Metal–Organic Frameworks as Fillers in Porous Organic Polymer-Based Hybrid Materials: Innovations in Composition, Processing, and Applications
Abstract
1. Introduction
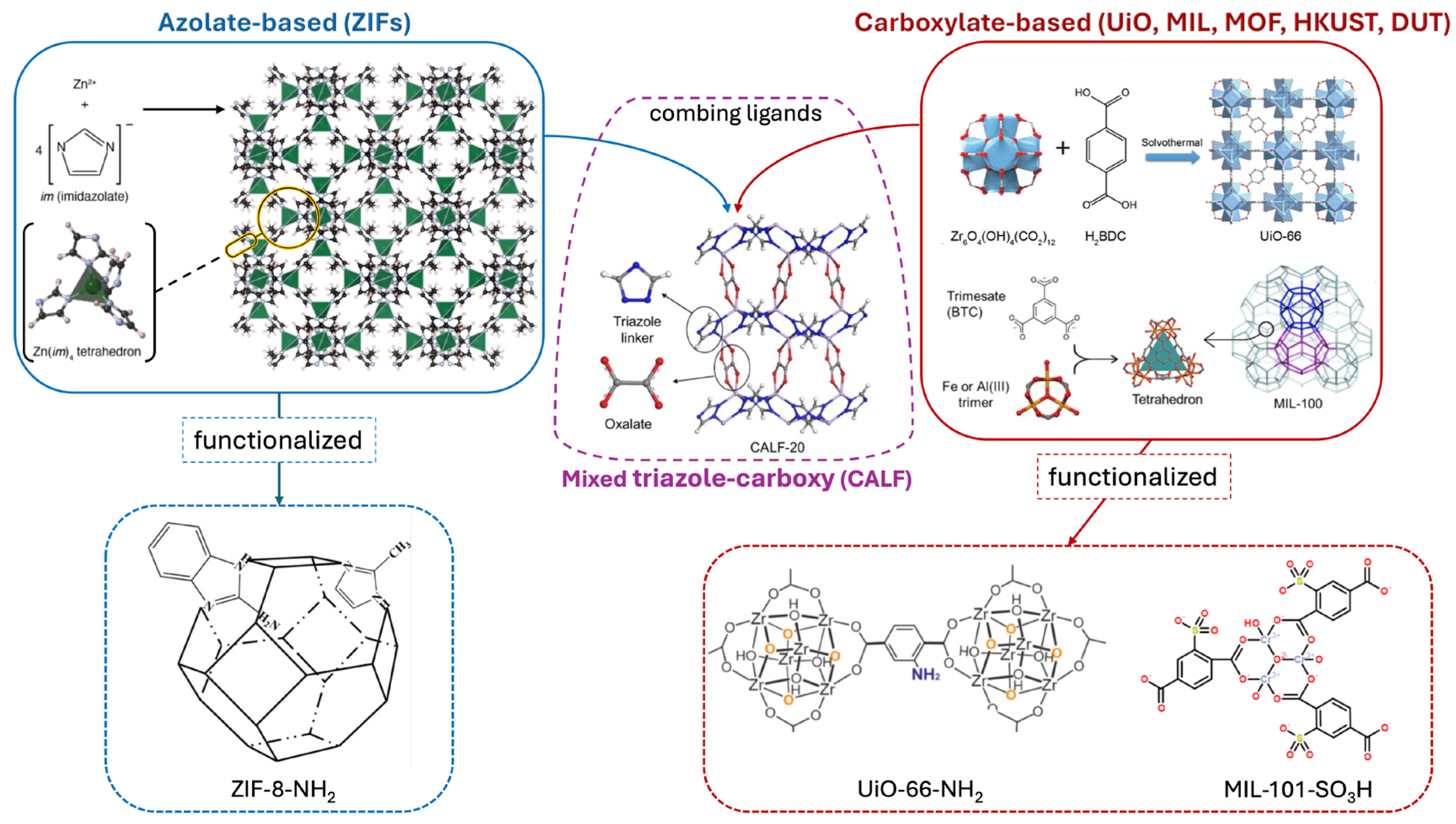
2. Diversity of MOF Structures and Their Functionalization Potential
2.1. Zeolitic Imidazolate Frameworks (ZIFs)
2.2. UiO-Series MOFs (UiO-66, UiO-67, UiO-68)
2.3. MIL-Series MOFs (MIL-101, MIL-53, MIL-100)
2.4. HKUST-1 (Cu3(BTC)2, Copper–Benzene Tricarboxylate MOF)
2.5. MOFs with Pillared-Layer Structures (MOF-5, DUT-Series)
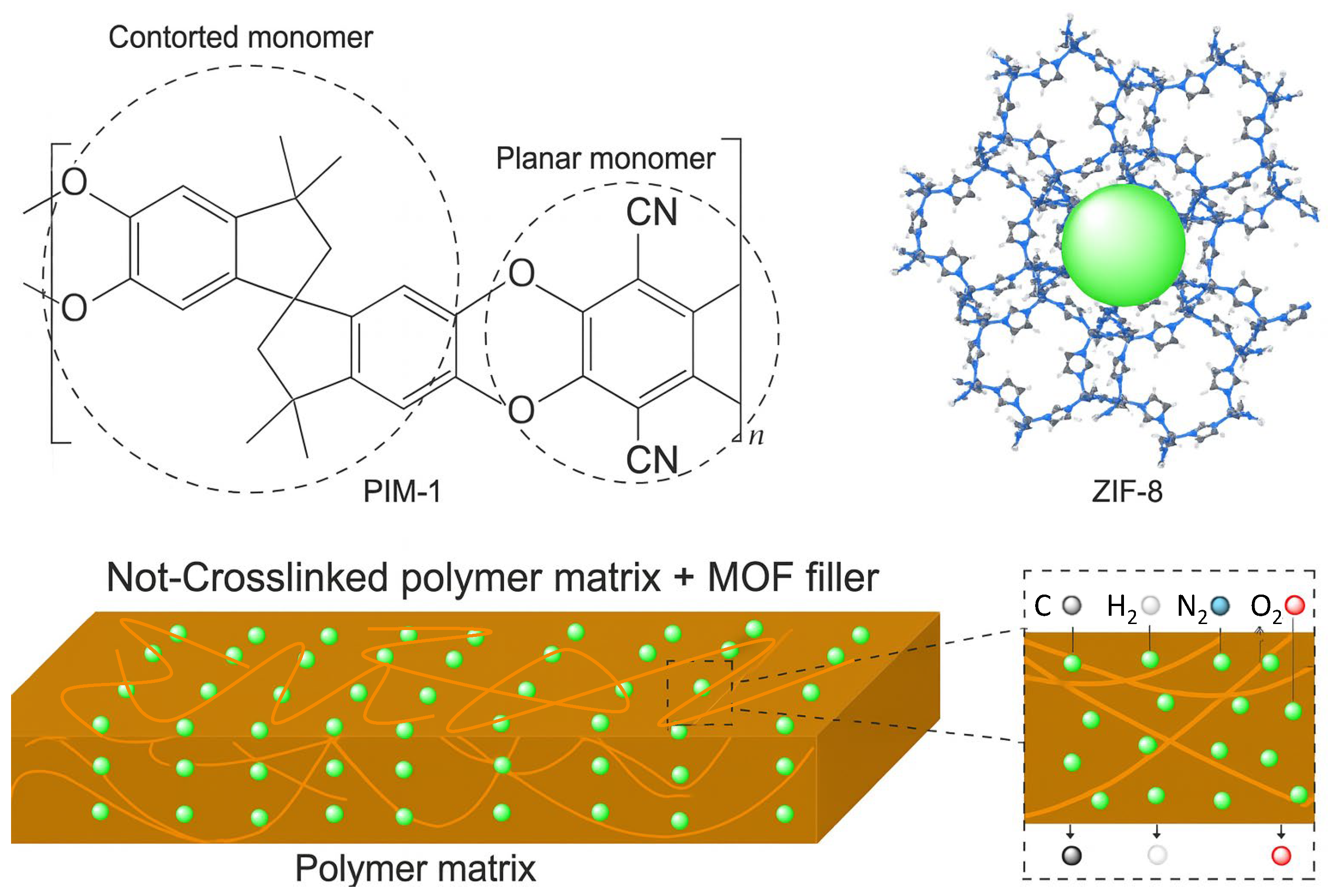
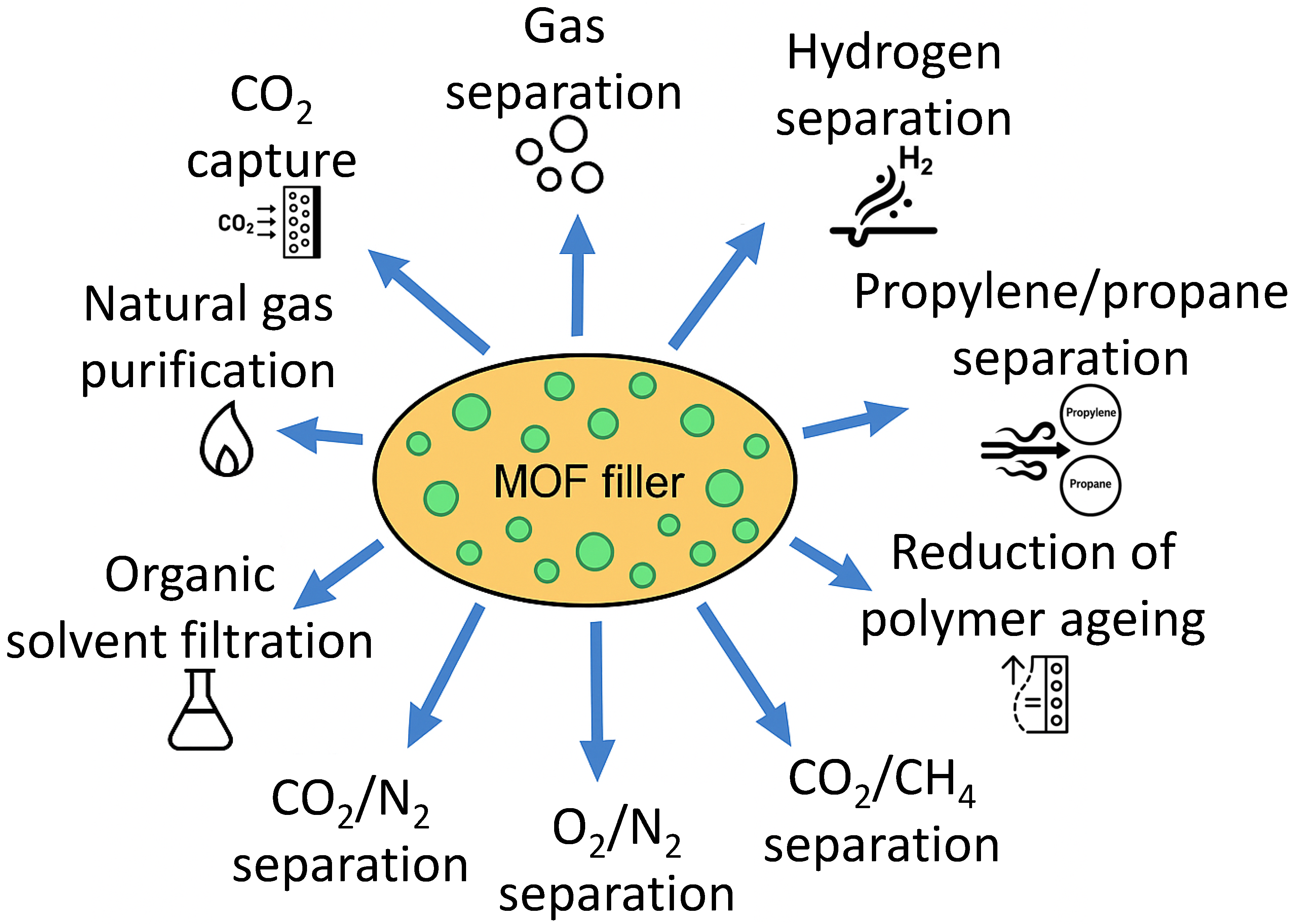
3. MOF-PIM Hybrids (MMMs)
- Solution mixing: The polymer is first dissolved in a suitable solvent, and the MOF filler is dispersed in the same solvent, often using ultrasonication to achieve a homogeneous mixture. This approach is simple and widely applied; however, it may involve risks such as partial loss of MOF crystallinity, especially under prolonged ultrasonication or in the presence of aggressive solvents, which can compromise the structural integrity of the framework and reduce its adsorption performance. Similarly, depending on the polymer chemistry, some POPs may undergo chemical degradation or swelling during extended exposure to polar solvents or elevated temperatures used in casting [127,128].
