Discovery of a High 3-Hydroxyhexanoate Containing Poly-3-hydroxybutyrate-co-3-hydroxyhexanoate Producer-, Cupriavidus sp. Oh_1 with Enhanced Fatty Acid Metabolism
Abstract
1. Introduction
| Strategies | Strains | Genetic Construct | Fatty Acids | DCW (g/L) | 3HHx (mol%) | Ref. |
|---|---|---|---|---|---|---|
| Introduction of phaC | H16dphaC1 | phaCAc | Octanoate | 2.6 | 2 | [17] |
| Bean | 4.1 | 11 | ||||
| H16dphaC1 | phaCAc | Palm kernel | 165 | 8 | [18] | |
| phaCAc + BktB | Palm kernel + butyrate | 170 | 13 | |||
| ΔphaC + phaC | C. necator DMS 541 | phaC1Re, phaBRe | Hexanoate | 1.9 | 10 | [19] |
| C. necator DMS 541 | phaCAc NSDG | Soybean | 2.3 ± 0.2 | 5.2 ± 0.3 | [7] | |
| Re2000 | ΔphaC1 + phaC1Ra | Palm | 7.3 ± 0.1 | 1.1 ± 0.3 | [10] | |
| Re2001 | ΔphaC2 + phaC1Ra | Palm | 2.2 ± 0.1 | 1.5 ± 0.2 | ||
| Re1034/pCB81 | ΔphaC1 + (phaCRa + phaACn + phaJPa) | Palm | 4.0 ± 0.2 | 11.6 ± 0.2 | ||
| Re2058/pCB113 | ΔphaC1, ΔproC + (phaCRa + phaACn + phaJPa + proCCn) | Palm | 3.6 ± 0.3 | 12.7 ± 0.3 | ||
| Re2135 | ΔphaC1, ΔphaB1, 2, 3 + phaC2Ra | Palm | 1.22 ± 0.08 | 31.4 ± 0.8 | ||
| ΔphaB,C + phaJ and/or phaC | Re2133/pCB81 | ΔphaC1, ΔphaB1, 2, 3 + (phaCRa + phaACn + phaJPa) | Palm | 2.9 ± 0.1 | 23.3 ± 0.2 | |
| Re2152 | ΔphaC1, ΔphaB1, 2, 3 + phaC2Ra + phaJ1Pa | Palm | 1.40 ± 0.02 | 22.4 ± 0.1 | ||
| NSDGΔAC | ΔphaC, phaA + (phaCNSDG + phaJAc) | Bean | 4.8 ± 0.05 | 9.9 ± 0.1 | [20] | |
| ΔphaCRe, phaA, fadB1 + (phaCNSDG + phaJAc + phaJ4aRe) | Bean | 4.7 ± 0.1 | 11.7 ± 0.8 | [21] | ||
| New strain | Oh_1 | phaCRa + phaRPa | Bean | 16.4 | 24.3 | This study |
2. Materials and Methods
2.1. Isolation and Screening of Strain
2.2. 16s rRNA Sequencing
2.3. Culture Conditions for PHA Synthesis
2.4. PHA Analytical Methods
2.5. RT-qPCR Analysis
2.6. Fed-Batch Fermentation Conditions
2.7. Analysis of Physical and Thermal Characteristics
3. Results and Discussion
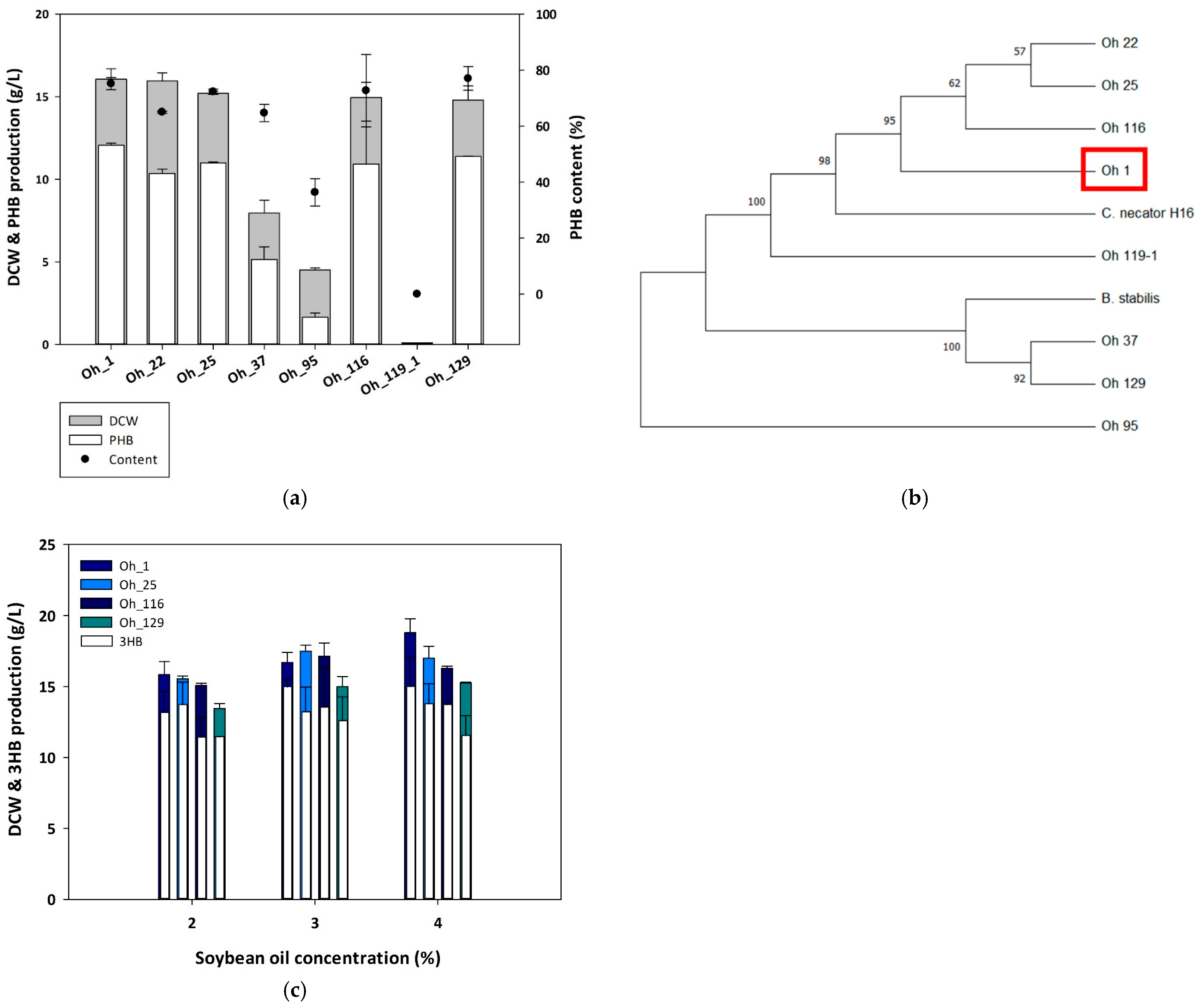
3.1. Identification of a High PHB-Producing Strain from Soybean Oil
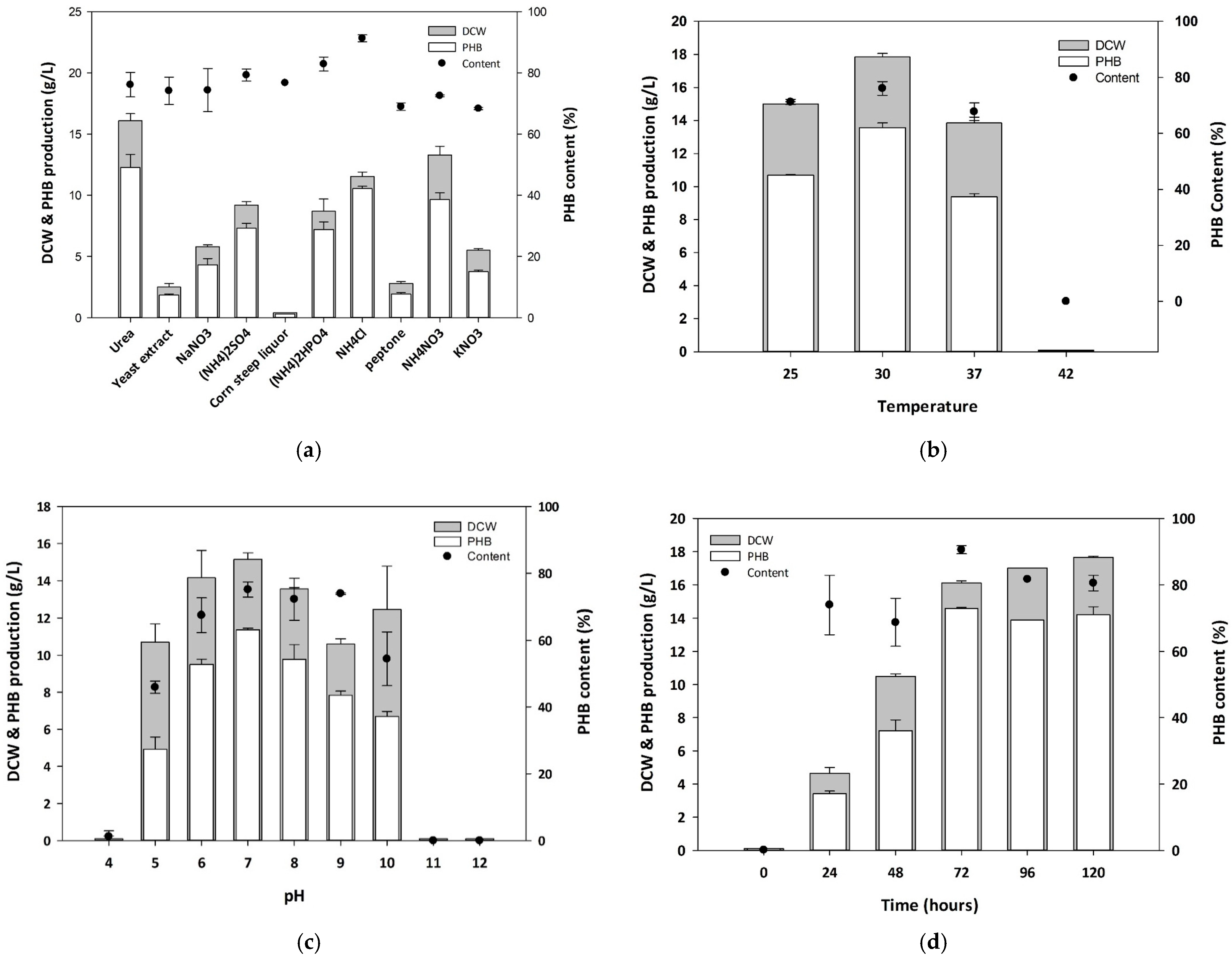
3.2. Optimization of Culture Conditions for PHB Production
