Solid-to-Solid Manufacturing Processes for High-Performance Li-Ion Solid-State Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Separator Design
2.3. Sample Composition
2.4. Cell Assembling
3. Results
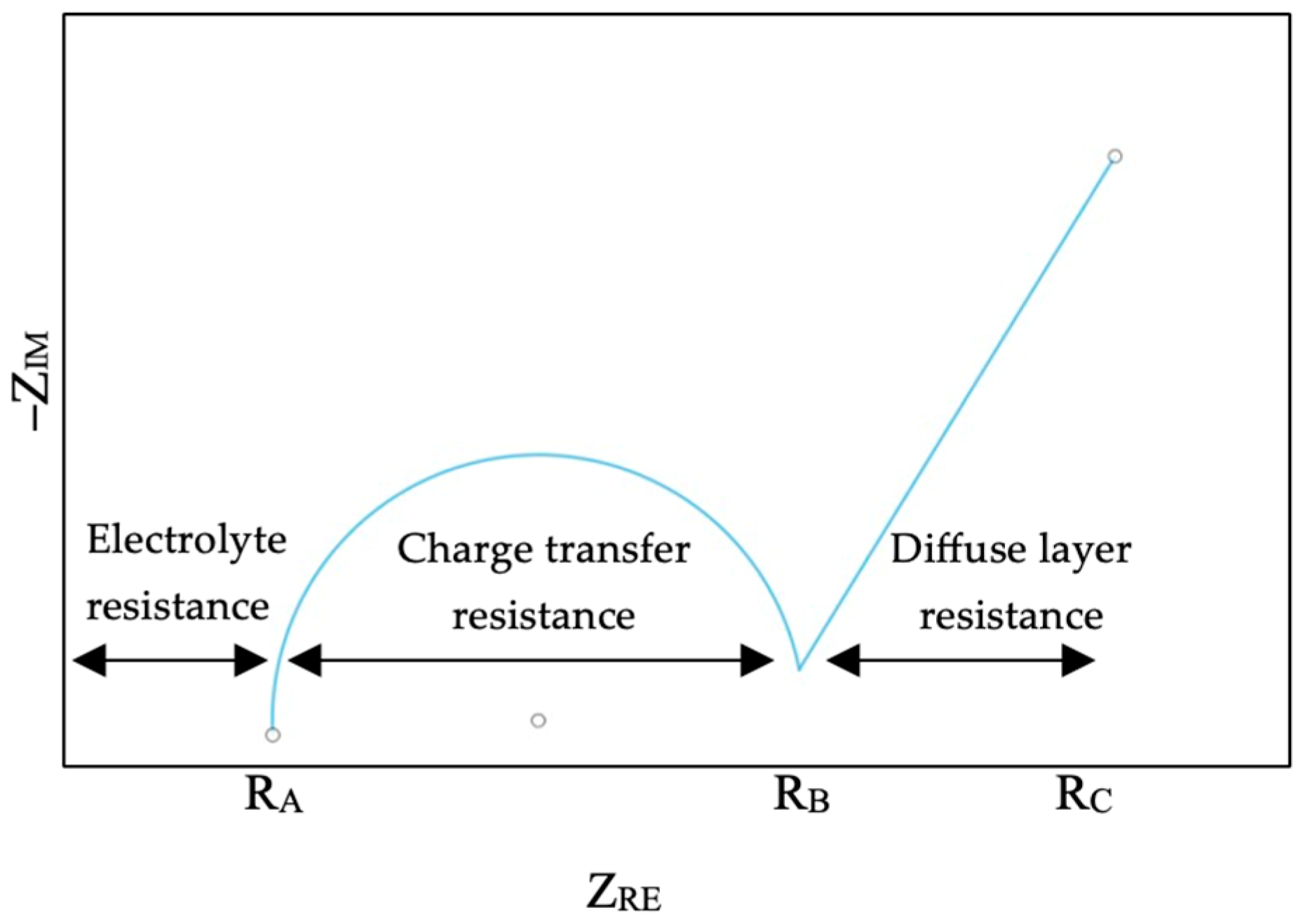
3.1. Electrochemical Impedance Spectroscopy (EIS) Analysis
3.2. Mechanical Property Analysis
4. Discussion
4.1. Ionic Conductivity
4.2. Mechanical Property
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSB | Solid-state battery |
| AM | Additive manufacturing |
| PLA | Polylactic acid |
| SE | Solid electrolyte |
| FFF | Fused filament fabrication |
| LiPF6 | Lithium hexafluorophosphate |
| EIS | Electrochemical impedance spectroscopy |
| I-C | Ionic conductivity |
References
- Chen, Y.; Kang, Y.; Zhao, Y. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Kuhn, H.; Seitz, A.; Lorenz, L.; Isikveren, A.T.; Sizmann, A. Progress and perspectives of electric air transport. In Proceedings of the 28th International Congress of the Aeronautical Sciences (ICAS 2012), Brisbane, Australia, 23–28 September 2012. [Google Scholar] [CrossRef]
- Airbus. Future of Electric Flight. Available online: https://www.airbus.com/en/newsroom/news/2017-06-future-of-electric-flight (accessed on 23 May 2024).
