Recent Advances in PEEK for Biomedical Applications: A Comprehensive Review of Material Properties, Processing, and Additive Manufacturing
Abstract
1. Introduction
2. Processing Techniques
2.1. Traditional Methods
2.2. 3D Printing
3. Applications
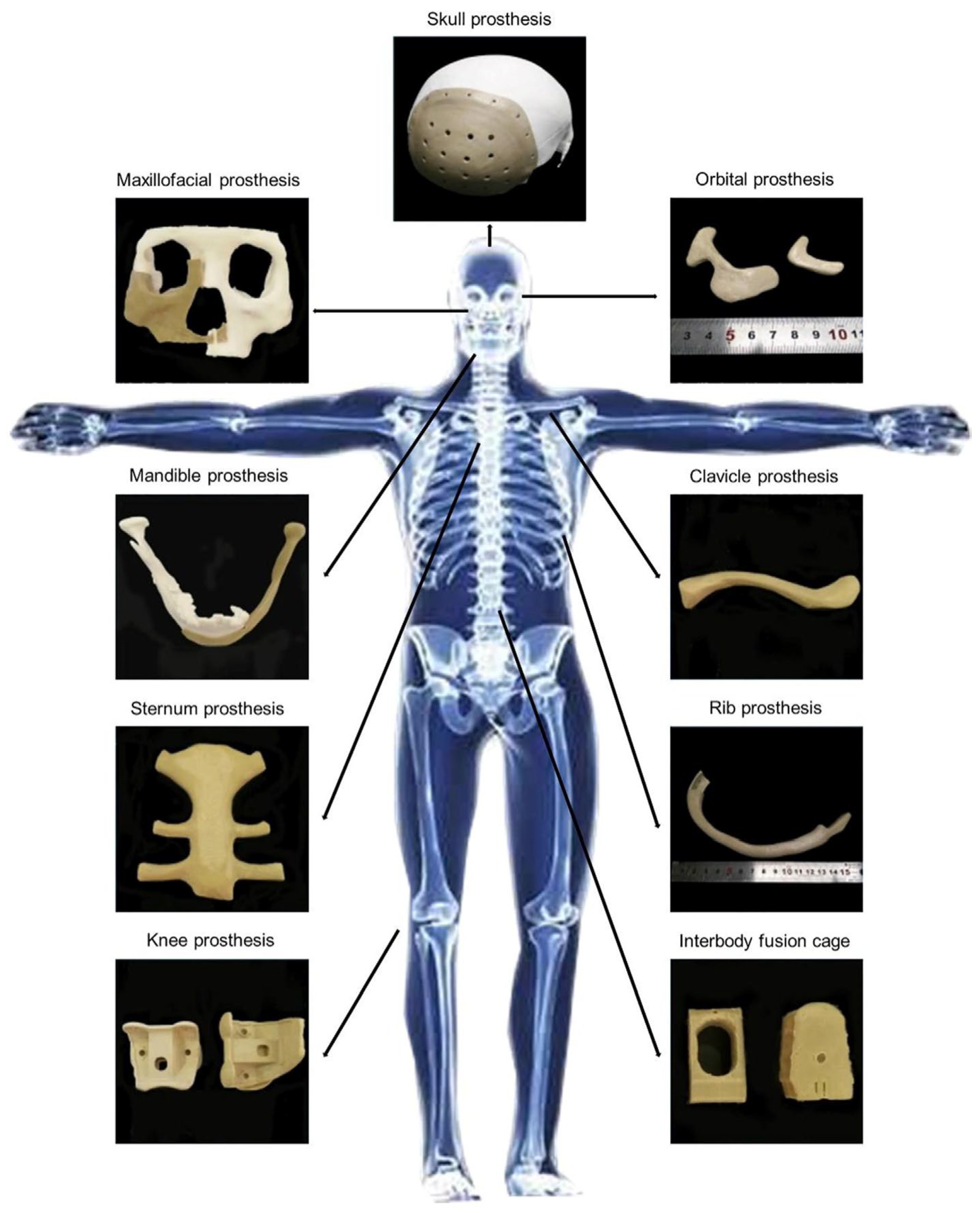
3.1. PEEK in Orthopedics Applications
3.1.1. Craniomaxillofacial Reconstruction
3.1.2. Spinal Implants
3.1.3. Joint Replacement
3.1.4. Rib Prostheses
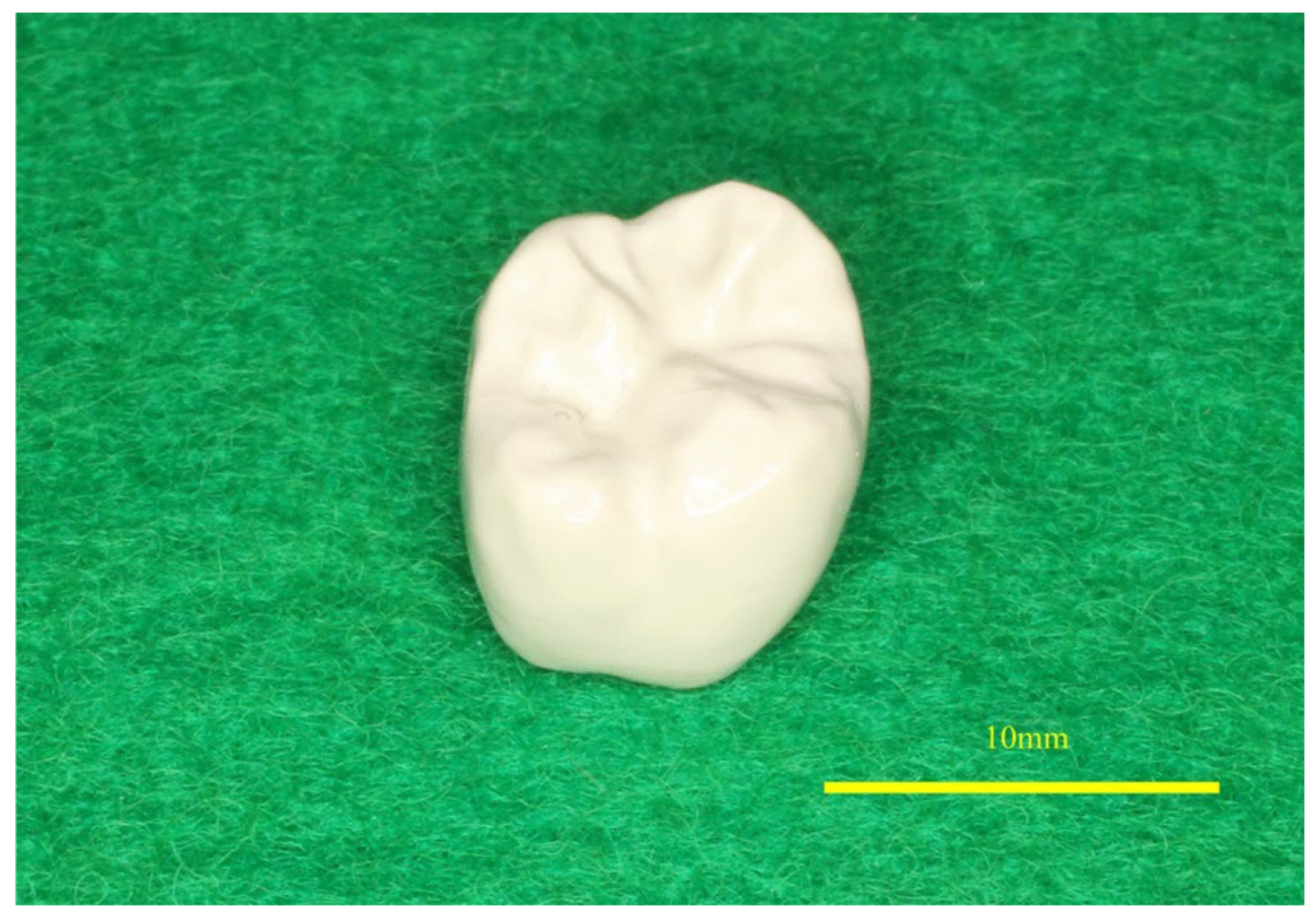
3.2. PEEK in Dental Implants
3.3. Other PEEK Applications
4. Properties of PEEK
4.1. Thermal and Radiative Properties
4.2. Porosity
4.3. Crystallinity
4.4. Fatigue and Fracture Behavior of PEEK
4.5. Biocompatibility and Physiological Toxicity
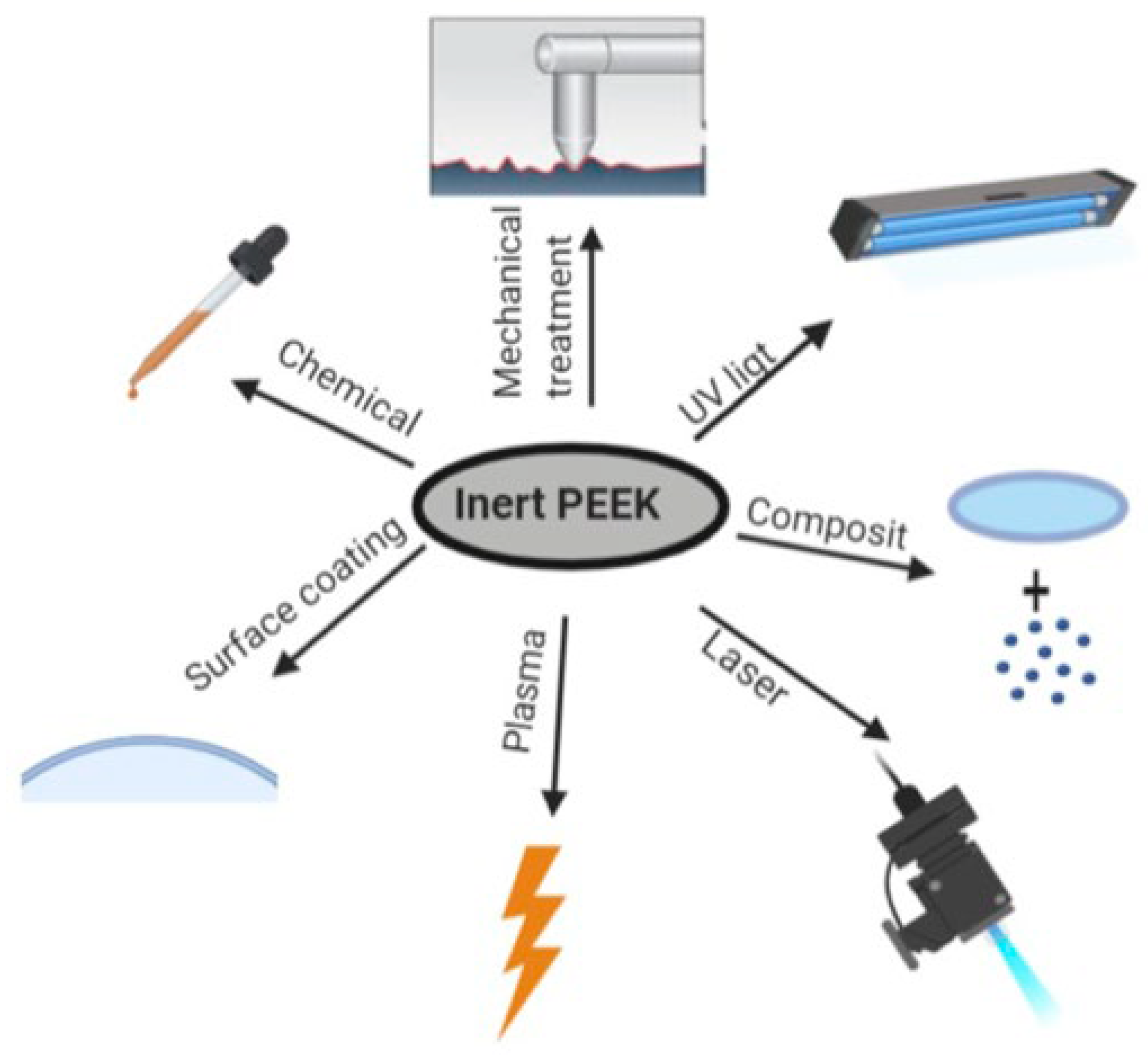
5. Limitations and Modifications of PEEK
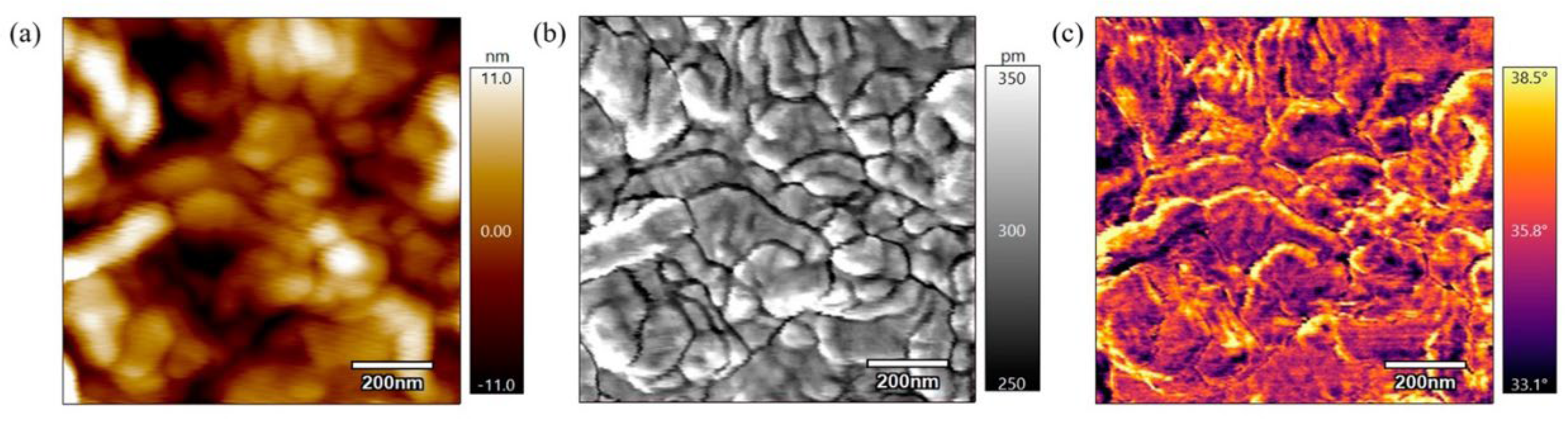
5.1. Hydrophilicity
5.1.1. Physical Modifications
5.1.2. Chemical Modifications
5.2. Bioactivity
6. Clinical Translation of PEEK Implants
6.1. Regulatory Considerations
6.2. Long-Term Clinical Performance and Failure Modes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mbogori, M.; Vaish, A.; Vaishya, R.; Haleem, A.; Javaid, M. Poly-Ether-Ether-Ketone (PEEK) in Orthopaedic Practice- A Current Concept Review. J. Orthop. Rep. 2022, 1, 3–7. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Ball, J.; Bremner, T.; Sue, H.-J. Crystallization Behavior and Morphological Characterization of Poly(Ether Ether Ketone). Polymer 2014, 55, 5255–5265. [Google Scholar] [CrossRef]
- Berretta, S.; Evans, K.; Ghita, O. Additive Manufacture of PEEK Cranial Implants: Manufacturing Considerations versus Accuracy and Mechanical Performance. Mater. Des. 2018, 139, 141–152. [Google Scholar] [CrossRef]
- Dua, R.; Rashad, Z.; Spears, J.; Dunn, G.; Maxwell, M. Applications of 3D-Printed PEEK via Fused Filament Fabrication: A Systematic Review. Polymers 2021, 13, 4046. [Google Scholar] [CrossRef]
- Verma, S.; Sharma, N.; Kango, S.; Sharma, S. Developments of PEEK (Polyetheretherketone) as a Biomedical Material: A Focused Review. Eur. Polym. J. 2021, 147, 110295. [Google Scholar] [CrossRef]
- Gummadi, S.K.; Saini, A.; Owusu-Danquah, J.S.; Sikder, P. Mechanical Properties of 3d-Printed Porous Poly-Ether-Ether-Ketone (Peek) Orthopedic Scaffolds. JOM 2022, 74, 3379–3391. [Google Scholar] [CrossRef]
- Lv, M.; Yang, X.; Gvetadze, S.R.; Gupta, A.; Li, J.; Sun, J. Accurate Reconstruction of Bone Defects in Orbital–Maxillary–Zygomatic (Omz) Complex with Polyetheretherketone (Peek). J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 1750–1757. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, C.; Ramakrishna, S. 3D Printing of Polyether-Ether-Ketone for Biomedical Applications. Eur. Polym. J. 2019, 114, 234–248. [Google Scholar] [CrossRef]
- Ma, H.; Suonan, A.; Zhou, J.; Yuan, Q.; Liu, L.; Zhao, X.; Lou, X.; Yang, C.; Li, D.; Zhang, Y. PEEK (Polyether-Ether-Ketone) and Its Composite Materials in Orthopedic Implantation. Arab. J. Chem. 2021, 14, 102977. [Google Scholar] [CrossRef]
- Jogi, B.F.; Tarekar, M.; Dhajekar, R.M.; Pawade, R. Multi Objective Optimization Using Taguchi Grey Relational Analysis (Gra) for Cnc Turning of Poly-Ether-Ether-Ketone (Peek) Polymer. Polym. Polym. Compos. 2016, 24, 523–528. [Google Scholar] [CrossRef]
- Moharil, S.; Reche, A.; Durge, K. Polyetheretherketone (PEEK) as a Biomaterial: An Overview. Cureus 2023, 15, e44307. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Kennedy, J.V.; Potgieter, J. A Comparison of Traditional Manufacturing vs Additive Manufacturing, the Best Method for the Job. Procedia Manuf. 2019, 30, 11–18. [Google Scholar] [CrossRef]
- Zarean, P.; Malgaroli, P.; Zarean, P.; Seiler, D.; de Wild, M.; Thieringer, F.M.; Sharma, N. Effect of Printing Parameters on Mechanical Performance of Material-Extrusion 3D-Printed PEEK Specimens at the Point-of-Care. Appl. Sci. 2023, 13, 1230. [Google Scholar] [CrossRef]
