Carbon:Nitrogen Ratio Affects Differentially the Poly-β-hydroxybutyrate Synthesis in Bacillus thuringiensis Isolates from México
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacillus thuringiensis Isolates
2.2. Selection of P(3HB) Producers Using Sudan Black Dye
2.3. Molecular Identification of Selected Bacillus thuringiensis Isolates
2.4. phaC Gene Amplification
2.5. Evaluation of C:N Ratio for P(3HB) Production During Flask and Bioreactor Cultures
2.6. Analytical Methods
2.7. P(3HB) Quantification by UV Spectrophotometry
2.8. P(3HB) Extraction and Characterization by FOURIER Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Selection and Identification of P(3HB) Producer Isolates by Sudan Black Dye
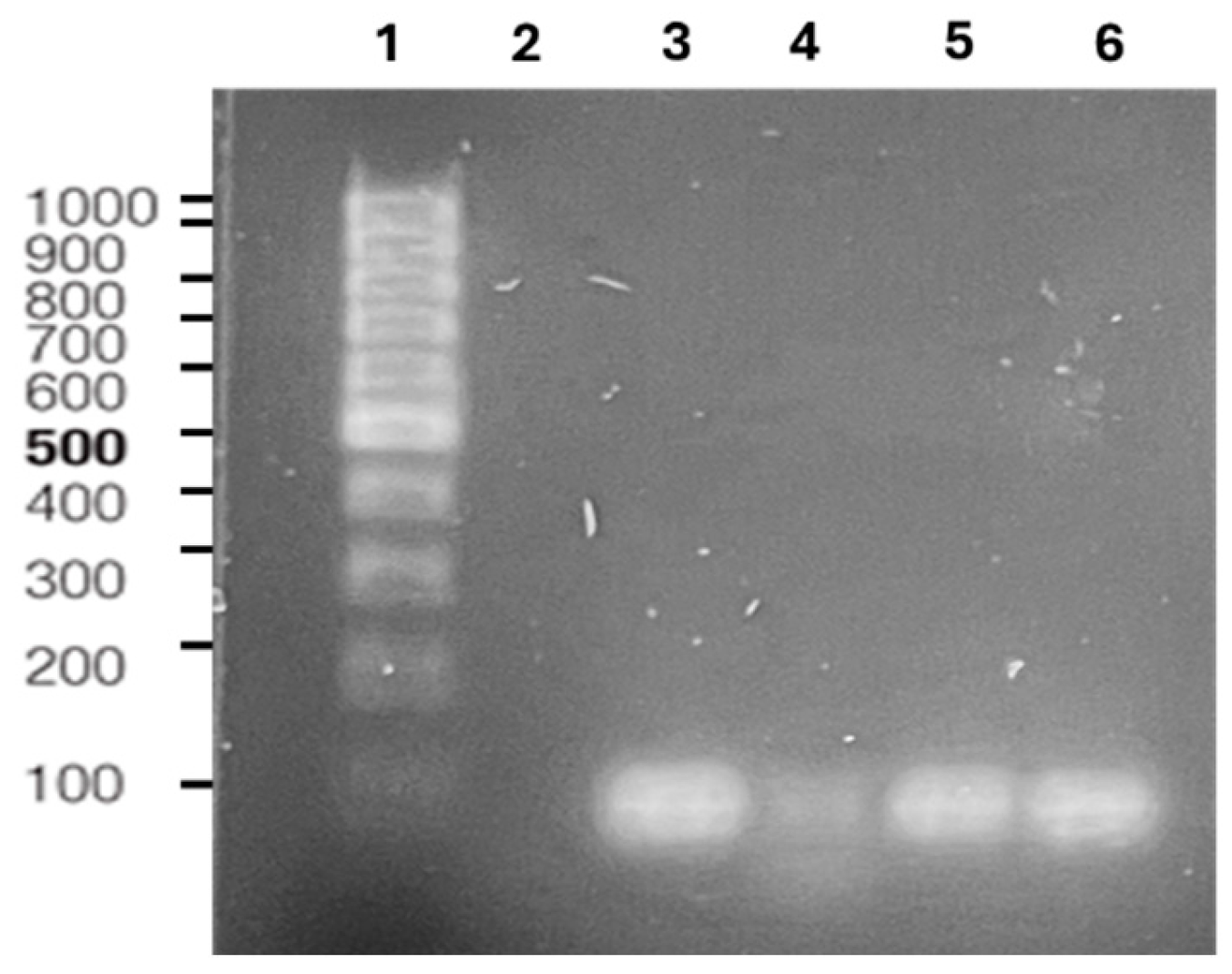
3.2. Bacillus thuringiensis Identification by 16S rRNA Gene Sequence and Identification of phaC Gene
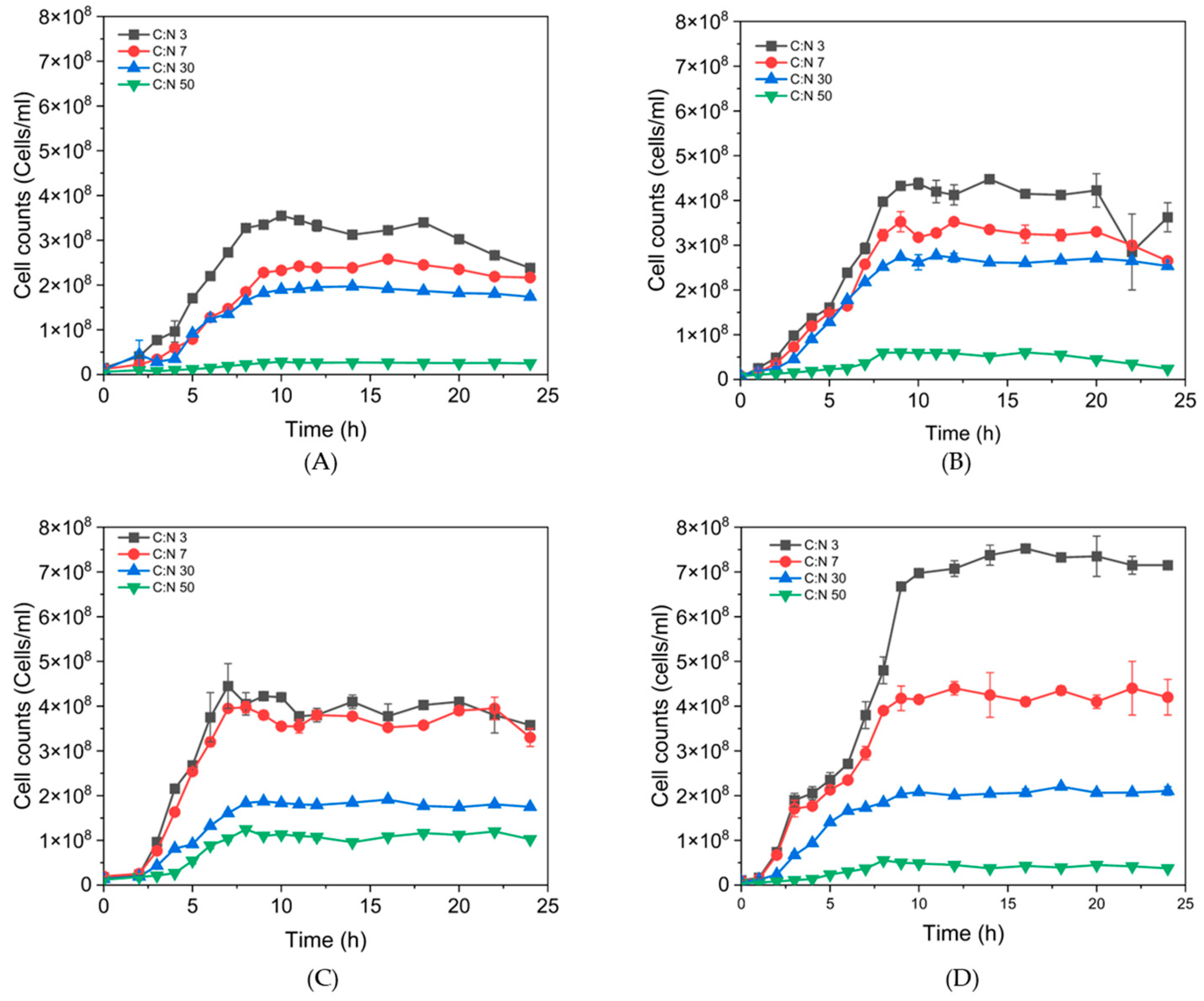
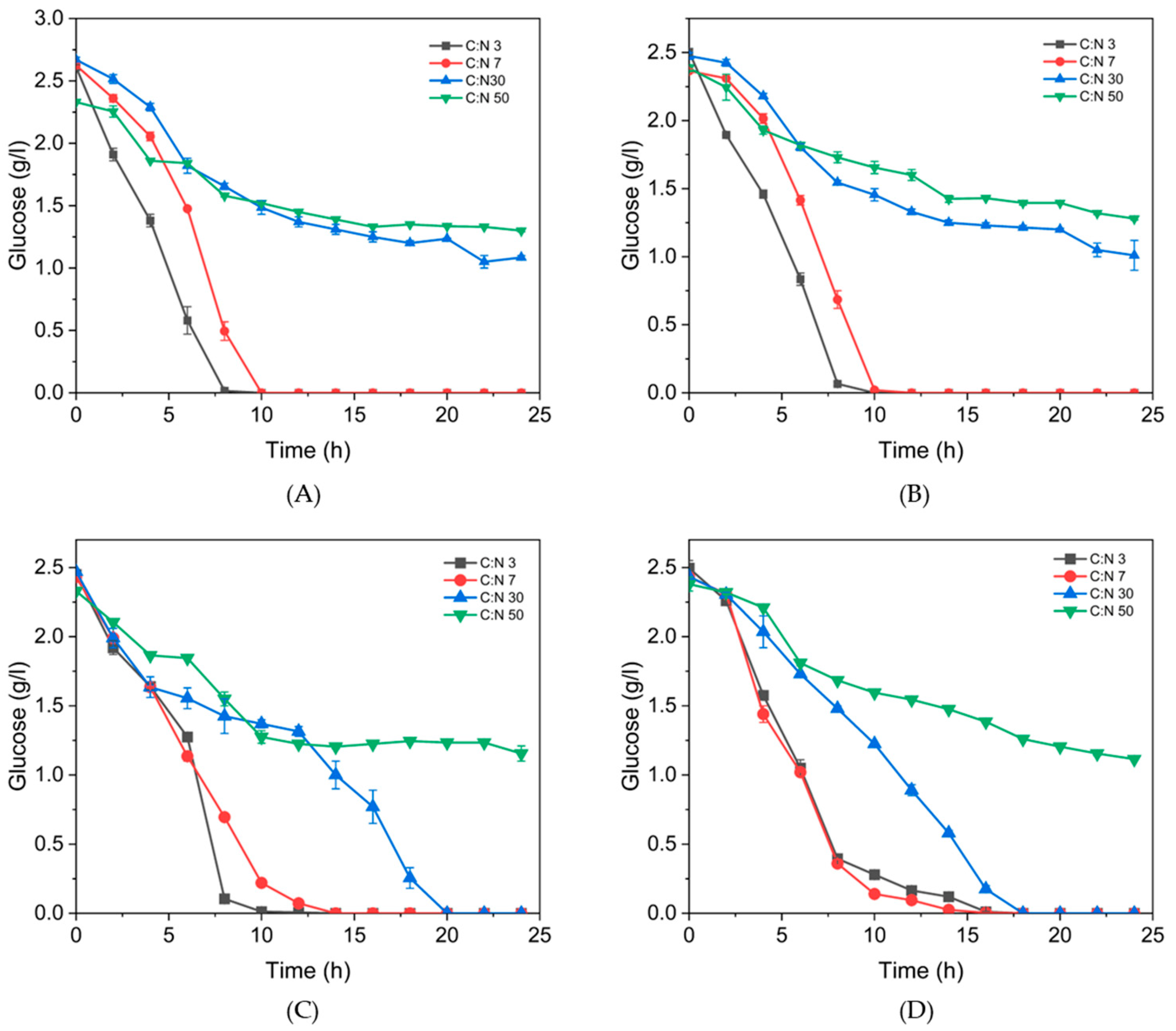
3.3. Effect of Different C:N Ratios on Growth and P(3HB) Accumulation in B. thuringiensis Isolates
3.4. Batch Production of P(3HB) at Bioreactor Scale
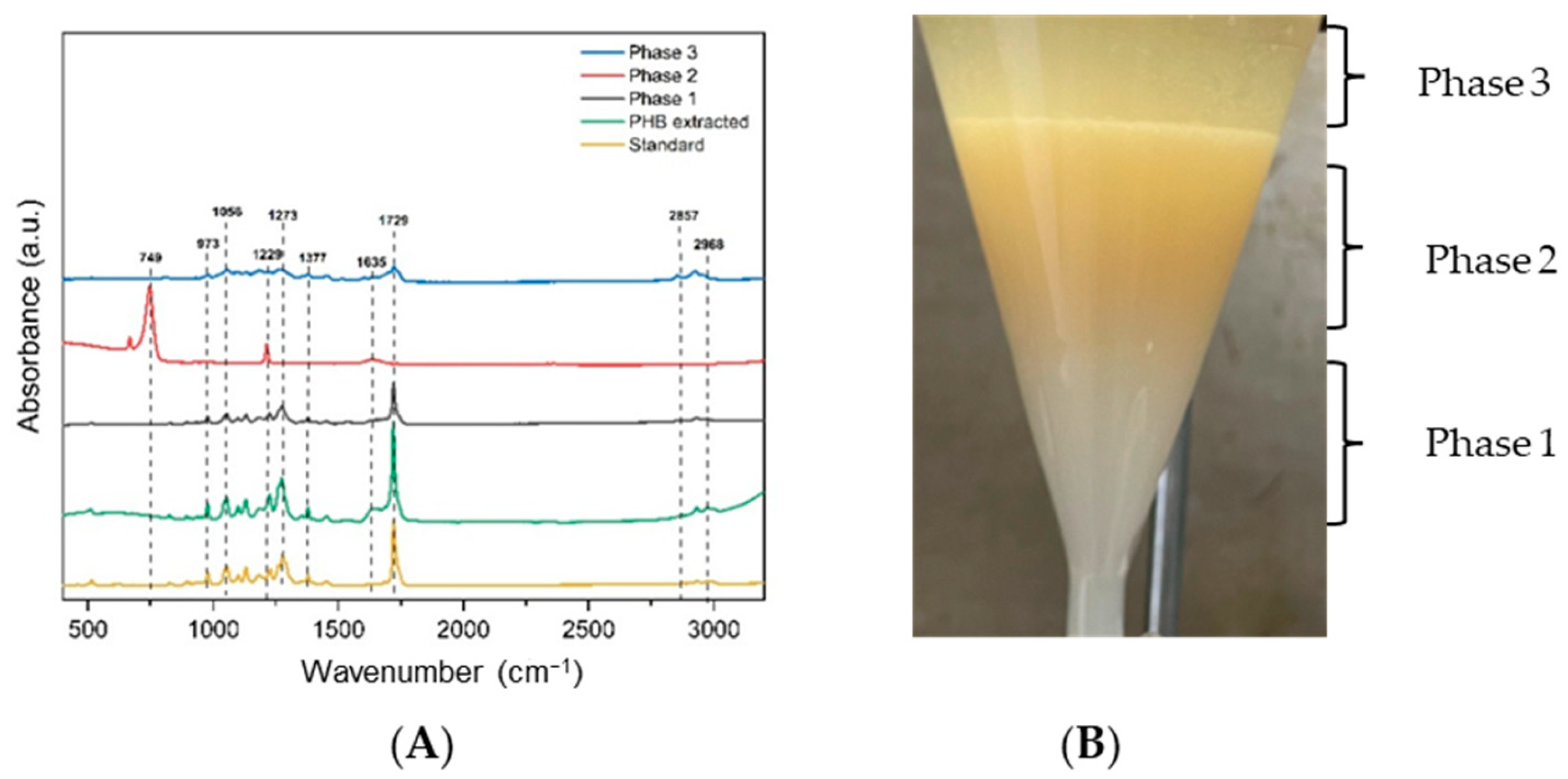
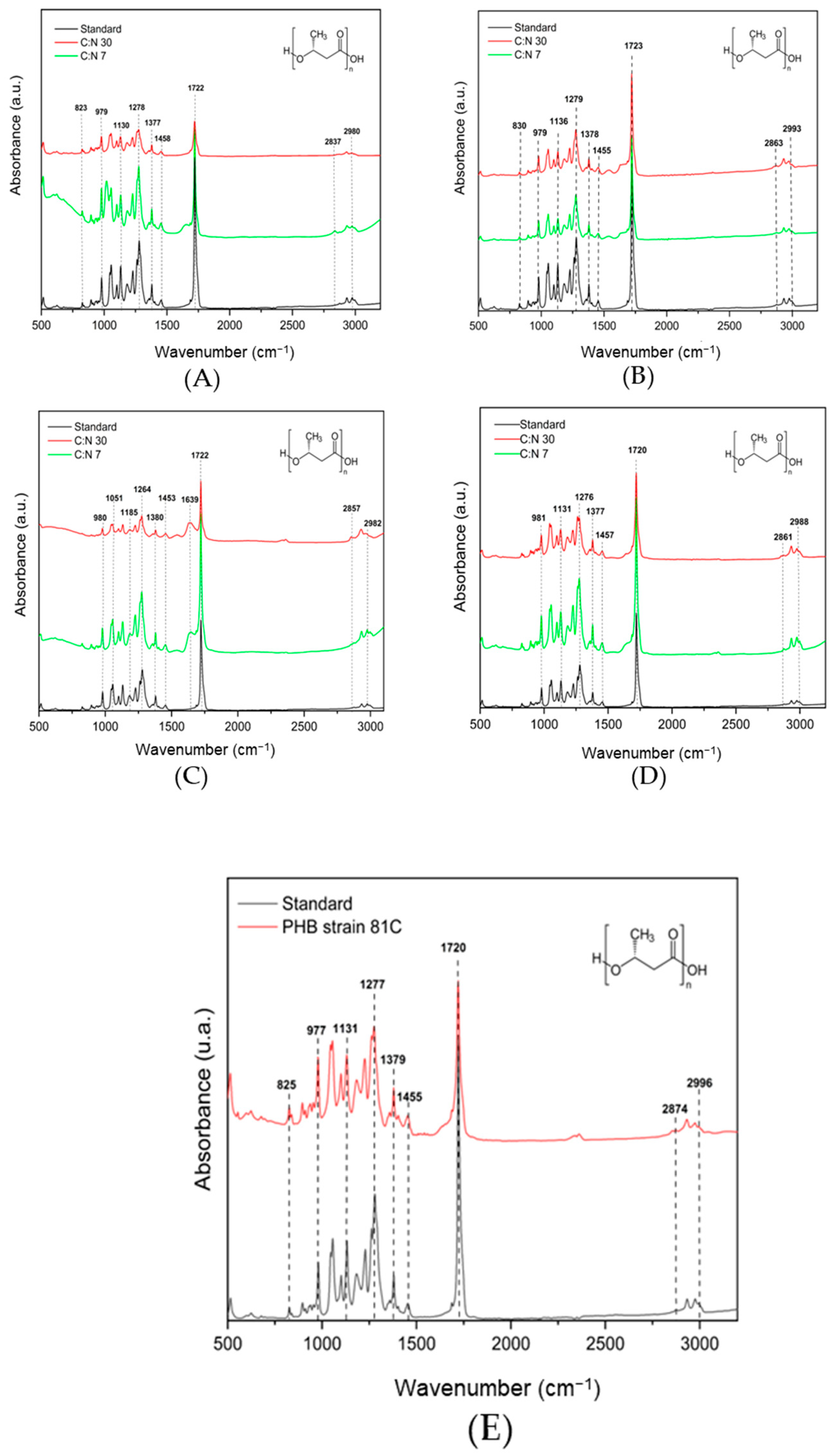
3.5. Characterization of P(3HB) Extracted from Bt by FTIR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gholamveisi, N.; Azar, S.M.; Moravej, R. Bacillus thuringiensis Strain NG, a Novel Isolated Strain for Production of Various Polyhydroxyalkanoates. J. Microb. Biol. 2018, 6, 13–20. [Google Scholar] [CrossRef]
- Campos, M.I.; Figueiredo, T.V.B.; Sousa, L.S.; Druzian, J.I. The Influence of Crude Glycerin and Nitrogen Concentrations on the Production of PHA by Cupriavidus necator Using a Response Surface Methodology and Its Characterizations. Ind. Crop Prod. 2014, 52, 338–346. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Pavan, F.A.; Bordignon, S.E.; de Aragão, G.M.F.; Poletto, P. Polyhydroxybutyrate (PHB) Production by Cupriavidus necator from Sugarcane Vinasse and Molasses as Mixed Substrate. Process Biochem. 