Plasticized Starch/Gelatin Blends with Humidity-Activated Shape-Memory Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Characterization Techniques
3. Results
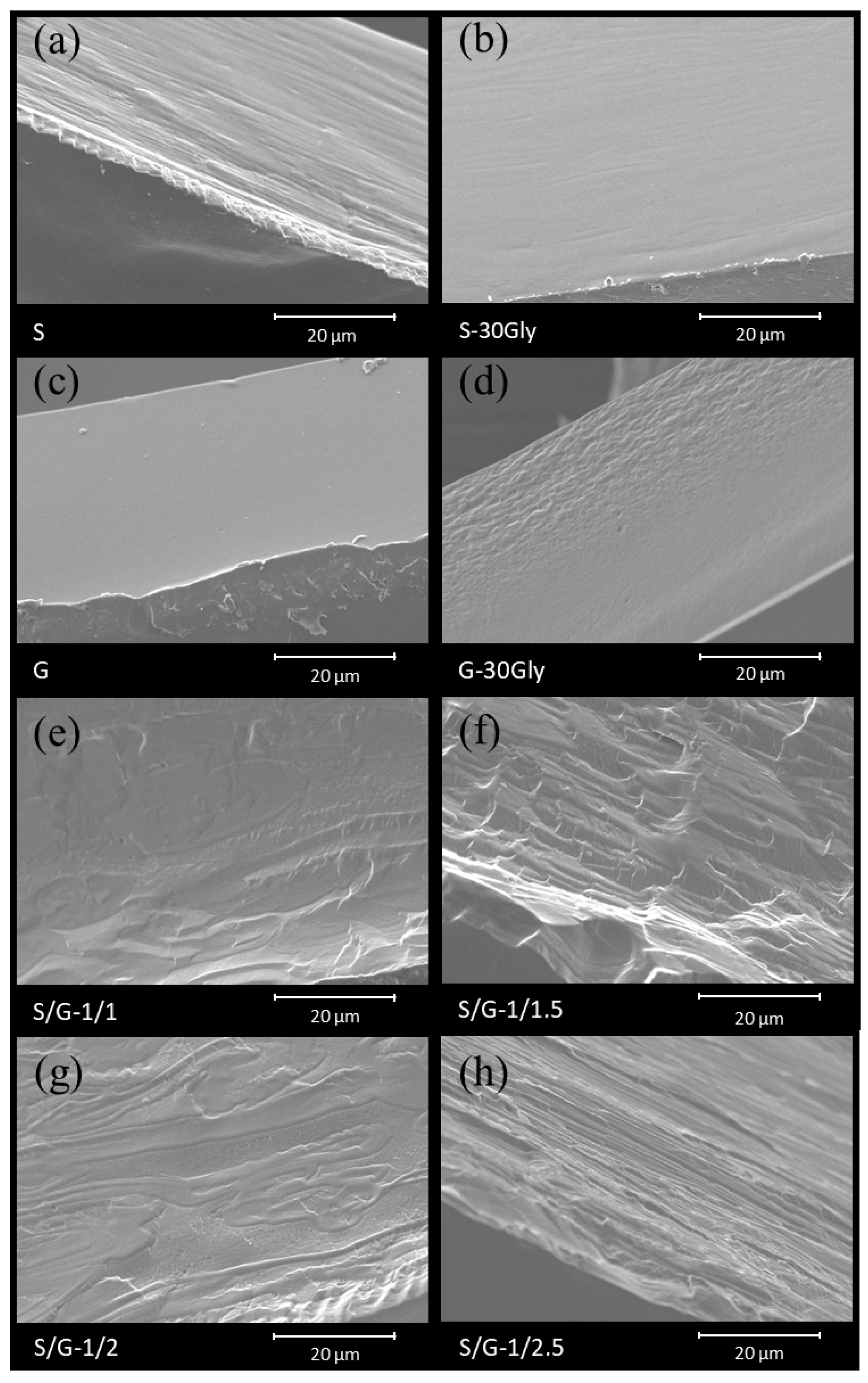
3.1. Scanning Electron Microscopy (SEM)
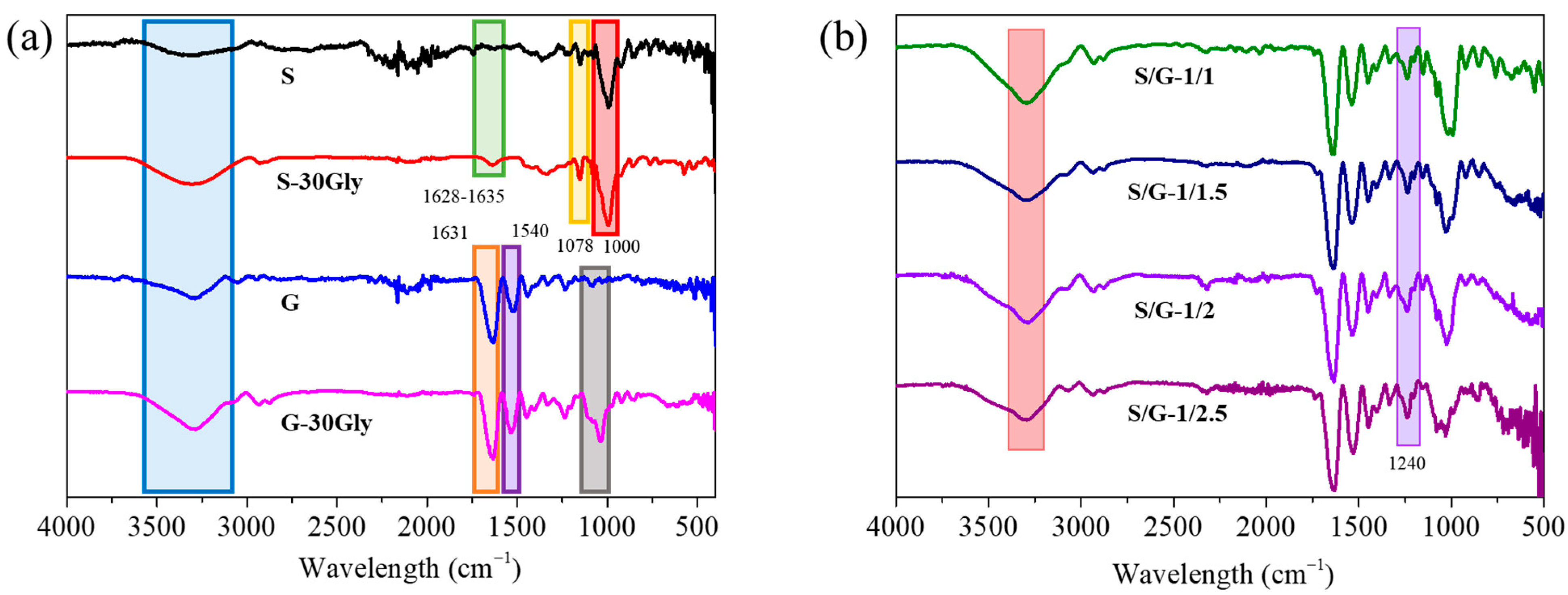
3.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.3. Raman Spectroscopy
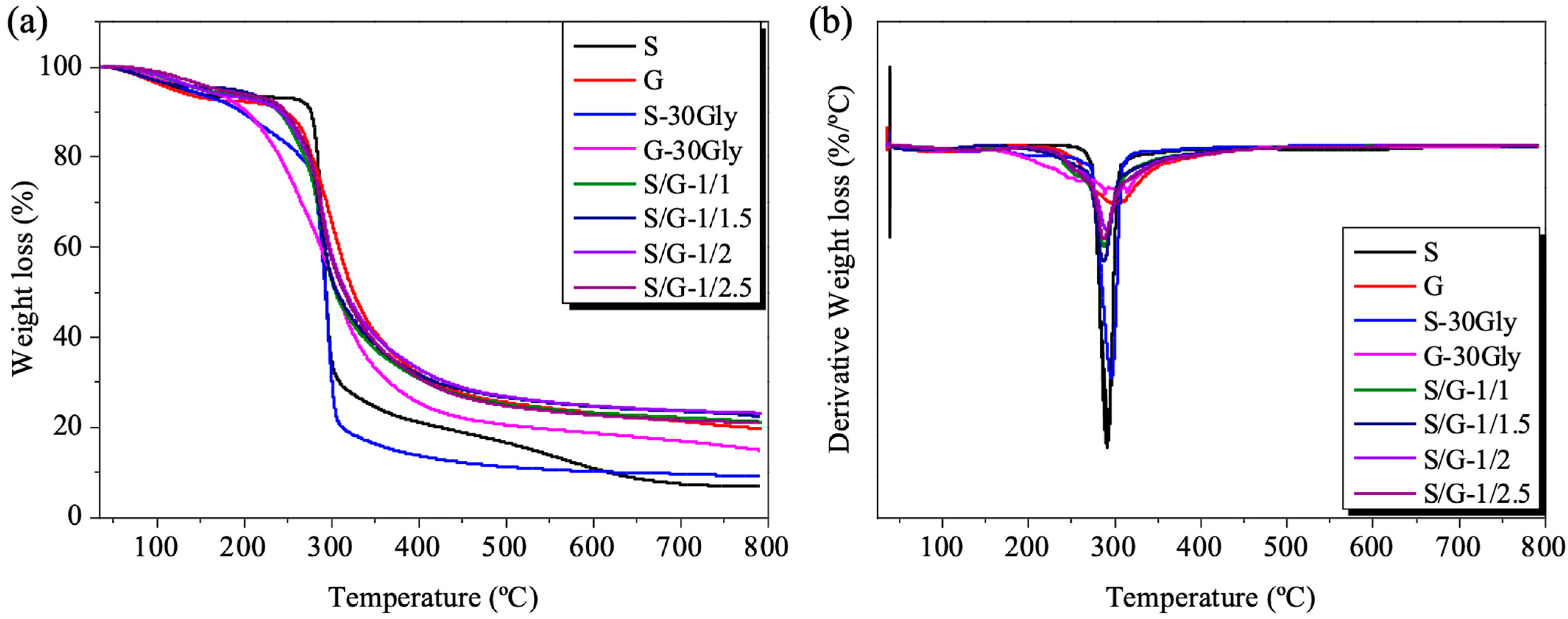
3.4. Thermogravimetric Analysis (TGA)
3.5. Dynamic Mechanical Thermal Analysis (DMTA)
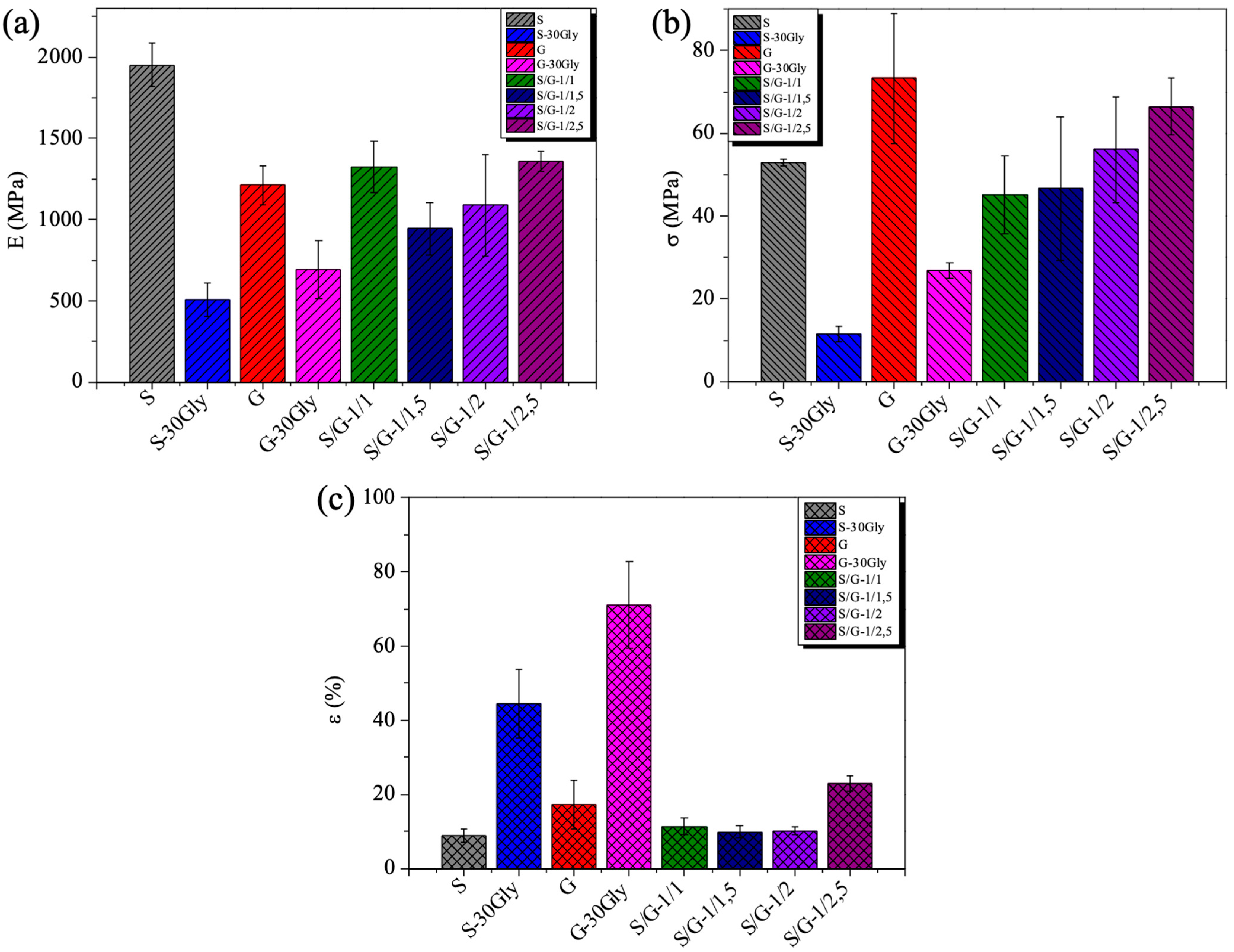
3.6. Mechanical Properties
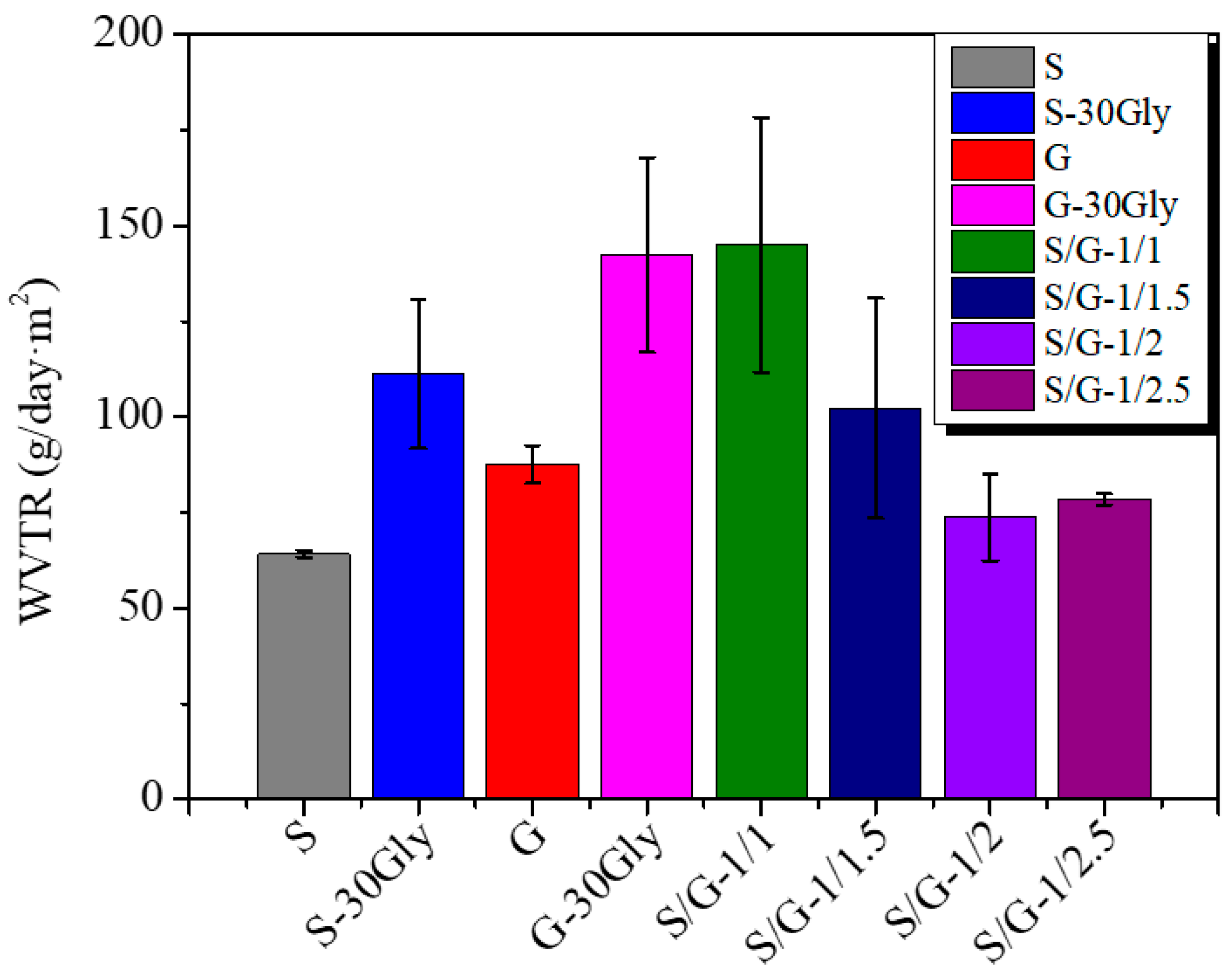
3.7. Water Vapor Transmission Rate (WVTR)
3.8. Surface Roughness
3.9. Water Contact Angle (WCA)
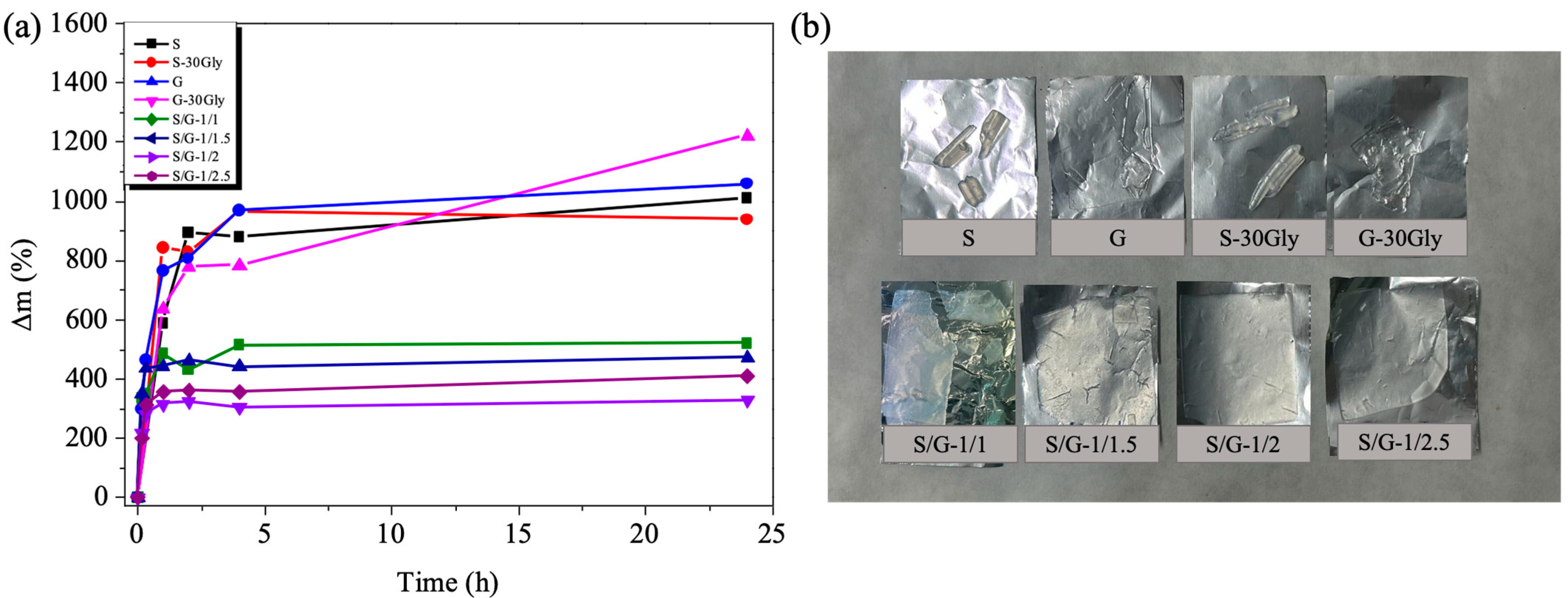
3.10. Moisture Absorption and Moisture Loss
3.11. Water Uptake Rate (WU)
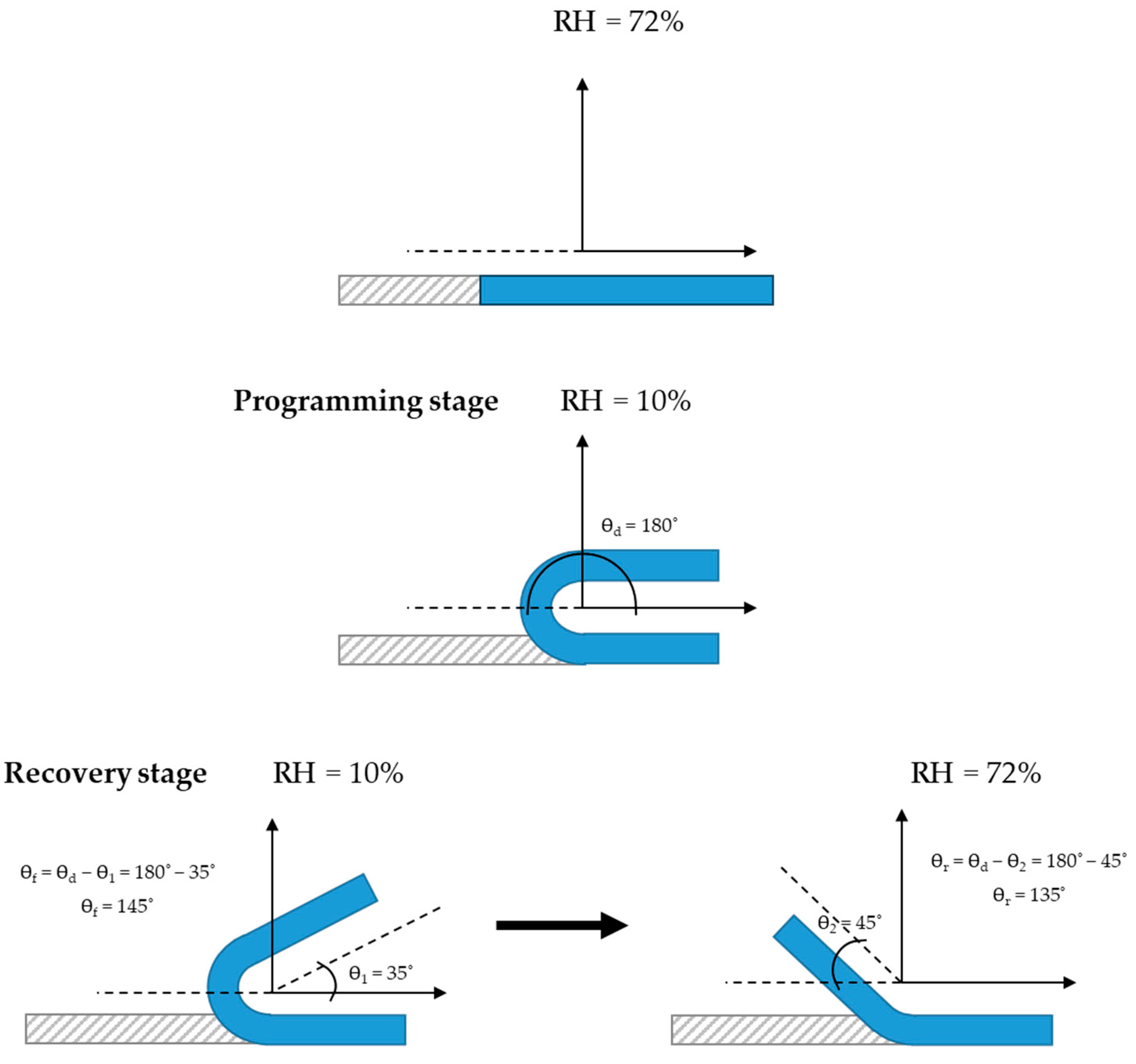
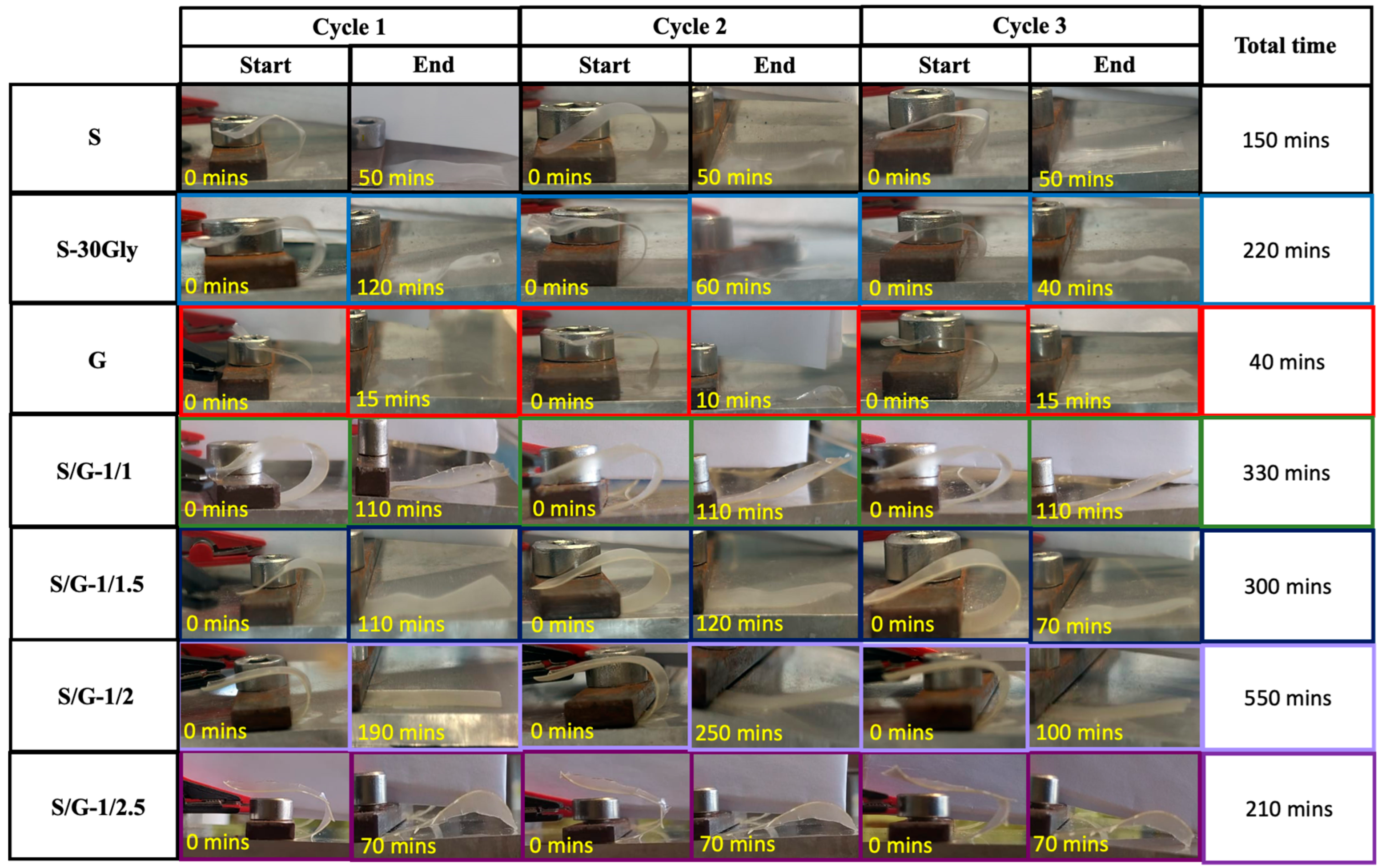
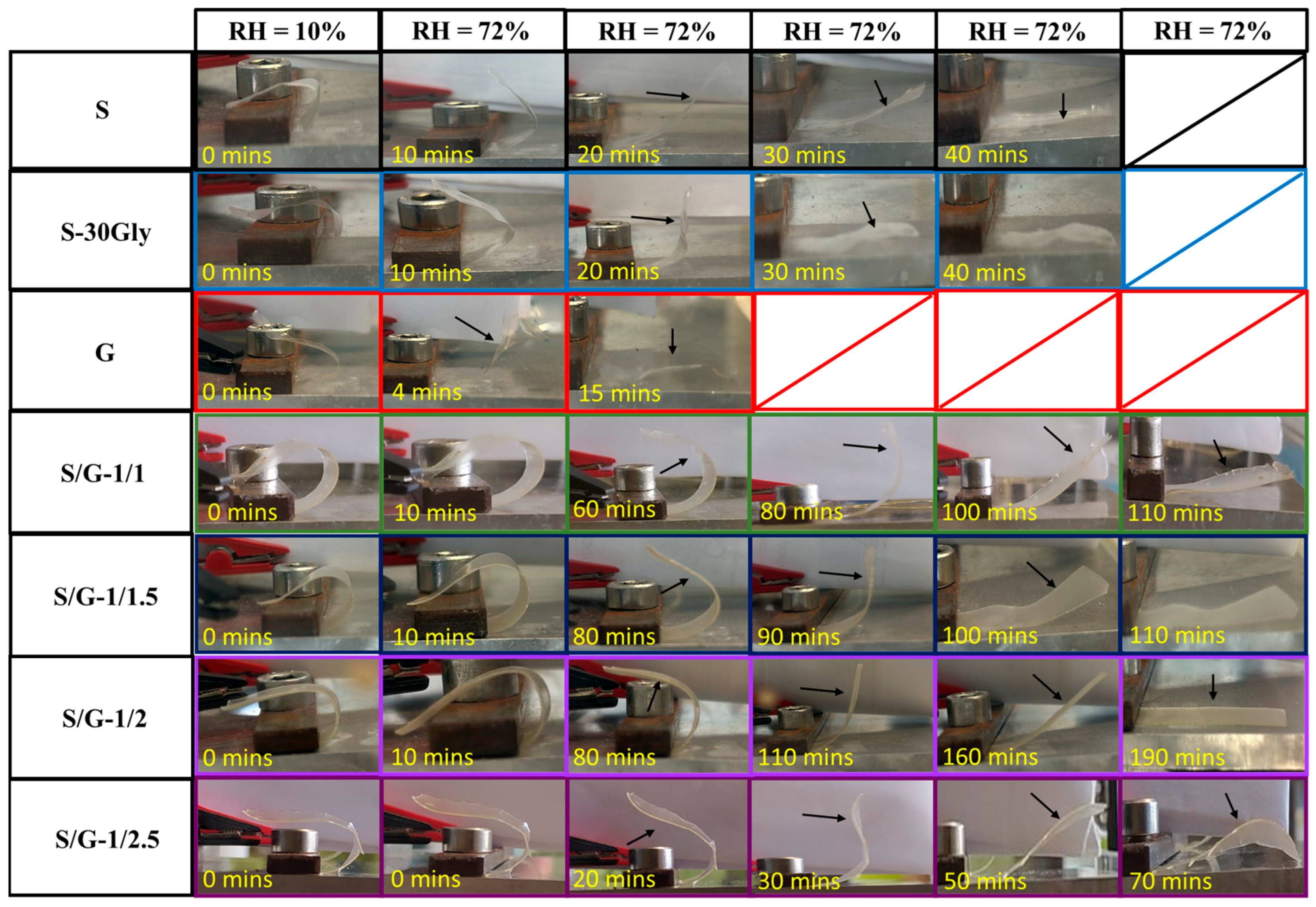