- IEA. Global EV Outlook 2024; IEA: Paris, France, 2025; Available online: https://www.iea.org/reports/global-ev-outlook-2025/executive-summary (accessed on 25 March 2025).
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Dammala, P.K.; Dermenci, K.B.; Kathribail, A.R.; Yadav, P.; Van Mierlo, J.; Berecibar, M. A critical review of future aspects of digitalization next generation Li-ion batteries manufacturing process. J. Energy Storage 2023, 74, 109209. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation ‘beyond Li-ion’ Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Chen, K.; Xue, D. Materials chemistry toward electrochemical energy storage. J. Mater. Chem. A 2016, 4, 7522–7537. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Prieto, A.L. Three-dimensional lithium-ion batteries with interdigitated electrodes. In Energy Harvesting and Storage: Materials, Devices, and Applications IV; SPIE: Baltimore, MD, USA, 2013; p. 872805. [Google Scholar] [CrossRef]
- Haas, O.; Cairns, E.J. Electrochemical energy storage. Annu. Rep. Prog. Chem. Sect. C 1999, 95, 163–197. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Yao, F.; Pham, D.T.; Lee, Y.H. Carbon-based materials for lithium-ion batteries, electrochemical capacitors, and their hybrid devices. ChemSusChem 2015, 8, 2284–2311. [Google Scholar] [CrossRef]
- Orisekeh, K.; Anye, V.; Oyewole, O.; Ahmed, R.; Orisekeh, D.K.; Oyelade, O.; Adeniji, S.; Umar, S.; Bello, A.; Soboyejo, W. Mechanical properties of polyvinylpyrrolidone/polyvinyl alcohol-based solid electrolytes. J. Appl. Polym. Sci. 2022, 139, e52379. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Baranowski, L.L.; Heveran, C.M.; Ferguson, V.L.; Stoldt, C.R. Multi-scale mechanical behavior of the Li3PS4 solid-phase electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 29573–29579. [Google Scholar] [CrossRef]
- Orisekeh, K.; Singh, B.; Olanrewaju, Y.; Kigozi, M.; Ihekweme, G.; Umar, S.; Anye, V.; Bello, A.; Parida, S.; Soboyejo, W. Processing of α-Fe2O3 nanoparticles on activated carbon cloth as binder-free electrode material for supercapacitor energy storage. J. Energy Storage 2021, 33, 102042. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Zhang, H.; Huang, K.; Yu, Y. Are solid-state batteries absolutely more environmentally friendly compared to traditional batteries-analyzing from the footprint family viewpoint. J. Clean. Prod. 2024, 447, 141452. [Google Scholar] [CrossRef]
- Zaman, W.; Hatzell, K.B. Processing and Manufacturing of Next Generation Lithium-Based All Solid-State Batteries. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101003. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1359028622000237 (accessed on 2 June 2024). [CrossRef]
- Randau, S.; Weber, D.A.; Kotz, O.; Koerver, R.; Braun, P.; Weber, A.; Ivers-Tiffee, E.; Adermann, T.; Kulisch, J.; Zeier, W.G.; et al. Benchmarking the performance of all-solid-state lithium batteries. Nat. Energy 2020, 5, 259–270. [Google Scholar] [CrossRef]
- Smith, L.; Ibn-Mohammed, T.; Astudillo, D.; Brown, S.; Reaney, I.M.; Koh, S.C.L. The role of cycle life on the environmental impact of Li6.4La3Zr1.4Ta0.6O12 based solid-state batteries. Adv. Sustain. Syst. 2021, 5, 2000241. Available online: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adsu.202000241 (accessed on 13 March 2024). [CrossRef]
- Troy, S.; Schreiber, A.; Reppert, T.; Gehrke, H.; Finsterbusch, M.; Uhlenbruck, S.; Stenzel, P. Life Cycle Assessment and resource analysis of all-solid-state batteries. Appl. Energy 2016, 169, 757–767. [Google Scholar] [CrossRef]
- Ke, J.Z.X.; Gu, Y.; Wang, F.; Zheng, D.; Shen, K.; Yuan, C. Cradle-to-gate life cycle assessment of all-solid-state lithium-ion batteries for sustainable design and manufacturing. Int. J. Life Cycle Assess. 2022, 27, 227–237. [Google Scholar] [CrossRef]
