Copolymerization Behavior of Acrylamide-Based Polymers in Ionic Liquid Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
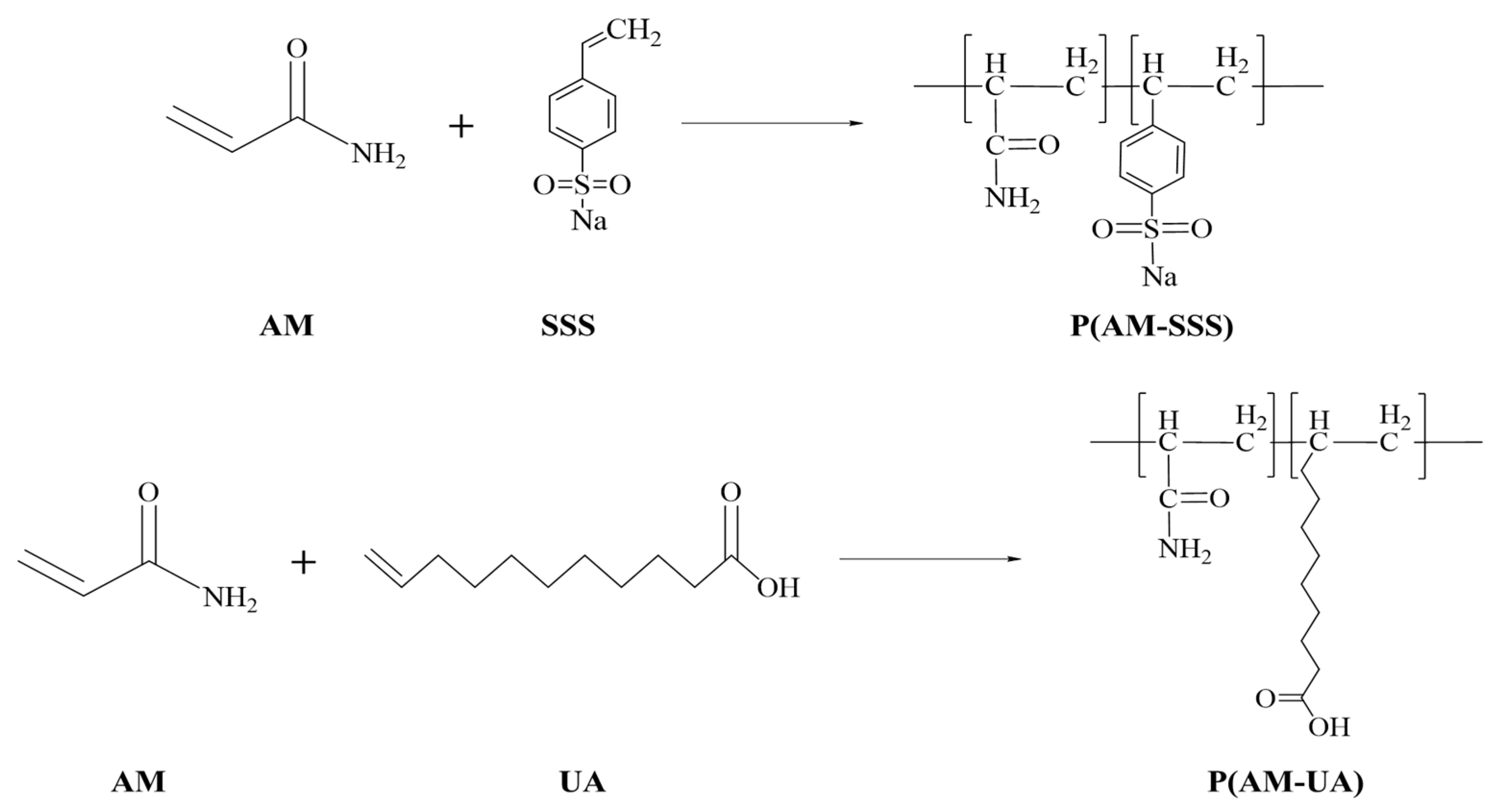
2.3. Preparation of Copolymers
2.4. FTIR and 1H NMR Characterization
2.5. Calculation of Monomer Copolymerization Rate and Chain Segment Sequence of Copolymers
2.6. Characterization of Microscopic Morphology
