Advances in Graphene-Based Flame-Retardant for Polystyrene Applications: Synthesis, Mechanisms, and Future Perspectives
Abstract
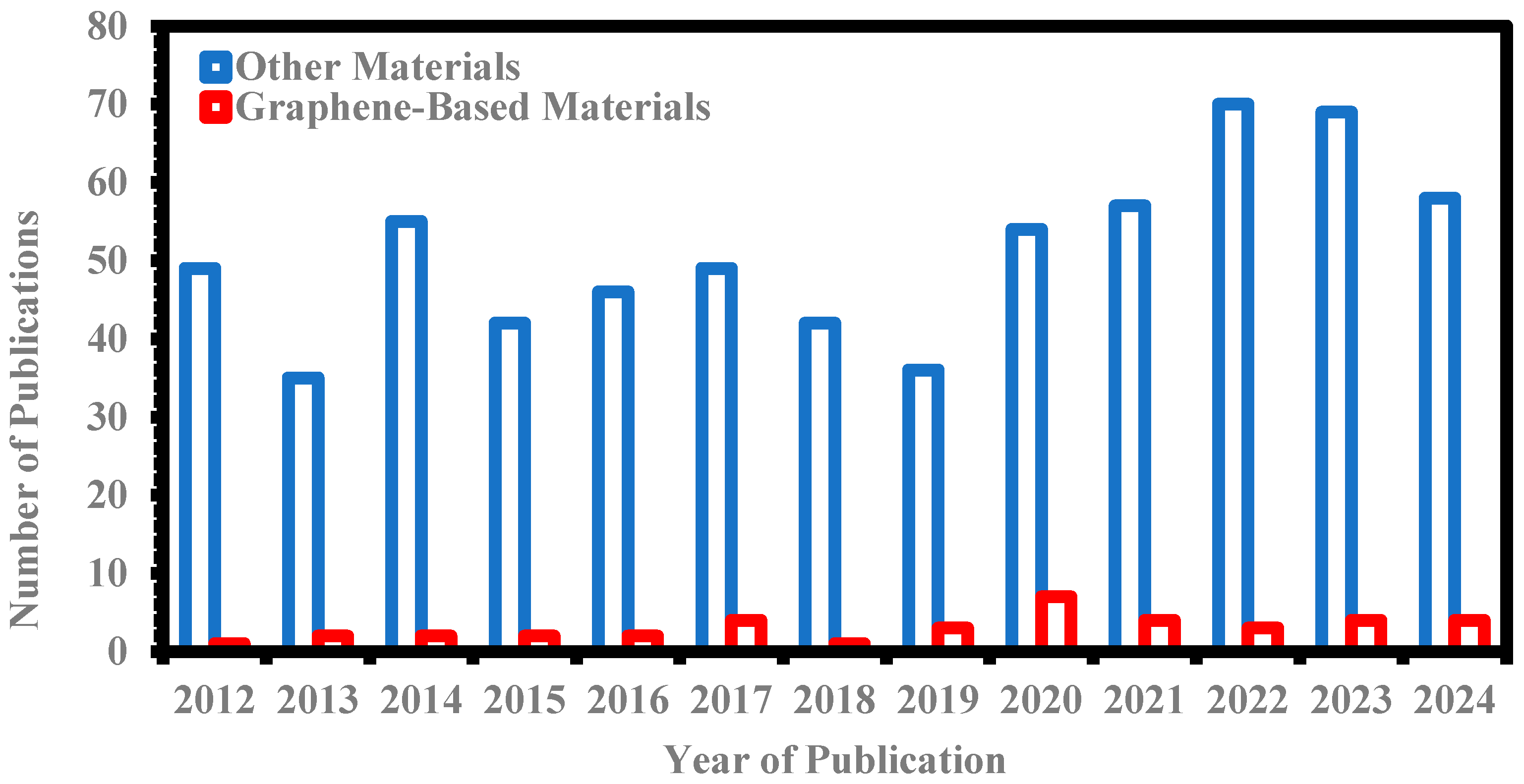
1. Introduction
2. Fundamentals of Flame Retardancy in Polymers
2.1. Combustion Mechanism
2.2. Flame-Retardant Mechanism
2.3. Conventional Flame Retardancy Tests
2.3.1. Limiting Oxygen Index (LOI)
2.3.2. Cone Calorimetry
- TTI (s): Under fixed irradiation intensity and sample thickness, a longer ignition time indicates greater resistance to ignition. However, in flame-retardant polymers, the presence of flame retardants may cause premature decomposition, reducing the TTI. Hence, a shorter TTI does not necessarily indicate reduced flame retardancy.
- HRR (kW/m2): The heat release rate quantifies the energy emitted per unit time and surface area during combustion, typically expressed in kilowatts per square meter. The peak heat release rate (PHRR), representing the maximum HRR observed, serves as a critical metric for assessing a material’s fire performance.
- THR (kJ/m2): The total heat released per unit area during combustion is calculated by integrating the HRR over the duration of the burning process.
- Fire growth rate (FGR, kW/(s·m2)): Defined as FGR = PHRR/tPHRR, where tPHRR is the time required to reach the peak HRR. A lower FGR value indicates better fire resistance.
- Mass loss rate (MLR, g/s): Represents the rate at which the material loses mass during combustion.
- Smoke production rate (SPR, m2/s) and total smoke production (TSP, m2): These parameters indicate the extent of combustion and the amount of smoke generated, providing insights into material flammability and smoke hazards.