- In situ method: This involves mixing the filler (typically pre-formed MOFs) with monomers prior to polymerization, allowing the filler to become embedded within the growing polymer network. A variation of this approach is in situ MOF growth, where MOF precursors are added to a pre-formed polymer solution, and crystallization is induced within the polymer matrix. This technique often enhances MOF dispersion and interfacial compatibility but requires precise control of synthesis conditions to avoid defective MOF growth or unintended interactions with functional groups in POPs, which might limit polymer performance or porosity development. In some cases, acidic or basic by-products generated during MOF crystallization can disrupt the polymer structure, especially in POPs containing hydrolytically sensitive linkages such as imines or boronate esters. Moreover, the thermal and chemical conditions required for successful MOF nucleation (such as solvothermal treatment or the presence of modulators) may exceed the stability window of certain polymers, resulting in chain scission, crosslinking, or partial collapse of the porous architecture. Therefore, careful optimization of the precursor ratios, reaction time, and solvent environment is essential to preserve both the structural integrity of the MOF and the intrinsic microporosity of the polymer phase [124,127,128].
Applications of MOF-PIM MMMs
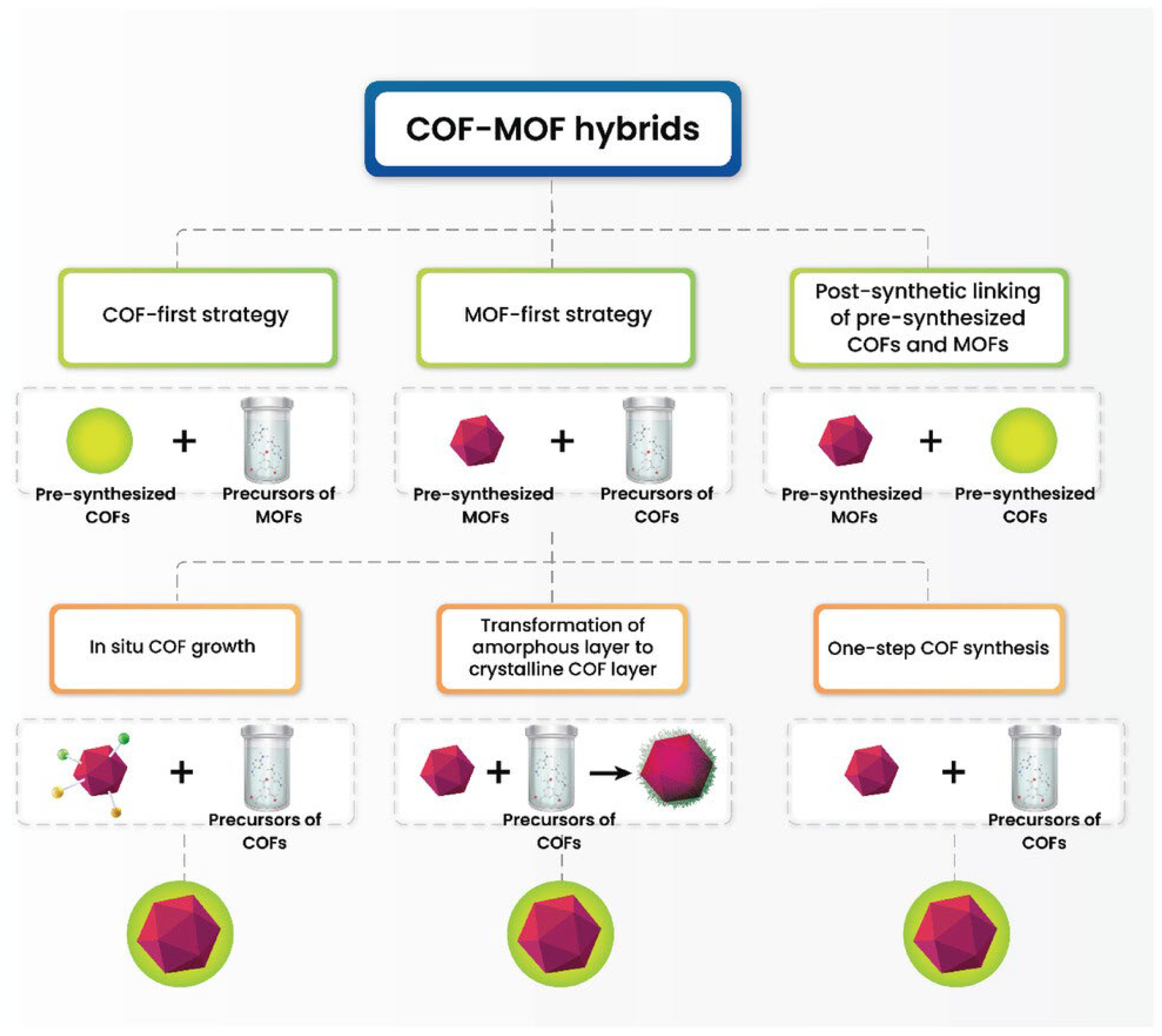
4. MOF-COF Hybrid Materials
4.1. Type of MOF/COF Hybrids
4.1.1. Strategies for the Synthesis of MOF-COF Hybrid Materials
4.1.2. MOF-First Strategy
4.1.3. COF-First Strategy
4.1.4. Post-Synthetic Linking of Pre-Synthesized COFs and MOFs
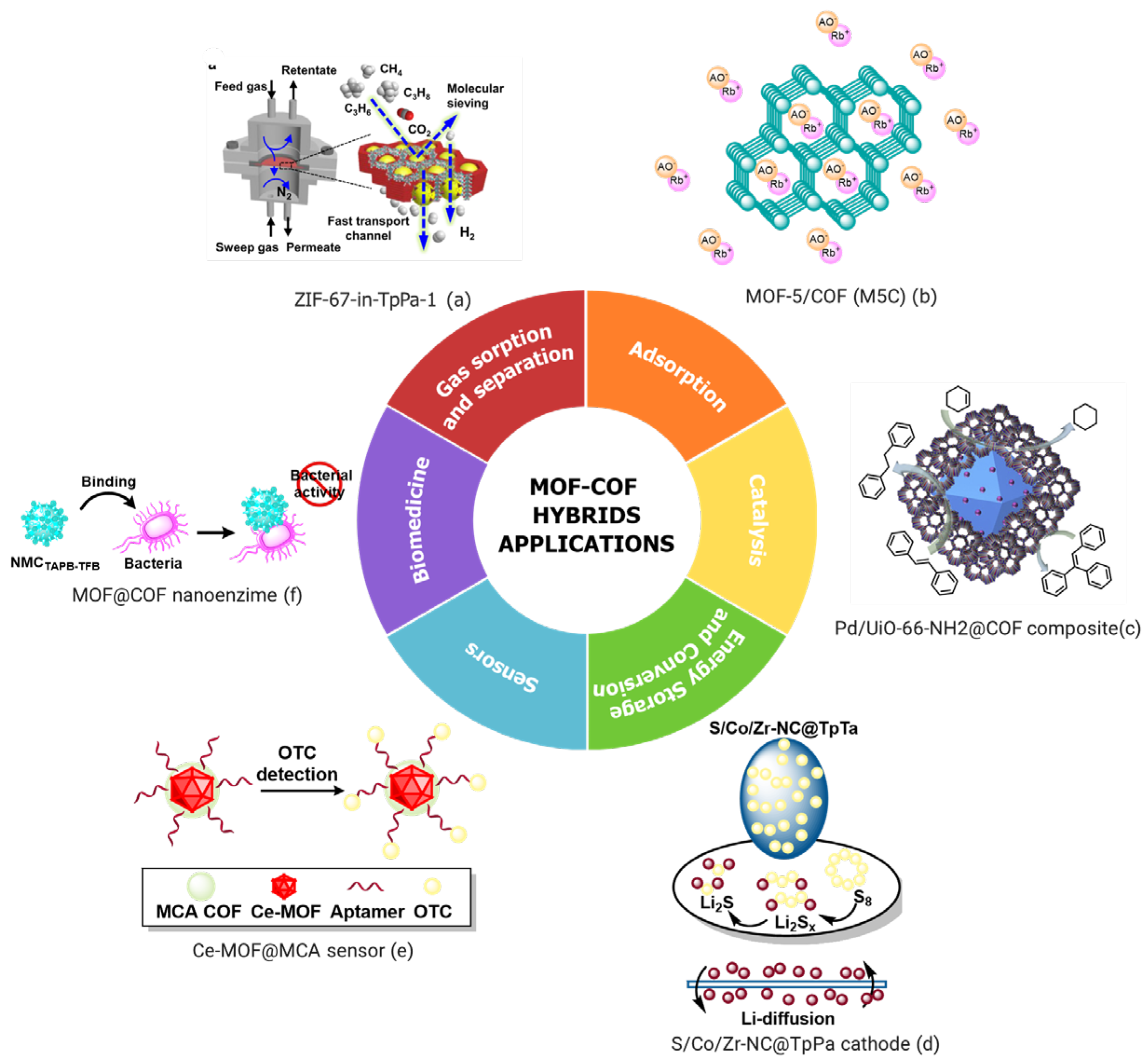
4.2. Applications of MOF/COF Hybrids
4.2.1. Sensors
4.2.2. Catalysis
4.2.3. Gas Sorption and Separation
4.2.4. Adsorption and Removal of Pollutants
4.2.5. Energy Storage and Conversion
4.2.6. Biomedical Applications
5. MOF/HCP, MOF/CTF, and MOF/CMP Hybrid Materials
- MOF/HCP hybrids:
- 2.
- MOF/CTF hybrids:
- 3.
