Cellulose Nanofibril-Based Biodegradable Polymers from Maize Husk: A Review of Extraction, Properties, and Applications
Abstract
1. Introduction
2. Maize Husk as a Renewable Source of Cellulose
3. Extraction Methods for CNF from Lignocellulosic Biomasses
| Extraction: Source and Cellulose | Method | Results | References |
|---|---|---|---|
| Cellulose fibres from the maize tassel | Alkali | Cellulose content increased from 41% to 56%, following alkali treatment. | [25] |
| Cellulose nano-whiskers (CNWs) from maize stalk | Chemical acid hydrolysis | CNWs have diameters between 3 and 7 nm, length between 150 and 450 nm. | [29] |
| CNCs from corn (Zea mays) | Alkali-treated, bleached, acid hydrolysis (sulfuric acid) | Increased Young’s modulus of natural rubber composites from 0.89 ± 0.15 MPa to 1.98 ± 0.73 MPa with the addition of 2 wt% CNCs. CNCs length of 940 ± 70 nm, width of 6 ± 2 nm, high aspect ratio of 157. | [26] |
| CNCs from corncobs | Chemical acid hydrolysis | Higher crystallinity (79%), Optimum yield of 81% at a temperature of 30 °C, 30.13 min reaction time, and 46 wt% sulfuric acid concentration. | [24] |
| CNCs from corncobs | Liquid hot water, Alkali treatment (temperature (150–200 °C), time (10–60 min), (3–10% w/w) NaOH (2 wt%) at 90 °C for 90 min | Yield of 56% at 200 °C, 10% w/w, and 60 min, surface morphology showed a more porous and rougher surface, and the crystallinity index of 57%. | [28] |
| α-cellulose from maize (Zea mays L.) husk | Chemical acid hydrolysis | 98% α-cellulose, 0.19% β-cellulose, and 1.86% γ-cellulose, 41% carbon, 3% hydrogen, 0.7% nitrogen, 0.07% sulphur, and 55.28% oxygen. | [27] |
4. Properties of Maize Husk-Derived CNF
| Cellulose | Source | Method of Extraction | Crystallinity (%) | Diameter (μm) | Length (nm) | Morphology | Reference |
|---|---|---|---|---|---|---|---|
| CNC | Maize husk | Alkali-treated, bleached, and hydrolysed to CNCs using sulfuric acid. | 940 ± 70 | [26] | |||
| CNC | Apple pomace | Alkaline NaOH (6–12%), (30–90 °C), (30–240 min) Response surface methodology (RSM) Acid hydrolysis Ultrasonication treatments | 78 | 0.0079 | 28 ± 2.03 | needle-like structure | [32] |
| CNC | Corncob | Acid hydrolysis Delignification (alkali and bleaching pretreatment) | 79 | needle-like | [24] | ||
| Cellulose acetate | Cajuput twigs, sugarcane bagasse | Pre-hydrolysis (NaOH) Pulping and elemental chlorine-free bleaching, iodine (I) as a catalyst | 76 | 30 | [34] | ||
| Cellulose nano-whiskers Cellulose nano-whiskers | Maize | Cutting mill (mechanical) Chemical extraction Bleaching | 0.007 | 50–450 | [29] | ||
| Cellulose | Sugarcane bagasse | Alkaline curing, (0.5, 1.5, 2.75, and 4%) NaOH, 120 °C, (15, 30, and 45 min) Optimum conditions: 2.75% NaOH, 120 °C, 45 min | 32 | 13 | [35] |
5. Processing of CNF-Reinforced Biodegradable Polymer Composites
5.1. Polymer Selection and Matrix–Filler Interactions
5.2. Surface Modification Strategies