2.3.3. Tests for Flammability of Plastic Materials UL-94
3. Graphene as a Flame Retardant: An Overview
3.1. Unique Properties of Graphene
3.2. Various Synthesis Methods for Graphene Sheets
3.3. Graphene as Flame-Retardant Material
4. Functionalization of Graphene for Enhanced Flame Retardancy
Functionalized Graphene-Based Materials for Polystyrene Flame Retardancy
5. Outlook on Graphene-Based Flame Retardants for Polystyrene Applications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eltaweil, A.S.; Elgarhy, G.S.; El-Subruiti, G.M.; Omer, A.M. Carboxymethyl Cellulose/Carboxylated Graphene Oxide Composite Microbeads for Efficient Adsorption of Cationic Methylene Blue Dye. Int. J. Biol. Macromol. 2020, 154, 307–318. [Google Scholar] [CrossRef]
- Omer, A.M.; El-Monaem, E.M.A.; El-Latif, M.M.A.; El-Subruiti, G.M.; Eltaweil, A.S. Facile Fabrication of Novel Magnetic ZIF-67 MOF@aminated Chitosan Composite Beads for the Adsorptive Removal of Cr(VI) from Aqueous Solutions. Carbohydr. Polym. 2021, 265, 118084. [Google Scholar] [CrossRef] [PubMed]
- Eltaweil, A.S.; El-Monaem, E.M.A.; Mohy-Eldin, M.S.; Omer, A.M. Fabrication of Attapulgite/Magnetic Aminated Chitosan Composite as Efficient and Reusable Adsorbent for Cr (VI) Ions. Sci. Rep. 2021, 11, 16598. [Google Scholar] [CrossRef]
- Attia, N.F.; Hassan, M.A.; Nour, M.A.; Geckeler, K.E. Flame-retardant materials: Synergistic effect of halloysite nanotubes on the flammability properties of acrylonitrile–butadiene–styrene composites. Polym. Int. 2014, 63, 1168–1173. [Google Scholar] [CrossRef]
- Isah, M.; Shehzad, F.; Daud, M.; Al-Harthi, M.A. Synthesis and Characterization of Phosphonium-Based Ionic Liquid Anchored Graphene Functionalized ZnAl-Ldh Nanocomposite for Polystyrene Applications. Macromol. Res. 2024, 33, 507–522. [Google Scholar] [CrossRef]
- Zhao, W.; Kundu, C.K.; Li, Z.; Li, X.; Zhang, Z. Flame Retardant Treatments for Polypropylene: Strategies and Recent Advances. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106382. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.-Y.; Zhang, M.; Zeng, H.-Y.; Wu, K.; Tian, X.-Y.; Chen, C.-R.; Pan, Y. Fabrication of Green Alginate-Based and Layered Double Hydroxides Flame Retardant for Enhancing the Fire Retardancy Properties of Polypropylene. Carbohydr. Polym. 2020, 234, 115891. [Google Scholar] [CrossRef]
- Attia, N.F.; Elashery, S.E.A.; Zakria, A.M.; Eltaweil, A.S.; Oh, H. Recent Advances in Graphene Sheets as New Generation of Flame Retardant Materials. Mater. Sci. Eng. B 2021, 274, 115460. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New Prospects in Flame Retardant Polymer Materials: From Fundamentals to Nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Attia, N.F.; Saleh, B.K. Novel Synthesis of Renewable and Green Flame-Retardant, Antibacterial and Reinforcement Material for Styrene–Butadiene Rubber Nanocomposites. J. Therm. Anal. Calorim. 2020, 139, 1817–1827. [Google Scholar] [CrossRef]
- Attia, N.F.; Hegazi, E.M.; Abdelmageed, A.A. Smart Modification of Inorganic Fibers and Flammability Mechanical and Radiation Shielding Properties of Their Rubber Composites. J. Therm. Anal. Calorim. 2018, 132, 1567–1578. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, Y.; Qiu, R.; Xu, W.; Hou, Y. Investigation of UiO-66 as Flame Retardant and Its Application in Improving Fire Safety of Polystyrene. Macromol. Res. 2020, 28, 42–50. [Google Scholar] [CrossRef]
- Attia, N.; Ahmed, H.; Yehia, D.; Hassan, M.; Zaddin, Y. Novel Synthesis of Nanoparticles-Based Back Coating Flame-Retardant Materials for Historic Textile Fabrics Conservation. J. Ind. Text. 2017, 46, 1379–1392. [Google Scholar] [CrossRef]
- Attia, N.F.; Mousa, M. Synthesis of Smart Coating for Furniture Textile and Their Flammability and Hydrophobic Properties. Prog. Org. Coat. 2017, 110, 204–209. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Hamerton, I. Recent Developments in the Chemistry of Halogen-Free Flame Retardant Polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Tirri, T.; Aubert, M.; Aziz, H.; Brusentsev, Y.; Pawelec, W.; Wilén, C.-E. Sulfenamides in Synergistic Combination with Halogen Free Flame Retardants in Polypropylene. Polym. Degrad. Stab. 2019, 164, 75–89. [Google Scholar] [CrossRef]
- Li, W.-X.; Zhang, H.-J.; Hu, X.-P.; Yang, W.-X.; Cheng, Z.; Xie, C.-Q. Highly Efficient Replacement of Traditional Intumescent Flame Retardants in Polypropylene by Manganese Ions Doped Melamine Phytate Nanosheets. J. Hazard. Mater. 2020, 398, 123001. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-Based Flame Retardants: When Nature Meets Fire Protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Salas, E.C.; Sun, Z.; Lüttge, A.; Tour, J.M. Reduction of Graphene Oxide via Bacterial Respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef]
- Nine, M.J.; Cole, M.A.; Tran, D.N.H.; Losic, D. Graphene: A Multipurpose Material for Protective Coatings. J. Mater. Chem. A Mater. 2015, 3, 12580–12602. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 1979, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Sang, B.; Li, Z.; Li, X.; Yu, L.; Zhang, Z. Graphene-Based Flame Retardants: A Review. J. Mater. Sci. 2016, 51, 8271–8295. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Burghard, M.; Kern, K. Elastic Properties of Chemically Derived Single Graphene Sheets. Nano Lett. 2008, 8, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Gao, J.; Wang, X.; Liang, H.; Ge, C. How Can Graphene Reduce the Flammability of Polymer Nanocomposites? Mater. Lett. 2012, 66, 187–189. [Google Scholar] [CrossRef]
- Yeoh, G.H.; De Cachinho Cordeiro, I.M.; Wang, W.; Wang, C.; Yuen, A.C.Y.; Chen, T.B.Y.; Vargas, J.B.; Mao, G.; Garbe, U.; Chua, H.T. Carbon-based Flame Retardants for Polymers: A Bottom-up Review. Adv. Mater. 2024, 36, 2403835. [Google Scholar] [CrossRef]
- Kausar, A. Advances in Polystyrene/Graphene Nanoplatelet Nanocomposites. J. Plast. Film Sheeting 2022, 38, 130–146. [Google Scholar] [CrossRef]
- Liu, S.; He, M.; Qin, Q.; Liu, W.; Liao, L.; Qin, S. Expanded Properties and Applications of Porous Flame-Retardant Polymers Containing Graphene and Its Derivatives. Polymers 2024, 16, 2053. [Google Scholar] [CrossRef] [PubMed]
- Baby, A.; Tretsiakova-McNally, S.; Arun, M.; Joseph, P.; Zhang, J. Reactive and Additive Modifications of Styrenic Polymers with Phosphorus-Containing Compounds and Their Effects on Fire Retardance. Molecules 2020, 25, 3779. [Google Scholar] [CrossRef]
- Joseph, P.; Tretsiakova-McNally, S. Melt-Flow Behaviours of Thermoplastic Materials under Fire Conditions: Recent Experimental Studies and Some Theoretical Approaches. Materials 2015, 8, 8793–8803. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, Y.; Li, X.; Shao, H.; Xu, W.; Li, D. A Facile, Environmentally and Friendly Flame-Retardant: Synergistic Flame Retardant Property of Polyurethane Rigid Foam. Mater. Lett. 2020, 267, 127542. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Yang, H.; Xing, W.; Kandola, B.; Hu, Y. Simultaneous Reduction and Surface Functionalization of Graphene Oxide with POSS for Reducing Fire Hazards in Epoxy Composites. J. Mater. Chem. 2012, 22, 22037. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Pornwannchai, W.; Hu, Y.; Kandola, B. The Effect of Graphene Presence in Flame Retarded Epoxy Resin Matrix on the Mechanical and Flammability Properties of Glass Fiber-Reinforced Composites. Compos. Part A Appl. Sci. Manuf. 2013, 53, 88–96. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent Developments in the Fire Retardancy of Polymeric Materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, W.; Wang, S.; Shen, Z.; Wang, Y.; Zhang, Q. A Novel Transposon, Tn, Mediated Transfer of Variant in ESBL-Producing Aeromonas veronii. Infect. Drug Resist. 2020, 13, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Wang, S. The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers 2021, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yuan, Y.; Xu, L.; Wang, W. Recent Progress in Two-Dimensional Nanomaterials for Flame Retardance and Fire-Warning Applications. Molecules 2024, 29, 1858. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Díaz Palencia, J.L.; Wang, N.; Jiang, Y.; Wang, D.-Y. Nanocarbon-Based Flame Retardant Polymer Nanocomposites. Molecules 2021, 26, 4670. [Google Scholar] [CrossRef]
- Hu, W.; Yu, B.; Jiang, S.-D.; Song, L.; Hu, Y.; Wang, B. Hyper-Branched Polymer Grafting Graphene Oxide as an Effective Flame Retardant and Smoke Suppressant for Polystyrene. J. Hazard. Mater. 2015, 300, 58–66. [Google Scholar] [CrossRef]
- ISO. ISO 4589-2:2017; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/60786.html (accessed on 16 June 2025).