3.12. Humidity-Activated Shape-Memory Behaviour
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, Y.V.; Čuček, L.; Si, C.; Jiang, P.; Vujanović, A.; Krajnc, D.; Lee, C.T. Uncovering Environmental Performance Patterns of Plastic Packaging Waste in High Recovery Rate Countries: An Example of EU-27. Environ. Res. 2024, 241, 117581. [Google Scholar] [CrossRef]
- Stegmann, P.; Daioglou, V.; Londo, M.; van Vuuren, D.P.; Junginger, M. Plastic Futures and Their CO2 Emissions. Nature 2022, 612, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Thivya, P.; Gururaj, P.N.; Reddy, N.B.P.; Rajam, R. Recent Advances in Protein-Polysaccharide Based Biocomposites and Their Potential Applications in Food Packaging: A Review. Int. J. Biol. Macromol. 2024, 268, 131757. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Kenny, J.M. Shape Memory Polymers: Properties, Synthesis and Applications. In Smart Polymers and Their Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 204–236. [Google Scholar]
- Olalla, A.S.; Sessini, V.; Torres, E.G.; Peponi, L. Smart Nanocellulose Composites with Shape-Memory Behavior. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements; Elsevier: Amsterdam, The Netherlands, 2016; pp. 277–312. [Google Scholar]
- Peponi, L.; Arrieta, M.P.; Mujica-Garcia, A.; López, D. Smart Polymers. In Modification of Polymer Properties; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–154. [Google Scholar]
- Sessini, V.; Arrieta, M.P.; Fernández-Torres, A.; Peponi, L. Humidity-Activated Shape Memory Effect on Plasticized Starch-Based Biomaterials. Carbohydr. Polym. 2018, 179, 93–99. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.-C.; Åman, P. The Influence of Amylose and Amylopectin Characteristics on Gelatinization and Retrogradation Properties of Different Starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Niranjana Prabhu, T.; Prashantha, K. A Review on Present Status