- Gonzalez Lugo, C.A.; Caputo, D.S.; Hutchinson, M.J.; Fouladi, K.; Eslami, B. Design and Development of an Environmentally Controlled Enclosure for a Commercial 3D Printer. Rapid Prototyp. J. 2022, 29, 780–791. [Google Scholar] [CrossRef]
- Mohammed Ali Al -Zaidi, A.; Al Gawhari, F. Types of Polymers Using in 3D Printing and Their Applications: A Brief Review. Eur. J. Theor. Appl. Sci. 2024, 1, 978–985. [Google Scholar] [CrossRef]
- Honigmann, P.; Sharma, N.; Okolo, B.; Popp, U.; Msallem, B.; Thieringer, F.M. Patient-Specific Surgical Implants Made of 3d Printed Peek: Material, Technology, and Scope of Surgical Application. BioMed Res. Int. 2018, 2018, 4520636. [Google Scholar] [CrossRef]
- Mendenhall, R.; Eslami, B. Experimental Investigation on Effect of Temperature on FDM 3D Printing Polymers: ABS, PETG, and PLA. Appl. Sci. 2023, 13, 11503. [Google Scholar] [CrossRef]
- Thiruchitrambalam, M.; Bubesh Kumar, D.; Shanmugam, D.; Jawaid, M. A Review on PEEK Composites–Manufacturing Methods, Properties and Applications. Mater. Today Proc. 2020, 33, 1085–1092. [Google Scholar] [CrossRef]
- Moby, V.; Dupagne, L.; Fouquet, V.; Attal, J.-P.; François, P.; Dursun, E. Mechanical Properties of Fused Deposition Modeling of Polyetheretherketone (PEEK) and Interest for Dental Restorations: A Systematic Review. Materials 2022, 15, 6801. [Google Scholar] [CrossRef]
- Li, T.; Song, Z.; Yang, X.; Du, J. Influence of Processing Parameters on the Mechanical Properties of Peek Plates by Hot Compression Molding. Materials 2023, 16, 36. [Google Scholar] [CrossRef]
- Czepiel, M.; Bańkosz, M.; Sobczak-Kupiec, A. Advanced Injection Molding Methods: Review. Materials 2023, 16, 5802. [Google Scholar] [CrossRef]
- Yang, J.; Xie, J.; Ji, K.; Wang, X.; Jiao, X.; Xu, Z.; Zhao, P. Microcellular Injection Molding of Polyether-Ether-Ketone. Polymer 2022, 251, 124866. [Google Scholar] [CrossRef]
- Siddiq, A.; Kennedy, A.R. Compression Moulding and Injection over Moulding of Porous PEEK Components. J. Mech. Behav. Biomed. Mater. 2020, 111, 103996. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for Medical Applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Khan, W. Comparison on Performance of Injection Moulding and 3D Printed Parts. Doctoral Dissertation, Mechanical Engineering, Edinburgh Napier University, Edinburgh, UK, 2020. [Google Scholar]
- Chen, M.; Luo, C.; Yuan, Y.; Zhou, H.; Li, Z.; Wang, Q.; Gong, B.; Li, Z.; Sun, H. Modification of PEEK for Implants: Strategies to Improve Mechanical, Antibacterial, and Osteogenic Properties. Rev. Adv. Mater. Sci. 2024, 63, 20240025. [Google Scholar] [CrossRef]
- Xiaoyong, S.; Liangcheng, C.; Honglin, M.; Peng, G.; Zhanwei, B.; Cheng, L. Experimental Analysis of High Temperature Peek Materials on 3d Printing Test. In Proceedings of the 2017 9th International Conference on Measuring Technology and Mechatronics Automation (ICMTMA), Changsha, China, 14–15 January 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 13–16. [Google Scholar]
- Baek, I.; Kwon, O.; Lim, C.-M.; Park, K.Y.; Bae, C.-J. 3D PEEK Objects Fabricated by Fused Filament Fabrication (FFF). Materials 2022, 15, 898. [Google Scholar] [CrossRef]
- Hu, B.; Duan, X.; Xing, Z.; Xu, Z.; Du, C.; Zhou, H.; Chen, R.; Shan, B. Improved Design of Fused Deposition Modeling Equipment for 3D Printing of High-Performance PEEK Parts. Mech. Mater. 2019, 137, 103139. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.E.; Park, J.; Lee, N.-K.; Son, Y.; Park, S.-H. High-Temperature 3D Printing of Polyetheretherketone Products: Perspective on Industrial Manufacturing Applications of Super Engineering Plastics. Mater. Des. 2021, 211, 110163. [Google Scholar] [CrossRef]
- Liu, G.; Hu, N.; Huang, J.; Tu, Q.; Xu, F. Experimental Investigation on the Mechanical and Dynamic Thermomechanical Properties of Polyether Ether Ketone Based on Fused Deposition Modeling. Polymers 2024, 16, 3007. [Google Scholar] [CrossRef]
- Chithambaram, K.; Senthilnathan, N. Effects of Printing Parameters on Hardness and Wear Characteristics of 3D Printed Polyetheretherketone (PEEK) Polymer. Mater. Lett. 2024, 356, 135588. [Google Scholar] [CrossRef]
