, 1476. [Google Scholar] [CrossRef]
- Van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Serini, S.; Calviello, G. Potential of Natural Phenolic Compounds against Doxorubicin-Induced Chemobrain: Biological and Molecular Mechanisms Involved. Antioxidants 2024, 13, 486. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Cen, J.; Dai, X.; Zhao, H.; Li, X.; Hu, X.; Wu, J.; Duan, S. Doxorubicin-Loaded Liposome with the Function of “Killing Two Birds with One Stone” against Glioma. ACS Appl. Mater. Interfaces 2023, 15, 46697–46709. [Google Scholar] [CrossRef]
- Kovrlija, I.; Pańczyszyn, E.; Demir, O.; Laizane, M.; Corazzari, M.; Locs, J.; Loca, D. Doxorubicin loaded octacalcium phosphate particles as controlled release drug delivery systems: Physico-chemical characterization, in vitro drug release and evaluation of cell death pathway. Int. J. Pharm. 2024, 653, 123932. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Raina, D.B.; Sebastian, S.; Nagesh, H.; Isaksson, H.; Engellau, J.; Lidgren, L.; Tägil, M. Sustained and controlled delivery of doxorubicin from an in-situ setting biphasic hydroxyapatite carrier for local treatment of a highly proliferative human osteosarcoma. Acta Biomater. 2021, 131, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef]
- Magnani, F.; Mattevi, A. Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef]
- Alam, M.; Ashraf, G.M.; Sheikh, K.; Khan, A.; Ali, S.; Ansari, M.M.; Adnan, M.; Pasupuleti, V.R.; Hassan, M.I. Potential therapeutic implications of caffeic acid in cancer signaling: Past, present and future. Front. Pharmacol. 2022, 13, 845871–845884. [Google Scholar] [CrossRef]
