Design of a Dual-Drug Delivery System for Local Release of Chlorhexidine and Dexketoprofen
Abstract
1. Introduction
2. Materials and Methods
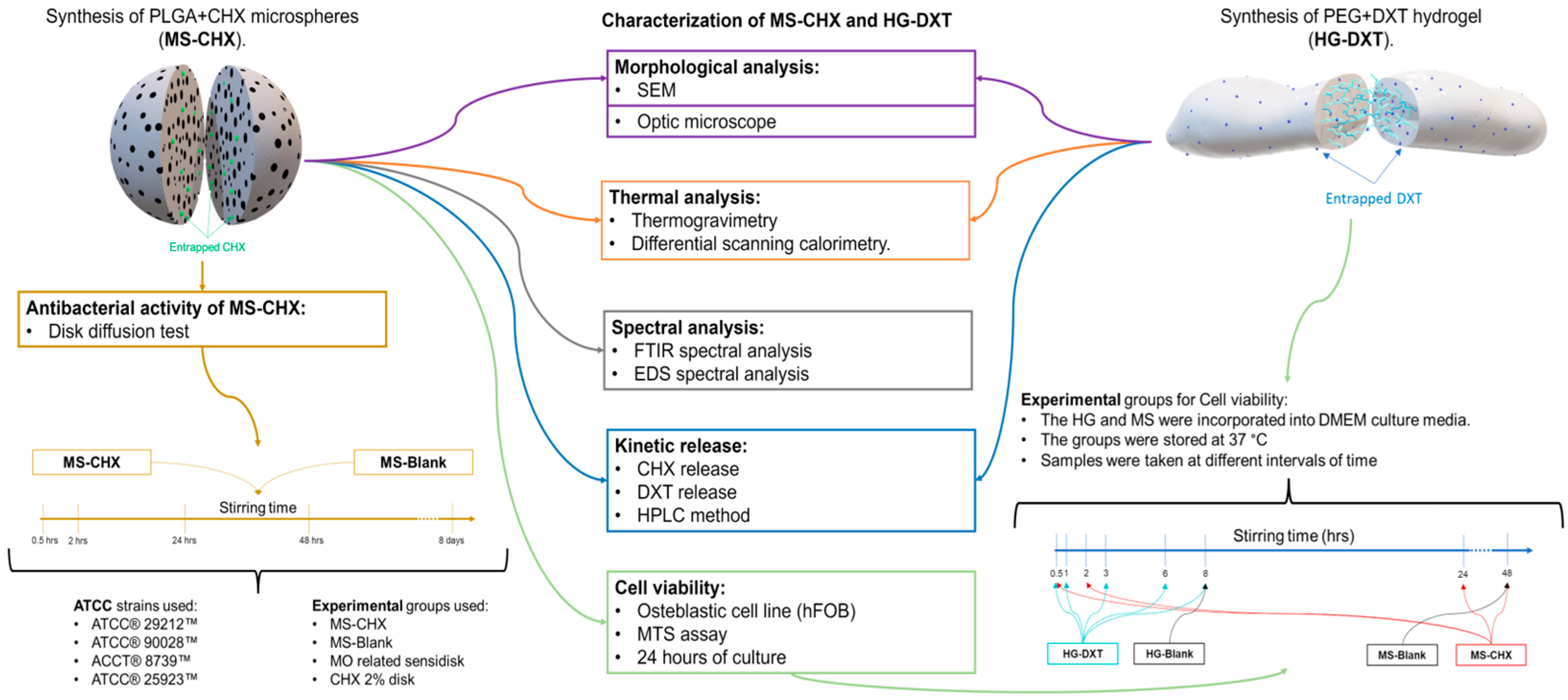
2.1. MS-CHX/HG-DXT DDS Preparation
2.1.1. PLGA MS-CHX-Loaded Fabrication
2.1.2. PEG HG-DXT-Loaded Preparation
2.2. Morphological Characterization of MS-CHX, HG-DXT, and MS-CHX/HG-DXT DDS
2.3. Thermal Characterization
2.4. Spectral Analysis of MS
2.5. Antibacterial Evaluation of MS
2.6. Cytotoxicity Evaluation of the MS-CHX and HG-DXT
2.7. Kinetics of CHX and DXT Release and Quantification from MS-CHX/HG-DXT DDS
2.8. Statistical Analysis
3. Results
3.1. Characterization of HG and MS
3.2. Thermal Characterization of MS-CHX, HG-DXT, and DDS
3.3. Spectral Analysis of MS-CHX
3.4. Antibacterial Effect of MS
3.5. Cytotoxic Effect of the MS-CHX and HG-DXT
3.6. Kinetics Release and Quantification of CHX and DXT from the DDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DDS | Drug delivery system |
| PLGA | Poly(lactic-co-glycolic acid |
| MS-CHX | Microspheres loaded with chlorhexidine |
| PEG | Polyethylene glycol |
| HG-DXT | Hydrogel containing dexketoprofen |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| HPLC | High-performance liquid chromatography |
| CHX | Chlorhexidine digluconate |
| DXT | Dexketoprofen |
| COXs | Cyclooxygenases |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| SSI | Surgical site infections |
| MS | Microspheres |
| DCM | Dichloromethane |
| PVA | Polyvinyl alcohol |
| HG | Hydrogel |
| PTFE | Polytetrafluoroethylene |
| Tp | Inflection point |
| Tg | Glass transition |
| MIC | Minimum inhibitory concentration |
References
- Pinto, D.; Marques, A.; Pereira, J.F.; Palma, P.J.; Santos, J.M. Long-term prognosis of endodontic microsurgery—A systematic review and meta-analysis. Medicina 2020, 56, 447. [Google Scholar] [CrossRef] [PubMed]
- Bieszczad, D.; Wichlinski, J.; Kaczmarzyk, T. Factors affecting the success of endodontic microsurgery: A cone-beam computed tomography study. J. Clin. Med. 2022, 11, 3391. [Google Scholar] [CrossRef] [PubMed]