- Ling, X.; Jing, X.; Zhang, C.; Chen, S. Polyether Ether Ketone (Peek) Properties and Its Application Status. IOP Conf. Ser. Earth Environ. Sci. 2020, 453, 012080. [Google Scholar] [CrossRef]
- Ahmad, K.; Batool, S.A.; Farooq, M.T.; Minhas, B.; Manzur, J.; Yasir, M.; Wadood, A.; Avcu, E.; Ur Rehman, M.A. Corrosion, Surface, and Tribological Behavior of Electrophoretically Deposited Polyether Ether Ketone Coatings on 316L Stainless Steel for Orthopedic Applications. J. Mech. Behav. Biomed. Mater. 2023, 148, 106188. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Wang, Y.; Deng, J.; Wang, X.; Wang, Q.; Liu, Y.; Ding, J.; Yu, L. Polyetheretherketone Implants with Hierarchical Porous Structure for Boosted Osseointegration. Biomater. Res. 2023, 27, 61. [Google Scholar] [CrossRef]
- Gassner, R.; Tuli, T.; Hächl, O.; Rudisch, A.; Ulmer, H. Cranio-Maxillofacial Trauma: A 10 Year Review of 9543 Cases with 21 067 Injuries. J. Cranio-Maxillo-Fac. Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Fac. Surg. 2003, 31, 51–61. [Google Scholar] [CrossRef]
- Slavin, B.V.; Ehlen, Q.T.; Costello, J.P.I.; Nayak, V.V.; Bonfante, E.A.; Benalcázar Jalkh, E.B.; Runyan, C.M.; Witek, L.; Coelho, P.G. 3D Printing Applications for Craniomaxillofacial Reconstruction: A Sweeping Review. ACS Biomater. Sci. Eng. 2023, 9, 6586–6609. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhang, Q.; Mai, Y.; Yang, H.; Li, Y.; Zhang, M.; Zhang, R. Outcome and Risk Factors of Complications after Cranioplasty with Polyetheretherketone and Titanium Mesh: A Single-Center Retrospective Study. Front. Neurol. 2022, 13, 926436. [Google Scholar] [CrossRef] [PubMed]
- Alkhaibary, A.; Alharbi, A.; Alnefaie, N.; Oqalaa Almubarak, A.; Aloraidi, A.; Khairy, S. Cranioplasty: A Comprehensive Review of the History, Materials, Surgical Aspects, and Complications. World Neurosurg. 2020, 139, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.B.; Alfarone, J.; Chernov, E.S.; Debick, N.A.; Jalal, M.; Kim, Y.; Suryadevara, A.; Krishnamurthy, S. Polyetheretherketone (Peek) into the Future: Lowering Infection Rates in Cranioplasty. Cureus 2025, 17, c214. [Google Scholar] [CrossRef]
- Lethaus, B.; Safi, Y.; Ter Laak-Poort, M.; Kloss-Brandstätter, A.; Banki, F.; Robbenmenke, C.; Steinseifer, U.; Kessler, P. Cranioplasty with Customized Titanium and Peek Implants in a Mechanical Stress Model. J. Neurotrauma 2012, 29, 1077–1083. [Google Scholar] [CrossRef]
- Sharma, N.; Zubizarreta-Oteiza, J.; Tourbier, C.; Thieringer, F.M. Can Steam Sterilization Affect the Accuracy of Point-of-Care 3d Printed Polyetheretherketone (Peek) Customized Cranial Implants? An Investigative Analysis. J. Clin. Med. 2023, 12, 2495. [Google Scholar] [CrossRef]
- Kauke-Navarro, M.; Knoedler, L.; Knoedler, S.; Deniz, C.; Safi, A.-F. Surface Modification of PEEK Implants for Craniofacial Reconstruction and Aesthetic Augmentation—Fiction or Reality? Front. Surg. 2024, 11, 1351749. [Google Scholar] [CrossRef] [PubMed]
- Basgul, C.; Yu, T.; MacDonald, D.W.; Siskey, R.; Marcolongo, M.; Kurtz, S.M. Structure–Property Relationships for 3D-Printed PEEK Intervertebral Lumbar Cages Produced Using Fused Filament Fabrication. J. Mater. Res. 2018, 33, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Basgul, C.; MacDonald, D.W.; Siskey, R.; Kurtz, S.M. Thermal Localization Improves the Interlayer Adhesion and Structural Integrity of 3D Printed PEEK Lumbar Spinal Cages. Materialia 2020, 10, 100650. [Google Scholar] [CrossRef] [PubMed]
- Lindtner, R.A.; Schmid, R.; Nydegger, T.; Konschake, M.; Schmoelz, W. Pedicle Screw Anchorage of Carbon Fiber-Reinforced PEEK Screws under Cyclic Loading. Eur. Spine J. 2018, 27, 1775–1784. [Google Scholar] [CrossRef]
- Cheppalli, N.; Metikala, S.; Albertson, B.S.; Yaw, K. Plastics in Total Knee Replacement: Processing to Performance. Cureus 2021, 13, e12969. [Google Scholar] [CrossRef]
