Thermal Characterization and Recycling of Polymers from Plastic Packaging Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. FTIR Analysis
2.2.2. Evolved Gas Analysis (EGA)
2.2.3. DSC Analysis
2.2.4. Recycling Method—Pyrolysis
3. Results and Discussion
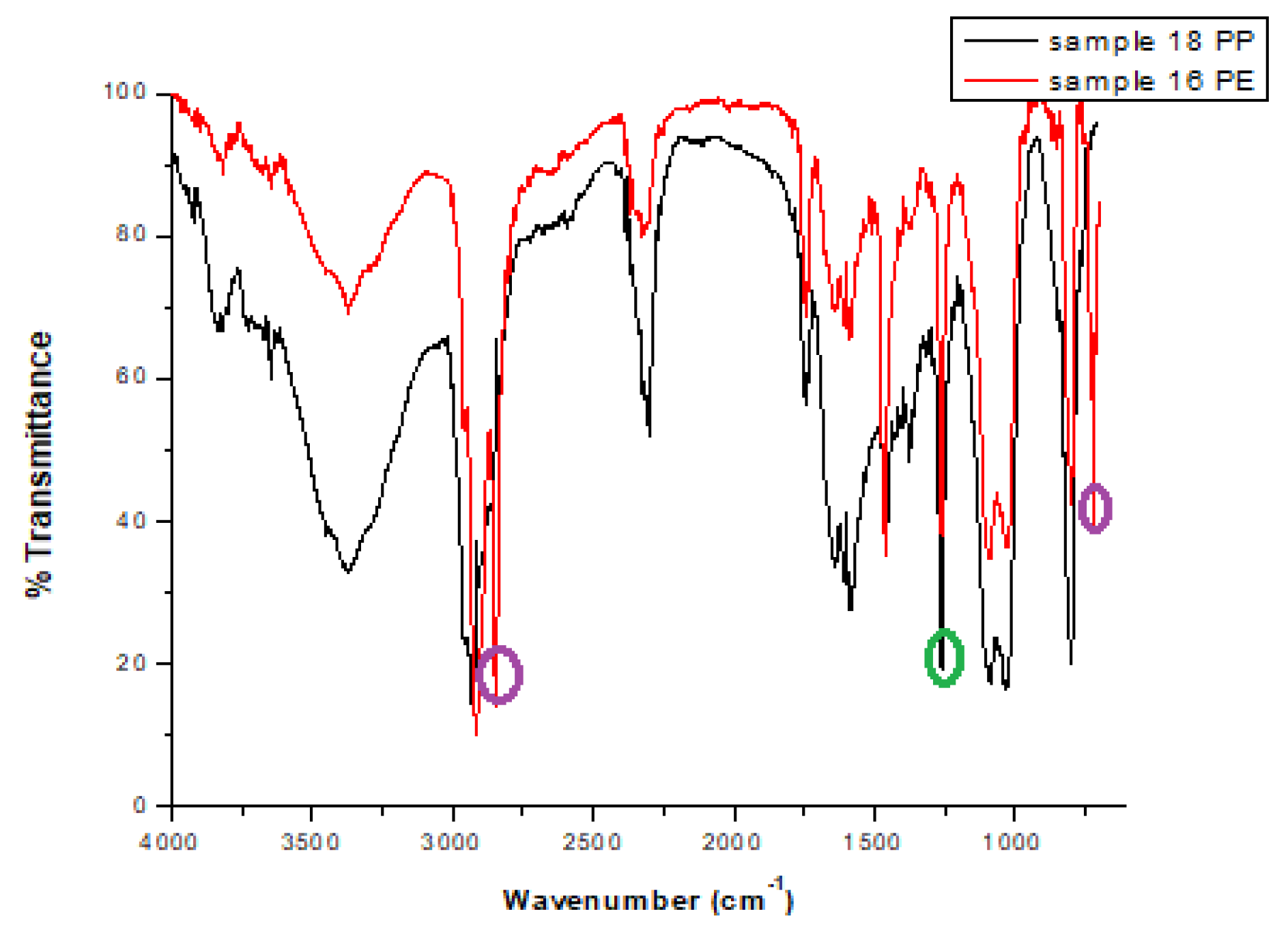
3.1. Spectroscopic Characterization
3.2. Thermal Characterization via Evolved Gas Analysis
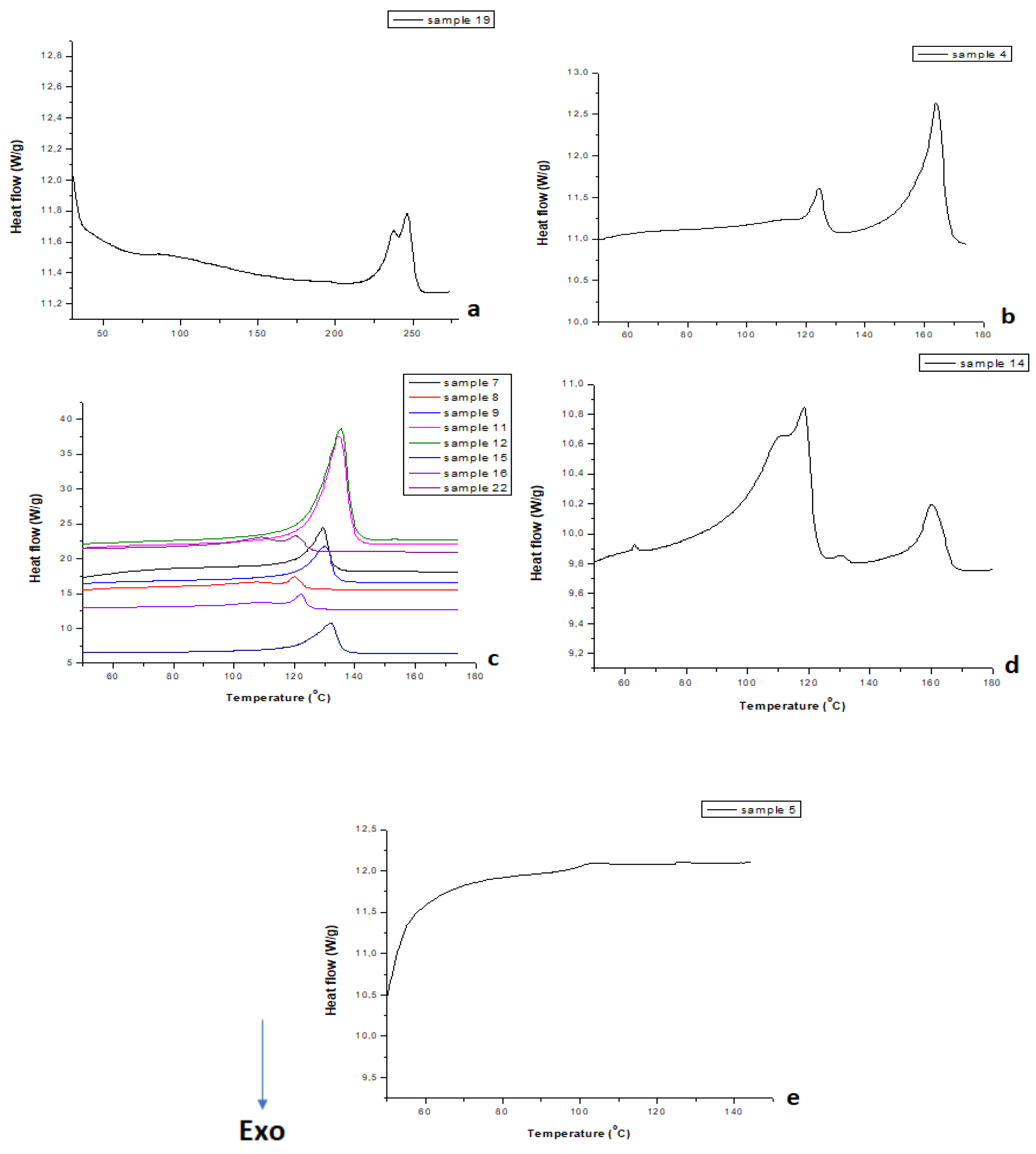