5.3. Processing Techniques and Dispersion Quality
5.4. Composite Property Enhancements
5.5. Biodegradability and Environmental Impact
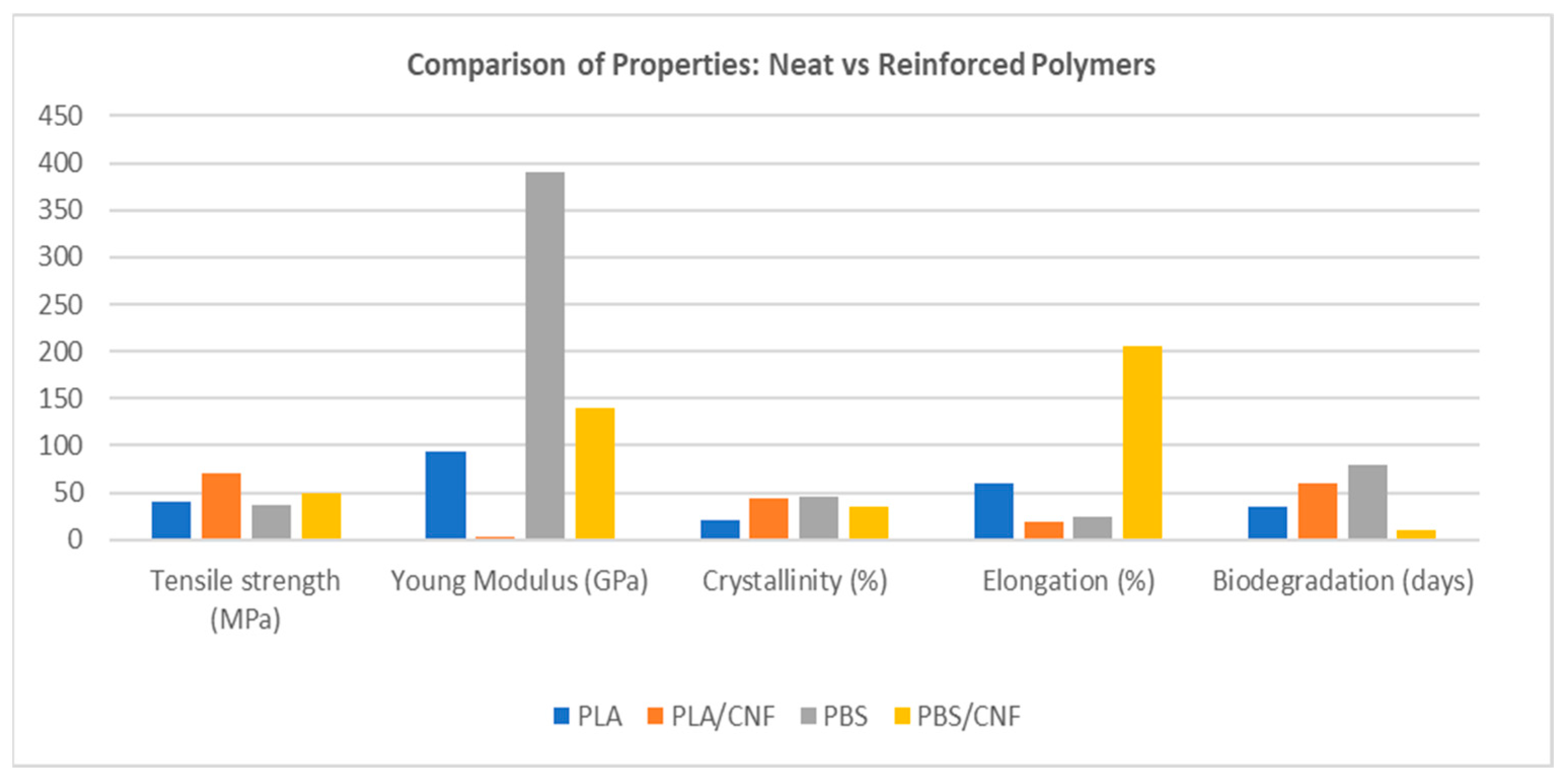
6. Cellulose Nanofibrils (CNFs) as Reinforcements for Biodegradable Polymers
7. Applications of CNF-Based Biocomposites in the South African Context
7.1. Packaging Sector and Plastic Waste Management
7.2. Agricultural Films and Biodegradable Mulch
7.3. Biomedical and Health Applications
7.4. Public Sector Procurement and Green Innovation
7.5. Challenges and Future Opportunities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNW | Cellulose nano-whiskers |
| EPR | Extended Producer Responsibility |
| LCA | Lifecycle assessment |
| PBS | Polybutylene succinate |
| PCL | Polycaprolactone |
| PLA | Polylactic acid |
| UV | Ultraviolet |
References
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.A.T. Environmental Impact of Bioplastic Use: A Review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Veerendra, G.T.N.; Babu, P.S.S.A.; Manoj, A.V.P.; Nagarjuna, K. Degradation of Plastics Waste and Its Effects on Biological Ecosystems: A Scientific Analysis and Comprehensive Review. Biomed. Mater. Devices 2024, 2, 70–112. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, I.; Khan, M.Q.S.; Jabeen, G.; Jabeen, H.S.; Işık, C. Household’s Perception-Based Factors Influencing Biogas Adoption: Innovation Diffusion Framework. Energy 2023, 263, 126155. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of Biodegradable Plastics: New Problem or Solution to Solve the Global Plastic Pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S. An Overview of Biopolymer-Derived Packaging Material. Polym. Renew. Resour. 2024, 15, 193–209. [Google Scholar] [CrossRef]
- Dutta, D.; Sit, N. A Comprehensive Review on Types and Properties of Biopolymers as Sustainable Bio-based Alternatives for Packaging. Food Biomacromol. 2024, 1, 58–87. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Antony Jose, S.; Cowan, N.; Davidson, M.; Godina, G.; Smith, I.; Xin, J.; Menezes, P.L. A Comprehensive Review on Cellulose Nanofibers, Nanomaterials, and Composites: Manufacturing, Properties, and Applications. Nanomaterials 2025, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.M.; Heo, Y.; Do, K.; Ghosh, M.; Son, Y.-O. Nanocellulose Derived from Agricultural Biowaste By-Products–Sustainable Synthesis, Biocompatibility, Biomedical Applications, and Future Perspectives: A Review. Carbohydr. Polym. Technol. Appl. 2024, 8, 100529. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, K.H.; Parvez, M.M.H.; Irizarry, N.; Uddin, M.N. Polymer Nanocomposites with Optimized Nanoparticle Dispersion and Enhanced Functionalities for Industrial Applications. Processes 2025, 13, 994. [Google Scholar] [CrossRef]
