Effect of Irradiated Nanocellulose on Enhancing the Functionality of Polylactic Acid-Based Composite Films for Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. The Materials and Chemicals
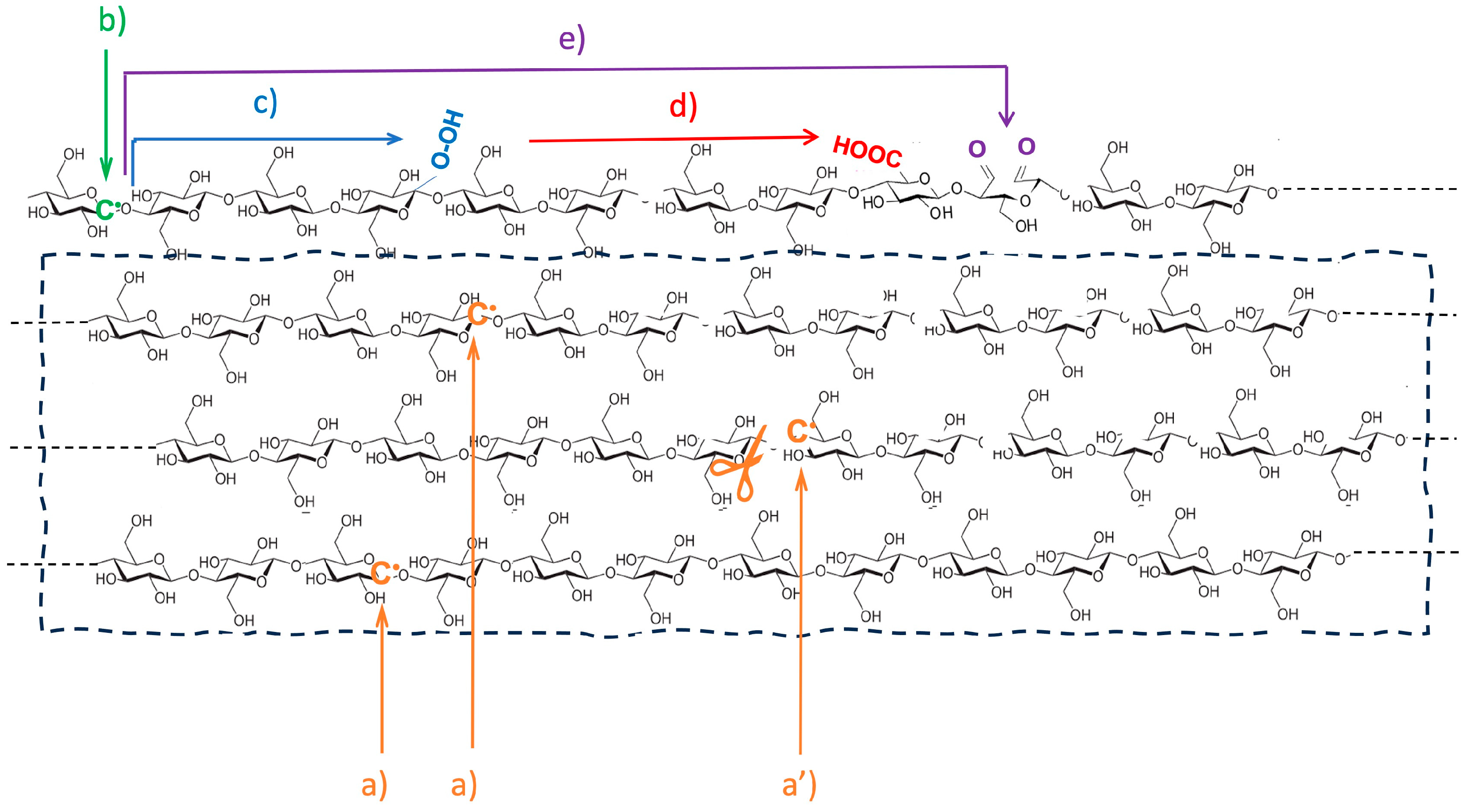
2.2. Irradiation Procedure
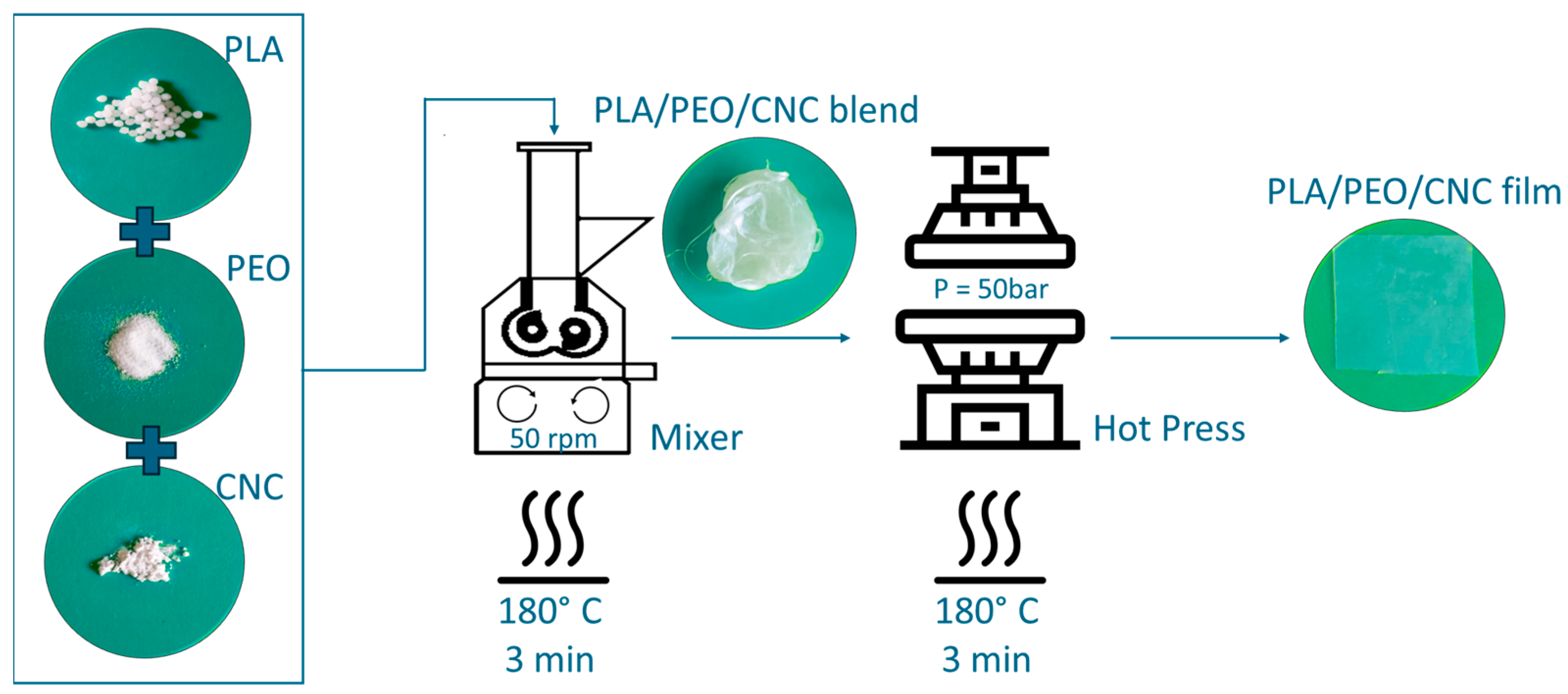
2.3. PLA-Based Film Preparation
2.4. Experimental Characterization of CNC Powder and PLA-Based Films
2.4.1. Scanning Electron Microscope (SEM)
2.4.2. Wide-Angle X-Ray Scattering (WAXS)
2.4.3. FTIR Analysis
2.4.4. Analysis Thermogravimetric Analysis (TGA)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Mechanical Properties Tests
2.4.7. Barrier Properties Tests
2.4.8. Surface Wettability: Contact Angle Measurements
2.4.9. Color Analysis Measurements
2.4.10. ABTS Radical Scavenging
3. Results
3.1. Powder Characterization
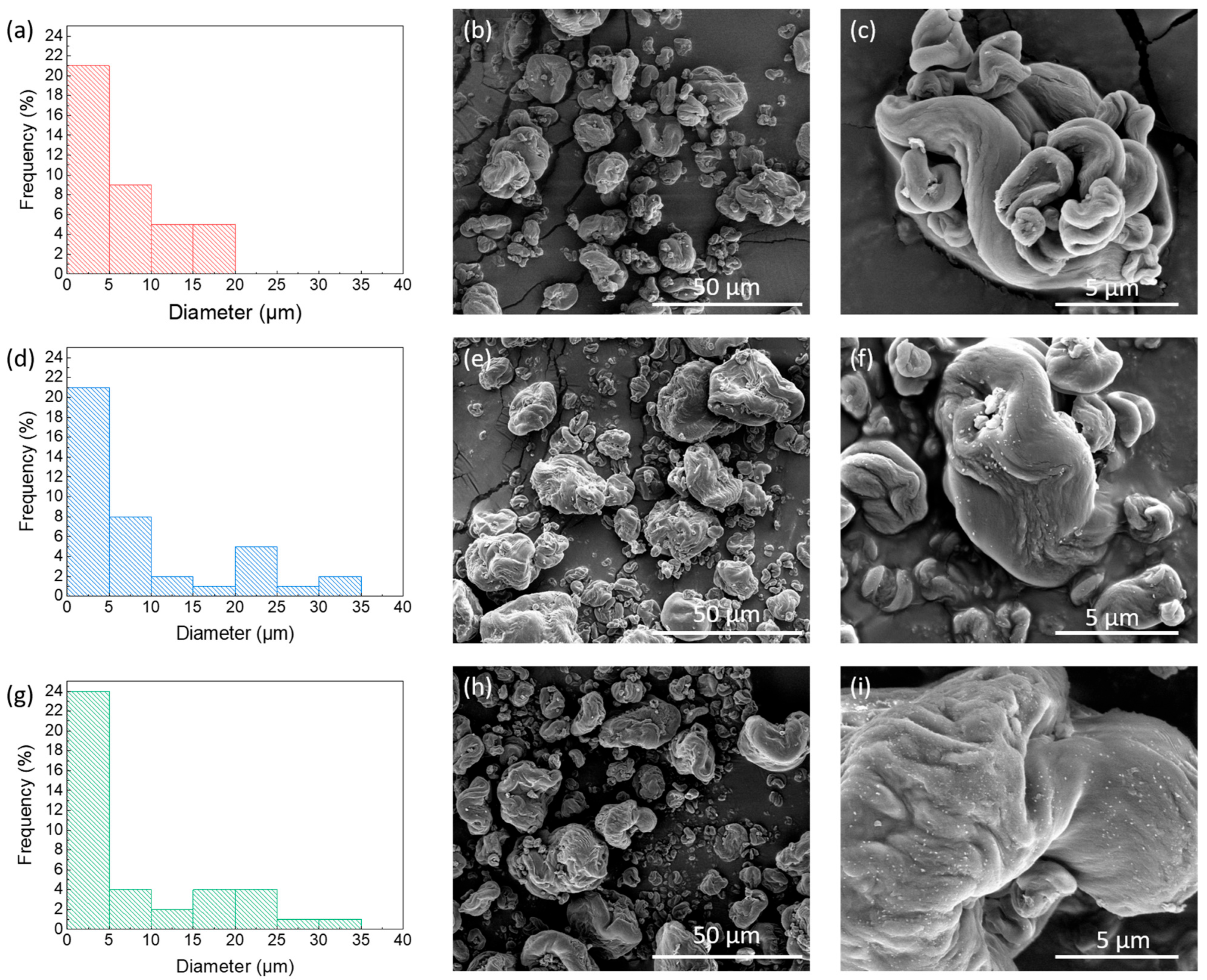
3.1.1. Morphological Powder Analyses
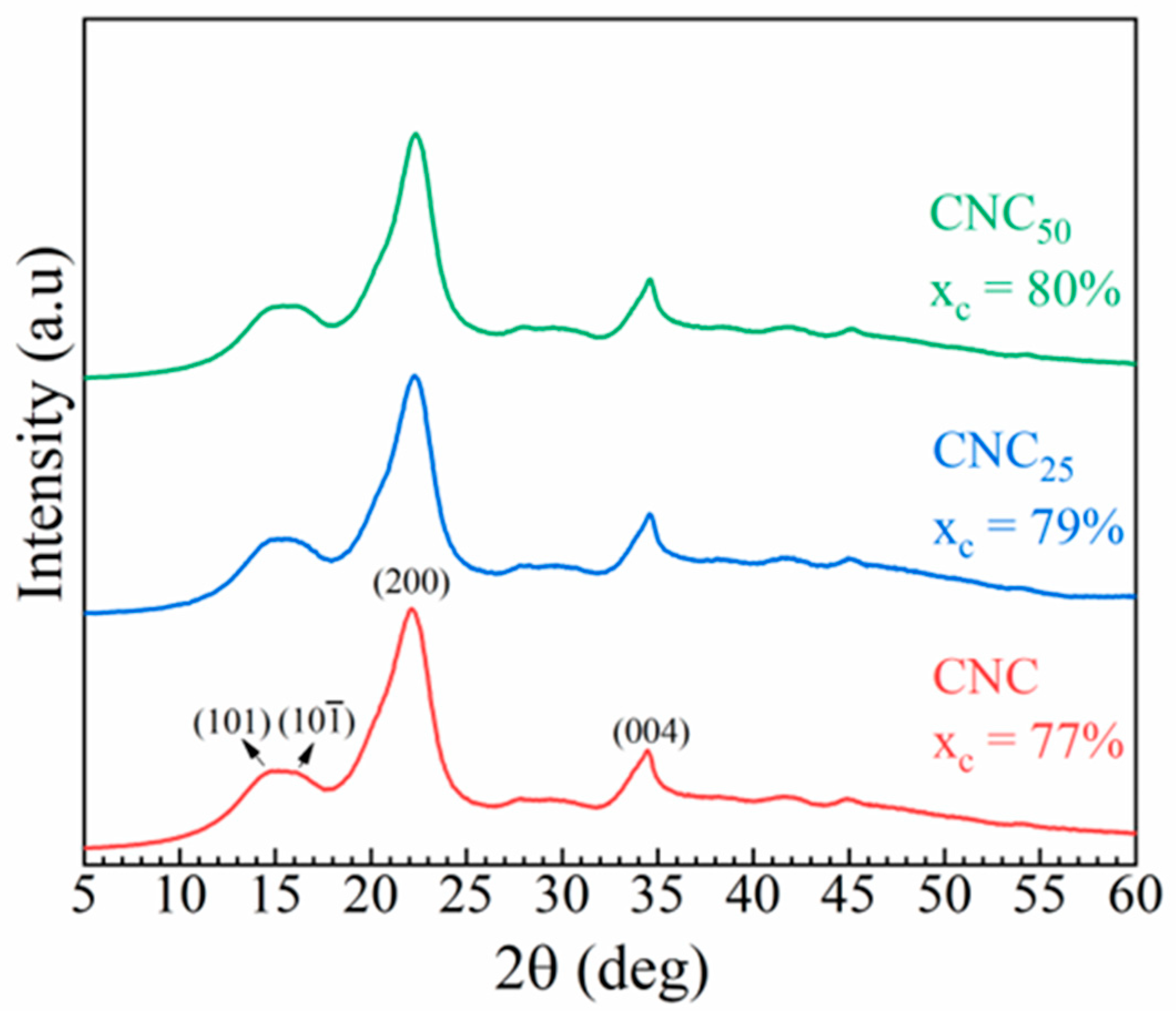
3.1.2. Structural Powder Analyses
3.2. Film Characterization
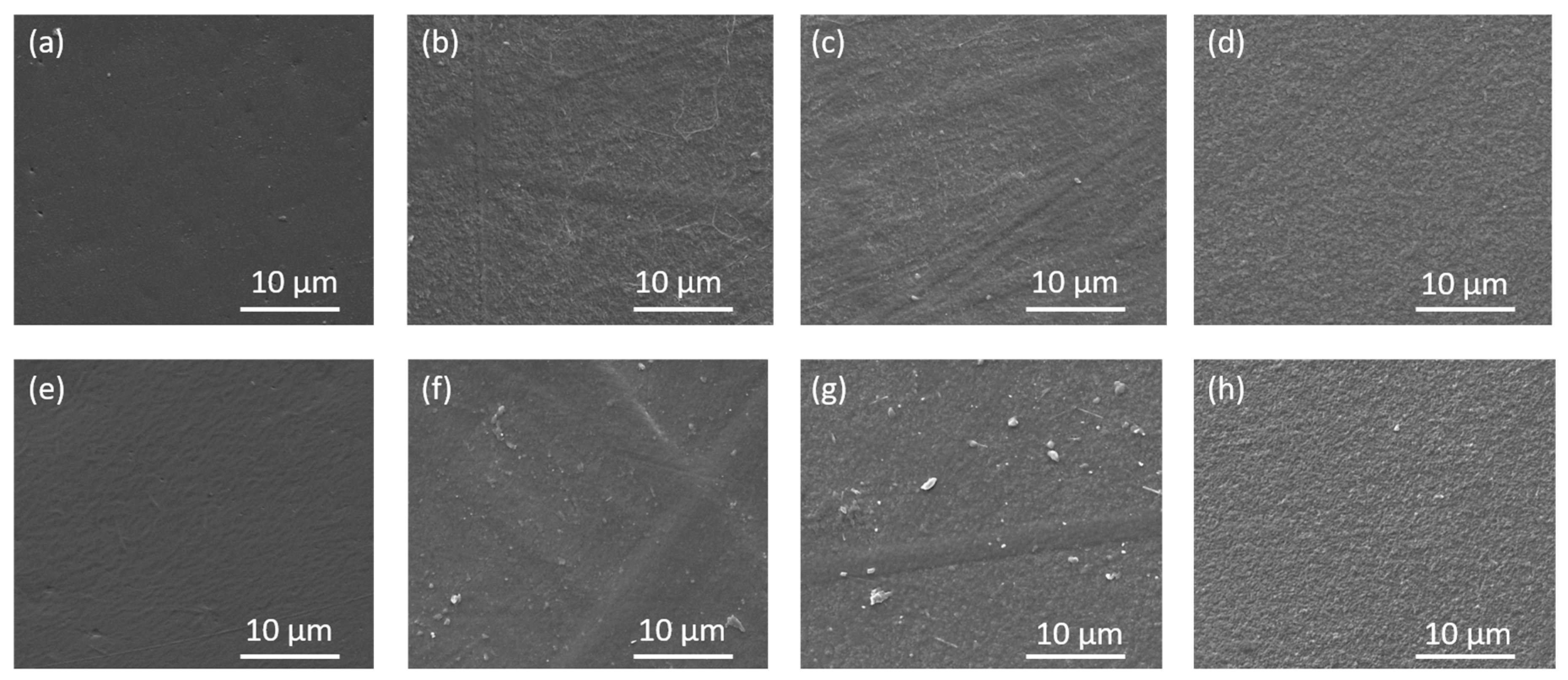
3.2.1. Morphological Analyses of Films
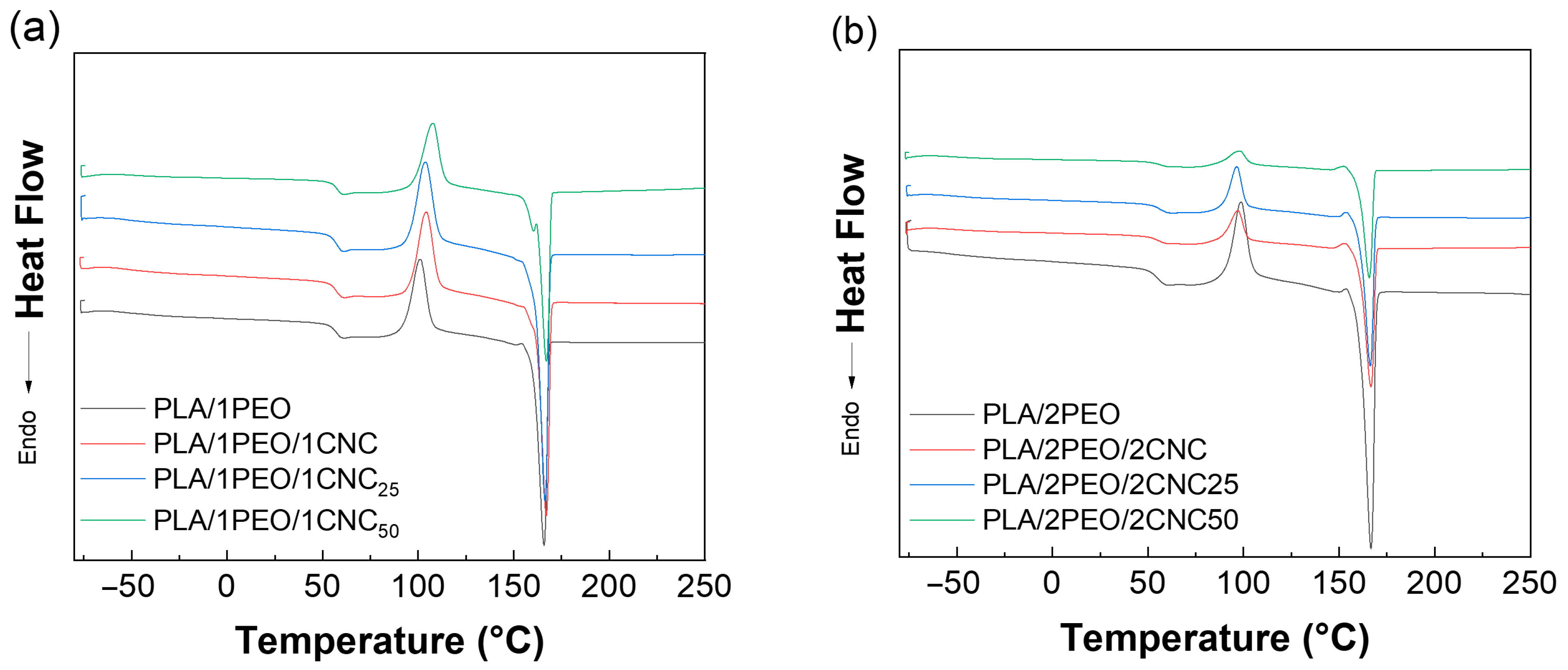
3.2.2. Structural Analyses of Films
3.2.3. Mechanical Properties
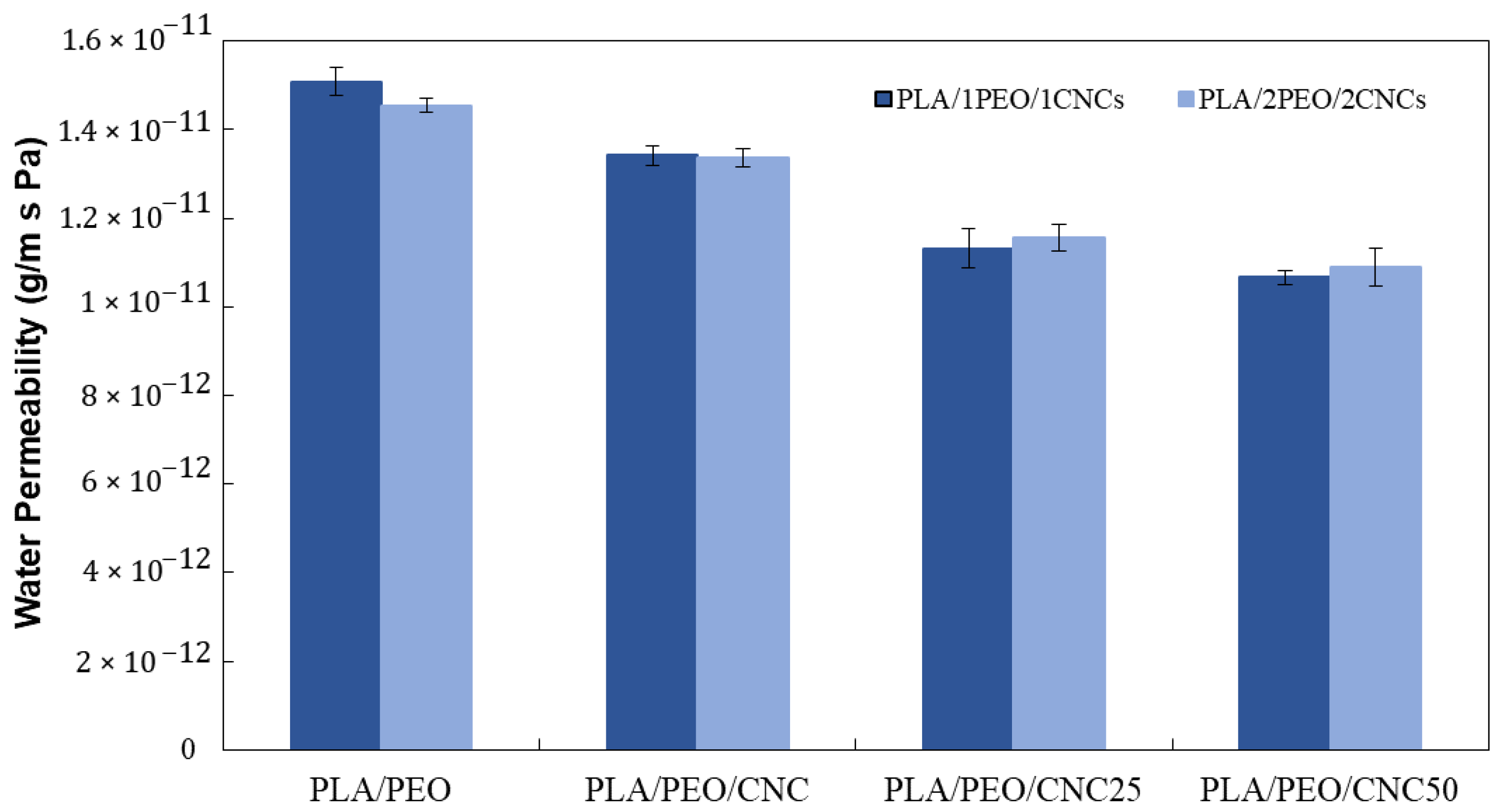
3.2.4. Water Permeability and Surface Wettability
3.2.5. Color Analysis
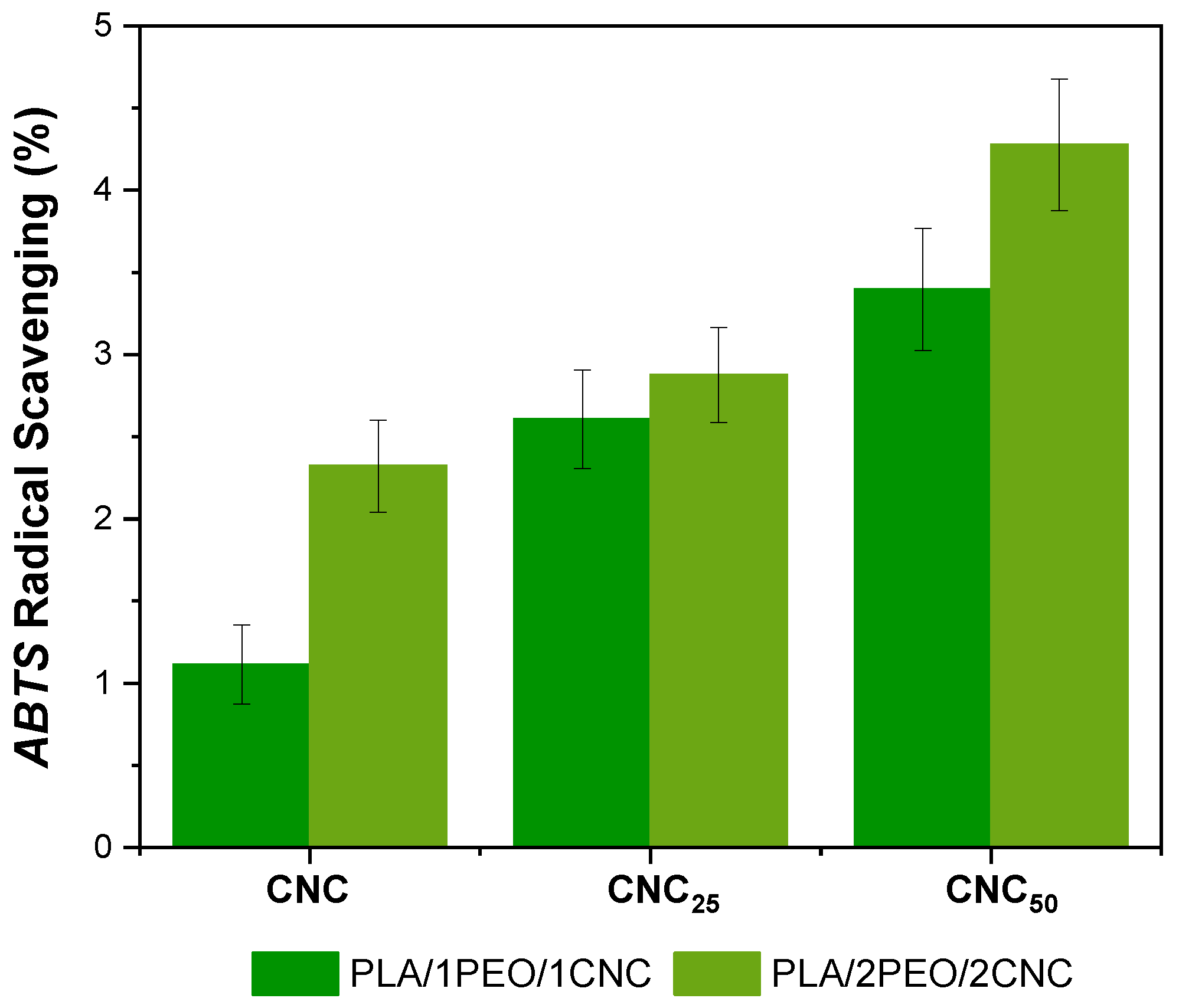
3.3. Evaluation of Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Fernando, A.L.; Silva, A.S. Active Edible Packaging. Encyclopedia 2021, 1, 360–370. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Mariño-Cortegoso, S.; Barbosa-Pereira, L.; Sendón, R.; Buonocore, G.G.; Stanzione, M.; Coelho, A.; Belo Correia, C.; Saraiva, M.; et al. LDPE and PLA Active Food Packaging Incorporated with Lemon by-Products Extract: Preparation, Characterization and Effectiveness to Delay Lipid Oxidation in Almonds and Beef Meat. Foods 2023, 12, 2450. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Aurrekoetxea, G.P.; Angulo, I.; Paseiro-Losada, P.; Cruz, J.M. Development of New Active Packaging Films Coated with Natural Phenolic Compounds to Improve the Oxidative Stability of Beef. Meat Sci. 2014, 97, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, J.; Bierbaumer, H.P.; Gratzl, F.; Kellauer, E. Decontamination of Food Packaging Using Electron Beam–Status and Prospects. Radiat. Phys. Chem. 2002, 63, 833–836. [Google Scholar] [CrossRef]