2019, 85, 12–18. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Wyman, I.; Auras, R.; Cheng, S. A Roadmap towards Green Packaging: The Current Status and Future Outlook for Polyesters in the Packaging Industry. Green Chem. 2017, 19, 4737–4753. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.-C. Bacterial Conversion of Waste into Polyhydroxybutyrate (PHB): A New Approach of Bio-Circular Economy for Treating Waste and Energy Generation. Bioresour. Technol. Rep. 2019, 7, 100246. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Song, H.-S.; Jeon, J.-M.; Kim, J.-S.; Lee, Y.-K.; Yang, Y.-H. Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Production from Engineered Ralstonia Eutropha Using Synthetic and Anaerobically Digested Food Waste Derived Volatile Fatty Acids. Int. J. Biol. Macromol. 2019, 133, 1–10. [Google Scholar] [CrossRef]
- Prasertsilp, P.; Pattaragulwanit, K.; Kim, B.S.; Napathorn, S.C. Microwave-Assisted Cassava Pulp Hydrolysis as Food Waste Biorefinery for Biodegradable Polyhydroxybutyrate Production. Front. Bioeng. Biotechnol. 2023, 11, 1131053. [Google Scholar] [CrossRef]
- Jae Park, S.; Hee Kang, K.; Lee, H.; Reum Park, A.; Eun Yang, J.; Hoon Oh, Y.; Keun Song, B.; Jegal, J.; Hwan Lee, S.; Yup Lee, S. Propionyl-CoA Dependent Biosynthesis of 2-Hydroxybutyrate Containing Polyhydroxyalkanoates in Metabolically Engineered Escherichia coli. J. Biotechnol. 2013, 165, 93–98. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Ghai, R.; Rashmi; Kalia, V.C. Polyhydroxyalkanoates an Overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Kumar, P.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Extending the Limits of Bacillus for Novel Biotechnological Applications. Biotechnol. Adv. 2013, 31, 1543–1561. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.-Y.; Yang, H.; Chen, J.-N.; Lin, Y.; Han, S.-Y.; Cao, Q.; Zeng, H.-S.; Ye, J.-W. A Polyhydroxyalkanoates-Based Carrier Platform of Bioactive Substances for Therapeutic Applications. Front. Bioeng. Biotechnol. 2022, 9, 798724. [Google Scholar] [CrossRef]