- ASTM. ASTM D2863-19; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index). ASTM International: West Conshohocken, PA, USA, 2019. Available online: https://store.astm.org/d2863-19.html (accessed on 16 June 2025).
- Xu, Z.; Deng, N.; Yan, L. Flame Retardancy and Smoke Suppression Properties of Transparent Intumescent Fire-Retardant Coatings Reinforced with Layered Double Hydroxides. J. Coat. Technol. Res. 2020, 17, 157–169. [Google Scholar] [CrossRef]
- Chavali, K.S.; Pethsangave, D.A.; Patankar, K.C.; Khose, R.V.; Wadekar, P.H.; Maiti, S.; Adivarekar, R.V.; Some, S. Graphene-Based Intumescent Flame Retardant on Cotton Fabric. J. Mater. Sci. 2020, 55, 14197–14210. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Zhao, Z.-Y.; Chen, H.; Wang, Y.-Z. Flame Retardation of Natural Rubber: Strategy and Recent Progress. Polymers 2020, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO 5660-1:2015; Reaction—Heat release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/57957.html (accessed on 16 June 2025).
- Shi, M.; Lin, T.; Hu, Y.; Peng, J.; Li, J.; Zhai, M. Functionalization of Graphene Oxide by Radiation Grafting Polyhedral Oligomeric Silsesquioxane with Improved Thermal Stability and Hydrophilicity. J. Mater. Sci. 2020, 55, 1489–1498. [Google Scholar] [CrossRef]
- Babrauskas, V.; Parker, W.J. Ignitability Measurements with the Cone Calorimeter. Fire Mater. 1987, 11, 31–43. [Google Scholar] [CrossRef]
- Mathews, L.D.; Capricho, J.C.; Peerzada, M.; Salim, N.V.; Parameswaranpillai, J.; Hameed, N. Recent Progress and Multifunctional Applications of Fire-Retardant Epoxy Resins. Mater. Today Commun. 2022, 33, 104702. [Google Scholar] [CrossRef]
- Kempel, F.; Schartel, B.; Marti, J.M.; Butler, K.M.; Rossi, R.; Idelsohn, S.R.; Oñate, E.; Hofmann, A. Modelling the Vertical UL 94 Test: Competition and Collaboration between Melt Dripping, Gasification and Combustion. Fire Mater. 2015, 39, 570–584. [Google Scholar] [CrossRef]
- IEC. IEC 60695-11-10; Fire Hazard Testing—Part 11–10: Test Flames—50 W Horizontal and Vertical Flame Test Methods, 2nd ed. International Electrotechnical Commission: Geneva, Switzerland, 2013. Available online: https://webstore.iec.ch/en/publication/2938 (accessed on 16 June 2025).
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. A High Performance Fully Bio-Based Epoxy Thermoset from a Syringaldehyde-Derived Epoxy Monomer Cured by Furan-Derived Amine. Green. Chem. 2021, 23, 501–510. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Chen, X.; Jin, Y.; Zhang, J. Burning and Dripping Behaviors of Polymers under the UL94 Vertical Burning Test Conditions. Fire Mater. 2010, 34, 203–215. [Google Scholar] [CrossRef]
- Hu, C.; Fontaine, G.; Tranchard, P.; Delaunay, T.; Collinet, M.; Marcille, S.; Bourbigot, S. In-Situ Investigation of Temperature Evolution of Drippings via an Optimized UL-94 Instrumentation: Application to Flame Retarded Polybutylene Succinate. Polym. Degrad. Stab. 2018, 155, 145–152. [Google Scholar] [CrossRef]
- Ng, Y.H.; Zope, I.S.; Dasari, A.; Tan, K.H. Correlating the Performance of a Fire-Retardant Coating across Different Scales of Testing. Polymers 2020, 12, 2271. [Google Scholar] [CrossRef]
- Marti, J.; Idelsohn, S.R.; Oñate, E. A Finite Element Model for the Simulation of the UL-94 Burning Test. Fire Technol. 2018, 54, 1783–1805. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Q.; Li, Y.; Zhang, M.; Wei, X. Preparation and Characterisation of Graphene. Mater. Res. Innov. 2015, 19, 344–350. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Shareena, T.P.D.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nanomicro Lett. 2018, 10, 53. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A. Graphene Synthesis, Characterization and Its Applications: A Review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Skoda, M.; Dudek, I.; Jarosz, A.; Szukiewicz, D. Graphene: One Material, Many Possibilities—Application Difficulties in Biological Systems. J. Nanomater. 2014, 2014, 890246. [Google Scholar] [CrossRef]
- Eigler, S. Graphene an Introduction to the Fundamentals and Industrial Applications Edited by Madhuri Sharon and Maheshwar Sharon. Angew. Chem. Int. Ed. 2016, 55, 5122. [Google Scholar] [CrossRef]
- Armano, A.; Agnello, S. Two-Dimensional Carbon: A Review of Synthesis Methods, and Electronic, Optical, and Vibrational Properties of Single-Layer Graphene. C 2019, 5, 67. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Faucheu, J.; Noël, A. Latex Routes to Graphene-Based Nanocomposites. Polym. Chem. 2015, 6, 5323–5357. [Google Scholar] [CrossRef]
- Burkholder, M.B.; Rahman, F.B.A.; Chandler, E.H.; Regalbuto, J.R.; Gupton, B.F.; Tengco, J.M.M. Metal Supported Graphene Catalysis: A Review on the Benefits of Nanoparticular Supported Specialty Sp2 Carbon Catalysts on Enhancing the Activities of Multiple Chemical Transformations. Carbon Trends 2022, 9, 100196. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of Graphene and Its Disorders: A Review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef]
- Trivedi, S.; Lobo, K.; Matte, H.S.S.R. Synthesis Properties, and Applications of Graphene. In Fundamentals and Sensing Applications of 2D Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–90. [Google Scholar]
