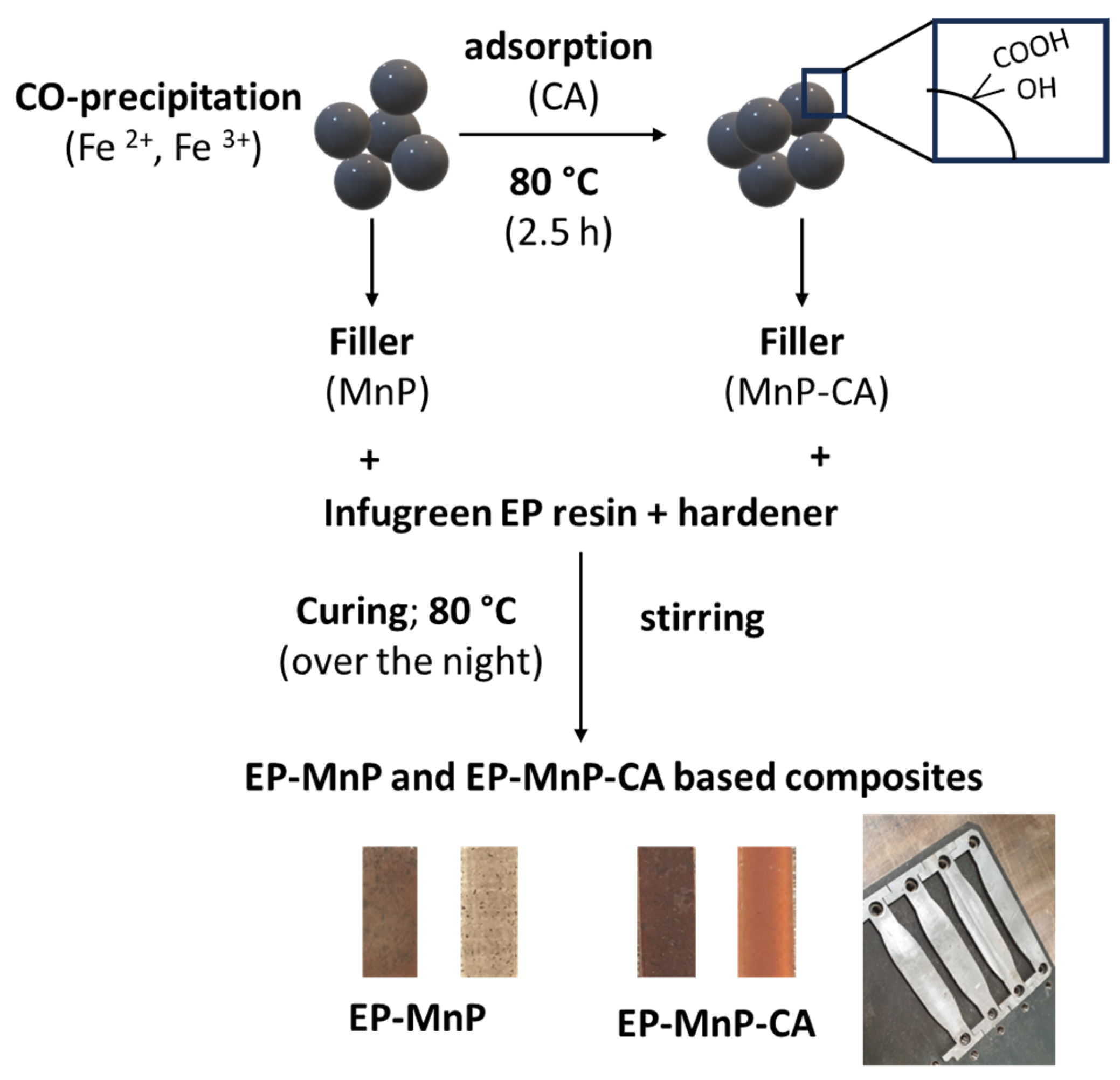
3.2. EP-MNP, EP-MNP-CA Characterization
To further quantify structural differences by identifying functional groups introduced or altered by the addition of nanoparticles in EP-MnP and EP-MnP-CA between neat bio-EP and EP-MnP resin composites, Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR) was employed. This technique effectively monitors changes resulting from nanoparticle incorporation into bio-EP resins, which subsequently enhance their final properties as well. ATR-FTIR enables detailed observation of chemical changes and functional group modifications arising from nanoparticle interactions within the bio-EP matrix. This understanding is crucial for developing advanced materials with tailored properties for specific applications.
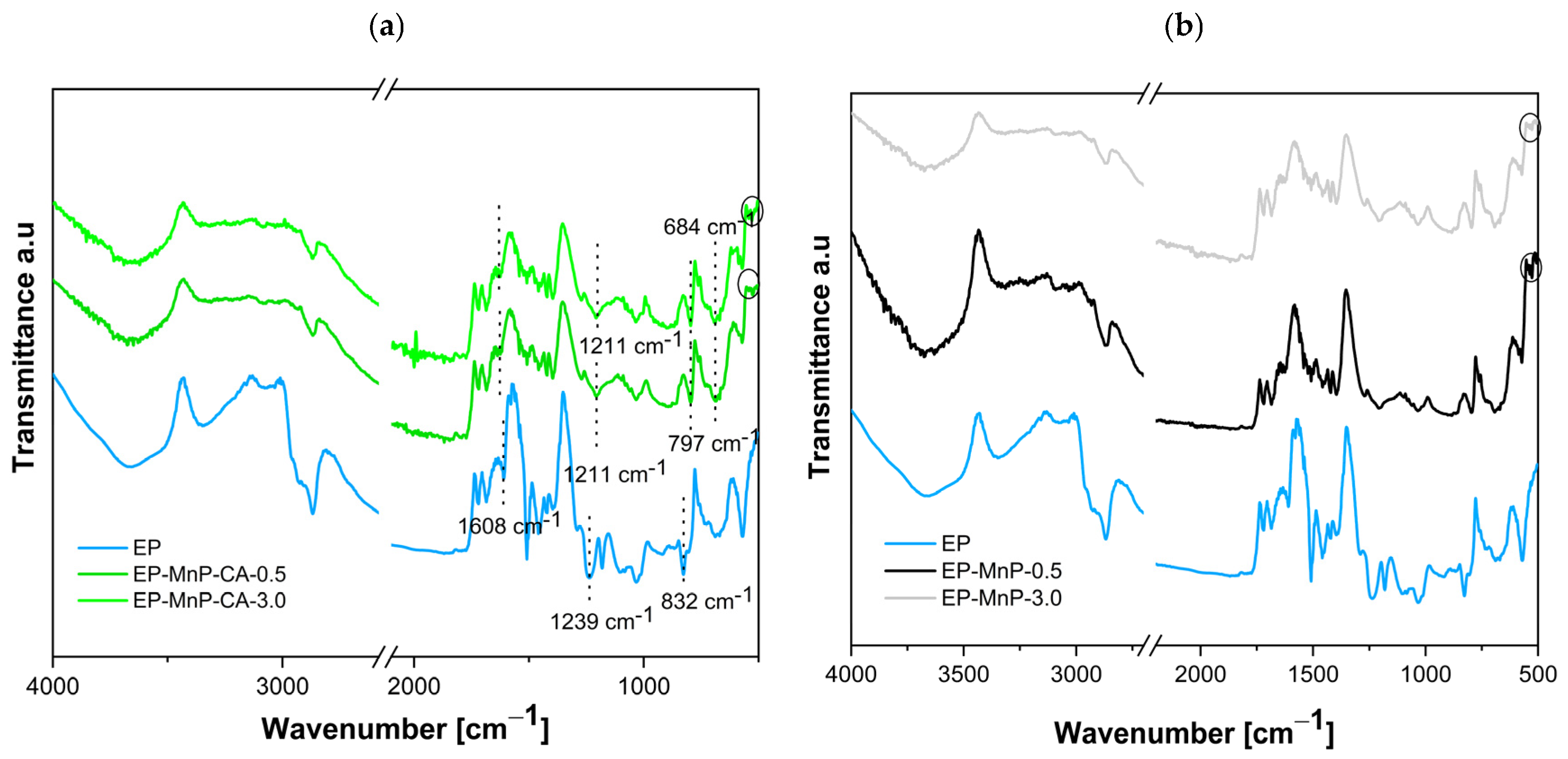
The ATR-FTIR analysis shown in
Figure 4a,b presents the spectra of neat bio-EP resin as well as all EP-MnP and EP-MnP-CA composites. The results of the ATR-FTIR analysis for neat bio-EP are given in
Figure 4a. A detailed assignment of the epoxy ATR-FTIR spectra is listed in
Table 3 [
11,
17,
18,
19] as well. The range at 3660 cm
−1 and 3401 cm
−1 corresponds to the overlapping –NH
2 and –OH stretching vibrations [
16]. Additionally, the ATR-FTIR spectra of the bio-EP display a broad band at 3401 cm
−1 and 1608 cm
−1, attributed to N–H stretching and N–H bending, respectively. The bands at 2966 cm
−1 and 2928 cm
−1 represent asymmetrical C–H stretching for CH
3 and CH
2 groups, while bands at 1511 cm
−1 and 1390 cm
−1 indicate CH
3 deformation, and the band at 1460 cm
−1 corresponds to CH
2 deformation. The aromatic nature of the epoxy is clearly visible in the structure, and ring substitution can be assessed within the (1300–1000) cm
−1 region when the epoxy resin chemically reacts with the hardener. Aldehyde C–H stretching appears at 2868 cm
−1, while C–O stretching is observed at 1330 cm
−1 [
20]. The curing reaction between the epoxy and the amine group of the hardener is further supported by the disappearance of the peak at 918 cm
−1, which is characteristic of the epoxide ring in uncured epoxy resin, as reported here [
21].