- Koh, Y.-G.; Park, K.-M.; Lee, J.-A.; Nam, J.-H.; Lee, H.-Y.; Kang, K.-T. Total Knee Arthroplasty Application of Polyetheretherketone and Carbon-Fiber-Reinforced Polyetheretherketone: A Review. Mater. Sci. Eng. C 2019, 100, 70–81. [Google Scholar] [CrossRef]
- Scholes, S.C.; Unsworth, A. Wear Studies on the Likely Performance of CFR-PEEK/CoCrMo for Use as Artificial Joint Bearing Materials. J. Mater. Sci. Mater. Med. 2009, 20, 163–170. [Google Scholar] [CrossRef]
- Zhao, G.; Yao, S.; Sun, X.; Ma, J.; Wang, J. Consequences of Using Poly-Ether-Ether-Ketone versus Traditional Implant on Tibial Cement Penetration and Short-Term Clinical Outcomes during Total Knee Arthroplasty: A Randomized Controlled Trial. J. Orthop. Surg. 2023, 18, 589. [Google Scholar] [CrossRef]
- Wang, A.; Lin, R.; Polineni, V.K.; Essner, A.; Stark, C.; Dumbleton, J.H. Carbon Fiber Reinforced Polyether Ether Ketone Composite as a Bearing Surface for Total Hip Replacement. Tribol. Int. 1998, 31, 661–667. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O. Mechanical Performances of Hip Implant Design and Fabrication with PEEK Composite. Polymer 2021, 227, 123865. [Google Scholar] [CrossRef]
- Alpkaya, A.; Mihcin, S. The Computational Approach to Predicting Wear: Comparison of Wear of Performance of CFR PEEK and XLPE Liners in Total Hip Replacement. Tribol. Trans. 2022, 66, 59–72. [Google Scholar] [CrossRef]
- Simal, I.; García-Casillas, M.; Cerdá, J.; Riquelme, Ó.; Lorca-García, C.; Pérez-Egido, L.; Fernández-Bautista, B.; Torre, M.; De Agustín, J. Three-Dimensional Custom-Made Titanium Ribs for Reconstruction of a Large Chest Wall Defect. Eur. J. Pediatr. Surg. Rep. 2016, 04, 026–030. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, L.; Yang, C.; Wang, L.; Yi, C.; He, J.; Li, D. Custom Design and Biomechanical Analysis of 3D-Printed PEEK Rib Prostheses. Biomech. Model. Mechanobiol. 2018, 17, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Kang, J.; Fuentes, O.M.; Li, D. Bionic Design and Verification of 3D Printed PEEK Costal Cartilage Prosthesis. J. Mech. Behav. Biomed. Mater. 2020, 103, 103561. [Google Scholar] [CrossRef]
- Akay, C.; Ersöz, M.B. PEEK in Dentistry, Properties and Application Areas. Int. Dent. Res. 2020, 10, 60–65. [Google Scholar] [CrossRef]
- Ouldyerou, A.; Merdji, A.; Aminallah, L.; Roy, S.; Mehboob, H.; Özcan, M. Biomechanical Performance of Ti-PEEK Dental Implants in Bone: An in-Silico Analysis. J. Mech. Behav. Biomed. Mater. 2022, 134, 105422. [Google Scholar] [CrossRef]
- Qin, L.; Yao, S.; Zhao, J.; Zhou, C.; Oates, T.W.; Weir, M.D.; Wu, J.; Xu, H.H.K. Review on Development and Dental Applications of Polyetheretherketone-Based Biomaterials and Restorations. Materials 2021, 14, 408. [Google Scholar] [CrossRef]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef]
- Kimura, H.; Morita, K.; Nishio, F.; Abekura, H.; Tsuga, K. Clinical Report of Six-Month Follow-up after Cementing PEEK Crown on Molars. Sci. Rep. 2022, 12, 19070. [Google Scholar] [CrossRef]
- Yoshida, E.; Nomoto, R.; Amitani, Y.; Hayakawa, T. PEEK Stress-Shielding with Artificial Bone for Dental Implants. Dent. Mater. J. 2025, 44, 121–127. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of Polyetheretherketone (PEEK) in Oral Implantology and Prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Benakatti, V.B.; Sajjanar, J.A.; Acharya, A. Polyetheretherketone (PEEK) in Dentistry. J. Clin. Diagn. Res. 2019, 13, ZE10–ZE12. [Google Scholar] [CrossRef]
- Nobrega, G.; Cardoso, B.; Souza, R.; Pereira, J.; Pontes, P.; Catarino, S.O.; Pinho, D.; Lima, R.; Moita, A. A Review of Novel Heat Transfer Materials and Fluids for Aerospace Applications. Aerospace 2024, 11, 275. [Google Scholar] [CrossRef]
- Pigliaru, L.; Rinaldi, M.; Ciccacci, L.; Norman, A.; Rohr, T.; Ghidini, T.; Nanni, F. 3D Printing of High Performance Polymer-Bonded PEEK-NdFeB Magnetic Composite Materials. Funct. Compos. Mater. 2020, 1, 4. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, G.; Huang, X.; Zhang, H.; Hao, X.; Tan, S.; Liu, K.; Ji, G. Optimized Pyramidal Honeycomb PEEK/CF Composites Metastructure through 3D Printing for Broadband Electromagnetic Wave Absorption. Mater. Today Phys. 2025, 50, 101620. [Google Scholar] [CrossRef]