2.7. Thermogravimetric Characterization (TGA)
2.8. Viscosity Increase Performance Testing
2.9. Temperature and Shear Resistance Testing
2.10. Salt Resistance Performance Testing
3. Results
3.1. Characterization of Copolymers in Ils
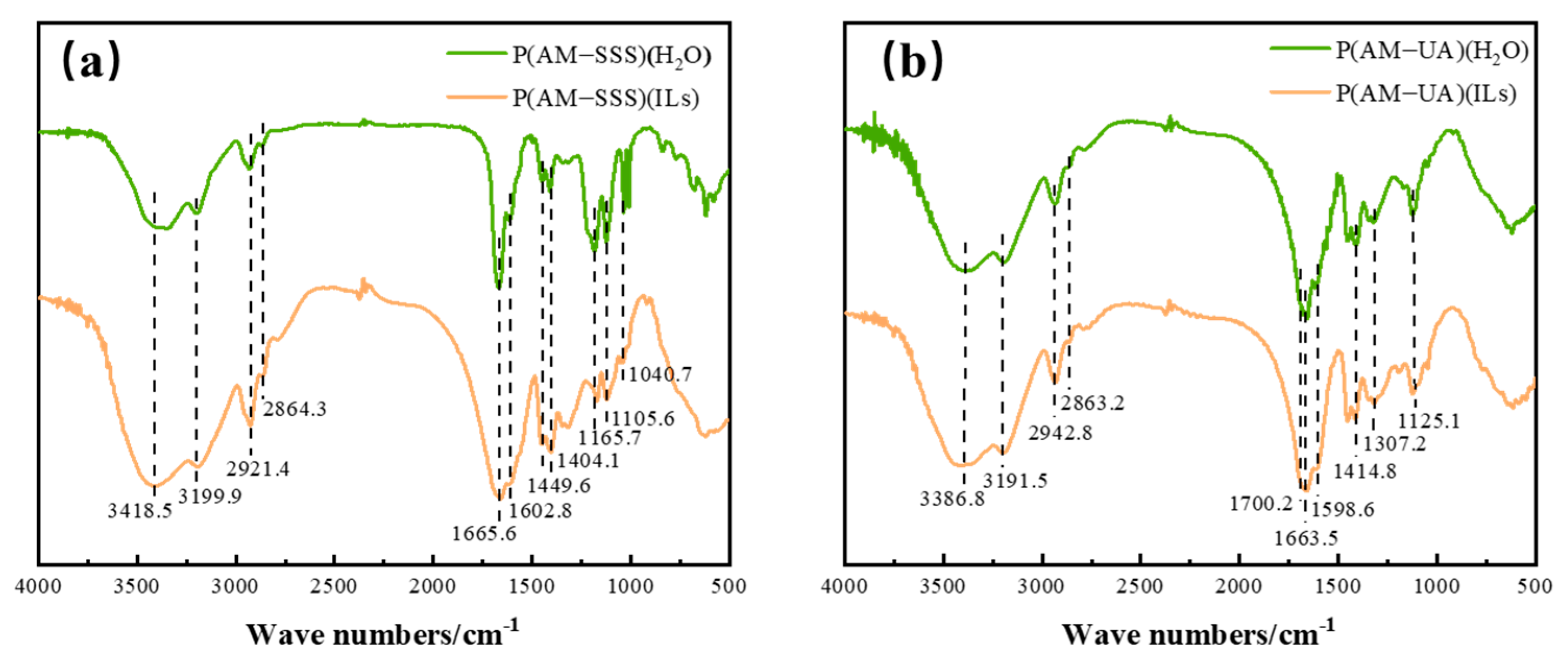
3.1.1. FTIR Analysis
3.1.2. 1H NMR Analysis
3.2. Monomer Polymerization Rate of Copolymers