- Malagise, C.J.; Khalighinejad, N.; Patel, Y.T.; Jalali, P.; He, J. Severe pain after endodontic surgery: An analysis of incidence and risk factors. J. Endod. 2021, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound healing: Biologic features and approaches to maximize healing trajectories. Curr. Probl. Surg. 2021, 38, 72–140. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- McCleane, G. Topical analgesics. Anesth. Clin. 2007, 25, 825–839. [Google Scholar] [CrossRef]
- Stanos, S.P. Topical agents for the management of musculoskeletal pain. J. Pain. Symptom Manag. 2007, 33, 342–355. [Google Scholar] [CrossRef]
- Derry, S.; Wiffen, P.J.; Kalso, E.A.; Bell, R.F.; Aldington, D.; Phillips, T.; Gaskell, H.; Moore, R.A. Topical analgesics for acute and chronic pain in adults—An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 5, CD008609. [Google Scholar] [CrossRef]
- Derry, S.; Moore, R.A.; Gaskell, H.; McIntyre, M.; Wiffen, P.J. Topical NSAIDs for acute musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2015, 11, CD007402. [Google Scholar] [CrossRef]
- Veys, E.M. 20 years’ experience with ketoprofen. Scand. J. Rheumatol. Suppl. 1991, 90, 1–44. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Arbos, R.M.; Amaro, S.R. Dexketoprofen trometamol: Clinical evidence supporting its role as a painkiller. Expert. Rev. Neurother. 2008, 8, 1625–1640. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Berrios-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Sheldon, A.T., Jr. Antiseptic “resistance”: Real or perceived threat? Clin. Infect. Dis. 2005, 40, 1650–1656. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and emerging topical antibacterials and antiseptics: Agents, action, and resistance patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Edmiston, C.E., Jr.; Okoli, O.; Graham, M.B.; Sinski, S.; Seabrook, G.R. Evidence for using chlorhexidine gluconate preoperative cleansing to reduce the risk of surgical site infection. AORN J. 2010, 92, 509–518. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable self-healing adhesive chitosan hydrogel with antioxidative, antibacterial, and hemostatic activities for rapid hemostasis and skin wound healing. ACS Appl. Mater. Interfac. 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, S.; Ai, D.; Qin, H.; Sun, Y.; Xia, X.; Xu, X.; Ji, W.; Song, J. Rational design of bioactive hydrogels toward periodontal delivery: From pathophysiology to therapeutic applications. Adv. Funct. Mater. 