Figure 4a,b presents the ATR-FTIR spectra for bio-EP containing 0.5 wt% and 3.0 wt% of MnP and MnP-CA, respectively. The incorporation of MnP-CA into the bio-EP resin is confirmed by a distinct vibration present in both the neat bio-EP and the MnP-CA composite. In the EP-MnP-CA-0.5 and EP-MnP-CA-3.0 samples, variations in the intensity and/or shifts in the characteristic vibration peaks are observed compared to the neat bio-EP. Common peaks in the EP-MnP-CA-0.5 and EP-MnP-CA-3.0 composites include 3660, 3340 cm
−1 (O–H, N–H stretching), 2966, 2882, 2868 cm
−1 (CH
2 asymmetric and symmetric stretching), 1722, 1684 cm
−1 (C=O stretching), 1511 to 1390 cm
−1 (C–H deformation, CH
2), 1293 cm
−1 (C–O stretching), 1179, 1080, 1033 cm
−1 (C–H bending), and 832, 532 cm
−1 (C–H and O–H deformation). The presence of characteristic vibrations for MnP-CA at 684 cm
−1 and peaks below 600 cm
−1 confirms the successful incorporation of MnP-CA in the EP-MnP-CA composites (sign with circle in
Figure 4a). In the ATR-FTIR spectra of MnP-CA, the appearance of new, intense absorption peaks at 1552, 1348, and 1244.6 cm
−1 indicates the formation of carboxylate groups from citric acid, which complex with Fe atoms on the maghemite surface, as reported in the previous chapter (
Figure 2d). Here, in the EP-MnP-CA composites, the N-H group of the bio-EP reduces the intensity of the characteristic vibration at 1608 cm
−1 in EP-MnP-CA-0.5 and EP-MnP-CA-3.0 samples. Additionally, the carbonyl absorption frequency is shifted from 1238 to 1211 cm
−1 (
Figure 4a), suggesting the formation of intermolecular hydrogen bonds between MnP-CA particles and the bio-EP matrix,and suggesting strong intermolecular hydrogen bonding. Conversely, the spectra of bio-EP containing unmodified MnP particles, which resemble those of neat bio-EP and neat MnP (see
Figure 4b), show no notable changes in peak formation, with peaks characteristic of MnP nanoparticles, such as Fe–O near 530 cm
−1, remaining unchanged (
Figure 4b, sign with circle).
The ATR-FTIR analysis was conducted to assess potential bonding between MnP-CA and bio-neat EP, arising from the interaction of nanoparticles within the bio-EP resin. Additionally, to evaluate the impact of nanoparticle interactions on the thermal properties and stability of all EP-MnP and EP-MnP-CA composites, thermogravimetric analysis (TGA) was performed at temperatures reaching up to 800 °C.
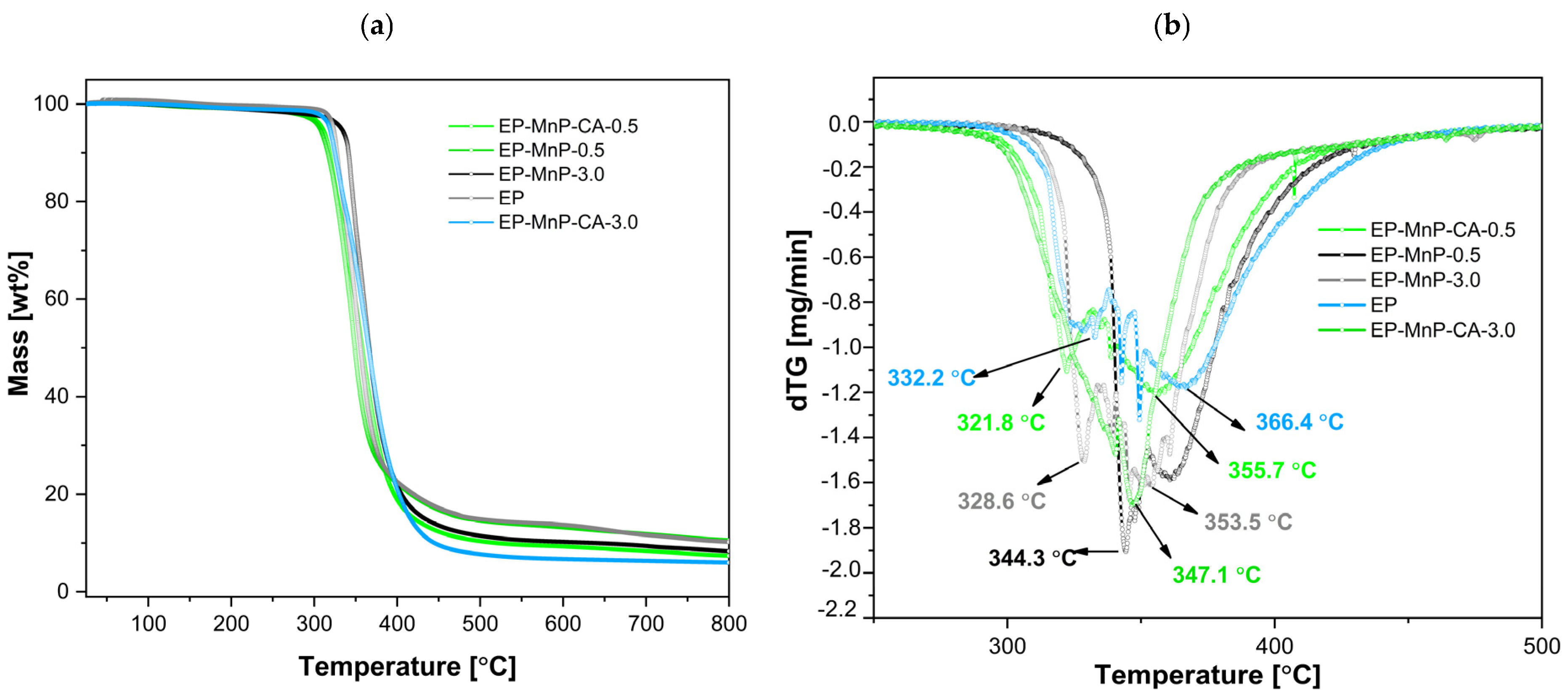
The obtained results are illustrated in
Figure 5a, b and represent the weight loss curves in relation to the temperature, with the peak/decomposition temperature (Td). These processes contributed to further degradation during the second stage of weight loss, which occurred between 200 °C and 400 °C. The successful removal of trace water and solvents was attained during fabrication, as no weight loss was found below 120 C. At around 300 °C to 400 °C, degradation began gradually through the homolytic cleavage of chemical bonds in the ester linkages within the network, triggered by the dehydration of the oxypropylene group, –CH
2-CH(OH), leading to the formation of double bonds. The decomposition temperature (
Figure 5b) for neat bio-EP resin was approximately (332.67 ± 0.25) °C. In this initial phase, isomerization reactions, chain transfers, intermolecular cyclization, and other radical reactions were triggered, marking the first stage of degradation. For bio-EP resins, thermal degradation typically involves the breakdown of its crosslinked network. Bio-based components, such as bio-derived hardeners or curing agents, often decompose at higher temperatures due to their complex chemical structures. This process includes the degradation of bio-based polyols or other functional groups derived from renewable sources, contributing to the material’s overall weight loss. As the degradation proceeds into the second stage, at 366.1 °C, further weight loss occurs due to the complete breakdown of the polymer backbone. This involves the scission of additional chemical bonds in both the epoxy network and bio-based additives. The release of volatile compounds and char formation is typical in this stage and depending on the specific formulation of the bio-epoxy resin, the thermal degradation may also involve reactions like oxidation or decomposition of remaining bio-functional groups. Bio-epoxy resins thermally degrade through a multi-stage process. In our samples, we performed a two-stage process, starting with bond cleavage and leading to the breakdown of both synthetic and bio-based components, with subsequent weight loss and the formation of volatile products.