3.3. Sequence Calculation of Copolymer Chain Segments
3.4. Performance Evaluation of Copolymers
3.4.1. SEM Characterization
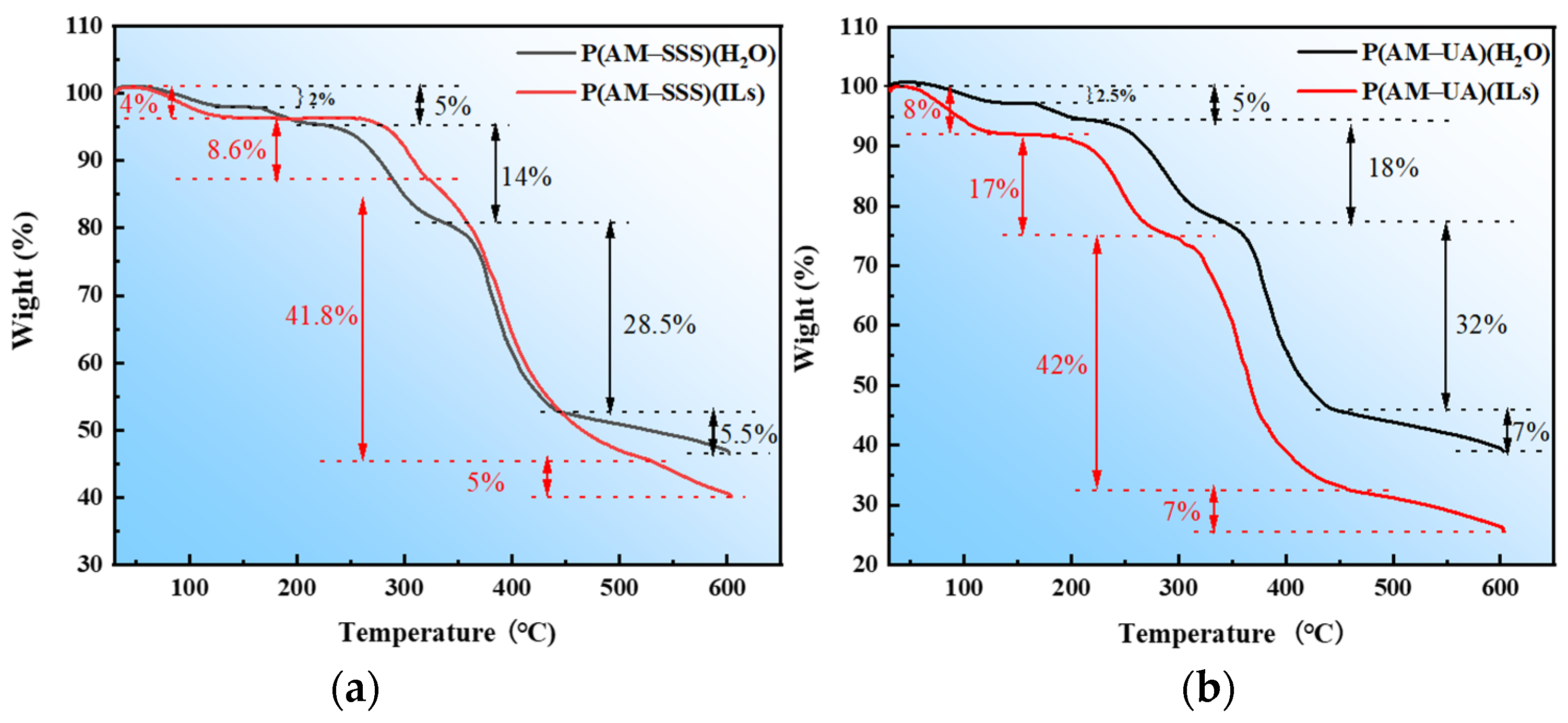
3.4.2. TGA Analysis
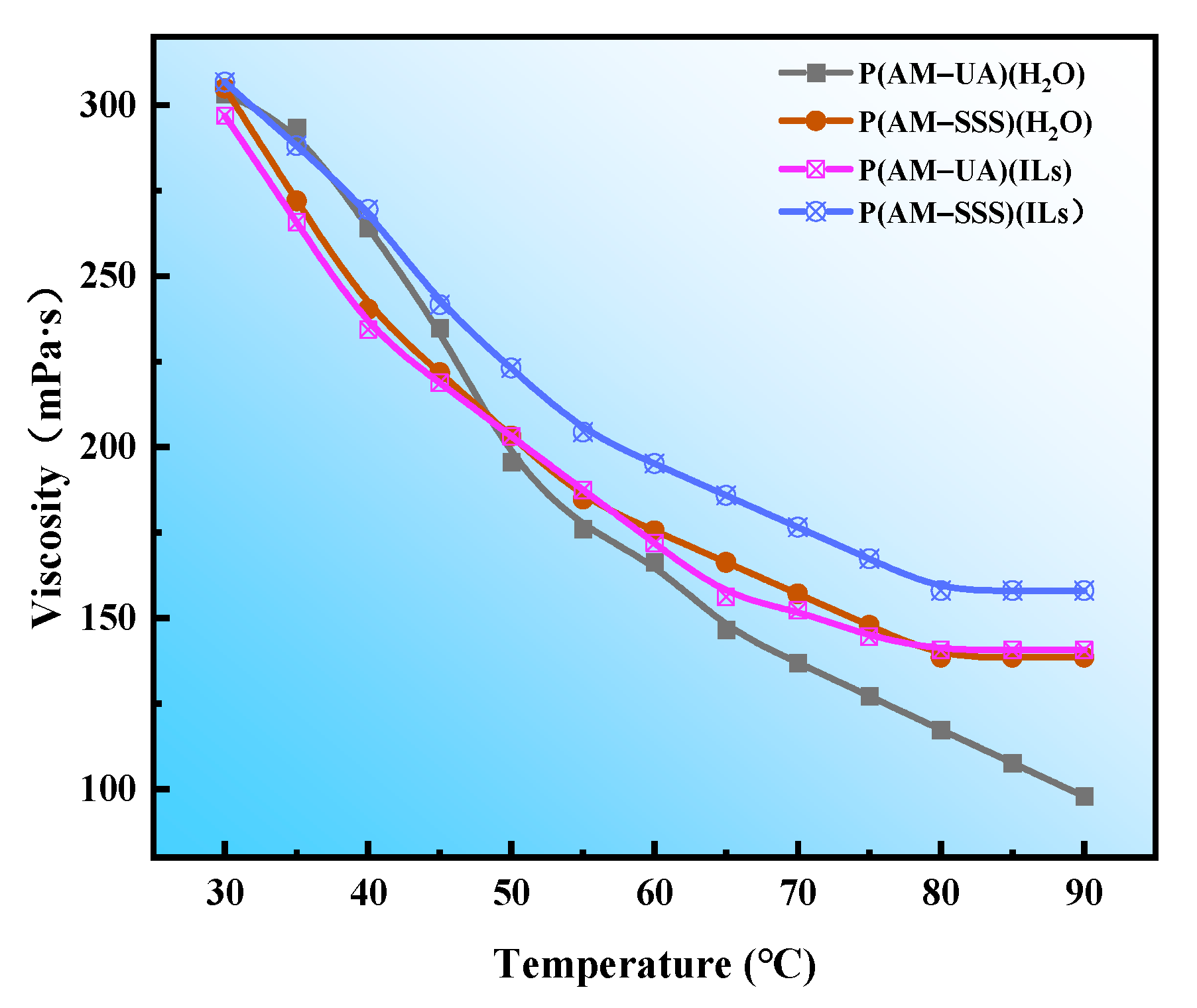
3.4.3. Evaluation of Increasing Viscosity
3.4.4. Evaluation of Temperature Resistance
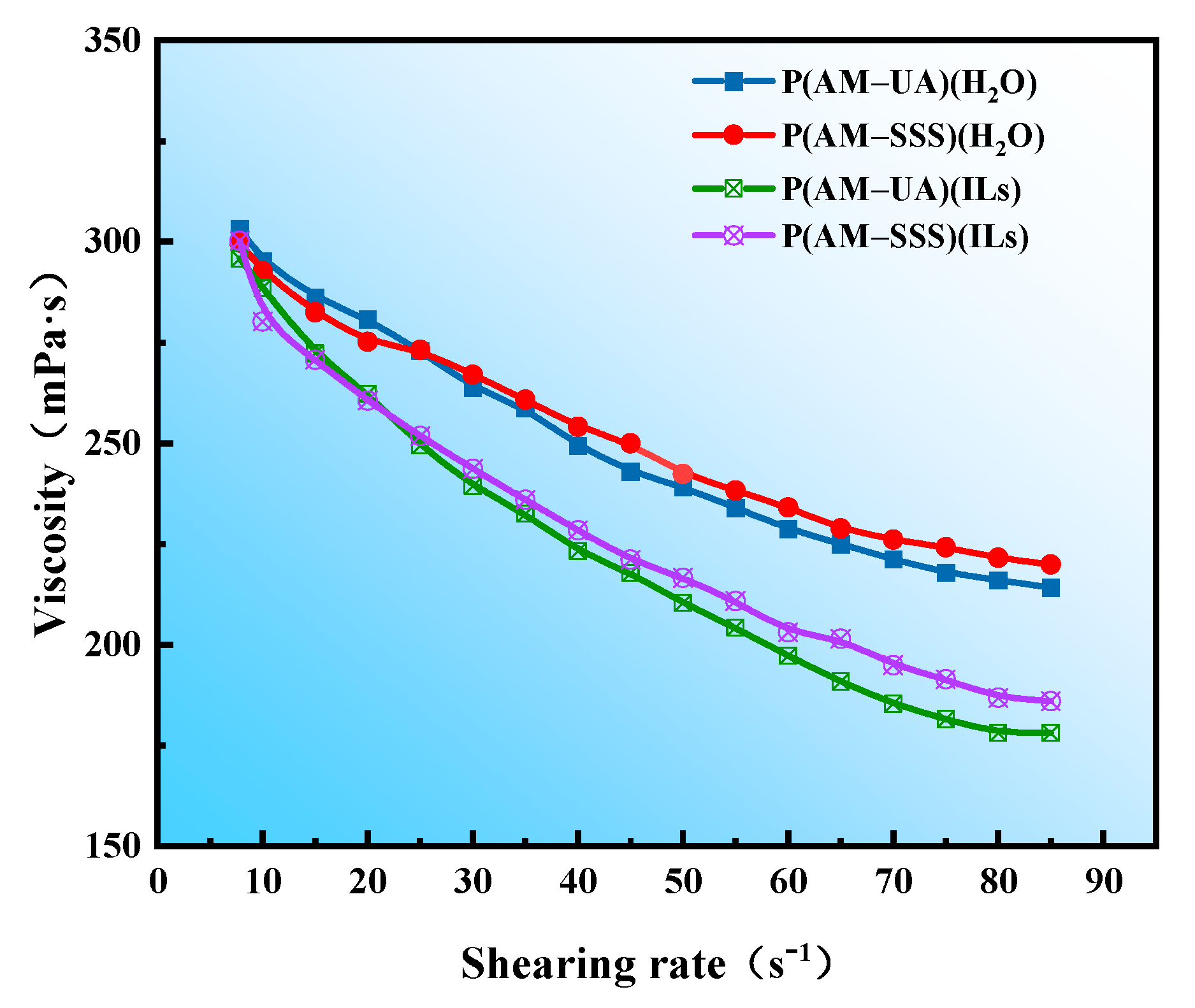