- Karaca, E.; Acaralı, N. Application of Graphene and Its Derivatives in Medicine: A Review. Mater. Today Commun. 2023, 37, 107054. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh Electron Mobility in Suspended Graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, Q.; Qiu, H.; LI, J.; Yang, J. A Simple Method for the Reduction of Graphene Oxide by Sodium Borohydride with CaCl2 as a Catalyst. New Carbon Mater. 2015, 30, 41–47. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Biswas, K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene, the New Nanocarbon. J. Mater. Chem. 2009, 19, 2457. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, B.; Qiu, W.; Li, W.; Zheng, X.; Xu, B. A Review of the Preparation and Applications of Wrinkled Graphene Oxide. New Carbon Mater. 2022, 37, 290–302. [Google Scholar] [CrossRef]
- Zhen, Z.; Zhu, H. Structure and Properties of Graphene. In Graphene; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lü, K.; Zhao, G.; Wang, X. A Brief Review of Graphene-Based Material Synthesis and Its Application in Environmental Pollution Management. Chin. Sci. Bull. 2012, 57, 1223–1234. [Google Scholar] [CrossRef]
- Bhuyan, M.; Uddin, M.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of Graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H.; Graphene-, R.; Oxide-, G. Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, B.C. An Overview on the Importance of Chemical Vapour Deposition Technique for Graphene Synthesis. Adv. Sci. Eng. Med. 2018, 10, 760–763. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-Dimensional Atomic Crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Liang, L.; Wang, J.; Lin, W.; Sumpter, B.G.; Meunier, V.; Pan, M. Electronic Bandgap and Edge Reconstruction in Phosphorene Materials. Nano Lett. 2014, 14, 6400–6406. [Google Scholar] [CrossRef] [PubMed]
- Ghany, N.A.A.; Elsherif, S.A.; Handal, H.T. Revolution of Graphene for Different Applications: State-of-the-Art. Surf. Interfaces 2017, 9, 93–106. [Google Scholar] [CrossRef]
- Kataria, S.; Wagner, S.; Ruhkopf, J.; Gahoi, A.; Pandey, H.; Bornemann, R.; Vaziri, S.; Smith, A.D.; Ostling, M.; Lemme, M.C. Chemical Vapor Deposited Graphene: From Synthesis to Applications: Chemical Vapor Deposited Graphene. Phys. Status Solidi A 2014, 211, 2439–2449. [Google Scholar] [CrossRef]
- Moharana, S.; Kar, S.K.; Mishra, M.K.; Mahaling, R.N. Synthesis and Properties of Graphene and Graphene Oxide-Based Polymer Composites. In Surface Engineering of Graphene; Sahoo, S., Tiwari, S.K., Nayak, G.C., Eds.; Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2019; pp. 175–201. ISBN 978-3-030-30206-1. [Google Scholar]
- Zhang, X.; Cai, L.; Xiang, Z.; Lu, W. Hollow CuS Microflowers Anchored Porous Carbon Composites as Lightweight and Broadband Microwave Absorber with Flame-Retardant and Thermal Stealth Functions. Carbon 2021, 184, 514–525. [Google Scholar] [CrossRef]
- Nabipour, H.; Nie, S.; Wang, X.; Song, L.; Hu, Y. Zeolitic Imidazolate Framework-8/Polyvinyl Alcohol Hybrid Aerogels with Excellent Flame Retardancy. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105720. [Google Scholar] [CrossRef]
- Li, Z.; Hu, M.; Shen, K.; Liu, Q.; Li, M.; Chen, Z.; Cheng, X.; Wu, X. Tuning Thermal Stability and Fire Hazards of Hydrophobic Silica Aerogels via Doping Reduced Graphene Oxide. J. Non Cryst. Solids 2024, 625, 122747. [Google Scholar] [CrossRef]
- Hong, N.; Song, L.; Hull, T.R.; Stec, A.A.; Wang, B.; Pan, Y.; Hu, Y. Facile Preparation of Graphene Supported Co3O4 and NiO for Reducing Fire Hazards of Polyamide 6 Composites. Mater. Chem. Phys. 2013, 142, 531–538. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Gao, S.; Yang, X.; Fan, R.; Zhi, M.; Fu, M. Recent Advances in the Flame Retardancy Role of Graphene and Its Derivatives in Epoxy Resin Materials. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106539. [Google Scholar] [CrossRef]
- Poutch, F. Flame Retardant Testing. In Flame Retardant Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 51–116. [Google Scholar] [CrossRef]
- Nabipour, H.; Rohani, S. Flame Retardant Properties of Polymer Nanocomposites Based on New Layered Structure Nanoparticles. In Flame Retardant Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 117–158. [Google Scholar] [CrossRef]
- Zheng, X.-T.; Dong, Y.-Q.; Liu, X.-D.; Xu, Y.-L.; Jian, R.-K. Fully Bio-Based Flame-Retardant Cotton Fabrics via Layer-by-Layer Self Assembly of Laccase and Phytic Acid. J. Clean. Prod. 2022, 350, 131525. [Google Scholar] [CrossRef]
- Cai, W.; Wang, B.-B.; Wang, X.; Zhu, Y.-L.; Li, Z.-X.; Xu, Z.-M.; Song, L.; Hu, W.-Z.; Hu, Y. Recent Progress in Two-Dimensional Nanomaterials Following Graphene for Improving Fire Safety of Polymer (Nano)Composites. Chin. J. Polym. Sci. 2021, 39, 935–956. [Google Scholar] [CrossRef]
- An, X.; Tang, N.; Liu, Y.; Song, S.; Chen, C.; Han, G.; Li, W.; Zhang, Y. Vascular Bundle-Structured Polymeric Composites with Fire-Safe, Self-Detecting and Heat Warning Capabilities for Power Batteries Thermal Management. Compos. Sci. Technol. 2025, 259, 110921. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A Comprehensive Review of Reactive Flame-Retardant Epoxy Resin: Fundamentals, Recent Developments, and Perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Tang, S.; Gao, J. A Novel Intumescent Flame Retardant-Functionalized Graphene: Nanocomposite Synthesis, Characterization, and Flammability Properties. Mater. Chem. Phys. 2012, 135, 938–947. [Google Scholar] [CrossRef]
- Sabet, M.; Soleimani, H.; Mohammadian, E.; Hosseini, S. Impact of Inclusion Graphene Oxide Nanosheets on Polystyrene Properties. Int. J. Plast. Technol. 2019, 23, 92–100. [Google Scholar] [CrossRef]
