Repurposing Torrefied Biomass as a Novel Feedstock for Microbial Bioprocessing—A Proof-of-Concept of Low-Cost Biosurfactant Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass
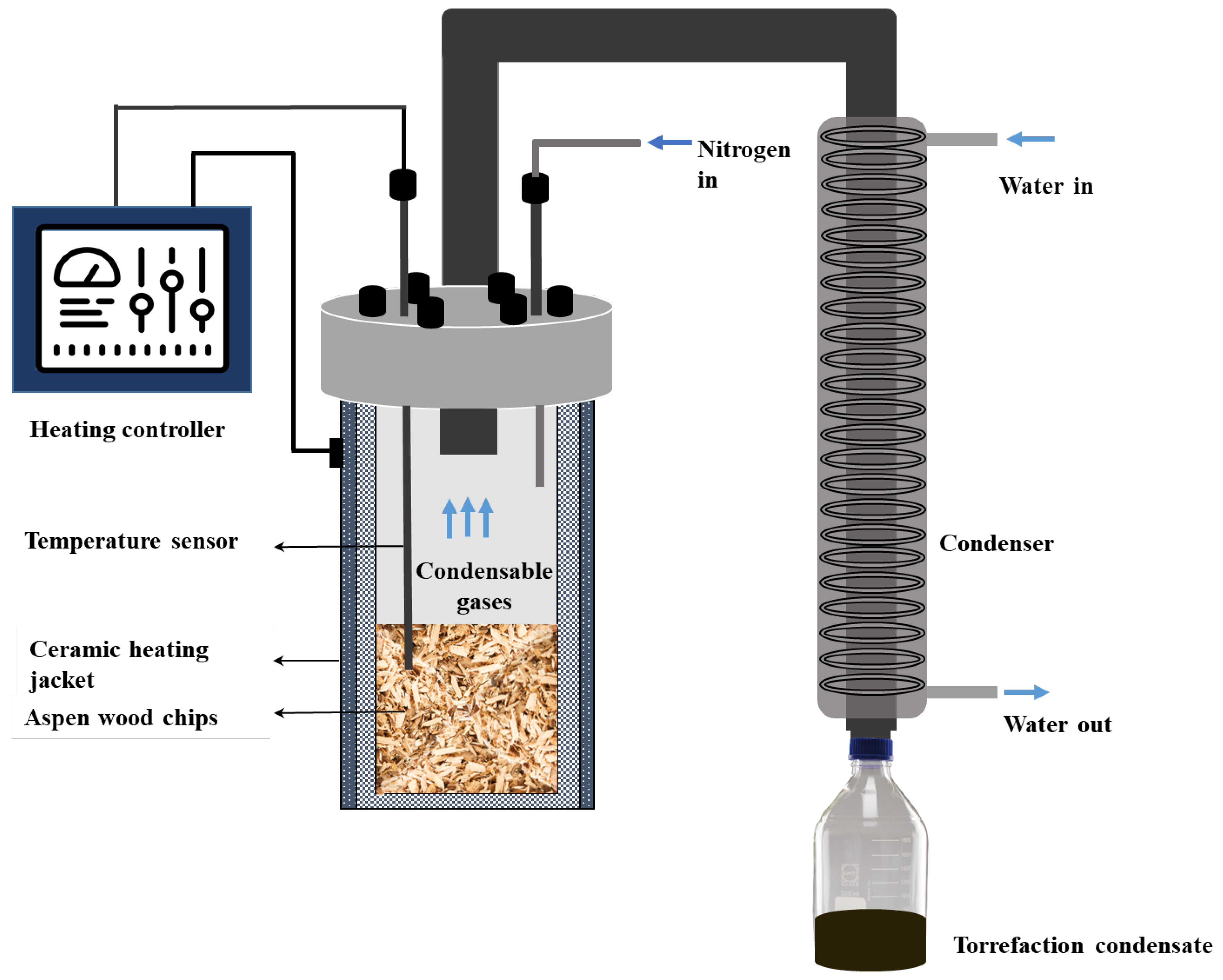
2.2. Torrefaction Experiments
2.3. Characterisation of Torrefied Biomass
2.3.1. Compositional Analysis
2.3.2. Thermogravimetric and Differential Thermogravimetric (TGA–DTG) Analyses
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. X-Ray Diffraction (XRD) Analyses
2.3.5. Scanning Electron Microscopy and Energy Dispersive X-Ray Analysis (SEM-EDX)
2.4. Biosurfactant Production Using Torrefied Biomass as Carbon Source
2.4.1. Microbial Culture and Media
2.4.2. Cell Separation and Evaluation of Tensio-Activity, Emulsification, and Biosurfactant Production
2.4.3. Qualitative Assays for Biosurfactant Production