- Singh, M.; Patel, S.K.; Kalia, V.C. Bacillus subtilis as Potential Producer for Polyhydroxyalkanoates. Microb. Cell Fact. 2009, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Siddiqui, A.J.; Ashraf, S.A.; Snoussi, M.; Badraoui, R.; Alreshidi, M.; Elasbali, A.M.; Al-Soud, W.A.; Alharethi, S.H.; Sachidanandan, M.; et al. Polyhydroxybutyrate (PHB)-Based Biodegradable Polymer from Agromyces Indicus: Enhanced Production, Characterization, and Optimization. Polymers 2022, 14, 3982. [Google Scholar] [CrossRef] [PubMed]
- Zafra, G.; Absalón, Á.E.; Cuevas, M.D.C.; Cortés-Espinosa, D.V. Isolation and Selection of a Highly Tolerant Microbial Consortium with Potential for PAH Biodegradation from Heavy Crude Oil-Contaminated Soils. Water Air Soil Pollut. 2014, 225, 1826. [Google Scholar] [CrossRef]
- Dinorín-Téllez-Girón, J.; Delgado-Macuil, R.J.; Larralde Corona, C.P.; Martínez Montes, F.J.; De La Torre Martínez, M.; López-Y-López, V.E. Reactance and Resistance: Main Properties to Follow the Cell Differentiation Process in Bacillus thuringiensis by Dielectric Spectroscopy in Real Time. Appl. Microbiol. Biotechnol. 2015, 99, 5439–5450. [Google Scholar] [CrossRef]
- Díaz Pacheco, A.; Delgado-Macuil, R.J.; Larralde-Corona, C.P.; Dinorín-Téllez-Girón, J.; Martínez Montes, F.; Martinez Tolibia, S.E.; López Y López, V.E. Two-Methods Approach to Follow up Biomass by Impedance Spectroscopy: Bacillus thuringiensis Fermentations as a Study Model. Appl. Microbiol. Biotechnol. 2022, 106, 1097–1112. [Google Scholar] [CrossRef]