- Attia, N.F. Sustainable and Efficient Flame Retardant Materials for Achieving High Fire Safety for Polystyrene Composites. J. Therm. Anal. Calorim. 2022, 147, 5733–5742. [Google Scholar] [CrossRef]
- Yuan, B.; Sheng, H.; Mu, X.; Song, L.; Tai, Q.; Shi, Y.; Liew, K.M.; Hu, Y. Enhanced Flame Retardancy of Polypropylene by Melamine-Modified Graphene Oxide. J. Mater. Sci. 2015, 50, 5389–5401. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, H.-B.; Cheng, J.-B.; Li, W.; Zhang, J.; Wang, Y.-Z. A Green, Durable and Effective Flame-Retardant Coating for Expandable Polystyrene Foams. Chem. Eng. J. 2022, 440, 135807. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Liu, P.; Xu, C.; Liu, Y.; Wang, Q. The Preparation and Application of a Graphene-Based Hybrid Flame Retardant Containing a Long-Chain Phosphaphenanthrene. Sci. Rep. 2017, 7, 8759. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.-W. Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped graphene. J. Hazard. Mater. 2018, 346, 140–151. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, W.; Huang, J.; He, Y.; Liang, X.; Su, Y.; Wu, W.; Li, R.K.Y. Interface Engineering of Graphene Oxide Containing Phosphorus/Nitrogen towards Fire Safety Enhancement for Thermoplastic Polyurethane. Compos. Commun. 2021, 27, 100821. [Google Scholar] [CrossRef]
- Yuan, B.; Fan, A.; Yang, M.; Chen, X.; Hu, Y.; Bao, C.; Jiang, S.; Niu, Y.; Zhang, Y.; He, S.; et al. The Effects of Graphene on the Flammability and Fire Behavior of Intumescent Flame Retardant Polypropylene Composites at Different Flame Scenarios. Polym. Degrad. Stab. 2017, 143, 42–56. [Google Scholar] [CrossRef]
- Attia, N.F.; El-Aal, N.S.A.; Hassan, M.A. Facile Synthesis of Graphene Sheets Decorated Nanoparticles and Flammability of Their Polymer Nanocomposites. Polym. Degrad. Stab. 2016, 126, 65–74. [Google Scholar] [CrossRef]
- Wu, F.; Bao, X.; Wang, J. One-Step Reduction of Graphene Oxide with Phosphorus/Silicon-Containing Compound and Its Flame Retardancy in Epoxy Resin. Polymers 2021, 13, 3985. [Google Scholar] [CrossRef]
- Li, L.; Shao, X.; Zhao, Z.; Liu, X.; Jiang, L.; Huang, K.; Zhao, S. Synergistic Fire Hazard Effect of a Multifunctional Flame Retardant in Building Insulation Expandable Polystyrene through a Simple Surface-Coating Method. ACS Omega 2020, 5, 799–807. [Google Scholar] [CrossRef]
- Huang, G.; Chen, W.; Wu, T.; Guo, H.; Fu, C.; Xue, Y.; Wang, K.; Song, P. Multifunctional Graphene-Based Nano-Additives toward High-Performance Polymer Nanocomposites with Enhanced Mechanical, Thermal, Flame Retardancy and Smoke Suppressive Properties. Chem. Eng. J. 2021, 410, 127590. [Google Scholar] [CrossRef]
- Ji, W.; Yao, Y.; Guo, J.; Fei, B.; Gu, X.; Li, H.; Sun, J.; Zhang, S. Toward an Understanding of How Red Phosphorus and Expandable Graphite Enhance the Fire Resistance of Expandable Polystyrene Foams. J. Appl. Polym. Sci. 2020, 137, 49045. [Google Scholar] [CrossRef]
- Deng, Z.; Tang, T.; Huo, J.; He, H.; Dai, K. Fabrication of Functionalized Graphene Oxide–Aluminum Hypophosphite Nanohybrids for Enhanced Fire Safety Performance in Polystyrene. Polymers 2024, 16, 3083. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.; Li, Y.; Tan, S.; Zhao, S.; Li, L. Synthesis of Bio-Based Toughening Phosphorus-Nitrogen Flame Retardant and Study on High Toughness EPS Flame Retardant Insulation Sheet. Polym. Degrad. Stab. 2023, 218, 110560. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Effect of Graphene on Flame Retardancy of Graphite Doped Intumescent Flame Retardant (IFR) Coatings: Synergy or Antagonism. Coatings 2019, 9, 94. [Google Scholar] [CrossRef]
- Sui, Y.; Qu, L.; Li, P.; Dai, X.; Fang, Q.; Zhang, C.; Wang, Y. Covalently Functionalized Graphene Oxide Wrapped by Silicon–Nitrogen-Containing Molecules: Preparation and Simultaneous Enhancement of the Thermal Stability, Flame Retardancy and Mechanical Properties of Epoxy Resin Nanocomposites. RSC Adv. 2020, 10, 13949–13959. [Google Scholar] [CrossRef]
- Wang, X.; Tu, H.; Xiao, H.; Lu, J.; Xu, J.; Gu, G. A Novel Halogen-Free Flame-Retardant Fabrication for the Study of Smoke Suppression and Flame Retardancy of Polystyrene. Polymers 2023, 283, 126240. [Google Scholar] [CrossRef]
- Zhao, Z.; Cai, W.; Xu, Z.; Mu, X.; Ren, X.; Zou, B.; Gui, Z.; Hu, Y. Multi-Role p-Styrene Sulfonate Assisted Electrochemical Preparation of Functionalized Graphene Nanosheets for Improving Fire Safety and Mechanical Property of Polystyrene Composites. Compos. B Eng. 2020, 181, 107544. [Google Scholar] [CrossRef]
- Shao, X.; Du, Y.; Zheng, X.; Wang, J.; Wang, Y.; Zhao, S.; Xin, Z.; Li, L. Reduced Fire Hazards of Expandable Polystyrene Building Materials via Intumescent Flame-Retardant Coatings. J. Mater. Sci. 2020, 55, 7555–7572. [Google Scholar] [CrossRef]
- Dai, K.; Sun, S.; Xu, W.; Song, Y.; Deng, Z.; Qian, X. Covalently-Functionalized Graphene Oxide via Introduction of Bifunctional Phosphorus-Containing Molecules as an Effective Flame Retardant for Polystyrene. RSC Adv. 2018, 8, 24993–25000. [Google Scholar] [CrossRef] [PubMed]