- Hamiruddin, N.A.; Othman, S.A. The Feasibility of Irradiated Bioplastics as Future Packaging Material. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 1422–1425. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, S.; Mishra, A.K.; Wang, X.H. Effects of Input Capacity Constraints on Food Quality and Regulation Mechanism Design for Food Safety Management. Ecol. Model. 2018, 385, 89–95. [Google Scholar] [CrossRef]
- Mariño-Cortegoso, S.; Stanzione, M.; Andrade, M.A.; Restuccia, C.; Rodríguez-Bernaldo de Quirós, A.; Buonocore, G.G.; Barbosa, C.H.; Vilarinho, F.; Silva, A.S.; Ramos, F.; et al. Development of Active Films Utilizing Antioxidant Compounds Obtained from Tomato and Lemon By-Products for Use in Food Packaging. Food Control 2022, 140, 109128. [Google Scholar] [CrossRef]
- Haji-Saeid, M.; Sampa, M.H.O.; Chmielewski, A.G. Radiation Treatment for Sterilization of Packaging Materials. Radiat. Phys. Chem. 2007, 76, 1535–1541. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Trends in Radiation Sterilization of Health Care Products; International Atomic Energy Agency: Vienna, Austria, 2008; ISBN 978-92-0-111007-7. [Google Scholar]
- Ferry, M.; Ngono-Ravache, Y.; Aymes-Chodur, C.; Clochard, M.C.; Coqueret, X.; Cortella, L.; Pellizzi, E.; Rouif, S.; Esnouf, S. Ionizing radiation effects in polymers. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: Oxford, UK, 2016; pp. 1–28. [Google Scholar] [CrossRef]
- Bryant, R. Irradiation of cables, wires and heat shrinkables. Radiat. Phys. Chem. 2020, 174, 108895. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.; Ulański, P.; Al-Sheikhly, M. Polymerization reactions and modifications of polymers by ionizing radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Criado, P.; Fraschini, C.; Jamshidian, M.; Salmieri, S.; Safrany, A.; Lacroix, M. Gamma-Irradiation of Cellulose Nanocrystals (CNCs): Investigation of Physicochemical and Antioxidant Properties. Cellulose 2017, 24, 2111–2124. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable Hybrid Nanocomposites of Chitosan/Gelatin and Silver Nanoparticles for Active Food Packaging Applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Khoo, R.Z.; Ismail, H.; Chow, W.S. Thermal and Morphological Properties of Poly (Lactic Acid)/Nanocellulose Nanocomposites. Procedia Chem. 2016, 19, 788–794. [Google Scholar] [CrossRef]