- López Y López, V.; Mártinez, S.; Díaz, A.; Lozano, A.; Sierra, P.; Téllez, J. Influence of Nutrient Feeding Variations on AbrB Accumulation, Sporulation and cry1Ac Expression during Fed-Batch Cultures of Bacillus thuringiensis. Mex. J. Biotechnol. 2023, 8, 46–67. [Google Scholar] [CrossRef]
- López-y-López, V.E.; De La Torre, M. Redirection of Metabolism during Nutrient Feeding in Fed-Batch Cultures of Bacillus thuringiensis. Appl. Microbiol. Biotechnol. 2005, 67, 254–260. [Google Scholar] [CrossRef]
- Lozano Goné, A.M.; Dinorín Téllez Girón, J.; Jiménez Montejo, F.E.; Hidalgo-Lara, M.E.; López Y López, V.E. Behavior of Transition State Regulator AbrB in Batch Cultures of Bacillus thuringiensis. Curr. Microbiol. 2014, 69, 725–732. [Google Scholar] [CrossRef]
- Monroy, M.R.; de la Torre, M. Effect of the Dilution Rate on the Biomass Yield of Bacillus thuringiensis and Determination of Its Rate Coefficients under Steady-State Conditions. Appl. Microbiol. Biotechnol. 1996, 45, 546–550. [Google Scholar] [CrossRef]
- Rajankar, M.P.; Ravindranathan, S.; Rajamohanan, P.R.; Raghunathan, A. Absolute Quantitation of Poly(R)-3-Hydroxybutyric Acid Using Spectrofluorometry in Recombinant Escherichia coli. Biol. Methods Protoc. 2018, 3, bpy007. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sithole, B.; Lekha, P.; Permaul, K.; Govinden, R. Optimization of Cultivation Medium and Cyclic Fed-Batch Fermentation Strategy for Enhanced Polyhydroxyalkanoate Production by Bacillus thuringiensis Using a Glucose-Rich Hydrolyzate. Bioresour. Bioprocess. 2021, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Thammasittirong, A.; Saechow, S.; Thammasittirong, S.N.-R. Efficient Polyhydroxybutyrate Production from Bacillus thuringiensis Using Sugarcane Juice Substrate. Turk. J. Biol. 2017, 41, 992–1002. [Google Scholar] [CrossRef]
- Narayanan, M.; Kandasamy, S.; Kumarasamy, S.; Gnanavel, K.; Ranganathan, M.; Kandasamy, G. Screening of Polyhydroxybutyrate Producing Indigenous Bacteria from Polluted Lake Soil. Heliyon 2020, 6, e05381. [Google Scholar] [CrossRef]
