Evaluation of Imidazolium Ionenes: Solid–Solid Phase Change Materials as Heat Sinks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization Techniques
2.2. Synthesis of Diimidazole Monomers and Ionenes
2.3. Characterization
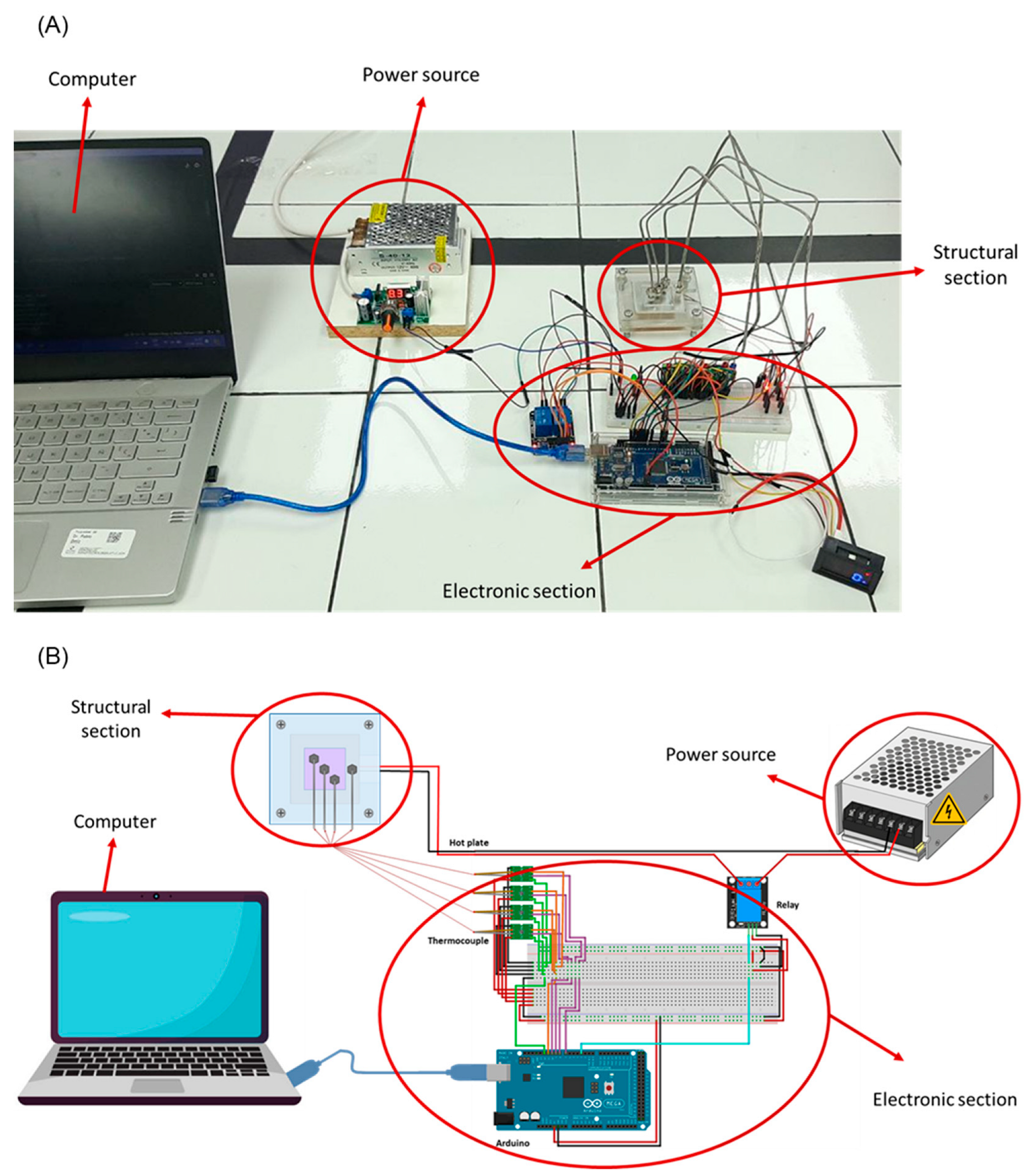
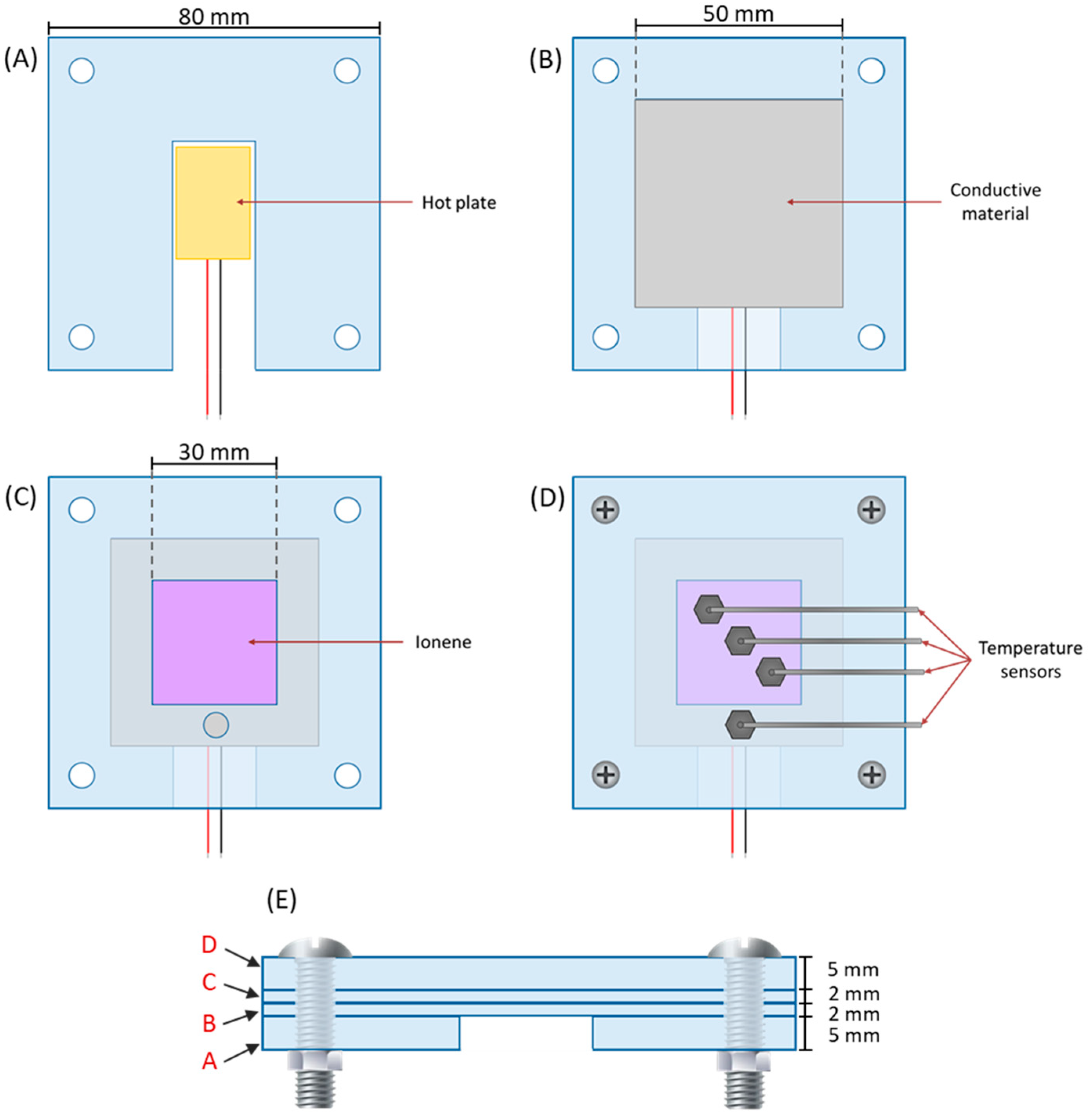
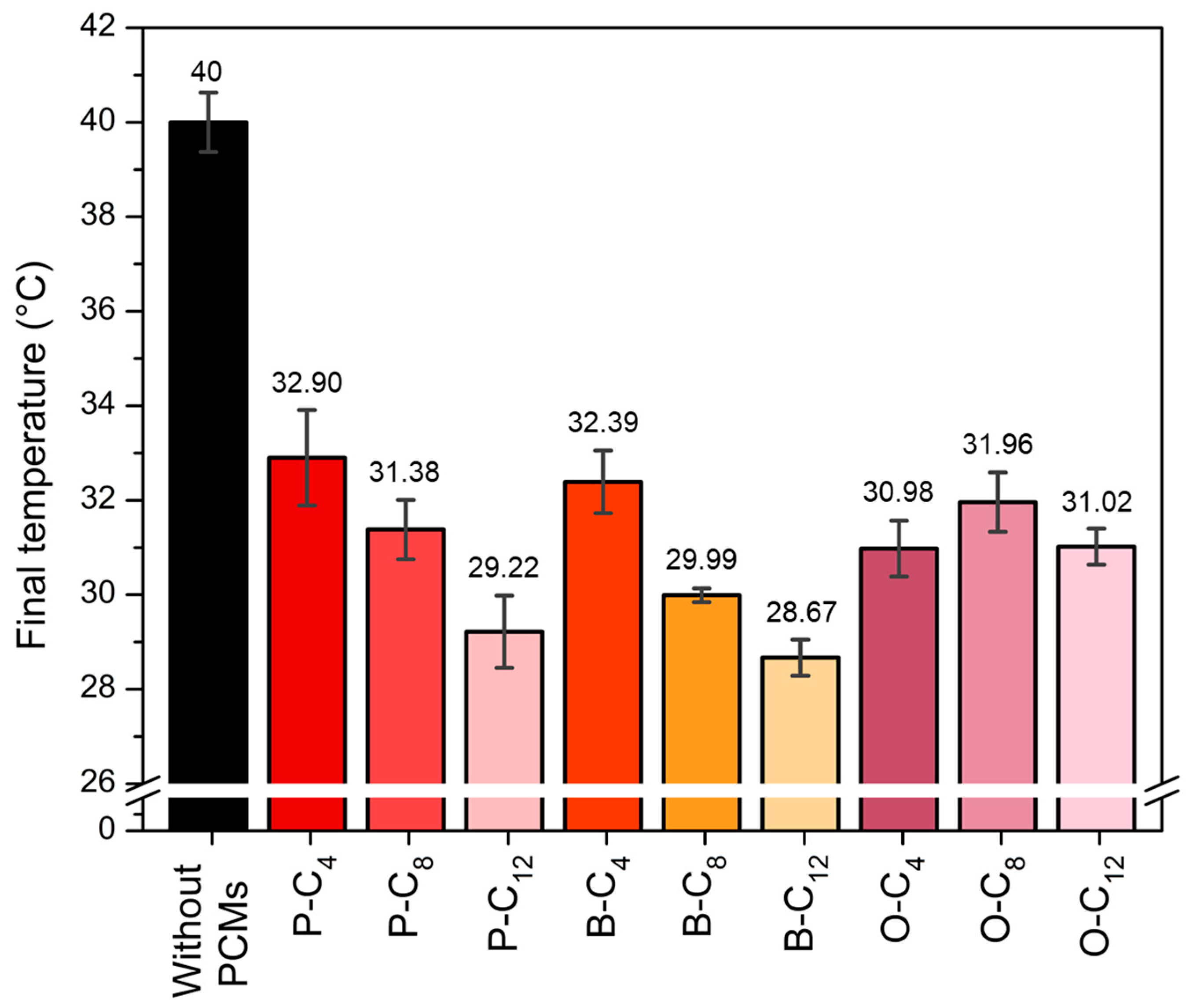
2.4. Evaluation of Ionenes as Heat Sinks
3. Results and Discussion
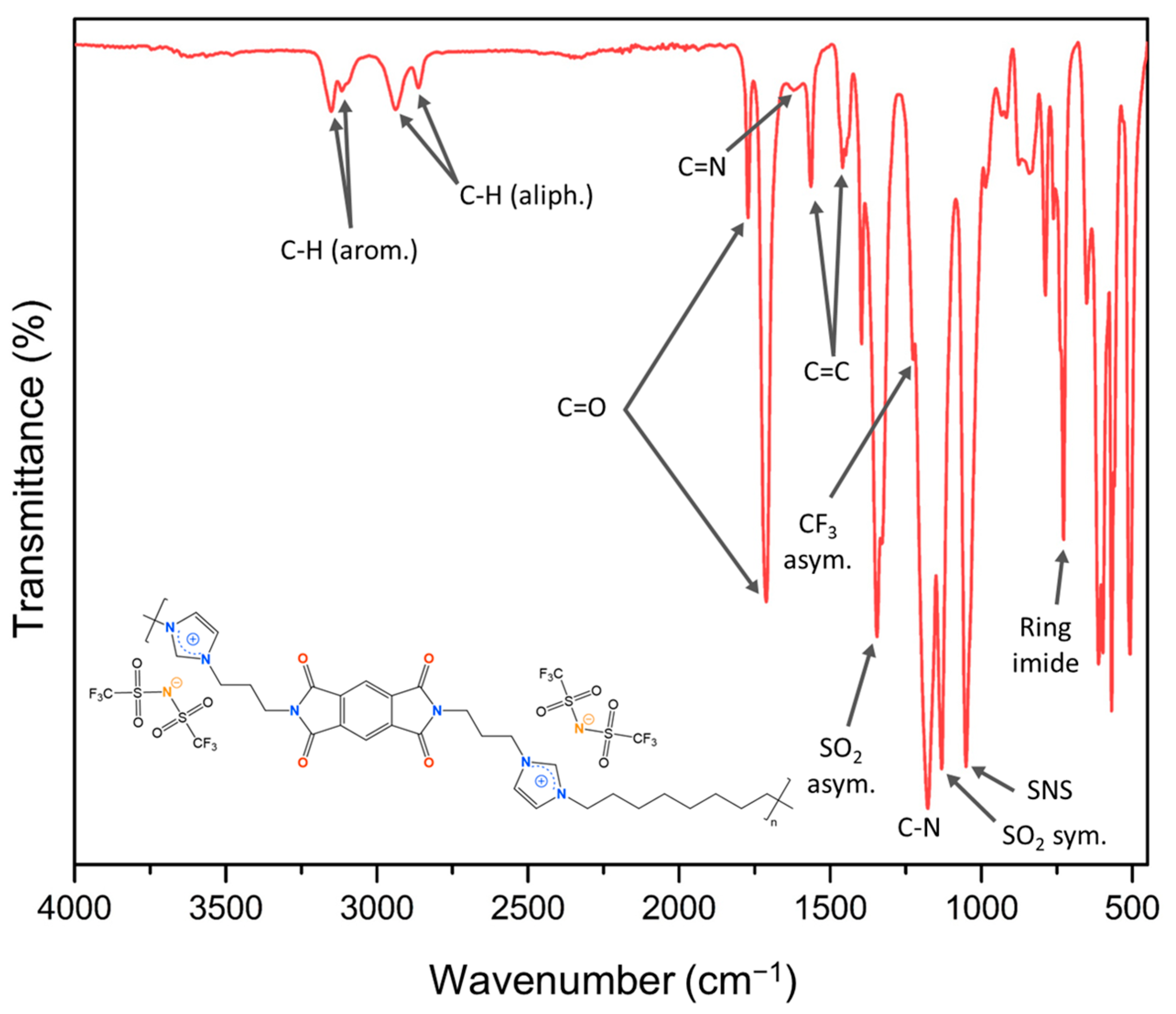
3.1. Synthesis and Structural Characterization of Ionenes
3.2. Properties of Ionenes
3.2.1. Solubility
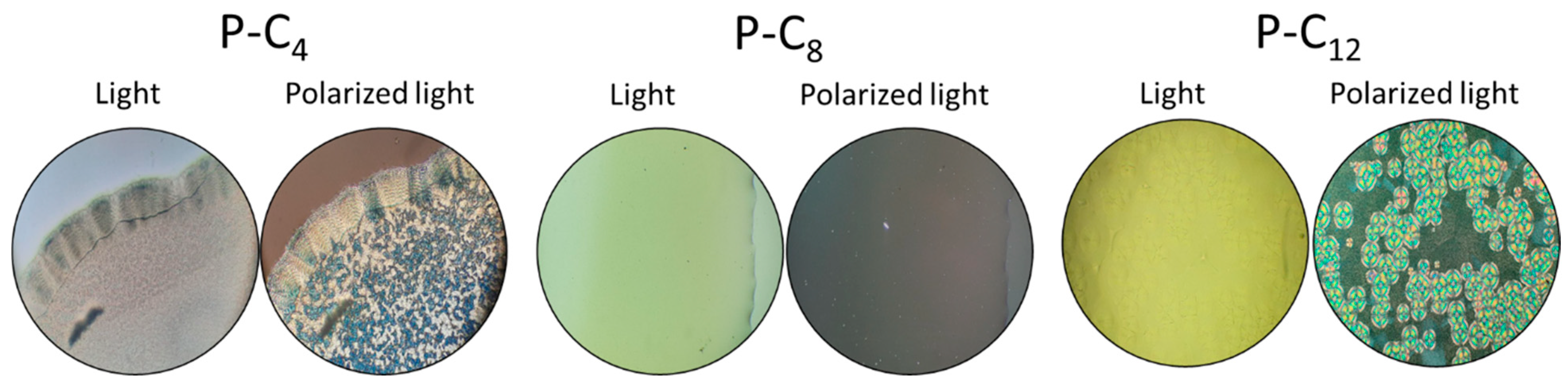
3.2.2. Crystalline Structure
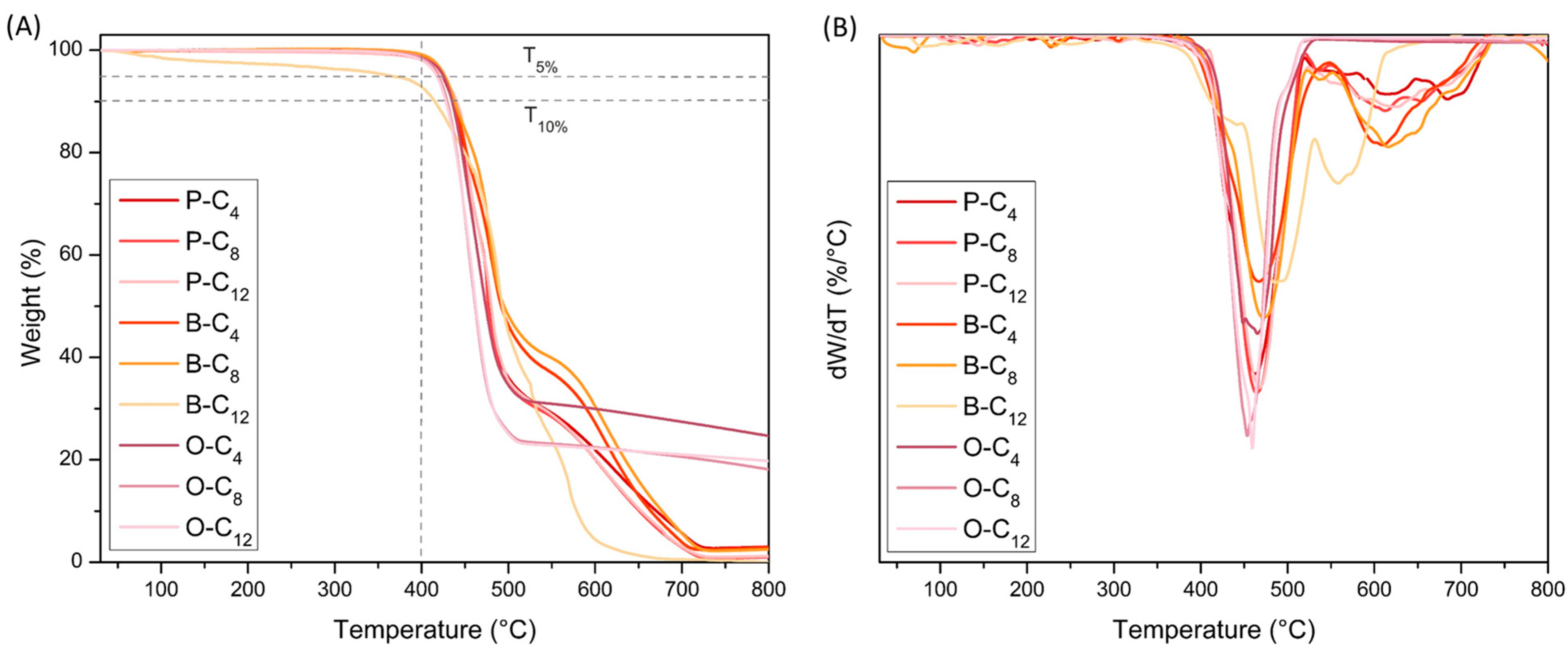
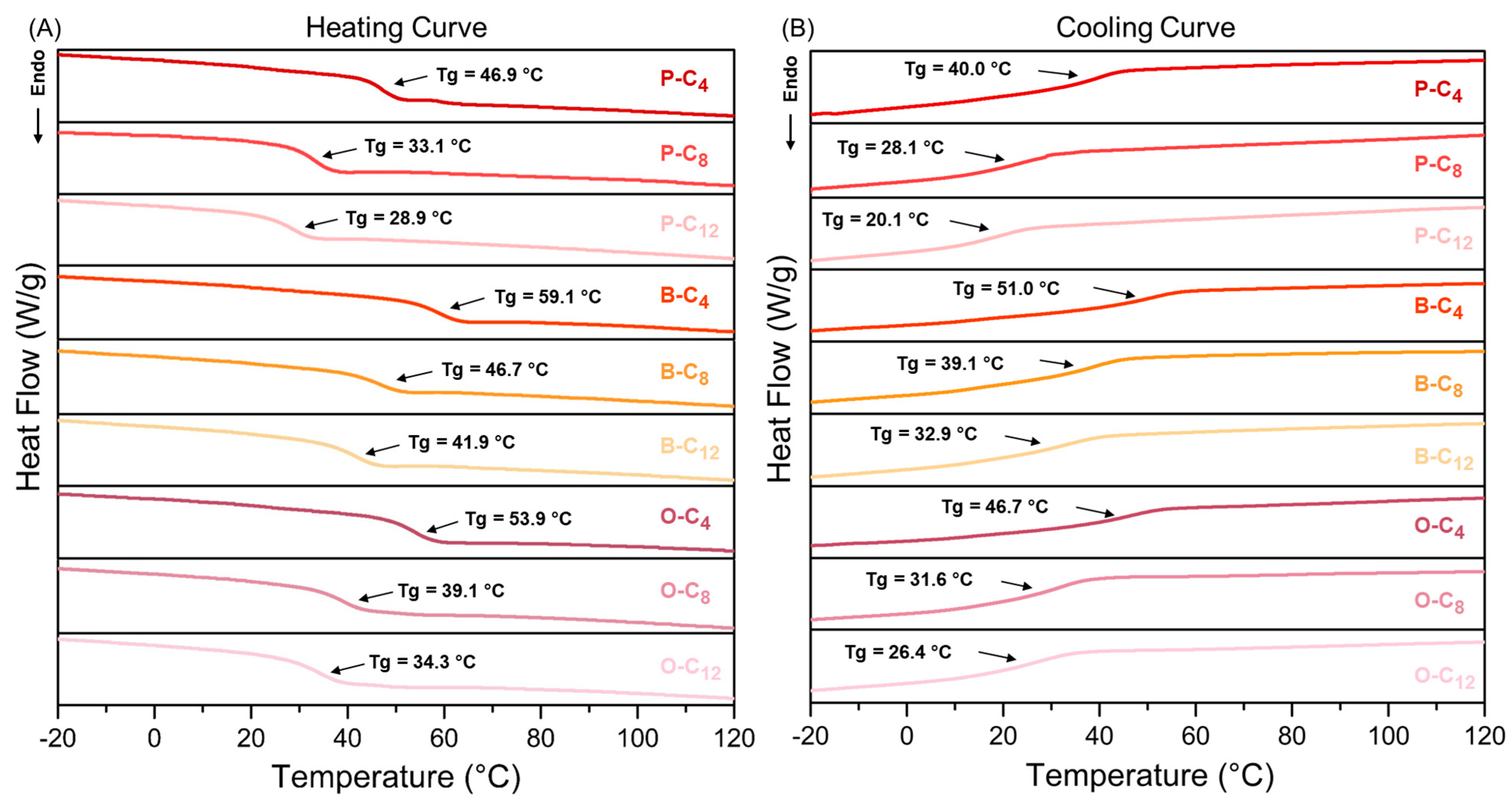
3.2.3. Thermal Properties
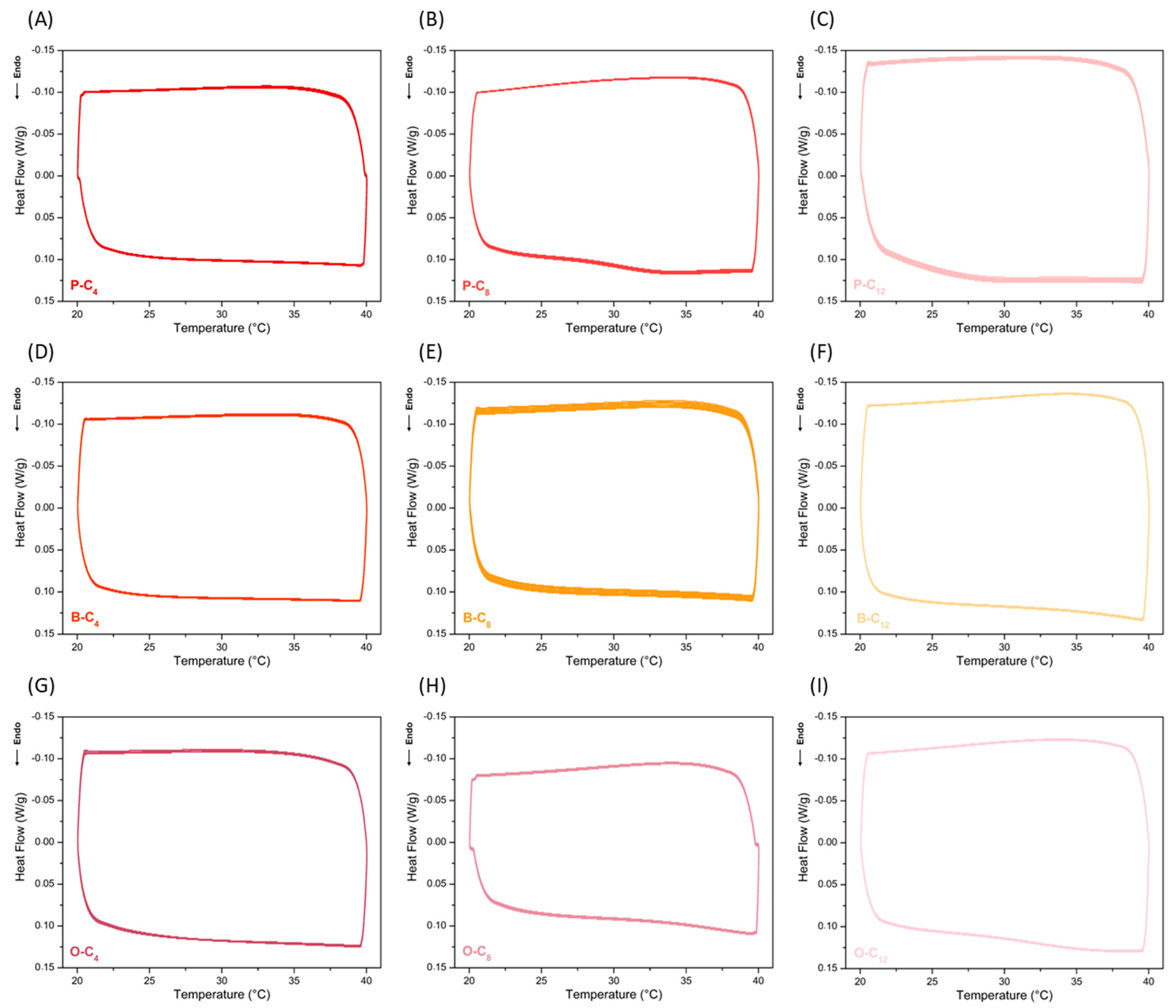
3.3. Characterization of Ionenes as SS-PCMs in Heat Sinks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filipovic, L.; Grasser, T. Special Issue on Miniaturized Transistors, Volume II. Micromachines 2022, 13, 603. [Google Scholar] [CrossRef] [PubMed]
- Kurhade, A.S.; Darade, M.M.; Siraskar, G.D.; Biradar, R.; Mahajan, R.G.; Kardile, C.S.; Waware, S.Y.; Yadav, R.S. State-of-the-Art Cooling Solutions for Electronic Devices Operating in Harsh Conditions. J. Mines Met. Fuels 2024, 72, 843–861. [Google Scholar] [CrossRef]
- Bailey, C. Modelling the effect of temperature on product reliability. In Proceedings of the Ninteenth Annual IEEE Semiconductor Thermal Measurement and Management Symposium, San Jose, CA, USA, 11–13 March 2003; IEEE: Piscataway, NJ, USA, 2003; pp. 324–331. [Google Scholar]
- Zhang, T.; Mo, Z.; Xu, X.; Liu, X.; Chen, H.; Han, Z.; Yan, Y.; Jin, Y. Advanced study of spray cooling: From theories to applications. Energies 2022, 15, 9219. [Google Scholar] [CrossRef]
- Nadjahi, C.; Louahlia, H.; Lemasson, S. A review of thermal management and innovative cooling strategies for data center. Sustain. Comput. Inform. Syst. 2018, 19, 14–28. [Google Scholar] [CrossRef]
- Savitha, Y.L.; Nanjundappa, C.E.; Shivakumara, I.S. Marangoni convection in a dielectric fluid layer with an AC electric field and nonuniform volumetric heat source due to incident radiation. Heat Transf. 2023, 52, 4529–4546. [Google Scholar] [CrossRef]
- Kumar, C.A.; Kumar, P.M. Review on electronics cooling systems. Adv. Nat. Appl. Sci. 2017, 11, 271–280. [Google Scholar]
- Rahman, M.A.; Hasnain, S.M.; Paramasivam, P.; Ayanie, A.G. Advancing thermal management in electronics: A review of innovative heat sink designs and optimization techniques. RSC Adv. 2024, 14, 31291–31319. [Google Scholar] [CrossRef]