The addition of nanoparticles, whether pristine or modified, results in an enhancement of the bio-EP resin’s thermal stability obtained from DTG curves (
Figure 5a,b), confirming changes in mass over a wide range of temperatures. In general, the degradation starts at around 300 °C, indicating a shift in thermal stability depending on the surface modification and its concentration. As shown in
Figure 5a,b, the Td for the EP-MnP-0.5 composites dips slightly below the benchmark, reaching a maximum of one peak of 344.2 °C (around 16 °C less than neat bio-EP), proving the increased average degradation temperature of EP-MnP-0.5 resin composites compared with neat bio-EP. With an increasing MnP loading content (3.0 wt%), the Td of the first peak was unretained at 328.62 °C, while the second peak appeared at 353.1 °C. In the subsequent temperature range, the composites underwent secondary decomposition between 400 and 500 °C, attributed to the further breakdown of the nanoparticle/epoxy/hardener network [
20,
49].
When reinforced with MnP-CA, the decomposition appeared in two stages, similar to neat bio-EP, but due to the MnP-CA concentration, it provided differences in the degradation T. The weight loss associated with the degradation of EP-MnP-CA-0.5 composites began at lower temperatures (a minor one at around 321.8 °C) and continued through the next stages up to higher temperatures, with a second degradation peak temperature at 355.1 °C, indicating multiple degradation phases influenced by the presence of nanoparticles. Contrarily, the addition of 3.0 wt% of modified nanoparticles led to a higher degradation temperature at 347.6 °C, leading almost to a one-step degradation phase. Notably, the introduction of 3.0 wt% of functionalized iron-oxide based nanoparticles provided a more available surface functional group, thus leading to an increase in interaction between the bio-EP and nanoparticles, providing activation energy for thermal degradation, which suggests improved thermal stability.
The dispersion of MnPs in bio-EP can significantly improve the thermal stability of epoxy composites. Properly dispersed nanoparticles enhance heat transfer throughout the matrix, resulting in increased thermal conductivity and greater resistance to thermal degradation [
50,
51]. This effect is especially notable with smaller nanoparticles, as their larger surface area allows for greater interaction with the bio-EP matrix. According to the literature, incorporating nanoparticles can increase the thermal stability of these composites, owing to the existence of a rigid part of nanoparticles, which acts as an interdiction to minimize the permeability of volatile degradation products from the epoxy nanocomposites [
52]. Enhanced thermal stability of EP-MnP and EP-Mnp-CA composites follow the effects studied in the literature due to synergistic effects, physical barriers, improved kinetics, and concentration effects [
20,
49,
53,
54].
In summary, the temperature of degradation for MnP- and MnP-CA-filled epoxy resins varies significantly based on the type and number of nanoparticles used. Generally, significant mass loss occurs between 300 °C and 480 °C, with complete degradation extending up to 700 °C. The incorporation of 0.5 wt% MnP and 3.0 wt% MnP-CA nanoparticles tends to improve thermal stability, shifting initial decomposition temperatures higher compared to unfilled bio-EP.
The heat evolution observed in epoxy resin using DSC is generally assumed to be proportional to the extent of reactive group consumption. The curing reaction of epoxy resin is a complex and critical process that transforms the liquid resin into a durable, solid thermoset material through a series of chemical reactions. Typically, the curing of epoxy resins involves a reaction between epoxy groups and curing agents—such as amines, anhydrides, or acids—which play a vital role in determining the crosslinking mechanism of the curing process [
55,
56]. These mechanisms may include ring-opening reactions, nucleophilic attacks, and crosslinking, all of which contribute to the development of a rigid, highly crosslinked network. Moreover, the curing reaction is temperature-dependent; higher temperatures generally accelerate the curing process.
Not just the selection of curing agents and curing conditions (such as temperature and time) but also the addition of nanoparticles and the overall composition of the resin system can significantly influence the curing kinetics and, consequently, the final properties of the cured material. The final factor—iron oxide nanoparticles—has a substantial impact on the curing reaction of epoxy resin, enhancing various properties, including the mechanical performance of the resulting composite material. The curing process, which involves the crosslinking of epoxy with a hardener, can be influenced by both the incorporation of bare and functionalized nanoparticles, as well as the concentration of nanoparticles used.Factors such as the degree of crosslinking and the resulting network structure affect the mechanical strength, thermal stability, chemical resistance, and durability of the epoxy. By carefully controlling the curing process, manufacturers can tailor the material properties to meet specific application requirements, making epoxy resins widely used in coatings, adhesives, electronics, and composites.