- Ratna, A.S.; Ghosh, A.; Mukhopadhyay, S. Advances and Prospects of Corn Husk as a Sustainable Material in Composites and Other Technical Applications. J. Clean. Prod. 2022, 371, 133563. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Vazvani, M.G.; Hassanisaadi, M.; Thakur, V.K. Agricultural Wastes: A Practical and Potential Source for the Isolation and Preparation of Cellulose and Application in Agriculture and Different Industries. Ind. Crops Prod. 2024, 208, 117904. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Kumar, V.V.; Elfaghi, A.M.; Ilyas, R.A.; Sapuan, S.M. Corn: Its Structure, Polymer, Fiber, Composite, Properties, and Applications. Polymers 2022, 14, 4396. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.K.; Gheshlagh, F.G.; Moezzi, M. Extraction and Characterization of Cellulose Microfibers from Cornhusk for Application as Reinforcing Agent in Biocomposite. Int. J. Biol. Macromol. 2024, 264, 130669. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Z.; Dou, J.; Liu, G.; Li, X.; Zhao, J. Structural Characterization of Corn Fiber Hemicelluloses Extracted by Organic Solvent and Screening of Degradation Enzymes. Carbohydr. Polym. 2023, 313, 120820. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, M.S.; Mondal, M.I.H. Synthesis of Highly Substituted Carboxymethyl Cellulose Depending on Cellulose Particle Size. Int. J. Biol. Macromol. 2015, 80, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Bakari, R.; Asha, R.; Hossein, M.; Huang, X.; Islam, N.F.; Liew, R.K.; Narayan, M.; Lam, S.S.; Sarma, H. Converting Food Waste to Biofuel: A Sustainable Energy Solution for Sub-Saharan Africa. Sustain. Chem. Environ. 2024, 7, 100126. [Google Scholar] [CrossRef]
- Mandree, P.; Thopil, G.A.; Ramchuran, S. Potential Opportunities to Convert Waste to Bio-Based Chemicals at an Industrial Scale in South Africa. Fermentation 2023, 9, 908. [Google Scholar] [CrossRef]
- Menon, M.P.; Selvakumar, R.; Suresh, P.; Ramakrishna, S. Extraction and Modification of Cellulose Nanofibers Derived from Biomass for Environmental Application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef]
- Cindradewi, A.W.; Bandi, R.; Park, C.W.; Park, J.S.; Lee, E.A.; Kim, J.K.; Kwon, G.J.; Han, S.Y.; Lee, S.H. Preparation and Characterization of Polybutylene Succinate Reinforced with Pure Cellulose Nanofibril and Lignocellulose Nanofibril Using Two-Step Process. Polymers 2021, 13, 3945. [Google Scholar] [CrossRef] [PubMed]
- Sadare, O.O.; Mabunda, N.; Ikegwu, U.M.; Keitemoge, M.K.; Daramola, M.O.; Moothi, K. Parametric Optimization of the Production of Cellulose Nanocrystals (CNCs) from South African Corncobs via an Empirical Modelling Approach. Sci. Rep. 2022, 12, 18665. [Google Scholar] [CrossRef] [PubMed]
- Maepa, C.E.; Jayaramudu, J.; Okonkwo, J.O.; Ray, S.S.; Sadiku, E.R.; Ramontja, J. Extraction and Characterization of Natural Cellulose Fibers from Maize Tassel. Int. J. Polym. Anal. Charact. 2015, 20, 99–109. [Google Scholar] [CrossRef]
- Smyth, M.; García, A.; Rader, C.; Foster, E.J.; Bras, J. Extraction and Process Analysis of High Aspect Ratio Cellulose Nanocrystals from Corn (Zea Mays) Agricultural Residue. Ind. Crops Prod. 2017, 108, 257–266. [Google Scholar] [CrossRef]