and Future Challenges of Starch Based Polymer Films and Their Composites in Food Packaging Applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Muñoz-Gimena, P.F.; Oliver-Cuenca, V.; Peponi, L.; López, D. A Review on Reinforcements and Additives in Starch-Based Composites for Food Packaging. Polymers 2023, 15, 2972. [Google Scholar] [CrossRef]
- Jane, J.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of Amylopectin Branch Chain Length and Amylose Content on the Gelatinization and Pasting Properties of Starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. Effect of Glycerol on the Morphology of Nanocomposites Made from Thermoplastic Starch and Starch Nanocrystals. Carbohydr. Polym. 2011, 84, 203–210. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J. Formamide as the Plasticizer for Thermoplastic Starch. J. Appl. Polym. Sci. 2004, 93, 1769–1773. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, P.; Li, Y.; Li, J.; Li, X.; Yang, J.; Ji, M.; Li, F.; Zhang, C. Recent Advances and Future Challenges of the Starch-Based Bio-Composites for Engineering Applications. Carbohydr. Polym. 2023, 307, 120627. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Garavito, J.; Peña-Venegas, C.P.; Castellanos, D.A. Production of Starch-Based Flexible Food Packaging in Developing Countries: Analysis of the Processes, Challenges, and Requirements. Foods 2024, 13, 4096. [Google Scholar] [CrossRef]
- Oliver-Cuenca, V.; Salaris, V.; Muñoz-Gimena, P.F.; Agüero, Á.; Peltzer, M.A.; Montero, V.A.; Arrieta, M.P.; Sempere-Torregrosa, J.; Pavon, C.; Samper, M.D.; et al. Bio-Based and Biodegradable Polymeric Materials for a Circular Economy. Polymers 2024, 16, 3015. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, R.; Han, J.; Ren, L.; Jiang, L. Protein-Based Packaging Films in Food: Developments, Applications, and Challenges. Gels 2024, 10, 418. [Google Scholar] [CrossRef]
- Toniciolli Rigueto, C.V.; Rosseto, M.; Alessandretti, I.; de Oliveira, R.; Wohlmuth, D.A.R.; Ferreira Menezes, J.; Loss, R.A.; Dettmer, A.; Pizzutti, I.R. Gelatin Films from Wastes: A Review of Production, Characterization, and Application Trends in Food Preservation and Agriculture. Food Res. Int. 2022, 162, 112114. [Google Scholar] [CrossRef]
- Tyuftin, A.A.; Kerry, J.P. Gelatin Films: Study Review of Barrier Properties and Implications for Future Studies Employing Biopolymer Films. Food Packag. Shelf Life 2021, 29, 100688. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Advances and Challenges in Biopolymer-Based Films. Polymers 2022, 14, 3920. [Google Scholar] [CrossRef]
- UNE 53097:2002; Sheet Materials—Determination of Water Vapour Transmission Rate—Gravimetric (Dish) Method. Asociación Española de Normalización—UNE: Madrid, Spain, 2002. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0026745 (accessed on 20 June 2025).