- Ordu, M.; Der, O. Polymeric materials selection for flexible pulsating heat pipe manufacturing using a comparative hybrid MCDM approach. Polymers 2023, 15, 2933. [Google Scholar] [CrossRef]
- Baiju, K.G.; Nandanwar, M.N.; Jayanarayanan, K.; Kumaresan, D. Numerical modelling and simulation of heat sink assisted thermal sintering of titania film on polymer substrates for the fabrication of high-performance flexible dye sensitized solar cells. Chem. Eng. Res. Des. 2022, 181, 209–219. [Google Scholar] [CrossRef]
- Rakshith, B.L.; Asirvatham, L.G.; Angeline, A.A.; Raj, J.A.P.S.; Bose, J.R.; Princess, P.J.B.; Gautam, S.; Mahian, O.; Ribatski, G.; Wongwises, S. Cooling of power electronic devices using rectangular flat heat pipes with externally and internally cooled condenser regions. Appl. Therm. Eng. 2024, 236, 121474. [Google Scholar] [CrossRef]
- Zahid, I.; Farhan, M.; Farooq, M.; Asim, M.; Imran, M. Experimental investigation for thermal performance enhancement of various heat sinks using Al2O3 NePCM for cooling of electronic devices. Case Stud. Therm. Eng. 2023, 41, 102553. [Google Scholar] [CrossRef]
- Ibrahim, A.; Salem, M.; Kamarol, M.; Delgado, M.T.; Desa, M.K.M. Review of active thermal control for power electronics: Potentials, limitations, and future trends. IEEE Open J. Power Electron. 2024, 5, 414–435. [Google Scholar] [CrossRef]
- Abedrabboh, O.; Koç, M.; Biçer, Y. Sustainability performance of space-cooling technologies and approaches. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 9017–9042. [Google Scholar] [CrossRef]
- Ersoy, K. Review of Electronic Cooling and Thermal Management in Space and Aerospace Applications. Eng. Proc. 2025, 89, 42. [Google Scholar]
- Italia, R. An Analysis of Heat Dissipation Techniques in Power Electronics. J. Eng. Appl. Sci. Technol. 2023, 5, 1–6. [Google Scholar]
- Zhang, X.; Yu, C.; Zhang, C. Advances in latent heat storage technology for electronic cooling. Renew. Sustain. Energy Rev. 2025, 215, 115614. [Google Scholar] [CrossRef]
- Martínez, F.R.; Borri, E.; Kala, S.M.; Ushak, S.; Cabeza, L.F. Phase change materials for thermal energy storage in industrial applications. Heliyon 2025, 11, e41025. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Nejat, P.; Fekri, Y.; Jomehzadeh, F. Phase change material (PCM) as the smart heat-storing concept: A brief review. Ann. Mar. Sci. 2022, 6, 34–38. [Google Scholar]
- McCord, M.R.Y.; Baniasadi, H. Advancements in form-stabilized phase change materials: Stabilization mechanisms, multifunctionalities, and applications-a comprehensive review. Mater. Today Energy 2024, 41, 101532. [Google Scholar] [CrossRef]