- Kowalska-Baron, A. Theoretical Insight into Antioxidant Mechanism of Caffeic Acid Against Hydroperoxyl Radicals in Aqueous Medium at Different pH-Thermodynamic and Kinetic Aspects. Int. J. Mol. Sci. 2024, 25, 12753. [Google Scholar] [CrossRef] [PubMed]
- Cortez, N.; Villegas, C.; Burgos, V.; Cabrera-Pardo, J.R.; Ortiz, L.; González-Chavarría, I.; Nchiozem-Ngnitedem, V.-A.; Paz, C. Adjuvant Properties of Caffeic Acid in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 7631. [Google Scholar] [CrossRef]
- Lim, S.C.; Lee, T.B.; Han, S.I. Caffeic Acid Enhances Anticancer Drug-induced Apoptosis in Acid-adapted HCT116 Colon Cancer Cells. Anticancer Res. 2024, 44, 2587–2595. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.B.M.; Pillat, M.M.; Mânica, A.; Stefanello, N.; Weis, G.C.C.; Alves, A.d.O.; de Andrade, C.M.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, M.; Mireskandari, E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomed. 2019, 9, 574–586. [Google Scholar] [PubMed]
- Su, Y.; Liu, Y.; Hu, X.; Lu, Y.; Zhang, J.; Jin, W.; Liu, W.; Shu, Y.; Cheng, Y.Y.; Li, W.; et al. Caffeic acid-grafted chitosan/sodium alginate/nanoclay-based multifunctional 3D-printed hybrid scaffolds for local drug release therapy after breast cancer surgery. Carbohydr. Polym. 2024, 324, 121441. [Google Scholar] [CrossRef] [PubMed]
- Colpan, R.D.; Erdemir, A. Co-delivery of quercetin and caffeic-acid phenethyl ester by polymeric nanoparticles for improved antitumor efficacy in colon cancer cells. J. Microencapsul. 2021, 38, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Pagnan, A.L.; Pessoa, A.S.; Tokuhara, C.K.; Fakhoury, V.S.; Oliveira, G.S.N.; Sanches, M.L.R.; Inacio, K.K.; Ximenes, V.F.; Oliveira, R.C. Anti-tumour potential and selectivity of caffeic acid phenethyl ester in osteosarcoma cells. Tissue Cell 2022, 74, 101705. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 4373–4382. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Wang, L.-Y.; Bu, F.-Z.; Li, Y.-T.; Wang, C.; Wu, Z.-Y. The supramolecular self-assembly of 5-fluorouracil and caffeic acid through cocrystallization strategy opens up a new way for the development of synergistic antitumor pharmaceutical cocrystal. CrystEngComm 2020, 22, 7992–8006. [Google Scholar] [CrossRef]
- Su, P.; Yang, Y.; Wang, G.; Chen, X.; Ju, Y. CUR Attenuates Resistance to Irinotecan via Induction of Apoptosis of Cancer Stem Cells in Chemoresistant Colon Cancer Cells. Internat. J. Oncol. 2018, 53, 1343–1353. [Google Scholar] [CrossRef]
- Jakobušić Brala, C.; Karković Marković, A.; Kugić, A.; Torić, J.; Barbarić, M. Combination Chemotherapy with Selected Polyphenols in Preclinical and Clinical Studies—An Update Overview. Molecules 2023, 28, 3746. [Google Scholar] [CrossRef]
- Cao, J.; Han, J.; Xiao, H.; Qiao, J.; Han, M. Effect of Tea Polyphenol Compounds on Anticancer Drugs in Terms of Anti-Tumor Activity, Toxicology, and Pharmacokinetics. Nutrients 2016, 8, 762. [Google Scholar] [CrossRef]
- Chisholm, K.; Bray, B.J.; Rosengren, R.J. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells. Anti-Cancer Drugs 2004, 15, 889–897. [Google Scholar] [CrossRef]
- Tutuianu, R.; Rosca, A.-M.; Albu Kaya, M.G.; Pruna, V.; Neagu, T.P.; Lascar, I.; Simionescu, M.; Titorencu, I. Mesenchymal stromal cell-derived factors promote the colonization of collagen 3D scaffolds with human skin cells. J. Cell. Mol. Med. 2020, 24, 9692–9704. [Google Scholar] [CrossRef]