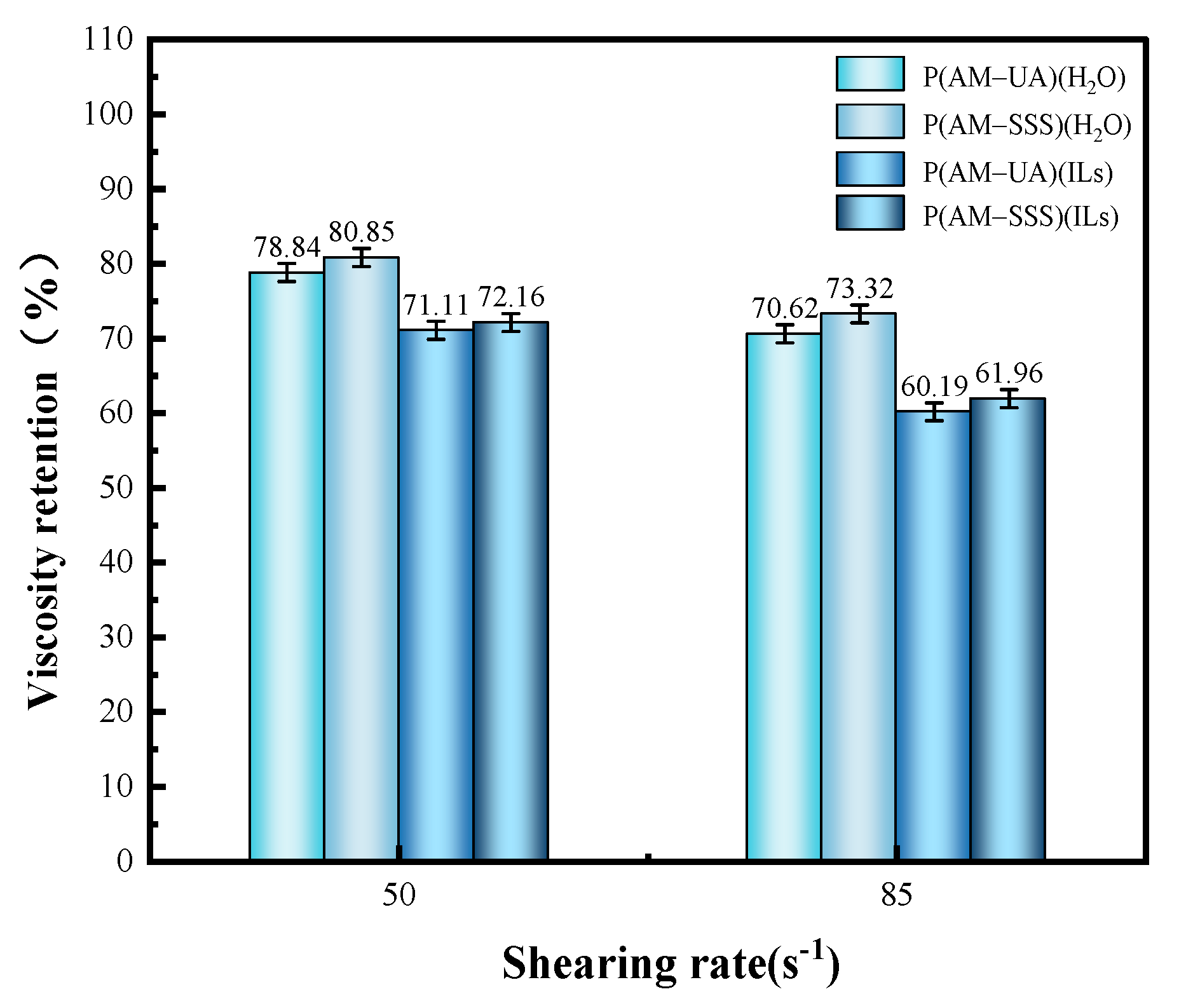
3.4.5. Evaluation of Shear Resistance
3.4.6. Evaluation of Salt Resistance
4. Conclusions
- (1)
- The calculation of polymerization rates for monomers and the sequences of chain segments reveal that the number-average lengths of AM chain segments in P(AM-SSS) (H2O), P(AM-SSS) (ILs), P(AM-UA) (H2O), and P(AM-UA) (ILs) are found to be 2.9170, 3.4606, 231.2866, and 91.1179, respectively. This indicates that [BMIM]Oac demonstrates substantial solubility for hydrophobic monomers, facilitating the dissolution of copolymer monomers with diverse characteristics. Consequently, a more homogenous distribution of hydrophobic monomers within the molecular chains of the copolymer products in [BMIM]Oac is achieved.