- Cheng, P.; Tang, Z.; Gao, Y.; Liu, P.; Liu, C.; Chen, X. Flexible engineering of advanced phase change materials. iScience 2022, 25, 104226. [Google Scholar] [CrossRef] [PubMed]
- Nandan, R.; Arumuru, V.; Das, M.K. PCM-based heat sink for thermal management of electronic chips. In Handbook of Thermal Management Systems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 703–725. [Google Scholar]
- Husainy, A.S.; Funde, A.M.; Sonalkar, A.B.; Mulla, S.I.; Gote, R.S. Review on PCM heat sink for electronic thermal management application. Asian Rev. Mech. Eng. 2023, 12, 9–14. [Google Scholar] [CrossRef]
- Ali, H.M.; Arshad, A.; Jabbal, M.; Verdin, P.G. Thermal management of electronics devices with PCMs filled pin-fin heat sinks: A comparison. Int. J. Heat Mass Transf. 2018, 117, 1199–1204. [Google Scholar] [CrossRef]
- Afaynou, I.; Faraji, H.; Choukairy, K.; Arshad, A.; Arıcı, M. Heat transfer enhancement of phase-change materials (PCMs) based thermal management systems for electronic components: A review of recent advances. Int. Commun. Heat Mass Transf. 2023, 143, 106690. [Google Scholar] [CrossRef]
- Lawag, R.A.; Ali, H.M. Phase change materials for thermal management and energy storage: A review. J. Energy Storage 2022, 55, 105602. [Google Scholar] [CrossRef]
- Huang, J.; Luo, Y.; Weng, M.; Yu, J.; Sun, L.; Zeng, H.; Liu, Y.; Zeng, W.; Min, Y.; Guo, Z. Advances and applications of phase change materials (PCMs) and PCMs-based technologies. ES Mater. Manuf. 2021, 13, 23–39. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [PubMed]
- Barbi, S.; Barbieri, F.; Marinelli, S.; Rimini, B.; Merchiori, S.; Bottarelli, M.; Montorsi, M. Phase change material evolution in thermal energy storage systems for the building sector, with a focus on ground-coupled heat pumps. Polymers 2022, 14, 620. [Google Scholar] [CrossRef]
- Elshaer, A.M.; Soliman, A.M.A.; Kassab, M.; Hawwash, A.A. Boosting the thermal management performance of a PCM-based module using novel metallic pin fin geometries: Numerical study. Sci. Rep. 2023, 13, 10955. [Google Scholar] [CrossRef]
- Adnin, R.J.; Lee, H.S. Advancing Thermal Energy Storage: Synthesis and Thermal Performance of Silica-Encapsulated Paraffin PCMs. Molecules 2025, 30, 1698. [Google Scholar] [CrossRef]