- Keshavarzmohammadian, A.; Cook, S.M.; Milford, J.B. Cradle-to-gate environmental impacts of sulfur-based solid-state lithium batteries for electric vehicle applications. J. Clean. Prod. 2018, 202, 770–778. [Google Scholar] [CrossRef]
- Lastoskie, C.M.; Dai, Q. Comparative life cycle assessment of laminated and vacuum vapor-deposited thin-film solid-state batteries. J. Clean. Prod. 2015, 91, 158–169. [Google Scholar] [CrossRef]
- Xiao, X.; Li, H. Predicting mechanical responses of additively manufactured metamaterials with computational efficiency. CIRP J. Manuf. Sci. Technol. 2024, 52, 149–158. [Google Scholar] [CrossRef]
- Johan, M.R.; Ibrahim, S. Optimization of neural network for ionic conductivity of nanocomposite solid polymer electrolyte system (PEO-LiPF6-EC-CNT). Commun. Nonlinear Sci. Numer. Simul. 2012, 17, 329–340. [Google Scholar] [CrossRef]
- Nava, D.P.; Guzmán, G.; Vazquez-Arenas, J.; Cardoso, J.; Gomez, B.; Gonzalez, I. An experimental and theoretical correlation to account for the effect of LiPF6 concentration on the ionic conductivity of poly(poly (ethylene glycol) methacrylate). Solid State Ion. 2016, 290, 98–107. [Google Scholar] [CrossRef]
- Ibrahim, S.; Johan, M.R. Conductivity, thermal and neural network model nanocomposite solid polymer electrolyte S LiPF6). Int. J. Electrochem. Sci. 2011, 6, 5565–5587. Available online: https://www.sciencedirect.com/science/article/pii/S1452398123184288 (accessed on 10 February 2024). [CrossRef]
- Orisekeh, D.K.; Jahan, M.P. Processing of bioceramics by additive manufacturing. In Advanced Bioceramics; CRC Press: Boca Raton, FL, USA, 2023; pp. 94–127. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003258353-5/processing-bioceramics-additive-manufacturing-david-orisekeh-jahan (accessed on 3 January 2023).
- Reyes, C.; Somogyi, R.; Niu, S.; Cruz, M.A.; Yang, F.; Catenacci, M.J.; Rhodes, C.P.; Wiley, B.J. Three-dimensional printing of a complete lithium-ion battery with fused filament fabrication. ACS Appl. Energy Mater. 2018, 1, 5268–5279. [Google Scholar] [CrossRef]
- Ragones, H.; Menkin, S.; Kamir, Y.; Gladkikh, A.; Mukra, T.; Kosa, G.; Golodnitsky, D. Towards smart free form-factor 3D printable batteries. Sustain. Energy Fuels 2018, 2, 1542–1549. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D printing of interdigitated Li-ion micro battery architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef]
- Pandeya, S.P.; Zou, S.; Roh, B.M.; Xiao, X. Programmable Thermo-Responsive Self-Morphing Structures Design and Performance. Materials 2022, 15, 8775. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.W.; Down, M.P.; Zhang, Y.; Ji, X.; Rowley-Neale, S.J.; Smith, G.C.; Kelly, P.J.; Banks, C.E. 3D printed graphene-based energy storage devices. Sci. Rep. 2017, 7, 42233. [Google Scholar] [CrossRef]
- Fu, K.; Wang, Y.; Yan, C.; Yao, Y.; Chen, Y.; Dai, J.; Lacey, S.; Wang, Y.; Wan, J.; Li, T.; et al. Graphene oxide-based electrode inks for 3d-printed lithium-ion batteries. Adv. Mater. 2016, 28, 2587–2594. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, F.; Wang, W.; Alexandridis, P.; Zhou, C.; Wu, G. 3D direct writing fabrication of electrodes for electrochemical storage devices. J. Power Sources 2017, 354, 134–147. [Google Scholar] [CrossRef]
- Santomartino, R.; Averesch, N.J.H.; Bhuiyan, M.; Cockell, C.S.; Colangelo, J.; Gumulya, Y.; Lehner, B.; Lopez-Ayala, I.; McMahon, S.; Mohanty, A.; et al. Toward sustainable space exploration: A roadmap for harnessing the power of microorganisms. Nat. Commun. 2023, 14, 1391. [Google Scholar] [CrossRef]
- Bush, G.W.; Cernan, E. The Vision for Space Exploration; National Aeronautics and Space Administration: Washington, DC, USA, 2003. Available online: http://www.nasa.gov (accessed on 2 April 2024).
