Biomass-Based Nanocomposites of Polydithioacetals Derived from Vanillin with Cellulose Nanocrystals: Synthesis, Thermomechanical and Reprocessing Properties
Abstract
1. Introduction
2. Materials and Methodology
2.1. Materials
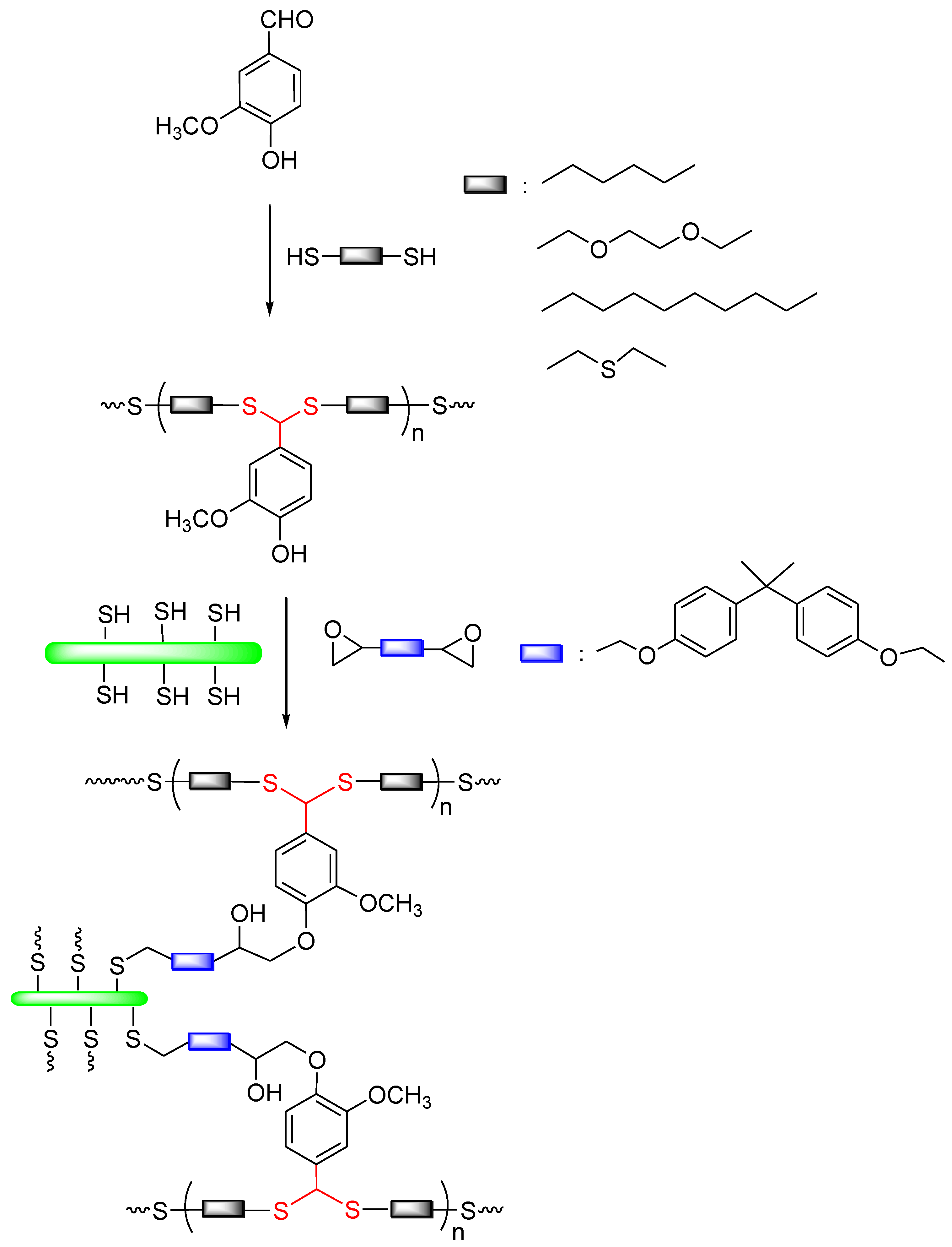
2.2. Synthesis of Polydithioacetals (PDTAs)
2.3. Surface Functionalization of CNCs