3.3. Thermal Characterization via DSC
3.4. Identification of the Samples Examined
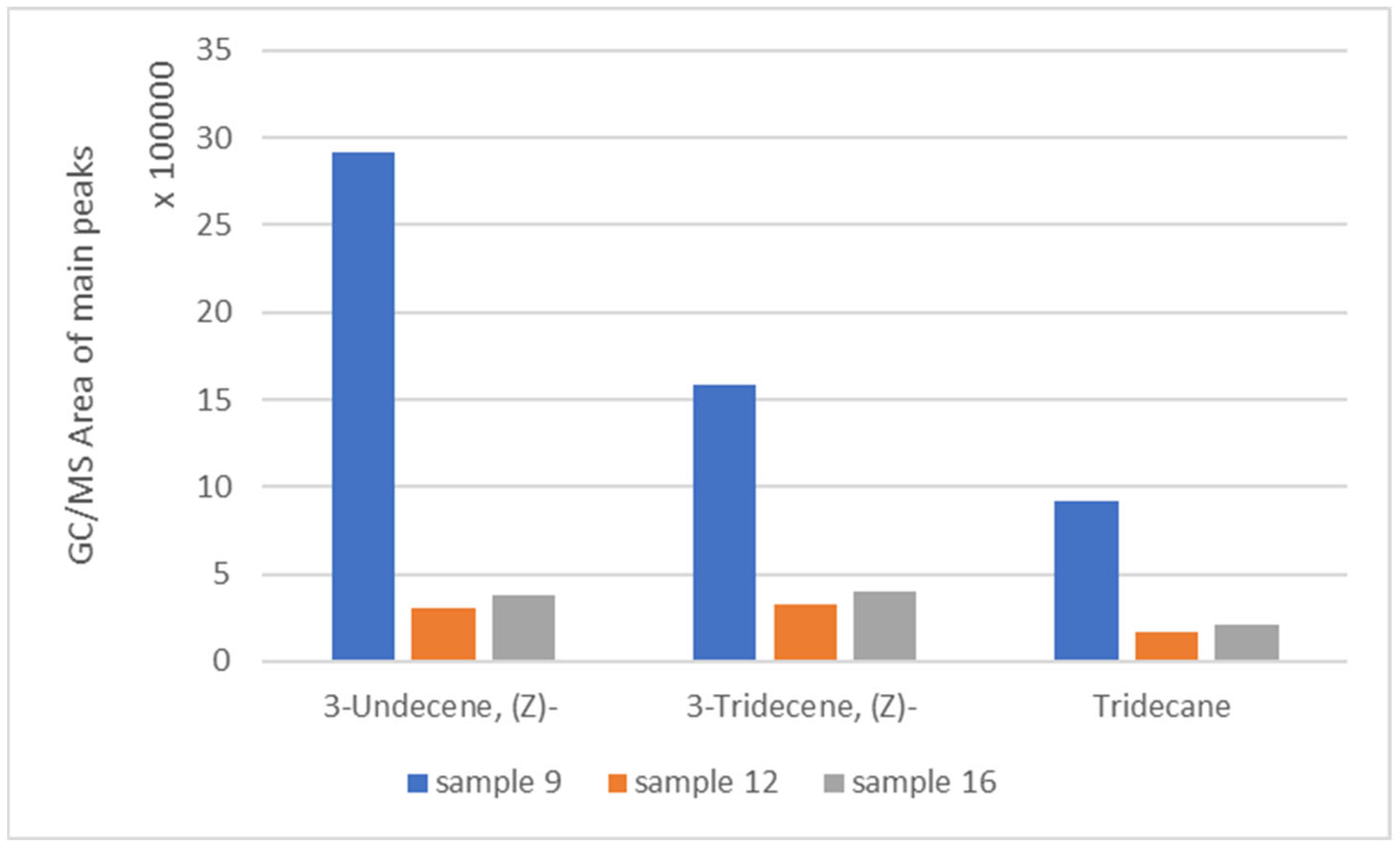
3.5. Pyrolysis Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A







References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Charitopoulou, M.A.; Alexopoulou, E.; Alexiou, P.; Achilias, D.S. Current Topics in Plastic Recycling. In Waste Material Recycling in the Circular Economy—Challenges and Developments; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total. Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- European Parliament. Plastic Waste and Recycling in the EU: Facts and Figures. 2024. Available online: https://www.europarl.europa.eu/topics/en/article/20181212STO21610/plastic-waste-and-recycling-in-the-eu-facts-and-figures (accessed on 14 May 2025).