2023, 33, 2301062. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, R.; Lei, L.; Yang, Y.; Hu, T. Drug delivery systems for oral disease applications. J. Appl. Oral. Sci. 2022, 30, 20210349. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Yue, I.C.; Poff, J.; Cortes, M.E.; Sinisterra, R.D.; Faris, C.B.; Hildgen, P.; Langer, R.; Shastri, V.P. A novel polymeric chlorhexidine delivery device for the treatment of periodontal disease. Biomaterials 2004, 25, 3743–3750. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, G.; Brey, E.M. Dual delivery of chlorhexidine and platelet-derived growth factor-BB for enhanced wound healing and infection control. Acta Biomater. 2013, 9, 4976–4984. [Google Scholar] [CrossRef]
- Priyadarshini, B.M.; Selvan, S.T.; Lu, T.B.; Xie, H.; Neo, J.; Fawzy, A.S. Chlorhexidine nanocapsule drug delivery approach to the resin-dentin interface. J. Dent. Res. 2016, 95, 1065–1072. [Google Scholar] [CrossRef]
- Chen, M.M.; Cao, H.; Liu, Y.Y.; Liu, Y.; Song, F.F.; Chen, J.D.; Zhang, Q.Q.; Yang, W.Z. Sequential delivery of chlorhexidine acetate and bFGF from PLGA-glycol chitosan core-shell microspheres. Colloids Surf. B Biointerfaces 2017, 151, 189–195. [Google Scholar] [CrossRef]
- Priyadarshini, B.M.; Mitali, K.; Lu, T.B.; Handral, H.K.; Dubey, N.; Fawzy, A.S. PLGA nanoparticles as chlorhexidine-delivery carrier to resin-dentin adhesive interface. Dent. Mater. 2017, 33, 830–846. [Google Scholar] [CrossRef]
- Faria, G.; Celes, M.R.; De Rossi, A.; Silva, L.A.; Silva, J.S.; Rossi, M.A. Evaluation of chlorhexidine toxicity injected in the paw of mice and added to cultured l929 fibroblasts. J. Endod. 2007, 33, 715–722. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D. Schubert US Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Lin, C.C.; Anseth, K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.D.; Felong, T.J.; Graunke, D.; Ovitt, C.E.; Benoit, D.S. Development of poly(ethylene glycol) hydrogels for salivary gland tissue engineering applications. Tissue Eng. Part A 2015, 21, 1733–1751. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Xiao, L.Q.; Tan, L.; Sun, W.; Wu, T.; Chen, L.W.; Mei, Y.; Shi, B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: In vitro and in vivo characteristics. J. Biomed. Mater. Res. A 2015, 103, 3580–3589. [Google Scholar] [CrossRef] [PubMed]