- Martin, R.G.; Johansson, C.; Tavares, J.R.; Dubé, M. CF/PEEK Skins Assembly by Induction Welding for Thermoplastic Composite Sandwich Panels. Compos. Part B Eng. 2024, 284, 111676. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, Y.; Wu, Y.; Li, D. Neutron Shielding Performance of 3d-Printed Boron Carbide Peek Composites. Materials 2020, 13, 2314. [Google Scholar] [CrossRef]
- Kumar, A.; Saxena, N.V. A Review on usage of peek material for wheels. Int. Res. J. Mod. Eng. Technol. Sci. 2021, 3, 844–846. [Google Scholar]
- Manivannan, R.; Rajasekar, R.; Vk, G. Modelling and Analysis of Four Wheel Rim by Using PEEK Composites. Mater. Sci. Eng. Int. J. 2018, 2, 177–183. [Google Scholar] [CrossRef]
- Badeghaish, W.; Wagih, A.; Rastogi, S.; Lubineau, G. Effect of High-Temperature Acid Aging on Microstructure and Mechanical Properties of PEEK. Polym. Test. 2024, 134, 108429. [Google Scholar] [CrossRef]
- Gupta, R.; Shinde, S.; Yella, A.; Subramaniam, C.; Saha, S.K. Thermomechanical Characterisations of PTFE, PEEK, PEKK as Encapsulation Materials for Medium Temperature Solar Applications. Energy 2020, 194, 116921. [Google Scholar] [CrossRef]
- Lu, C.; Xu, N.; Zheng, T.; Zhang, X.; Lv, H.; Lu, X.; Xiao, L.; Zhang, D. The Optimization of Process Parameters and Characterization of High-Performance CF/PEEK Composites Prepared by Flexible CF/PEEK Plain Weave Fabrics. Polymers 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Duan, M.; Qin, S.; Zhang, Z. Preparation and Modification of Porous Polyetheretherketone (PEEK) Cage Material Based on Fused Deposition Modeling (FDM). Polymers 2022, 14, 5403. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical Applications of Polymer-Composite Materials: A Review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Peng, T.-Y.; Shih, Y.-H.; Hsia, S.-M.; Wang, T.-H.; Li, P.-J.; Lin, D.-J.; Sun, K.-T.; Chiu, K.-C.; Shieh, T.-M. In Vitro Assessment of the Cell Metabolic Activity, Cytotoxicity, Cell Attachment, and Inflammatory Reaction of Human Oral Fibroblasts on Polyetheretherketone (PEEK) Implant–Abutment. Polymers 2021, 13, 2995. [Google Scholar] [CrossRef]
- Kumar, A.; Yap, W.T.; Foo, S.L.; Lee, T.K. Effects of Sterilization Cycles on Peek for Medical Device Application. Bioengineering 2018, 5, 18. [Google Scholar] [CrossRef]
- Chai, L.; Zhang, B.; Qiao, L.; Wang, P.; Weng, L. Influence of Gamma Irradiation-Induced Surface Oxidation on Tribological Property of Polyetheretherketone (Peek). Polym. Bull. 2022, 79, 6513–6531. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Ormanbekov, K.; Zhassulan, A.; Andybayeva, G.; Shynarbek, A.; Yeldos Mukhametov, Y.M. Influence of Irradiation Doses on the Mechanical and Tribological Properties of Polyetheretherketone Exposed to Electron Beam Treatment. Adv. Sci. Technol. Res. J. 2024, 19, 121–131. [Google Scholar] [CrossRef]
- Evans, N.T.; Torstrick, F.B.; Lee, C.S.D.; Dupont, K.M.; Safranski, D.L.; Chang, W.A.; Macedo, A.E.; Lin, A.; Boothby, J.M.; Whittingslow, D.C.; et al. High Strength, Surface Porous Polyether-Ether-Ketone for Load-Bearing Orthopaedic Implants. Acta Biomater. 2015, 13, 159–167. [Google Scholar] [CrossRef]
- Wong, K.I.; Zhong, Y.; Li, D.; Cheng, Z.; Yu, Z.; Wei, M. Modified Porous Microstructure for Improving Bone Compatibility of Poly-Ether-Ether-Ketone. J. Mech. Behav. Biomed. Mater. 2021, 120, 104541. [Google Scholar] [CrossRef]
- Vaezi, M.; Yang, S. Extrusion-Based Additive Manufacturing of PEEK for Biomedical Applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Gao, S.; Liu, R.; Xin, H.; Liang, H.; Wang, Y.; Jia, J. The Surface Characteristics, Microstructure and Mechanical Properties of PEEK Printed by Fused Deposition Modeling with Different Raster Angles. Polymers 2022, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.; Zhao, B.; Quan, L.; Fu, J. Effect of 3d Printing Process Parameters and Heat Treatment Conditions on the Mechanical Properties and Microstructure of Peek Parts. Polymers 2023, 15, 2209. [Google Scholar] [CrossRef] [PubMed]