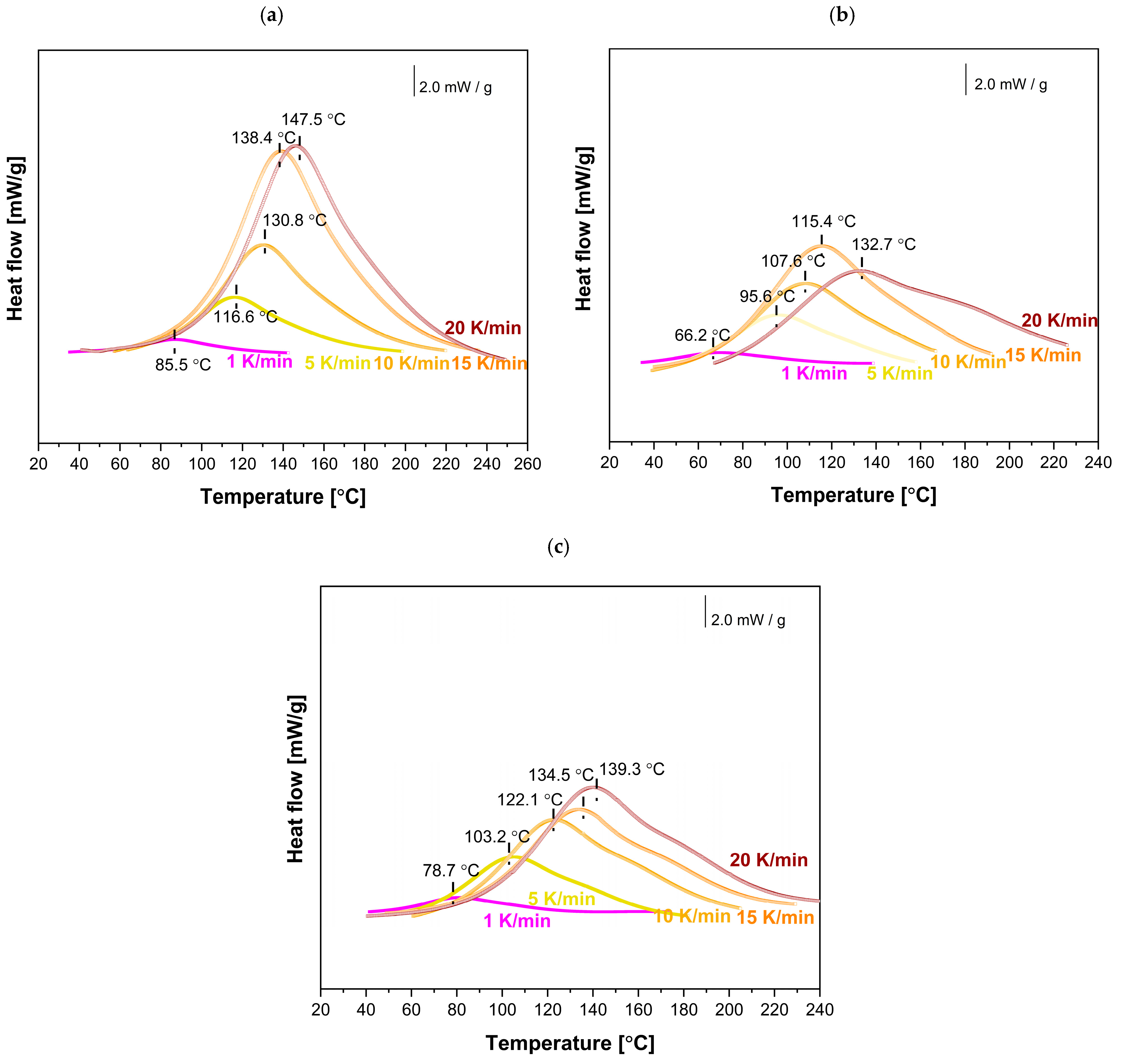
To investigate the effect of MnP and MnP-CA nanoparticle addition on the curing reactions of the EP-MnP and EP-MnP-CA systems, the initial step involved selecting the optimal curing temperatures based on the analysis of dynamic cure DSC curves obtained from non-isothermal DSC measurements (
Figure 6 and
Figure 7).Subsequently, a series of measurements of the non-isothermal curing reactions at different heating rates, ranging from 1 to 20 K/min, was conducted (
Figure 6 and
Figure 7) for EP, EP-filled MnP, and EP-filled MnP-CA, which plots the heat flow (dH/dt) against temperature (T). The key information that can be directly extracted from the DSC curve includes the onset temperature (Ti), peak temperature (Tp), and the values of dH/dt.
For neat bio-EP, as expected (
Figure 6 and
Figure 7a), the DSC is determined by a broad exothermic peak, and with the increasing heating rate, the magnitude of the exotherm increases as well, and the temperature of the peak shifts to a higher temperature value due to the increasing heating rate.
Due to the MnP addition (0.5 wt% and 3.0 wt%), the heat flow is enhanced with the increasing heating rate, as shown in
Figure 6b,c. It is evident from
Figure 6b,c that even for the EP-MnP-0.5 epoxy resin composite or EP-MnP-3.0, the peak temperature of curing shifts. As the heating rate increases, the curing start temperature (Ti), peak temperature (Tp), and end temperature (Tf) shift to lower values compared to neat bio-EP. The results suggest that MnP acts as a trigger for the fastest curing process of EP-MnP composites. Thus, the % of the loading of MnP impacts the reaction kinetics of all EP-MnP composites. Higher heating rates tend to shift the curing start temperature (Ti), peak temperature (Tp), and end temperature (Tf) to lower values, indicating that the EP-MnP resin reacts more quickly at elevated temperatures than neat bio-EP, providing lower activation energy to be utilized in a shorter time frame.
When nanoparticles are functionalized with citric acid (CA), the curing process of all EP-MnP-CA epoxy resin composites is further enhanced compared to EP-MnP composites filled with bare iron oxide nanoparticles..
Figure 7 represents the comparison of curing temperatures of neat bio-EP, MnP-CA-0.5, and EP-MnP-3.0 resin composites by analyzing the dynamic cure DSC curves, a non-isothermal curing reaction at different heating rates ranging from 1 to 20 K/min focusing on the onset temperature (Ti), peak temperature (Tp), terminal temperature (Tf), and the values of dH/dt. It is evident that due to the incorporation of 0.5 wt% or 3.0 wt%, the temperature of curing is enhanced and shifted to the lowest value of temperature. More evident with the increasing content of nanoparticles is that the temperature of curing decreases. The critical factors influencing the curing process and the resulting material properties include the addition of nanoparticles and their interaction mechanisms. It is well known that, at low concentrations, these nanoparticles enhance the curing reaction due to their high surface area, which promotes better interaction with the epoxy matrix. They can act as catalysts in the curing process, promoting the ring-opening of epoxy groups and leading to a denser crosslinked network [
30,
38,
57]. This is particularly effective when using amine-functionalized Fe
3O
4 nanoparticles, which actively participate in the curing reaction by attacking epoxy groups.
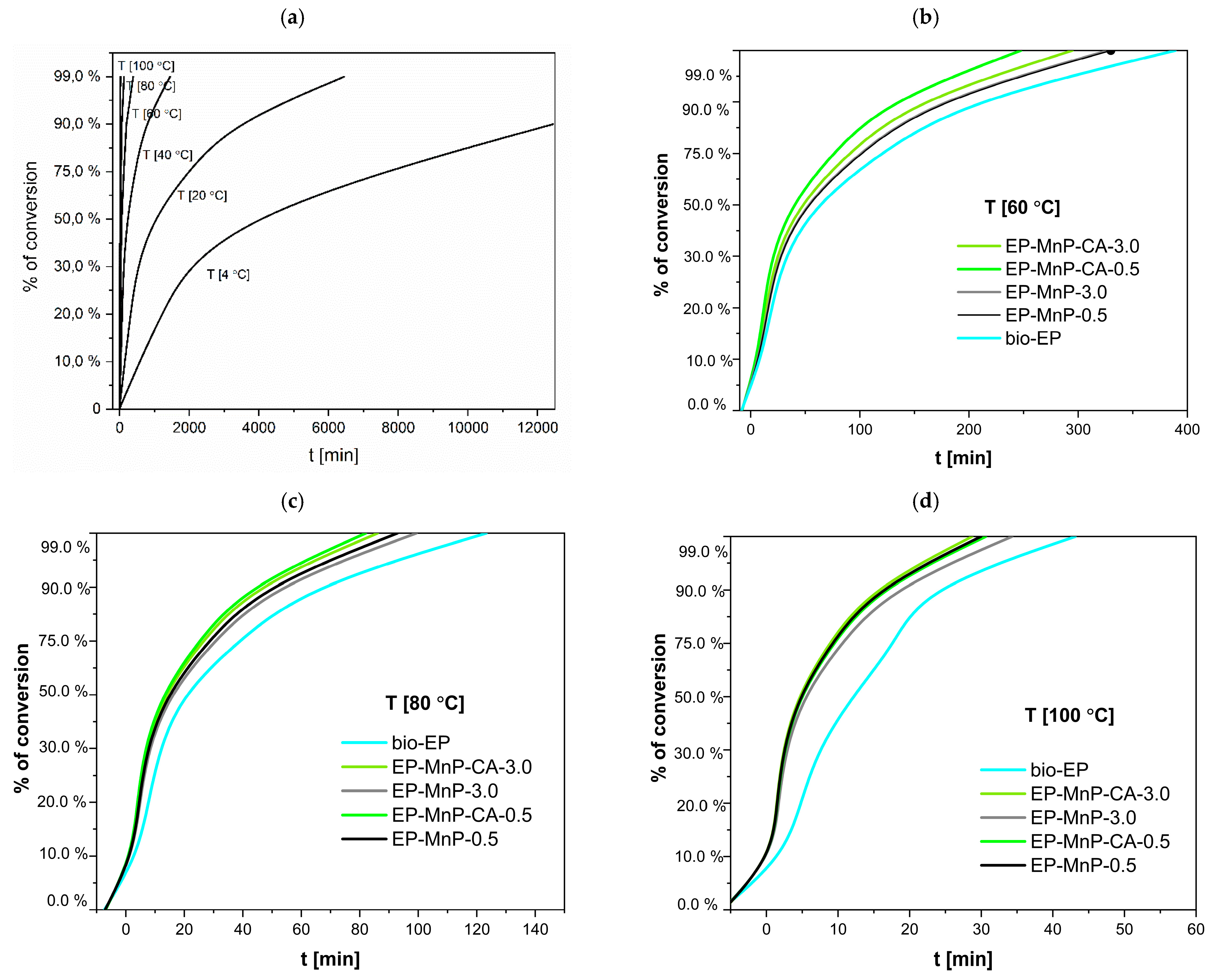
The dispersion of bare nanoparticles differs from that of surface-modified nanoparticles in the bio-EP resin, resulting in variations in the degree of curing that depend on the temperature heating rate, as shown in
Figure 8 and
Table 3. The curing degree of neat bio-EP increased rapidly during the initial reaction stage with the increasing temperature heating rate, then increased slowly, and finally tended to a certain value. Nevertheless, the time for curing the formulations loaded with MnP and MnP-CA greatly varies depending on the amount of MnP (
Table 3). Nevertheless, this effect is more visible at low temperatures for neat bio-EP. The time needed for 99% curing conversion at room temperature, is 80.9 h. With an increasing temperature to 60 °C, the curing conversion decreases to shorten the time to 320.41 min, and with a further increasing temperature, at 100 °C, in only 50.98 min, 99% bio-EP-neat is cured. Compared with EP-MnP filled with 0.5 wt.% and 3.0 wt.% MnP, the curing conversion of all composites is fastened, providing less time for 99% of cured EP-MnP composite samples. The 0.5 wt% MnP-loaded nanoparticles exhibit the fastest curing conversion of EP-MnP composites compared to neat bio-EP at temperatures of 60 °C, 80 °C, and 99 °C, with curing times of 328.92 min and 29 min for EP-MnP-0.5, and 327.99 min and 34 min for EP-MnP-3.0, respectively. At higher heating rates, the conversion of curing curves becomes faster than that of neat bio-EP. This indicates that, due to the MnP addition, more heat is released during the curing process, which can enhance crosslinking.
