Next-Generation Nafion Membranes: Synergistic Enhancement of Electrochemical Performance and Thermomechanical Stability with Sulfonated Siliceous Layered Material (sSLM)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Sulfonated Siliceous Layered Materials (sSLMs)
2.3. Preparation of Nanocomposite Membrane
2.4. Characterization Techniques
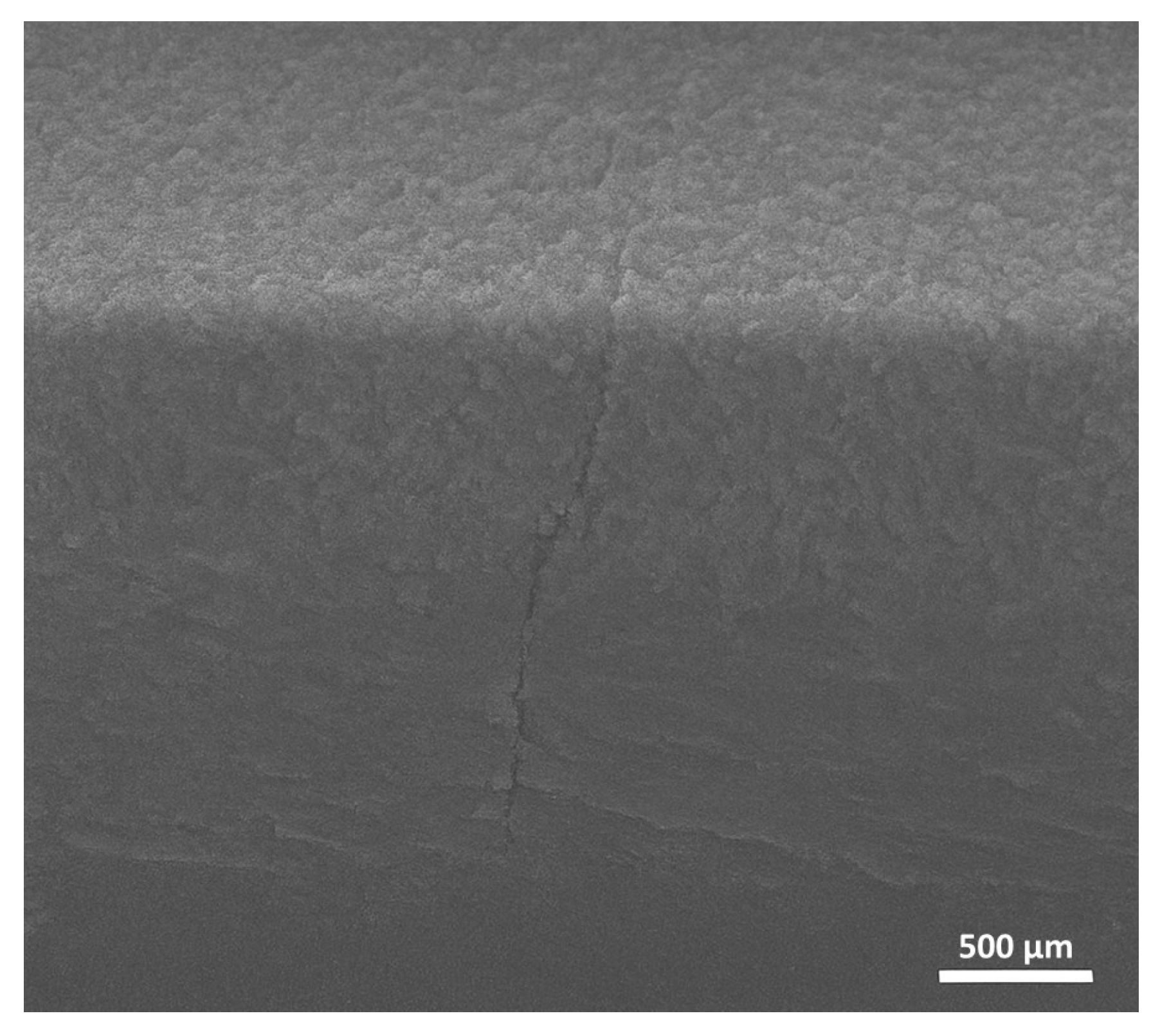
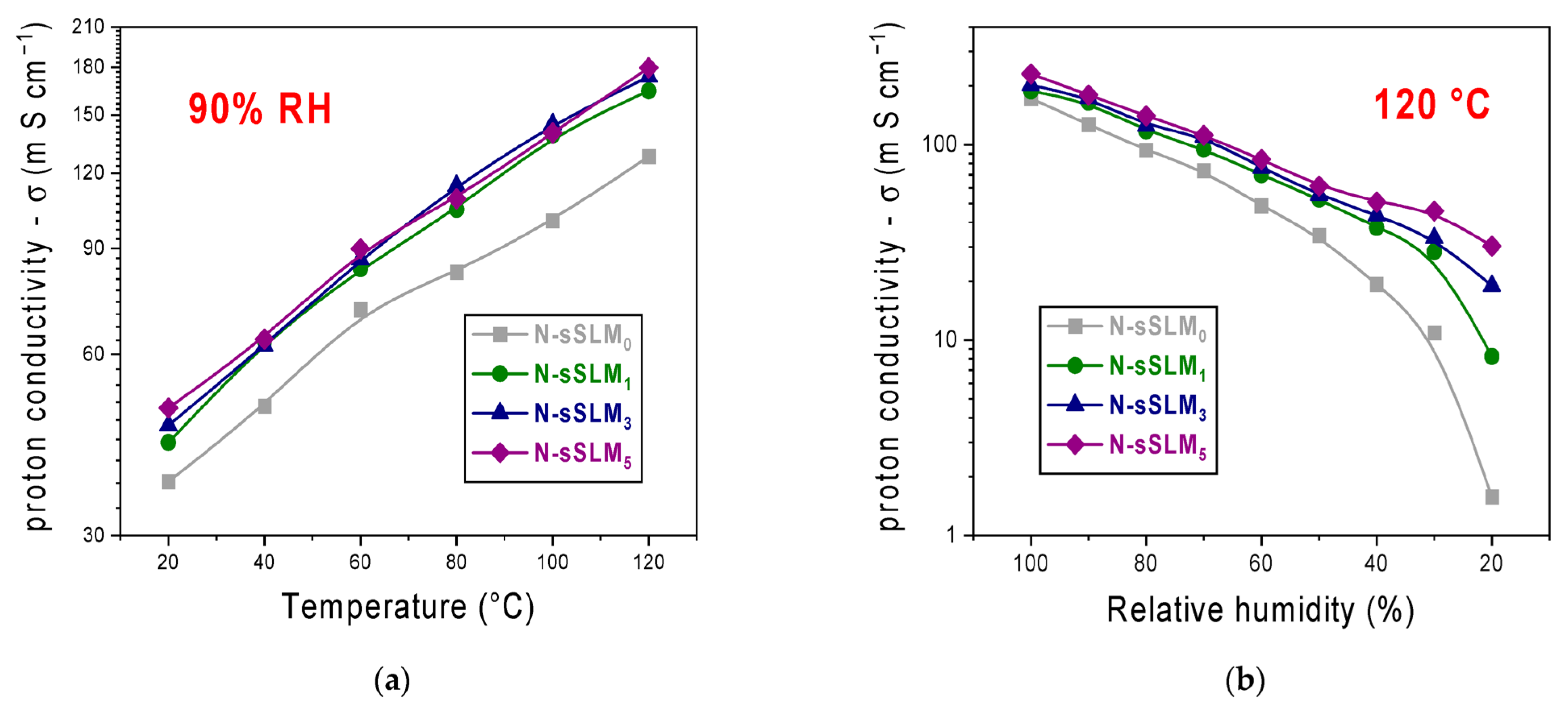
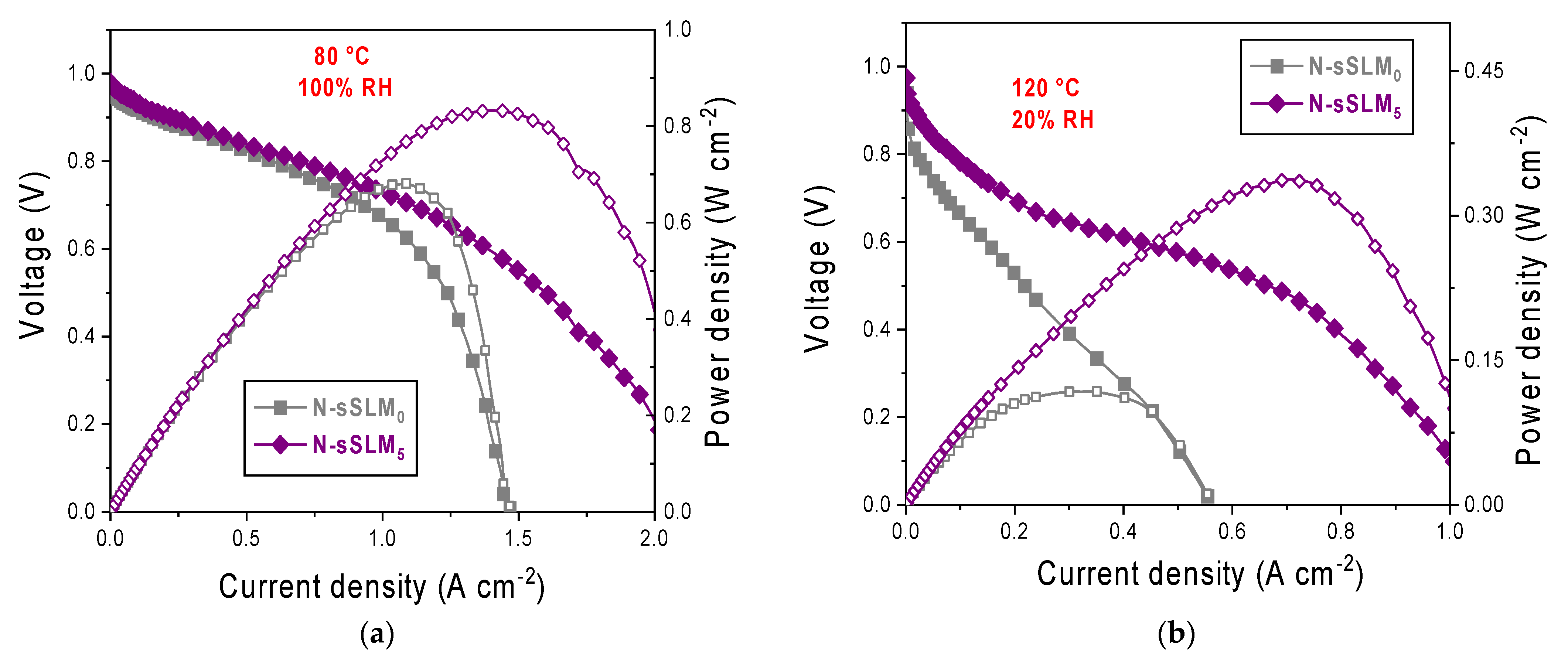
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent Approaches to Improve Nafion Performance for Fuel Cell Applications: A Review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Sengupta, S.; Lyulin, A.V. Molecular Modeling of Structure and Dynamics of Nafion Protonation States. J. Phys. Chem. B 2019, 123, 6882–6891. [Google Scholar] [CrossRef] [PubMed]
- Privalov, A.F.; Sinitsyn, V.V.; Vogel, M. Transport Mechanism in Nafion Revealed by Detailed Comparison of 1H and 17O Nuclear Magnetic Resonance Diffusion Coefficients. J. Phys. Chem. Lett. 2023, 14, 9335–9340. [Google Scholar] [CrossRef]
- Sun, H.; Sun, Z.; Wu, Y. Proton Transfer Mechanism in Perfluorinated Sulfonic Acid Polytetrafluoroethylene. Int. J. Hydrogen Energy 2012, 37, 12821–12826. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the Proton Exchange Membranes for Fuel Cell Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; Volume 35, ISBN 2177491223. [Google Scholar]
- Hooshyari, K.; Amini Horri, B.; Abdoli, H.; Fallah Vostakola, M.; Kakavand, P.; Salarizadeh, P. A Review of Recent Developments and Advanced Applications of High-Temperature Polymer Electrolyte Membranes for Pem Fuel Cells. Energies 2021, 14, 5440. [Google Scholar] [CrossRef]
- Ozen, D.N.; Timurkutluk, B.; Altinisik, K. Effects of Operation Temperature and Reactant Gas Humidity Levels on Performance of PEM Fuel Cells. Renew. Sustain. Energy Rev. 2016, 59, 1298–1306. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific Aspects of Polymer Electrolyte Fuel Cell Durability and Degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Pham, T.A.; Nam, L.V.; Choi, E.; Lee, M.S.; Jun, T.S.; Jang, S.; Kim, S.M. Mechanically Stable Thinned Membrane for a High-Performance Polymer Electrolyte Membrane Fuel Cell via a Plasma-Etching and Annealing Process. Energy Fuels 2021, 35, 11525–11532. [Google Scholar] [CrossRef]