- (2)
- An analysis of the thermal stability and micro-morphology of the copolymers reveals that the three-dimensional spatial structures of the copolymers synthesized in [BMIM]Oac possess both robust and loose characteristics. The copolymer derived from SSS in ionic liquids displays considerable thermal stability, achieving a decomposition temperature as high as 260 °C, while the copolymer synthesized from UA in water exhibits strong thermal stability with a decomposition temperature of 240 °C.
- (3)
- In comparison to aqueous solutions, the copolymers derived from AM with SSS and UA in ionic liquids show notable thickening properties. Specifically, the copolymer P(AM-SSS) (ILs) exhibits remarkable shear resistance, whereas P(AM-UA) (ILs) showcases exceptional resistance to salt, sustaining a viscosity retention rate exceeding 100% even in high concentrations of Na+ and Ca2+.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.; Yang, H.; Zheng, L.; Yang, J.; Zhu, J.; Nan, Y.; Su, G.; Yu, X. Preparation of Amphiphilic Janus Nanosheets Based on Thermally Expandable Microspheres and Evaluation of Their Physical and Chemical Properties. Fuel 2024, 358, 130253. [Google Scholar] [CrossRef]
- Xin, X.; Yu, G.; Chen, Z.; Wu, K.; Dong, X.; Zhu, Z. Effect of Polymer Degradation on Polymer Flooding in Heterogeneous Reservoirs. Polymers 2018, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.J.; Leonhardt, B.; Azri, N. Status of Polymer-Flooding Technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Afolabi, R.O.; Oluyemi, G.F.; Officer, S.; Ugwu, J.O. Hydrophobically Associating Polymers for Enhanced Oil Recovery—Part B: A Review of Modelling Approach to Flow in Porous Media. J. Mol. Liq. 2019, 293, 111495. [Google Scholar] [CrossRef]
- Hua, X.; Han, K.; Lin, Z.; Yin, B.; Wang, P.; Qi, D.; Hou, D.; Chen, J. Modification of In-Situ Polymerization of Acrylamide and Synergies with Fiber in Enhancing Cement-Based Composite. J. Build. Eng. 2024, 85, 108605. [Google Scholar] [CrossRef]
- Li, C.; Wu, C.; Jiang, Z.; Su, G.; Yang, H.; Yu, X.; Wang, J. Effect of Ionic Liquids on the Polymerization Behavior of Acrylamide-based Copolymers. J. Appl. Polym. Sci. 2024, 141, e56103. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, Z. Self-Assembly of Hydrophobic Associating Polyacrylamide Prepared by Aqueous Dispersion Polymerization. J. Dispers. Sci. Technol. 2019, 40, 1317–1325. [Google Scholar] [CrossRef]
- El-hoshoudy, A.N.; Desouky, S.E.M.; Al-Sabagh, A.M.; Betiha, M.A.; El-kady, M.Y.; Mahmoud, S. Evaluation of Solution and Rheological Properties for Hydrophobically Associated Polyacrylamide Copolymer as a Promised Enhanced Oil Recovery Candidate. Egypt. J. Pet. 2017, 26, 779–785. [Google Scholar] [CrossRef]
- Han, Y.; Yu, Z.; Guan, Z.; Ma, R.; Hu, Y. Hydrophobic Association Supramolecular Gel Suitable for Oil and Gas Drilling in Fractured Formation. Front. Chem. 2024, 12, 1468766. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Lai, X.; Li, J.; Wang, T.; Wang, L.; Gao, J.; Wen, X.; Liu, G.; Liu, Y. Rheological Properties and Ultra-High Salt Resistance of Novel Hydrophobically Associating Copolymers for Fracturing Fluids. Polym. Bull. 2023, 80, 8725–8743. [Google Scholar] [CrossRef]
- Torres-Martínez, J.G.; Pérez-Alvarez, M.; Jiménez-Regalado, E.J.; St Thomas, C.; Alonso-Martínez, F. Behavior Study of a Thermo-responsive Hydrophobically Associative Water-soluble Terpolymer in Laboratory Test with Heavy Crude Oil. J. Appl. Polym. Sci. 2022, 139, e52860. [Google Scholar] [CrossRef]
- Liu, R.; Pu, W.; Sheng, J.J.; Du, D. Star-like Hydrophobically Associative Polyacrylamide for Enhanced Oil Recovery: Comprehensive Properties in Harsh Reservoir Conditions. J. Taiwan Inst. Chem. Eng. 2017, 80, 639–649. [Google Scholar] [CrossRef]