- MOF/CMP hybrids:
6. Perspectives on MOF/PIM, MOF/COF, MOF/HCP, MOF/CTF, and MOF/CMP Hybrids
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MOF | Metal–organic framework |
| POP | Porous organic polymer |
| COF | Covalent organic Framework |
| MMMs | Mixed-matrix membranes |
| ZIF | Zeolitic imidazole framework |
| HCP | Hyper-crosslinked polymers |
| CMP | Conjugated microporous polymers |
| SBET | Specific surface area (Brunauer–Emmett–Teller) |
| FFV | Free fraction volume |
| UiO | Universitetet i Oslo |
| MIL | Materials Institute Lavoisier |
| HKUST | Hong Kong University of Science and Technology |
| DUT | Dresden University of Technology |
| PSM | Post-synthetic modification |
| BDC | Benzene dicarboxylate |
| BPDC | Biphenyl dicarboxylate |
| BTC | Benzene tricarboxylate |
| TPDC | Terphenyl dicarboxylate |
| MTN | Mobil Thirty-Nine, related to the structure of the zeolite ZSM-39 |
| VOC | Volatile organic compound |
| NGA | Negative gas adsorption |
References
- Kitagawa, S.; Uemura, K. Dynamic Porous Properties of Coordination Polymers Inspired by Hydrogen Bonds. Chem. Soc. Rev. 2005, 34, 109–119. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kawata, S. Coordination Compounds of 1,4-Dihydroxybenzoquinone and Its Homologues. Structures and Properties. Coord. Chem. Rev. 2002, 224, 11–34. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Henke, S. Metal-Organic Frameworks with Additional Flexible Substituents-Modulating Responsiveness, Gas Sorption Selectivity & Network Topologies. Ph.D. Thesis, Technical University Dortmund, Dortmund, Germany, 2011. [Google Scholar]
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Chizallet, C.; Quoineaud, A.A.; Pirngruber, G.D. Comparison of the Behavior of Metal-Organic Frameworks and Zeolites for Hydrocarbon Separations. J. Am. Chem. Soc. 2012, 134, 8115–8126. [Google Scholar] [CrossRef] [PubMed]
- Malekshah, R.E.; Moharramnejad, M.; Gharanli, S.; Shahi, M.; Ehsani, A.; Haribabu, J.; Ouachtak, H.; Mirtamizdoust, B.; Kamwilaisak, K.; Sillanpää, M.; et al. MOFs as Versatile Catalysts: Synthesis Strategies and Applications in Value-Added Compound Production. ACS Omega 2023, 8, 31600–31619. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. Rational Design of MOF-Based Materials for Next-Generation Rechargeable Batteries. Nano-Micro Lett. 2021, 13, 203. [Google Scholar] [CrossRef]
- Zuliani, A.; Khiar, N.; Carrillo-Carrión, C. Recent Progress of Metal–Organic Frameworks as Sensors in (Bio)Analytical Fields: Towards Real-World Applications. Anal. Bioanal. Chem. 2023, 415, 2005–2023. [Google Scholar] [CrossRef]
- Avci, C.; Imaz, I.; Carné-Sánchez, A.; Pariente, J.A.; Tasios, N.; Pérez-Carvajal, J.; Alonso, M.I.; Blanco, A.; Dijkstra, M.; López, C.; et al. Self-Assembly of Polyhedral Metal-Organic Framework Particles into Three-Dimensional Ordered Superstructures. Nat. Chem. 2018, 10, 78–84. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Duan, S.; Qian, L.; Zheng, Y.; Zhu, Y.; Liu, X.; Dong, L.; Yan, W.; Zhang, J. Mechanisms of the Accelerated Li+ Conduction in MOF-Based Solid-State Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. Adv. Mater. 2024, 36, 2314120. [Google Scholar] [CrossRef]
- Pander, M.; Gil-San-Millan, R.; Delgado, P.; Perona-Bermejo, C.; Kostrzewa, U.; Kaczkowski, K.; Kubicki, D.J.; Navarro, J.A.R.; Bury, W. MOF/Polymer Hybrids through in Situ Free Radical Polymerization in Metal-Organic Frameworks. Mater. Horiz. 2023, 10, 1301–1308. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Yuan, S.; Zhou, J.; Wang, B. Challenges and Recent Advances in MOF-Polymer Composite Membranes for Gas Separation. Inorg. Chem. Front. 2016, 3, 896–909. [Google Scholar] [CrossRef]
- Husna, A.; Hossain, I.; Jeong, I.; Kim, T.H. Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer. Polymers 2022, 14, 655. [Google Scholar] [CrossRef]
- Barcus, K.; Lin, P.A.; Zhou, Y.; Arya, G.; Cohen, S.M. Influence of Polymer Characteristics on the Self-Assembly of Polymer-Grafted Metal-Organic Framework Particles. ACS Nano 2022, 16, 18168–18177. [Google Scholar] [CrossRef]
- Uemura, T.; Uchida, N.; Higuchi, M.; Kitagawa, S. Effects of Unsaturated Metal Sites on Radical Vinyl Polymerization in Coordination Nanochannels. Macromolecules 2011, 44, 2693–2697. [Google Scholar] [CrossRef]
- Zhang, Z.; Nguyen, H.T.H.; Miller, S.A.; Cohen, S.M. PolyMOFs: A Class of Interconvertible Polymer-Metal-Organic-Framework Hybrid Materials. Angew. Chem. 2015, 127, 6250–6255. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, M.; Zhang, W.; Pearson, M.A.; Johnson, J.A. PolyMOF Nanoparticles: Dual Roles of a Multivalent PolyMOF Ligand in Size Control and Surface Functionalization. Angew. Chem. 2019, 131, 16829–16834. [Google Scholar] [CrossRef]
- Yazaki, K.; Takahashi, M.; Miyajima, N.; Obata, M. Construction of a PolyMOF Using a Polymer Ligand Bearing the Benzenedicarboxylic Acid Moiety in the Side Chain. New J. Chem. 2020, 44, 5182–5185. [Google Scholar] [CrossRef]
- Yu, S.B.; Lin, F.; Tian, J.; Yu, J.; Zhang, D.W.; Li, Z.T. Water-Soluble and Dispersible Porous Organic Polymers: Preparation, Functions and Applications. Chem. Soc. Rev. 2022, 51, 434–449. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Tran, C.H.; Choi, H.K.; Yu, D.G.; Kim, I. Porous Organic Polymers with Defined Morphologies: Synthesis, Assembly, and Emerging Applications. Prog. Polym. Sci. 2023, 142, 101691. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, Z. Covalent Organic Frameworks as Electrode Materials for Rechargeable Metal-ion Batteries. Interdiscip. Mater. 2023, 2, 231–259. [Google Scholar] [CrossRef]
- Li, Y.; Karimi, M.; Gong, Y.N.; Dai, N.; Safarifard, V.; Jiang, H.L. Integration of Metal-Organic Frameworks and Covalent Organic Frameworks: Design, Synthesis, and Applications. Matter 2021, 4, 2230–2265. [Google Scholar] [CrossRef]
- Altintas, C.; Erucar, I.; Keskin, S. MOF/COF Hybrids as next Generation Materials for Energy and Biomedical Applications. CrystEngComm 2022, 24, 7360–7371. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated Microporous Polymers: Design, Synthesis and Application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cheng, Z.; Trunk, M.; Thomas, A. Targeted Control over the Porosities and Functionalities of Conjugated Microporous Polycarbazole Networks for CO2-Selective Capture and H2 Storage. Polym. Chem. 2017, 8, 7240–7247. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Z.; Liu, C.; Song, Q.; Liu, Y.; Zhang, Z.; Han, B. Bipyridine-Based Conjugated Microporous Polymers for Boosted Photocatalytic U(VI) Separation. Chem. Commun. 2024, 60, 13348–13351. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zeng, Z.; Wang, H.; Xiong, W.; Song, B.; Zhou, C.; Duan, A.; Tan, X.; He, Q.; Zeng, G.; et al. Recent Progress in Conjugated Microporous Polymers for Clean Energy: Synthesis, Modification, Computer Simulations, and Applications. Prog. Polym. Sci. 2021, 115, 101374. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Chen, P.; Luan, X.; Dou, J.; Bai, J.; Fang, H.; Shi, W.; Zhou, B. CMP-on-MOF Bimetallic Hybrids Derived Sheet-on-Rod Heterostructure as Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Batteries. Microporous Mesoporous Mater. 2022, 331, 111639. [Google Scholar] [CrossRef]
- Moradi, M.R.; Torkashvand, A.; Ramezanipour Penchah, H.; Ghaemi, A. Amine Functionalized Benzene Based Hypercrosslinked Polymer as an Adsorbent for CO2/N2 Adsorption. Sci. Rep. 2023, 13, 9214. [Google Scholar] [CrossRef]
- Song, W.; Tang, Y.; Moon, B.Y.; Liao, Q.; Xu, H.; Hou, Q.; Zhang, H.; Yu, D.G.; Liao, Y.; Kim, I. Green Synthesis of Hypercrosslinked Polymers for CO2 Capture and Conversion: Recent Advances, Opportunities, and Challenges. Green. Chem. 2024, 26, 2476–2504. [Google Scholar] [CrossRef]
- Bauzá, M.; Palomino, G.T.; Cabello, C.P. Hypercrosslinked Polymer Derived Carbon@MIL-100 Magnetic Material for the Enhanced Extraction of Diclofenac. Separ. Purif. Technol. 2022, 303, 122211. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, Z.J.; Zhang, K.A.I. Covalent Triazine Frameworks as Emerging Heterogeneous Photocatalysts. Chem. Mater. 2021, 33, 1909–1926. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, S. Recent Advancements in the Synthesis of Covalent Triazine Frameworks for Energy and Environmental Applications. Polymers 2018, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Liu, X.; Kong, X.; Dong, F.; Yu, Y.; Wang, L.; Wang, D.; He, Z.; Song, S. Boosting Photocatalytic H2 Evolution UIO-66-NH2/Covalent Triazine-Based Frameworks Composites by Constructing a Covalent Heterojunction. Environ. Sci. Pollut. Res. 2023, 30, 111039–111050. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.H.; Huang, A.X.; Yan, X.C.; Liu, Y.H.; Wang, Y.; Jin, X.; Zhang, F.M. Constructing Tightly Integrated Conductive Metal-Organic Framework/Covalent Triazine Framework Heterostructure by Coordination Bonds for Photocatalytic Hydrogen Evolution. J. Colloid. Interface Sci. 2023, 633, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Zhang, C.; Zhang, X.; Zhang, R. Covalent Integration of Fe-Based Metal–Organic Framework and Triazine-Containing Covalent Organic Framework for Enhanced Adsorptive Removal of Antibiotics. J. Clean. Prod. 2024, 434, 140259. [Google Scholar] [CrossRef]
- Li, S.; Dai, Y.; Ye, P.; Liu, F.; Li, L.; Zhang, D.; Zhu, M. Hierarchical Porous MOF/CTF Hybrid Frameworks Used as Protection against Acidic Harmful Gases. J. Chem. Eng. 2024, 491, 152035. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Kim, J.Y.; Bae, J.H.; Nam, K.W. Imidazole Linker-Induced Covalent Triazine Framework–ZIF Hybrids for Confined Hollow Carbon Super-Heterostructures toward a Long-Life Supercapacitor. Carbon. Energy 2023, 5, e344. [Google Scholar] [CrossRef]