- Dafalla, A.M.; Jiang, F. Stresses and Their Impacts on Proton Exchange Membrane Fuel Cells: A Review. Int. J. Hydrogen Energy 2018, 43, 2327–2348. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in Hybrid Composite Nafion®-Based Membranes for Proton Exchange Fuel Cell Application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Lu, J.L.; Fang, Q.H.; Li, S.L.; Jiang, S.P. A Novel Phosphotungstic Acid Impregnated Meso-Nafion Multilayer Membrane for Proton Exchange Membrane Fuel Cells. J. Membr. Sci. 2013, 427, 101–107. [Google Scholar] [CrossRef]
- Sizov, V.E.; Zefirov, V.V.; Abramchuk, S.S.; Korlyukov, A.A.; Kondratenko, M.S.; Vasil’ev, V.G.; Gallyamov, M.O. Composite Nafion-Based Membranes with Nanosized Tungsten Oxides Prepared in Supercritical Carbon Dioxide. J. Membr. Sci. 2020, 609, 118244. [Google Scholar] [CrossRef]
- Krathumkhet, N.; Vongjitpimol, K.; Chuesutham, T.; Changkhamchom, S.; Phasuksom, K.; Sirivat, A.; Wattanakul, K. Preparation of Sulfonated Zeolite ZSM-5/Sulfonated Polysulfone Composite Membranes as PEM for Direct Methanol Fuel Cell Application. Solid State Ion. 2018, 319, 278–284. [Google Scholar] [CrossRef]
- Prapainainar, P.; Du, Z.; Theampetch, A.; Prapainainar, C.; Kongkachuichay, P.; Holmes, S.M. Properties and DMFC Performance of Nafion/Mordenite Composite Membrane Fabricated by Solution-Casting Method with Different Solvent Ratio. Energy 2020, 190, 116451. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Y.; Wu, J.; Zhang, F.; Baker, A.P.; Lavorgna, M.; Wu, Q.; Tang, Q.; Lu, J.; Xiao, Z.; et al. Porous Silicon-Aluminium Oxide Particles Functionalized with Acid Moieties: An Innovative Filler for Enhanced Nafion-Based Membranes of Direct Methanol Fuel Cell. J. Power Sources 2018, 403, 118–126. [Google Scholar] [CrossRef]