- James, A.; Sekomeng Johannes, M.; Faks Fanyana, M.; Ojo, F.; Joseph Bamidele, O. Extraction and characterization of α-cellulose-rich residue from maize (Zea mays L.) husk. Cellulose 2023, 57, 14. [Google Scholar]
- Olawuni, O.A.; Sadare, O.O.; Moothi, K. Optimization of Liquid Hot Water Pretreatment for Extraction of Nanocellulose Crystal from South African Waste Corncobs. Chem. Eng. Commun. 2024, 211, 26–39. [Google Scholar] [CrossRef]
- Motaung, T.E.; Mtibe, A. Alkali Treatment and Cellulose Nanowhiskers Extracted from Maize Stalk Residues. Mater. Sci. Appl. 2015, 06, 1022–1032. [Google Scholar] [CrossRef]
- Ngo, A.T.; Mori, Y.; Bui, L.T. Effects of Cellulose Nanofibers on Soil Water Retention and Aggregate Stability. Env. Technol. Innov. 2024, 35, 103650. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.; Chen, X.; Zhang, Y.; Yang, H.; Li, Q.; Jiang, J. Isolation and Characteristics of Nanocellulose from Hardwood Pulp via Phytic Acid Pretreatment. Ind. Crops Prod. 2022, 182, 114921. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum Alkaline Treatment Parameters for the Extraction of Cellulose and Production of Cellulose Nanocrystals from Apple Pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Azubuike, C.P.; Mgboko, M.S.; Ologunagba, M.O.; Oseni, B.A.; Madu, S.J.; Igwilo, C.I. Preparation and Characterization of Corn Cob Cellulose Acetate for Potential Industrial Applications. Niger. J. Pharm. 2023, 57, 709–718. [Google Scholar] [CrossRef]
- Maryana, R.; Yanto, M.; Triwahyuni, E.; Oktaviani, O.; Prasetia, H.; Das, A.K.; Sudiyani, Y. Extraction of Cellulose Acetate from Cajuput (Melaleuca leucadendron) Twigs and Sugarcane (Saccharum officinarum) Bagasse by Environmentally Friendly Approach. Waste Biomass Valorization 2022, 13, 1535–1545. [Google Scholar] [CrossRef]
- Melesse, G.T.; Hone, F.G.; Mekonnen, M.A. Extraction of Cellulose from Sugarcane Bagasse Optimization and Characterization. Adv. Mater. Sci. Eng. 2022, 2022, 1712207. [Google Scholar] [CrossRef]
- Agbakoba, V.C.; Mokhena, T.C.; Ferg, E.E.; Hlangothi, S.P.; John, M.J. PLA Bio-Nanocomposites Reinforced with Cellulose Nanofibrils (CNFs) for 3D Printing Applications. Cellulose 2023, 30, 11537–11559. [Google Scholar] [CrossRef]
- Baraka, F.; Robles, E.; Labidi, J. Microwave-Assisted Esterification of Bleached and Unbleached Cellulose Nanofibers. Ind. Crops Prod. 2023, 191, 115970. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Si, J.; Cui, Z.; Peng, K. Morphological, Mechanical and Thermal Properties of Poly(Lactic Acid) (PLA)/Cellulose Nanofibrils (CNF) Composites Nanofiber for Tissue Engineering. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 207–215. [Google Scholar] [CrossRef]
- Islam, M.M.; Islam, M.A.; Rahman, A.N.M.M.; Xuepeng, Z.; Li, J.; Liu, L. Development of Hybrid Nanocellulose-Reinforced PLA Biocomposites from Waste Jute Bags and Ramie Fabric with Enhanced Mechanical and Thermal Properties. Biomass Convers. Biorefin. 2025, 15, 19263–19281. [Google Scholar] [CrossRef]
