Hydrosilylation vs. Piers–Rubinsztajn: Synthetic Routes to Chemically Cross-Linked Hybrid Phosphazene-Siloxane 3D-Structures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
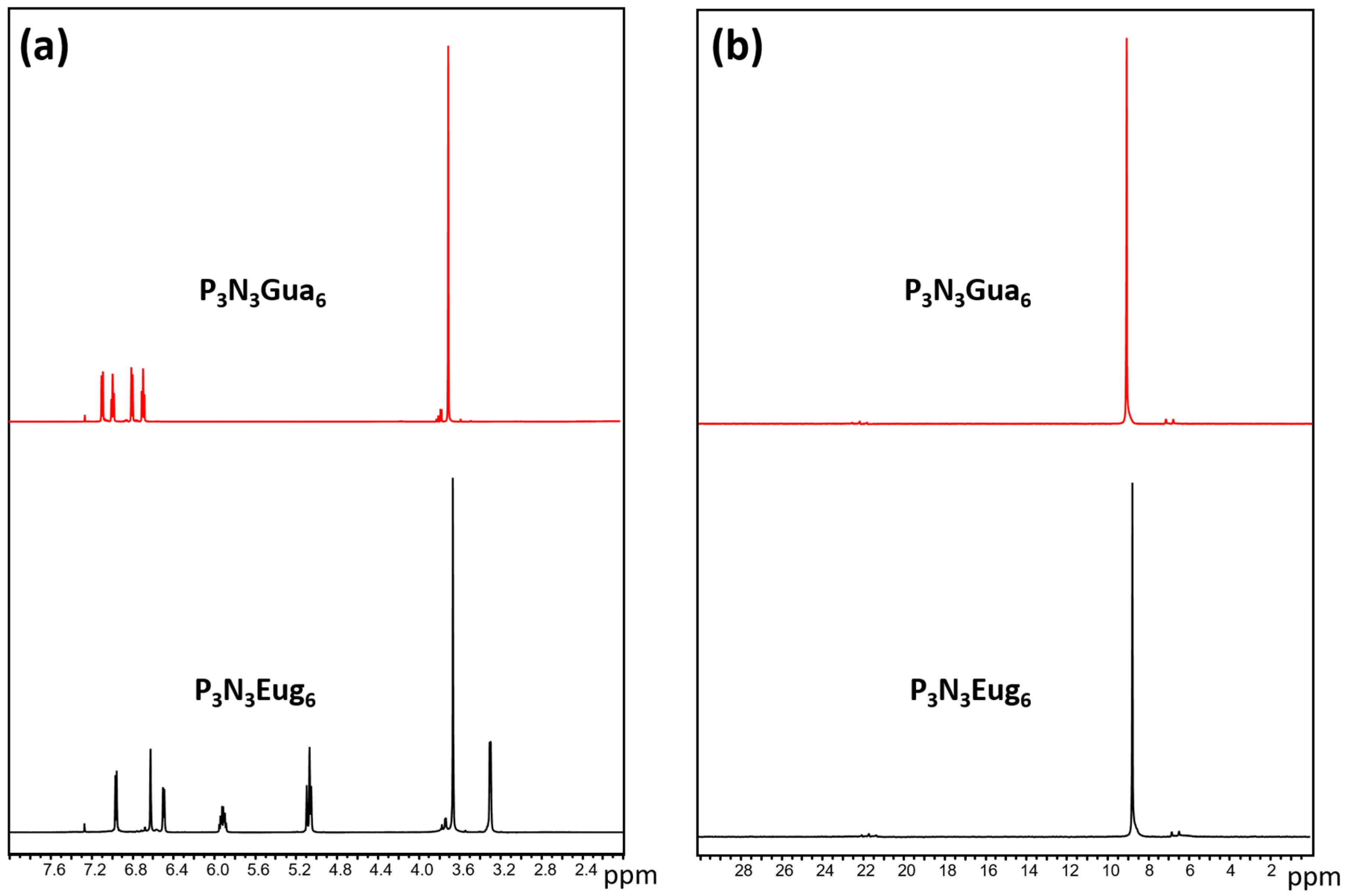
2.3. Synthesis of Cyclic Phosphazene P3N3Gua6 or P3N3Eug6
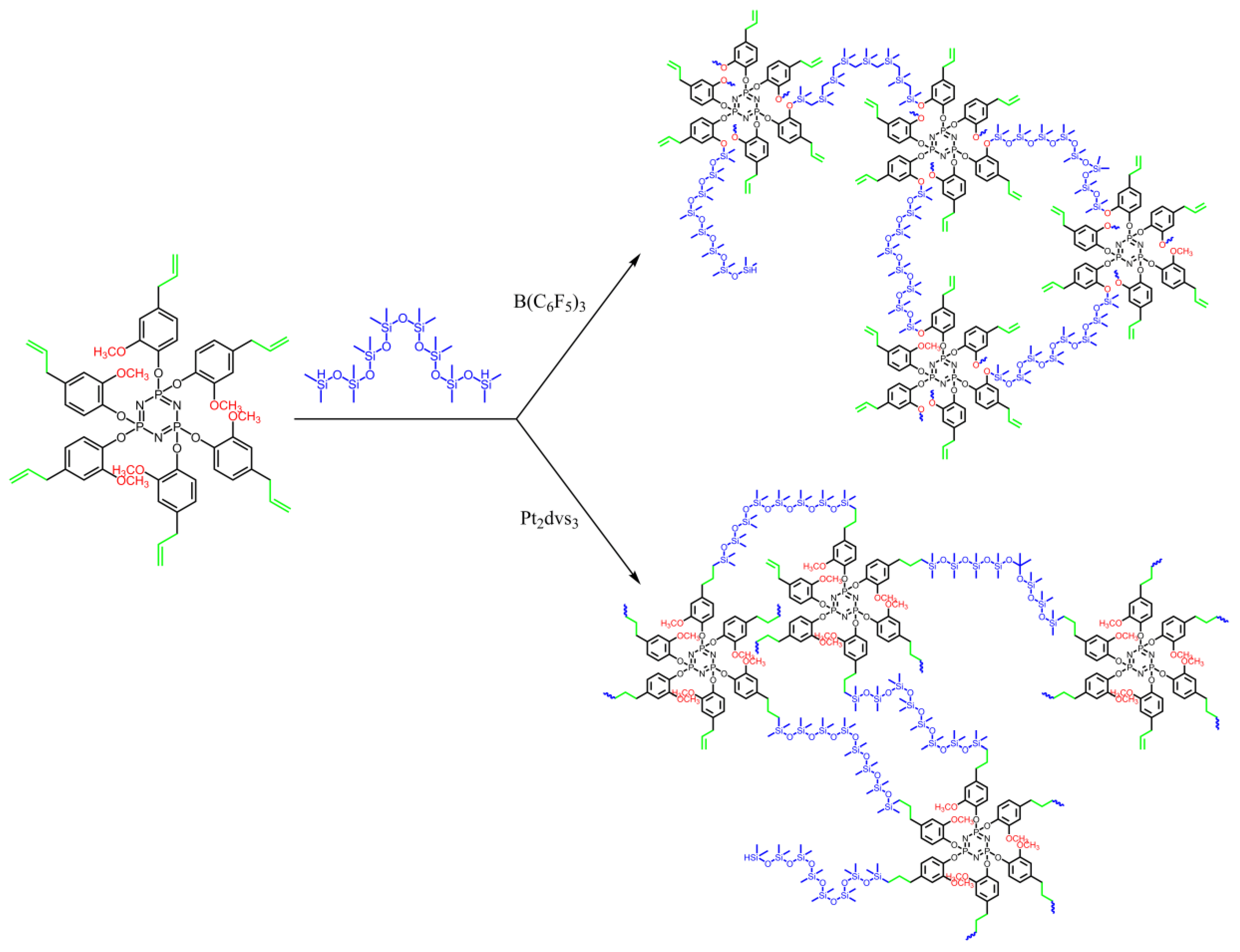
2.4. Preparation of Hybrid Cross-Linked Phosphazene-Siloxane Materials
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2016, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R.; Chen, C. Polyphosphazenes: Phosphorus in Inorganic-Organic Polymers. J. Org. Chem. 2020, 85, 14286–14297. [Google Scholar] [CrossRef] [PubMed]
- Bredov, N.S.; Gorlov, M.V.; Esin, A.S.; Bykovskaya, A.A.; Kireev, V.V.; Sinegribova, O.A.; Ryabochenko, M.D. Linear 2-ethylhexyl imidophosphoric esters as effective rare-earth element extractants. Appl. Sci. 2020, 10, 1229. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Sarychev, I.A.; Vorobyeva, V.V.; Kuzmich, A.A.; Bornosuz, N.V.; Onuchin, D.V.; Gorbunova, I.Y.; Kireev, V.V. Synthesis of Phosphazene-Containing, Bisphenol A-Based Benzoxazines and Properties of Corresponding Polybenzoxazines. Polymers 2020, 12, 1225. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R.; Crane, C.A.; Morrissey, C.T.; Olshavsky, M.A. A New Route to the Phosphazene Polymerization Precursors, Cl3PNSiMe3 and (NPCl2)3. Inorg. Chem. 1999, 38, 280–283. [Google Scholar] [CrossRef]