- Truffier-Boutry, D.; De Geyer, A.; Guetaz, L.; Diat, O.; Gebel, G. Structural Study of Zirconium Phosphate-Nafion Hybrid Membranes for High-Temperature Proton Exchange Membrane Fuel Cell Applications. Macromolecules 2007, 40, 8259–8264. [Google Scholar] [CrossRef]
- Donnadio, A.; Narducci, R.; Casciola, M.; Marmottini, F.; D’Amato, R.; Jazestani, M.; Chiniforoshan, H.; Costantino, F. Mixed Membrane Matrices Based on Nafion/UiO-66/SO3H-UiO-66 Nano-MOFs: Revealing the Effect of Crystal Size, Sulfonation, and Filler Loading on the Mechanical and Conductivity Properties. ACS Appl. Mater. Interfaces 2017, 9, 42239–42246. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, H.J.; Ren, Q.; Luo, H.B.; Ren, X.M.; Tian, Z.F.; Lu, S. Extra Water- and Acid-Stable MOF-801 with High Proton Conductivity and Its Composite Membrane for Proton-Exchange Membrane. ACS Appl. Mater. Interfaces 2018, 10, 28656–28663. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Luo, X.; Xiong, J.; Liu, Z.; Cai, W. Effect of Nano-Size of Functionalized Silica on Overall Performance of Swelling-Filling Modified Nafion Membrane for Direct Methanol Fuel Cell Application. Appl. Energy 2018, 213, 408–414. [Google Scholar] [CrossRef]
- Oh, K.; Kwon, O.; Son, B.; Lee, D.H.; Shanmugam, S. Nafion-Sulfonated Silica Composite Membrane for Proton Exchange Membrane Fuel Cells under Operating Low Humidity Condition. J. Membr. Sci. 2019, 583, 103–109. [Google Scholar] [CrossRef]
- Matos, B.R.; Santiago, E.I.; Rey, J.F.Q.; Ferlauto, A.S.; Traversa, E.; Linardi, M.; Fonseca, F.C. Nafion-Based Composite Electrolytes for Proton Exchange Membrane Fuel Cells Operating above 120 °C with Titania Nanoparticles and Nanotubes as Fillers. J. Power Sources 2011, 196, 1061–1068. [Google Scholar] [CrossRef]
- Jang, S.; Kang, Y.S.; Choi, J.; Yeon, J.H.; Seol, C.; Nam, L.V.; Choi, M.; Kim, S.M.; Yoo, S.J. Prism Patterned TiO2 Layers/Nafion® Composite Membrane for Elevated Temperature/Low Relative Humidity Fuel Cell Operation. J. Ind. Eng. Chem. 2020, 90, 327–332. [Google Scholar] [CrossRef]