- Yang, Z.L.; Walvekar, R.; Wong, W.P.; Sharma, R.K.; Dharaskar, S.; Khalid, M. Advances in phase change materials, heat transfer enhancement techniques, and their applications in thermal energy storage: A comprehensive review. J. Energy Storage 2024, 87, 111329. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Palacios, A.; Navarro-Rivero, M.E.; Zou, B.; Jiang, Z.; Harrison, M.T.; Ding, Y. A perspective on Phase Change Material encapsulation: Guidance for encapsulation design methodology from low to high-temperature thermal energy storage applications. J. Energy Storage 2023, 72, 108597. [Google Scholar] [CrossRef]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados-Focil, S.; Van Dessel, S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- Usman, A.; Xiong, F.; Aftab, W.; Qin, M.; Zou, R. Emerging solid-to-solid phase-change materials for thermal-energy harvesting, storage, and utilization. Adv. Mater. 2022, 34, 2202457. [Google Scholar] [CrossRef]
- Zhi, M.; Yue, S.; Zheng, L.; Su, B.; Fu, J.; Sun, Q. Recent developments in solid-solid phase change materials for thermal energy storage applications. J. Energy Storage 2024, 89, 111570. [Google Scholar] [CrossRef]
- Raj, C.R.; Suresh, S.; Bhavsar, R.R.; Singh, V.K. Recent developments in thermo-physical property enhancement and applications of solid solid phase change materials: A review. J. Therm. Anal. Calorim. 2020, 139, 3023–3049. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Li, C.; Deng, J.; Huang, Q.; Jia, W.; Mao, Y.; Zou, Y.; Wu, Y.; Tian, J.; et al. Flexible solid-solid phase change material with zero leakage via in-situ preparation for battery thermal management. Innov. Energy 2024, 1, 100034. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Aftab, W. Advanced solid–solid phase change thermal storage material. Nano Res. Energy 2024, 3, e9120103. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, T.; Zhao, J.; Liu, Q.; Sun, W.; Guo, C.; Ali, H.M.; Chen, X.; Gu, Y. Polymer engineering in phase change thermal storage materials. Renew. Sustain. Energy Rev. 2023, 188, 113814. [Google Scholar] [CrossRef]
- Guo, X.; Wei, K.; Ni, T.; Shi, W.; Dai, C.; Zhao, Z.; Gu, Z. Preparation and performance analysis of polyethylene glycol/epoxy resin composite phase change material. J. Energy Storage 2024, 88, 111525. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Dai, J.; Yang, K.; Wang, S.; Liu, X. Integration of sustainable polymers with phase change materials. Prog. Mater. Sci. 2025, 151, 101447. [Google Scholar] [CrossRef]
- Paberit, R.; Rilby, E.; Gohl, J.; Swenson, J.; Refaa, Z.; Johansson, P.; Jansson, H. Cycling stability of poly (ethylene glycol) of six molecular weights: Influence of thermal conditions for energy applications. ACS Appl. Energy Mater. 2020, 3, 10578–10589. [Google Scholar] [CrossRef]