2.4. Preparation of Nanocomposites of PDTAs with CNCs
3. Results and Discussion
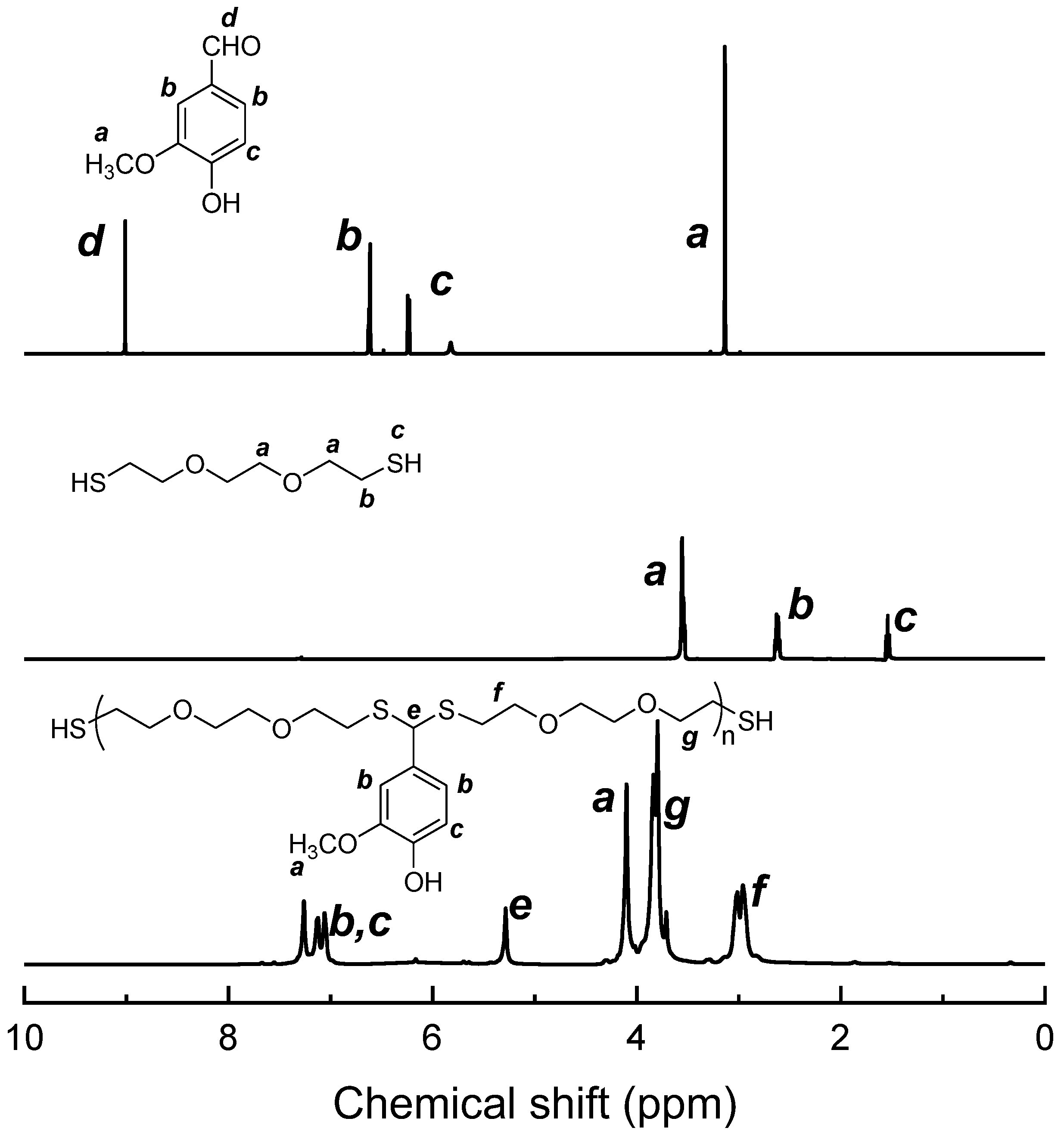
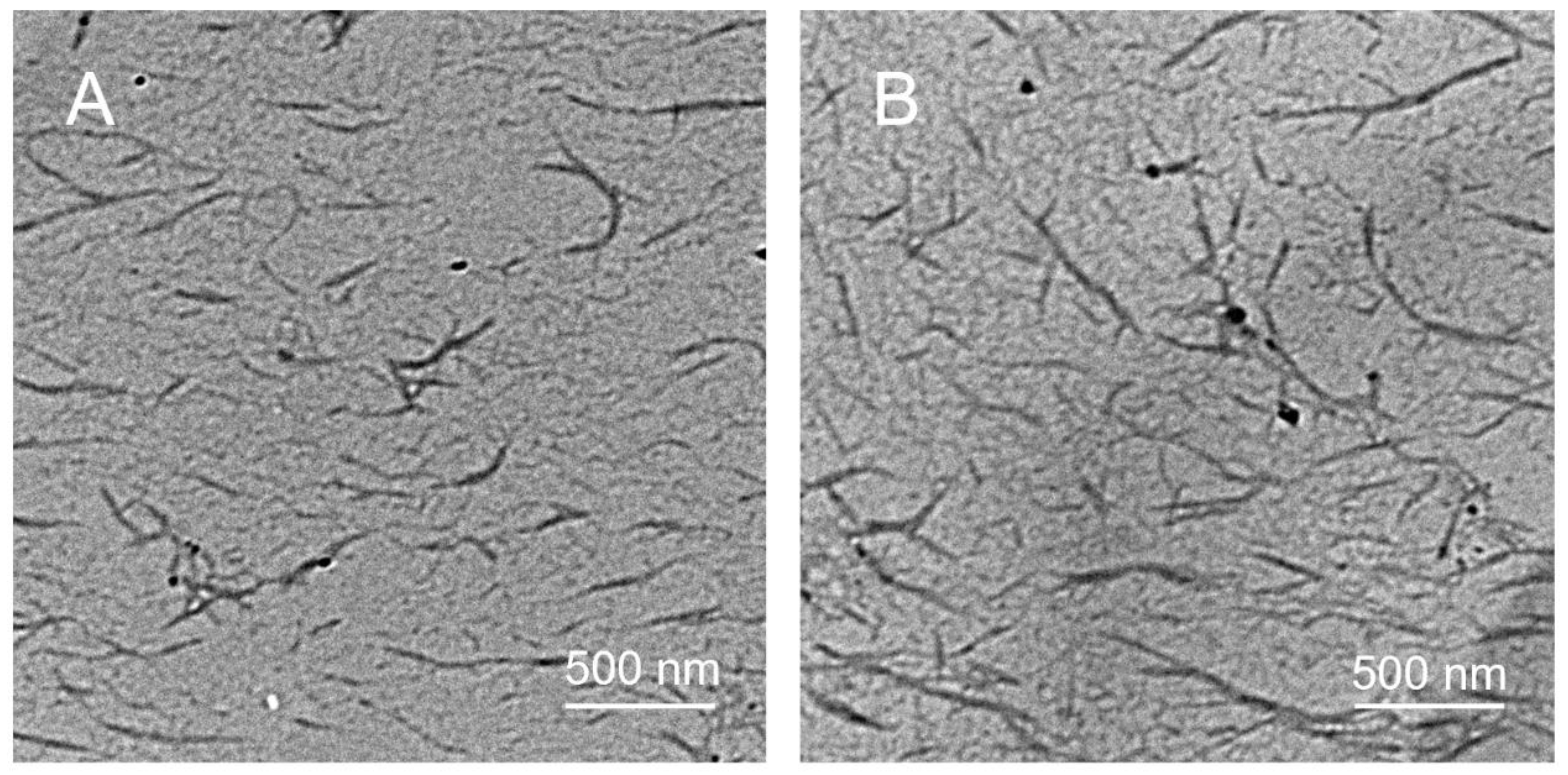