- Vladu, A.F.; Albu Kaya, M.G.; Truşcă, R.D.; Motelica, L.; Surdu, V.-A.; Oprea, O.C.; Constantinescu, R.R.; Cazan, B.; Ficai, D.; Andronescu, E.; et al. The Role of Crosslinking Agents in the Development of Collagen–Hydroxyapatite Composite Materials for Bone Tissue Engineering. Materials 2025, 18, 998. [Google Scholar] [CrossRef]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications: The Obtaining and Characterization of Collagen-Based Biomaterials as Support for Local Release; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2011; pp. 9–12. [Google Scholar]
- Albu Kaya, M.G.; Ferdes, M.; Kaya, D.A.; Ghica, M.V.; Titorencu, I.; Popa, L.; Albu, L. Collagen wound dressings with anti-inflamatory activity. Mol. Cryst. Liq. Cryst. 2012, 555, 271–279. [Google Scholar] [CrossRef]
- Fecteau, K.A.; Eiler, H. Evaluation of inhibitory effects of doxorubicin on collagenase using a bovine placentome model. In Vivo 1998, 12, 485–488. [Google Scholar]
- Mao, H.; Kawazoe, N.; Chen, G. Cellular Uptake of Single-Walled Carbon Nanotubes in 3D Extracellular Matrix-Mimetic Composite Collagen Hydrogels. J. Nanosci. Nanotechnol. 2014, 14, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Ficai, A.; Trusca, R.D.; Ilie, C.I.; Oprea, O.C.; Andronescu, E. Innovative antimicrobial chitosan/ZnO/Ag NPs/citronella essential oil nanocomposite—potential coating for grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Busuioc, C.; Isopencu, G.; Banciu, A.; Banciu, D.D.; Oprea, O.; Mocanu, A.; Deleanu, I.; Zaulet, M.; Popescu, L.; Tanasuica, R.; et al. Bacterial cellulose hybrid composites with calcium phosphate for bone tissue regeneration. Int. J. Mol. Sci. 2022, 23, 16180. [Google Scholar] [CrossRef]