- Bejan, D.; Cojocariu, N.; Cherecheş, E.I.; Minea, A.A. Studies on several mixtures of PEG based phase change materials for heat transfer Applications: An experimental approach. J. Mol. Liq. 2025, 429, 127652. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, T.; Wei, Z.; Yuan, A.; Chen, Y.; Jiang, L.; Lei, J.; Fu, X. Polymeric phase change material networks based on multi-telechelic polyethylene glycol-derived multimer structures for thermal energy storage. Chem. Eng. J. 2023, 462, 142164. [Google Scholar] [CrossRef]
- Marcos, M.A.; Cabaleiro, D.; Guimarey, M.J.; Comuñas, M.J.; Fedele, L.; Fernández, J.; Lugo, L. PEG 400-based phase change materials nano-enhanced with functionalized graphene nanoplatelets. Nanomaterials 2017, 8, 16. [Google Scholar] [CrossRef]
- Kim, A.; Wert, N.A.; Gowd, E.B.; Patel, R. Recent progress in PEG-based composite phase change materials. Polym. Rev. 2023, 63, 1078–1129. [Google Scholar] [CrossRef]
- Bara, J.E.; O’Harra, K.E. Recent advances in the design of ionenes: Toward convergence with high-performance polymers. Macromol. Chem. Phys. 2019, 220, 1900078. [Google Scholar] [CrossRef]
- Hotton, C.; Sakhawoth, Y.; Rollet, A.L.; Sirieix-Plénet, J.; Tea, L.; Combet, S.; Sharp, M.; Hoffmann, I.; Nallet, F.; Malikova, N. Ion-specific effects in polyelectrolyte solutions: Chain–chain interactions, chain rigidity and dynamics. Comptes Rendus Chim. 2024, 27, 1–13. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Santos, D.M. Applications of polymer electrolytes in lithium-ion batteries: A review. Polymers 2023, 15, 3907. [Google Scholar] [CrossRef]
- O’Harra, K.; Kammakakam, I.; Shinde, P.; Giri, C.; Tuan, Y.; Jackson, E.M.; Bara, J.E. Poly (ether ether ketone) Ionenes: Ultrahigh-Performance Polymers Meet Ionic Liquids. ACS Appl. Polym. Mater. 2022, 4, 8365–8376. [Google Scholar] [CrossRef]
- Wanghofer, F.; Wolfberger, A.; Wolfahrt, M.; Schlögl, S. Cross-Linking and Evaluation of the Thermo-Mechanical Behavior of Epoxy Based Poly (ionic Liquid) Thermosets. Polymers 2021, 13, 3914. [Google Scholar] [CrossRef]
- Shin, J.C.; Kim, T.Y.; Choi, U.H.; Lee, M. Pyrrolidinium-Based Polyurethane Ionenes: Influence of Counterions, Chain Extenders, and PEG Blocks on Thermal Properties and Ion Conduction. ACS Appl. Polym. Mater. 2025, 7, 3067–3074. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Zhang, H.; Ji, X. Self-healing composite solid electrolytes with enhanced Li+ transport and mechanical properties for safe lithium metal batteries. Chem. Eng. J. 2022, 438, 135418. [Google Scholar] [CrossRef]