- Tahir, Z.; Alomar, M.; Sarfraz, M.; Waheed, A.; Ayub, H.M.U. Carbon Capturing Composite Membranes Comprising Cu-MOF and PIM-1. J. Appl. Polym. Sci. 2024, 141, e55709. [Google Scholar] [CrossRef]
- Mondal, S.K.; Aina, P.O.; Rownaghi, A.A.; Rezaei, F. Design and Development of UiO-67-Coated PIM-1-Based Composites and Demonstration of Their Detoxification Performance. J. Chem. Eng. 2024, 493, 152269. [Google Scholar] [CrossRef]
- Du, X.; Shao, D.; Wan, Y. PIM-1 Based Mixed Matrix Membranes with MOF-808(Ce) for CO2 Separations. Gas. Sci. Eng. 2024, 131, 205476. [Google Scholar] [CrossRef]
- Rong, S.; Su, P.; Chen, S.; Jia, M.; Li, W. Sub-5 Nm Porous Polymer Decoration toward Superhydrophobic MOFs with Enhanced Stability and Processability. Chin. Chem. Lett. 2022, 33, 2134–2138. [Google Scholar] [CrossRef]
- Yu, S.; Li, C.; Zhao, S.; Chai, M.; Hou, J.; Lin, R. Recent Advances in the Interfacial Engineering of MOF-Based Mixed Matrix Membranes for Gas Separation. Nanoscale 2024, 16, 7716–7733. [Google Scholar] [CrossRef] [PubMed]
- Doǧan, E.B.; Maurin, G.; Ahunbay, M.G. Atomistic Insight into the Interfacial Structuring of ZIF-67 MOF/Polymer Composites and Their Propylene-Propane Adsorption Properties. J. Phys. Chem. C 2023, 127, 20491–20502. [Google Scholar] [CrossRef]
- Fan, D.; Ozcan, A.; Shekhah, O.; Semino, R.; Eddaoudi, M.; Maurin, G. Engineering MOF Surface Defects in Mixed Matrix Membranes: An Effective Strategy to Enhance MOF/Polymer Adhesion and Control Interfacial Gas Transport. J. Membr. Sci. Lett. 2022, 2, 100029. [Google Scholar] [CrossRef]
- Jaid, G.M.; AbdulRazak, A.A.; Meskher, H.; Al-Saadi, S.; Alsalhy, Q.F. Metal-Organic Frameworks (MOFs), Covalent Organic Frameworks (COFs), and Hydrogen-Bonded Organic Frameworks (HOFs) in Mixed Matrix Membranes. Mater. Today Sustain. 2024, 25, 100672. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef]
- Pu, Y.; Yang, Z.; Wee, V.; Wu, Z.; Jiang, Z.; Zhao, D. Amino-Functionalized NUS-8 Nanosheets as Fillers in PIM-1 Mixed Matrix Membranes for CO2 Separations. J. Membr. Sci. 2022, 641, 119912. [Google Scholar] [CrossRef]
- Carja, I.D.; Tavares, S.R.; Shekhah, O.; Ozcan, A.; Semino, R.; Kale, V.S.; Eddaoudi, M.; Maurin, G. Insights into the Enhancement of MOF/Polymer Adhesion in Mixed-Matrix Membranes via Polymer Functionalization. ACS Appl. Mater. Interfaces 2021, 13, 29041–29047. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Kitagawa, H. Ion-Conductive Metal-Organic Frameworks. Dalton Trans. 2021, 50, 5385–5397. [Google Scholar] [CrossRef]
- Mínguez Espallargas, G.; Coronado, E. Magnetic Functionalities in MOFs: From the Framework to the Pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef]
- Morris, W.; Stevens, C.J.; Taylor, R.E.; Dybowski, C.; Yaghi, O.M.; Garcia-Garibay, M.A. NMR and X-Ray Study Revealing the Rigidity of Zeolitic Imidazolate Frameworks. J. Phys. Chem. C 2012, 116, 13307–13312. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic Imidazolate Framework Membrane with Molecular Sieving Properties by Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef]
- Hu, L.; Yan, Z.; Mo, X.; Peng, X.; Chen, L. Morphology Control Synthesis of ZIF-8 as Highly Efficient Catalyst for the Cycloaddition of CO2 to Cyclic Carbonate. ChemCatChem 2019, 11, 3212–3219. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cô, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Qian, J.; Sun, F.; Qin, L. Hydrothermal Synthesis of Zeolitic Imidazolate Framework-67 (ZIF-67) Nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- Zhao, P.; Fu, S.; Cheng, L.; Jiao, Z.; Wu, M. Modulating Zeolitic Imidazolate Framework-67 and Its Derivatives as Advanced Oxygen Evolution Reaction Electrocatalysts. Coord. Chem. Rev. 2024, 498, 215452. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; McIntyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal-Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Øien, S.; Wragg, D.; Reinsch, H.; Svelle, S.; Bordiga, S.; Lamberti, C.; Lillerud, K.P. Detailed Structure Analysis of Atomic Positions and Defects in Zirconium Metal-Organic Frameworks. Cryst. Growth Des. 2014, 14, 5370–5372. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhou, Z.; Shen, Y.; Jiang, L. Pore Size Effect of Zirconium-Based Metal-Organic Frameworks for Encapsulation and Release of Volatile Monoterpenes. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131907. [Google Scholar] [CrossRef]
- Ye, X.; Liu, D. Metal-Organic Framework UiO-68 and Its Derivatives with Sufficiently Good Properties and Performance Show Promising Prospects in Potential Industrial Applications. Cryst. Growth Des. 2021, 21, 4780–4804. [Google Scholar] [CrossRef]
- Dong, J.; Wee, V.; Zhao, D. Stimuli-Responsive Metal–Organic Frameworks Enabled by Intrinsic Molecular Motion. Nat. Mater. 2022, 21, 1334–1340. [Google Scholar] [CrossRef]
- Férey, C.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. Chemistry: A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; De Weireld, G.; Chang, J.S.; Hong, D.Y.; Hwang, Y.K.; et al. High Uptakes of CO2 and CH4 in Mesoporous Metal-Organic Frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250. [Google Scholar] [CrossRef] [PubMed]
- Boutin, A.; Coudert, F.X.; Springuel-Huet, M.A.; Neimark, A.V.; Férey, G.; Fuchs, A.H. The Behavior of Flexible MIL-53(Al) upon CH4 and CO2 Adsorption. J. Phys. Chem. C 2010, 114, 22237–22244. [Google Scholar] [CrossRef]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.Y.; Seo, Y.K.; Chang, J.S.; Grenèche, J.M.; Margiolaki, I.; Férey, G. Synthesis and Catalytic Properties of MIL-100(Fe), an Iron(III) Carboxylate with Large Pores. ChemComm. 2007, 27, 2820–2822. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; To, T.D.; Dao, Q.T.; Nghiem, L.D. Facile Synthesis of MIL-100(Fe) Adsorbents for Boosting Organic Dye Removal. Environ. Sci. Pollut. Res. 2025, 32, 4975–4988. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-Organic Frameworks: A New Class of Porous Materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- S-Y Chui, S.; M-F Lo, S.; H Charmant, J.P.; Guy Orpen, A.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Krause, S.; Bon, V.; Senkovska, I.; Stoeck, U.; Wallacher, D.; Többens, D.M.; Zander, S.; Pillai, R.S.; Maurin, G.; Coudert, F.X.; et al. A Pressure-Amplifying Framework Material with Negative Gas Adsorption Transitions. Nature 2016, 532, 348–352. [Google Scholar] [CrossRef]
- Kouser, S.; Hezam, A.; Khadri, M.J.N.; Khanum, S.A. A Review on Zeolite Imidazole Frameworks: Synthesis, Properties, and Applications. J. Porous Mater. 2022, 29, 663–681. [Google Scholar] [CrossRef]
- Troyano, J.; Carné-Sánchez, A.; Avci, C.; Imaz, I.; Maspoch, D. Colloidal Metal-Organic Framework Particles: The Pioneering Case of ZIF-8. Chem. Soc. Rev. 2019, 48, 5534–5546. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Ullah, S.; Shahid, M.U.; Hossain, I.; Najam, T.; Ismail, M.A.; ur Rehman, A.; Karim, M.R.; Shah, S.S.A. Zeolitic Imidazolate Frameworks (ZIF-8 & ZIF-67): Synthesis and Application for Wastewater Treatment. Sep. Purif. Technol. 2025, 356, 129828. [Google Scholar]
- Duan, C.; Yu, Y.; Hu, H. Recent Progress on Synthesis of ZIF-67-Based Materials and Their Application to Heterogeneous Catalysis. Green Energy Environ. 2022, 7, 3–15. [Google Scholar] [CrossRef]
- Xiong, Y.; Deng, N.; Wu, X.; Zhang, Q.; Liu, S.; Sun, G. De Novo Synthesis of Amino-Functionalized ZIF-8 Nanoparticles: Enhanced Interfacial Compatibility and Pervaporation Performance in Mixed Matrix Membranes Applying for Ethanol Dehydration. Sep. Purif. Technol. 2022, 285, 120321. [Google Scholar] [CrossRef]
- Neubertová, V.; Švorčík, V.; Kolská, Z. Amino-Modified ZIF-8 for Enhanced CO2 Capture: Synthesis, Characterization and Performance Evaluation. Microporous Mesoporous Mater. 2024, 366, 112956. [Google Scholar] [CrossRef]
- Behera, D.; Priyadarshini, P.; Parida, K. ZIF-8 Metal-Organic Frameworks and Their Hybrid Materials: Emerging Photocatalysts for Energy and Environmental Applications. Dalton Trans. 2025, 54, 2681–2708. [Google Scholar] [CrossRef]
- Ye, C.; Wu, X.; Wu, H.; Yang, L.; Ren, Y.; Wu, Y.; Liu, Y.; Guo, Z.; Zhao, R.; Jiang, Z. Incorporating Nano-Sized ZIF-67 to Enhance Selectivity of Polymers of Intrinsic Microporosity Membranes for Biogas Upgrading. Chem. Eng. Sci. 2020, 216, 115497. [Google Scholar] [CrossRef]
- Wu, R.; Li, Y.; Huang, A. Synthesis of High-Performance Co-Based ZIF-67 Membrane for H2 Separation by Using Cobalt Ions Chelated PIM-1 as Interface Layer. J. Membr. Sci. 2021, 620, 118841. [Google Scholar] [CrossRef]
- Li, C.; Chandresh, A.; Zhang, Z.; Moulai, S.; Heinke, L. Stability and Degradation of Metal–Organic-Framework Films under Ambient Air Explored by Uptake and Diffusion Experiments. Adv. Mater. Interfaces 2022, 9, 2101947. [Google Scholar] [CrossRef]
- Kadhom, M.; Al-Furaiji, M.; Salih, S.; Al-Obaidi, M.A.; Abdullah, G.H.; Albayati, N. A Review on UiO-66 Applications in Membrane-Based Water Treatment Processes. J. Water Process. Eng. 2023, 51, 103402. [Google Scholar] [CrossRef]
- Sun, Y. UiO-66 Metal-Organic Framework Membranes: Structural Engineering for Separation Applications. Membranes 2025, 15, 8. [Google Scholar] [CrossRef]