- Lee, M.; Heo, M.H.; Lee, H.; Lee, H.H.; Jeong, H.; Kim, Y.W.; Shin, J. Facile and Eco-Friendly Extraction of Cellulose Nanocrystals: Via Electron Beam Irradiation Followed by High-Pressure Homogenization. Green Chem. 2018, 20, 2596–2610. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and Modification of Nanofibrillated Cellulose Using Various Mechanical Processes: A Review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Vilarinho, F.; Stanzione, M.; Buonocore, G.G.; Barbosa-Pereira, L.; Sendón, R.; Vaz, M.F.; Sanches Silva, A. Green Tea Extract and Nanocellulose Embedded into Polylactic Acid Film: Properties and Efficiency on Retarding the Lipid Oxidation of a Model Fatty Food. Food Packag. Shelf Life 2021, 27, 100609. [Google Scholar] [CrossRef]
- Lizundia, E.; Fortunati, E.; Dominici, F.; Vilas, J.L.; León, L.M.; Armentano, I.; Torre, L.; Kenny, J.M. PLLA-Grafted Cellulose Nanocrystals: Role of the CNC Content and Grafting on the PLA Bionanocomposite Film Properties. Carbohydr. Polym. 2016, 142, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.V.; Seo, Y.B. Characterization of Cellulose Nanocrystal Obtained from Electron Beam Treated Cellulose Fiber. Nord. Pulp Pap. Res. J. 2017, 32, 170–178. [Google Scholar] [CrossRef]
- Furtak-Wrona, K.; Kozik-Ostrówka, P.; Jadwiszczak, K.; Maigret, J.E.; Aguié-Béghin, V.; Coqueret, X. Polyurethane acrylate networks including cellulose nanocrystals: A comparison between UV and EB- curing. Radiat. Phys. Chem. 2018, 14, 94–99. [Google Scholar] [CrossRef]
- Mohamad, S.F.; Aguié-Béghin, V.; Kurek, B.; Coqueret, X. Radiation-induced graft polymerization of N-isopropyl acrylamide onto microcrystalline cellulose: Assessing the efficiency of the peroxidation method. Radiat. Phys. Chem. 2022, 194, 110038. [Google Scholar] [CrossRef]
- Gomri, C.; Cretin, M.; Semsarilar, M. Recent progress on chemical modification of cellulose nanocrystal (CNC) and its application in nanocomposite films and membranes–A comprehensive review. Carbohydr. Polym. 2022, 294, 119790. [Google Scholar] [CrossRef] [PubMed]