3.3. Copolymer Synthesis via Supplementation with Various Monomers and the Comparison of P(3HB-co-3HHx) Production of Cupriavidus sp. Oh_1 and H16
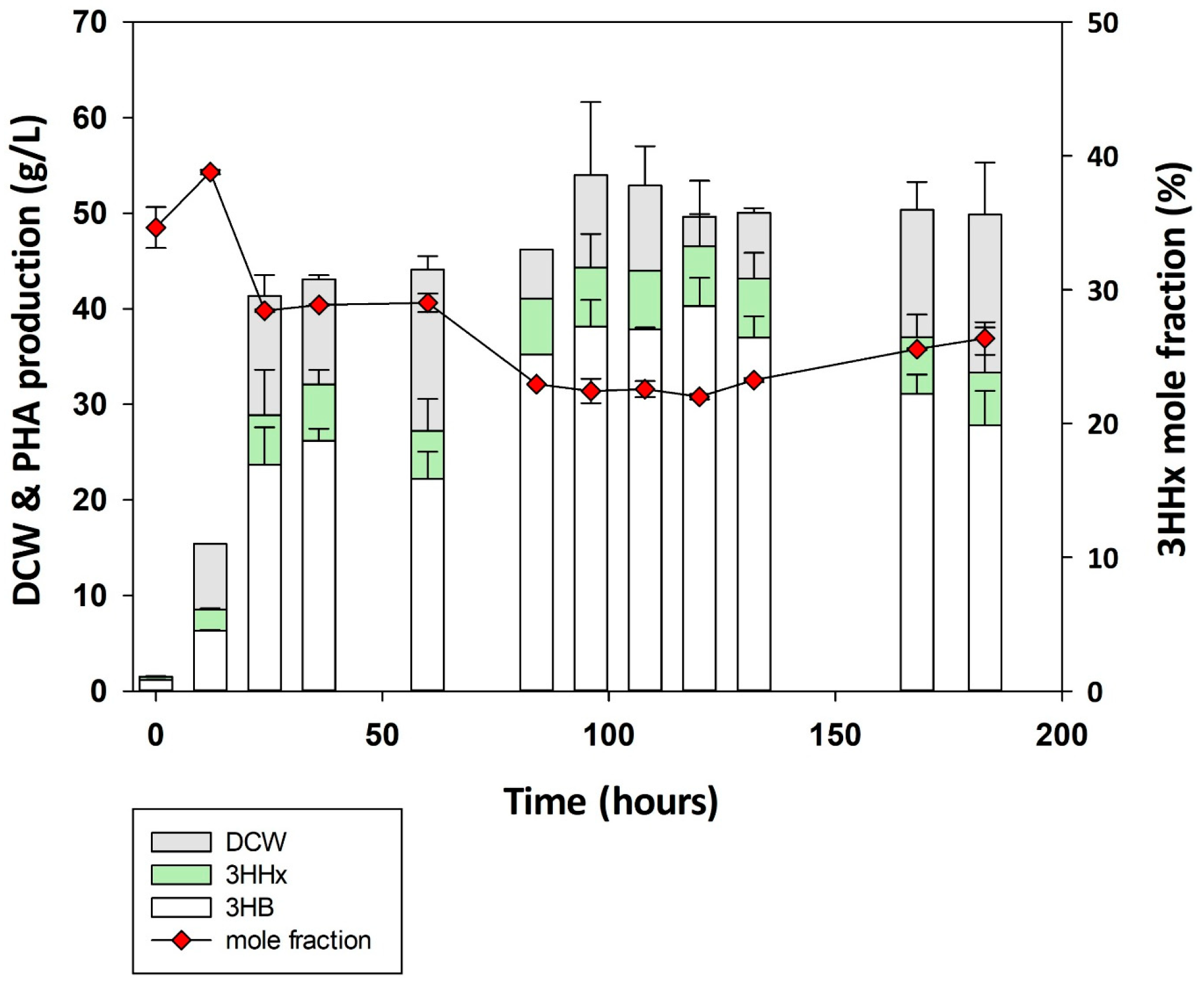
3.4. Time-Dependent Monitoring of P(3HB-co-3HHx) Production by Cupriavidus sp. Oh_1
3.5. Fed-Batch Fermentation Using Cupriavidus sp. Oh_1/phaCJ
3.6. Copolymer of the Physical Properties of PHB and PHBHHx Produced by Cupriavidus sp. Oh_1 and H16
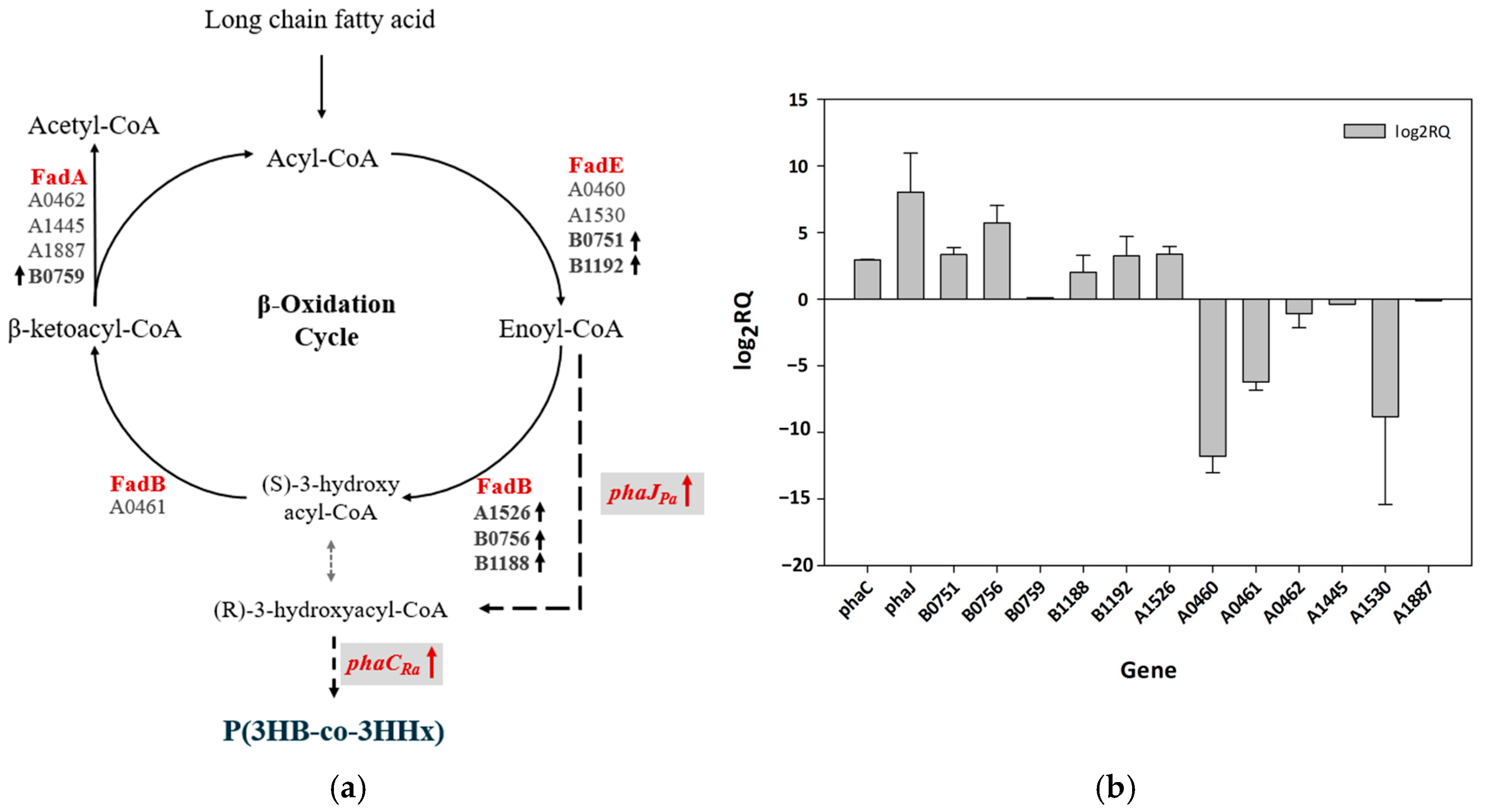
3.7. mRNA Expression Analysis of Fatty Acid β-Oxidation-Encoding Genes in Cupriavidus sp. Oh_1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DSC | Differential scanning calorimetry |
| GPC | Gel permeation chromatography |
| MCL | Medium-chain length |
| ReMM | Ralstonia eutropha minimal media |
| SCL | Short-chain length |
| TSA | Tryptic soy agar |
| TSB | Tryptic soy broth |
References
- Park, Y.L.; Bhatia, S.K.; Gurav, R.; Choi, T.R.; Kim, H.J.; Song, H.S.; Park, J.Y.; Han, Y.H.; Lee, S.M.; Park, S.L.; et al. Fructose Based Hyper Production of Poly-3-Hydroxybutyrate from Halomonas Sp. YLGW01 and Impact of Carbon Sources on Bacteria Morphologies. Int. J. Biol. Macromol. 2020, 154, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and Potential Application of Short-Chain-Length Polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Janchai, P.; Neoh, S.Z.; Sudesh, K.; Vaithanomsat, P. Statistical Optimization of P(3HB-Co-3HHx) Copolymers Production by Cupriavidus Necator PHB−4/PBBR_CnPro-PhaCRp and Its Properties Characterization. Sci. Rep. 2023, 13, 9005. [Google Scholar] [CrossRef]
- Tang, H.J.; Neoh, S.Z.; Sudesh, K. A Review on Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) [P(3HB-Co-3HHx)] and Genetic Modifications That Affect Its Production. Front. Bioeng. Biotechnol. 2022, 10, 1057067. [Google Scholar] [CrossRef]