- Moorkoth, D.; Madhavan, K. Production and Characterization of Poly(3-Hydroxy Butyrate-Co-3 Hydroxyvalerate) (PHBV) by a Novel Halotolerant Mangrove Isolate. Bioresour. Technol. 2016, 201, 253–260. [Google Scholar] [CrossRef]
- Choi, S.Y.; Rhie, M.N.; Kim, H.T.; Joo, J.C.; Cho, I.J.; Son, J.; Jo, S.Y.; Sohn, Y.J.; Baritugo, K.-A.; Pyo, J.; et al. Metabolic Engineering for the Synthesis of Polyesters: A 100-Year Journey from Polyhydroxyalkanoates to Non-Natural Microbial Polyesters. Metab. Eng. 2020, 58, 47–81. [Google Scholar] [CrossRef]
- Peña-Jurado, E.; Pérez-Vega, S.; Zavala-Díaz De La Serna, F.J.; Pérez-Reyes, I.; Gutiérrez-Méndez, N.; Vazquez-Castillo, J.; Salmerón, I. Production of Poly (3-Hydroxybutyrate) from a Dairy Industry Wastewater Using Bacillus Subtilis EPAH18: Bioprocess Development and Simulation. Biochem. Eng. J. 2019, 151, 107324. [Google Scholar] [CrossRef]
- Saravanan, K.; Umesh, M.; Kathirvel, P. Microbial Polyhydroxyalkanoates (PHAs): A Review on Biosynthesis, Properties, Fermentation Strategies and Its Prospective Applications for Sustainable Future. J. Polym. Environ. 2022, 30, 4903–4935. [Google Scholar] [CrossRef]
- Zhou, W.; Colpa, D.I.; Geurkink, B.; Euverink, G.-J.W.; Krooneman, J. The Impact of Carbon to Nitrogen Ratios and pH on the Microbial Prevalence and Polyhydroxybutyrate Production Levels Using a Mixed Microbial Starter Culture. Sci. Total Environ. 2022, 811, 152341. [Google Scholar] [CrossRef]
- Kumar, P.; Ray, S.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Bioconversion of Crude Glycerol to Polyhydroxyalkanoate by Bacillus thuringiensis under Non-Limiting Nitrogen Conditions. Int. J. Biol. Macromol. 2015, 78, 9–16. [Google Scholar] [CrossRef]
- Farrera, R.; Pérez, F.; De La Torre, M. Carbon:Nitrogen Ratio Interacts with Initial Concentration of Total Solids on Insecticidal Crystal Protein and Spore Production in Bacillus thuringiensis HD-73. Appl. Microbiol. Biotechnol. 1998, 49, 758–765. [Google Scholar] [CrossRef]