- Crawford, R.J.; Martin, P.J. Plastics Engineering, 4th ed.; Butterworth-Heinemann–Elsevier: Oxford, UK, 2020. [Google Scholar]
- Geyer, R. Production, Use, and Fate of Synthetic Polymers, Plastic Waste and Recycling; Academic Press: Cambridge, MA, USA, 2020; pp. 13–32. [Google Scholar] [CrossRef]
- Hoseini, M.; Bond, T. Predicting the global environmental distribution of plastic polymers. Environ. Pollut. 2022, 300, 118966. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Leslie, H.A. Plastic Debris is a Human Health Issue. Environ. Sci. Technol. 2016, 50, 6825–6826. [Google Scholar] [CrossRef] [PubMed]
- Jeswani, H.K.; Azapagic, A. Assessing the environmental sustainability of energy recovery from municipal solid waste in the UK. Waste Manag. 2016, 50, 346–363. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Jeslin Drusila Nesamalar, J.; Venkatesh, P.; Charles Raja, S. The drive of renewable energy in Tamilnadu: Status, barriers and future prospect. Renew. Sustain. Energy Rev. 2017, 73, 115–124. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Monteiro, B.; Maria, L.; Cruz, A.; Carretas, J.M.; Marçalo, J.; Leal, J.P. Thermal stability and specific heats of coordinating ionic liquids. Thermochim. Acta 2020, 684, 178482. [Google Scholar] [CrossRef]
- Pereira, A.P.; Silva, M.; Édio, P., Jr.; Paula, A.; Tommasini, F. Processing and Characterization of PET Composites Reinforced with Geopolymer Concrete Waste. Mater. Res. 2017, 20, 411–420. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly(ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]
- Sukumaran, S.; Chen, R. Characterization of Contaminants in Recycled PET and HDPE Using FTIR Microscopy; Thermo Fisher Scientific: Waltham, MA USA, 2024. [Google Scholar]
- Endo, K.; Hayashi, K.; Ida, T.; Takemura, T. IR and Py–GC/MS Spectral Simulation of Polymer Film by Quantum Chemical and Quantum Molecular Dynamics Calculations Using the Polymer Models. Russ. J. Phys. Chem. A 2014, 88, 2370–2379. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless melt electrospinning of polypropylene nanofibers. J. Nanomater. 2012, 2012, 382639. [Google Scholar] [CrossRef]
- Abo, H. Distinction of Polyethylene and Polypropylene by Infrared Spectrum; Shimadzu Corporation: Kyoto, Japan, 2024. [Google Scholar]
- D’Amelia, R.; Gentile, S.; Nirode, W.; Huang, L. Quantitative Analysis of Copolymers and Blends of Polyvinyl Acetate (PVAc) Using Fourier Transform Infrared Spectroscopy (FTIR) and Elemental Analysis (EA). World J. Chem. Educ. 2016, 4, 25–31. [Google Scholar] [CrossRef]
- Al-Kadhemy, M.F.H.; Abaas, W.H.; Fakher, I. The Effect of Gamma Radiation on the FTIR Spectrum of Crystal Violet Doped Polystyrene Films. Casp. J. Appl. Sci. Res. 2013, 2, 11–17. [Google Scholar]
- Thinh, P.X.; Basavaraja, C.; Kim, D.G.; Huh, D.S. Characterization and electrochemical behaviors of honeycomb-patterned poly(N-vinylcarbazole)/polystyrene composite films. Polym. Bull. 2012, 69, 81–94. [Google Scholar] [CrossRef]
- Lauschke, T.; Dierkes, G.; Ternes, T.A. Challenges in the quantification of poly(ethylene terephthalate) microplastics via thermoanalytical methods posed by inorganic matrix components. J. Anal. Appl. Pyrolysis 2023, 174, 106108. [Google Scholar] [CrossRef]