- Criado, P.; Fraschini, C.; Salmieri, S.; Becher, D.; Safrany, A.; Lacroix, M. Free Radical Grafting of Gallic Acid (GA) on Cellulose Nanocrystals (CNCs) and Evaluation of Antioxidant Reinforced Gellan Gum Films. Radiat. Phys. Chem. 2016, 118, 61–69. [Google Scholar] [CrossRef]
- Chanda, S.; Bajwa, D.S.; Holt, G.A.; Stark, N.; Bajwa, S.G.; Quadir, M. Silane Compatibilzation to Improve the Dispersion, Thermal and Mechanical Properties of Cellulose Nanocrystals in Poly (Ethylene Oxide). Nanocomposites 2021, 7, 87–96. [Google Scholar] [CrossRef]
- Arias, A.; Heuzey, M.C.; Huneault, M.A.; Ausias, G.; Bendahou, A. Enhanced Dispersion of Cellulose Nanocrystals in Melt-Processed Polylactide-Based Nanocomposites. Cellulose 2015, 22, 483–498. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Coccia, F.; Gryshchuk, L.; Moimare, P.; Bossa, F.D.L.; Santillo, C.; Barak-Kulbak, E.; Verdolotti, L.; Boggioni, L.; Lama, G.C. Chemically Functionalized Cellulose Nanocrystals as Reactive Filler in Bio-Based Polyurethane Foams. Polymers 2021, 13, 2556. [Google Scholar] [CrossRef] [PubMed]
- Łojewski, T.; Miśkowiec, P.; Missori, M.; Lubańska, A.; Proniewicz, L.M.; Łojewska, J. FTIR and UV/Vis as Methods for Evaluation of Oxidative Degradation of Model Paper: DFT Approach for Carbonyl Vibrations. Carbohydr. Polym. 2010, 82, 370–375. [Google Scholar] [CrossRef]
- Łojewski, T.; Zięba, K.; Knapik, A.; Bagniuk, J.; Lubańska, A.; Łojewska, J. Evaluating Paper Degradation Progress. Cross-Linking between Chromatographic, Spectroscopic and Chemical Results. Appl. Phys. A 2010, 100, 809–821. [Google Scholar] [CrossRef]
- Zotti, A.; Zuppolini, S.; Tábi, T.; Grasso, M.; Ren, G.; Borriello, A.; Zarrelli, M. Effects of 1D and 2D Nanofillers in Basalt/Poly(Lactic Acid) Composites for Additive Manufacturing. Compos. Part B Eng. 2018, 153, 364–375. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- Luis Orellana, J.; Wichhart, D.; Kitchens, C.L. Mechanical and Optical Properties of Polylactic Acid Films Containing Surfactant-Modified Cellulose Nanocrystals. J. Nanomater. 2018, 2018, 7124260. [Google Scholar] [CrossRef]