- Riaz, T.; Rabia, Z.; Faiza, Z.; Kanwal, I.; Nawshad, M.; Zaman, S.S.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Tihăuan, B.M.; Grădișteanu Pîrcălăbioru, G.; Axinie, M.; Marinas, I.C.; Nicoară, A.C.; Măruțescu, L.; Oprea, O.; Matei, E.; Maier, S.S. Crosslinked Collagenic Scaffold Behavior Evaluation by Physico-chemical, Mechanical and Biological Assessments in an In Vitro Microenvironment. Polymers 2022, 14, 2430. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Petrisor, G.; Oprea, O.C.; Trușcǎ, R.D.; Ficai, A.; Andronescu, E.; Hudita, A.; Holban, A.M. Antimicrobial Hydroxyethyl-Cellulose-Based Composite Films with Zinc Oxide and Mesoporous Silica Loaded with Cinnamon Essential Oil. Pharmaceutics 2024, 16, 1225. [Google Scholar] [CrossRef]
- Sudarsanan, K.T.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Struct. Sci. 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Okuda, Y.; Shigemasa, R.; Hirota, K.; Mizutani, T. In Situ Crystallization of Hydroxyapatite on Carboxymethyl Cellulose as a Biomimetic Approach to Biomass-Derived Composite Materials. ACS Omega 2022, 7, 12127–12137. [Google Scholar] [CrossRef]
- Bai, X.; Li, S.; Liu, X.; An, H.; Kang, X.; Guo, S. Caffeic Acid, an Active Ingredient in Coffee, Combines with DOX for Multitarget Combination Therapy of Lung Cancer. J. Agric. Food Chem. 2022, 70, 8326–8337. [Google Scholar] [CrossRef] [PubMed]
- Sandra, F.; Rizal, M.I.; Aliwarga, C.C.; Hadimartana, J.C.; Celinna, M. Caffeic Acid Induces Apoptosis in MG-63 Osteosarcoma Cells via Protein Kinase C Delta (PKCδ) Translocation and Mitochondrial Membrane Potential Reduction. Indones. Biomed. J. 2022, 14, 358–364. [Google Scholar] [CrossRef]