- Wang, B.; Rivard, E.; Manners, I. A New High-Yield Synthesis of Cl3P=NSiMe3, a Monomeric Precursor for the Controlled Preparation of High Molecular Weight Polyphosphazenes. Inorg. Chem. 2002, 41, 1690–1691. [Google Scholar] [CrossRef] [PubMed]
- Gorlov, M.V.; Bredov, N.S.; Esin, A.S.; Kireev, V.V. A direct synthesis of Cl3P=NSiMe3 from PCl5 and hexamethyldisilazane. J. Organomet. Chem. 2016, 818, 82–84. [Google Scholar] [CrossRef]
- Honeyman, C.H.; Manners, I.; Morrissey, C.T.; Allcock, H.R. Ambient Temperature Synthesis of Poly(dichlorophosphazene) with Molecular Weight Control. J. Am. Chem. Soc. 1995, 117, 7035–7036. [Google Scholar] [CrossRef]
- Allcock, H.R.; Crane, C.A.; Morrissey, C.T.; Nelson, J.M.; Reeves, S.D.; Honeyman, C.H.; Manners, I. “Living” Cationic Polymerization of Phosphoranimines as an Ambient Temperature Route to Polyphosphazenes with Controlled Molecular Weights. Macromolecules 1996, 29, 7740–7747. [Google Scholar] [CrossRef]
- Van Wüllen, L.; Köster, T.K.-J.; Wiemhöfer, H.-D.; Kaskhedikar, N. Local Cation Coordination Motifs in Polyphosphazene Based Composite Electrolytes. Chem. Mater. 2008, 20, 7399–7407. [Google Scholar] [CrossRef]
- Blonsky, P.M.; Shriver, D.F.; Austin, P.; Allcock, H.R. Polyphosphazene Solid Electrolytes. J. Am. Chem. Soc. 1984, 106, 6854–6855. [Google Scholar] [CrossRef]