- Bhubalan, K.; Rathi, D.N.; Abe, H.; Iwata, T.; Sudesh, K. Improved Synthesis of P(3HB-Co-3HV-Co-3HHx) Terpolymers by Mutant Cupriavidus Necator Using the PHA Synthase Gene of Chromobacterium Sp. USM2 with High Affinity towards 3HV. Polym. Degrad. Stab. 2010, 95, 1436–1442. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, H.J.; Choi, T.R.; Oh, S.J.; Kim, S.; Lee, Y.; Choi, S.; Oh, J.; Kim, S.Y.; Lee, Y.S.; et al. Identification of Oil-Loving Cupriavidus Necator BM3-1 for Polyhydroxyalkanoate Production and Assessing Contribution of Exopolysaccharide for Vegetable Oil Utilization. Polymers 2024, 16, 1639. [Google Scholar] [CrossRef]
- Mifune, J.; Nakamura, S.; Fukui, T. Engineering of Pha Operon on Cupriavidus Necator Chromosome for Efficient Biosynthesis of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) from Vegetable Oil. Polym. Degrad. Stab. 2010, 95, 1305–1312. [Google Scholar] [CrossRef]
- Wong, Y.M.; Brigham, C.J.; Rha, C.K.; Sinskey, A.J.; Sudesh, K. Biosynthesis and Characterization of Polyhydroxyalkanoate Containing High 3-Hydroxyhexanoate Monomer Fraction from Crude Palm Kernel Oil by Recombinant Cupriavidus necator. Bioresour. Technol. 2012, 121, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.Y.; Lee, W.H.; Tsuge, T.; Doi, Y.; Sudesh, K. Biosynthesis and Characterization of Poly(3-Hydroxybutyrate-Co-3- Hydroxyhexanoate) from Palm Oil Products in a Wautersia Eutropha Mutant. Biotechnol. Lett. 2005, 27, 1405–1410. [Google Scholar] [CrossRef]
- Budde, C.F.; Riedel, S.L.; Willis, L.B.; Rha, C.K.; Sinskey, A.J. Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) from Plant Oil by Engineered Ralstonia Eutropha Strains. Appl. Environ. Microbiol. 2011, 77, 2847–2854. [Google Scholar] [CrossRef]
- Insomphun, C.; Mifune, J.; Orita, I.; Numata, K.; Nakamura, S.; Fukui, T. Modification of β-Oxidation Pathway in Ralstonia Eutropha for Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) from Soybean Oil. J. Biosci. Bioeng. 2014, 117, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, J.H.; Kim, E.J.; Kim, K.J. Crystal Structure of (R)-3-Hydroxybutyryl-CoA Dehydrogenase PhaB from Ralstonia Eutropha. Biochem. Biophys. Res. Commun. 2014, 443, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Cheng, W.; Mifune, J.; Orita, I.; Nakamura, S.; Fukui, T. Characterization and Functional Analyses of R-Specific Enoyl Coenzyme a Hydratases in Polyhydroxyalkanoate-Producing Ralstonia Eutropha. Appl. Environ. Microbiol. 2012, 78, 493–502. [Google Scholar] [CrossRef]