- Häring, M.; Grijalvo, S.; Haldar, D.; Saldías, C.; Díaz, D.D. Polymer topology-controlled self-healing properties of polyelectrolyte hydrogels based on DABCO-containing aromatic ionenes. Eur. Polym. J. 2019, 115, 221–224. [Google Scholar] [CrossRef]
- O’Harra, K.E.; Kammakakam, I.; Bara, J.E.; Jackson, E.M. Understanding the effects of backbone chemistry and anion type on the structure and thermal behaviors of imidazolium polyimide-ionenes. Polym. Int. 2019, 68, 1547–1556. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, Y. Poly (ionic liquid) s: An emerging platform for green chemistry. Green Chem. 2024, 26, 5022–5102. [Google Scholar] [CrossRef]
- Li, X.; Drockenmuller, E.; Stiernet, P.; Zhang, W.; Yuan, J. Poly (1, 2, 4-triazolium) s as the rising generation of functional poly (ionic liquid) s. Prog. Polym. Sci. 2025, 165, 101969. [Google Scholar] [CrossRef]
- Yang, B.; Yang, G.; Zhang, Y.M.; Zhang, S.X.A. Recent advances in poly (ionic liquid) s for electrochromic devices. J. Mater. Chem. C 2021, 9, 4730–4741. [Google Scholar] [CrossRef]
- Tröger-Müller, S.; Liedel, C. Sustainable polyimidazolium networks as versatile hydrogel materials. ACS Appl. Polym. Mater. 2019, 1, 2606–2612. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; Ezzat, A.O. Synthesis and application of geminal dicationic ionic liquids and poly (ionic liquids) combined imidazolium and pyridinium cations as demulsifiers for petroleum crude oil saline water emulsions. J. Mol. Liq. 2021, 325, 115264. [Google Scholar] [CrossRef]
- Corzo, B.A.; Hernández-Martínez, H.; Aldeco-Pérez, E.J.; Cárdenas, J.; Lara, V.; Olvera, L.I. Functionalized Imidazolium Ether-Free Polymer Backbones with Ion Transport Channels and Catalytic Activity. ACS Mater. Au. 2025, 5, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, E.; Dinarès, I.; Ibáñez, A.; Mesquida, N. A simple halide-to-anion exchange method for heteroaromatic salts and ionic liquids. Molecules 2012, 17, 4007–4027. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.G.; Matsumoto, A.; Shen, A.Q. Dilute polyelectrolyte solutions: Recent progress and open questions. Soft Matter 2024, 20, 2635–2687. [Google Scholar] [CrossRef]
- Arriaza-Echanes, C.; Velázquez-Tundidor, M.V.; Angel-López, A.; Norambuena, Á.; Palay, F.E.; Terraza, C.A.; Tundidor-Camba, A.; Ortiz, P.A.; Coll, D. Ionenes as Potential Phase Change Materials with Self-Healing Behavior. Polymers 2023, 15, 4460. [Google Scholar] [CrossRef]
- Zhuo, Y.; Cheng, H.L.; Zhao, Y.G.; Cui, H.R. Ionic liquids in pharmaceutical and biomedical applications: A review. Pharmaceutics 2024, 16, 151. [Google Scholar] [CrossRef]