- Allcock, H.R.; Ravikiran, R.; O’Connor, S.J.M. Effect of Oligo(ethyleneoxy)cyclotriphosphazenes, Tetraglyme, and Other Small Molecules on the Ionic Conductivity of the Poly[bis(methoxyethoxyethoxy)phosphazene] (MEEP)/Lithium Triflate System. Macromolecules 1997, 30, 3184–3190. [Google Scholar] [CrossRef]
- Stalin, S.; Johnson, H.E.; Biswal, P.; Vu, D.; Zhao, Q.; Yin, J.; Abel, B.A.; Deng, Y.; Coates, G.W.; Archer, L.A. Achieving Uniform Lithium Electrodeposition in Cross-Linked Poly(ethylene oxide) Networks: “Soft” Polymers Prevent Metal Dendrite Proliferation. Macromolecules 2020, 53, 5445. [Google Scholar] [CrossRef]
- Wang, G.-J.N.; Shaw, L.; Xu, J.; Kurosawa, T.; Schroeder, B.C.; Oh, J.Y.; Benight, S.J.; Bao, Z. Inducing Elasticity through Oligo-Siloxane Crosslinks for Intrinsically Stretchable Semiconducting Polymers. Adv. Funct. Mater. 2016, 26, 7254–7262. [Google Scholar] [CrossRef]
- Shaik, A.; Narayan, R.; Raju, K.V.S.N. Synthesis and properties of siloxane-crosslinked polyurethaneurea/silica hybrid films from castor oil. J. Coat. Technol. Res. 2014, 11, 397–407. [Google Scholar] [CrossRef]
- Kolel-Veetil, M.K.; Keller, T.M. Formation of Elastomeric Network Polymers from Ambient Heterogeneous Hydrosilations of Carboranylenesiloxane and Branched Siloxane Monomers. J. Polym. Sci. A Polym. Chem. 2006, 44, 147–155. [Google Scholar] [CrossRef]