- Vogt, B.D.; Soles, C.L.; Lee, H.-J.; Lin, E.K.; Wu, W. Moisture Absorption into Ultrathin Hydrophilic Polymer Films on Different Substrate Surfaces. Polymer 2005, 46, 1635–1642. [Google Scholar] [CrossRef]
- Chen, S.; Hu, J.; Yuen, C.; Chan, L. Novel Moisture-Sensitive Shape Memory Polyurethanes Containing Pyridine Moieties. Polymer 2009, 50, 4424–4428. [Google Scholar] [CrossRef]
- Tan, S.X.; Ong, H.C.; Andriyana, A.; Lim, S.; Pang, Y.L.; Kusumo, F.; Ngoh, G.C. Characterization and Parametric Study on Mechanical Properties Enhancement in Biodegradable Chitosan-Reinforced Starch-Based Bioplastic Film. Polymers 2022, 14, 278. [Google Scholar] [CrossRef]
- Dzeikala, O.; Prochon, M.; Marzec, A.; Szczepanik, S. Preparation and Characterization of Gelatin-Agarose and Gelatin-Starch Blends Using Alkaline Solvent. Int. J. Mol. Sci. 2023, 24, 1473. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ghoshal, G.; Goyal, M. Biodegradable Composite Films/Coatings of Modified Corn Starch/Gelatin for Shelf Life Improvement of Cucumber. J. Food Sci. Technol. 2021, 58, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tu, Z.; Sha, X.; Hu, Y.; Chen, N.; Wang, H. Fabrication and Performance Evaluation of Pectin–Fish Gelatin–Resveratrol Preservative Films. Food Chem. 2021, 361, 129832. [Google Scholar] [CrossRef] [PubMed]
- Sow, L.C.; Peh, Y.R.; Pekerti, B.N.; Fu, C.; Bansal, N.; Yang, H. Nanostructural Analysis and Textural Modification of Tilapia Fish Gelatin Affected by Gellan and Calcium Chloride Addition. LWT—Food Sci. Technol. 2017, 85, 137–145. [Google Scholar] [CrossRef]
- Syahputra, R.A.; Rani, Z.; Ridwanto, R.; Miswanda, D.; Pulungan, A.F. Isolation and Characterization of Glycerol by Transesterification of Used Cooking Oil. RASAYAN J. Chem. 2023, 16, 648–652. [Google Scholar] [CrossRef]
- Duconseille, A.; Gaillard, C.; Santé-Lhoutellier, V.; Astruc, T. Molecular and Structural Changes in Gelatin Evidenced by Raman Microspectroscopy. Food Hydrocoll. 2018, 77, 777–786. [Google Scholar] [CrossRef]
- Frushour, B.G.; Koenig, J.L. Raman Scattering of Collagen, Gelatin, and Elastin. Biopolymers 1975, 14, 379–391. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N.; Yu, L.; Zhou, S.; Shanks, R.; Zheng, J. Imaging the Phase of Starch–Gelatin Blends by Confocal Raman Microscopy. Food Hydrocoll. 2016, 60, 7–10. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Gobinet, C.; Feru, J.; -Pasco, S.B.; Manfait, M.; Piot, O. Characterization of Type I and IV Collagens by Raman Microspectroscopy: Identification of Spectral Markers of the Dermo-Epidermal Junction. J. Spectrosc. 2012, 27, 421–427. [Google Scholar] [CrossRef]
- Alimova, A.; Chakraverty, R.; Muthukattil, R.; Elder, S.; Katz, A.; Sriramoju, V.; Lipper, S.; Alfano, R.R. In Vivo Molecular Evaluation of Guinea Pig Skin Incisions Healing after Surgical Suture and Laser Tissue Welding Using Raman Spectroscopy. J. Photochem. Photobiol. B 2009, 96, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, J.; Oba, K.; Arase, Y.; Kaneshiro, J.; Tate, S.; Watanabe, T.M. Quantitative Prediction of Rice Starch Digestibility Using Raman Spectroscopy and Multivariate Calibration Analysis. Food Chem. 2024, 435, 137505. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Zhu, W.; Chikaguchi, H.; Marin, E.; Masumura, T.; Sato, Y.; Nakazaki, T. Raman Spectroscopic Analysis of Polysaccharides in Popular Japanese Rice Cultivars. Food Chem. 2021, 354, 129434. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Siddiqui, M.A.; Chandio, A.D.; Shar, M.A.; Alhazaa, A. Multi-Shaded Edible Films Based on Gelatin and Starch for the Packaging Applications. Polymers 2022, 14, 5020. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-Mechanical, Rheological, Structural and Antimicrobial Properties of Bionanocomposite Films Based on Fish Skin Gelatin and Silver-Copper Nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Barreto, P.L.M.; Pires, A.T.N.; Soldi, V. Thermal Degradation of Edible Films Based on Milk Proteins and Gelatin in Inert Atmosphere. Polym. Degrad. Stab. 2003, 79, 147–152. [Google Scholar] [CrossRef]
- Prochoń, M.; Marzec, A.; Szadkowski, B. Preparation and Characterization of New Environmentally Friendly Starch-Cellulose Materials Modified with Casein or Gelatin for Agricultural Applications. Materials 2019, 12, 1684. [Google Scholar] [CrossRef]
- Lourdin, D.; Bizot, H.; Colonna, P. “Antiplasticization” in Starch-Glycerol Films? J. Appl. Polym. Sci. 1997, 63, 1047–1053. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Unexpected Plasticization Effects on the Structure and Properties of Polyelectrolyte Complexed Chitosan/Alginate Materials. ACS Appl. Polym. Mater. 2020, 2, 2957–2966. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Ye, R.; Xiao, J.; Liu, Y.; Ding, J.; Zhang, S.; Liu, A. Mechanical and Barrier Properties of Maize Starch–Gelatin Composite Films: Effects of Amylose Content. J. Sci. Food Agric. 2017, 97, 3613–3622. [Google Scholar] [CrossRef]