- Valle-Prieto, A.; Conget, P.A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef]
- Yagi, H.; Tan, J.; Tuan, R.S. Polyphenols suppress hydrogen peroxide-induced oxidative stress in human bone-marrow derived mesenchymal stem cells. J. Cell Biochem. 2013, 114, 1163–1173. [Google Scholar] [CrossRef]
- Shifa Ul Haq, H.M.; Ashfaq, R.; Mehmood, A.; Shahid, W.; Ghufran Azam, H.; Azam, M.; Tasneem, S.; Akram, S.J.; Malik, K.; Riazuddin, S. Priming with caffeic acid enhances the potential and survival ability of human adipose-derived stem cells to counteract hypoxia. Regen. Ther. 2023, 22, 115–127. [Google Scholar] [CrossRef]
- Rong, Z.-J.; Yang, L.-J.; Cai, B.-T.; Zhu, L.-X.; Cao, Y.-L.; Wu, G.-F.; Zhang, Z.-J. Porous nano-hydroxyapatite/collagen scaffold containing drug-loaded ADM–PLGA microspheres for bone cancer treatment. J. Mater. Sci. Mater. Med. 2016, 27, 89. [Google Scholar] [CrossRef]
- Lu, Y.; Wan, Y.; Gan, D.; Zhang, Q.; Luo, H.; Deng, X.; Li, Z.; Yang, Z. Enwrapping Polydopamine on Doxorubicin-Loaded Lamellar Hydroxyapatite/Poly(lactic-co-glycolic acid) Composite Fibers for Inhibiting Bone Tumor Recurrence and Enhancing Bone Regeneration. ACS Appl. Bio Mater. 2021, 4, 6036–6045. [Google Scholar] [CrossRef]
- Wang, L.; Dai, Z.; Bi, J.; Chen, Y.; Wang, Z.; Sun, Z.; Ji, Z.; Wang, H.; Zhang, Y.; Wang, L.; et al. Polydopamine-functionalized calcium-deficient hydroxyapatite 3D-printed scaffold with sustained doxorubicin release for synergistic chemo-photothermal therapy of osteosarcoma and accelerated bone regeneration. Mater. Today Bio 2024, 29, 101253. [Google Scholar] [CrossRef]
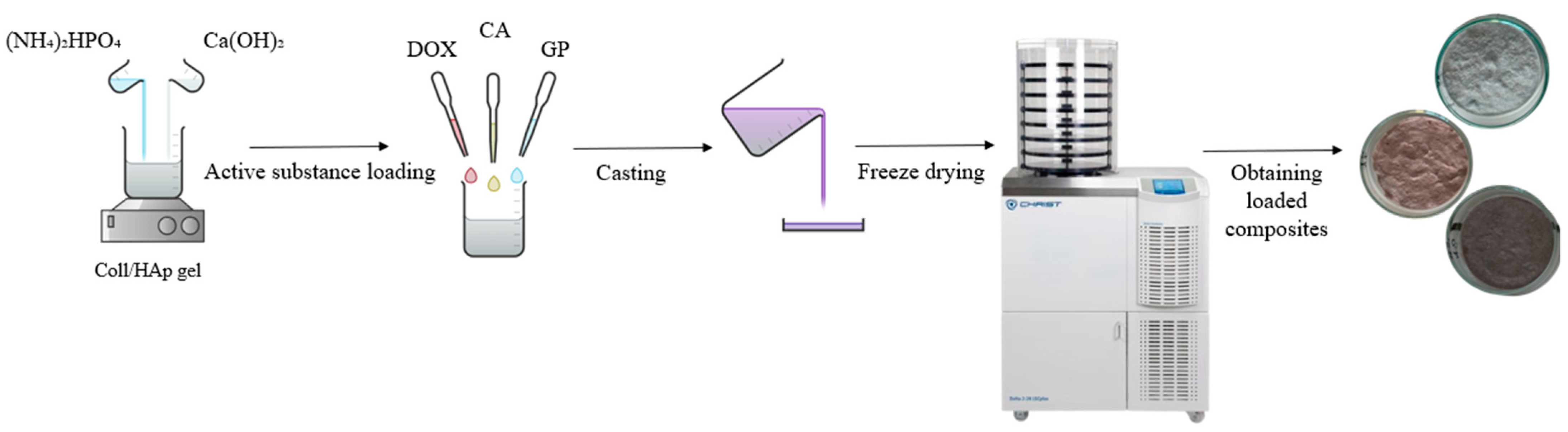