- Sagir, O.; Sunay, F.B.; Yildirim, H.; Aksoz, E.; Ozaslan, S.; Koroglu, A.; Aydemir, T.; Ulusal, A.E.; Kockar, F. Evaluation of the effects of dexketoprofen trometamol on knee joint: An in vivo & in vitro study. Indian. J. Med. Res. 2013, 138, 912–918. [Google Scholar]
- Liu, G.; McEnnis, K. Glass Transition temperature of PLGA particles and the influence on drug delivery applications. Polymers 2022, 14, 993. [Google Scholar] [CrossRef]
- Fredenberg, S.; Jonsson, M.; Laakso, T.; Wahlgren, M.; Reslow, M.; Axelsson, A. Development of mass transport resistance in poly(lactide-co-glycolide) films and particles--a mechanistic study. Int. J. Pharm. 2011, 409, 194–202. [Google Scholar] [CrossRef]
- Ford Versypt, A.N.; Pack, D.W.; Braatz, R.D. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres—A review. J. Control. Release 2013, 165, 29–37. [Google Scholar] [CrossRef]
- Brunner, A.; Mäder, K.; Göpferich, A. pH and osmotic pressure inside biodegradable microspheres during erosion. Pharm. Res. 1999, 16, 847–853. [Google Scholar] [CrossRef]
- Fu, K.; Pack, D.W.; Klibanov, A.M.; Langer, R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm. Res. 2000, 17, 100–106. [Google Scholar] [CrossRef]
- Beckert, S.; Farrahi, F.; Aslam, R.S.; Scheuenstuhl, H.; Königsrainer, A.; Hussain, M.Z.; Hunt, T.K. Lactate stimulates endothelial cell migration. Wound Repair. Regen. 2006, 14, 321–324. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Vandermeulen, G.; Preat, V. PLGA based drug delivery systems: Promising carriers for wound healing activity. Wound Repair. Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Hu, C.C.; Lee, S.S.; Chou, M.Y.; Chang, Y.C. Cytotoxicity of chlorhexidine on human osteoblastic cells is related to intracellular glutathione levels. Int. Endod. J. 2010, 43, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Klose, D.; Siepmann, F.; Elkharraz, K.; Siepmann, J. PLGA-based drug delivery systems: Importance of the type of drug and device geometry. Int. J. Pharm. 