- Anderson, T. Efects of Carbon:Nitrogen Ratio and Oxygen on the Growth Kinetics of Bacillus thuringiensis and Yield of Bioinsecticidal Crystal Protein. Master’s Thesis, University of Western, London, ON, Canada, 1990. [Google Scholar]
- Gowda, V.; Shivakumar, S. Agrowaste-Based Polyhydroxyalkanoate (PHA) Production Using Hydrolytic Potential of Bacillus thuringiensis IAM 12077. Braz. Arch. Biol. Technol. 2014, 57, 55–61. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Alrumman, S.A.; Otaif, K.A.; Alamri, S.A.; Mostafa, M.S.; Sahlabji, T. Production and Characterization of Bioplastic by Polyhydroxybutyrate Accumulating Erythrobacter Aquimaris Isolated from Mangrove Rhizosphere. Molecules 2020, 25, 179. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Patel, P.; Desai, R. Detection & Characterization of PHB (Polyhydroxybutyrate) Producers Halophilic Bacteria Isolated from Marine Water Sample of Valsad District. Int. J. Pharma Bio Sci. 2017, 8, 1100–1108. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Geerts, C.; Furtos, A.; Waters, P.; Cyr, D.; Wang, S.; Mitchell, G.A. The Multiple Facets of Acetyl-CoA Metabolism: Energetics, Biosynthesis, Regulation, Acylation and Inborn Errors. Mol. Genet. Metab. 2022, 138, 106966. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Flores-Rodríguez, P.; Almaguer-Cantú, V.; Valencia-Vázquez, R.; Rosas-Flores, W.; Medrano-Roldán, H.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M. Utilization of Agave Durangensis Leaves by Bacillus Cereus 4N for Polyhydroxybutyrate (PHB) Biosynthesis. Int. J. Biol. Macromol. 2021, 175, 199–208. [Google Scholar] [CrossRef]
- Lovely; Kumar, S.; Srivastava, A.K.; Shivakumar, S. Optimized Batch Cultivation and Scale-up of Bacillus thuringiensis for High-Yield Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate). Bioresour. Technol. 2024, 409, 131220. [Google Scholar] [CrossRef]