Hemolysis Assay
CTAB-Agar Assay
Drop-Collapse Assay
Oil Displacement Assay
Analysis of Interfacial Tension
2.5. Statistical Analyses
3. Results and Discussion
3.1. Chemical and Physical Characterisation of Torrefied Biomass
3.1.1. Elemental Composition of Raw and Torrefied Aspen Biomass
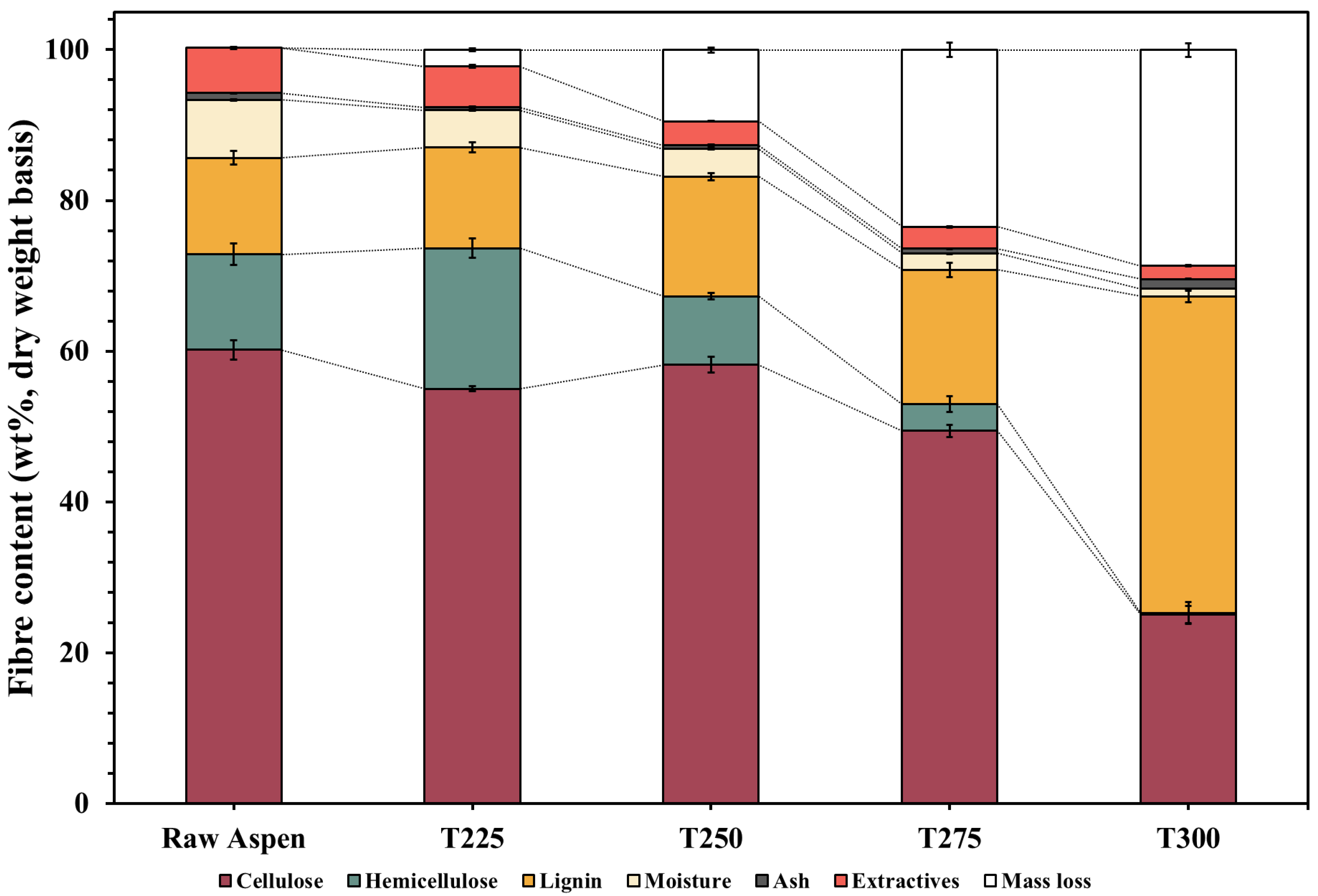
3.1.2. Fibre Analysis of Raw and Torrefied Aspen Biomass
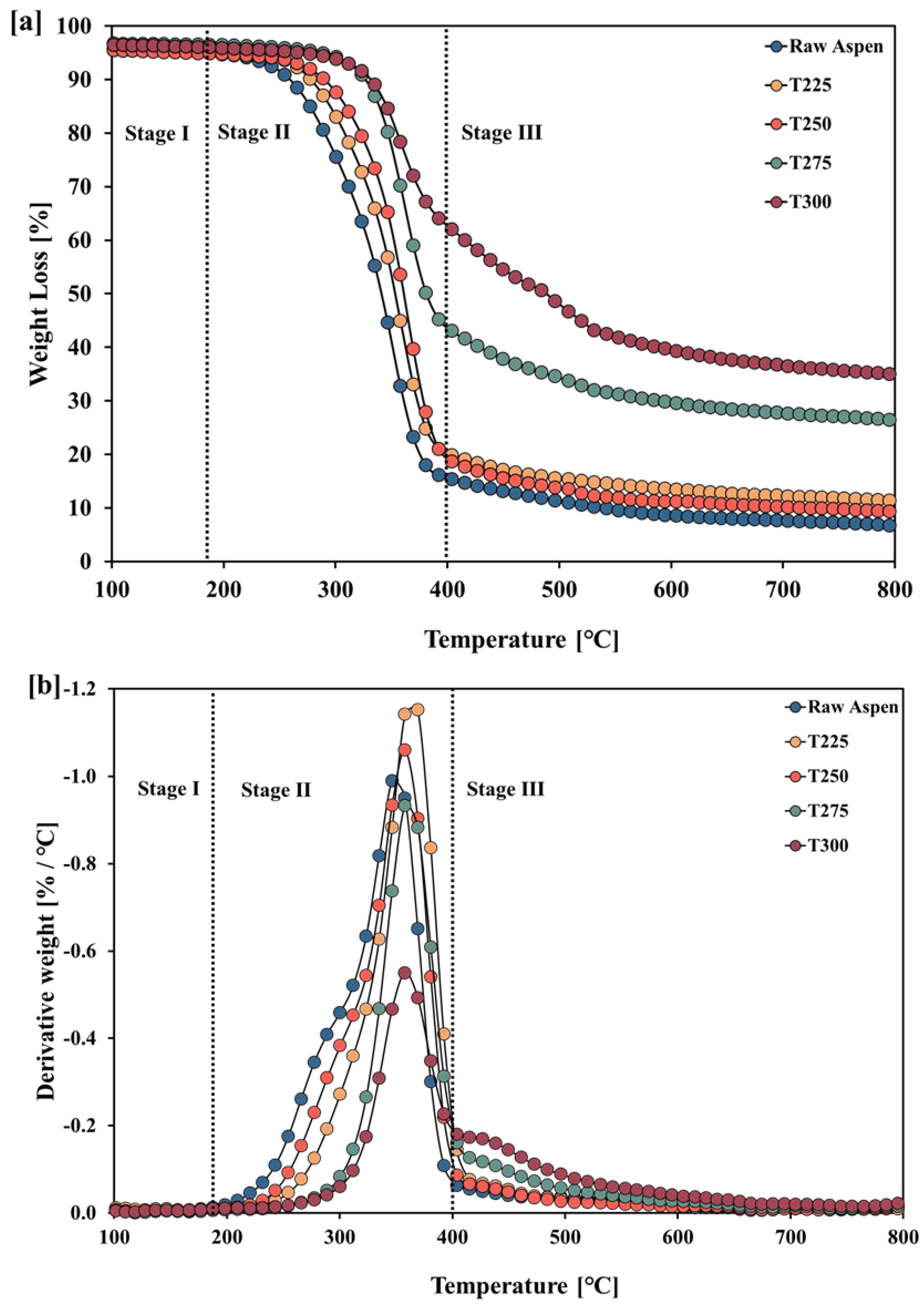
3.1.3. TGA–DTG Analyses
3.1.4. FTIR Analysis
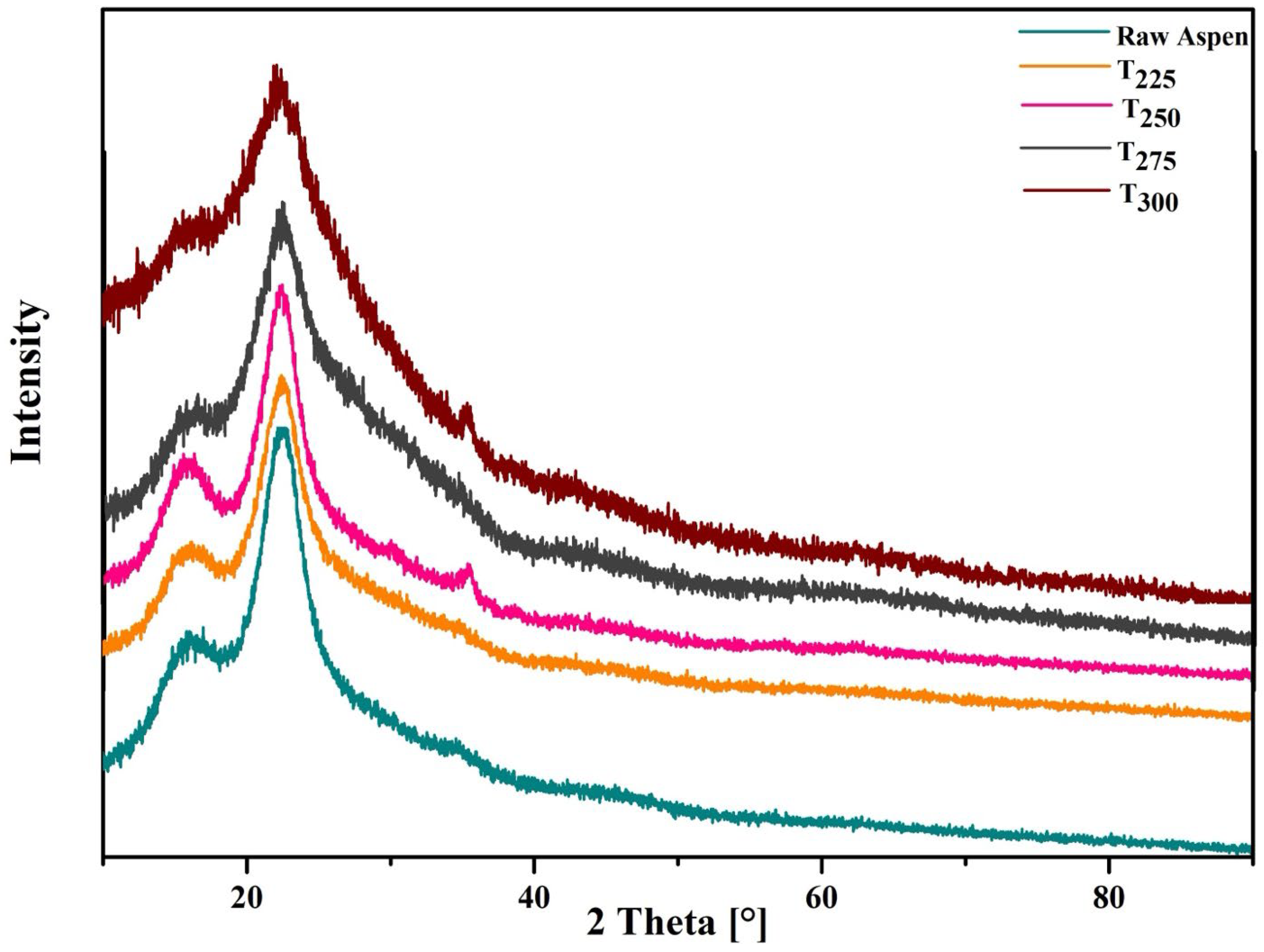
3.1.5. XRD Analysis
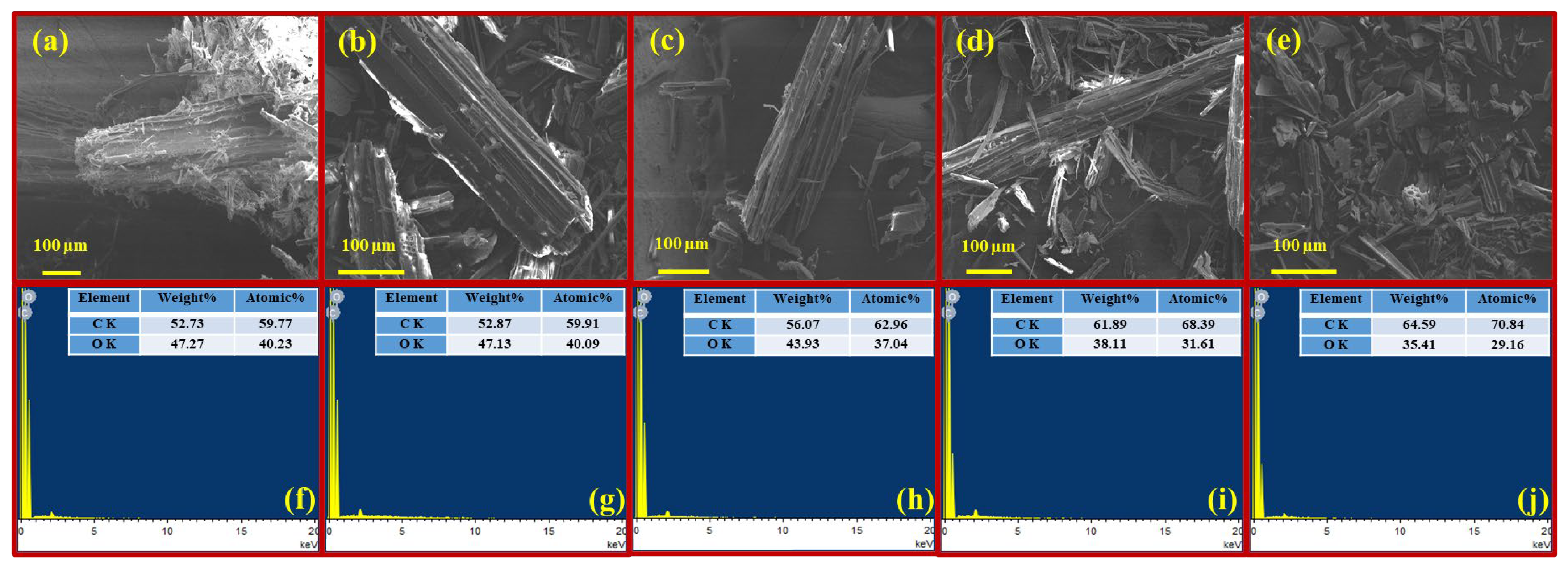
3.1.6. SEM-EDX Analysis
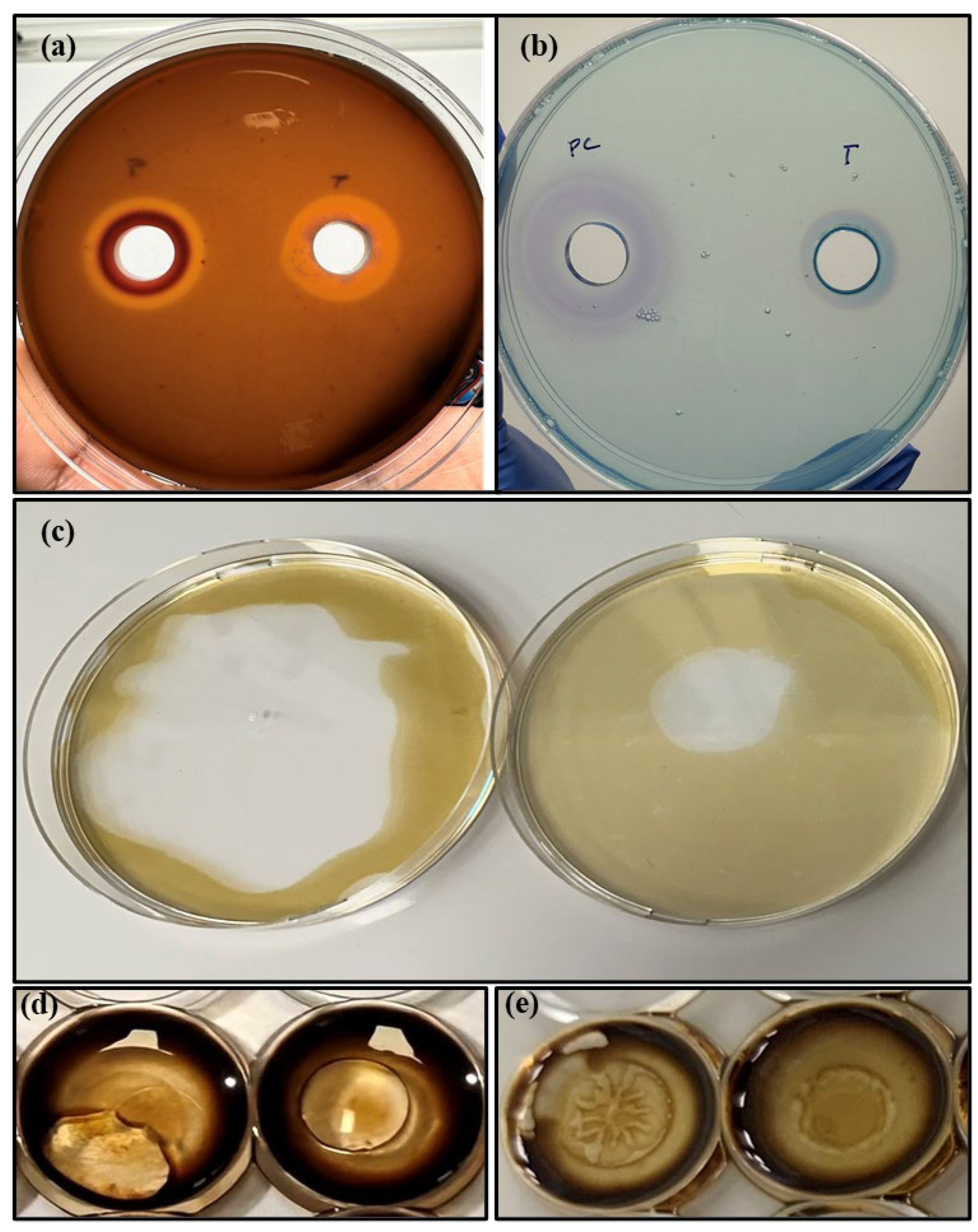
3.2. Biosurfactant Production from Torrefied Biomass
Preliminary Characterisation of Biosurfactant from Torrefied Biomass