- Budde, C.F.; Mahan, A.E.; Lu, J.; Rha, C.K.; Sinskey, A.J. Roles of Multiple Acetoacetyl Coenzyme A Reductases in Polyhydroxybutyrate Biosynthesis in Ralstonia Eutropha H16. J. Bacteriol. 2010, 192, 5319–5328. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, B.F.; Pitera, D.J.; Smolke, C.D.; Keasling, J.D. Combinatorial Engineering of Intergenic Regions in Operons Tunes Expression of Multiple Genes. Nat. Biotechnol. 2006, 24, 1027–1032. [Google Scholar] [CrossRef]
- Jeon, J.M.; Brigham, C.J.; Kim, Y.H.; Kim, H.J.; Yi, D.H.; Kim, H.; Rha, C.K.; Sinskey, A.J.; Yang, Y.H. Biosynthesis of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (P(HB-Co-HHx)) from Butyrate Using Engineered Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2014, 98, 5461–5469. [Google Scholar] [CrossRef]
- Mifune, J.; Nakamura, S.; Fukui, T. Targeted Engineering of Cupriavidus Necator Chromosome for Biosynthesis of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) from Vegetable Oil. Can. J. Chem. 2008, 86, 621–627. [Google Scholar] [CrossRef]
- Sato, S.; Maruyama, H.; Fujiki, T.; Matsumoto, K. Regulation of 3-Hydroxyhexanoate Composition in PHBH Synthesized by Recombinant Cupriavidus Necator H16 from Plant Oil by Using Butyrate as a Co-Substrate. J. Biosci. Bioeng. 2015, 120, 246–251. [Google Scholar] [CrossRef]
- Dennis, D.; Mccoy, M.; Stangl, A.; Valentin, H.E.; Wu, Z. Formation of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) by PHA Synthase from Ralstonia Eutropha. J. Biotechnol. 1998, 64, 177–186. [Google Scholar] [CrossRef]
- Murugan, P.; Chhajer, P.; Kosugi, A.; Arai, T.; Brigham, C.J.; Sudesh, K. Production of P(3HB-Co-3HHx) with Controlled Compositions by Recombinant Cupriavidus Necator Re2058/PCB113 from Renewable Resources. Clean 2016, 44, 1234–1241. [Google Scholar] [CrossRef]