- Orlo, E.; Russo, C.; Nugnes, R.; Lavorgna, M.; Isidori, M. Natural Methoxyphenol Compounds: Antimicrobial Activity against Foodborne Pathogens and Food Spoilage Bacteria, and Role in Antioxidant Processes. Foods 2021, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Orlo, E.; Nerín, C.; Lavorgna, M.; Wrona, M.; Russo, C.; Stanzione, M.; Nugnes, R.; Isidori, M. Antioxidant Activity of Coatings Containing Eugenol for Flexible Aluminium Foils to Preserve Food Shelf-Life. Food Packag. Shelf Life 2023, 39, 101145. [Google Scholar] [CrossRef]
- Wan Ishak, W.H.; Ahmad, I.; Ramli, S.; Mohd Amin, M.C.I. Gamma Irradiation-Assisted Synthesis of Cellulose Nanocrystal-Reinforced Gelatin Hydrogels. Nanomater 2018, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, K.; Abramowska, A. The Effect of the Addition of Crystalline Nanocellulose (CNC) and Radiation Treatment on the Properties of Edible Films Based on a Cornstarch–Poly(Vinyl Alcohol) System. Coatings 2025, 15, 452. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2011, 18, 717–728. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, W.J. The Influence of Cellulose Nanocrystal Characteristics on Regenerative Silk Composite Fiber Properties. Materials 2023, 16, 2323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y. High Energy Oxidation and Organosolv Solubilization for High Yield Isolation of Cellulose Nanocrystals (CNC) from Eucalyptus Hardwood. Sci. Rep. 2018, 8, 16505. [Google Scholar] [CrossRef] [PubMed]
- Sabara, Z.; Mutmainnah, A.; Kalsum, U.; Afiah, I.N.; Husna, I.; Saregar, A.; Irzaman; Umam, R. Sugarcane Bagasse as the Source of Nanocrystalline Cellulose for Gelatin-Free Capsule Shell. Int. J. Biomater. 2022, 2022, 9889127. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, A.V.; Ershov, B.G. Radiation-Induced Formation of Peroxides in Cellulose. Radiat. Phys. Chem. 2018, 152, 63–68. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Coqueret, X.; Zaman, H.M.K.; Sen, M.; Ulanski, P. The Radiation Chemistry of Polysaccharides; IAEA: Vienna, Austria, 2016; ISBN 978–92–0–101516–7. [Google Scholar]
- Kubota, H.; Ogiwara, Y. Cellulose peroxides derived from carbonylated cellulose and hydrogen. J. Appl. Polym. Sci. 1980, 25, 683–689. [Google Scholar] [CrossRef]