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics-Estonia. Turnover of Manufacturing, Annual|2019–2023. Available online: https://www.stat.ee/en/find-statistics/statistics-theme/economy/industry (accessed on 29 May 2025).
- Forest Management & Bioenergy|Ministry of Climate. Available online: https://kliimaministeerium.ee/forest-management-and-bioenergy (accessed on 14 May 2025).
- Besserer, A.; Troilo, S.; Girods, P.; Rogaume, Y.; Brosse, N. Cascading recycling of wood waste: A review. Polymers 2021, 13, 1752. [Google Scholar] [CrossRef] [PubMed]
- Ehrenpreis, P. Synthetic biology opens up a new development path for the wood industry. Available online: https://researchinestonia.eu/2020/09/22/5909-2/ (accessed on 14 May 2025).
- Becker, A.; Scherer, V. A comparison of the torrefaction behavior of wood, miscanthus and palm kernel shells: Measurements on single particles with geometries of technical relevance. Fuel 2018, 224, 507–520. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Madissoo, M.; Pärn, L.; Virro, I.; Kikas, T. Torrefaction of agricultural and wood waste: Comparative analysis of selected fuel characteristics. Energies 2021, 14, 2774. [Google Scholar] [CrossRef]
- Doddapaneni, T.R.K.C.; Kikas, T. Advanced applications of torrefied biomass: A perspective view. Energies 2023, 16, 1635. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.M.I.; Kim, C.H.; Park, H.J.; Kim, S.H.; Kim, G.C.; Lee, J.Y.; Sim, S.W.; Kim, J.W. Effect of torrefaction for the pretreatment of rice straw for ethanol production. J. Sci. Food Agric. 2013, 93, 3198–3204. [Google Scholar] [CrossRef]
- Tripathi, J.; Richard, T.L.; Memis, B.; Demirci, A.; Ciolkosz, D. Interactions of torrefaction and alkaline pretreatment with respect to glucose yield of hydrolyzed wheat straw. Biomass 2022, 2, 264–278. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Shanmugam, S.; Kikas, T. Synergistic effects of torrefaction and alkaline pretreatment on sugar and bioethanol production from wood waste. Energies 2023, 16, 7606. [Google Scholar] [CrossRef]
- Sarker, T.R.; German, C.S.; Borugadda, V.B.; Meda, V.; Dalai, A.K. Techno-economic analysis of torrefied fuel pellet production from agricultural residue via integrated torrefaction and pelletization process. Heliyon 2023, 9, e16359. [Google Scholar] [CrossRef]
- Astute-Analytica. Furfural Market to Hit Valuation of US$ 1,379.2 Million By 2033. Available online: https://www.globenewswire.com/news-release/2025/01/30/3018213/0/en/Furfural-Market-to-Hit-Valuation-of-US-1-379-2-Million-By-2033-Astute-Analytica.html (accessed on 26 June 2025).