- Okba, D.; Hassan, S.; Abdel Aleem, A.A.H.; Shehab El-Din, M.T.; El Tantawy El Sayed, I.; Abou-Elyazed, A.S. Enhanced Performance of UiO-66 for Supercapacitor Applications through Oxidation via the Hummers’ Method. RSC Adv. 2025, 15, 795–805. [Google Scholar] [CrossRef]
- Tatay, S.; Martínez-Giménez, S.; Rubio-Gaspar, A.; Gómez-Oliveira, E.; Castells-Gil, J.; Dong, Z.; Mayoral, Á.; Almora-Barrios, N.; Padial, M.N.; Martí-Gastaldo, C. Synthetic Control of Correlated Disorder in UiO-66 Frameworks. Nat. Commun. 2023, 14, 6962. [Google Scholar] [CrossRef]
- Miao, C.L.; Wang, X.X.; Guan, D.H.; Li, J.X.; Wang, H.F.; Xu, J.J. Directional Modification-Functionalized Metal–Organic Framework Solid-State Electrolytes for Highly Stable Li–O2 Batteries. Adv. Funct. Mater. 2024, 34, 2307150. [Google Scholar] [CrossRef]
- Timofeev, K.L.; Kulinich, S.A.; Kharlamova, T.S. NH2-Modified UiO-66: Structural Characteristics and Functional Properties. Molecules 2023, 28, 3916. [Google Scholar] [CrossRef]
- Surblé, S.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Férey, G. A New Isoreticular Class of Metal-Organic-Frameworks with the MIL-88 Topology. ChemComm. 2006, 3, 284–286. [Google Scholar] [CrossRef]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’Arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the Optical Response of the Titanium-MIL-125 Metal-Organic Framework through Ligand Functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef]
- Palomino Cabello, C.; Gómez-Pozuelo, G.; Opanasenko, M.; Nachtigall, P.; Čejka, J. Metal–Organic Frameworks M-MOF-74 and M-MIL-100: Comparison of Textural, Acidic, and Catalytic Properties. Chempluschem 2016, 81, 828–835. [Google Scholar] [CrossRef]
- Le Vuong, M.D.; Christodoulou, I.; Porcino, M.; Dong, S.T.; Lassalle-Kaiser, B.; Haouas, M.; Gref, R.; Martineau-Corcos, C. Degradation Mechanism of Metal-Organic Framework Drug Nanocarriers Studied by Solid-State Nuclear Magnetic Resonance and X-Ray Absorption Near-Edge Structure Spectroscopy. Chem. Mater. 2022, 34, 8178–8189. [Google Scholar] [CrossRef]
- Férey, G.; Millange, F.; Morcrette, M.; Serre, C.; Doublet, M.L.; Grenèche, J.M.; Tarascon, J.M. Mixed-Valence Li/Fe-Based Metal-Organic Frameworks with Both Reversible Redox and Sorption Properties. Angew. Chem. Int. Ed. 2007, 46, 3259–3263. [Google Scholar] [CrossRef] [PubMed]
- Celeste, A.; Paolone, A.; Itié, J.P.; Borondics, F.; Joseph, B.; Grad, O.; Blanita, G.; Zlotea, C.; Capitani, F. Mesoporous Metal-Organic Framework MIL-101 at High Pressure. J. Am. Chem. Soc. 2020, 142, 15012–15019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhou, M.L.; Cui, Y.H.; Yang, M.; Bao, Y.S.; Ye, Y.; Tian, D.M.; Liu, L.Y.; Han, Z.B. Polymelamine Formaldehyde-Coated MIL-101 as an Efficient Dual-Functional Core-Shell Composite to Catalyze the Deacetalization-Knoevenagel Tandem Reaction. Inorg. Chem. 2022, 61, 13678–13684. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Rasaily, S.; Pradhan, S.; Baruah, K.; Tamang, S.; Pariyar, A. HKUST-1 Metal Organic Framework as an Efficient Dual-Function Catalyst: Aziridination and One-Pot Ring-Opening Transformation for Formation of β-Aryl Sulfonamides with C-C, C-N, C-S, and C-O Bonds. Inorg. Chem. 2021, 60, 7794–7802. [Google Scholar] [CrossRef]
- Grande, C.A.; Kaiser, A.; Andreassen, K.A. Methane Storage in Metal-Organic Framework HKUST-1 with Enhanced Heat Management Using 3D Printed Metal Lattices. ChERD 2023, 192, 362–370. [Google Scholar] [CrossRef]
- Lin, K.S.; Adhikari, A.K.; Ku, C.N.; Chiang, C.L.; Kuo, H. Synthesis and Characterization of Porous HKUST-1 Metal Organic Frameworks for Hydrogen Storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Shen, T.; Liu, T.; Mo, H.; Yuan, Z.; Cui, F.; Jin, Y.; Chen, X. Cu-Based Metal-Organic Framework HKUST-1 as Effective Catalyst for Highly Sensitive Determination of Ascorbic Acid. RSC Adv. 2020, 10, 22881–22890. [Google Scholar] [CrossRef]
- Fuoco, A.; Khdhayyer, M.R.; Attfield, M.P.; Esposito, E.; Jansen, J.C.; Budd, P.M. Synthesis and Transport Properties of Novel MOF/PIM-1/MOF Sandwich Membranes for Gas Separation. Membranes 2017, 7, 7. [Google Scholar] [CrossRef]
- Bönisch, N.; Maliuta, M.; Senkovska, I.; Bon, V.; Petkov, P.; Plätzer, C.; Müller, P.; Kaskel, S. Linker Expansion and Its Impact on Switchability in Pillared-Layer MOFs. Inorg. Chem. 2021, 60, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, Y.; Sun, M.; Xu, P.; Wang, C.; Huang, K.; Jiang, H.; Mintova, S.; Guo, H. Is Amino-Modification of HKUST-1 in PEI Mixed-Matrix Membranes Always Favorable to CO2 Separation? Microporous Mesoporous Mater. 2023, 359, 112649. [Google Scholar] [CrossRef]
- Drwęska, J.; Roztocki, K.; Janiak, A.M. Advances in Chemistry of CALF-20, a Metal-Organic Framework for Industrial Gas Applications. Chem. Commun. 2024, 61, 1032–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Yang, Y.; Li, C.C.; Tang, H.L.; Zhang, F.M.; Zhang, G.L.; Yan, H. A New Strategy for Constructing Covalently Connected MOF@COF Core-Shell Heterostructures for Enhanced Photocatalytic Hydrogen Evolution. J. Mater. Chem. A Mater. 2021, 9, 16743–16750. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, D. Post-Synthetic Modification of Metal-Organic Framework-Based Membranes for Enhanced Molecular Separations. Coord. Chem. Rev. 2023, 491, 215259. [Google Scholar] [CrossRef]
- Jue, M.L.; Lively, R.P. PIM Hybrids and Derivatives: How to Make a Good Thing Better. Curr. Opin. Chem. Eng. 2022, 35, 100750. [Google Scholar] [CrossRef]
- Ma, C.; Urban, J.J. Polymers of Intrinsic Microporosity (PIMs) Gas Separation Membranes: A Mini Review. Proc. Nat. Res. Soc. 2018, 2, 02002. [Google Scholar] [CrossRef]
- McKeown, N.B. Polymers of Intrinsic Microporosity. ISRN Mater. Sci. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Mc Keown, N.B.; Budd, P.M. Polymers of Intrinsic Microporosity (PIMs): Organic Materials for Membrane Separations, Heterogeneous Catalysis and Hydrogen Storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Ghanem, B.S.; Alghunaimi, F.; Pinnau, I.; Han, Y. Polymers of Intrinsic Microporosity for Energy-Intensive Membrane-Based Gas Separations. Mater. Today Nano 2018, 3, 69–95. [Google Scholar] [CrossRef]
- Low, Z.X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas Permeation Properties, Physical Aging, and Its Mitigation in High Free Volume Glassy Polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef]
- Gao, G.K.; Wang, Y.R.; Zhu, H.J.; Chen, Y.; Yang, R.X.; Jiang, C.; Ma, H.; Lan, Y.Q. Rapid Production of Metal–Organic Frameworks Based Separators in Industrial-Level Efficiency. Adv. Sci. 2020, 7, 2002190. [Google Scholar] [CrossRef]
- Paul, D.R.; Kemp, D.R. Diffusion Time Lag in Polymer Membranes Containing Adsorptive Fillers. J. Polym. Sci. Part. C Polym. Symp. 1973, 41, 79–93. [Google Scholar] [CrossRef]
- Yong, W.F.; Kwek, K.H.A.; Liao, K.S.; Chung, T.S. Suppression of Aging and Plasticization in Highly Permeable Polymers. Polymer 2015, 77, 377–386. [Google Scholar] [CrossRef]
- Salahshoori, I.; Seyfaee, A.; Babapoor, A. Recent Advances in Synthesis and Applications of Mixed Matrix Membranes. Synth. Sinter. 2021, 1, 1–27. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V.; Dung, C.T. Mixed Matrix Membranes Based on PIMs for Gas Permeation: Principles, Synthesis, and Current Status. Chem. Eng. Commun. 2017, 204, 295–309. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Castro-Munõz, R.; Budd, P.M. Boosting Gas Separation Performance and Suppressing the Physical Aging of Polymers of Intrinsic Microporosity (PIM-1) by Nanomaterial Blending. Nanoscale 2020, 12, 23333–23370. [Google Scholar] [CrossRef] [PubMed]
- Hossain, I.; Kim, K.I.; Husna, A.; Kang, J.H.; Kim, T.H.; Park, H.B. Metal-Coordinated, Dual-Crosslinked PIM Polymer Membranes for Upgraded CO2 Separation: Aging and Plasticization Resistance. Small 2024, 21, 2407973. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; Mckeown, N.B.; Fritsch, D. Gas Separation Membranes from Polymers of Intrinsic Microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Monsalve-Bravo, G.M.; Bhatia, S.K. Modeling Permeation through Mixed-Matrix Membranes: A Review. Processes 2018, 6, 172. [Google Scholar] [CrossRef]
- Goh, S.H.; Lau, H.S.; Yong, W.F. Metal–Organic Frameworks (MOFs)-Based Mixed Matrix Membranes (MMMs) for Gas Separation: A Review on Advanced Materials in Harsh Environmental Applications. Small 2022, 18, 2107536. [Google Scholar] [CrossRef] [PubMed]
- Knebel, A.; Bavykina, A.; Datta, S.J.; Sundermann, L.; Garzon-Tovar, L.; Lebedev, Y.; Durini, S.; Ahmad, R.; Kozlov, S.M.; Shterk, G.; et al. Solution Processable Metal–Organic Frameworks for Mixed Matrix Membranes Using Porous Liquids. Nat. Mater. 2020, 19, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Swaidan, R.J.; Wang, Y.; Hsiung, C.E.; Han, Y.; Pinnau, I. Highly Compatible Hydroxyl-Functionalized Microporous Polyimide-Zif-8 Mixed Matrix Membranes for Energy Efficient Propylene/Propane Separation. ACS Appl. Nano Mater. 2018, 1, 3541–3547. [Google Scholar] [CrossRef]
- Zhuang, G.L.; Tseng, H.H.; Wey, M.Y. Preparation of PPO-Silica Mixed Matrix Membranes by in-Situ Sol-Gel Method for H2/CO2 Separation. Int. J. Hydrogen Energy 2014, 39, 17178–17190. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Tan, X. High Performance of PIM-1/ZIF-8 Composite Membranes for O2/N2 Separation. ACS Omega 2019, 4, 16572–16577. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, B.; Wang, G.; Yu, G.; Zou, X.; Zhu, G. Small-Pore CAU-21 and Porous PIM-1 in Mixed-Matrix Membranes for Improving Selectivity and Permeability in Hydrogen Separation. Chem. Commun. 2019, 55, 7101–7104. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced Selectivity in Mixed Matrix Membranes for CO2 Capture through Efficient Dispersion of Amine-Functionalized MOF Nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Zhang, C.; Lively, R.P.; Zhang, K.; Johnson, J.R.; Karvan, O.; Koros, W.J. Unexpected Molecular Sieving Properties of Zeolitic Imidazolate Framework-8. J. Phys. Chem. Lett. 2012, 3, 2130–2134. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Düren, T. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef]