- Burgaz, E.; Lian, H.; Alonso, R.H.; Estevez, L.; Kelarakis, A.; Giannelis, E.P. Nafion–Clay Hybrids with a Network Structure. Polymer 2009, 50, 2384–2392. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Kabiri, K. Nafion®/Bio-Functionalized Montmorillonite Nanohybrids as Novel Polyelectrolyte Membranes for Direct Methanol Fuel Cells. J. Power Sources 2009, 190, 318–321. [Google Scholar] [CrossRef]
- Rhee, C.H.; Kim, H.K.; Chang, H.; Lee, J.S. Nafion/Sulfonated Montmorillonite Composite: A New Concept Electrolyte Membrane for Direct Methanol Fuel Cells. Chem. Mater. 2005, 17, 1691–1697. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.K.S.; Yue, P.L.; Gao, P. Exfoliated Pt-Clay/Nafion Nanocomposite Membrane for Self-Humidifying Polymer Electrolyte Fuel Cells. Langmuir 2008, 24, 2663–2670. [Google Scholar] [CrossRef]
- Adjemian, K.T.; Dominey, R.; Krishnan, L.; Ota, H.; Majsztrik, P.; Zhang, T.; Mann, J.; Kirby, B.; Gatto, L.; Velo-Simpson, M.; et al. Function and Characterization of Metal Oxide-Nafion Composite Membranes for Elevated-Temperature H 2/O 2 PEM Fuel Cells. Chem. Mater. 2006, 18, 2238–2248. [Google Scholar] [CrossRef]
- Seo, D.C.; Jeon, I.; Jeong, E.S.; Jho, J.Y. Mechanical Properties and Chemical Durability of Nafion/Sulfonated Graphene Oxide/Cerium Oxide Composite Membranes for Fuel-Cell Applications. Polymers 2020, 12, 1375. [Google Scholar] [CrossRef]
- Berning, T.; Bessarabov, D. GOMEA: A Conceptual Design of a Membrane Electrode Assembly for a Proton Exchange Membrane Electrolyzer. Membranes 2023, 13, 614. [Google Scholar] [CrossRef]
- Yin, C.; Xiong, B.; Liu, Q.; Li, J.; Qian, L.; Zhou, Y.; He, C. Lateral-Aligned Sulfonated Carbon-Nanotubes/Nafion Composite Membranes with High Proton Conductivity and Improved Mechanical Properties. J. Membr. Sci. 2019, 591, 117356. [Google Scholar] [CrossRef]
- Nicotera, I.; Kosma, V.; Simari, C.; D’Urso, C.; Aricò, A.S.; Baglio, V. Methanol and Proton Transport in Layered Double Hydroxide and Smectite Clay-Based Composites: Influence on the Electrochemical Behavior of Direct Methanol Fuel Cells at Intermediate Temperatures. J. Solid State Electrochem. 2015, 19, 2053–2061. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Li, Y.C.; Liu, J.; He, J.; Wang, L.Y.; Lei, J.D. Recent Developments in High-Performance Nafion Membranes for Hydrogen Fuel Cells Applications. Pet. Sci. 2022, 19, 1371–1381. [Google Scholar] [CrossRef]