The same trend of curing conversion behavior is evident for the MnP-CA 0.5 and MnP-CA-3.0 composites’ resin (
Figure 8 and
Table 4). With the incorporation of 0.5 wt% MnP-CA, the curing process is faster than in neat bio-EP and all EP-MnP composites. At 60 °C, the curing conversion is achieved in 247 minutes, approximately 100 minutes faster than neat bio-EP. At 100 °C, EP-MnP-0.5 reaches curing conversion in only 30 minutes. The addition of 3.0 wt% MnP-CA further accelerates the curing process, with conversion occurring at 294.86 °C and in 28 minutes. EP-MnP-3.0 also cures efficiently at 60 °C, 80 °C, and 99 °C.
The results shown in
Figure 8 and
Table 4 highlight several critical factors that influence the curing of bio-EP resin filled with bare and surface-modified MnP nanoparticles. The recent research findings focus on the following aspects. The incorporation of iron-oxide nanoparticles, bare or surface-modified, into bio-epoxy resin significantly influences its curing reaction and kinetics. The curing process, which involves the crosslinking of epoxy with a hardener, can be tuned: first, by the concentration of nanoparticles used; second, by the functionalization of surfaces of nanoparticles; third, by the dispersion in EP, resulting in an enhanced interaction between n EP and MnP, providing faster curing than neat bio-EP. At lower loadings (e.g., 1–5 wt%), the nanoparticles remain well-dispersed, enhancing the curing process without significant aggregation. This results in improved mechanical and thermal properties of the cured epoxy [
38,
57]. Bare Fe
2O
3 nanoparticles tend to agglomerate, especially at higher concentrations. This clustering leads to voids within the epoxy matrix, which can hinder effective crosslinking during the curing process. The tendency for bare nanoparticles to form clusters is attributed to van der Waals forces and magnetic interactions, which can deteriorate the mechanical properties and barrier performance of the cured epoxy. Surface-modified nanoparticles enhance curing kinetics by participating more effectively in crosslinking reactions due to their reactive functional groups acting as a curing promoter as well. This leads to a more effective curing process, especially at lower concentrations. Similar results found in the literature reveal that the surface modification (e.g., with SiO
2 or amine groups) enhances the chemical stability and compatibility of Fe
3O
4 nanoparticles with the epoxy resin. This results in a more uniform dispersion throughout the matrix and enhanced interaction. Functionalized nanoparticles can participate in chemical interactions with the epoxy, promoting better integration into the matrix.This leads to a denser crosslinked network and fewer voids, improving mechanical properties and corrosion resistance. As reported in Ref. [
57], the addition of bare iron oxide nanoparticles (particularly Fe
3O
4, magnetite) at low contents of nanoparticles has been shown to decrease the curing enthalpy of epoxy systems. This suggests that the presence of these nanoparticles can facilitate the curing reaction, potentially leading to a more efficient crosslinking process, demonstrating a similar trend in our study when MnP-CA is incorporated in bio-EP composites’ resin. Additionally, the published work investigated the effect of bare, SiO
2/chitosan-, and SiO
2/chitosan/imide/phenylalanine-modified Fe
3O
4 nanoparticles in EP resin, demonstrating a curing-promoting effect. This effect was more pronounced in samples where Fe
3O
4; was functionalized with chitosan [
38]. Previous studies have shown that nanoparticle chemistry significantly affects curing behavior. For example, Co
2+-doped Fe
3O
4 was reported to increase the heat released during epoxy curing, while AuNPs were found to reduce the exothermic response—likely due to steric hindrance effects. These findings support the notion that nanoparticle composition and surface reactivity play a key role in tailoring crosslinking kinetics, although such doped systems were not part of the present study. Even more, it is clear that nanoparticles play a dual role as curing promoters, resulting in increased viscosity of the system and leading to the gelation process due to uniform dispersion of nanoparticles in bio-EP resin.
Dynamic mechanical properties (DMA) of bio-based EP resin composites filled with the nanoparticle filler, including storage modulus, loss modulus, and glass transition, depend on the addition of either neat or functionalized fillers and provide crucial knowledge for applications requiring enhanced mechanical performance and thermal stability. Here, the morphology and size of the fillers play a crucial role in determining the mechanical properties, where the smaller particles more uniformly distribute in the bio-EP resin matrix, leading to more sufficient reinforcement and minor agglomeration [
58,
59,
60]. It is well established that the DMA properties of epoxy-based nanocomposites vary depending on the type of filler, its surface modification, and temperature. These factors influence the viscoelastic behavior of the composites. Moreover, the incorporation of fillers into bio-based epoxy generally leads to an increase in storage modulus, indicating enhanced stiffness and load-bearing capacity.