- Kireev, V.V.; Bilichenko, Y.V.; Borisov, R.S.; Sirotin, I.S.; Filatov, S.N. Laser Mass Spectrometry Analysis of the Formation of Phosphazene-Containing Epoxy Oligomers. Polym. Sci. Ser. B 2018, 60, 243–262. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Solodukhin, A.N.; Kireev, V.V.; Buzin, M.I.; Borisov, R.S. Eugenol Derivatives of Higher Chlorocyclophosphazenes and Related Epoxy Oligomers. Polym. Sci. Ser. B 2013, 55, 241–251. [Google Scholar] [CrossRef]
- Kireev, V.V.; Bredov, N.S.; Prudskov, B.M.; Mu, J.; Borisov, R.S.; Sokol’skaya, I.B.; Chuev, V.P. Oligo and Polysiloxanephosphazenes Based on Eugenol Cyclotriphosphazene Derivatives. Polym. Scie. Ser. B 2011, 53, 64–67. [Google Scholar] [CrossRef]
- Laengert, S.E.; Schneider, A.F.; Lovinger, E.; Chen, Y.; Brook, M.A. Sequential Functionalization of a Natural Crosslinker Leads to Designer Silicone Networks. Chem. Asian J. 2017, 12, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Elsevier: Burlington, MA, USA, 2009; 760p. [Google Scholar]
- Grande, J.B.; Thompson, D.B.; Gonzaga, F.; Brook, M.A. Testing the functional tolerance of the Piers–Rubinsztajn reaction: A new strategy for functional silicones. Chem. Commun. 2010, 46, 4988–4990. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, C.S.; Bhavsar, R.; Dash, B.P.; Satapathy, R. Cobaltabisdicarbollide based metallodendrimers with cyclotriphosphazene core. J. Organomet. Chem. 2018, 865, 183–188. [Google Scholar] [CrossRef]