2008, 354, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Villalpando, V.; Chavarria-Bolanos, D.; Gordillo-Moscoso, A.; Masuoka-Ito, D.; Martinez-Rider, R.; Isiordia-Espinoza, M.; Pozos-Guillen, A. Comparison of the analgesic efficacy of preoperative/postoperative oral dexketoprofen trometamol in third molar surgery: A randomized clinical trial. J. Craniomaxillofac. Surg. 2016, 44, 1350–1355. [Google Scholar] [CrossRef]
- Barbanoj, M.J.; Antonijoan, R.M.; Gich, I. Clinical pharmacokinetics of dexketoprofen. Clin. Pharmacokinet. 2001, 40, 245–262. [Google Scholar] [CrossRef]
- Wyganowska-Swiatkowska, M.; Kotwicka, M.; Urbaniak, P.; Nowak, A.; Skrzypczak-Jankun, E.; Jankun, J. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int. J. Mol. Med. 2016, 37, 1594–1600. [Google Scholar] [CrossRef]
- Tu, Y.Y.; Yang, C.Y.; Chen, R.S.; Chen, M.H. Effects of chlorhexidine on stem cells from exfoliated deciduous teeth. J. Formos. Med. Assoc. 2015, 114, 17–22. [Google Scholar] [CrossRef]
- Esparza-Villalpando, V.; Chavarria-Bolanos, D.; Zapata-Morales, J.R.; Vertiz-Hernandez, A.; Pozos-Guillen, A. Antinociceptive local effect of the combination of dexketoprofen trometamol and chlorhexidine gluconate in a formalin test: An additive effect. Braz. J. Pharm. Sci. 2018, 54, e17799. [Google Scholar] [CrossRef]
- Esparza-Villalpando, V.; Pozos-Guillen, A.; Zapata-Morales, J.R.; Vertiz-Hernandez, A.; Martinez-Aguilar, V.M.; Chavarria-Bolanos, D. Evaluation of the local synergistic effect of a dexketoprofen and chlorhexidine combination in the formalin test. Puerto Rico Health Sci. J. 2023, 42, 35–42. [Google Scholar]




| Abbreviature | Description | Content | Manufacturer |
|---|---|---|---|
| CHX | Chlorhexidine | Chlorhexidine digluconate solution 20% in H2O | Sigma-Aldrich |
| PLGA | Poly(lactic-co-glycolic acid) | 50:50; Molecular weight range ∼66,000–110,000 | Sigma-Aldrich |
| MS-Blank | Empty microspheres (MS) | Only PLGA | NA |
| MS-CHX | CHX-Loaded microspheres | PLGA + CHX | NA |
| DXT | Dexketoprofen Trometamol | Dexketoprofen Trometamol powder | STEIN labs |
| PEG | Polyethylene glycol | PEG (MW: 400 and 4000) | Sigma-Aldrich |
| HG-Blank | Empty Hydrogel (HG) | Only PEG | NA |
| HG-DXT | DXT-loaded hydrogel | PEG + DXT | NA |