- Roy, B.; Kleine-Möllhoff, P.; Dalibard, A. Superheated steam Torrefaction of biomass residues with valorisation of platform chemicals part—2: Economic assessment and commercialisation opportunities. Sustainability 2022, 14, 2338. [Google Scholar] [CrossRef]
- Sałek, K.; Euston, S.R. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem. 2019, 85, 143–155. [Google Scholar] [CrossRef]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Hari, A.; Doddapaneni, T.R.K.C.; Kikas, T. Common operational issues and possible solutions for sustainable biosurfactant production from lignocellulosic feedstock. Environ. Res. 2024, 251, 118665. [Google Scholar] [CrossRef] [PubMed]
- Hrůzová, K.; Patel, A.; Masák, J.; Maťátková, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. A novel approach for the production of green biosurfactant from Pseudomonas aeruginosa using renewable forest biomass. Sci. Total Environ. 2020, 711, 135099. [Google Scholar] [CrossRef] [PubMed]
- Noll, P.; Solarte-Toro, J.C.; Restrepo-Serna, D.L.; Treinen, C.; Poveda-Giraldo, J.A.; Henkel, M.; Alzate, C.A.C.; Hausmann, R. Limits for sustainable biosurfactant production: Techno-economic and environmental assessment of a rhamnolipid production process. Bioresour. Technol. Rep. 2024, 25, 101767. [Google Scholar] [CrossRef]
- Dubeau, D.; Déziel, E.; Woods, D.E.; Lépine, F. Burkholderia thailandensis harbors two identical rhl gene clusters responsible for the biosynthesis of rhamnolipids. BMC. Microbiol. 2009, 9, 263. [Google Scholar] [CrossRef]
- Chen, C.; Su, J.; Ali, A.; Zhai, Z. Cornstalk biochar promoted the denitrification performance and cellulose degradation rate of Burkholderia sp. CF6. J. Environ. Chem. Eng. 2022, 10, 106998. [Google Scholar] [CrossRef]
- Akita, H.; Kimura, Z.-i.; Mohd Yusoff, M.Z.; Nakashima, N.; Hoshino, T. Isolation and characterization of Burkholderia sp. strain CCA53 exhibiting ligninolytic potential. SpringerPlus 2016, 5, 596. [Google Scholar] [CrossRef]
- Morya, R.; Kumar, M.; Singh, S.S.; Thakur, I.S. Genomic analysis of Burkholderia sp. ISTR5 for biofunneling of lignin-derived compounds. Biotechnol. Biofuels 2019, 12, 277. [Google Scholar] [CrossRef]
- Morya, R.; Salvachúa, D.; Thakur, I.S. Burkholderia: An untapped but promising bacterial genus for the conversion of aromatic compounds. Trends Biotechnol. 2020, 38, 963–975. [Google Scholar] [CrossRef]
- ISO18122:2015; Solid Biofuels–Determination of Ash Content. ISO: Geneva, Switzerland, 2015.