- Wood, D.; Rathnasabapathy, M.; Stober, K.J.; Menon, P. Challenges and progress in applying space technology in support of the sustainable development goals. Acta Astronaut. 2024, 219, 678–692. [Google Scholar] [CrossRef]
- Galli, A.; Losch, A. Beyond planetary protection: What is planetary sustainability and what are its implications for space research? Life Sci. Space Res. 2019, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.; Weigel, A. Charting the evolution of satellite programs in developing countries—The Space Technology Ladder. Space Policy 2012, 28, 15–24. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Costa, C.M.; Lanceros-Méndez, S. Polymer composites and blends for battery separators: State of the art, challenges, and future trends. J. Power Sources 2015, 281, 378–398. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium-ion battery applications. RSC Adv. 2013, 3, 11404–11417. Available online: https://pubs.rsc.org/en/content/articlelanding/2013/ra/c3ra40732b (accessed on 3 March 2024). [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.H.; Kim, J.H.; Lee, S.Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Miranda, D.; Costa, C.M.; Almeida, A.M.; Lanceros-Méndez, S. Modeling separator membranes physical characteristics for optimized lithium-ion battery performance. Solid State Ion. 2015, 278, 78–84. [Google Scholar] [CrossRef]
- Deng, Y.; Song, X.; Ma, Z.; Zhang, X.; Shu, D.; Nan, J. Al2O3/PVdF-HFP-CMC/PE separator prepare using aqueous slurry and post-hot-pressing method for polymer lithium-ion batteries with enhanced safety. Electrochim. Acta 2016, 212, 416–425. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; Zhou, Y.; Zhang, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. The Application of Al2O3 in Separators and Solid Electrolytes of Lithium-ion Battery: A Review. Energy Storage Mater. 2024, 71, 103575. [Google Scholar] [CrossRef]
- Orisekeh, D.K.; Corti, G.; Jahan, M.P. Enhancing thermo-mechanical properties of additively manufactured PLA using eggshell microparticle fillers. J. Manuf. Process 2025, 133, 782–797. [Google Scholar] [CrossRef]