- Adamson, M.; Eslami, B. Post-Processing PEEK 3D-Printed Parts: Experimental Investigation of Annealing on Microscale and Macroscale Properties. Polymers 2025, 17, 744. [Google Scholar] [CrossRef]
- Lee, A.; Wynn, M.; Quigley, L.; Salviato, M.; Zobeiry, N. Effect of Temperature History during Additive Manufacturing on Crystalline Morphology of PEEK. Adv. Ind. Manuf. Eng. 2022, 4, 100085. [Google Scholar] [CrossRef]
- Zanjanijam, A.R.; Major, I.; Lyons, J.G.; Lafont, U.; Devine, D.M. Fused Filament Fabrication of Peek: A Review of Process-Structure-Property Relationships. Polymers 2020, 12, 1665. [Google Scholar] [CrossRef]
- Shirani Bidabadi, B.; Motta De Castro, E.; Carrola, M.; Koirala, P.; Tehrani, M.; Asadi, A. Engineering the Crystalline Architecture for Enhanced Properties in Fast-Rate Processing of Poly(Ether Ether Ketone) (Peek) Nanocomposites. ACS Appl. Eng. Mater. 2024, 2, 2038–2054. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Gache, C.C.L.; Cascolan, H.M.S.; Cancino, L.T.; Advincula, R.C. Post-Processing of 3D-Printed Polymers. Technologies 2021, 9, 61. [Google Scholar] [CrossRef]
- Tang, S. Tension–Tension Fatigue Behavior of Hydroxyapatite Reinforced Polyetheretherketone Composites. Int. J. Fatigue 2004, 26, 49–57. [Google Scholar] [CrossRef]
- Sobieraj, M.C.; Murphy, J.E.; Brinkman, J.G.; Kurtz, S.M.; Rimnac, C.M. Notched Fatigue Behavior of PEEK. Biomaterials 2010, 31, 9156–9162. [Google Scholar] [CrossRef]
- Chen, F.; Gatea, S.; Ou, H.; Lu, B.; Long, H. Fracture Characteristics of PEEK at Various Stress Triaxialities. J. Mech. Behav. Biomed. Mater. 2016, 64, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Rendas, P.; Imperadeiro, A.; Martins, R.F.; Soares, B.A.R. High-Cycle Fatigue Behaviour of Polyetheretherketone (Peek) Produced by Additive Manufacturing. Polymers 2023, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B.; Turner, A.S. Polyetheretherketone as a Biomaterial for Spinal Applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Tang, T. Current Strategies to Improve the Bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef]
- Choudhury, S.S.; Pandey, M.; Bhattacharya, S. Recent Developments in Surface Modification of PEEK Polymer for Industrial Applications: A Critical Review. Rev. Adhes. Adhes. 2021, 9, 410–433. [Google Scholar]
- Li, W.; Sang, L.; Jian, X.; Wang, J. Influence of Sanding and Plasma Treatment on Shear Bond Strength of 3D-Printed PEI, PEEK and PEEK/CF. Int. J. Adhes. Adhes. 2020, 100, 102614. [Google Scholar] [CrossRef]
- Omrani, M.M.; Hadjizadeh, A.; Milani, A.; Kim, K. PEEK Surface Modification Methods and Effect of the Laser Method on Surface Properties. Biointerface Res. Appl. Chem. 2020, 10, 5132–5140. [Google Scholar] [CrossRef]
- Münstedt, H.; Grossmann, J. Surface Modifications of Polyetheretherketone (Peek): Results from the Literature and Special Studies of Copper-Coated Films. Polymers 2022, 14, 4797. [Google Scholar] [CrossRef]
- Wang, B.; Huang, M.; Dang, P.; Xie, J.; Zhang, X.; Yan, X. Peek in Fixed Dental Prostheses: Application and Adhesion Improvement. Polymers 2022, 14, 2323. [Google Scholar] [CrossRef]
- YU, D.; LEI, X.; ZHU, H. Modification of Polyetheretherketone (PEEK) Physical Features to Improve Osteointegration. J. Zhejiang Univ. Sci. B 2022, 23, 189–203. [Google Scholar] [CrossRef]
- Elawadly, T.; Radi, I.A.W.; El Khadem, A.; Osman, R.B. Can PEEK Be an Implant Material? Evaluation of Surface Topography and Wettability of Filled Versus Unfilled PEEK With Different Surface Roughness. J. Oral Implantol. 2017, 43, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Mithran, A.; Upwanshi, V.R.; Alqahtani, I.M.; Alqahtani, S.J.M.; Nayak, P.P.; Alsharif, A.A.; Babu, J.S.; Swarnalatha, C.; Nayyar, A.S. Evaluation of the Impact of Surface Modification with Air Abrasion (Sandblasting) and Ultraviolet Radiation on the Retention Strength of Polyetherether Ketone (Peek) Coping in Relation to the Underlying Dentin Abutments: An in Vitro Study. J. Microsc. Ultrastruct. 2024. [Google Scholar] [CrossRef]