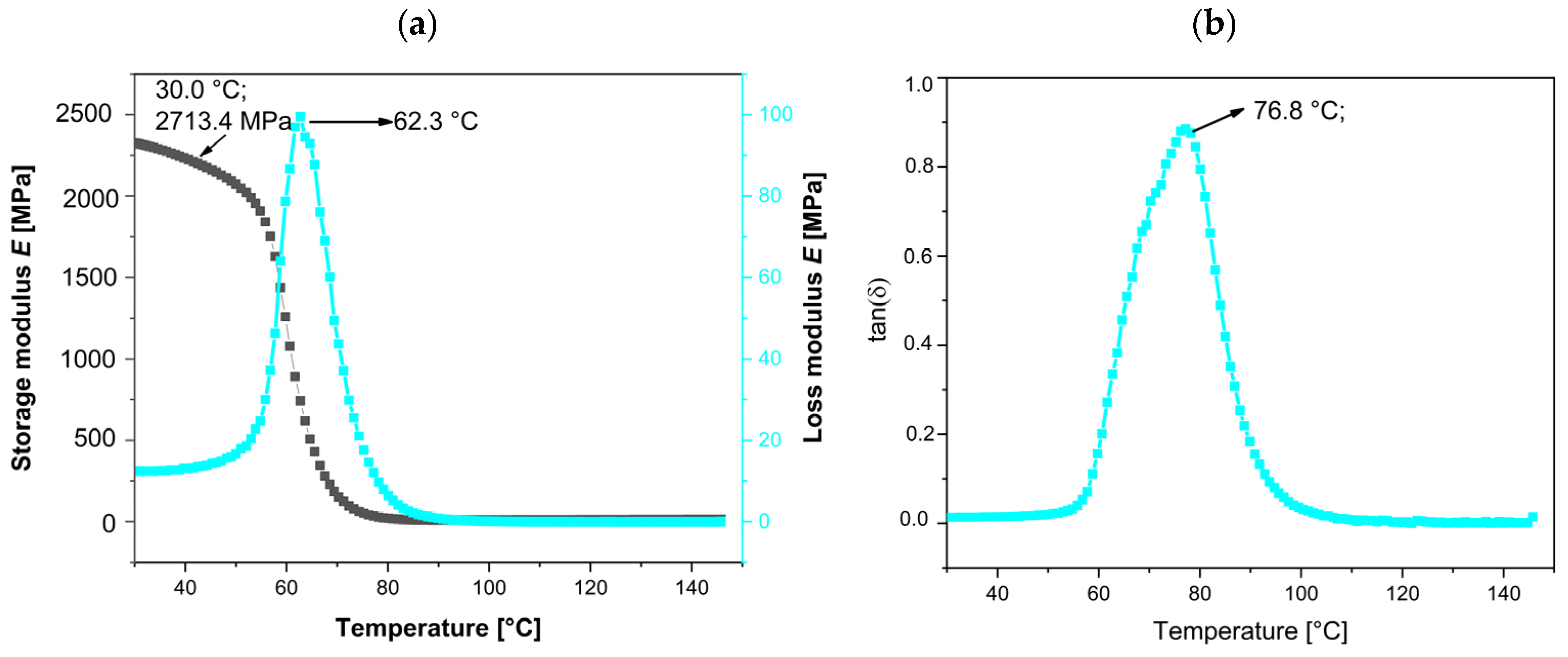
The neat bio-EP resin, as a thermoset material, exhibits a high storage modulus (E′), loss modulus (E″), and damping factor (tanδ), as shown in
Figure 9a,b. The initial values of E′, E″ at the peak, and maximum tanδ are summarized in
Table 5. At 25 °C, the initial E′ value is 2710.4 MPa, while the tan delta (tanδ), representing both the elastic and viscous components of this viscoelastic polymer, peaks at 76.8 °C.
The evolution of storage modulus (E′) in MPa, loss modulus (E″) in MPa, and tangent delta (tanδ), or loss factor/damping factor (non-dimensional) for the EP, EP-filled MnP, and MnP-CA samples is summarized in
Figure 9,
Figure 10 and
Figure 11 and
Table 5 to further illustrate the dynamic mechanical properties.
Figure 10 shows the effect of different MnP loadings (0.5 and 3.0 wt%) on the storage modulus, loss modulus, and tan delta of all EP-MnP composites. Adding either 0.5 or 3.0 wt% of MnP enhances the storage modulus of EP composites at 20 °C: a 14.1% increase with 0.5 wt% MnP and a 24.7% increase with 3.0 wt% MnP, as shown in
Figure 10a,b and summarized in
Table 5. The addition of rigid MnP particles makes the EP-MnP composites tougher and stiffer than the neat epoxy composites. Thus,
Figure 10 clearly shows that the tensile strength of the epoxy composites improves with the addition of an MnP filler. This enhancement in storage modulus is attributed to the reinforcing effect of MnP particles, which also results in changed mobility of the EP-MnP composites, as indicated by the glass transition temperature (Tg). Additionally, the ratio of the elastic to viscous portions in EP-MnP composites, represented by tanδ, is shown in
Figure 10b. With increasing MnP content, the Tg value initially rises to 79.4 °C for the 0.5 wt.% MnP addition but slightly decreases to 72.1 °C at 3.0 wt% compared to the neat bio-EP material. This increase in Tg is likely due to enhanced movement within the EP-polymer network chains caused by the nanoparticle addition, which leads to the formation of free volume that restricts the chain’s mobility, as it was found in ZnO-EP composites and Fe
3O
4/GPTMS/Epoxy composites.
On the other hand, the storage modulus, loss modulus, and tan delta of all EP-MnP-CA composites, as shown in
Figure 10a and
Figure 11a,b, exhibit higher values compared to both neat bio-EP and EP-MnP composites. Specifically, as the content of modified nanoparticles increases from 0.5 wt% to 3.0 wt%, the storage modulus of the EP composites increases by 20.1% and 22.4%, respectively. This indicates the successful transfer of applied stresses from the polymer matrix to the reinforcement effect. As can be seen in
Figure 11a, all the samples for EP-MNP-CA, independent of the proportion, present slight increments in the rigidity (storage modulus) in both glassy (20 °C) and rubbery states (120 °C).
The tan delta of EP-MnP-CA composites was also determined. It was observed that the Tg notably decreases when the EP is reinforced with modified nanoparticles. The results summarized in
Table 5 and
Figure 11b indicate an increase in Tg from 62.2 °C for neat bio-EP to 67.1 °C for EP-MnP-CA-0.5 composites and further for EP-MnP-CA-3.0 composites, demonstrating the enhanced thermal performance with increasing MnP-CA content. It was also observed that the height of the tanδ peak (which is related to the mobility of the network structure) changes when increasing the proportion of 0.5 wt% and decreases to 3.0 wt% (
Table 5). Similar results have reported a similar trend, with none of the proposed theories being conclusive. In a study on EP-based magnetite nanoparticle composites, when functionalized with either PDA, GPTMS, or APTES on maghemite nanoparticles, the tan delta (Tg) shifted to a lower temperature. It has been suggested that the interaction between the polymer matrix and the reinforcements leads to the formation of multiple layers around the particles. A secondary polymer nanolayer that forms around the nanoparticles is believed to relax more quickly than the immobile layer directly bound to the NPs. This portion of the matrix can move more freely at higher temperatures, potentially reducing the composite’s Tg [
61]. Another study on EP–organoclay composites found that chain entanglement and excess free volume within the matrix system lead to a decrease in Tg values. This reduction is associated with increased molecular mobility, which in turn lowers the reaction rate acceleration and effective crosslink density, ultimately reducing the curing temperature [
18].
To summarize, the reinforcing effect of either neat or functionalized nanoparticles is attributed to improved storage modulus and is more pronounced in functionalized nanoparticles. First, the functionalizing of nanoparticles is crucial for optimizing the interaction with the epoxy matrix, leading to better dispersion and load transfer, which also restricts macromolecular mobility near the nanoparticles. It is clearly measured that as the nanoparticles were further modified, Tg decreased to lower values. This decrease in Tg is a clear indication of hindrance in more uniformly distributed nanoparticles that provide better adhesion of nanoparticles in the epoxy matrix, which may result in restricted chain mobility. However, when unmodified MnP is incorporated into EP composites, it does not significantly hinder dispersion, initiate agglomeration, or cause notable changes in the Tg value.With respect to the storage modulus in the glassy and rubbery state, the tendency is similar to the peak of tanδ. As the nanoparticles are modified, the storage modulus at 120 °C reaches 14.4 MPa compared to non-modified MnP nanoparticles, which decreases the storage modulus to 4.4 MPa. The results of the storage modulus at the rubbery state indicate better distribution in the polymer matrix and pronounced crosslinking density. The storage modulus in the rubbery state is also related to the crosslinking density, reaching the highest value at 120 °C at 19.1 MPa when the nanoparticles are modified with CA, and 4.3 MPa when EP-composites consist of non-modified nanoparticles [
18]. This highlights the advantageous role of nanoparticle functionalization, which introduces polar groups—such as hydroxyl and carboxyl—on the nanoparticle surface, thereby enhancing their interaction with the epoxy matrix. The modified MnP-CA nanoparticles provide these functional groups and play a critical role in improving the properties of epoxy composites through multiple mechanisms, primarily involving enhanced interfacial interactions and physical reinforcement. It also allows for the formation of hydrogen bonds between the filler and the epoxy matrix and creates an interface between bio and EP. These interactions are crucial for enhancing filler–matrix adhesion, which is essential for transferring stress effectively during mechanical loading. Stronger interfacial bonding leads to improved tensile properties and overall durability of the composite. In addition, hydroxyl groups in fillers can catalyze the polymerization process of epoxy resins curing. This catalytic effect facilitates the diffusion of epoxy and curing agent molecules, leading to improved crosslinking within the matrix. Enhanced crosslinking results in a more robust network structure, contributing to higher mechanical strength and thermal stability of the composite. In the literature, it was also known that fillers with hydroxyl functional groups can significantly enhance the mechanical properties of epoxy composites. For instance, EP composites reinforced with silicate nanofillers containing hydroxyl groups exhibited increased tensile strength and storage modulus compared to those without such functionalization. This enhancement is attributed to better dispersion and interaction at the filler–matrix interface [
50,
62,
63]. Although the functionalization of MnP nanoparticles with CA enhances dispersion and filler–matrix interactions, the observed decrease in Tg for EP-MnP-CA composites may be attributed to a plasticization effect induced by the hydrophilic functional groups (–OH, –COOH) of citric acid. These groups can increase local free volume or form loosely bound interfacial regions with greater chain mobility. Similar behavior has been reported in epoxy systems functionalized with organic modifiers, where improved dispersion is often accompanied by a reduction in Tg due to enhanced segmental motion in interfacial zones [
62]. Additionally, variations in local curing behavior and crosslinking density near the functionalized nanoparticle surfaces may further contribute to this effect.