- Liu, G.; Chernikova, V.; Liu, Y.; Zhang, K.; Belmabkhout, Y.; Shekhah, O.; Zhang, C.; Yi, S.; Eddaoudi, M.; Koros, W.J. Mixed Matrix Formulations with MOF Molecular Sieving for Key Energy-Intensive Separations. Nat. Mater. 2018, 17, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, E.; Warfsmann, J.; Tokay, B.; Shishatskiy, S.; Lee, Y.J.; Lillepaerg, J.; Champness, N.R.; Filiz, V. Gas Transport Properties of the Metal-Organic Framework (MOF)-Assisted Polymer of Intrinsic Microporosity (PIM-1) Thin-Film Composite Membranes. ACS Sustain. Chem. Eng. 2021, 9, 684–694. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Mizrahi Rodriguez, K.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.; Gaffney, P.R.J.; Peeva, L.G.; Livingston, A.G. Roll-to-Roll Dip Coating of Three Different PIMs for Organic Solvent Nanofiltration. J. Membr. Sci. 2018, 558, 52–63. [Google Scholar] [CrossRef]
- Li, N.; Ma, C.; Li, D.; Li, P.; Ye, M.; Wang, Z.; Qiao, Z.; Zhong, C. In-Situ Glass Transition of ZIF-62 Based Mixed Matrix Membranes for Enhancing H2 Fast Separation. Sep. Purif. Technol. 2025, 353, 128500. [Google Scholar] [CrossRef]
- Baloochiyan, A.; Öztürk, H.; Erucar, I. Atomistic Investigation of Porous Amorphous Materials for CH4/H2 Separation. Chem. Eng. Sci. 2025, 301, 120741. [Google Scholar] [CrossRef]
- Xiong, S.; Pan, C.; Dai, G.; Liu, C.; Tan, Z.; Chen, C.; Yang, S.; Ruan, X.; Tang, J.; Yu, G. Interfacial Co-Weaving of AO-PIM-1 and ZIF-8 in Composite Membranes for Enhanced H2 Purification. J. Membr. Sci. 2022, 645, 120217. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of Separation Factor versus Permeability for Polymeric Membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson Upper Bounds for CO2/CH4 and CO2/N2 Separations Using a Series of Ultrapermeable Benzotriptycene-Based Polymers of Intrinsic Microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Tanh Jeazet, H.B.; Staudt, C.; Janiak, C. Metal-Organic Frameworks in Mixed-Matrix Membranes for Gas Separation. Dalton Trans. 2012, 41, 14003–14027. [Google Scholar] [CrossRef]
- Guo, X.; Qiao, Z.; Liu, D.; Zhong, C. Mixed-Matrix Membranes for CO2 Separation: Role of the Third Component. J. Mater. Chem. A Mater. 2019, 7, 24738–24759. [Google Scholar] [CrossRef]
- Wu, W.-N. Microporous Polymer-Metal Organic Framework (MOF) Hybrid Materials for Separations. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2024. [Google Scholar]
- Zhang, H.; Sheng, L.; Chen, J.; Wang, X.; Tao, P.; Ren, D.; Cui, H.; Yang, K.; Tang, Z.; Zhang, Z.; et al. Scalable MOF-Based Mixed Matrix Membranes with Enhanced Permeation Processes Facilitate the Scale Application of Membrane-Based Carbon Capture Technologies. Carbon Capture Sci. Technol. 2024, 13, 100276. [Google Scholar] [CrossRef]
- Tien-Binh, N.; Vinh-Thang, H.; Chen, X.Y.; Rodrigue, D.; Kaliaguine, S. Crosslinked MOF-Polymer to Enhance Gas Separation of Mixed Matrix Membranes. J. Membr. Sci. 2016, 520, 941–950. [Google Scholar] [CrossRef]
- Huang, G.; Liu, T.; Peng, H.; Xie, Y.; Chen, Q.; Li, J.R. Enhancement of CO2/N2 Separation in MMMs by the Regulation of Nanofiller Microenvironment. Sep. Purif. Technol. 2025, 354, 128737. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Hou, L.; Yang, C.; Shen, H.; Yang, K.; Wang, Z. Metal-Organic Framework MOF-801/PIM-1 Mixed-Matrix Membranes for Enhanced CO2/N2 Separation Performance. Sep. Purif. Technol. 2020, 250, 117198. [Google Scholar] [CrossRef]
- Yin, H.; Alkaş, A.; Zhang, Y.; Zhang, Y.; Telfer, S.G. Mixed Matrix Membranes (MMMs) Using an Emerging Metal-Organic Framework (MUF-15) for CO2 Separation. J. Membr. Sci. 2020, 609, 118245. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Wu, H.; Wu, X.; Yang, H.; Yang, L.; Wang, X.; Wu, Y.; Liu, Y.; Jiang, Z. Amino-Functionalized ZIF-7 Embedded Polymers of Intrinsic Microporosity Membrane with Enhanced Selectivity for Biogas Upgrading. J. Membr. Sci. 2020, 602, 117970. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, W.; Kang, Z.; Zou, X.; Fan, W.; Jiang, Y.; Fan, L.; Wang, R.; Sun, D. Thermal Treatment Optimization of Porous MOF Glass and Polymer for Improving Gas Permeability and Selectivity of Mixed Matrix Membranes. J. Chem. Eng. 2023, 465, 142873. [Google Scholar] [CrossRef]
- Budd, P.M.; Foster, A.B. Seeking Synergy in Membranes: Blends and Mixtures with Polymers of Intrinsic Microporosity. Curr Opin. Chem. Eng. 2022, 36, 100792. [Google Scholar] [CrossRef]
- Zhou, H.C.J.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Wang, Y.; Jiang, H.L.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, 1703663. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Agamendran, N.; Sekar, K.; Natarajan, T.S. Metal–Organic Frameworks (MOFs) for Energy Production and Gaseous Fuel and Electrochemical Energy Storage Applications. Phys. Chem. Chem. Phys. 2023, 25, 30116–30144. [Google Scholar] [CrossRef]
- Virender, V.; Pandey, V.; Singh, G.; Sharma, P.K.; Bhatia, P.; Solovev, A.A.; Mohan, B. Hybrid Metal-Organic Frameworks (MOFs) for Various Catalysis Applications. Top. Curr. Chem. 2024, 383, 1–36. [Google Scholar] [CrossRef]
- Wei, Y.S.; Zhang, M.; Zou, R.; Xu, Q. Metal-Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, H.; Xu, Y. Etching Strategies Induced Multihierarchical Structures of MOFs and Their Derivatives for Gas Sensing Applications: A Review. J. Mater. Chem. C Mater. 2025, 13, 3653–3668. [Google Scholar] [CrossRef]
- Healy, C.; Patil, K.M.; Wilson, B.H.; Hermanspahn, L.; Harvey-Reid, N.C.; Howard, B.I.; Kleinjan, C.; Kolien, J.; Payet, F.; Telfer, S.G.; et al. The Thermal Stability of Metal-Organic Frameworks. Coord. Chem. Rev. 2020, 419, 213388. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.L. Improving MOF Stability: Approaches and Applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Ding, S.Y.; Wang, W. Covalent Organic Frameworks (COFs): From Design to Applications. Chem. Soc. Rev. 2012, 42, 548–568. [Google Scholar] [CrossRef]
- Beagle, L.K.; Fang, Q.; Tran, L.D.; Baldwin, L.A.; Muratore, C.; Lou, J.; Glavin, N.R. Synthesis and Tailored Properties Towards Designer Covalent Organic Framework Thin Films and Heterostructures. arXiv 2021, arXiv:2105.12904. [Google Scholar]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Bennett, T.; Butler, K.; Easun, T.L.; Eddaoudi, M.; Forgan, R.; Gagliardi, L.; Hendon, C.; Jorge, M.; Lamberti, C.; et al. Electronic, Magnetic and Photophysical Properties of MOFs and COFs: General Discussion. Faraday Discuss. 2017, 201, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Duan, F.; Zhang, S.; He, L.; Wang, M.; Chen, J.; Zhang, J.; Jia, Q.; Zhang, Z.; Du, M. Heterostructured Hybrids of Metal–Organic Frameworks (MOFs) and Covalent–Organic Frameworks (COFs). J. Mater. Chem. A Mater. 2022, 10, 475–507. [Google Scholar] [CrossRef]
- Xue, R.; Guo, H.; Yang, W.; Huang, S.L.; Yang, G.Y. Cooperation between Covalent Organic Frameworks (COFs) and Metal Organic Frameworks (MOFs): Application of COFs-MOFs Hybrids. Adv. Compos. Hybrid Mater. 2022, 5, 1595–1611. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Ghaffarkhah, A.; Molavi, H.; Dutta, S.; Lu, Y.; Wuttke, S.; Kamkar, M.; Rojas, O.J.; Arjmand, M. COF and MOF Hybrids: Advanced Materials for Wastewater Treatment. Adv. Funct. Mater. 2024, 34, 2305527. [Google Scholar] [CrossRef]
- Ma, M.; Lu, X.; Guo, Y.; Wang, L.; Liang, X. Combination of Metal-Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs): Recent Advances in Synthesis and Analytical Applications of MOF/COF Composites. TrAC 2022, 157, 116741. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Yang, C.; Cheng, K.; Tan, T.; Lv, Y.; Liu, Y. Hybrid Porous Crystalline Materials from Metal Organic Frameworks and Covalent Organic Frameworks. Adv. Sci. 2021, 8, 2101883. [Google Scholar] [CrossRef]
- Wu, M.X.; Wang, Y.; Zhou, G.; Liu, X. Sparks from Different Worlds: Collaboration of MOFs and COFs. Coord. Chem. Rev. 2021, 430, 213735. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, M.; Chen, B.; Zhang, Z.; Huang, Y.; Dai, F.; Lai, Z.; Cui, X.; Tan, C.; Zhang, H. Hybridization of MOFs and COFs: A New Strategy for Construction of MOF@COF Core-Shell Hybrid Materials. Adv. Mater. 2018, 30, 1705454. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Zhai, L.; Liu, G.; Dong, J.; Wang, Y.; Christopher, M.P.; Long, S.; Wang, Y.; Zhao, D. Mixed Matrix Membranes Containing MOF@COF Hybrid Fillers for Efficient CO2/CH4 Separation. J. Membr. Sci. 2019, 573, 97–106. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Teo, W.L.; Chen, B.; Jin, F.; Zhang, L.; Zeng, Y.; Zhao, Y. Construction of a Sandwiched MOF@COF Composite as a Size-Selective Catalyst. Cell Rep. Phys. Sci. 2020, 1, 100272. [Google Scholar] [CrossRef]
- Sun, D.; Kim, D.P. Hydrophobic MOFs@Metal Nanoparticles@COFs for Interfacially Confined Photocatalysis with High Efficiency. ACS Appl. Mater. Interfaces 2020, 12, 20589–20595. [Google Scholar] [CrossRef]
- Firoozi, M.; Rafiee, Z.; Dashtian, K. New MOF/COF Hybrid as a Robust Adsorbent for Simultaneous Removal of Auramine O and Rhodamine B Dyes. ACS Omega 2020, 5, 9420–9428. [Google Scholar] [CrossRef]