- Lee, S.-H.; Teramoto, Y.; Endo, T. Cellulose Nanofiber-Reinforced Polycaprolactone/Polypropylene Hybrid Nanocomposite. Compos. Part A Appl. Sci. Manuf. 2011, 42, 151–156. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-Based Hydrogels as Versatile Materials with Interesting Functional Properties for Tissue Engineering Applications. J. Mater. Chem. B. 2024, 12, 7692–7759. [Google Scholar] [CrossRef] [PubMed]
- Senkum, H.; Kelly, P.V.; Ahmad, A.A.L.; Shams Es-haghi, S.; Gramlich, W.M. Strengthening Polylactic Acid (PLA) Composites with Poly(Methyl Methacrylate)-Functionalized Cellulose Nanofibrils Created through Grafting-through Emulsion Polymerization. RSC Appl. Polym. 2024, 2, 224–237. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, J.; Wu, Y.; Yin, D.; Chen, S.; Tu, H.; Wei, T.; Zhang, C.; Zhu, H.; Jin, H. Highly Enhanced Mechanical, Thermal, and Crystallization Performance of PLA/PBS Composite by Glass Fiber Coupling Agent Modification. Polymers 2023, 15, 3164. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.S.; Behera, D.; Nanda, D.; Das, N.; Behera, A.K. Green Chemistry Approaches in Materials Science: Physico-Mechanical Properties and Sustainable Applications of Grass Fiber-Reinforced Composites. Green Chem. 2025, 27, 2629–2660. [Google Scholar] [CrossRef]
- Sridhara, P.K.; Vilaseca, F. High Performance PA 6/Cellulose Nanocomposites in the Interest of Industrial Scale Melt Processing. Polymers 2021, 13, 1495. [Google Scholar] [CrossRef] [PubMed]
- El-Sakhawy, M.; Tohamy, H.-A.S.; AbdelMohsen, M.M.; El-Missiry, M. Biodegradable Carboxymethyl Cellulose Based Material for Sustainable/Active Food Packaging Application. J. Thermoplast. Compos. Mater. 2024, 37, 2035–2050. [Google Scholar] [CrossRef]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Kumar, L.; Gaikwad, K.K. Halloysite Nanotubes for Food Packaging Application: A Review. Appl. Clay. Sci. 2023, 234, 106856. [Google Scholar] [CrossRef]
- Nonkrathok, W.; Trongsatitkul, T.; Suppakarn, N. Role of Maleic Anhydride-Grafted Poly(Lactic Acid) in Improving Shape Memory Properties of Thermoresponsive Poly(Ethylene Glycol) and Poly(Lactic Acid) Blends. Polymers 2022, 14, 3923. [Google Scholar] [CrossRef]
- Shi, K.; Liu, G.; Sun, H.; Yang, B.; Weng, Y. Effect of Biomass as Nucleating Agents on Crystallization Behavior of Polylactic Acid. Polymers 2022, 14, 4305. [Google Scholar] [CrossRef] [PubMed]
- Taleb, K.; Saidi-Besbes, S.; Pillin, I.; Grohens, Y. Biodegradable Poly(Butylene Succinate) Nanocomposites Based on Dimeric Surfactant Organomodified Clays with Enhanced Water Vapor Barrier and Mechanical Properties. ACS Omega 2022, 7, 43254–43264. [Google Scholar] [CrossRef] [PubMed]