- Huang, Q.A.; Shen, Y.; Huang, Y.; Zhang, L.; Zhang, J. Impedance Characteristics and Diagnoses of Automotive Lithium-Ion Batteries at 7.5% to 93.0% State of Charge. Electrochim. Acta 2016, 219, 751–765. [Google Scholar] [CrossRef]
- Tröltzsch, U.; Kanoun, O.; Tränkler, H.R. Characterizing aging effects of lithium-ion batteries by impedance spectroscopy. Electrochim. Acta 2006, 51, 1664–1672. [Google Scholar] [CrossRef]
- Middlemiss, L.A.; Rennie, A.J.R.; Sayers, R.; West, A.R. Characterisation of batteries by electrochemical impedance spectroscopy. Energy Rep. 2020, 6, 232–241. [Google Scholar] [CrossRef]
- Love, C.T. Thermomechanical analysis and durability of commercial micro-porous polymer Li-ion battery separators. J. Power Sources 2011, 196, 2905–2912. [Google Scholar] [CrossRef]
- Cannarella, J.; Liu, X.; Leng, C.Z.; Sinko, P.D.; Gor, G.Y.; Arnold, C.B. Mechanical Properties of a Battery Separator under Compression and Tension. J. Electrochem. Soc. 2014, 161, F3117–F3122. [Google Scholar] [CrossRef]
- Sheidaei, A.; Xiao, X.; Huang, X.; Hitt, J. Mechanical behavior of a battery separator in electrolyte solutions. J. Power Sources 2011, 196, 8728–8734. [Google Scholar] [CrossRef]