- Olsson, C.; Idström, A.; Nordstierna, L.; Westman, G. Influence of Water on Swelling and Dissolution of Cellulose in 1-Ethyl-3-Methylimidazolium Acetate. Carbohydr. Polym. 2014, 99, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jian, O.; Paillet, S.; Desbrières, J.; Grassl, B. Hydrophobically Modified Associating Polyacrylamide (HAPAM) Synthesized by Micellar Copolymerization at High Monomer Concentration. Eur. Polym. J. 2007, 43, 824–834. [Google Scholar] [CrossRef]
- Afolabi, R.O.; Oluyemi, G.F.; Officer, S.; Ugwu, J.O. Hydrophobically Associating Polymers for Enhanced Oil Recovery—Part A: A Review on the Effects of Some Key Reservoir Conditions. J. Pet. Sci. Eng. 2019, 180, 681–698. [Google Scholar] [CrossRef]
- Garcia, H.; Ferreira, R.; Petkovic, M.; Ferguson, J.L.; Leitão, M.C.; Gunaratne, H.Q.N.; Seddon, K.R.; Rebelo, L.P.N.; Silva Pereira, C. Dissolution of Cork Biopolymers in Biocompatible Ionic Liquids. Green Chem. 2010, 12, 367. [Google Scholar] [CrossRef]
- Kubisa, P. Kinetics of Radical Polymerization in Ionic Liquids. Eur. Polym. J. 2020, 133, 109778. [Google Scholar] [CrossRef]
- Nordness, O.; Brennecke, J.F. Ion Dissociation in Ionic Liquids and Ionic Liquid Solutions. Chem. Rev. 2020, 120, 12873–12902. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, Y.; Zhao, T.; Wang, H. Free Radical Polymerization of Acrylonitrile in Green Ionic Liquids. Macromol. Symp. 2004, 216, 9–16. [Google Scholar] [CrossRef]
- Low, K.; Wylie, L.; Scarborough, D.L.A.; Izgorodina, E.I. Is It Possible to Control Kinetic Rates of Radical Polymerisation in Ionic Liquids? Chem. Commun. 2018, 54, 11226–11243. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gou, S.; Wu, Q.; Yang, X.; He, Y.; Zhou, L.; Tang, L.; Liu, L.; Duan, M. Ionic Liquids as an Effective Additive for Improving the Solubility and Rheological Properties of Hydrophobic Associating Polymers. J. Mol. Liq. 2019, 296, 111833. [Google Scholar] [CrossRef]
- Eftekhari, A.; Saito, T. Synthesis and Properties of Polymerized Ionic Liquids. Eur. Polym. J. 2017, 90, 245–272. [Google Scholar] [CrossRef]
- Jiménez-Victoria, A.; Peralta-Rodríguez, R.D.; Saldívar-Guerra, E.; Cortez-Mazatán, G.Y.; Soriano-Melgar, L.d.A.A.; Guerrero-Sánchez, C. Emulsion Polymerization Using an Amphiphilic Oligoether Ionic Liquid as a Surfactant. Polymers 2022, 14, 3475. [Google Scholar] [CrossRef] [PubMed]
- Salas, R.; Villa, R.; Velasco, F.; Cirujano, F.G.; Nieto, S.; Martin, N.; Garcia-Verdugo, E.; Dupont, J.; Lozano, P. Ionic Liquids in Polymer Technology. Green Chem. 2025, 27, 1620–1651. [Google Scholar] [CrossRef]
- Durga, G.; Kalra, P.; Kumar Verma, V.; Wangdi, K.; Mishra, A. Ionic Liquids: From a Solvent for Polymeric Reactions to the Monomers for Poly(Ionic Liquids). J. Mol. Liq. 2021, 335, 116540. [Google Scholar] [CrossRef]
- Wu, C.; Dong, H.; Su, G.; Yang, H.; Yu, X. The Effect of the Structure of Functional Monomers on the Resistance of Copolymers to Fe2+ and S2-. J. Pet. Sci. Eng. 2022, 212, 110309. [Google Scholar] [CrossRef]
- Huo, J.; Peng, Z.; Ye, Z.; Feng, Q.; Zheng, Y.; Zhang, J.; Liu, X. Preparation, Characterization, and Investigation of Poly(AMPS/AM/SSS) on Application Performance of Water-based Drilling Fluid. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Acebo, C.; Fernández-Francos, X.; de la Flor, S.; Ramis, X.; Serra, À. New Anhydride/Epoxy Thermosets Based on Diglycidyl Ether of Bisphenol A and 10-Undecenoyl Modified Poly(Ethyleneimine) with Improved Impact Resistance. Prog. Org. Coat. 2015, 85, 52–59. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, H.; Huang, F.; Li, X.; He, S.; Zhao, C. Template Polymerization of a Novel Cationic Polyacrylamide: Sequence Distribution, Characterization, and Flocculation Performance. Ind. Eng. Chem. Res. 2016, 55, 9819–9828. [Google Scholar] [CrossRef]