- Enotiadis, A.; Boutsika, L.G.; Spyrou, K.; Simari, C.; Nicotera, I. A Facile Approach to Fabricating Organosilica Layered Material with Sulfonic Groups as an Efficient Filler for Polymer Electrolyte Nanocomposites. New J. Chem. 2017, 41, 9489–9496. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Boutsika, L.G.; Coppola, L.; Spyrou, K.; Enotiadis, A. NMR Investigation on Nanocomposite Membranes Based on Organosilica Layered Materials Bearing Different Functional Groups for PEMFCs. Int. J. Hydrogen Energy 2017, 42, 27940–27949. [Google Scholar] [CrossRef]
- Simari, C.; Enotiadis, A.; Nicotera, I. Transport Properties and Mechanical Features of Sulfonated Polyether Ether Ketone/Organosilica Layered Materials Nanocomposite Membranes for Fuel Cell Applications. Membranes 2020, 10, 87. [Google Scholar] [CrossRef]
- Cossari, P.; Simari, C.; Cannavale, A.; Gigli, G.; Nicotera, I. Advanced Processing and Characterization of Nafion Electrolyte Films for Solid-State Electrochromic Devices Fabricated at Room Temperature on Single Substrate. Solid State Ion. 2018, 317, 46–52. [Google Scholar] [CrossRef]
- Lufrano, E.; Simari, C.; Di Vona, M.L.; Nicotera, I.; Narducci, R. How the Morphology of Nafion-Based Membranes Affects Proton Transport. Polymers 2021, 13, 359. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer Electrolyte Fuel Cell Model. J. Electrochem. Soc. 2008, 138, 2334. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Nicotera, I.; Kosma, V.; Simari, C.; Angioni, S.; Mustarelli, P.; Quartarone, E. Ion Dynamics and Mechanical Properties of Sulfonated Polybenzimidazole Membranes for High-Temperature Proton Exchange Membrane Fuel Cells. J. Phys. Chem. C 2015, 119, 9745–9753. [Google Scholar] [CrossRef]