- Podshivalov, A.; Zakharova, M.; Glazacheva, E.; Uspenskaya, M. Gelatin/Potato Starch Edible Biocomposite Films: Correlation between Morphology and Physical Properties. Carbohydr. Polym. 2017, 157, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water Vapor Permeability of Edible Starch Based Films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- EFSA Supporting Publications. Principles That Could Be Applicable to the Safety Assessment of the Use of Mixtures of Natural Origin to Manufacture Food Contact Materials; EFSA: Parma, Italy, 2013. [Google Scholar]
- Averous, L.; Boquillon, N. Biocomposites Based on Plasticized Starch: Thermal and Mechanical Behaviours. Carbohydr. Polym. 2004, 56, 111–122. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Correlations between Structural, Barrier, Thermal and Mechanical Properties of Plasticized Gelatin Films. Innov. Food Sci. Emerg. Technol. 2010, 11, 369–375. [Google Scholar] [CrossRef]
- Chivrac, F.; Angellier-Coussy, H.; Guillard, V.; Pollet, E.; Avérous, L. How Does Water Diffuse in Starch/Montmorillonite Nano-Biocomposite Materials? Carbohydr. Polym. 2010, 82, 128–135. [Google Scholar] [CrossRef]
- Goument, C.; Gerges, T.; Lombard, P.; Lakhdar, H.; Arli, M.; Gilmus, V.M.; Lambert, S.A.; Allard, B.; Charmeau, J.-Y.; Cabrera, M. In-Mold Electronics on Poly(Lactic Acid): Towards a More Sustainable Mass Production of Plastronic Devices. Int. J. Adv. Manuf. Technol. 2023, 125, 2643–2660. [Google Scholar] [CrossRef]
- Rodrigues, M.Á.V.; Bertolo, M.R.V.; Marangon, C.A.; Martins, V.d.C.A.; Plepis, A.M.d.G. Chitosan and Gelatin Materials Incorporated with Phenolic Extracts of Grape Seed and Jabuticaba Peel: Rheological, Physicochemical, Antioxidant, Antimicrobial and Barrier Properties. Int. J. Biol. Macromol. 2020, 160, 769–779. [Google Scholar] [CrossRef]
- Biazuz, J.; Zardo, P.; Rodrigues-Junior, S.A. Water Sorption, Solubility and Surface Roughness of Resin Surface Sealants. Braz. J. Oral. Sci. 2015, 14, 27–30. [Google Scholar] [CrossRef]




| Sample | Composition (w%) | ||
|---|---|---|---|
| Starch | Glycerol | Gelatin | |
| S | 100 | - | - |
| S-30Gly | 70 | 30 | - |
| G | 100 | - | - |
| G-30Gly | - | 30 | 70 |
| S/G-1/1 | 43.5 | 13 | 43.5 |
| S/G-1/1.5 | 35.7 | 10.7 | 53.6 |
| S/G-1/2 | 30.3 | 9.1 | 60.6 |
| S/G-1/2.5 | 26.3 | 7.9 | 65.8 |
| Sample | Tmax (°C) |
|---|---|
| S | 291 |
| S-30Gly | 297 |
| G | 301 |
| G-30Gly | 289 |
| S/G-1/1 | 289 |
| S/G-1/1.5 | 288 |
| S/G-1/2 | 288 |
| S/G-1/2.5 | 291 |
| Sample | E (MPa) | σ (MPa) | ε (%) |
|---|---|---|---|
| S | 1951 ± 134 a | 53 ± 1 a | 9 ± 2 a |
| S-30Gly | 507 ± 105 b | 11 ± 2 b | 44 ± 9 b |
| G | 1213 ± 118 c | 73 ± 16 a | 17 ± 7 a |
| G-30Gly | 693 ± 181 b | 27 ± 2 b | 71 ± 12 c |
| S/G-1/1 | 1326 ± 160 c | 45 ± 9 c | 11 ± 2 a |
| S/G-1/1.5 | 945 ± 162 b, c | 47 ± 17 c | 10 ± 2 a |
| S/G-1/2 | 1090 ± 312 c | 56 ± 13 d | 10 ± 1 a |
| S/G-1/2.5 | 1361 ± 61 c | 66 ± 7 d | 23 ± 2 a |
| F ratio | 2.5543 | 8.30623 | 3.63972 |
| p-Value | 0.03224 * | 0.0000083 * | 0.00766 * |
| Sample | Sa (µm) | Height Ratio |
|---|---|---|
| S | 1.2 ± 0.1 | 1.49 ± 0.03 |
| S-30Gly | 0.36 ± 0.06 | 1.183 ± 0.005 |
| G | 0.35 ± 0.03 | 1.19 ± 0.01 |
| G-30Gly | 0.32 ± 0.01 | 1.198 ± 0.003 |
| S/G-1/1 | 1.4 ± 0.3 | 1.87 ± 0.06 |
| S/G-1/1.5 | 2.5 ± 0.3 | 1.93 ± 0.07 |
| S/G-1/2 | 2.5 ± 0.5 | 1.52 ± 0.02 |
| S/G-1/2.5 | 4.0 ± 0.2 | 1.62 ± 0.06 |
| Sample | Cycle | Rf (%) | Rr (%) |
|---|---|---|---|
| S | 1 | 100 | 97.6 |
| 2 | 100 | 96.6 | |
| 3 | 100 | 99.0 | |
| S-30Gly | 1 | 100 | 94.9 |
| 2 | 100 | 96.8 | |
| 3 | 100 | 99.1 | |
| G | 1 | 85.2 | 99.1 |
| 2 | 94.5 | 96.5 | |
| 3 | 100 | 91.2 | |
| S/G-1/1 | 1 | 100 | 94.8 |
| 2 | 100 | 89.4 | |
| 3 | 100 | 92.1 | |
| S/G-1/1.5 | 1 | 100 | 91.5 |
| 2 | 100 | 96.0 | |
| 3 | 100 | 94.0 | |
| S/G-1/2 | 1 | 100 | 99.7 |
| 2 | 100 | 93.2 | |
| 3 | 100 | 97.9 | |
| S/G-1/2.5 | 1 | 95.8 | 97.9 |
| 2 | 88.9 | 94.1 | |
| 3 | 89.5 | 97.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver-Cuenca, V.; Muñoz-Menzinger, A.; Arrieta, M.P.; López, D.; Peponi, L. Plasticized Starch/Gelatin Blends with Humidity-Activated Shape-Memory Behavior. Polymers 2025, 17, 1763. https://doi.org/10.3390/polym17131763
Oliver-Cuenca V, Muñoz-Menzinger A, Arrieta MP, López D, Peponi L. Plasticized Starch/Gelatin Blends with Humidity-Activated Shape-Memory Behavior. Polymers. 2025; 17(13):1763. https://doi.org/10.3390/polym17131763
Chicago/Turabian StyleOliver-Cuenca, Victor, Ana Muñoz-Menzinger, Marina P. Arrieta, Daniel López, and Laura Peponi. 2025. "Plasticized Starch/Gelatin Blends with Humidity-Activated Shape-Memory Behavior" Polymers 17, no. 13: 1763. https://doi.org/10.3390/polym17131763
APA StyleOliver-Cuenca, V., Muñoz-Menzinger, A., Arrieta, M. P., López, D., & Peponi, L. (2025). Plasticized Starch/Gelatin Blends with Humidity-Activated Shape-Memory Behavior. Polymers, 17(13), 1763. https://doi.org/10.3390/polym17131763









