The Role of Artificial Weathering Protocols on Abiotic and Bacterial Degradation of Polyethylene
Abstract
1. Introduction
2. Results and Discussion
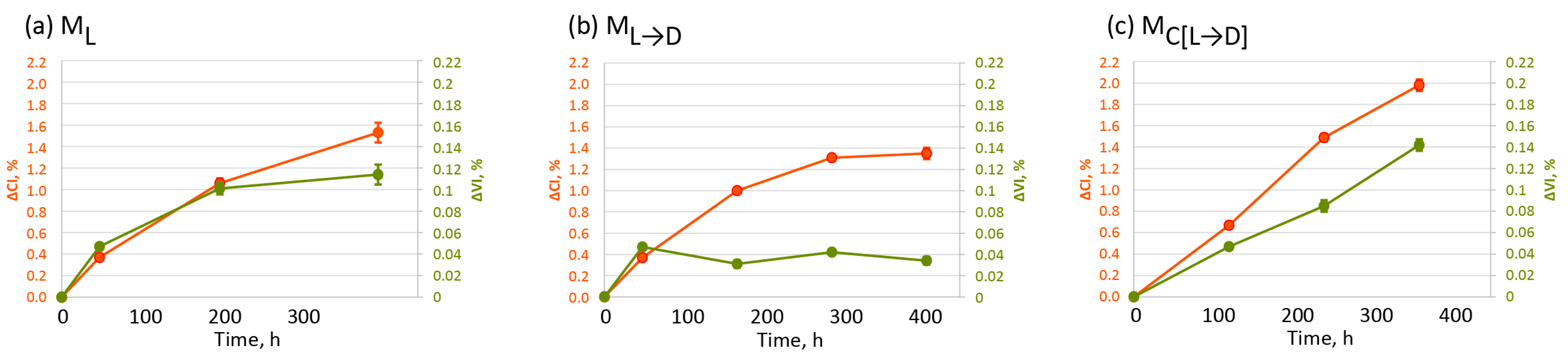
2.1. Changes in Surface Chemistry of Oxo-LDPE Under Various Artificial Weathering Protocols
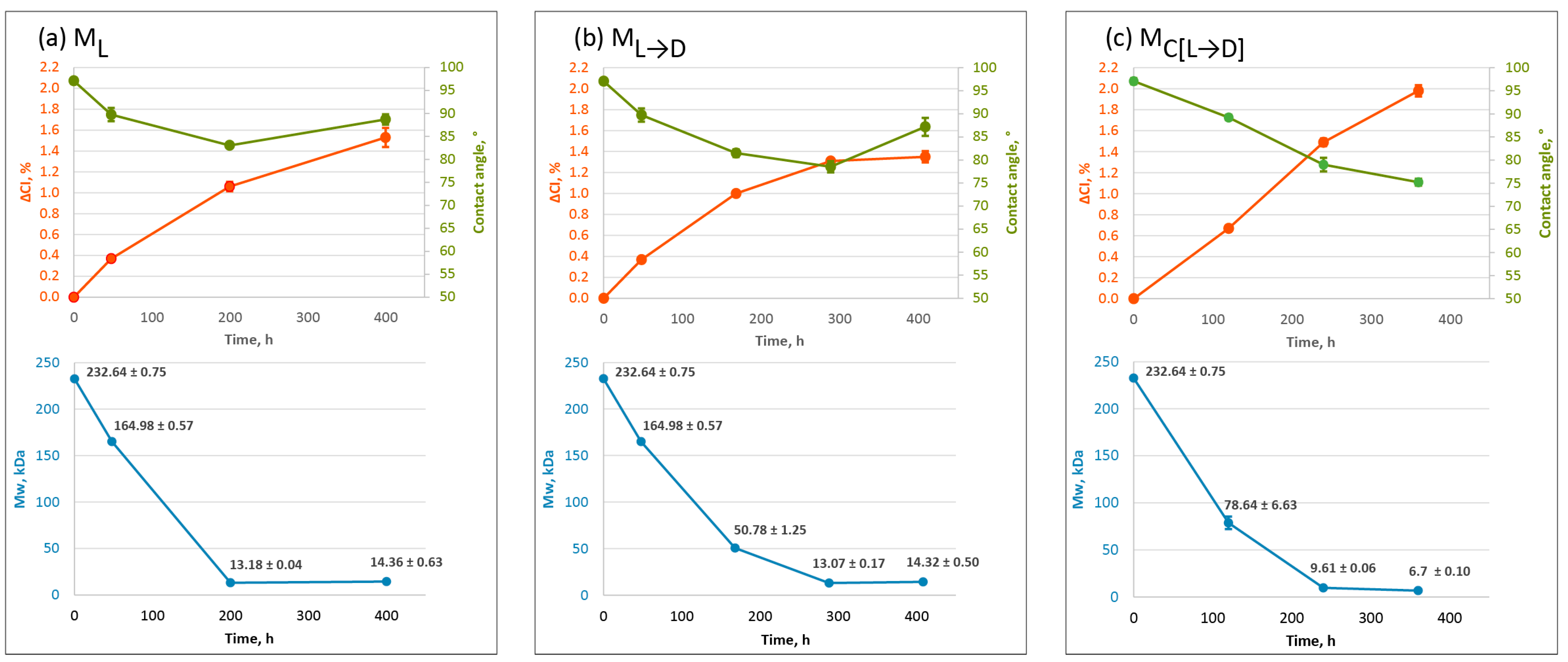
2.2. Physicochemical Changes in Oxo-LDPE with Different Weathering Protocols
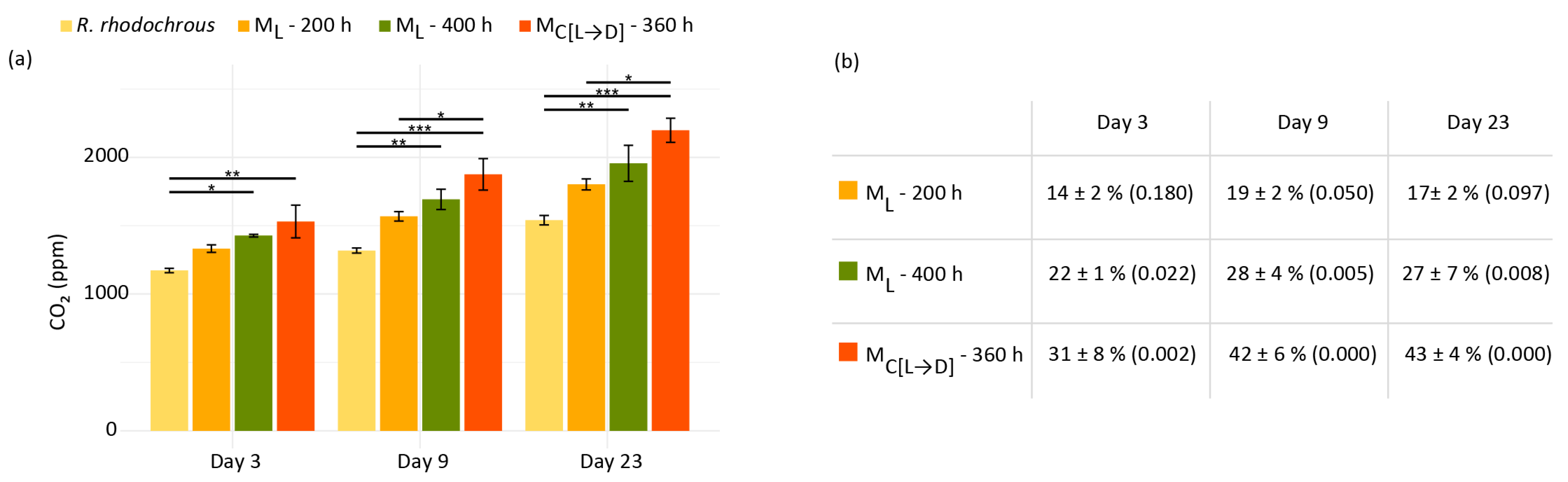
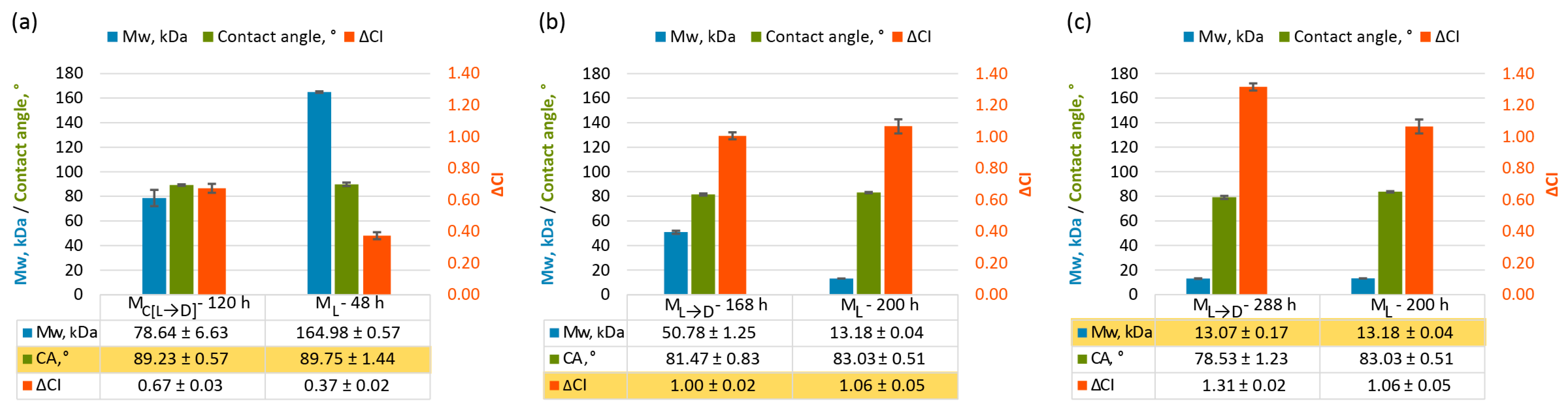
2.3. Can Plastic Degradation Be Accurately Assessed Through a Single Metric?
3. Materials and Methods
3.1. Materials
3.2. Accelerated Artificial Weathering
3.3. Analysis of Surface Chemistry
3.4. Analysis of Surface Hydrophilicity
3.5. Analysis of Molecular Weight
3.6. Analysis of Bacterial Biodegradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iroegbu, A.O.C.; Ray, S.S.; Mbarane, V.; Bordado, J.C.; Sardinha, J.P. Plastic Pollution: A Perspective on Matters Arising: Challenges and Opportunities. ACS Omega 2021, 6, 19343–19355. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Collard, F.; Fabres, J.; Gabrielsen, G.W.; Provencher, J.F.; Rochman, C.M.; van Sebille, E.; Tekman, M.B. Plastic pollution in the Arctic. Nat. Rev. Earth Environ. 2022, 3, 323–337. [Google Scholar] [CrossRef]
- The Birmingham Plastics Network. Plastic a Call to Action; University of Birmingham: Birmingham, UK, 2023. [Google Scholar] [CrossRef]
- Jones, J.I.; Vdovchenko, A.; Cooling, D.; Murphy, J.F.; Arnold, A.; Pretty, J.L.; Spencer, K.L.; Markus, A.A.; Vethaak, A.D.; Resmini, M. Systematic analysis of the relative abundance of polymers occurring as microplastics in freshwaters and estuaries. Int. J. Environ. Res. Public Health 2020, 17, 9304. [Google Scholar] [CrossRef] [PubMed]
- Vdovchenko, A.; Resmini, M. Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7074. [Google Scholar] [CrossRef]
- Persiani, E.; Cecchettini, A.; Ceccherini, E.; Gisone, I.; Morales, M.A.; Vozzi, F. Microplastics: A Matter of the Heart (and Vascular System). Biomedicines 2023, 11, 264. [Google Scholar] [CrossRef]