- Edenharter, A.; Feicht, P.; Diar-Bakerly, B.; Beyer, G.; Breu, J. Superior Flame Retardant by Combining High Aspect Ratio Layered Double Hydroxide and Graphene Oxide. Polymers 2016, 91, 41–49. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Gao, X.; Li, T.; Zhang, Q. Preparation of Thermally Reduced Graphene Oxide and the Influence of Its Reduction Temperature on the Thermal, Mechanical, Flame Retardant Performances of PS Nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 336–343. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, Y.; Zhang, H.; Cui, J.; Guo, J.; Yang, B. Butyltriphenylphosphine-based Chelate Borates Influenced on Flame Retardancy of Polystyrene Composite Containing Self-expanded Intumescent Flame Retardants. J. Appl. Polym. Sci. 2021, 138, 50650. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Bai, S.; Wang, Q. Synergistic Effects of Flame Retardants on the Flammability and Foamability of PS Foams Prepared by Supercritical Carbon Dioxide Foaming. ACS Omega 2019, 4, 9306–9315. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Peng, S.; Pan, B.; Lu, C.; Liu, H.; Ma, J.; Niu, Q. Fire Property and Charring Behavior of High Impact Polystyrene Containing Expandable Graphite and Microencapsulated Red Phosphorus. Polym. Degrad. Stab. 2015, 121, 261–270. [Google Scholar] [CrossRef]
- Wang, G.; Bai, S. Synergistic Effect of Expandable Graphite and Melamine Phosphate on Flame-retardant Polystyrene. J. Appl. Polym. Sci. 2017, 134, 45474. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Méndez-Lozano, N.; Pérez-Reynoso, F.; González-Gutiérrez, C. Eco-Friendly Approach for Graphene Oxide Synthesis by Modified Hummers Method. Materials 2022, 15, 7228. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Crespilho, F.N. Graphene Oxide: The Schrödinger’s Cat of Nanomaterials. ACS Appl. Nano Mater. 2025, 8, 1680–1682. [Google Scholar] [CrossRef]
- Yoosuf, S.; Kuthirummal, N.; Tharayil, S.B. Fabrication of Reduced Graphene Oxide Decorated with Nonmetal-Doped Nanotitania: An Efficient Visible Light–Driven Photocatalyst and Sterilizing Agent for Microbial Cells. ACS Omega 2025, 10, 5296–5311. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-R.; Jung, S.-M.; Jeon, I.-Y.; Baek, J.-B. The Oxidation Mechanism of Highly Ordered Pyrolytic Graphite in a Nitric Acid/Sulfuric Acid Mixture. Carbon 2013, 52, 493–498. [Google Scholar] [CrossRef]
- Sorokina, N.E.; Khaskov, M.A.; Avdeev, V.V.; Nikol’skaya, I.V. Reaction of Graphite with Sulfuric Acid in the Presence of KMnO4. Russ. J. Gen. Chem. 2005, 75, 162–168. [Google Scholar] [CrossRef]
- ASTM. ASTM E1354; Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products Using an Oxygen Consumption Calorimeter. ASTM: West Conshohocken, PA, USA. Available online: https://store.astm.org/e1354-23.html (accessed on 16 June 2025).
- Korucu, H. Multi Response Optimization of Synthesis of Boron Compounds by Dopting to Graphene Oxide in the Modified Hummers Method. Mater. Sci. Eng. B 2025, 311, 117839. [Google Scholar] [CrossRef]
- Korobeinichev, O.; Shaklein, A.; Trubachev, S.; Karpov, A.; Paletsky, A.; Chernov, A.; Sosnin, E.; Shmakov, A. The Influence of Flame Retardants on Combustion of Glass Fiber-Reinforced Epoxy Resin. Polymers 2022, 14, 3379. [Google Scholar] [CrossRef]
- Sun, S.; Yu, Q.; Yu, B.; Zhou, F. New Progress in the Application of Flame-Retardant Modified Epoxy Resins and Fire-Retardant Coatings. Coatings 2023, 13, 1663. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, L.; Han, J.-M.; Li, H. Molding Fabrication of Copper Azide/Porous Graphene with High Electrostatic Safety by Self-Assembly of Graphene Oxide. Nanotechnology 2021, 32, 385704. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, J.; Long, J.; Liang, B. Synthesis of a Novel DOPO-Based Ionic Liquid Flame Retardant and Its Application in Epoxy Resin. J. Mater. Chem. C Mater. 2025, 13, 1844–1856. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; Zhao, J.; Tian, Y.; Yan, X.; Li, Y. Fabrication, Structure and Mechanism of Reduced Graphene Oxide-Based Carbon Composite Films. J. Mater. Chem. A Mater. 2014, 2, 10502. [Google Scholar] [CrossRef]
- Dai, K.; Song, L.; Hu, Y. Study of the Flame Retardancy and Thermal Properties of Unsaturated Polyester Resin via Incorporation of a Reactive Cyclic Phosphorus-Containing Monomer. High. Perform. Polym. 2013, 25, 938–946. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, Z.; Yang, X.; Zhang, Z.; Wang, X.; Chen, X.; Yang, L. The Mechanism Study on the Cooperative Flame Resistance Effect between HMP and NP in ABS by TG–FTIR. J. Therm. Anal. Calorim. 2017, 129, 303–314. [Google Scholar] [CrossRef]
- Zhang, H.; Farris, R.J.; Westmoreland, P.R. Low Flammability and Thermal Decomposition Behavior of Poly(3,3‘-Dihydroxybiphenylisophthalamide) and Its Derivatives. Macromolecules 2003, 36, 3944–3954. [Google Scholar] [CrossRef]
- Xiao, X.-X.; Bai, T.-Y.; Zhang, Q.; Chen, Z.-X.; Wang, Z.-N.; Bai, J.-H.; Chen, L.; Liu, B.-W.; Wang, Y.-Z. Machine Learning-Assisted Design and Scalable Fabrication of High-Performance Fire-Safe Polycarbonate for Advanced Applications. Chem. Eng. J. 2024, 484, 149565. [Google Scholar] [CrossRef]
- Tai, Q.; Song, L.; Lv, X.; Lu, H.; Hu, Y.; Yuen, R.K.K. Flame-retarded Polystyrene with Phosphorus- and Nitrogen-containing Oligomer: Preparation and Thermal Properties. J. Appl. Polym. Sci. 2012, 123, 770–778. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, D.; Lin, Y.; Li, K.; Fan, G.; Li, F. Hierarchical Nano/Micro-Array Structured CuMgAl-LDH/rGO Hybrids for Remarkably Improved Flame Retardancy and Smoke Suppression Performance of Flexible Polyvinyl Chloride. ACS Appl. Mater. Interfaces 2024, 16, 61224–61238. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. The Thermal Degradation of Polystyrene Nanocomposite. Polymers 2005, 46, 2933–2942. [Google Scholar] [CrossRef]