- Kimiaei, E.; Kwon, S.; Meinander, K.; Österberg, M.; Lavoine, N.; Venditti, R. Biodegradation of Lignocellulose-Polyester Composite Films in Freshwater and Seawater Conditions. J. Polym. Environ. 2024, 32, 5560–5575. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, Z.; Xie, W.; Wu, D. Cellulose Nanofibers Reinforced Biodegradable Polyester Blends: Ternary Biocomposites with Balanced Mechanical Properties. Carbohydr. Polym. 2020, 233, 115845. [Google Scholar] [CrossRef] [PubMed]
- Palacios, H.H.; Urena-Saborio, H.; Zurita, F.; Guerrero de León, A.A.; Sundaram, G.; Sulbarán-Rangel, B. Nanocellulose and Polycaprolactone Nanospun Composite Membranes and Their Potential for the Removal of Pollutants from Water. Molecules 2020, 25, 683. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Tajvidi, M. Cellulose Nanofibrils Dewatered with Poly(Lactic Acid) for Improved Bio-Polymer Nanocomposite Processing. Nanomaterials 2024, 14, 1419. [Google Scholar] [CrossRef] [PubMed]
- Wagh, S.S.; Shelake, H.D.; Chougale, A.S.; Topare, N.S.; Gunnasegaran, P.; Syed, A. Nanoparticles and Nanofillers: A Promising Future Drug Delivery Industry. In Handbook of Nanofillers; Springer Nature: Singapore, 2024; pp. 1–28. [Google Scholar]
- Shazleen, S.S.; Foong Ng, L.Y.; Ibrahim, N.A.; Hassan, M.A.; Ariffin, H. Combined Effects of Cellulose Nanofiber Nucleation and Maleated Polylactic Acid Compatibilization on the Crystallization Kinetic and Mechanical Properties of Polylactic Acid Nanocomposite. Polymers 2021, 13, 3226. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, X.; Bai, Y.; Fang, Z.; Zhang, S.; Wang, X.; Yang, Y.; Guo, Y. Recent Advances in Cellulose Nanofiber Modification and Characterization and Cellulose Nanofiber-Based Films for Eco-Friendly Active Food Packaging. Foods 2024, 13, 3999. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lin, X.; Yu, M.; Mondal, A.K.; Wu, H. Recent Advances in TEMPO-Oxidized Cellulose Nanofibers: Oxidation Mechanism, Characterization, Properties and Applications. Int. J. Biol. Macromol. 2024, 259, 129081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, J.; Zhang, M.; Xu, T.; Zheng, C.; Yuan, Z.; Si, C. Cellulose-Based Aerogels, Films, and Fibers for Advanced Biomedical Applications. Chem. Eng. J. 2024, 497, 154434. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, Z.; Deng, T.; Zhang, L.; Li, C. Simultaneous Improvement of Tensile Strength and Toughness of Polylactic Acid by Incorporating a Biodegradable Core-shell Nanofiller with Double Polymer Layers. Polym. Compos. 2024, 45, 12660–12674. [Google Scholar] [CrossRef]
- Chen, H.; Chen, P.; Qi, Z.; Sun, C. Cross-Linked Structures Reinforced the Bamboo Fiber/Poly(Lactic Acid) Composites with High Heat Resistance and Their Environmental Impact through the Life Cycle Assessment Analysis. Front. Mater. 2024, 11, 1484677. [Google Scholar] [CrossRef]