- Vallada da Silva, D.; Moraes, C.A.M.; Santos da Silva, P.R. Thermal Pyrolysis of LDPE and LLDPE Films in Post-Consumer Packaging. Special Edition 10 years: Advances in environmental engineering. Rev. Eletrônica Gestão Educ. Tecnol. Ambient. 2020, 24, 1. [Google Scholar] [CrossRef]
- Demirel, B.; Yaraş, A.; ELÇİÇEK, H. Crystallization Behavior of PET Materials. BAÜ Fen Bil. Enst. Dergisi Cilt 2011, 13, 26–35. [Google Scholar]
- Jaziri, M.; Barhoumi, N.; Massardier, V.; Mélis, F. Blending PP with PA6 industrial wastes: Effect of the composition and the compatibilization. J. Appl. Polym. Sci. 2008, 107, 3451–3458. [Google Scholar] [CrossRef]
- Achilias, D.S.; Giannoulis, A.; Papageorgiou, G.Z. Recycling of polymers from plastic packaging materials using the dissolution–reprecipitation technique. Polym. Bull. 2009, 63, 449–465. [Google Scholar] [CrossRef]
- Najmi, S.; Vance, B.C.; Selvam, E.; Huang, D.; Vlachos, D.G. Controlling PET oligomers vs monomers via microwave-induced heating and swelling. Chem. Eng. J. 2023, 471, 144712. [Google Scholar] [CrossRef]
- Antonakou, E.V.; Kalogiannis, K.G.; Stephanidis, S.D.; Triantafyllidis, K.S.; Lappas, A.A.; Achilias, D.S. Pyrolysis and catalytic pyrolysis as a recycling method of waste CDs originating from polycarbonate and HIPS. Waste Manag. 2014, 34, 2487–2493. [Google Scholar] [CrossRef]
- Brebu, M.; Bhaskar, T.; Murai, K.; Muto, A.; Sakata, Y.; Uddin, M.A. Removal of nitrogen, bromine, and chlorine from PP/PE/PS/PVC/ABS-Br pyrolysis liquid products using Fe- and Ca-based catalysts. Polym. Degrad. Stab. 2005, 87, 225–230. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Papadopoulou, L.; Achilias, D.S. Effect of brominated flame retardant on the pyrolysis products of polymers originating in WEEE. Environ. Sci. Pollut. Res. 2022, 29, 29570–29582. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]





| Sample Description | Suspected Polymer Type | Sample Category | Sample Name |
|---|---|---|---|
| See-through cap (of food container) | PET | Food packaging | Sample 1 |
| Disposable plastic cup (drink container) | PS | Food packaging | Sample 2 |
| See-through cap | PP | Food packaging | Sample 3 |
| White cap | PP | Food packaging | Sample 4 |
| Black matte (coffee drink) cap | PS | Food packaging | Sample 5 |
| Black glossy (coffee drink) cap | PS | Food packaging | Sample 6 |
| Small transparent food bag | PE | Food packaging | Sample 7 |
| Small transparent zip lock bag | PE | Food packaging | Sample 8 |
| Transparent food bag | PE | Food packaging | Sample 9 |
| Disposable water bottle (firm 1) | PET | Food packaging | Sample 10 |
| Water bottle cap (firm 1) | PE | Food packaging | Sample 11 |
| Water bottle cap (firm 2) | PE | Food packaging | Sample 12 |
| Disposable water bottle (firm 2) | PET | Food packaging | Sample 13 |
| Frozen food packaging | PE | Food packaging | Sample 14 |
| Face cream packaging | PE | Non-food packaging | Sample 15 |
| Diaper packaging | PE | Non-food packaging | Sample 16 |
| Yogurt pot (firm 1) | PS | Food packaging | Sample 17 |
| Yogurt pot (firm 2) | PP | Food packaging | Sample 18 |
| Yogurt lid | PET | Food packaging | Sample 19 |
| Shampoo bottle | PET | Non-food packaging | Sample 20 |
| Face wash bottle | PP | Non-food packaging | Sample 21 |
| Sanitary pad packaging | PE | Non-food packaging | Sample 22 |