- Li, S.; Keenan, J.I.; Shaw, I.C.; Frizelle, F.A. Could Microplastics Be a Driver for Early Onset Colorectal Cancer? Cancers 2023, 15, 3323. [Google Scholar] [CrossRef]
- Lu, J.; Yu, Z.; Ngiam, L.; Guo, J. Microplastics as potential carriers of viruses could prolong virus survival and infectivity. Water Res. 2022, 225, 119115. [Google Scholar] [CrossRef] [PubMed]
- Moyal, J.; Dave, P.H.; Wu, M.; Karimpour, S.; Brar, S.K.; Zhong, H.; Kwong, R.W.M. Impacts of Biofilm Formation on the Physicochemical Properties and Toxicity of Microplastics: A Concise Review. Rev. Environ. Contam. Toxicol. 2023, 261, 8. [Google Scholar] [CrossRef]
- Joo, S.H.; Liang, Y.; Kim, M.; Byun, J.; Choi, H. Microplastics with adsorbed contaminants: Mechanisms and Treatment. Environ. Chall. 2021, 3, 100042. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U.; Schawaller, D. Kinetic study on the thermal degradation of polypropylene and polyethylene. J. Anal. Appl. Pyrolysis 1999, 48, 93–109. [Google Scholar] [CrossRef]
- Fernández-González, V.; Andrade-Garda, J.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Impact of weathering on the chemical identification of microplastics from usual packaging polymers in the marine environment. Anal. Chim. Acta 2021, 1142, 179–188. [Google Scholar] [CrossRef]
- Quade, J.; López-Ibáñez, S.; Beiras, R. UV Dosage Unveils Toxic Properties of Weathered Commercial Bioplastic Bags. Environ. Sci. Technol. 2023, 57, 14807–14816. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Hof, D.; Dombrowski, A.; Schweyen, P.; Dierkes, G.; Ternes, T.; Schulte-Oehlmann, U.; Oehlmann, J. Enhanced in vitro toxicity of plastic leachates after UV irradiation. Water Res. 2021, 199, 117203. [Google Scholar] [CrossRef]
- Schefer, R.B.; Armanious, A.; Mitrano, D.M. Eco-Corona Formation on Plastics: Adsorption of Dissolved Organic Matter to Pristine and Photochemically Weathered Polymer Surfaces. Environ. Sci. Technol. 2023, 57, 14707–14716. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, P.; Wu, X.; Shi, H.; Huang, H.; Wang, H.; Gao, S. Insight into chain scission and release profiles from photodegradation of polycarbonate microplastics. Water Res. 2021, 195, 116980. [Google Scholar] [CrossRef]