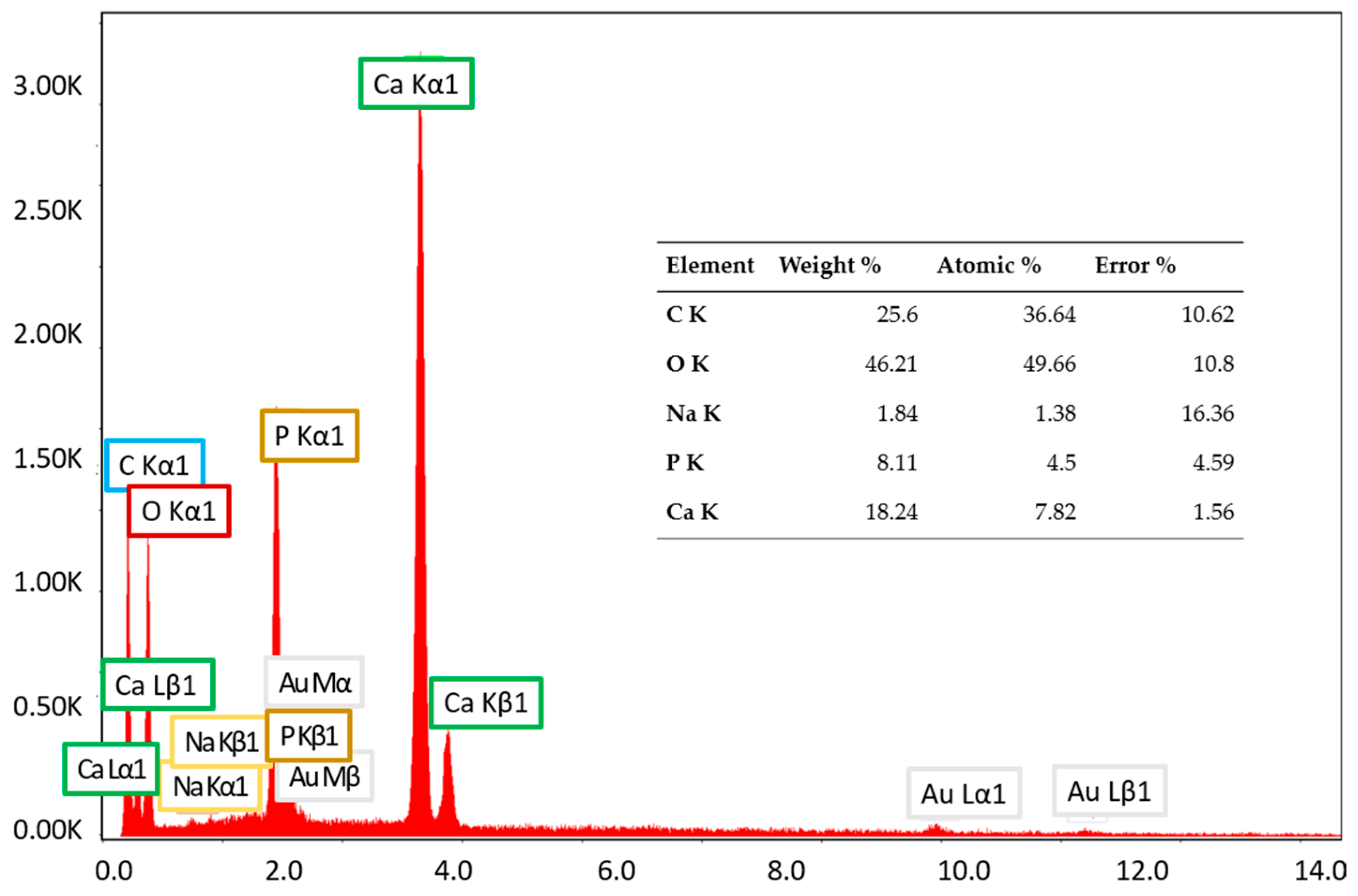
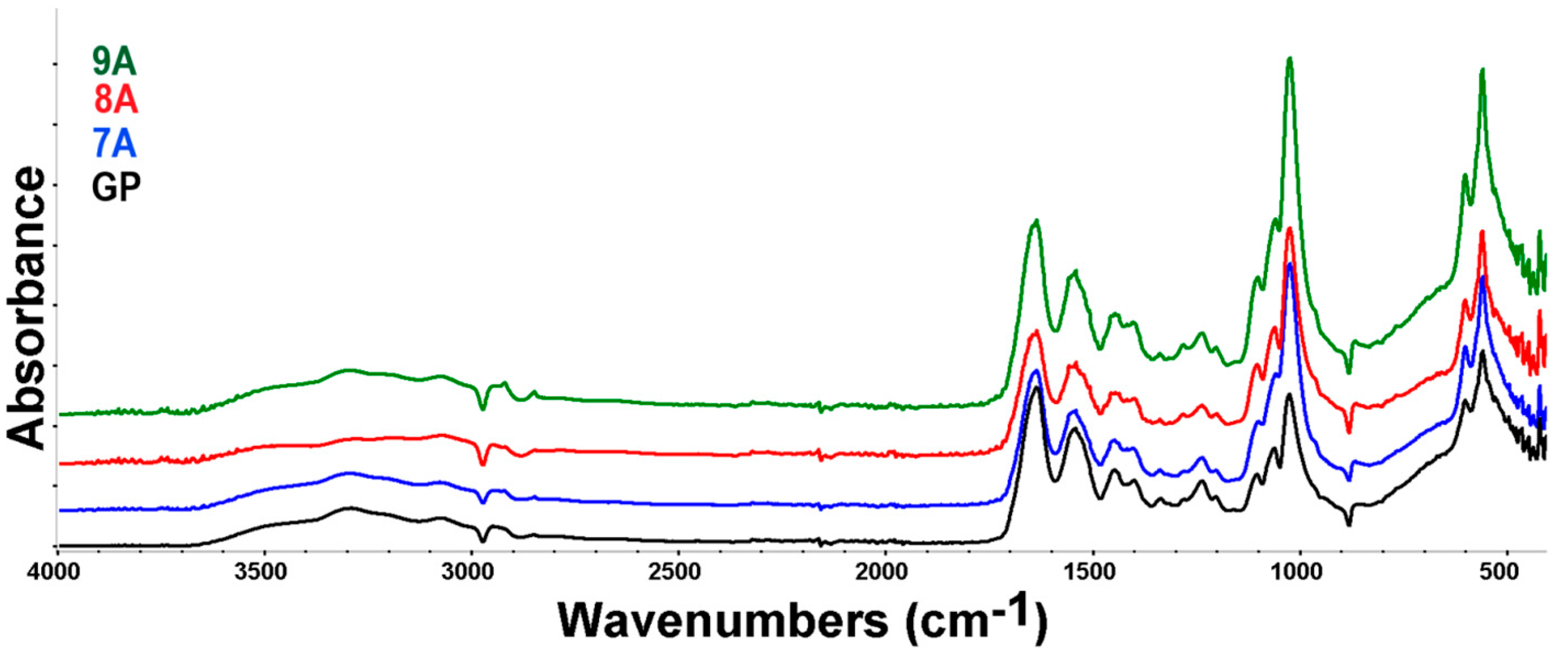
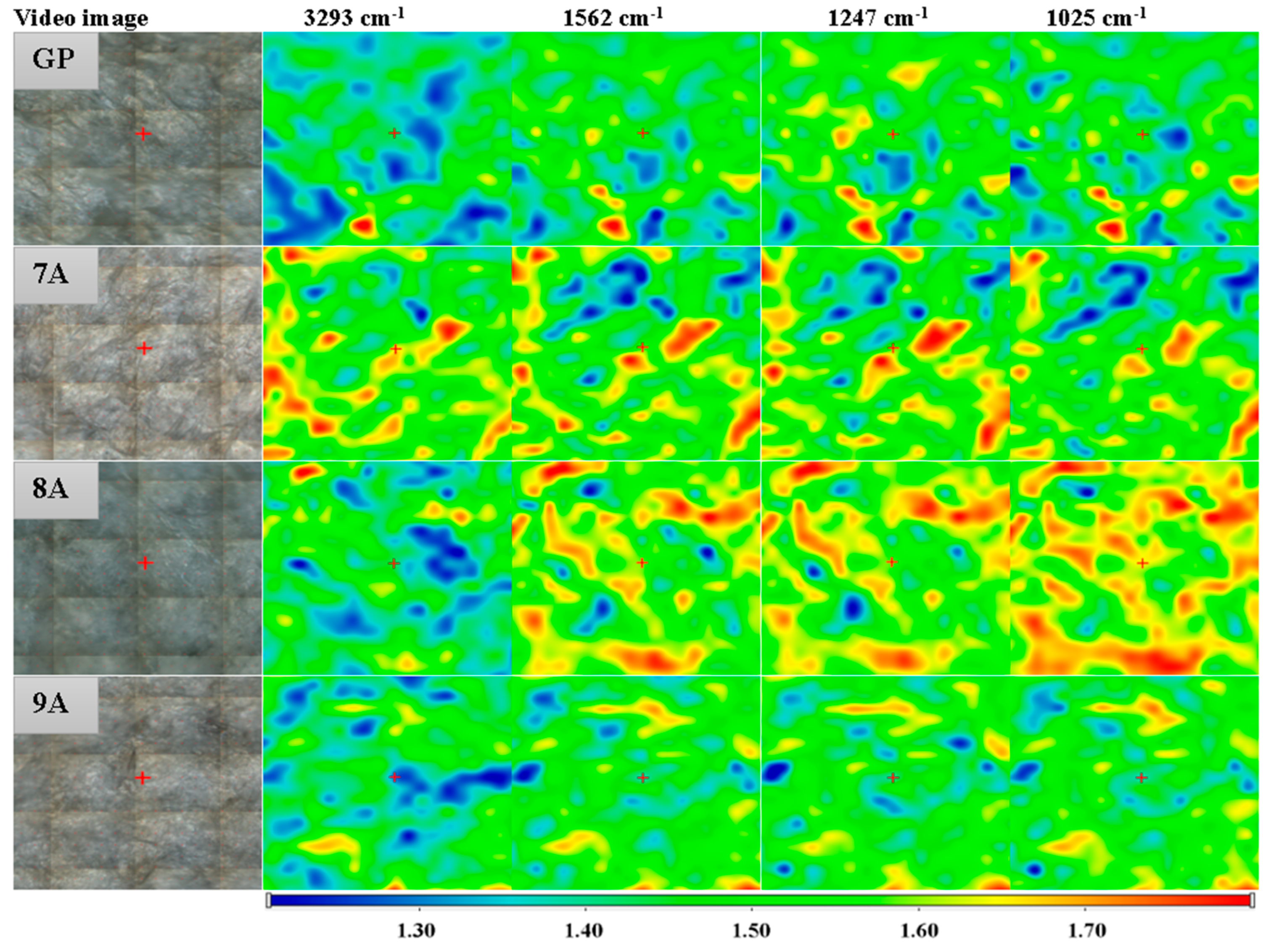
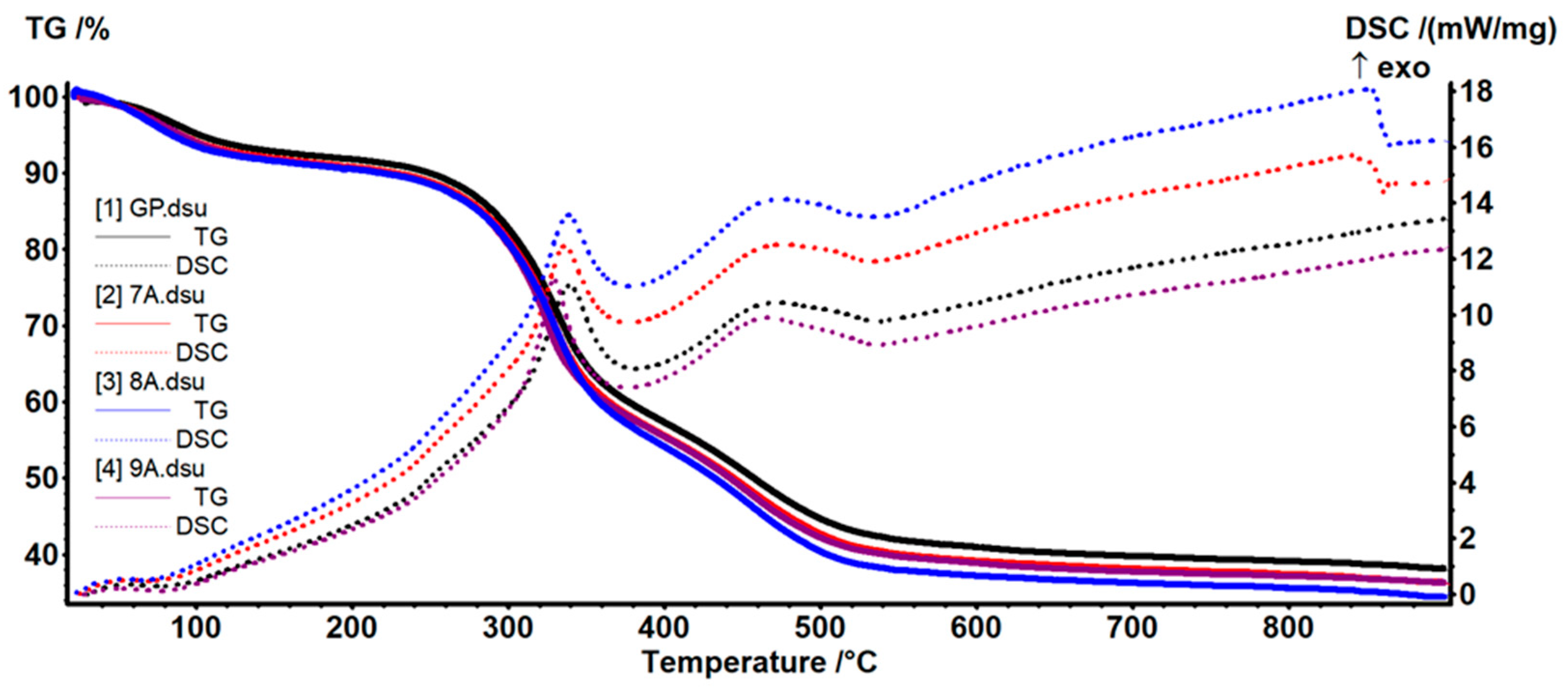

| Sample Code | Coll/HAp 1:1 Mineralized Gel | DOX | CA | GP |
|---|---|---|---|---|
| 7A | 9 mL | 0.001 g | - | 0.0075 g |
| 8A | 9 mL | - | 0.003 g | 0.0075 g |
| 9A | 9 mL | 0.001 g | 0.003 g | 0.0075 g |
| GP | 9 mL | - | - | 0.0075 g |
| Sample | Mass Loss (%) | Residual Mass (%) | Endo | Exo | Exo | ||
|---|---|---|---|---|---|---|---|
| RT–200 °C | 200–540 °C | 540–900 °C | |||||
| GP | 7.89 | 49.51 | 4.13 | 38.13 | 75.2 °C | 339.8 °C | 470.4 °C |
| 7A | 9.29 | 50.40 | 3.95 | 36.40 | 68.2 °C | 336.3 °C | 475.3 °C |
| 8A | 9.54 | 52.28 | 3.84 | 34.36 | 64.5 °C | 338.7 °C | 475.2 °C |
| 9A | 9.43 | 50.52 | 3.74 | 36.29 | 75.5 °C | 329.5 °C | 465.6 °C |
| Scaffold or Study | Composition | Structure and Porosity | MG-63 Viability | MSC Viability | Distinctive Characteristics |
|---|---|---|---|---|---|
| 9A (this study) | Collagen–HAp + DOX + CA | 20–250-µm pores, interconnected structure | ~20% (3 days) | ~17% | Selective effect: cytotoxic to MG63, partial protection of BMSCs |
| Rong et al. (2016) [60] | Collagen–HAp + PLGA/DOX microspheres | 100–200-µm pores, 3D interconnected structure | ~50% (3 days) | Not reported | Controlled DOX release, in vivo osteointegration, injectable structures |
| Lu et al. (2021) [61] | Poly(lactic-co-glycolic acid)–HAp + DOX + Polydopamine nanofibers | Oriented nanostructure, nanoscale pores | Significantly reduced | Enhanced proliferation | Fibrous structure, regenerative applicability, osteoinductive effects |