- Rodrigues, S.C.S.; de Mesquita, F.A.S.; de Carvalho, L.H.; Alves, T.S.; Folkersma, R.; dos R M Araújo, R.S.; Oliveira, A.D.; Barbosa, R. Preparation and Characterization of Polymeric Films Based on PLA, PBAT and Corn Starch and Babassu Mesocarp Starch by Flat Extrusion. Mater. Res. Express 2021, 8, 35305. [Google Scholar] [CrossRef]
- Maréchal, Y.; Chanzy, H. The Hydrogen Bond Network in Iβ Cellulose as Observed by Infrared Spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Iijima, M.; Hatakeyama, H. Role of Bound Water on Structural Change of Water Insoluble Polysaccharides. Food Hydrocoll. 2016, 53, 62–68. [Google Scholar] [CrossRef]
- Varma, A.J.; Chavan, V.B. Thermal Properties of Oxidized Cellulose. Cellulose 1995, 2, 41–49. [Google Scholar] [CrossRef]
- Wu, J.; Gao, N.; Jiang, L.; Zhong, G.; Deng, C.; Gao, X. The Coupling Effect of Cellulose Nanocrystal and Strong Shear Field Achieved the Strength and Toughness Balance of Polylactide. Int. J. Biol. Macromol. 2022, 207, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Choi, B.; Park, S.I. A Study on Mechanical and Thermal Properties of PLA/PEO Blends. J. Polym. Environ. 2019, 27, 256–262. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/Cellulose Based Films: Mechanical, Barrier and Disintegration Properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Kovalev, G.V.; Bugaenko, L.T. On the Crosslinking of Cellulose under Exposure to Radiation. Polymers 2003, 37, 209–215. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. Polymers 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, G.; Panea, B.; Latorre, M.Á. A Machine Learning Approach Investigating Consumers’ Familiarity with and Involvement in the Just Noticeable Color Difference and Cured Color Characterization Scale. Foods 2023, 12, 4426. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Rüegg, N.; Yildirim, S. Evaluation of the Antimicrobial Activity of Sodium Alginate Films Integrated with Cinnamon Essential Oil and Citric Acid on Sliced Cooked Ham. Packag. Technol. Sci. 2023, 36, 647–656. [Google Scholar] [CrossRef]
- Panchal, P.; Mekonnen, T.H. Tailored Cellulose Nanocrystals as a Functional Ultraviolet Absorbing Nanofiller of Epoxy Polymers. Polymers 2019, 11, 2612–2623. [Google Scholar] [CrossRef] [PubMed]