- Evans, A.; Morris, L.J.; Turner, Z.R.; O’Hare, D. Phosphonate-Functionalized Polypropylenes: Single-Component Flame Retardants. ACS Appl. Polym. Mater. 2025, 7, 2508–2516. [Google Scholar] [CrossRef]
- Banks, S.W.; Nowakowski, D.J.; Bridgwater, A.V. Impact of Potassium and Phosphorus in Biomass on the Properties of Fast Pyrolysis Bio-Oil. Energy Fuels 2016, 30, 8009–8018. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, S.; Gui, Z.; Li, G.; Shi, X.; Chen, G.; Peng, X. Synthesis of a Novel Highly Effective Flame Retardant Containing Multivalent Phosphorus and Its Application in Unsaturated Polyester Resins. RSC Adv. 2016, 6, 86632–86639. [Google Scholar] [CrossRef]
- Yang, T.; Wu, Y.; Cheng, Y.; Huang, T.; Yu, B.; Zhu, M.; Yu, H. Synthesis of a Charring Agent Containing Triazine and Benzene Groups and Its Intumescent Flame Retardant Performance for Polypropylene. Polym. Degrad. Stab. 2022, 204, 110107. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Wang, Y. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef]
- Lim, Q.F.; Tan, M.Y.; Lin, E.M.J.; Toh, J.P.W.; Wang, S.; Zhu, Q.; Thitsartarn, W.; Ho, K.-H.; He, C.; Liu, S.; et al. DOPO-Grafted Nitrogen-Rich Polymeric Backbones for Polypropylene: An Efficient Intumescent Synergist to Simultaneously Enhance Flame Retardancy, Mechanical Properties and Aging Resistance. Eur. Polym. J. 2025, 222, 113609. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Wang, J.; Yang, S.; Chen, K.; Zhu, L.; Huo, S.; Song, P.; Wang, H. High-Performance, Intrinsically Fire-Safe, Single-Component Epoxy Resins and Carbon Fiber Reinforced Epoxy Composites Based on Two Phosphorus-Derived Imidazoliums. Polym. Degrad. Stab. 2023, 208, 110261. [Google Scholar] [CrossRef]
- Zou, J.; Duan, H.; Zhang, J.; Cao, J.; Zhang, C.; Wan, C.; Ma, H. Multi-element Heterocyclic Compound Derived from DOPO and Thiadiazole toward Flame-retardant Epoxy Resin with Satisfactory Mechanical Properties. J. Appl. Polym. Sci. 2022, 139, 52036. [Google Scholar] [CrossRef]
- Qian, L.; Feng, F.; Tang, S. Bi-Phase Flame-Retardant Effect of Hexa-Phenoxy-Cyclotriphosphazene on Rigid Polyurethane Foams Containing Expandable Graphite. Polymers 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Liu, Y.; Wang, D.-Y.; Wang, D.-L.; Wang, Y.-Z. Synergistic Effect of Ammonium Polyphosphate and Layered Double Hydroxide on Flame Retardant Properties of Poly(Vinyl Alcohol. Polym. Degrad. Stab. 2008, 93, 1323–1331. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic Effect between Expandable Graphite and Ammonium Polyphosphate on Flame Retarded Polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Kaur, A.; Morton, J.A.; Tyurnina, A.V.; Priyadarshi, A.; Ghorbani, M.; Mi, J.; Porfyrakis, K.; Eskin, D.G.; Tzanakis, I. Dual Frequency Ultrasonic Liquid Phase Exfoliation Method for the Production of Few Layer Graphene in Green Solvents. Ultrason. Sonochem 2024, 108, 106954. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Hu, P.; Hu, F.; Tian, Z.; Tang, J.; Zhang, P.; Pan, L.; Barsoum, M.W.; Cai, L.; Sun, Z. Multifunctional MXene/C Aerogels for Enhanced Microwave Absorption and Thermal Insulation. Nanomicro Lett. 2023, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, J.; Li, H.; Li, J.; Jin, Q.; Ren, K.; Ji, J. Mussel-Inspired Polydopamine: A Biocompatible and Ultrastable Coating for Nanoparticles in Vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef]
- Amin, M.M.; Mohamed, L.S.; Hossain, M.M.; Razzak, S.A.; Siddiquee, M.N. Prospects and Challenges of Carbon Fiber Production from Heavy Petroleum Fractions. Ind. Eng. Chem. Res. 2024, 63, 15613–15636. [Google Scholar] [CrossRef]
- Ma, J.; Cao, B.; Dong, S.; Tian, Y.; Wang, M.; Xiong, J.; Sun, S. MLMD: A programming-free AI platform to predict and design materials. NPJ Comput. Mater. 2024, 10, 59. [Google Scholar] [CrossRef]

















| S/N | Evaluation Standards | Fire Classification | ||
|---|---|---|---|---|
| V-0 | V-1 | V-2 | ||
| 1 | Number of samples | 5 | 5 | 5 |
| 2 | Maximum flame burning time (s) per sample after flame removal | 10 | 30 | 30 |
| 3 | Maximum total flaming time (s) for 5 samples (10 ignitions) | 50 | 250 | 250 |
| 4 | Maximum afterglow time (s) per sample after second flame application | 30 | 60 | 60 |
| 5 | Flames or afterglow reaching the holding clamp | No | No | No |
| 6 | Dripping of flaming particles igniting cotton | No | No | Yes |
| 7 | Burning time (s) | 2 | 2 | 2 |
| Method | Lateral | Descriptions | Thickness | Advantage | Disadvantage |
|---|---|---|---|---|---|
| Micromechanical exfoliation | μm to cm | This technique involves using adhesive tape to strip graphene layers from a graphite source. | Few layers | Pristine, unaltered graphene sheets of substantial size. | Extremely limited production scale, rendering it unsuitable for large-scale industrial manufacturing. |
| Electrochemical exfoliation | 500–700 nm | This technique utilizes an electric field applied to graphite within an electrolyte solution to separate graphene layers. | Single to few layers | Potential for large-scale production and yields functionalized graphene with high electrical conductivity. | High cost associated with ionic liquids. |