- Menzel, T.; Meides, N.; Mauel, A.; Mansfeld, U.; Kretschmer, W.; Kuhn, M.; Herzig, E.M.; Altstädt, V.; Strohriegl, P.; Senker, J.; et al. Degradation of low-density polyethylene to nanoplastic particles by accelerated weathering. Sci. Total Environ. 2022, 826, 154035. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, M.N. Isolation of a thermophilic bacterium capable of low-molecular-weight polyethylene degradation. Biodegradation 2013, 24, 89–98. [Google Scholar] [CrossRef]
- Madras, G. Molecular weight effect on the dynamics of polystyrene degradation. Ind. Eng. Chem. Res. 1997, 36, 2019–2024. [Google Scholar] [CrossRef]
- Hamzah, M.; Khenfouch, M.; Rjeb, A.; Sayouri, S.; Houssaini, D.S.; Darhouri, M.; Srinivasu, V.V. Surface chemistry changes and microstructure evaluation of low density nanocluster polyethylene under natural weathering: A spectroscopic investigation. J. Phys. Conf. Ser. 2018, 984, 012010. [Google Scholar] [CrossRef]
- Du, F.; Cai, H.; Su, L.; Wang, W.; Zhang, L.; Sun, C.; Yan, B.; Shi, H. The missing small microplastics: Easily generated from weathered plastic pieces in labs but hardly detected in natural environments. Environ. Sci. Adv. 2023, 3, 227–238. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Benke, A.; Sonnenberg, J.; Oelschlägel, K.; Schneider, M.; Lux, M.; Potthoff, A. Wettability after Artificial and Natural Weathering of Polyethylene Terephthalate. Environments 2022, 9, 134. [Google Scholar] [CrossRef]
- Palanisamy, N.; Ragunathan, R.; Pandiyaraj, K.N.; Muralidharan, V.S. Investigation on biodegradability of polyethylene by Bacillus cereus strain Ma-Su isolated from compost. Int. Res. J. Microbiol. 2011, 2, 292–302. [Google Scholar]
- Rose, R.S.; Richardson, K.H.; Latvanen, E.J.; Hanson, C.A.; Resmini, M.; Sanders, I.A. Microbial degradation of plastic in aqueous solutions demonstrated by CO2 evolution and quantification. Int. J. Mol. Sci. 2020, 21, 1176. [Google Scholar] [CrossRef]