| DDS | Drug delivery system | MS-CHX + HG-DXT | NA |
| Groups (n = 3) | Strain | Inhibition Zone (mm) Mean (sd) | p-Value * |
|---|---|---|---|
| MS-CHX | E. faecalis | 15.6 (0.53) | p < 0.05 |
| MS-Blank | 6 (0) | ||
| CHX 2% | 15.5 (0.5) | ||
| Sensi-disk | 20.03 (0.06) | ||
| MS-CHX | C. albicans | 16.3 (1.42) | p < 0.05 |
| MS-Blank | 6 (0) | ||
| CHX 2% | 27.5( 1.2) | ||
| Sensi-disk | 25.6 (0.58) | ||
| MS-CHX | E. coli | 14.23 (0.45) | p < 0.05 |
| MS-Blank | 6 (0) | ||
| CHX 2% | 15.5 (0.5) | ||
| Sensi-disk | 28.1 (0.1) | ||
| MS-CHX | S. aureus | 17.83 (1.61) | p < 0.05 |
| MS-Blank | 6 (0) | ||
| CHX 2% | 27.5 (1.32) | ||
| Sensi-disk | 25.67 (0.2) |
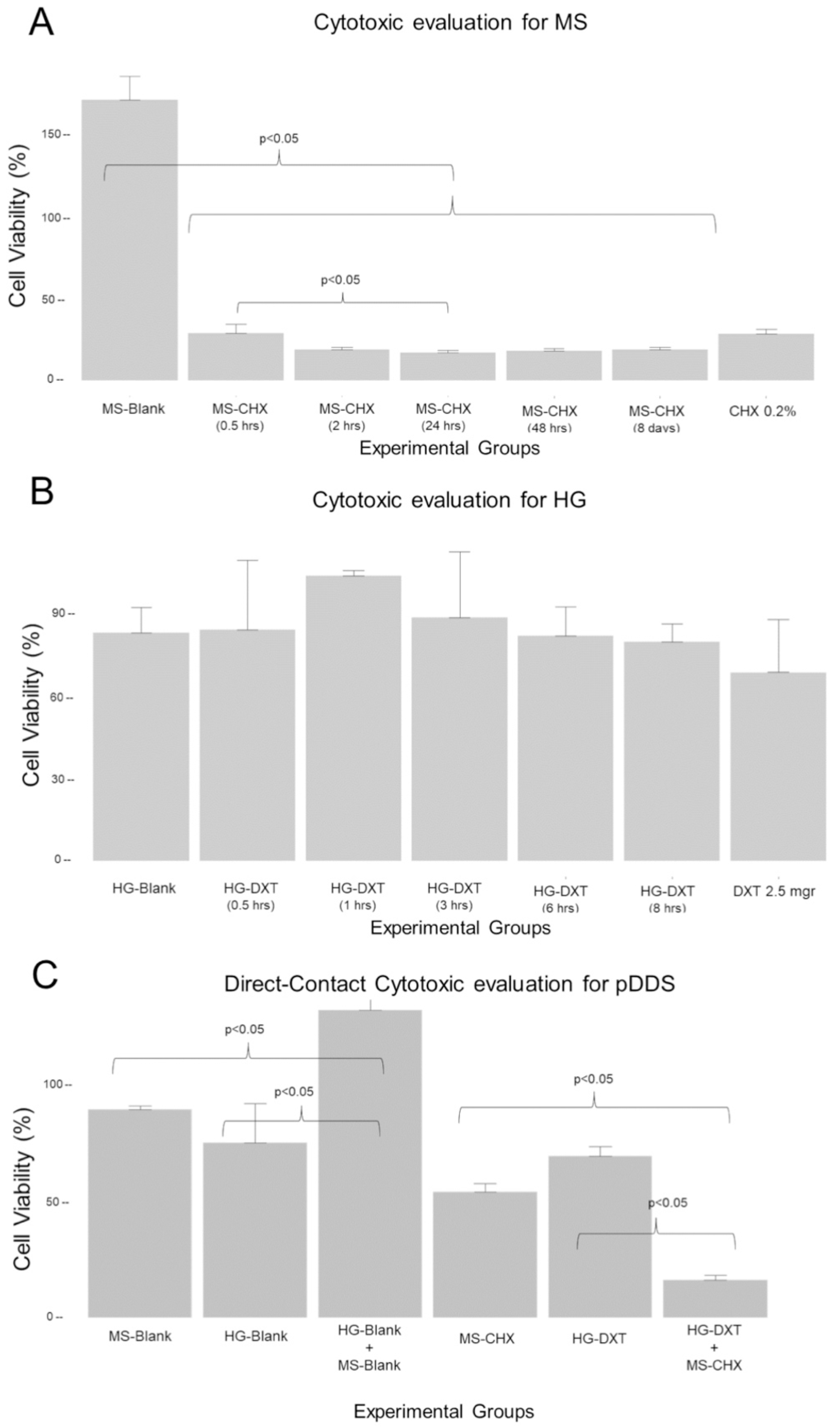
| Cytotoxicity Evaluation of the MS | ||
| Groups (n = 5) | Cell Viability (%) Mean (SD) | Kruskal–Wallis Test |
| MS-Blank | 172 (14.2) | p < 0.05 |
| MS-CHX (0.5 h) | 29.2 (5.35) | |
| MS-CHX (2 h) | 19 (1.94) | |
| MS-CHX (24 h) | 17.3 (1.49) | |
| MS-CHX (48 h) | 18.7 (1.16) | |
| MS-CHX (8 days) | 19 (1.8) | |
| CHX 0.2% | 29 (2.87) | |
| Cytotoxicity evaluation of the HG | ||
| Groups (n = 5) | Cell viability (%) Mean (SD) | ANOVA test |
| HG-Blank | 82.4 (9.15) | p > 0.05 |
| HG-DXT (0.5 h) | 83.4 (25.2) | |
| HG-DXT (1 h) | 103 (1.94) | |
| HG-DXT (3 h) | 87.7 (24.1) | |
| HG-DXT (6 h) | 81.3 (10.5) | |
| HG-DXT (8 h) | 79.2 (6.38) | |
| DXT 2.5 mg | 68 (19.2) | |
| Cytotoxicity evaluation of the MS, HG and DDS at direct contact with cells | ||
| Groups (n = 5) | Cell Viability (%) Mean (SD) | ANOVA test |
| MS-Blank | 89.2 (1.77) | p < 0.05 |
| HG-Blank | 74.8 (17.0) | |
| HG-Blank+MS-Blank (2:1) | 132 (6.41) | |
| MS-CHX | 53.8 (3.71) | |
| HG-DXT | 69.2 (4.02) | |
| HG-DXT+MS-CHX (2:1) | 16.2 (2.13) | |
| Total Amount of Drug Release in the DDS | |||||||
| Group | Mean of CHX Content in µgr (% of Drug Release) | Mean of DXT Content in µgr (% of Drug Release) | n | ||||
| MS-CHX | ~536.74 µgr (~91.57%) | NA | 3 | ||||
| HG-DXT | NA | ~242.35 µgr (96.94%) | 3 | ||||
| MS:HG 1:1 | ~246.26 µgr (~84.06%) | ~60.65 µgr (48.52%) | 3 | ||||
| MS:HG 1:2 | ~156.99 µgr (~80.38%) | ~150.39 µgr (90.24%) | 3 | ||||
| Detection points in the kinetic release of CHX and DXT | |||||||