- Porrelli, D.; Mardirossian, M.; Crapisi, N.; Urban, M.; Ulian, N.A.; Bevilacqua, L.; Turco, G.; Maglione, M. Polyetheretherketone and Titanium Surface Treatments to Modify Roughness and Wettability–Improvement of Bioactivity and Antibacterial Properties. J. Mater. Sci. Technol. 2021, 95, 213–224. [Google Scholar] [CrossRef]
- Comyn, J.; Mascia, L.; Xiao, G.; Parker, B.M. Corona-Discharge Treatment of Polyetheretherketone (PEEK) for Adhesive Bonding. Int. J. Adhes. Adhes. 1996, 16, 301–304. [Google Scholar] [CrossRef]
- Khoury, J.; Maxwell, M.; Cherian, R.E.; Bachand, J.; Kurz, A.C.; Walsh, M.; Assad, M.; Svrluga, R.C. Enhanced Bioactivity and Osseointegration of PEEK with Accelerated Neutral Atom Beam Technology. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 531–543. [Google Scholar] [CrossRef]
- Chayanun, S.; Chanamuangkon, T.; Boonsuth, B.; Boccaccini, A.R.; Lohwongwatana, B. Enhancing PEEK Surface Bioactivity: Investigating the Effects of Combining Sulfonation with Sub-Millimeter Laser Machining. Mater. Today Bio 2023, 22, 100754. [Google Scholar] [CrossRef]
- Zhao, X.; Karthik, N.; Xiong, D.; Liu, Y. Bio-Inspired Surface Modification of PEEK through the Dual Cross-Linked Hydrogel Layers. J. Mech. Behav. Biomed. Mater. 2020, 112, 104032. [Google Scholar] [CrossRef]
- Luo, F.; Li, D.; Huang, Y.; Mao, R.; Wang, L.; Lu, J.; Ge, X.; Fan, Y.; Zhang, X.; Chen, Y.; et al. Efficient Osteogenic Activity of PEEK Surfaces Achieved by Femtosecond Laser–Hydroxylation. ACS Appl. Mater. Interfaces 2023, 15, 37232–37246. [Google Scholar] [CrossRef]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK Surface Modification by Fast Ambient-Temperature Sulfonation for Bone Implant Applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef]
- Dos Santos, F.S.F.; Vieira, M.; da Silva, H.N.; Tomás, H.; Fook, M.V.L. Surface Bioactivation of Polyether Ether Ketone (PEEK) by Sulfuric Acid and Piranha Solution: Influence of the Modification Route in Capacity for Inducing Cell Growth. Biomolecules 2021, 11, 1260. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, J.; Sun, S.; Meng, W.; Zhang, Y.; Wu, J. Current Treatment Methods to Improve the Bioactivity and Bonding Strength of PEEK for Dental Application: A Systematic Review. Eur. Polym. J. 2023, 183, 111757. [Google Scholar] [CrossRef]
- Feng, X.; Ma, L.; Liang, H.; Liu, X.; Lei, J.; Li, W.; Wang, K.; Song, Y.; Wang, B.; Li, G.; et al. Osteointegration of 3d-Printed Fully Porous Polyetheretherketone Scaffolds with Different Pore Sizes. ACS Omega 2020, 5, 26655–26666. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Barkarmo, S.; Hawthan, M.; Peruzzi, N.; Kjellin, P.; Wennerberg, A. Biomechanical, Histological, and Computed X-ray Tomographic Analyses of Hydroxyapatite Coated PEEK Implants in an Extended Healing Model in Rabbit. J. Biomed. Mater. Res. A 2018, 106, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Guo, F.; Chen, J.; Wang, X.; Zhao, X.; Xiao, P. Bioactive Glass–Chitosan Composite Coatings on PEEK: Effects of Surface Wettability and Roughness on the Interfacial Fracture Resistance and in Vitro Cell Response. Appl. Surf. Sci. 2018, 440, 514–523. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Toita, R.; Tsuru, K.; Ishikawa, K. Enhanced Osseointegration Capability of Poly(Ether Ether Ketone) via Combined Phosphate and Calcium Surface-Functionalization. Int. J. Mol. Sci. 2019, 21, 198. [Google Scholar] [CrossRef]
- Roskies, M.; Jordan, J.O.; Fang, D.; Abdallah, M.-N.; Hier, M.P.; Mlynarek, A.; Tamimi, F.; Tran, S.D. Improving PEEK Bioactivity for Craniofacial Reconstruction Using a 3D Printed Scaffold Embedded with Mesenchymal Stem Cells. J. Biomater. Appl. 2016, 31, 132–139. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. 510(k) Premarket Notification; FDA: Silver Spring, MD, USA, 1998.