- Andrade, J.; Fernández-González, V.; López-Mahía, P.; Muniategui, S. A low-cost system to simulate environmental microplastic weathering. Mar. Pollut. Bull. 2019, 149, 110663. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Karkanorachaki, K.; Kalogerakis, G.C.; Triantafyllidi, E.I.; Gotsis, A.D.; Partsinevelos, P.; Fava, F. Microplastics generation: Onset of fragmentation of polyethylene films in marine environment mesocosms. Front. Mar. Sci. 2017, 4, 84. [Google Scholar] [CrossRef]
- Meides, N.; Mauel, A.; Menzel, T.; Altstädt, V.; Ruckdäschel, H.; Senker, J.; Strohriegl, P. Quantifying the fragmentation of polypropylene upon exposure to accelerated weathering. Microplast. Nanoplast. 2022, 2, 23. [Google Scholar] [CrossRef]
- ASTM D2508-01; Standard Practice for Fluroescent (UV) Exposure of Photodegradable Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2022.
- ISO/TS 19022:2016; Plastics—Method of Controlled Acceleration of Laboratory Weathering by Increased Irradiance. ISO—International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/63749.html (accessed on 11 December 2024).
- ISO 4582:2017; Plastics—Determination of Changes in Colour and Variations in Properties After Exposure to Glass-Filtered Solar Radiation, Natural Weathering or Laboratory Radiation Sources. ISO—International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/67791.html (accessed on 11 December 2024).
- Vulic, I.; Stretanski, J.; Sanders, B. UV stabilization of polyolefin systems. Polym. Polym. Compos. 2000, 8, 529–535. [Google Scholar]
- Cao, H.; Ding, P.; Li, X.; Huang, C.; Li, X.; Chen, X.; Zhang, L.; Qi, J. Environmentally persistent free radicals on photoaged microplastics from disposable plastic cups induce the oxidative stress-associated toxicity. J. Hazard. Mater. 2024, 464, 132990. [Google Scholar] [CrossRef]