- García, A.; Segura, D.; Espín, G.; Galindo, E.; Castillo, T.; Peña, C. High Production of Poly-β-Hydroxybutyrate (PHB) by an Azotobacter Vinelandii Mutant Altered in PHB Regulation Using a Fed-Batch Fermentation Process. Biochem. Eng. J. 2014, 82, 117–123. [Google Scholar] [CrossRef]
- Ghosh, S.B.; Bhattacharya, K.; Nayak, S.; Mukherjee, P.; Salaskar, D.; Kale, S.P. Identification of Different Species of Bacillus Isolated from Nisargruna Biogas Plant by FTIR, UV–Vis and NIR Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 148, 420–426. [Google Scholar] [CrossRef]
- Naumann, D.; Helm, D.; Labischinski, H. Microbiological Characterizations by FT-IR Spectroscopy. Nature 1991, 351, 81–82. [Google Scholar] [CrossRef]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Almaguer-Cantú, V.; Rosas-Flores, W.; Martínez-Gómez, V.J.; Quintero-Zapata, I.; Rivera, G.; Rutiaga-Quiñones, O.M. Efficient Recovery of Thermostable Polyhydroxybutyrate (PHB) by a Rapid and Solvent-Free Extraction Protocol Assisted by Ultrasound. Int. J. Biol. Macromol. 2020, 164, 771–782. [Google Scholar] [CrossRef]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Kosugi, A.; Arai, T.; Sudesh, K.; Vaithanomsat, P. Enhanced Polyhydroxybutyrate (PHB) Production by Newly Isolated Rare Actinomycetes Rhodococcus Sp. Strain BSRT1-1 Using Response Surface Methodology. Sci. Rep. 2021, 11, 1896. [Google Scholar] [CrossRef]





| C:N Ratio | Glucose (g/L) | Soy Peptone (g/L) | Yeast Extract (g/L) |
|---|---|---|---|
| 3 | 2.5 | 2.5 | 4.8 |
| 7 | 2.5 | 2.5 | 0.32 |
| 30 | 2.5 | 0.16 | 0.20 |
| 50 | 2.5 | 0.10 | 0.10 |
| Isolate | C:N | Maximal Biomass (g/L) | YX/S | * YP/S |
|---|---|---|---|---|
| 3 | 1.73 | 0.692 | Undetectable | |
| 7 | 1.01 | 0.411 | 0.039 | |
| 81C | 30 | 0.50 | 0.208 | 0.056 |
| 50 | 0.14 | 0.085 | 0.008 | |