| PLA-Based Sample | % PEO | % CNC | % Irradiated CNC |
|---|---|---|---|
| PLA/1PEO | 1 | - | - |
| PLA/1PEO/1CNC | 1 | 1 | - |
| PLA/1PEO/1CNC25 | 1 | 1 | (25 kGy) |
| PLA/1PEO/1CNC50 | 1 | 1 | (50 kGy) |
| PLA/2PEO | 2 | - | - |
| PLA/2PEO/2CNC | 2 | 2 | - |
| PLA/2PEO/2CNC25 | 2 | 2 | (25 kGy) |
| PLA/2PEO/2CNC50 | 2 | 2 | (50 kGy) |
| Bands | Wavenumber (cm−1) |
|---|---|
| –CH2 antisymmetric and symmetric stretching | 2997–2945 |
| –C=0 stretching | 1747 |
| –CH3 antisymmetric and symmetric bending | 1300–1500 |
| C–O–C stretching of esters | 1266–1209 |
| C–O–C stretching of PLA | 1180 |
| C–O stretching | 1125, 1080, 1040 |
| Amorphous and crystalline phases of PLA | 868–755 |
| Tg (°C) | ΔHc (J/(g·°C) | Tc Max (°C) | ΔHm (J/g) | Tm Max (°C) | Xc (%) | |
|---|---|---|---|---|---|---|
| PLA | 61 | 35.30 | 112 | 43.70 | 167 | 9.03 |
| PLA/1PEO | 57 | 54.40 | 101 | 82.60 | 166 | 30.62 |
| PLA/1PEO/1CNC | 57 | 29.60 | 104 | 49.90 | 167 | 22.27 |
| PLA/1PEO/1CNC25 | 57 | 31.10 | 104 | 51.10 | 166 | 21.94 |
| PLA/1PEO/1CNC50 | 57 | 34.40 | 108 | 49.90 | 167 | 17.01 |
| PLA/2PEO | 55 | 21.40 | 113 | 56.60 | 183 | 38.62 |
| PLA/2PEO/2CNC | 56 | 18.80 | 97 | 56.40 | 167 | 42.11 |
| PLA/2PEO/2CNC25 | 53 | 21.00 | 97 | 53.80 | 166 | 36.73 |
| PLA/2PEO/2CNC50 | 56 | 11.20 | 98 | 43.70 | 166 | 36.40 |
| Stress at Break (MPa) | Elongation at Break (%) | Young Modulus (MPa) | |
|---|---|---|---|
| PLA/1PEO | 21.6 ± 2.4 | 1.0 ± 0.0 | 4387.0 ± 430.9 |
| PLA/1PEO/1CNC | 21.7 ± 1.6 | 1.3 ± 0.2 | 4994.4 ± 353.2 |
| PLA/1PEO/1CNC25 | 14.7 ± 2.3 | 1.3 ± 0.2 | 4125.0 ± 279.6 |
| PLA/1PEO/1CNC50 | 25.4 ± 2.5 | 2.2 ± 0.5 | 3769.6 ± 371.4 |
| PLA/2PEO | 19.7 ± 2.4 | 1.1 ± 0.1 | 4700.4 ± 285.4 |
| PLA/2PEO/2CNC | 17.7 ± 2.2 | 1.2 ± 0.1 | 4320.9 ± 257.6 |
| PLA/2PEO/2CNC25 | 24.0 ± 1.6 | 2.1 ± 0.4 | 4385.9 ± 184.9 |
| PLA/2PEO/2CNC50 | 20.0 ± 1.3 | 2.0 ± 0.5 | 4143.1 ± 260.2 |
| Water Contact Angle (°) | Diiodiomethane Contact Angle (°) | (mN/m) | (mN/m) | ||
|---|---|---|---|---|---|
| PLA/1PEO | 87.4 ± 1.7 | 56.8 ± 1.7 | 30.42 | 2.97 | 33.38 |
| PLA/1PEO/1CNC | 88.8 ± 2.3 | 58.2 ± 1.8 | 29.61 | 2.71 | 32.32 |
| PLA/1PEO/1CNC25 | 91.6 ± 1.3 | 52.7 ± 1.6 | 32.76 | 1.47 | 34.23 |
| PLA/1PEO/1CNC50 | 89.9 ± 2.2 | 56.9 ± 1.4 | 30.36 | 2.26 | 32.62 |