- Simari, C.; Lo Vecchio, C.; Baglio, V.; Nicotera, I. Sulfonated Polyethersulfone/Polyetheretherketone Blend as High Performing and Cost-Effective Electrolyte Membrane for Direct Methanol Fuel Cells. Renew. Energy 2020, 159, 336–345. [Google Scholar] [CrossRef]
- Poiana, R.; Lufrano, E.; Tsurumaki, A.; Simari, C.; Nicotera, I.; Navarra, M.A. Safe Gel Polymer Electrolytes for High Voltage Li-Batteries. Electrochim. Acta 2021, 401, 139470. [Google Scholar] [CrossRef]
- Simari, C.; Vecchio, C.L.; Enotiadis, A.; Davoli, M.; Baglio, V.; Nicotera, I. Toward Optimization of a Robust Low-Cost Sulfonated-Polyethersulfone Containing Layered Double Hydroxide for PEM Fuel Cells. J. Appl. Polym. Sci. 2019, 136, 47884. [Google Scholar] [CrossRef]
- Simari, C.; Enotiadis, A.; Lo Vecchio, C.; Baglio, V.; Coppola, L.; Nicotera, I. Advances in Hybrid Composite Membranes Engineering for High-Performance Direct Methanol Fuel Cells by Alignment of 2D Nanostructures and a Dual-Layer Approach. J. Membr. Sci. 2020, 599, 117858. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loise, V.; Simari, C. Next-Generation Nafion Membranes: Synergistic Enhancement of Electrochemical Performance and Thermomechanical Stability with Sulfonated Siliceous Layered Material (sSLM). Polymers 2025, 17, 1866. https://doi.org/10.3390/polym17131866
Loise V, Simari C. Next-Generation Nafion Membranes: Synergistic Enhancement of Electrochemical Performance and Thermomechanical Stability with Sulfonated Siliceous Layered Material (sSLM). Polymers. 2025; 17(13):1866. https://doi.org/10.3390/polym17131866
Chicago/Turabian StyleLoise, Valeria, and Cataldo Simari. 2025. "Next-Generation Nafion Membranes: Synergistic Enhancement of Electrochemical Performance and Thermomechanical Stability with Sulfonated Siliceous Layered Material (sSLM)" Polymers 17, no. 13: 1866. https://doi.org/10.3390/polym17131866
APA StyleLoise, V., & Simari, C. (2025). Next-Generation Nafion Membranes: Synergistic Enhancement of Electrochemical Performance and Thermomechanical Stability with Sulfonated Siliceous Layered Material (sSLM). Polymers, 17(13), 1866. https://doi.org/10.3390/polym17131866