- Murugan, P.; Gan, C.Y.; Sudesh, K. Biosynthesis of P(3HB-Co-3HHx) with Improved Molecular Weights from a Mixture of Palm Olein and Fructose by Cupriavidus Necator Re2058/PCB113. Int. J. Biol. Macromol. 2017, 102, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kim, H.J.; Kim, H.J.; Shin, N.; Oh, S.J.; Park, J.H.; Cho, W.D.; Ahn, J.; Bhatia, S.K.; Yang, Y.H. Galactose-Based Biohydrogen Production from Seaweed Biomass by Novel Strain Clostridium Sp. JH03 from Anaerobic Digester Sludge. Biotechnol. Bioprocess Eng. 2024, 29, 219–231. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Jeon, J.M.; Yoon, J.J.; Bhatia, S.K.; Gurav, R.; et al. Screening of the Strictly Xylose-Utilizing Bacillus Sp. SM01 for Polyhydroxybutyrate and Its Co-Culture with Cupriavidus Necator NCIMB 11599 for Enhanced Production of PHB. Int. J. Biol. Macromol. 2021, 181, 410–417. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, B.S. Production of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) by Ralstonia Eutropha from Soybean Oil. New Biotechnol. 2011, 28, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Abouseoud, M.; Maachi, R.; Amrane, A.; Boudergua, S.; Nabi, A. Evaluation of Different Carbon and Nitrogen Sources in Production of Biosurfactant by Pseudomonas Fluorescens. Desalination 2008, 223, 143–151. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Jin, D.; Jia, X. Optimization of Medium-Chain-Length Polyhydroxyalkanoate Production by Pseudomonas Putida KT2440 from Co-Metabolism of Glycerol and Octanoate. Can. J. Chem. Eng. 2021, 99, 657–666. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of Microorganisms to Cold Temperatures, Weak Acid Preservatives, Low PH, and Osmotic Stress: A Review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef]
- Zhou, W.; Colpa, D.I.; Geurkink, B.; Euverink, G.J.W.; Krooneman, J. The Impact of Carbon to Nitrogen Ratios and PH on the Microbial Prevalence and Polyhydroxybutyrate Production Levels Using a Mixed Microbial Starter Culture. Sci. Total Environ. 2022, 811, 152341. [Google Scholar] [CrossRef]