| PLA/2PEO | 86.9 ± 3.2 | 61.6 ± 1.4 | 27.65 | 3.74 | 31.4 |
| PLA/2PEO/2CNC | 83.9 ± 1.0 | 55.7 ± 2.0 | 31.05 | 3.98 | 35.03 |
| PLA/2PEO/2CNC25 | 90.9 ± 2.7 | 57.4 ± 1.4 | 30.07 | 2.05 | 32.12 |
| PLA/2PEO/2CNC50 | 94.8 ± 2.8 | 61.4 ± 1.4 | 27.77 | 1.5 | 29.27 |
| L* | a* | b* | Total Color (ΔE) | |
|---|---|---|---|---|
| Reference | 96.30 ± 0.09 | 0.50 ± 0.04 | 2.90 ± 0.14 | 0.00 |
| PLA/1PEO | 94.70 ± 0.06 | 0.50 ± 0.00 | 3.20 ± 0.16 | 1.63 |
| PLA/1PEO/1CNC | 95.30 ± 0.06 | 0.50 ± 0.06 | 2.90 ± 0.45 | 1.00 |
| PLA/1PEO/1CNC25 | 94.10 ± 0.16 | 0.50 ± 0.06 | 4.70 ± 0.26 | 2.84 |
| PLA/1PEO/1CNC50 | 94.5 ± 0.23 | 0.50 ± 0.06 | 3.7 ± 0.23 | 1.97 |
| PLA/2PEO | 95.00 ± 0.15 | 0.50 ± 0.00 | 3.1 ± 0.06 | 1.32 |
| PLA/2PEO/2CNC | 94.70 ± 0.06 | 0.40 ± 0.06 | 3.80 ± 0.26 | 1.84 |
| PLA/2PEO/2CNC25 | 93.60 ± 0.06 | 0.30 ± 0.00 | 4.50 ± 0.35 | 3.14 |
| PLA/2PEO/2CNC50 | 95.10 ± 0.21 | 0.50 ± 0.00 | 4.50 ± 0.67 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Improta, I.; Stanzione, M.; Orlo, E.; Tescione, F.; Lavorgna, M.; Coqueret, X.; Buonocore, G.G. Effect of Irradiated Nanocellulose on Enhancing the Functionality of Polylactic Acid-Based Composite Films for Packaging Applications. Polymers 2025, 17, 1939. https://doi.org/10.3390/polym17141939
Improta I, Stanzione M, Orlo E, Tescione F, Lavorgna M, Coqueret X, Buonocore GG. Effect of Irradiated Nanocellulose on Enhancing the Functionality of Polylactic Acid-Based Composite Films for Packaging Applications. Polymers. 2025; 17(14):1939. https://doi.org/10.3390/polym17141939
Chicago/Turabian StyleImprota, Ilaria, Mariamelia Stanzione, Elena Orlo, Fabiana Tescione, Marino Lavorgna, Xavier Coqueret, and Giovanna G. Buonocore. 2025. "Effect of Irradiated Nanocellulose on Enhancing the Functionality of Polylactic Acid-Based Composite Films for Packaging Applications" Polymers 17, no. 14: 1939. https://doi.org/10.3390/polym17141939
APA StyleImprota, I., Stanzione, M., Orlo, E., Tescione, F., Lavorgna, M., Coqueret, X., & Buonocore, G. G. (2025). Effect of Irradiated Nanocellulose on Enhancing the Functionality of Polylactic Acid-Based Composite Films for Packaging Applications. Polymers, 17(14), 1939. https://doi.org/10.3390/polym17141939