- Mulligan, C.N.; Cooper, D.G.; NEUFELD, R.J. Selection of microbes producing biosurfactants in media without hydrocarbons. J. Ferment. Technol. 1984, 62, 311–314. [Google Scholar]
- Siegmund, I.; Wagner, F. New method for detecting rhamnolipids excreted by Pseudomonas species during growth on mineral agar. Biotechnol. Tech. 1991, 5, 265–268. [Google Scholar] [CrossRef]
- Jain, D.; Collins-Thompson, D.; Lee, H.; Trevors, J. A drop-collapsing test for screening surfactant-producing microorganisms. J. Microbiol. Methods. 1991, 13, 271–279. [Google Scholar] [CrossRef]
- Morikawa, M.; Hirata, Y.; Imanaka, T. A study on the structure–function relationship of lipopeptide biosurfactants. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1488, 211–218. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, J.P.; Mondal, M.K. Torrefaction of Acacia nilotica: Oxygen distribution and carbon densification mechanism based on in-depth analyses of solid, liquid, and gaseous products. Energy Fuel 2020, 34, 12586–12597. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Zhao, M.; Feng, X.; Wang, L.; Yang, H.; Ma, H.; Zhou, J. Effects of torrefaction on the lignin of apricot shells and its catalytic conversion to aromatics. ACS Omega 2021, 6, 25742–25748. [Google Scholar] [CrossRef]
- Singh, A.K.; Chakraborty, J.P. Torrefaction of woody biomass (Acacia nilotica) in fluidized bed reactor: Study of solid fuel properties. Biomass Convers. Biorefin. 2025. [Google Scholar] [CrossRef]
- Barzegar, R.; Yozgatligil, A.; Olgun, H.; Atimtay, A.T. TGA and kinetic study of different torrefaction conditions of wood biomass under air and oxy-fuel combustion atmospheres. J. Energy Inst. 2020, 93, 889–898. [Google Scholar] [CrossRef]
- Pushkin, S.A.; Kozlova, L.V.; Makarov, A.A.; Grachev, A.N.; Gorshkova, T.A. Cell wall components in torrefied softwood and hardwood samples. J. Anal. Appl. Pyrol. 2015, 116, 102–113. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Sun, Q.; Ma, J.; Xia, A.; Huang, Y.; Zhu, X.; Liao, Q. Elucidation of the interaction effects of cellulose, hemicellulose and lignin during degradative solvent extraction of lignocellulosic biomass. Fuel 2022, 327, 125141. [Google Scholar] [CrossRef]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Podgorbunskikh, E.M.; Bychkov, A.L.; Ryabchikova, E.I.; Lomovsky, O.I. The effect of thermomechanical pretreatment on the structure and properties of lignin-rich plant biomass. Molecules 2020, 25, 995. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Thilakarathna, W.W.; He, Q.S.; Rupasinghe, H.V. A review: Depolymerization of lignin to generate high-value bio-products: Opportunities, challenges, and prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Tripathi, J.; Ciolkosz, D. Changes in torrefied cellulose and their relationship with glucose yield: A mechanistic study. Cellulose 2025, 32, 3737–3757. [Google Scholar] [CrossRef]
- Bert, V.; Allemon, J.; Sajet, P.; Dieu, S.; Papin, A.; Collet, S.; Gaucher, R.; Chalot, M.; Michiels, B.; Raventos, C. Torrefaction and pyrolysis of metal-enriched poplars from phytotechnologies: Effect of temperature and biomass chlorine content on metal distribution in end-products and valorization options. Biomass Bioenergy 2017, 96, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Bonvicini, G.; Tognotti, L.; Yang, W.; Blasiak, W. High-temperature rapid devolatilization of biomasses with varying degrees of torrefaction. Fuel 2014, 122, 261–269. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goricanec, D.; Krope, J.; Urbancl, D. Torrefaction pretreatment of lignocellulosic biomass for sustainable solid biofuel production. Energy 2022, 240, 122483. [Google Scholar] [CrossRef]
- Morales, D.A.G. Studies of the Torrefaction of Sugarcane Bagasse and Poplar Wood. Ph.D. Thesis, Universidad Nacional de Colombia, Medellín, Colombia, 2017. [Google Scholar]
- Toscano, G.; Maceratesi, V.; Leoni, E.; Stipa, P.; Laudadio, E.; Sabbatini, S. FTIR spectroscopy for determination of the raw materials used in wood pellet production. Fuel 2022, 313, 123017. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Martínez Cortizas, A. Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci. Technol. 2018, 52, 487–504. [Google Scholar] [CrossRef]
- de Oliveira Brotto, J.; Cruz, T.A.; de Oliveira Pereira, I.; Ienczak, J.L.; Peralta, R.A.; Lázaro-Martínez, J.M.; José, H.J.; Rodríguez-Castellón, E.; Moreira, R.d.F.P.M. Mechanistic insights and kinetics of torrefaction of pine wood biomasses using solid-state NMR. J. Anal. Appl. Pyrol. 2023, 172, 106019. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M.; Ahmed, N.A.; Ogunkunle, O. Biomethane production from Arachis hypogea shells: Effect of thermal pretreatment on substrate structure and yield. Biomass. Convers. Biorefin. 2024, 14, 6925–6938. [Google Scholar] [CrossRef]
- Sarker, T.R.; Azargohar, R.; Dalai, A.K.; Meda, V. Enhancement of fuel and physicochemical properties of canola residues via microwave torrefaction. Energy Rep. 2021, 7, 6338–6353. [Google Scholar] [CrossRef]