- Narupai, B.; Willenbacher, J.; Bates, M.W.; Barbon, S.M.; Zerdan, R.B.; McGrath, A.J.; Lee, I.; Anastasaki, A.; Discekici, E.H.; Laitar, D.S.; et al. Low-Temperature, Rapid Copolymerization of Acrylic Acid and Sodium Acrylate in Water. J. Polym. Sci. A Polym. Chem. 2019, 57, 1414–1419. [Google Scholar] [CrossRef]
- Sepehrianazar, A.; Güven, O. Synthesis and Characterization of (Allylamine Hydrochloride-Vinyl Sulfonic Acid) Copolymer and Determination of Monomer Reactivity Ratios. J. Polym. Res. 2022, 29, 330. [Google Scholar] [CrossRef]
- Selen, F.; Can, V.; Temel, G. Preparation of Photodegradable Polyacrylamide Hydrogels via Micellar Copolymerization and Determination of Their Phototunable Elasticity and Swelling Behaviors. RSC Adv. 2016, 6, 31692–31697. [Google Scholar] [CrossRef]
- Peng, C.; Gou, S.; Wu, Q.; Zhou, L.; Zhang, H.; Fei, Y. Modified Acrylamide Copolymers Based on β-Cyclodextrin and Twin-Tail Structures for Enhanced Oil Recovery through Host–Guest Interactions. New J. Chem. 2019, 43, 5363–5373. [Google Scholar] [CrossRef]
- Chen, T.-L.; Lathrop, P.M.; Sun, R.; Elabd, Y.A. Lithium-Ion Transport in Poly (Ionic Liquid) Diblock Copolymer Electrolytes: Impact of Salt Concentration and Cation and Anion Chemistry. Macromolecules 2021, 54, 8780–8797. [Google Scholar] [CrossRef]






| P(AM-SSS) (ILs)/P(AM-SSS) (H2O) | P(AM-UA) (ILs)/P(AM-UA) (H2O) | Monomer Feed Ratio | ||
|---|---|---|---|---|
| AM | SSS | AM | UA | |
| 0.0456 | 0.0051 | 0.0468 | 0.0052 | 9:1 |
| 0.0349 | 0.0087 | 0.0366 | 0.0091 | 8:2 |
| 0.0269 | 0.0115 | 0.0286 | 0.0122 | 7:3 |
| 0.0206 | 0.0137 | 0.0221 | 0.0147 | 6:4 |
| 0.0155 | 0.0155 | 0.0168 | 0.0168 | 5:5 |
| Copolymer | Reaction Medium | C, wt% | N, wt% | O, wt% | H, wt% | S, wt% |
|---|---|---|---|---|---|---|
| P(AM-SSS) | H2O | 44.502 | 7.876 | 21.063 | 4.691 | 8.065 |
| ILS | 40.601 | 7.796 | 19.161 | 4.314 | 6.697 | |
| P(AM-UA) | H2O | 47.775 | 18.282 | 21.079 | 6.355 | / |
| ILS | 48.500 | 18.108 | 21.101 | 6.561 | / |
| Category | Method | P(AM-SSS) | P(AM-UA) | ||
|---|---|---|---|---|---|
| r1 | r2 | r1 | r2 | ||
| H2O | F-RA | 0.2137 | 2.4645 | 25.2373 | 0.0643 |
| M-L | 0.2109 | 2.3988 | 25.9375 | 0.0699 | |
| Average value | 0.2123 | 2.4316 | 25.5874 | 0.0671 | |
| ILs | F-RA | 0.2668 | 0.1938 | 10.0330 | 0.0779 |
| M-L | 0.2837 | 0.1895 | 9.9932 | 0.0733 | |
| Average value | 0.2752 | 0.1916 | 10.0131 | 0.0756 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, G.; Cui, J.; Li, C.; Chen, P.; Li, Y.; Jiang, W.; Yang, H.; Yu, X.; Wang, L. Copolymerization Behavior of Acrylamide-Based Polymers in Ionic Liquid Media. Polymers 2025, 17, 1963. https://doi.org/10.3390/polym17141963
Su G, Cui J, Li C, Chen P, Li Y, Jiang W, Yang H, Yu X, Wang L. Copolymerization Behavior of Acrylamide-Based Polymers in Ionic Liquid Media. Polymers. 2025; 17(14):1963. https://doi.org/10.3390/polym17141963
Chicago/Turabian StyleSu, Gaoshen, Jingyi Cui, Chaoyang Li, Ping Chen, Yong Li, Wenxue Jiang, Huan Yang, Xiaorong Yu, and Liangliang Wang. 2025. "Copolymerization Behavior of Acrylamide-Based Polymers in Ionic Liquid Media" Polymers 17, no. 14: 1963. https://doi.org/10.3390/polym17141963
APA StyleSu, G., Cui, J., Li, C., Chen, P., Li, Y., Jiang, W., Yang, H., Yu, X., & Wang, L. (2025). Copolymerization Behavior of Acrylamide-Based Polymers in Ionic Liquid Media. Polymers, 17(14), 1963. https://doi.org/10.3390/polym17141963