- Jung, H.J.; Shin, Y.; Hwang, J.H.; Shin, N.; Kim, H.J.; Oh, S.J.; Choi, T.R.; Park, H.J.; Jung, J.H.; Bhatia, S.K.; et al. Establishment of an Optimized Electroporation Method for Halomonas Sp. YK44 and Its Application in the Coproduction of PHB and Isobutanol. Biotechnol. Bioprocess Eng. 2024, 29, 339–351. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Mozumder, M.S.I.; Dubreuil, M.; Volcke, E.I.P.; De Wever, H. Sustainable Autotrophic Production of Polyhydroxybutyrate (PHB) from CO2 Using a Two-Stage Cultivation System. Catal. Today 2015, 257, 237–245. [Google Scholar] [CrossRef]
- Haase, S.M.; Huchzermeyer, B.; Rath, T. PHB Accumulation in Nostoc Muscorum under Different Carbon Stress Situations. J. Appl. Phycol. 2012, 24, 157–162. [Google Scholar] [CrossRef]
- Min Song, H.; Chan Joo, J.; Hyun Lim, S.; Jin Lim, H.; Lee, S.; Jae Park, S. Production of Polyhydroxyalkanoates Containing Monomers Conferring Amorphous and Elastomeric Properties from Renewable Resources: Current Status and Future Perspectives. Bioresour. Technol. 2022, 366, 128144. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yoon, J.J.; Kim, H.J.; Hong, J.W.; Gi Hong, Y.; Song, H.S.; Moon, Y.M.; Jeon, J.M.; Kim, Y.G.; Yang, Y.H. Engineering of Artificial Microbial Consortia of Ralstonia Eutropha and Bacillus Subtilis for Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Copolymer Production from Sugarcane Sugar Without Precursor Feeding. Bioresour. Technol. 2018, 257, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Nemtsev, I.V.; Lukyanenko, A.V.; Shishatskaya, E.I.; Volova, T.G. Biosynthesis and Properties of a P(3HB-Co-3HV-Co-4HV) Produced by Cupriavidus Necator B-10646. Polymers 2022, 14, 4226. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, S.; Lee, Y.; Shin, Y.; Choi, S.; Oh, J.; Bhatia, S.K.; Joo, J.C.; Yang, Y.H. Controlled Production of a Polyhydroxyalkanoate (PHA) Tetramer Containing Different Mole Fraction of 3-Hydroxybutyrate (3HB), 3-Hydroxyvalerate (3 HV), 4 HV and 5 HV Units by Engineered Cupriavidus Necator. Int. J. Biol. Macromol. 2024, 266, 131332. [Google Scholar] [CrossRef]
- Asrar, J.; Valentin, H.E.; Berger, P.A.; Tran, M.; Padgette, S.R.; Garbow, J.R. Biosynthesis and Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Polymers. Biomacromolecules 2002, 3, 1006–1012. [Google Scholar] [CrossRef]