| Direct sonication of graphene | μm | This technique uses ultrasonic waves to exfoliate graphite into graphene within a solvent medium. It is simple but yields mixed-quality graphene and is better for small-scale use. | Includes both monolayer and multilayer structures. | Cost-effective and pristine graphene. | Produces low yield and quality graphene, making it ideal for small-scale or research use. |
| Reduction in carbon monoxide | Sub-μm | GO can be produced by oxidizing graphite and subsequently reducing it to graphene using chemical or thermal reduction methods. This approach is ideal for generating graphene that retains functional groups. | Multiple layers | Un-oxidized sheets | Contamination with α-Al2S and α-Al2O3 |
| Epitaxial growth on SIC | Up to cm size | This involves heating silicon carbide to evaporate silicon, forming high-quality graphene on the surface. It is ideal for electronics but requires high temperatures. | Few layers | Very large area of pure graphene | Very small scale, require expensive equipment, and the graphene remains substrate-bound. |
| CVD | Very large (cm) | CVD entails the deposition of a carbon-based gas onto a substrate under high temperatures, where methane is commonly used as a precursor gas. | Few layers | Large size and area; high-quality graphene. | Small production scale and require high temperature. |
| Unzipping of carbon nanotubes | Few μm long nano ribbons | This technique involves chemically or physically splitting nanotubes into graphene nanoribbons or sheets. It produces tailored graphene with defined edges, useful for electronics. | Multiple layers | Size is determined by the initial nanotube dimensions. | Expensive and oxidized graphene. Additionally, it requires precise control for consistent quality. |
| Material | Polymer | Material Loading (wt%) | LOI (%) | UL-94 | PHRR (kW/m2) | THR (MJ/m2) | TTI (s) | Ref. |
|---|---|---|---|---|---|---|---|---|
| G | PA6 | 2 | __ | __ | 1257 | 133.1 | 79 | [84] |
| GNS-CoO4 | 1282 | 141.4 | 81 | |||||
| GNS-NiO | 1105 | 130 | 75 | |||||
| GO | PS | 2 | 18.9 | __ | 384 | 131 | 36 | [92] |
| GO | PS | 2 | 18.8 | __ | 385 | 130 | 37 | [93] |
| GO | PVA | 5 | __ | __ | 133 | 38 | 45 | [24] |
| PS-S-GRF | PS | 30 | 24 | HB | 479 | 89 | 23 | [94] |
| ZnAl-G-PCL | PS | 15 | 20.01 | __ | __ | __ | 5 | [5] |
| FGO | PP | 2 | __ | __ | 739 | 98.7 | 33 | [95] |
| EPS-44%DG/EG | EPS | 44 | 36.0 | V-0 | 138.2 | 12.6 | 3 | [96] |
| DPP-GO | EP | 4 | 25.2 | V-0 | 301.9 | 13.2 | 3.2 | [97] |
| P-N-rGO | EP | 5 | 30.5 | V-1 | 785.7 | 57.7 | 56 | [98] |
| G-DOPO | EP | 5 | __ | HB | 538 | 36.5 | 32 | [32] |
| GO-DOPO | TPU | 2 | __ | __ | 362 | 48 | __ | [99] |
| APP-CFA-G | PP | 25 | 32 | V-0 | 140 | 90.4 | 35 | [100] |
| G-MDP-TiO2NP | ABS | 30 | __ | HB | 720 | 75 | 35 | [101] |
| GO-DOPO-V | EP | 2 | __ | __ | 1552.78 | 78.97 | __ | [102] |
| DGO | EPS | 20 | 29 | V-0 | 304.6 | 39.9 | 31 | [103] |
| Mo5/PN-rGO | ABS | 1 | __ | __ | 362 | 99 | 59 | [104] |
| EG | EPS | 33 | 22 | No | 144.90 | 28.22 | __ | [105] |
| RP-EG (1:2) | 25 | V-0 | __ | __ | 2.5/4.5 | |||
| RP-EG (1:1) | 26.9 | V-0 | 180.67 | 61.01 | 1.5/7.5 | |||
| RP-EG (2:1) | 26.1 | V-1 | __ | __ | 7.5/3 | |||
| FGO–AHP | PS | 5 | __ | __ | 639 | 34.9 | __ | [106] |
| PAUCG | EPS | __ | 48.0 | V-0 | 36.5 | 4.56 | 22.0 | [107] |
| SD8 + graphene | PR | 0.5 | __ | __ | 31.5 | 10.36 | 288 | [108] |
| FR-fGO | EP | 1 | 29.2 | V-1 | 927.23 | 70 | __ | [109] |
| PON-EG | PS | 20 | 25.8 | V-0 | 242.0 | 90.6 | 34 | [110] |
| PON-GN | 23.5 | V-1 | 321.8 | 90.7 | 42 | |||
| PSS@GNS | PS | 1 | __ | __ | 1036.2 | 77.6 | 45 | [111] |
| 2 | 1007.6 | 65.0 | 51 | |||||
| 4 | 763.7 | 62.1 | 56 | |||||
| ATG | EPS | 20 | 35.5 | V-0 | 201.4 | 9.0 | 227 | [112] |
| GO | PS | 1 | 21 | __ | 441 | 24.2 | __ | [113] |
| FGO | 1 | 22.5 | 445 | 23.4 | ||||
| 2 | 24.0 | 438 | 22.7 | |||||
| 3 | 25.0 | 436 | 21.4 | |||||
| LDH-DBP-5 wt%/GO-DDA-1.0 wt% | PS | 6 | 20 | __ | 456 | 124 | 65 | [114] |
| TGO | PS | 8 | __ | __ | 452 | 69 | 17 | [115] |
| PS1 | PS | 17 | 24.1 | V-2 | 334.2 | 79.0 | 28 | [116] |
| PS2 | 17 | 24.8 | V-2 | 351.6 | 79.2 | 34 | ||
| PS3 | 17 | 24.8 | V-2 | 415.7 | 85.2 | 33 | ||
| PS4 | 17 | 27.0 | V-0 | 319.6 | 74.6 | 31 | ||
| HPCTP/MP/EG | PS | 25 | 29.6 | HF1 and V-0 | 169 | 18.63 | 14 | [117] |
| EG15/MRP5 | HIPS | 20 | 26.8 | V-0 | 191 | 59 | 54 | [118] |
| FGO2.0 | PS | 30 | __ | __ | 514 W/g | 26.9 kJ/g | __ | [38] |
| RPEG | EPS | 33 | 26.9 | V-0 | 180.67 | 61.01 | __ | [105] |
| PSS@GNS | PS | 4 | __ | __ | 763.7 | 62.1 | 56 | [111] |
| MP/EG(1:2) | PS | 20 | 28 | V-0 | 209.7 | 104.9 | 44 | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isah, M.; Shehzad, F.; Al-Harthi, M.A. Advances in Graphene-Based Flame-Retardant for Polystyrene Applications: Synthesis, Mechanisms, and Future Perspectives. Polymers 2025, 17, 1811. https://doi.org/10.3390/polym17131811
Isah M, Shehzad F, Al-Harthi MA. Advances in Graphene-Based Flame-Retardant for Polystyrene Applications: Synthesis, Mechanisms, and Future Perspectives. Polymers. 2025; 17(13):1811. https://doi.org/10.3390/polym17131811
Chicago/Turabian StyleIsah, Mutawakkil, Farrukh Shehzad, and Mamdouh A. Al-Harthi. 2025. "Advances in Graphene-Based Flame-Retardant for Polystyrene Applications: Synthesis, Mechanisms, and Future Perspectives" Polymers 17, no. 13: 1811. https://doi.org/10.3390/polym17131811
APA StyleIsah, M., Shehzad, F., & Al-Harthi, M. A. (2025). Advances in Graphene-Based Flame-Retardant for Polystyrene Applications: Synthesis, Mechanisms, and Future Perspectives. Polymers, 17(13), 1811. https://doi.org/10.3390/polym17131811