The incorporation of iron-oxide nanoparticles, specifically magnetite (Fe3O4) and hematite (Fe2O3), into EP composites not only affects the curing process, providing differences in thermal properties, but significantly influences their surface properties as well. In the following study, the surface properties of bio-EP, EP-MnP, and EP-MnP-CA were evaluated with surface zeta potential measurements, resulting in differences in the surface charge.
Surface charge plays an essential role in describing how EP-based nanocomposites interact with other materials in aqueous environments, particularly solid surfaces. This charge characteristic becomes critical for understanding how bio-EP resin composites behave before and after introducing iron-oxide-based fillers. The addition of such fillers can significantly alter the material’s interfacial properties, compatibility, and potential uses in various applications, such as biomedical devices, aerospace, automotive, construction, and coatings. To explore these changes, zeta potential measurements were conducted, targeting the surface properties of neat EP composites while varying the type and concentration of bare MnP and MnP-CA. These measurements provide a quantitative view of the surface charge across a pH range, enabling a better understanding of how different fillers influence composite surface behavior.
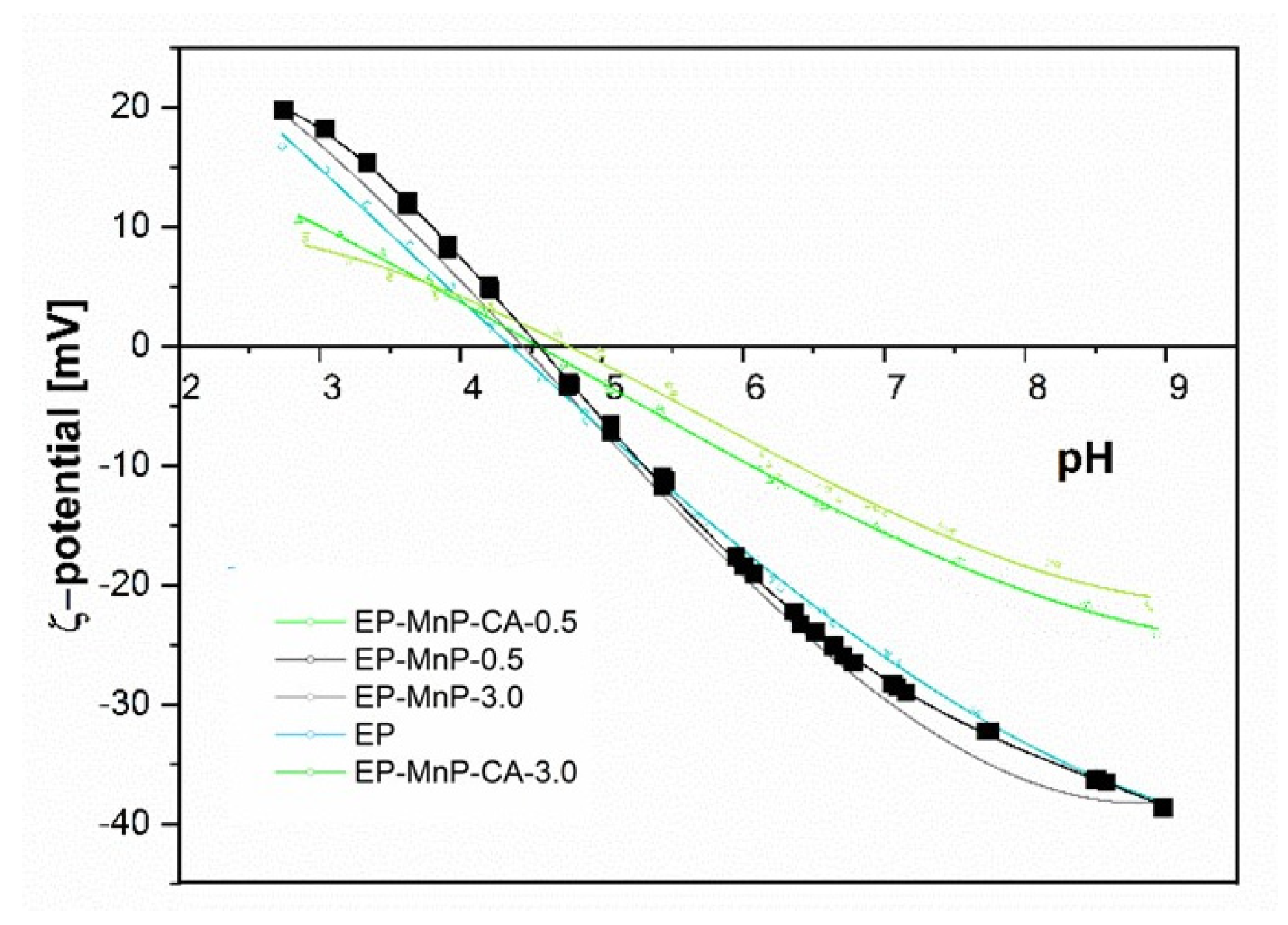
Figure 12 presents the surface zeta potential values for neat bio-EP, EP-MnP, and EP-MnP-CA composites across a pH spectrum from 2 to 12, demonstrating how MnP filler additions shift the surface charge. These zeta potential and isoelectric point (IEP) values reveal essential details about the functional groups on the composite surface. For instance, the isoelectric point serves as a valuable indicator of surface acidity or basicity, with a lower IEP suggesting an increase in acidic functional groups, while a higher IEP points toward additional basic groups. As shown in
Figure 12, the IEP for neat EP occurs at a pH of approximately 4.3, a result that aligns with existing literature on EP resin composites. Interestingly, with the incorporation of MnP and MnP-CA fillers, the isoelectric point (IEP) remains relatively unchanged across different concentrations, showing only slight variations: EP-MnP-0.5 = 2.90, EP-MnP-3.0 = 3.05, EP-MnP-CA-0.5 = 3.21, and EP-MnP-CA-3.0 = 3.21. For instance, the IEP values for EP-MnP and EP-MnP-CA composites range from pH 4.5 to pH 4.7, indicating a slight shift toward the right (less acidity) but overall stability in the IEP. The stable IEP values suggest that the additions of MnP and MnP-CA do not significantly alter the fundamental surface charge properties of the EP matrix. This modest change suggests that the filler particles are not significantly altering the surface chemistry of the composites, likely because they are embedded within the epoxy matrix rather than being predominantly present at the surface. The hydrogen bonding or other interfacial interactions (e.g., with carbonyl groups of EP reported by ATR-FTIR results) between the fillers and the matrix might also contribute to this observation. These interactions stabilize the filler within the matrix without dramatically altering the external surface charge properties.