- Mistry, A.N.; Kachenchart, B.; Wongthanaroj, A.; Somwangthanaroj, A.; Luepromchai, E. Rapid Biodegradation of High Molecular Weight Semi-Crystalline Polylactic Acid at Ambient Temperature via Enzymatic and Alkaline Hydrolysis by a Defined Bacterial Consortium. Polym. Degrad. Stab. 2022, 202, 110051. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical Properties of Cellulose Nanofiber (CNF) Reinforced Polylactic Acid (PLA) Prepared by Twin Screw Extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Szabo, P.; Daugaard, A.E.; Giacinti Baschetti, M. Effect of Crystallinity on Water Vapor Sorption, Diffusion, and Permeation of PLA-Based Nanocomposites. ACS Omega 2020, 5, 15362–15369. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Wu, M.; Wang, L.; Zheng, W.; Hikima, Y.; Semba, T.; Ohshima, M. Cellulose Nanofiber Reinforced Poly (Lactic Acid) with Enhanced Rheology, Crystallization and Foaming Ability. Carbohydr. Polym. 2022, 286, 119320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, B.; Jiang, N. The Mechanical Properties and Degradation Behavior of 3D-Printed Cellulose Nanofiber/Polylactic Acid Composites. Materials 2023, 16, 6197. [Google Scholar] [CrossRef] [PubMed]
- Dmitruk, A.; Ludwiczak, J.; Skwarski, M.; Makuła, P.; Kaczyński, P. Influence of PBS, PBAT and TPS Content on Tensile and Processing Properties of PLA-Based Polymeric Blends at Different Temperatures. J. Mater. Sci. 2023, 58, 1991–2004. [Google Scholar] [CrossRef]
- Deng, Y.; Thomas, N.L. Blending Poly(Butylene Succinate) with Poly(Lactic Acid): Ductility and Phase Inversion Effects. Eur. Polym. J. 2015, 71, 534–546. [Google Scholar] [CrossRef]
- Righetti, M.C.; Cinelli, P.; Aliotta, L.; Bianchi, E.; Tricoli, F.; Seggiani, M.; Lazzeri, A. Immiscible PHB/PBS and PHB/PBSA Blends: Morphology, Phase Composition and Modelling of Elastic Modulus. Polym. Int. 2022, 71, 47–56. [Google Scholar] [CrossRef]
- Hu, X.; Su, T.; Pan, W.; Li, P.; Wang, Z. Difference in Solid-State Properties and Enzymatic Degradation of Three Kinds of Poly(Butylene Succinate)/Cellulose Blends. RSC Adv. 2017, 7, 35496–35503. [Google Scholar] [CrossRef]
- Cho, H.S.; Moon, H.S.; Kim, M.; Nam, K.; Kim, J.Y. Biodegradability and Biodegradation Rate of Poly(Caprolactone)-Starch Blend and Poly(Butylene Succinate) Biodegradable Polymer under Aerobic and Anaerobic Environment. Waste Manag. 2011, 31, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yin, X.; Chen, H.; Fu, X.; Sun, Y.; Chen, Q.; Liu, W.; Shen, X. Mechanical, Crystallization, Rheological, and Supercritical CO2 Foaming Properties of Polybutylene Succinate Nanocomposites: Impact of Carbon Nanofiber Content. Polymers 2023, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- El-Hadi, A.M. Increase the Elongation at Break of Poly (Lactic Acid) Composites for Use in Food Packaging Films. Sci. Rep. 2017, 7, 46767. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Jung, S.; Yun, H.; Choi, K.; Heo, G.; Jin, H.-J.; Park, S.; Kwak, H.W. Biodegradation Behavior of Polybutylene Succinate (PBS) Fishing Gear in Marine Sedimentary Environments for Ghost Fishing Prevention. Polym. Degrad Stab. 2023, 216, 110490. [Google Scholar] [CrossRef]