- Schwarz, W.; Wegener, S.; Schertzinger, G.; Pannekens, H.; Schweyen, P.; Dierkes, G.; Klein, K.; Ternes, T.A.; Oehlmann, J.; Dopp, E. Chemical and toxicological assessment of leachates from UV-degraded plastic materials using in-vitro bioassays. PeerJ 2023, 11, e15192. [Google Scholar] [CrossRef] [PubMed]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Degradation profile of polyethylene after artificial accelerated weathering. Polym. Degrad. Stab. 2003, 79, 385–397. [Google Scholar] [CrossRef]
- Doğan, M. Ultraviolet light accelerates the degradation of polyethylene plastics. Microsc. Res. Tech. 2021, 84, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Shashoua, Y.; Peydaei, A.; Mortensen, M.N.; Kanstrup, A.B.; Gregory, D.J. Physio-chemical degradation of single-use plastics in natural weather and marine environments. Environ. Pollut. 2024, 357, 124414. [Google Scholar] [CrossRef]
- WRAP Statement on PAS 9017 Plastics. Biodegradation of Polyolefins in an Open-Air Terrestrial Environment; Targeted News Service: Washington, DC, USA, 2020. [Google Scholar]
- Hill, G.; Moreira, C.; Huynh, F.; Trufasila, A.; Ly, F.; Lloyd, R.; Sawal, H.; Wallis, C.J. Correlation of a temperate uv-weathering cycle to outdoor exposure for the determination of the environmental instability of polyethylene films using ht-gpc analysis. Polymers 2021, 13, 591. [Google Scholar] [CrossRef]
- Vazquez, Y.V.; Ressia, J.A.; Cerrada, M.L.; Barbosa, S.E.; Vallés, E.M. Prodegradant Additives Effect onto Comercial Polyolefins. J. Polym. Environ. 2019, 27, 464–471. [Google Scholar] [CrossRef]
- Elkori, R.; Lamarti, A.; Had, K.E.; Hachim, A.; Yamari, I. Evaluation of the impact of natural weathering on the properties of high-density polyethylene bottles by experimental approach. Polym. Eng. Sci. 2024, 64, 2975–2987. [Google Scholar] [CrossRef]
- Rotchell, J.M.; Jenner, L.C.; Chapman, E.; Bennett, R.T.; Bolanle, I.O.; Loubani, M.; Sadofsky, L.; Palmer, T.M. Detection of microplastics in human saphenous vein tissue using μFTIR: A pilot study. PLoS ONE 2023, 18, e0280594. [Google Scholar] [CrossRef]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Vittoria, V.; Camino, G.; Cagnani, C. Chemical and morphologial modifications of irradiated linear low density polyethylene (LLDPE). Polym. Degrad. Stab. 2001, 72, 175–186. [Google Scholar] [CrossRef]