- Heston, A.J.; Panzner, M.J.; Youngs, W.J.; Tessier, C.A. Lewis Acid Adducts of [PCl2N]3. Inorg. Chem. 2005, 44, 6518–6520. [Google Scholar] [CrossRef] [PubMed]
- Mathias, L.J.; Lewis, C.M. Unexpectedly Rapid Hydrosilation Polymerization of the Diallyl Derivative of Bisphenol A and 2,6-Diallylphenol. Macromolecules 1993, 26, 4070–4071. [Google Scholar] [CrossRef]
- Both, E.M.; Tersteeg, S.M.B.; Boom, R.M.; Schutyser, M.A.I. Drying kinetics and viscoelastic properties of concentrated thin films as a model system for spray drying. Colloids Surf. A. 2020, 585, 124075. [Google Scholar] [CrossRef]



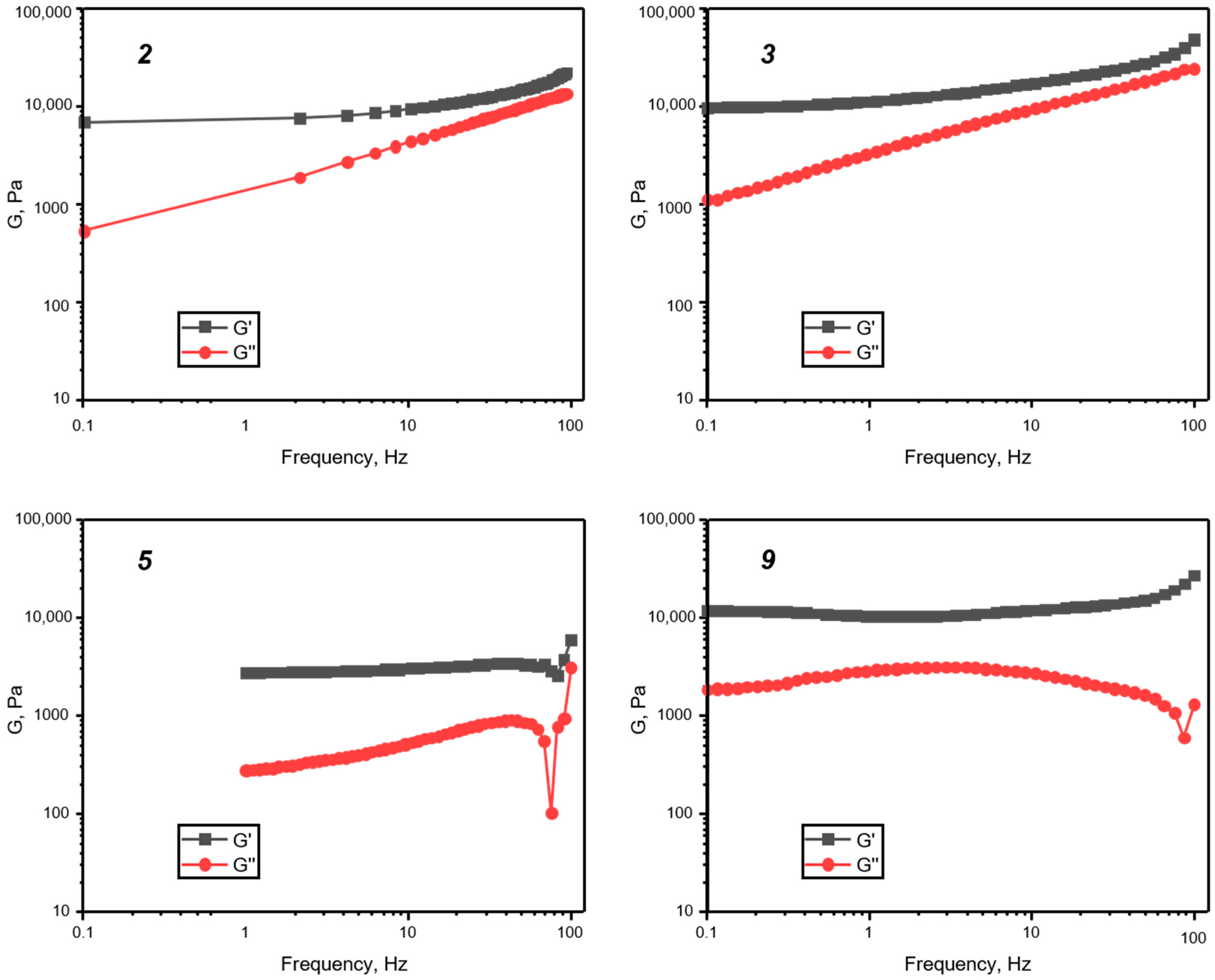
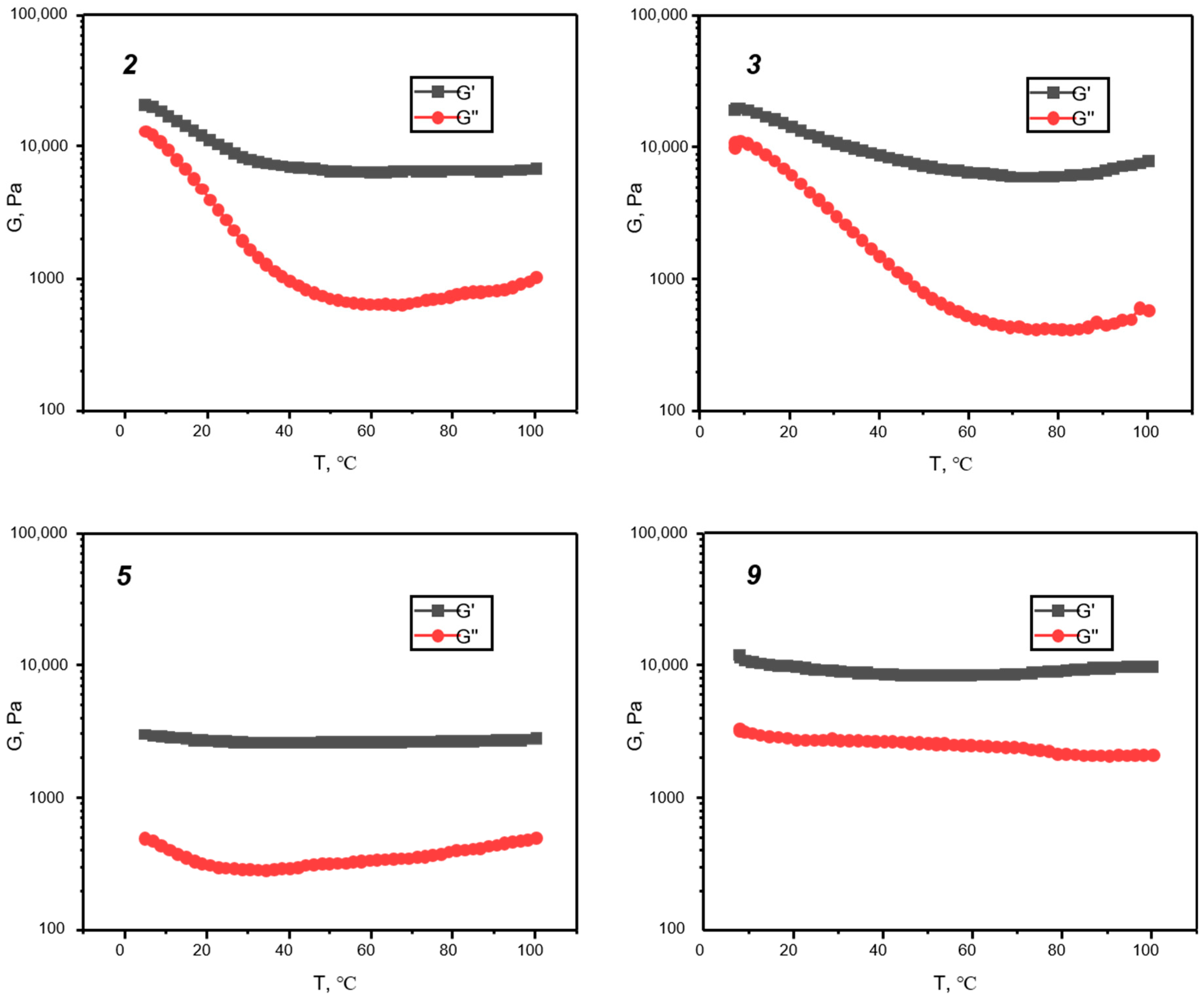
| # of Sample | Siloxane | Molar Ratio P3N3Eug6: Siloxane | Catalyst Amount, mol. % | Concentration of P3N3Eug6 in Toluene, mg/mL | Result | Tg, °C |
|---|---|---|---|---|---|---|
| 1 | Si6 | 1:3 | 0.0015 | 50 | Yellow sticky film * | 5.3 |
| 2 | Si6 | 1:3 | 0.003 | 50 | Yellow non-sticky film | −5.1 |
| 3 | Si6 | 1:3 | 0.006 | 50 | Yellow non-sticky film | −9.2 |
| 4 | Si6 | 1:3 | 0.003 | 25 | Yellow sticky film | −13.7 |
| 5 | Si6 | 1:3 | 0.003 | 100 | Yellow non-sticky film | −7.5 |
| 6 | Si6 | 1:2 | 0.003 | 100 | Yellow sticky film | −5.3 |
| 7 | Si6 | 1:1 | 0.003 | 100 | Yellow soluble resin | −4.8 |
| 8 | TMDS | 1:3 | 0.003 | 100 | Yellow soluble resin | 8.6 |