| 3 | 0.82 | 0.312 | 0.004 | |
| 7 | 0.59 | 0.225 | 0.034 | |
| 32A | 30 | 0.45 | 0.287 | 0.043 |
| 50 | 0.07 | 0.063 | 0.024 | |
| 3 | 1.02 | 0.417 | Undetectable | |
| 7 | 0.91 | 0.376 | 0.049 | |
| 73B | 30 | 0.44 | 0.178 | 0.046 |
| 50 | 0.29 | 0.253 | 0.049 | |
| 3 | 1.01 | 0.397 | Undetectable | |
| 7 | 0.81 | 0.345 | 0.031 | |
| 42A | 30 | 0.51 | 0.203 | 0.022 |
| 50 | 0.14 | 0.057 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero Sanchez, M.T.; Martínez Tolibia, S.E.; García Barrera, L.J.; Sierra Martínez, P.; Gracida Rodríguez, J.N.; López Gayou, V.; López y López, V.E. Carbon:Nitrogen Ratio Affects Differentially the Poly-β-hydroxybutyrate Synthesis in Bacillus thuringiensis Isolates from México. Polymers 2025, 17, 1978. https://doi.org/10.3390/polym17141978
Romero Sanchez MT, Martínez Tolibia SE, García Barrera LJ, Sierra Martínez P, Gracida Rodríguez JN, López Gayou V, López y López VE. Carbon:Nitrogen Ratio Affects Differentially the Poly-β-hydroxybutyrate Synthesis in Bacillus thuringiensis Isolates from México. Polymers. 2025; 17(14):1978. https://doi.org/10.3390/polym17141978
Chicago/Turabian StyleRomero Sanchez, Marco Tulio, Shirlley Elizabeth Martínez Tolibia, Laura Jeannette García Barrera, Pavel Sierra Martínez, Jorge Noel Gracida Rodríguez, Valentín López Gayou, and Víctor Eric López y López. 2025. "Carbon:Nitrogen Ratio Affects Differentially the Poly-β-hydroxybutyrate Synthesis in Bacillus thuringiensis Isolates from México" Polymers 17, no. 14: 1978. https://doi.org/10.3390/polym17141978
APA StyleRomero Sanchez, M. T., Martínez Tolibia, S. E., García Barrera, L. J., Sierra Martínez, P., Gracida Rodríguez, J. N., López Gayou, V., & López y López, V. E. (2025). Carbon:Nitrogen Ratio Affects Differentially the Poly-β-hydroxybutyrate Synthesis in Bacillus thuringiensis Isolates from México. Polymers, 17(14), 1978. https://doi.org/10.3390/polym17141978