| Wang et al. (2024) [62] | Polydopamine-functionalized calcium-deficient HAp 3D-printed scaffold + DOX | 3D-printed scaffold, defined porosity | Considerably reduced | Promoted proliferation | Combined chemo–photothermal therapy, accelerated bone regeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladu, A.F.; Albu Kaya, M.G.; Ficai, A.; Ficai, D.; Tutuianu, R.; Motelica, L.; Surdu, V.A.; Oprea, O.-C.; Truşcă, R.D.; Titorencu, I. Localized Combination Therapy Using Collagen–Hydroxyapatite Bone Grafts for Simultaneous Bone Cancer Inhibition and Tissue Regeneration. Polymers 2025, 17, 2239. https://doi.org/10.3390/polym17162239
Vladu AF, Albu Kaya MG, Ficai A, Ficai D, Tutuianu R, Motelica L, Surdu VA, Oprea O-C, Truşcă RD, Titorencu I. Localized Combination Therapy Using Collagen–Hydroxyapatite Bone Grafts for Simultaneous Bone Cancer Inhibition and Tissue Regeneration. Polymers. 2025; 17(16):2239. https://doi.org/10.3390/polym17162239
Chicago/Turabian StyleVladu, Alina Florentina, Madalina Georgiana Albu Kaya, Anton Ficai, Denisa Ficai, Raluca Tutuianu, Ludmila Motelica, Vasile Adrian Surdu, Ovidiu-Cristian Oprea, Roxana Doina Truşcă, and Irina Titorencu. 2025. "Localized Combination Therapy Using Collagen–Hydroxyapatite Bone Grafts for Simultaneous Bone Cancer Inhibition and Tissue Regeneration" Polymers 17, no. 16: 2239. https://doi.org/10.3390/polym17162239
APA StyleVladu, A. F., Albu Kaya, M. G., Ficai, A., Ficai, D., Tutuianu, R., Motelica, L., Surdu, V. A., Oprea, O.-C., Truşcă, R. D., & Titorencu, I. (2025). Localized Combination Therapy Using Collagen–Hydroxyapatite Bone Grafts for Simultaneous Bone Cancer Inhibition and Tissue Regeneration. Polymers, 17(16), 2239. https://doi.org/10.3390/polym17162239