- Tan, H.T.; Chek, M.F.; Miyahara, Y.; Kim, S.Y.; Tsuge, T.; Hakoshima, T.; Sudesh, K. Characterization of an (R)-Specific Enoyl-CoA Hydratase from Streptomyces Sp. Strain CFMR 7: A Metabolic Tool for Enhancing the Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). J. Biosci. Bioeng. 2022, 134, 288–294. [Google Scholar] [CrossRef]
- Oh, S.J.; Choi, T.R.; Kim, H.J.; Shin, N.; Hwang, J.H.; Kim, H.J.; Bhatia, S.K.; Kim, W.; Yeon, Y.J.; Yang, Y.H. Maximization of 3-Hydroxyhexanoate Fraction in Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Using Lauric Acid with Engineered Cupriavidus Necator H16. Int. J. Biol. Macromol. 2024, 256, 128376. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, L.; Ma, X.; Zhu, L.; Li, J.; Meng, L.; Han, M.; Wang, D.; Shen, H.; Liu, C. Stability Assessment of Housekeeping Genes for qRT-PCR in Yersinia enterocolitica Cultured at 22 °C and 37 °C. Microbiol. Spectr. 2024, 12, e0114624. [Google Scholar] [CrossRef]
- Potvin, E.; Sanschagrin, F.; Levesque, R.C. Sigma Factors in Pseudomonas aeruginosa: A Review of Their Roles in Regulation and Virulence. FEMS Microbiol. Rev. 2008, 32, 38–55. [Google Scholar] [CrossRef]
- Oliveira, P.A.A.d.; Baboghlian, J.; Ramos, C.O.A.; Mançano, A.S.F.; Porcari, A.M.; Girardello, R.; Ferraz, L.F.C. Selection and Validation of Reference Genes Suitable for Gene Expression Analysis by Reverse Transcription Quantitative Real-Time PCR in Acinetobacter baumannii. Sci. Rep. 2024, 14, 3830. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome Sequence of the Bioplastic-Producing “Knallgas” Bacterium Ralstonia Eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef] [PubMed]


| Strain | Precursor (0.1%) | DCW (g/L) | PHA (g/L) | PHA Mole Fraction (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 3HB | 3HP | 4HB | 3HV | 4HV | 5HV | ||||
| Oh_1 | 3HP | 15.2 | 14.3 | 99.7 | - | - | 0.3 | - | - |
| 4HB | 16.3 | 15.4 | 99.2 | - | 0.5 | 0.3 | - | - | |
| 4HV | 14.95 | 13.7 | 98.5 | - | - | 1.3 | 0.2 | - | |
| 5HV | 16.9 | 15.1 | 99.2 | - | - | 0.4 | - | 0.4 | |
| C. necator H16 | 3HP | 21.5 | 17.8 | 99.8 | - | - | 0.2 | - | - |
| 4HB | 19 | 16.99 | 99.4 | - | 0.3 | 0.2 | - | - | |