- Gulati, A.; Douglas, J.F.; Matsarskaia, O.; Lopez, C.G. Influence of counterion type on the scattering of a semiflexible polyelectrolyte. Soft Matter 2024, 20, 8610–8620. [Google Scholar] [CrossRef]
- Fernández-d’Arlas, B.; Baumann, R.P.; Pöselt, E.; Müller, A.J. Influence of composition on the isothermal crystallisation of segmented thermoplastic polyurethanes. CrystEngComm 2017, 19, 4720–4733. [Google Scholar] [CrossRef]
- Muthukumar, M. Trends in polymer physics and theory. Prog. Polym. Sci. 2020, 100, 101184. [Google Scholar] [CrossRef]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Yang, Y.; Zou, X.; Ye, H.; Zhu, W.; Dong, H.; Bi, M. Modified group contribution scheme to predict the glass-transition temperature of homopolymers through a limiting property dataset. ACS Omega 2020, 5, 29538–29546. [Google Scholar] [CrossRef] [PubMed]
- Angel-López, A.; Norambuena, Á.; Arriaza-Echanes, C.; Terraza, C.A.; Tundidor-Camba, A.; Coll, D.; Ortiz, P.A. Development of Novel Phase-Change Materials Derived from Methoxy Polyethylene Glycol and Aromatic Acyl Chlorides. Polymers 2023, 15, 3069. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Niu, J.; Wu, J.Y. Preparation of stable phase change material emulsions for thermal energy storage and thermal management applications: A review. Materials 2021, 15, 121. [Google Scholar] [CrossRef]
- Ahmed, T.; Bhouri, M.; Groulx, D.; White, M.A. Passive thermal management of tablet PCs using phase change materials: Intermittent operation. Appl. Sci. 2019, 9, 902. [Google Scholar] [CrossRef]
- Ungar, E.; Stroud, K. A new approach to defining human touch temperature standards. In Proceedings of the 40th International Conference on Environmental Systems, Barcelona, Spain, 11–15 July 2010; p. 6310. [Google Scholar]
- Goo, B.; Kim, D.S. impact of Contactless Apoptosis-inducing RF on Temperature of Human Skin Surface and Subcutaneous layer as well as porcine Histology: A pilot Study. Med. Lasers 2016, 5, 29–33. [Google Scholar] [CrossRef][Green Version]
- Emam, M.; Ookawara, S.; Ahmed, M. Thermal management of electronic devices and concentrator photovoltaic systems using phase change material heat sinks: Experimental investigations. Renew. Energy 2019, 141, 322–339. [Google Scholar] [CrossRef]



| Solvent | P-C4 | P-C8 | P-C12 | B-C4 | B-C8 | B-C12 | O-C4 | O-C8 | O-C12 |
|---|---|---|---|---|---|---|---|---|---|
| Water | − | − | − | − | − | − | − | − | − |
| DMSO | + | + | + | + | + | + | + | + | + |
| DMF | + | + | + | + | + | + | + | + | + |
| Acetonitrile | +/− | + | + | + | + | + | + | + | + |
| Acetone | +/− | + | + | + | + | + | + | + | + |
| Ethanol | − | − | − | − | − | − | − | − | − |