- Zhang, F.M.; Sheng, J.L.; Di Yang, Z.; Sun, X.J.; Tang, H.L.; Lu, M.; Dong, H.; Shen, F.C.; Liu, J.; Lan, Y.Q. Rational Design of MOF/COF Hybrid Materials for Photocatalytic H2 Evolution in the Presence of Sacrificial Electron Donors. Angew. Chem. Int. Ed. Engl. 2018, 57, 12106–12110. [Google Scholar] [CrossRef]
- Troyano, J.; Çamur, C.; Garzón-Tovar, L.; Carné-Sánchez, A.; Imaz, I.; Maspoch, D.; Maspoch, D. Spray-Drying Synthesis of MOFs, COFs, and Related Composites. Acc. Chem. Res. 2020, 53, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Das, S.; Xing, G.; Ben, T.; Valtchev, V.; Qiu, S. Fabrication of COF-MOF Composite Membranes and Their Highly Selective Separation of H2/CO2. J. Am. Chem. Soc. 2016, 138, 7673–7680. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, M.; Wang, M.; Song, Y.; Zhou, N.; He, L.; Zhang, Z. Novel Nanoarchitecture of Co-MOF-on-TPN-COF Hybrid: Ultralowly Sensitive Bioplatform of Electrochemical Aptasensor toward Ampicillin. Biosens. Bioelectron. 2019, 123, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Ma, Y.; Hu, B.; He, L.; Wang, S.; Zhang, Z.; Lu, S. Construction of Ce-MOF@COF Hybrid Nanostructure: Label-Free Aptasensor for the Ultrasensitive Detection of Oxytetracycline Residues in Aqueous Solution Environments. Biosens. Bioelectron. 2019, 127, 92–100. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Shi, B.; Dai, W.; Ren, H.; An, K.; Zhou, Z.; Zhao, Z.; Wang, W.; Jiang, Z. In Situ Construction of Hydrazone-Linked COF-Based Core–Shell Hetero-Frameworks for Enhanced Photocatalytic Hydrogen Evolution. J. Mater. Chem. A Mater. 2020, 8, 7724–7732. [Google Scholar] [CrossRef]
- Sun, D.; Jang, S.; Yim, S.J.; Ye, L.; Kim, D.P. Metal Doped Core–Shell Metal-Organic Frameworks@Covalent Organic Frameworks (MOFs@COFs) Hybrids as a Novel Photocatalytic Platform. Adv. Funct. Mater. 2018, 28, 1707110. [Google Scholar] [CrossRef]
- Cai, M.; Li, Y.; Liu, Q.; Xue, Z.; Wang, H.; Fan, Y.; Zhu, K.; Ke, Z.; Su, C.-Y.; Li, G.; et al. One-Step Construction of Hydrophobic MOFs@COFs Core–Shell Composites for Heterogeneous Selective Catalysis. Adv. Sci. 2019, 6, 1802365. [Google Scholar] [CrossRef]
- Yang, R.G.; Fu, Y.M.; Wang, H.N.; Zhang, D.P.; Zhou, Z.; Cheng, Y.Z.; Meng, X.; He, Y.O.; Su, Z.M. ZIF-8/Covalent Organic Framework for Enhanced CO2 Photocatalytic Reduction in Gas-Solid System. J. Chem. Eng. 2022, 450, 138040. [Google Scholar] [CrossRef]
- Yue, B.; Liu, J.; Li, G.; Wu, Y. Synthesis of Magnetic Metal Organic Framework/Covalent Organic Framework Hybrid Materials as Adsorbents for Magnetic Solid-Phase Extraction of Four Endocrine-Disrupting Chemicals from Milk Samples. Rapid Commun. Mass Spectrom. 2020, 34, e8909. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Peng, M.; Strauss, I.; Mundstock, A.; Meng, H.; Caro, J. MOF-in-COF Molecular Sieving Membrane for Selective Hydrogen Separation. Nat. Commun. 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Wang, H.; Du, X.; Zhang, D.; Zhang, Z.; Liu, G. A COF-Coated MOF Framework Polysulfide Barrier Design for Enhanced Performance in Lithium-Sulfur Batteries. Electrochim. Acta 2022, 412, 140156. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Deng, Q.; Sang, Y.; Dong, K.; Ren, J.; Qu, X. Nature-Inspired Construction of MOF@COF Nanozyme with Active Sites in Tailored Microenvironment and Pseudopodia-Like Surface for Enhanced Bacterial Inhibition. Angew. Chem. 2020, 60, 3469–3474. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhu, Q.Q.; Yuan, R.; He, H. Crystal Engineering of MOF@COF Core-Shell Composites for Ultra-Sensitively Electrochemical Detection. Sens. Actuators B Chem. 2021, 329, 129144. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.; Xing, J.; Wang, F.; Shi, Y.; Zhao, X.; Liu, J.; Yang, Y.; Zhao, Z. A Fluorescent Dual-Emissions UiO-66-NH2@TpTt-COF Core-Shell Composite for Sensitive and Optosmart Sensing of Tetracycline. Appl. Surf. Sci. 2023, 616, 156455. [Google Scholar] [CrossRef]
- Gao, M.L.; Qi, M.H.; Liu, L.; Han, Z.B. An Exceptionally Stable Core–Shell MOF/COF Bifunctional Catalyst for a Highly Efficient Cascade Deacetalization–Knoevenagel Condensation Reaction. Chem. Commun. 2019, 55, 6377–6380. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, B.; Peng, R. New Core-Shell Hybrid Material IR-MOF3@COF-LZU1 for Highly Efficient Visible-Light Photocatalyst Degrading Nitroaromatic Explosives. Langmuir 2020, 36, 5665–5670. [Google Scholar] [CrossRef]
- Abeysinghe, A.K.; Peng, Y.-P.; Huang, P.-J.; Chen, K.-F.; Chen, C.-H.; Chen, W.-X.; Liang, F.Y.; Chien, P.-Y. Enhancing Visible-Light-Driven Photocatalysis: Unveiling the Remarkable Potential of H2O2-Assisted MOF/COF Hybrid Material for Organic Pollutant Degradation. Environ. Sci. Pollut. Res. 2024, 31, 50983–50999. [Google Scholar] [CrossRef]
- Wu, Z.; Li, W.; Hou, L.; Wei, Q.; Yang, H.; Jiang, Y.; Tang, D. A Novel Sunflower-like MOF@COF for Improved Photocatalytic CO2 Reduction. Sep. Purif. Technol. 2023, 311, 123322. [Google Scholar] [CrossRef]
- Garzón-Tovar, L.; Pérez-Carvajal, J.; Yazdi, A.; Hernández-Muñoz, J.; Tarazona, P.; Imaz, I.; Zamora, F.; Maspoch, D. A MOF@COF Composite with Enhanced Uptake through Interfacial Pore Generation. Angew. Chem. Int. Ed. Engl. 2019, 58, 9512–9516. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ben, T. A [COF-300]-[UiO-66] Composite Membrane with Remarkably High Permeability and H2/CO2 Separation Selectivity. Dalton Trans. 2018, 47, 7206–7212. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cai, K.; Tao, D.J.; Zhao, T. The Mega-Merger Strategy: M@COF Core-Shell Hybrid Materials for Facilitating CO2 Capture and Conversion to Monocyclic and Polycyclic Carbonates. Appl. Catal. B 2024, 341, 123317. [Google Scholar] [CrossRef]
- Fakhraie, S.; Rajabi, H.R.; Rashidi, A. Fabrication and Application of Novel Core–Shell MIL-101(Cr)@UiO-66(Zr) Nanocrystals for Highly Selective Separation of H2S and CO2. J. Chem. Eng. 2023, 452, 139001. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, Y.; Liang, W.; Zhu, Y.; Hu, B. Construction of Core-Shell MOFs@COF Hybrids as a Platform for the Removal of UO22+and Eu3+Ions from Solution. ACS Appl. Mater. Interfaces 2021, 13, 13883–13895. [Google Scholar] [CrossRef]
- Peng, H.; Huang, S.; Tranca, D.; Richard, F.; Baaziz, W.; Zhuang, X.; Samorì, P.; Ciesielski, A. Quantum Capacitance through Molecular Infiltration of 7,7,8,8-Tetracyanoquinodimethane in Metal-Organic Framework/Covalent Organic Framework Hybrids. ACS Nano 2021, 15, 18580–18589. [Google Scholar] [CrossRef]
- Pakulski, D.; Veró Nica Montes-Garcí, A.; Czepa, W.; Marcinkowski, D.; Peng, H.; Chudziak, T.; Gorczyński, A.; Gorczyński, G.; Kukułka, W.; Valentini, C.; et al. MOF (UiO-66-NH2)@COF (TFP-TABQ) Hybrids via on-Surface Condensation Reactions for Sustainable Energy Storage. Chem. Commun. 2024, 60, 412. [Google Scholar] [CrossRef]
- Peng, H.; Raya, J.; Richard, F.; Baaziz, W.; Ersen, O.; Ciesielski, A.; Samorì, P. Synthesis of Robust MOFs@COFs Porous Hybrid Materials via an Aza-Diels-Alder Reaction: Towards High-Performance Supercapacitor Materials. Angew. Chem. Int. Ed. Engl. 2020, 59, 19602–19609. [Google Scholar] [CrossRef]
- Sun, W.; Tang, X.; Yang, Q.; Xu, Y.; Wu, F.; Guo, S.; Zhang, Y.; Wu, M.; Wang, Y.; Sun, W.; et al. Coordination-Induced Interlinked Covalent- and Metal–Organic-Framework Hybrids for Enhanced Lithium Storage. Adv. Mater. 2019, 31, 1903176. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Lu, W.; Zhao, M.; Xiao, H.; Hu, T.; Ma, J.; Zheng, Z.; Jia, J.; Wu, H. A Label-Free Electrochemical Aptasensor Based on the Core-Shell Cu-MOF@TpBD Hybrid Nanoarchitecture for the Sensitive Detection of PDGF-BB. Analyst 2021, 146, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.L.; Atar, N. Amperometric Galectin-3 Immunosensor-Based Gold Nanoparticle-Functionalized Graphitic Carbon Nitride Nanosheets and Core-Shell Ti-MOF@COFs Composites. Nanoscale 2020, 12, 19824–19832. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, K.; Zhu, L.; Zhang, S.; Wang, M.; He, L.; Zhang, Z.; Du, M. MOF@COF Heterostructure Hybrid for Dual-Mode Photoelectrochemical-Electrochemical HIV-1 DNA Sensing. Langmuir 2021, 37, 13479–13492. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.M.; Wang, H.; Wu, H.; Tyagi, M.; Yildirim, T.; Zhou, W.; Chen, B. A Porous Metal-Organic Framework with Dynamic Pyrimidine Groups Exhibiting Record High Methane Storage Working Capacity. J. Am. Chem. Soc. 2014, 136, 6207–6210. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dincǎ, M. Electrically Conductive Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal-Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF Hybrids: Synthesis and Applications. Coord. Chem. Rev. 2021, 432, 213743. [Google Scholar] [CrossRef]
- Daliran, S.; Oveisi, A.R.; Peng, Y.; López-Magano, A.; Khajeh, M.; Mas-Ballesté, R.; Alemán, J.; Luque, R.; Garcia, H. Metal–Organic Framework (MOF)-, Covalent-Organic Framework (COF)-, and Porous-Organic Polymers (POP)-Catalyzed Selective C–H Bond Activation and Functionalization Reactions. Chem. Soc. Rev. 2022, 51, 7810–7882. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ding, X.; Jiang, D. Covalent Organic Frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef]
- Liu, M.; Guo, L.; Jin, S.; Tan, B. Covalent Triazine Frameworks: Synthesis and Applications. J. Mater. Chem. A Mater. 2019, 7, 5153–5172. [Google Scholar] [CrossRef]