| Group | Chlorhexidine gluconate | Dexketoprofen trometamol | n | ||||
| FS | LS | Rate | FS | LS | Rate | ||
| MS-CHX | 72 h | 840 h | 0.698 µgr/h | NA | NA | NA | 3 |
| HG-DXT | NA | NA | NA | 0.5 h | 336 h | 0.722 µgr/h | 3 |
| MS:HG 1:1 | 120 h | 768 h | 0.38 µgr/h | 24 h | 624 h | 0.101 µgr/h | 3 |
| MS:HG 1:2 | 120 h | 696 h | 0.272 µgr/h | 0.5 h | 240 h | 0.627 µgr/h | 3 |
| Kinetic models for CHX release | |||||||
| Group | Zero Order | First Order | Korsmeyer-Peppas | ||||
| R2 | Kµgr·h−1 | R2 | Kh−1 | R2 | c | ||
| MS-CHX | 0.969 | 0.135 | 0.976 | −0.0008 | 0.988 | 0.698 | |
| MS:HG 1:1 | 0.974 | 0.093 | 0.883 | −0.0007 | 0.996 | 1.668 | |
| MS:HG 1:2 | 0.949 | 0.089 | 0.783 | −0.0007 | 0.997 | 2.128 | |
| Kinetic models for DXT release | |||||||
| Group | Zero Order | First Order | Korsmeyer-Peppas | ||||
| R2 | Kµgr·h−1 | R2 | Kh−1 | R2 | c | ||
| HG-DXT | 0.889 | 0.883 | 0.961 | −0.007 | 0.989 | 0.358 | |
| MS:HG 1:1 | 0.941 | 0.097 | 0.92 | −0.0005 | 0.979 | 0.598 | |
| MS:HG 1:2 | 0.921 | 0.699 | 0.947 | −0.0043 | 0.994 | 0.398 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esparza-Villalpando, V.; Pozos-Guillén, A.; Vértiz-Hernández, Á.A.; Vega-Baudrit, J.; Chavarría-Bolaños, D. Design of a Dual-Drug Delivery System for Local Release of Chlorhexidine and Dexketoprofen. Polymers 2025, 17, 1771. https://doi.org/10.3390/polym17131771
Esparza-Villalpando V, Pozos-Guillén A, Vértiz-Hernández ÁA, Vega-Baudrit J, Chavarría-Bolaños D. Design of a Dual-Drug Delivery System for Local Release of Chlorhexidine and Dexketoprofen. Polymers. 2025; 17(13):1771. https://doi.org/10.3390/polym17131771
Chicago/Turabian StyleEsparza-Villalpando, Vicente, Amaury Pozos-Guillén, Ángel Antonio Vértiz-Hernández, Jose Vega-Baudrit, and Daniel Chavarría-Bolaños. 2025. "Design of a Dual-Drug Delivery System for Local Release of Chlorhexidine and Dexketoprofen" Polymers 17, no. 13: 1771. https://doi.org/10.3390/polym17131771
APA StyleEsparza-Villalpando, V., Pozos-Guillén, A., Vértiz-Hernández, Á. A., Vega-Baudrit, J., & Chavarría-Bolaños, D. (2025). Design of a Dual-Drug Delivery System for Local Release of Chlorhexidine and Dexketoprofen. Polymers, 17(13), 1771. https://doi.org/10.3390/polym17131771