| 9 | Si30 | 1:3 | 0.003 | 100 | Yellow non-sticky film | −51.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esin, A.S.; Chernysheva, A.I.; Yurasova, E.A.; Karpova, E.A.; Shutov, V.V.; Sirotin, I.S.; Soldatov, M.A.; Gorlov, M.V.; Raitman, O.A. Hydrosilylation vs. Piers–Rubinsztajn: Synthetic Routes to Chemically Cross-Linked Hybrid Phosphazene-Siloxane 3D-Structures. Polymers 2025, 17, 1967. https://doi.org/10.3390/polym17141967
Esin AS, Chernysheva AI, Yurasova EA, Karpova EA, Shutov VV, Sirotin IS, Soldatov MA, Gorlov MV, Raitman OA. Hydrosilylation vs. Piers–Rubinsztajn: Synthetic Routes to Chemically Cross-Linked Hybrid Phosphazene-Siloxane 3D-Structures. Polymers. 2025; 17(14):1967. https://doi.org/10.3390/polym17141967
Chicago/Turabian StyleEsin, Andrey S., Anna I. Chernysheva, Ekaterina A. Yurasova, Ekaterina A. Karpova, Vyacheslav V. Shutov, Igor S. Sirotin, Mikhail A. Soldatov, Mikhail V. Gorlov, and Oleg A. Raitman. 2025. "Hydrosilylation vs. Piers–Rubinsztajn: Synthetic Routes to Chemically Cross-Linked Hybrid Phosphazene-Siloxane 3D-Structures" Polymers 17, no. 14: 1967. https://doi.org/10.3390/polym17141967
APA StyleEsin, A. S., Chernysheva, A. I., Yurasova, E. A., Karpova, E. A., Shutov, V. V., Sirotin, I. S., Soldatov, M. A., Gorlov, M. V., & Raitman, O. A. (2025). Hydrosilylation vs. Piers–Rubinsztajn: Synthetic Routes to Chemically Cross-Linked Hybrid Phosphazene-Siloxane 3D-Structures. Polymers, 17(14), 1967. https://doi.org/10.3390/polym17141967