- Luo, H.; Xia, Y.; Zhou, Q. Mechanical damage in a lithium-ion pouch cell under indentation loads. J. Power Sources 2017, 357, 61–70. [Google Scholar] [CrossRef]
- Cláudio, R.A.; Dupont, J.; Baptista, R.; Leite, M.; Reis, L. Behaviour evaluation of 3D printed polylactic acid under compression. J. Mater. Res. Technol. 2022, 21, 4052–4066. [Google Scholar] [CrossRef]
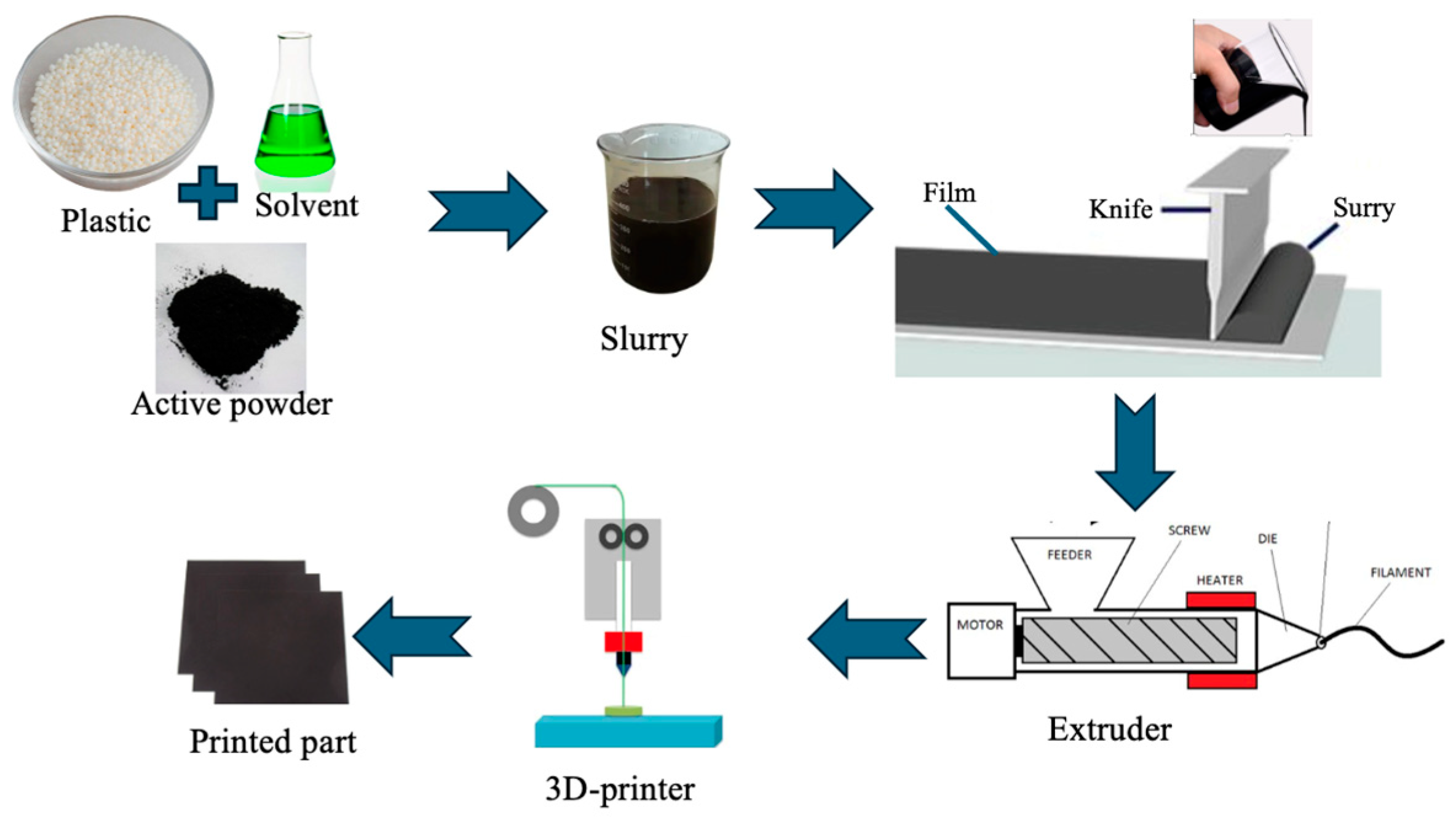
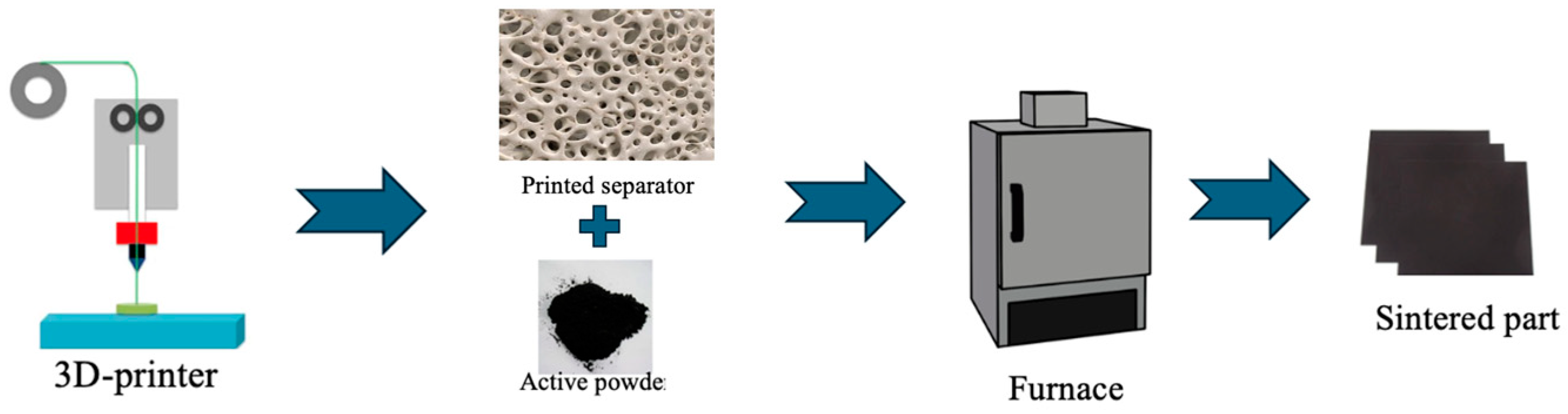
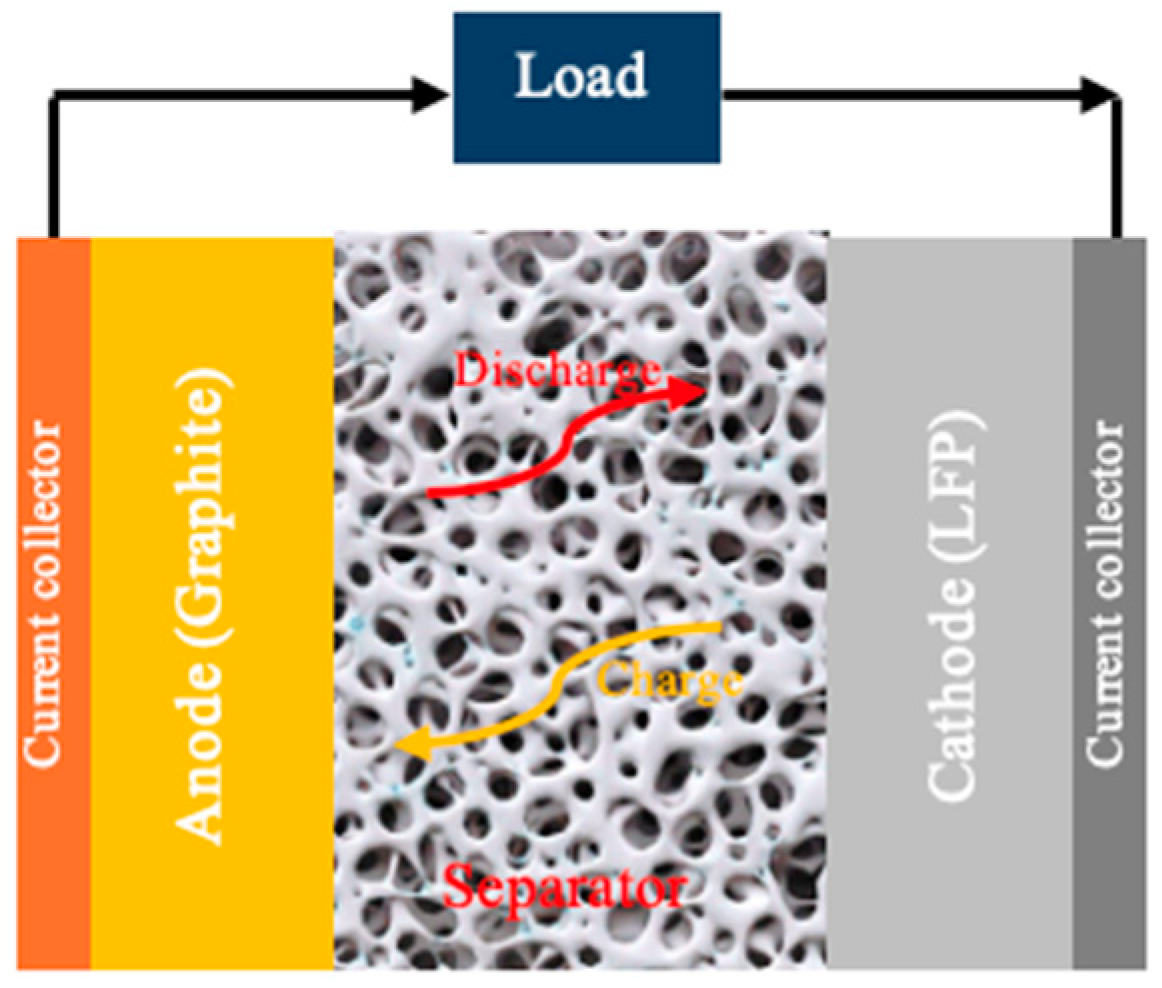


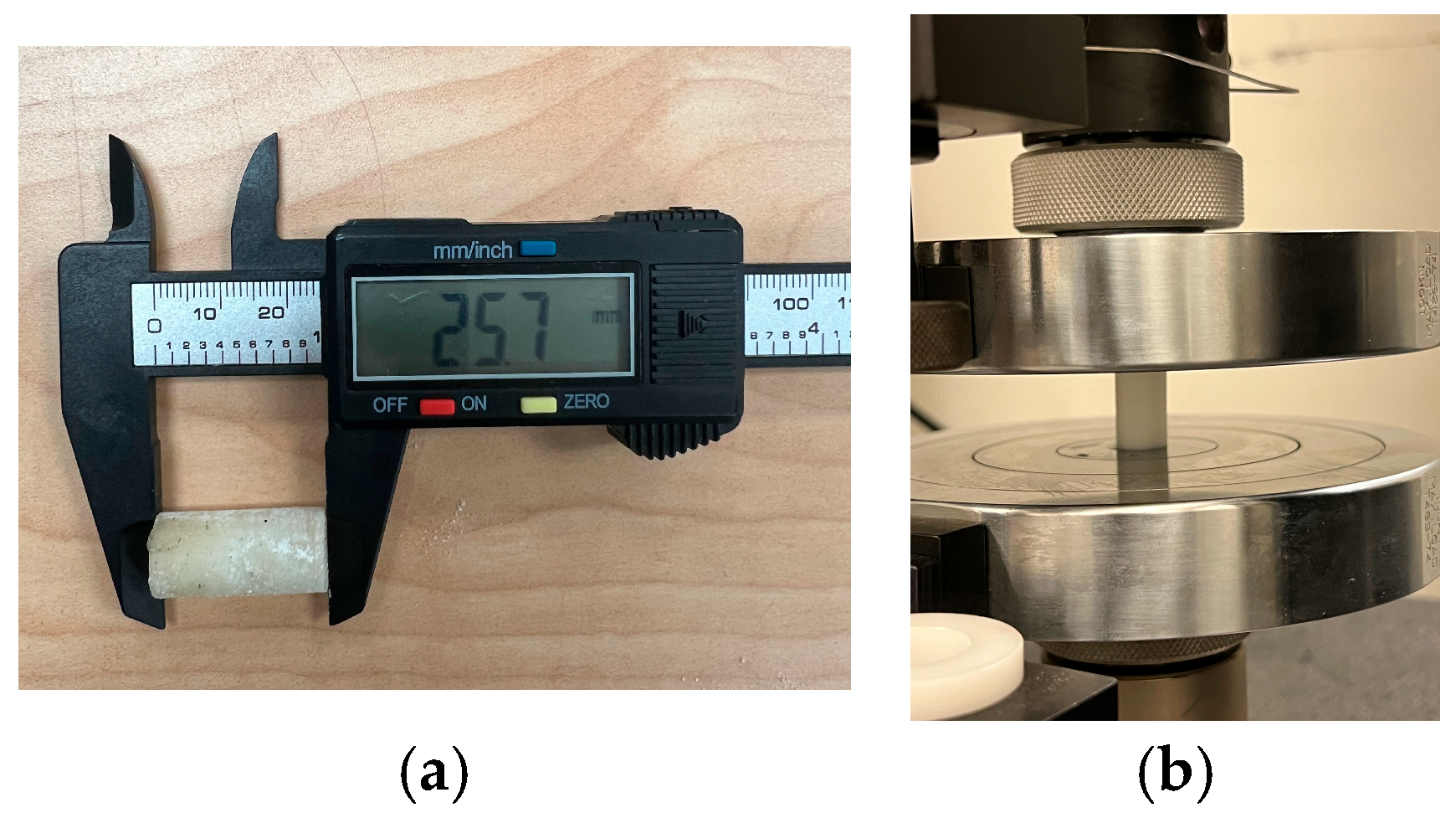

| Parameter | Value |
|---|---|
| Printer nozzle diameter | 0.4 mm |
| Printer nozzle temperature | 210 °C |
| Printer bed temperature | 45 °C |
| Printing speed | 40% |
| Infill line distance | 0.8 mm |
| Infill density | 20% |
| Infill pattern | Gyroid |
| Infill line direction | [0, 90] |
| PLA (Vol. %) | LiPF6 (Vol. %) | |
|---|---|---|
| Sample | 81 | 19 |
| Ionic Conductivity (S·cm−1) | Reference | |
|---|---|---|
| PLA/LiPF6 | 2.529 × 10−5 | This study |
| pPEGMA/LiPF6 | 2.07 × 10−5 | D.P. Nava et al. [30] |
| PEG/LC/LiPF6 | 3.22 × 10−4 | C.M. Costa et al. [52] |