- Raza, S.; Nazeer, S.; Abid, A.; Kanwal, A. Recent Research Progress in the Synthesis, Characterization and Applications of Hyper Cross-Linked Polymer. J. Polym. Res. 2023, 30, 1–26. [Google Scholar] [CrossRef]
- Gonte, R.R.; Deb, P.C.; Balasubramanian, K. Hydrogen Sorption onto Hypercrosslinked Polymer Decorated with Metal-Organic Framework. J. Polym. 2013, 2013, 684584. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zhao, S.; Zhang, L.; Qiao, S.; Zhou, J. New Design to Enhance Phosphonate Selective Removal from Water by MOF Confined in Hyper-Cross-Linked Resin. Sci. Total Environ. 2024, 914, 169760. [Google Scholar] [CrossRef]
- Karimi, Z.; Karami, B.; Farahi, M.; Mahmoudi Asl, A. Reinforced MOF/Polymer Composite Based on a Copper Metal-Organic Framework Supported on Nanoporous Hyper Cross-Linked Polycalix[4]Resorcinarene for the Preparation of Phthalazine and 4H-Pyran Heterocycles. Mater. Today Chem. 2024, 38, 102085. [Google Scholar] [CrossRef]
- Ci, E.; Chen, Q.; Wang, Y.; Liu, T.; Xu, Z.; Liu, F.; Chen, P.; Zhao, T. The Hyper-Crosslinked Aniline Polymer@MOFs Hybrid Materials Reinforced with Active Ionic Sites for Efficient and Fast Cr(VI) Removal. Sep. Purif. Technol. 2025, 352, 128144. [Google Scholar] [CrossRef]
- Bariki, R.; Joseph, R.G.; El-Kadri, O.M.; Al-Sayah, M.H. The Development of Metal-Free Porous Organic Polymers for Sustainable Carbon Dioxide Photoreduction. Nanomaterials 2024, 14, 1432. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Shi, W.; Zhang, Z.; Zhao, Y.; Luo, X.; Liu, X. Pyridine-Based Conjugated Microporous Polymers as Adsorbents for CO2 Uptake via Weak Supramolecular Interaction. New J. Chem. 2022, 46, 6394–6397. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. MOF–Graphite Oxide Composites: Combining the Uniqueness of Graphene Layers and Metal–Organic Frameworks. Adv. Mater. 2009, 21, 4753–4757. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal-Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef]
- Ning, H.; Yang, Z.; Yin, Z.; Wang, D.; Meng, Z.; Wang, C.; Zhang, Y.; Chen, Z. A Novel Strategy to Enhance the Performance of CO2Adsorption Separation: Grafting Hyper-Cross-Linked Polyimide onto Composites of UiO-66-NH2 and GO. ACS Appl. Mater. Interfaces 2021, 13, 17781–17790. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lei, Y.; Ke, L.; Bai, L.; Zheng, Y.; Xu, Z.; Li, T.; Chen, S.; Zhang, D. Construction of Self-Healing Multifunctional Hyper-Crosslinked Hyperbranched Polymer @ Metal–Organic Polyhedra (HHMOP) Membranes. Eur. Polym. J. 2024, 211, 112966. [Google Scholar] [CrossRef]
- Naz, N.; Manzoor, M.H.; Naqvi, S.M.G.; Ehsan, U.; Aslam, M.; Verpoort, F. Porous Organic Polymers; an Emerging Material Applied in Energy, Environmental and Biomedical Applications. Appl. Mater. Today 2024, 38, 102198. [Google Scholar] [CrossRef]
- Chen, B.; Chen, W.; Wang, M.; Peng, C.; Chen, B.; Chen, W.; Wang, M.; Peng, C. Unravelling the Multiple Synergies in MOF/CMP Supramolecular Heterojunction for Enhanced Artificial Photosynthesis. Adv. Mater. Interfaces 2023, 10, 2201971. [Google Scholar] [CrossRef]
| MOF | Surface Area (m2/g) | Characteristics | Advantages | Disadvantages | Applications | Ref. |
|---|---|---|---|---|---|---|
| ZIF-8 | ~1600 | Zn2+ + 2-methylimidazolate; sodalite-type | High thermal/chemical stability; simple synthesis | Hydrophobicity limits polar gas capture | CO2/CH4 separation, membranes, catalysis | [53,54,55,56] |
| ZIF-67 | ~1500 | Co2+ analog of ZIF-8; same topology | Redox-active; magnetic; electrocatalytic | Prone to oxidation | Electrocatalysis, batteries, sensors | [57,58] |
| UiO-66 | ~1100 | Zr6O4(OH)4 + BDC; fcu topology | High defect tolerance; stable in aqueous/acidic media | Smaller pore size | Drug delivery, catalysis, CO2 capture | [59,60] |
| UiO-67 | ~2500 | Zr6 + BPDC linker; larger pores | Accommodates larger guests | Costlier than UiO-66 | Guest encapsulation, pollutant adsorption | [61,62] |
| UiO-68 | ~3300 | Zr6 + TPDC; very large pores | Superior pore volume; tunable linker | Increased structural defects | Photocatalysis, dye adsorption, enzyme carriers | [63,64] |
| MIL-101(Cr) | ~4100 | Cr3+ + BDC; mesoporous cages | Very high porosity; chemical stability | Chromium toxicity; expensive | Methane/H2 storage, catalysis | [65,66] |
| MIL-53(Al) | ~1100 | Al3+ + BDC; breathing framework | Flexible; scalable | Pore instability on activation | CO2 separation, VOC adsorption | [67] |
| MIL-100(Fe) | ~2200–2900 | Fe3+ + BTC; MTN topology; mesoporous | Biocompatible; water-stable | Moderate crystallinity | Drug delivery, wastewater treatment | [68,69] |
| MOF-5 (IRMOF-1) | ~3800 | Zn4O + BDC; cubic pores | Historic benchmark; easy to modify | Very moisture-sensitive | H2 storage, small-molecule adsorption | [70,71] |
| HKUST-1 | ~1800 | Cu2+ paddlewheels + BTC | Open metal sites; strong π-interactions | Moisture instability | CO2/CH4 storage, catalysis, sensing | [72,73] |
| DUT-49 | ~5470 | Cu+ framework; flexible with NGA behavior | Very high surface area; responsive pores | Mechanically unstable | Gas adsorption, negative gas adsorption (NGA) | [74] |
| Hybrid Material | Type of Gas Separation | Permeability (Barrer) | Selectivity | Ref. nº |
|---|---|---|---|---|
| CAU-12-ODB/PIM-1 | H2/N2 | 7199 (H2) | 127 | [129] |
| ZIF-8/AO-PIM-1 | H2/CO2 | 5688 (H2) | 11.97 | [139] |
| ZIF-8/PIM-1 | O2/N2 | 1287 (O2) | 3.7 | [128] |
| ZIF-8/PIM-6FDA-OH MMMs | C3H6/C3H8 (propylene/propane) | 3.5 (propylene) | 30 | [126] |
| CALF-20/PIM-1 MMM | CO2/N2 | 8003 (CO2) | 25 | [105] |
| Mg-MOF-74 (CPO-27)/PIM-1 MMM | CO2/CH4 | 21,000 (CO2) | 19.1 | [124,146] |
| Mg-MOF-74 (CPO-27)/PIM-1 MMM | CO2/N2 | 21,000 (CO2) | 15.4 | [124,146] |
| MIL-101-HNO3/PIM-1 MMM | CO2/N2 | 14,879 (CO2) | 24.3 | [147] |
| MOF-801/PIM-1 MMM (5% load) | CO2/N2 | 9686 (CO2) | 27 | [148] |
| MOF-808/PIM-1 MMM (2% load) | CO2/N2 | 6854 (CO2) | 23.2 | [42] |
| MUF-15/PIM-1 MMM (optimal 5% load) | CO2/N2 | (38% increase over pure PIM-1) * | 17.72 | [149] |
| NH2-ZIF-7/PIM-1 MMM (20% load) | CO2/CH4 | 2953 (CO2) | 20.6 | [150,152] |
| agZIF-62/PIM-1 MMM (30% load) | CO2/CH4 | 5914 (CO2) | 67 | [151] |
| ZIF-S/PIM-1 MMM (15% load) | CO2/CH4 | 2805 (CO2) | 21.09 | [82] |
| Property/Aspect | MOFs | COFs | MOF–COF Hybrids |
|---|---|---|---|
| Composition | Metal ions or clusters linked by organic ligands | Covalent bonds between light elements (C, H, N, O) | Combination of metal ions (MOFs) and covalent organic frameworks (COFs) |
| Porosity | High, tunable | Permanent, highly ordered | Synergistic, with hierarchical porosity |
| Thermal/chemical stability | Moderate to high, but limited in harsh environments | High, with chemical robustness | Enhanced stability due to combined frameworks |
| Electronic properties | Strongly tunable via metal centers | Limited electronic properties | Tunable properties combining MOF’s metal nodes and COF’s organic framework |
| Functionalization | Broad, via choice of metal ions and linkers | High, via diverse organic chemistry | Enhanced functionalization through integration of metal centers and organic scaffolds |
| Synthesis challenges | Complex, often requiring specific solvothermal conditions | Moderate, with solvent-based or solid-state approaches | Complex, requiring careful hybridization strategies |
| Applications | Gas storage, catalysis, sensing | Energy storage, membranes, photocatalysis | Advanced applications: catalysis, gas separation, energy storage, and biomedicine |
| Advantages of hybrids | - | - | Synergy of MOF and COF properties: high surface area, tunable functionality, stability, and hierarchical pores |
| Strategy | Example Material | Application | Reference |
|---|---|---|---|
| MOF-first (in situ COF growth) | NH2-UiO-66@TFPT-DETH | Photocatalyst for hydrogen evolution | [183] |
| MOF-first (amorphous → crystalline COF) | NH2-MIL-125(Ti)@COF-LZU1 | Photocatalyst for hydrogenation of styrene | [184] |
| MOF-first (one-step COF synthesis) | NH2-MIL-101(Fe)@NTU-COF | Styrene oxidation catalyst | [185] |
| COF-first (layer-by-layer growth) | COF-300@ZIF-8 | Gas separation | [180] |
| COF-first (sequential MOF-on-COF growth) | Tp-Pa-1 COF@ZIF-8 | Photocatalyst for CO2 reduction | [186] |
| Post-synthetic linking | Fe3O4@A-TpBD@NH2-MIL-125(Ti) | EDC absorption and selectivity | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán-Egido, V.; García-Giménez, D.; Martínez-López, J.C.; Pérez-Vidal, L.; Carretero-González, J. Metal–Organic Frameworks as Fillers in Porous Organic Polymer-Based Hybrid Materials: Innovations in Composition, Processing, and Applications. Polymers 2025, 17, 1941. https://doi.org/10.3390/polym17141941
Durán-Egido V, García-Giménez D, Martínez-López JC, Pérez-Vidal L, Carretero-González J. Metal–Organic Frameworks as Fillers in Porous Organic Polymer-Based Hybrid Materials: Innovations in Composition, Processing, and Applications. Polymers. 2025; 17(14):1941. https://doi.org/10.3390/polym17141941
Chicago/Turabian StyleDurán-Egido, Victor, Daniel García-Giménez, Juan Carlos Martínez-López, Laura Pérez-Vidal, and Javier Carretero-González. 2025. "Metal–Organic Frameworks as Fillers in Porous Organic Polymer-Based Hybrid Materials: Innovations in Composition, Processing, and Applications" Polymers 17, no. 14: 1941. https://doi.org/10.3390/polym17141941
APA StyleDurán-Egido, V., García-Giménez, D., Martínez-López, J. C., Pérez-Vidal, L., & Carretero-González, J. (2025). Metal–Organic Frameworks as Fillers in Porous Organic Polymer-Based Hybrid Materials: Innovations in Composition, Processing, and Applications. Polymers, 17(14), 1941. https://doi.org/10.3390/polym17141941