- Code of Federal Regulations. Title 21 CFR 820: Quality System Regulation; U.S. Government Publishing Office: Washington, DC, USA, 2016.
- U.S. Food and Drug Administration. Technical Considerations for Additive Manufactured Medical Devices: Guidance for Industry and FDA Staff; FDA: Silver Spring, MD, USA, 2017.
- ISO 10993-1:2018; Biological Evaluation of Medical Devices–Part 1: Evaluation and Testing Within a Risk Management Process. ISO: Geneva, Switzerland, 2018.
- MohanaSundaram, A.; Kamalakannan, Y.; Raja, V.; Mofatteh, M.; Haque, M.A. The World’s First 3D-Printed PEEK Cranial Implant: A New Horizon in Precision and Personalized Neurosurgery. Neurosurg. Rev. 2024, 47, 616. [Google Scholar] [CrossRef]
- Rendas, P.; Figueiredo, L.; Machado, C.; Mourão, A.; Vidal, C.; Soares, B. Mechanical Performance and Bioactivation of 3D-Printed PEEK for High-Performance Implant Manufacture: A Review. Prog. Biomater. 2023, 12, 89–111. [Google Scholar] [CrossRef]





| References | Property | PEEK | Titanium | UHMWPE |
|---|---|---|---|---|
| [2,20,47,60] | Elasticity Modulus (GPa) | 3–4 | 102–113 | 0.8–1.6 |
| [2,14,60] | Tensile Strength (MPa) | 80–97 | 954–976 | 20–49 |
| [2] | Elongation at break (ductility) | ~30% | - | - |
| [2] | Flexural Strength (MPa) | ~140–160 | - | - |
| [50,54] | Wear Resistance | Good | Excellent | Excellent |
| [1,10,60,61] | Biocompatibility | Excellent | Excellent | Good |
| [1,2,10,47,56] | Radiolucency | Radiolucent | Radiopaque | Radiolucent |
| [1,5,47] | Osteointegration | Poor | Good | Poor |
| [1,78] | Cytotoxicity | Low | Low | Moderate |
| [1,2] | Raw Material Cost | High | Moderate | Low |
| [1,2] | Manufacturing Cost | Moderate | High | Low |
| Reference | Focus | Findings | Conclusion |
|---|---|---|---|
| [92] | Understanding the fatigue behavior of PEEK and its composites in implant devices subjected to repeated loading. | PEEK composites, such as HA/PEEK materials, exhibit promising fatigue resistance properties suitable for biomedical applications. | Highlighted the promising future of PEEK composites in biomedical implants and stressed the need for further research into the development of fatigue damage in these materials. |
| [93] | Investigating the stress-life fatigue behavior and fracture characteristics of notched PEEK specimens. | More severe notch geometries and higher cyclic stress levels significantly reduced the number of cycles to failure. Majority of the fatigue lifetime was spent in the crack initiation phase. | Emphasized the importance of considering design-related stress concentrations when developing PEEK-based implants undergoing cyclic loading. |
| [94] | Examining the fracture behavior of PEEK specimens with varying notch radii under different stress triaxialities. | Smaller notch radii resulted in higher stress triaxiality. Designs with reduced stress concentrations demonstrated improved performance under cyclic loading conditions. | Highlighted stress triaxiality as a key design parameter for optimizing the performance of notched PEEK implants. |
| [95] | Investigating the high-cycle fatigue behavior of 3D-printed PEEK specimens subjected to stress-controlled tension–tension cyclic loading, highlighting the significance of cyclic loading in load-bearing implant design. | Three-dimensionally printed PEEK endured high cyclic stress levels up to 75% of its ultimate tensile strength before failure. Fracture surface analysis revealed that crack initiation occurred in voids formed during the printing process. | Highlighted PEEK’s potential for load-bearing applications. Emphasized the need for further research to understand the correlation between printing parameters and fatigue behavior. |
| Reference. | Surface Modification Technique | Summary |
|---|---|---|
| [101,104,105] | Sandblasting/Blasting | Increased surface roughness without significantly altering the chemical composition of PEEK. Increased wettability and adhesion. |
| [101,107] | Corona treatment | Increased surface polarity and O/C ratio, improving adhesion and wettability. However, it decreased surface roughness. |
| [101] | Oxygen plasma treatment | Significantly increased the O/C ratio, enhancing adhesion and wettability. However, it decreased surface roughness. |
| [101] | Argon plasma treatment | Enhanced surface adhesion without increasing oxygen content. Also reduced surface roughness. |
| [101,105,111] | Laser treatment | Reduced surface roughness and contact angle without oxygen content. Enhanced surface hydrophilicity. May introduce surface degradation |
| [101] | UV radiation | Increased oxygen content on the surface PEEK, enhancing wettability and bond strength. |
| [101] | Chemical treatment | Strongly increased the O/C ratio and surface roughness, significantly improving adhesion. |
| [110] | Hydrogel-coated PEEK | Improved surface wettability and lubrication. Demonstrated self-healing properties, and reduced wear in implants. |
| [112,113] | Sulfonation | Improved hydrophilicity by significantly reducing the water contact angle. Created a nano-porous PEEK surface for enhanced adhesion and bioactivity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallal, S.; Eslami, B.; Tiari, S. Recent Advances in PEEK for Biomedical Applications: A Comprehensive Review of Material Properties, Processing, and Additive Manufacturing. Polymers 2025, 17, 1968. https://doi.org/10.3390/polym17141968
Dallal S, Eslami B, Tiari S. Recent Advances in PEEK for Biomedical Applications: A Comprehensive Review of Material Properties, Processing, and Additive Manufacturing. Polymers. 2025; 17(14):1968. https://doi.org/10.3390/polym17141968
Chicago/Turabian StyleDallal, Samreen, Babak Eslami, and Saeed Tiari. 2025. "Recent Advances in PEEK for Biomedical Applications: A Comprehensive Review of Material Properties, Processing, and Additive Manufacturing" Polymers 17, no. 14: 1968. https://doi.org/10.3390/polym17141968
APA StyleDallal, S., Eslami, B., & Tiari, S. (2025). Recent Advances in PEEK for Biomedical Applications: A Comprehensive Review of Material Properties, Processing, and Additive Manufacturing. Polymers, 17(14), 1968. https://doi.org/10.3390/polym17141968