| PEO/LiPF6 | 10−5 | J. Nunes-Pereira et al. [45] |
| Samples | Compression Strength (MPa) | Compression Modulus (GPa) |
|---|---|---|
| PLA 100% infill | 61.7 | 2.14 |
| PLA 20% infill | 23.5 | 0.9 |
| PLA 100% infill/LiPF6 | 22.1 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orisekeh, D.; Roh, B.-M.; Xiao, X. Solid-to-Solid Manufacturing Processes for High-Performance Li-Ion Solid-State Batteries. Polymers 2025, 17, 1788. https://doi.org/10.3390/polym17131788
Orisekeh D, Roh B-M, Xiao X. Solid-to-Solid Manufacturing Processes for High-Performance Li-Ion Solid-State Batteries. Polymers. 2025; 17(13):1788. https://doi.org/10.3390/polym17131788
Chicago/Turabian StyleOrisekeh, David, Byeong-Min Roh, and Xinyi Xiao. 2025. "Solid-to-Solid Manufacturing Processes for High-Performance Li-Ion Solid-State Batteries" Polymers 17, no. 13: 1788. https://doi.org/10.3390/polym17131788
APA StyleOrisekeh, D., Roh, B.-M., & Xiao, X. (2025). Solid-to-Solid Manufacturing Processes for High-Performance Li-Ion Solid-State Batteries. Polymers, 17(13), 1788. https://doi.org/10.3390/polym17131788