| Methanol | − | − | − | − | − | − | − | − | − |
| Ethyl acetate | − | − | − | − | − | − | − | − | − |
| THF | − | − | − | − | − | − | − | − | − |
| Chloroform | − | − | − | − | − | − | − | − | − |
| Ethyl ether | − | − | − | − | − | − | − | − | − |
| Ionene | Tonset (°C) a | T5% (°C) b | T10% (°C) b | Td1 (°C) c | Td2 (°C) c | R800 (%) d |
|---|---|---|---|---|---|---|
| P-C4 | 426 | 424 | 436 | 462 | 618 | 2.98 |
| P-C8 | 430 | 426 | 438 | 464 | 614 | 1.01 |
| P-C12 | 432 | 424 | 440 | 468 | 626 | 1.18 |
| B-C4 | 424 | 427 | 438 | 467 | 606 | 2.83 |
| B-C8 | 427 | 427 | 438 | 473 | 618 | 2.52 |
| B-C12 | 421 | 365 | 415 | 491 | 561 | 0.01 |
| O-C4 | 427 | 425 | 435 | 459 | - | 24.7 |
| O-C8 | 429 | 419 | 429 | 453 | - | 18.1 |
| O-C12 | 428 | 417 | 428 | 459 | - | 19.8 |
| Heating Curve | Cooling Curve | ||||||
|---|---|---|---|---|---|---|---|
| Ionene | Tg (°C) | Cp (J/g°C) | ∆HTg (J/g) | Tg (°C) | Cp (J/g°C) | ∆HTg (J/g) | ∆Tg (°C) |
| P-C4 | 46.9 | 0.247 | 3.74 | 40.0 | 0.258 | 2.89 | 6.9 |
| P-C8 | 33.1 | 0.248 | 4.08 | 28.1 | 0.319 | 3.63 | 5.0 |
| P-C12 | 28.9 | 0.229 | 3.42 | 20.1 | 0.317 | 2.41 | 8.8 |
| B-C4 | 59.1 | 0.222 | 4.11 | 51.0 | 0.220 | 2.99 | 8.1 |
| B-C8 | 46.7 | 0.249 | 3.99 | 39.1 | 0.299 | 3.39 | 7.6 |
| B-C12 | 41.9 | 0.281 | 5.00 | 32.9 | 0.362 | 4.44 | 9.0 |
| O-C4 | 53.9 | 0.243 | 3.24 | 46.7 | 0.247 | 2.23 | 7.2 |
| O-C8 | 39.1 | 0.307 | 3.87 | 31.6 | 0.341 | 3.37 | 7.5 |
| O-C12 | 34.3 | 0.294 | 4.51 | 26.4 | 0.333 | 3.28 | 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arriaza-Echanes, C.; Krüger, G.I.; Comesaña-Gándara, B.; Terraza, C.A.; Sanhueza, L.; Ortiz, P.A. Evaluation of Imidazolium Ionenes: Solid–Solid Phase Change Materials as Heat Sinks. Polymers 2025, 17, 1782. https://doi.org/10.3390/polym17131782
Arriaza-Echanes C, Krüger GI, Comesaña-Gándara B, Terraza CA, Sanhueza L, Ortiz PA. Evaluation of Imidazolium Ionenes: Solid–Solid Phase Change Materials as Heat Sinks. Polymers. 2025; 17(13):1782. https://doi.org/10.3390/polym17131782
Chicago/Turabian StyleArriaza-Echanes, Carolina, Gabriel I. Krüger, Bibiana Comesaña-Gándara, Claudio A. Terraza, Loreto Sanhueza, and Pablo A. Ortiz. 2025. "Evaluation of Imidazolium Ionenes: Solid–Solid Phase Change Materials as Heat Sinks" Polymers 17, no. 13: 1782. https://doi.org/10.3390/polym17131782
APA StyleArriaza-Echanes, C., Krüger, G. I., Comesaña-Gándara, B., Terraza, C. A., Sanhueza, L., & Ortiz, P. A. (2025). Evaluation of Imidazolium Ionenes: Solid–Solid Phase Change Materials as Heat Sinks. Polymers, 17(13), 1782. https://doi.org/10.3390/polym17131782