| Shampoo bottle cap | PP | Non-food packaging | Sample 23 |
| Sample Name | Tmax (°C) |
|---|---|
| Sample 1 | 444 ± 1 |
| Sample 2 | 423 ± 2 |
| Sample 3 | 466 ± 2 |
| Sample 4 | 468 ± 3 |
| Sample 5 | 440 ± 1 |
| Sample 6 | 444 ± 1 |
| Sample 7 | 482 ± 2 |
| Sample 8 | 480 ± 1 |
| Sample 9 | 481 ± 1 |
| Sample 10 | 443 ± 1 |
| Sample 11 | 489 ± 2 |
| Sample 12 | 485 ± 3 |
| Sample 13 | 444 ± 1 |
| Sample 14 | 476 ± 2 |
| Sample 15 | 491 ± 1 |
| Sample 16 | 482 ± 1 |
| Sample 17 | 433 ± 3 |
| Sample 18 | 456 ± 3 |
| Sample 19 | 444 ± 1 |
| Sample 20 | 443 ± 1 |
| Sample 21 | 463 ± 2 |
| Sample 22 | 488 ± 2 |
| Sample 23 | 468 ± 1 |
| Sample Name | Tm (°C) | ΔHm (J/g) | Tc (°C) | PE Type |
|---|---|---|---|---|
| Sample 7 | 129.5 | 141.6 | 115 | HDPE |
| Sample 8 | 109; 120.3 | 114.4 | 107 | LDPE/LLDPE |
| Sample 9 | 129.4 | 139.8 | 115 | HDPE |
| Sample 11 | 134 | 160.2 | 117 | HDPE |
| Sample 12 | 135 | 162.1 | 116 | HDPE |
| Sample 15 | 132.2 | 144.2 | 115 | HDPE |
| Sample 16 | 109; 122 | 122.5 | 108 | LDPE/LLDPE |
| Sample 22 | 110; 121 | 118.7 | 105 | LDPE/LLDPE |
| Sample Name | Polymer Type |
|---|---|
| Samples 1, 10, 13, 19, and 20 | PET |
| Samples 3, 4, 18, 21, and 23 | PP |
| Samples 8, 16, and 22 | LDPE/LLDPE |
| Samples 7, 9, 11, 12, and 15 | HDPE |
| Samples 2, 5, 6, and 17 | PS |
| Sample 14 | PP/LLDPE |
| Sample—Polymer Type | Pyrolysis Products | Pyrolysis Temperature—Tmax (°C) |
|---|---|---|
| Sample 1—PET | Benzoic acid (8.7 min); Methyl terephthalate (13.5 min); Vinyl benzoate (17.8 min); 2-Formylbenzoic acid (21.6 min) | 444 |
| Sample 2—PS | Styrene (2.9 min); 3-Butynylbenzene (13.4 min); Cyclohexane, 1,3,5-triphenyl- (20.2 min) | 423 |
| Sample 3—PP | Cyclobutane (1.3 min); 2-Octene (2.3 min); 1-Nonene, 4,6,8-trimethyl- (8.2 min); 1,6-Octadiene, 5,7-dimethyl- (9.1 min); 1-Undecene, 7-methyl- (10.9 min); 1-Decene, 5-methyl- (11.8 min); Cyclooctane, 1,4-dimethyl-, cis- (14.1 min); Cyclopentane, 1,2-dibutyl- (20 min) | 466 |
| Sample 9—PE | 1,6-Octadiene (6.5 min); Octane (6.8 min); 2-Octene (8.1 min); 3-Undecene, (Z)- (9.4 min); 3-Tridecene, (Z)- (10.7 min); Tridecane (14.2 min) | 481 |
| Sample 14—PE/PP Blend | 2-Octene (2.2 min); 1-Nonene (8.2 min); 3-Decene (9.3 min); 4-Dodecene, (E)- (10.6 min); Undecane, 4-methyl- (10.7 min); 3-Tridecene, (Z)- (10.9 min); 3-Tetradecene, (E)- (16.0 min); Tridecane, 3-ethyl- (16.1 min) | 476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charitopoulou, M.-A.; Koutroumpi, S.; Achilias, D.S. Thermal Characterization and Recycling of Polymers from Plastic Packaging Waste. Polymers 2025, 17, 1786. https://doi.org/10.3390/polym17131786
Charitopoulou M-A, Koutroumpi S, Achilias DS. Thermal Characterization and Recycling of Polymers from Plastic Packaging Waste. Polymers. 2025; 17(13):1786. https://doi.org/10.3390/polym17131786
Chicago/Turabian StyleCharitopoulou, Maria-Anna, Stavri Koutroumpi, and Dimitrios S. Achilias. 2025. "Thermal Characterization and Recycling of Polymers from Plastic Packaging Waste" Polymers 17, no. 13: 1786. https://doi.org/10.3390/polym17131786
APA StyleCharitopoulou, M.-A., Koutroumpi, S., & Achilias, D. S. (2025). Thermal Characterization and Recycling of Polymers from Plastic Packaging Waste. Polymers, 17(13), 1786. https://doi.org/10.3390/polym17131786