| 4HV | 18.5 | 16.51 | 98.8 | - | - | 1.2 | 0.1 | - | |
| 5HV | 22.3 | 20.45 | 99.5 | - | - | 0.3 | - | 0.2 | |
| 3HHx (mol%) | UTM | DSC | GPC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TS (MPa) | EL (%) | YM (MPa) | Tg (°C) | Tc (°C) | Tm (°C) | ΔH (J/g) | Mn (105) | Mw (105) | PDI | ||
| Oh_1 wt | 0 | 9.9 ± 5.9 | 11.7 ± 5.2 | 488.6 ± 122.6 | n.a. | n.a. | 170.9 | 28.6 | 10.9 | 17.4 | 1.66 |
| Oh_1/phaCJ | 27.2 | 3.6 ± 0.1 | 179.7 ± 10.2 | 22.2 ± 3.5 | −6.29 ± 3.0 | 58.77 | 176.1 | 16.3 | 8.7 | 12.3 | 1.41 |
| H16/phaCJ | 22.5 | 5.8 ± 0.3 | 152.3 ± 6.2 | 1225 ± 172 | −0.37 | 71.61 ± 0.2 | 174.2 | 16.6 | 6.7 | 14.4 | 2.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, G.; Oh, S.-J.; Han, Y.; Yun, J.; Joo, J.C.; Kim, H.-T.; Koh, H.G.; Park, S.-H.; Park, K.; Yang, Y.-H. Discovery of a High 3-Hydroxyhexanoate Containing Poly-3-hydroxybutyrate-co-3-hydroxyhexanoate Producer-, Cupriavidus sp. Oh_1 with Enhanced Fatty Acid Metabolism. Polymers 2025, 17, 1824. https://doi.org/10.3390/polym17131824
Lim G, Oh S-J, Han Y, Yun J, Joo JC, Kim H-T, Koh HG, Park S-H, Park K, Yang Y-H. Discovery of a High 3-Hydroxyhexanoate Containing Poly-3-hydroxybutyrate-co-3-hydroxyhexanoate Producer-, Cupriavidus sp. Oh_1 with Enhanced Fatty Acid Metabolism. Polymers. 2025; 17(13):1824. https://doi.org/10.3390/polym17131824
Chicago/Turabian StyleLim, Gaeun, Suk-Jin Oh, Yebin Han, Jeonghee Yun, Jeong Chan Joo, Hee-Taek Kim, Hyun Gi Koh, See-Hyoung Park, Kyungmoon Park, and Yung-Hun Yang. 2025. "Discovery of a High 3-Hydroxyhexanoate Containing Poly-3-hydroxybutyrate-co-3-hydroxyhexanoate Producer-, Cupriavidus sp. Oh_1 with Enhanced Fatty Acid Metabolism" Polymers 17, no. 13: 1824. https://doi.org/10.3390/polym17131824
APA StyleLim, G., Oh, S.-J., Han, Y., Yun, J., Joo, J. C., Kim, H.-T., Koh, H. G., Park, S.-H., Park, K., & Yang, Y.-H. (2025). Discovery of a High 3-Hydroxyhexanoate Containing Poly-3-hydroxybutyrate-co-3-hydroxyhexanoate Producer-, Cupriavidus sp. Oh_1 with Enhanced Fatty Acid Metabolism. Polymers, 17(13), 1824. https://doi.org/10.3390/polym17131824