- Van Os, E.; De Kock, L. Plastics: From Recycling to (Post-Consumer) Recyclate Industry Views on Barriers and Opportunities in South Africa; WWF South Africa: Cape Town, South Africa, 2021. [Google Scholar]
- Chen, S.; Wu, M.; Wang, C.; Yan, S.; Lu, P.; Wang, S. Developed Chitosan/Oregano Essential Oil Biocomposite Packaging Film Enhanced by Cellulose Nanofibril. Polymers 2020, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Baloyi, O.; Masinga, K. The New National Environmental Management: Waste Act; a Shift in Waste Management Approach in South Africa. WIT Trans. Ecol. Environ. 2010, 142, 311–322. [Google Scholar] [CrossRef]
- Chang, F.-L.; Hu, B.; Huang, W.-T.; Chen, L.; Yin, X.-C.; Cao, X.-W.; He, G.-J. Improvement of Rheology and Mechanical Properties of PLA/PBS Blends by in-Situ UV-Induced Reactive Extrusion. Polymers 2022, 259, 125336. [Google Scholar] [CrossRef]
- Borbolla-Jiménez, F.V.; Peña-Corona, S.I.; Farah, S.J.; Jiménez-Valdés, M.T.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Magaña, J.J.; Leyva-Gómez, G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics 2023, 15, 1914. [Google Scholar] [CrossRef] [PubMed]
- Darmenbayeva, A.; Zhussipnazarova, G.; Rajasekharan, R.; Massalimova, B.; Zharlykapova, R.; Nurlybayeva, A.; Mukazhanova, Z.; Aubakirova, G.; Begenova, B.; Manapova, S.; et al. Applications and Advantages of Cellulose–Chitosan Biocomposites: Sustainable Alternatives for Reducing Plastic Dependency. Polymers 2024, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Banda, F.; Robb, G.; Roberts, S. Review Paper Two: The Links Between Competition Policy, Regulatory Policy and Trade Industrial Policies; Working Paper 5/2015; University of Johannesburg, Centre of Competition Regulation and Economic Development: Johannesburg, South Africa, 2015. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motshabi, N.; Lenetha, G.G.; Malimabe, M.A.; Gumede, T.P. Cellulose Nanofibril-Based Biodegradable Polymers from Maize Husk: A Review of Extraction, Properties, and Applications. Polymers 2025, 17, 1947. https://doi.org/10.3390/polym17141947
Motshabi N, Lenetha GG, Malimabe MA, Gumede TP. Cellulose Nanofibril-Based Biodegradable Polymers from Maize Husk: A Review of Extraction, Properties, and Applications. Polymers. 2025; 17(14):1947. https://doi.org/10.3390/polym17141947
Chicago/Turabian StyleMotshabi, Nthabiseng, Gaofetoge Gobodiwang Lenetha, Moipone Alice Malimabe, and Thandi Patricia Gumede. 2025. "Cellulose Nanofibril-Based Biodegradable Polymers from Maize Husk: A Review of Extraction, Properties, and Applications" Polymers 17, no. 14: 1947. https://doi.org/10.3390/polym17141947
APA StyleMotshabi, N., Lenetha, G. G., Malimabe, M. A., & Gumede, T. P. (2025). Cellulose Nanofibril-Based Biodegradable Polymers from Maize Husk: A Review of Extraction, Properties, and Applications. Polymers, 17(14), 1947. https://doi.org/10.3390/polym17141947