- Szufa, S.; Unyay, H.; Piersa, P.; Kędzierska-Sar, A.; Romanowska-Duda, Z.; Likozar, B. Reduction of spruce phytotoxicity by superheated steam torrefaction and its use in stimulating the growth of ecological bio-products: Lemna minor L. Biomass Convers. Biorefin. 2025. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Gu, J.; Zheng, Z. Effect of the Torrefaction Temperature on the Structural Properties and Pyrolysis Behavior of Biomass. BioResour. 2017, 12, 3425–3447. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, W.; Chen, W.-H.; Ho, S.-H.; Pétrissans, A.; Pétrissans, M. Effect of torrefaction on the structure and reactivity of rice straw as well as life cycle assessment of torrefaction process. Energy. 2022, 240, 122470. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Niu, C.; Dalai, A.K. Experimental and modeling studies of torrefaction of spent coffee grounds and coffee husk: Effects on surface chemistry and carbon dioxide capture performance. ACS Omega 2021, 7, 638–653. [Google Scholar] [CrossRef]
- Saini, J.K.; Kaur, A.; Mathur, A. Strategies to enhance enzymatic hydrolysis of lignocellulosic biomass for biorefinery applications: A review. Bioresour. Technol. 2022, 360, 127517. [Google Scholar] [CrossRef]
- Maiorano, G.; Ramires, F.A.; Durante, M.; Palamà, I.E.; Blando, F.; De Rinaldis, G.; Perbellini, E.; Patruno, V.; Gadaleta Caldarola, C.; Vitucci, S. The controlled semi-solid fermentation of seaweeds as a strategy for their stabilization and new food applications. Foods 2022, 11, 2811. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, D.; Zhou, Y.; Wang, H.; Xia, L.; Tan, C.; Guo, F.; Zhang, X.; Yang, R.; Yu, Y. Lignin’s complex role in lignocellulosic biomass Recalcitrance: A case study on bamboo. Chem. Eng. J. 2024, 490, 151422. [Google Scholar] [CrossRef]
- Beladhadi, R.; Shankar, K.; Jayalakshmi, S.; Sreeramulu, K. Production of cocktail of lignolytic, cellulolytic and hemicellulolytic enzymes by the novel bacterium Burkholderia sp SMB1 utilizing rice bran and straw: Application in the saccharification of untreated agro-wastes for bioethanol production. Waste. Biomass. Valori. 2022, 13, 1565–1577. [Google Scholar] [CrossRef]
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnol. 2017, 36, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.V.; Rebocho, A.T.; Esmail, A.; Sevrin, C.; Grandfils, C.; Torres, C.A.; Reis, M.A.; Freitas, F. Characterization of the thermostable biosurfactant produced by burkholderia thailandensis DSM 13276. Polymers 2022, 14, 2088. [Google Scholar] [CrossRef]
- Willumsen, P.A.; Karlson, U. Screening of bacteria, isolated from PAH-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation 1996, 7, 415–423. [Google Scholar] [CrossRef]
- Rani, M.; Weadge, J.T.; Jabaji, S. Isolation and characterization of biosurfactant-producing bacteria from oil well batteries with antimicrobial activities against food-borne and plant pathogens. Front. Microbiol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Thavasi, R.; Sharma, S.; Jayalakshmi, S. Evaluation of screening methods for the isolation of biosurfactant producing marine bacteria. J. Pet. Environ. Biotechnol. 2011, 1. [Google Scholar] [CrossRef]
- Balseiro-Romero, M.; Gkorezis, P.; Kidd, P.S.; Van Hamme, J.; Weyens, N.; Monterroso, C.; Vangronsveld, J. Characterization and degradation potential of diesel-degrading bacterial strains for application in bioremediation. Int. J. Phytoremediation 2017, 19, 955–963. [Google Scholar] [CrossRef]


| Sample | C (%) | H (%) | N (%) | O (%) | S (%) | Atomic Ratio | |
|---|---|---|---|---|---|---|---|
| H/C | O/C | ||||||
| Raw aspen | 47.7 ± 0.1 | 7.0 ± 0.0 | 0.2 ± 0.0 | 45.0 ± 0.1 | 0.04 | 0.15 | 0.94 |
| T225 | 49.4 ± 0.2 | 7.1 ± 0.2 | 0.6 ± 0.7 | 42.9 ± 0.8 | 0.02 | 0.14 | 0.87 |
| T250 | 49.6 ± 0.3 | 6.7 ± 0.1 | 0.1 ± 0.0 | 43.6 ± 0.3 | 0.01 | 0.14 | 0.88 |
| T275 | 55.6 ± 0.1 | 6.3 ± 0.1 | 0.1 ± 0.1 | 38 ± 0.0 | 0.01 | 0.11 | 0.68 |
| T300 | 61.4 ± 0.5 | 5.9 ± 0.1 | 0.1 ± 0.0 | 32.6 ± 0.5 | 0.01 | 0.10 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hari, A.; Rooni, V.; Veerabagu, U.; Sarker, S.; Konist, A.; Kikas, T. Repurposing Torrefied Biomass as a Novel Feedstock for Microbial Bioprocessing—A Proof-of-Concept of Low-Cost Biosurfactant Production. Polymers 2025, 17, 1808. https://doi.org/10.3390/polym17131808
Hari A, Rooni V, Veerabagu U, Sarker S, Konist A, Kikas T. Repurposing Torrefied Biomass as a Novel Feedstock for Microbial Bioprocessing—A Proof-of-Concept of Low-Cost Biosurfactant Production. Polymers. 2025; 17(13):1808. https://doi.org/10.3390/polym17131808
Chicago/Turabian StyleHari, Anjana, Vahur Rooni, Udayakumar Veerabagu, Shiplu Sarker, Alar Konist, and Timo Kikas. 2025. "Repurposing Torrefied Biomass as a Novel Feedstock for Microbial Bioprocessing—A Proof-of-Concept of Low-Cost Biosurfactant Production" Polymers 17, no. 13: 1808. https://doi.org/10.3390/polym17131808
APA StyleHari, A., Rooni, V., Veerabagu, U., Sarker, S., Konist, A., & Kikas, T. (2025). Repurposing Torrefied Biomass as a Novel Feedstock for Microbial Bioprocessing—A Proof-of-Concept of Low-Cost Biosurfactant Production. Polymers, 17(13), 1808. https://doi.org/10.3390/polym17131808