This negligible effect on the surface charge results in a relatively consistent IEP value, highlighting the compatibility and cohesive interaction of EP with fillers.
The overall zeta potential of EP-MnP and EP-MnP-CA composites, which shift from high negative values at an elevated pH towards more positive values, is shown in
Figure 12. Above a pH of 4.7, all EP-MnP and EP-MnP-CA composites exhibit increasingly negative zeta potentials. The impact of MnP and MnP-CA incorporation on the surface and interfacial charge of these composites is reflected in the negative zeta potential values at a pH of 8 (
Figure 12). This may primarily reflect charge redistribution within the matrix rather than a wholesale alteration of surface functionality. However, although no significant changes in IEP occurred, it is evident that as MnP-CA is incorporated into EP, the rise in the negative zeta potential plateau values occurred. With higher levels of MnP-CA, the zeta potential at a pH of 8 becomes more negative than that of neat EP. This effect is attributed to the addition or redistribution of polar groups on the composite surfaces, which enhances surface hydrophilicity. Overall, zeta potential measurements for all EP-MnP and EP-MnP-CA composites exhibit an anionic charge above their respective IEP values, indicating a high capacity for adhesion or adsorption of cationic substances and the repulsion of anionic substances. Nevertheless, the incorporation of CA-functionalized MnP (MnP-CA) results in a more negative ζ-potential at neutral-to-basic pH values compared to neat EP and EP-MnP composites. This behavior can be attributed to the presence of ionizable carboxyl and hydroxyl groups introduced by citric acid on the nanoparticle surface. These groups enhance the hydrophilicity and polarity of the filler surfaces and facilitate stronger interfacial interactions (e.g., hydrogen bonding) with the epoxy matrix. Although the isoelectric point remains relatively unchanged—likely due to the encapsulation of fillers within the matrix—the increase in a negative surface charge above the IEP confirms that functionalization leads to a redistribution of surface-active groups and improved matrix compatibility.
The incorporation of MNPs is also significant for the practical applicability of magnetic bio-epoxy resin composite materials. The iron oxide nanoparticles, synthesized via coprecipitation with an appropriate ratio of precursor salts (iron sulfates), exhibit a structural composition that is a mixture of maghemite (γ-Fe
2O
3) and magnetite (Fe
3O
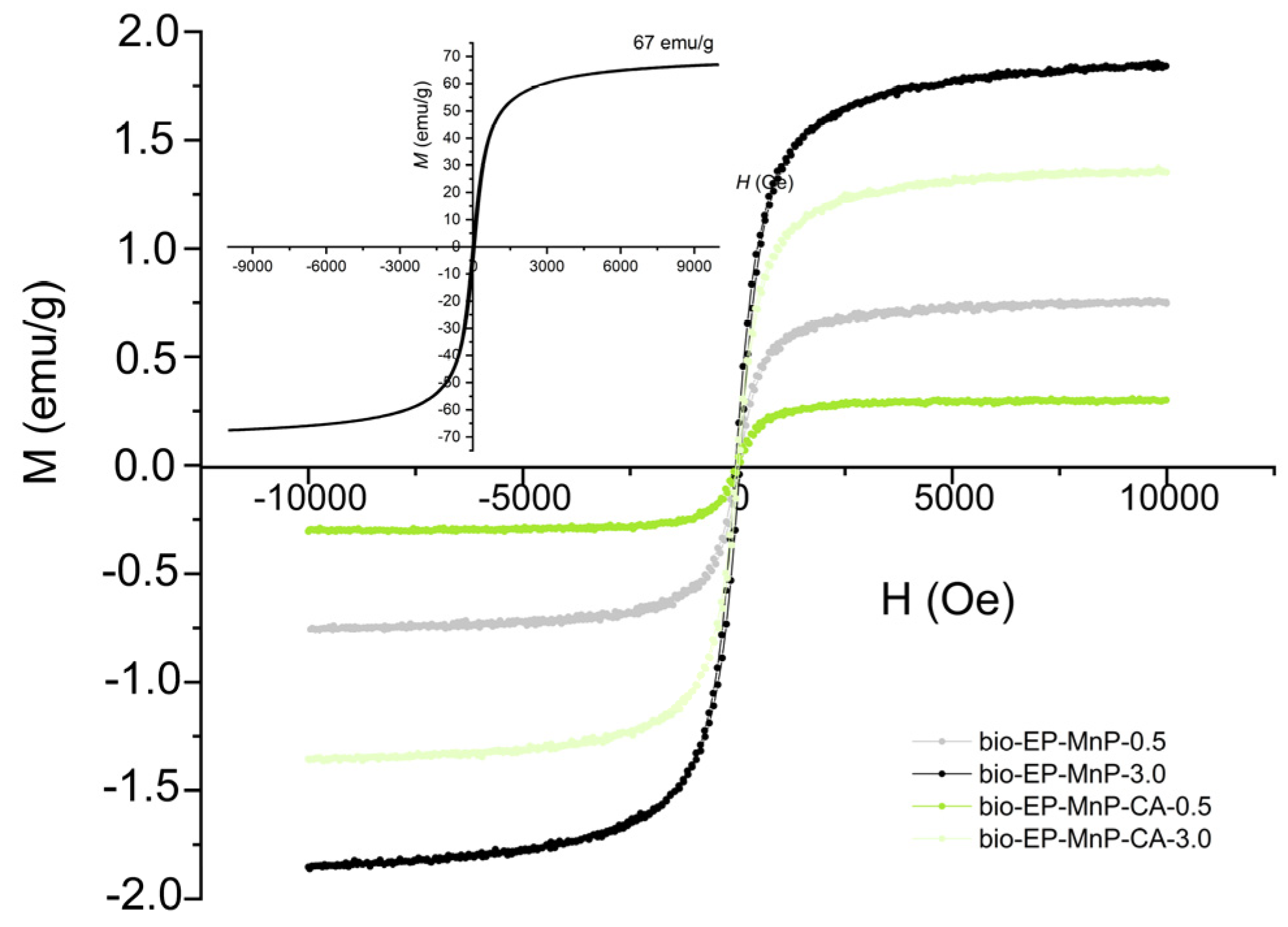
4). These nanoparticles are characterized by superparamagnetic properties, which depend on their particle size. While the magnetic moments of individual atoms remain aligned, the overall magnetic moment of the nanoparticle fluctuates rapidly under the influence of thermal energy. In the absence of an external magnetic field, superparamagnetic nanoparticles do not exhibit magnetization, with their remanence being equal to zero. This behavior results in an S-shaped (sigmoidal) M-H curve, as illustrated in the inset of
Figure 13.
Figure 13 presents the magnetization (M–H) curves for bio-EP-MnP/MnP-CA composites containing iron oxide nanoparticles coated with citric acid (CA), illustrating their magnetic response as a function of the applied magnetic field. The sigmoidal shape of the magnetization curves confirms the presence of superparamagnetic nanoparticles within the polymer matrix. It is observed that the saturation magnetization (
Ms) increases proportionally with nanoparticle content, regardless of whether functionalization has occurred. In the bio-EP-MnP composites, the saturation magnetization increases with higher MnP content. A similar trend is observed in the bio-EP composites filled with MnP-CA particles; however, due to better dispersion, the saturation magnetization values are slightly lower compared to those with unmodified MnP nanoparticles. It is well established that saturation magnetization is related to the nanoparticle size and tends to increase with the particle size. The observed trend suggests that nanoparticle agglomeration occurs at higher concentrations in the composites containing bare MnP nanoparticles, likely due to dipole–dipole interactions.
In general, the improved dispersion of MnP-CA nanoparticles in the bio-EP matrix is supported by multiple complementary analyses, including zeta potential, DMA, TGA, and ATR-FTIR. These results collectively indicate that citric acid functionalization enhances nanoparticle compatibility with the epoxy matrix, reducing agglomeration and facilitating stronger filler–matrix interactions. This improved dispersion is a key factor behind the enhanced mechanical, thermal, and curing behaviors observed in EP-MnP-CA composites.