- Yagoubi, W.; Abdelhafidi, A.; Sebaa, M.; Chabira, S.F. Identification of carbonyl species of weathered LDPE films by curve fitting and derivative analysis of IR spectra. Polym. Test. 2015, 44, 37–48. [Google Scholar] [CrossRef]
- Starkova, O.; Gagani, A.I.; Karl, C.W.; Rocha, I.B.C.M.; Burlakovs, J.; Krauklis, A.E. Modelling of environmental ageing of polymers and polymer composites—Durability prediction methods. Polymers 2022, 14, 907. [Google Scholar] [CrossRef] [PubMed]
- Lee, Q.Y.; Li, H. Photocatalytic degradation of plastic waste: A mini review. Micromachines 2021, 12, 907. [Google Scholar] [CrossRef]
- Grause, G.; Chien, M.F.; Inoue, C. Changes during the weathering of polyolefins. Polym. Degrad. Stab. 2020, 181, 109364. [Google Scholar] [CrossRef]
- Hamerton, I.; Howlin, B.J.; Yeung, S.Y.C. Studying structure-property relationships in oligomeric engineering thermoplastics by controlled preparation of low molecular weight polymers. React. Funct. Polym. 2014, 81, 22–32. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bigault De Cazanove, P.F.; Vdovchenko, A.; Rose, R.S.; Resmini, M. The Role of Artificial Weathering Protocols on Abiotic and Bacterial Degradation of Polyethylene. Polymers 2025, 17, 1798. https://doi.org/10.3390/polym17131798
De Bigault De Cazanove PF, Vdovchenko A, Rose RS, Resmini M. The Role of Artificial Weathering Protocols on Abiotic and Bacterial Degradation of Polyethylene. Polymers. 2025; 17(13):1798. https://doi.org/10.3390/polym17131798
Chicago/Turabian StyleDe Bigault De Cazanove, Pauline F., Alena Vdovchenko, Ruth S. Rose, and Marina Resmini. 2025. "The Role of Artificial Weathering Protocols on Abiotic and Bacterial Degradation of Polyethylene" Polymers 17, no. 13: 1798. https://doi.org/10.3390/polym17131798
APA StyleDe Bigault De Cazanove, P. F., Vdovchenko, A., Rose, R. S., & Resmini, M. (2025). The Role of Artificial Weathering Protocols on Abiotic and Bacterial Degradation of Polyethylene. Polymers, 17(13), 1798. https://doi.org/10.3390/polym17131798