3.1. Synthesis of PDTAs and Surface Functionalization of CNCs
3.2. Generation of Nanocomposites of PDTAs with CNCs
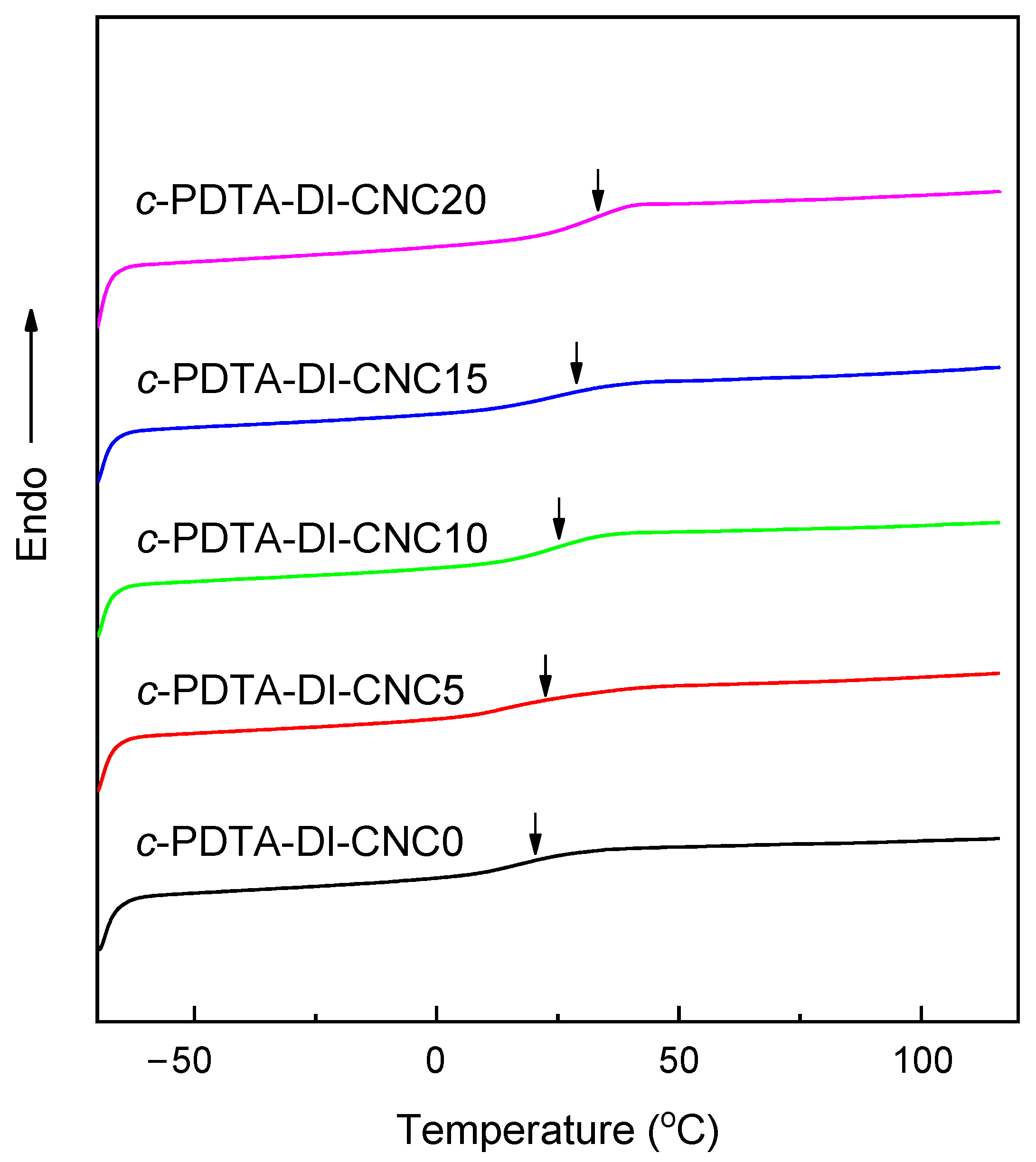
3.3. Thermal and Mechanical Properties
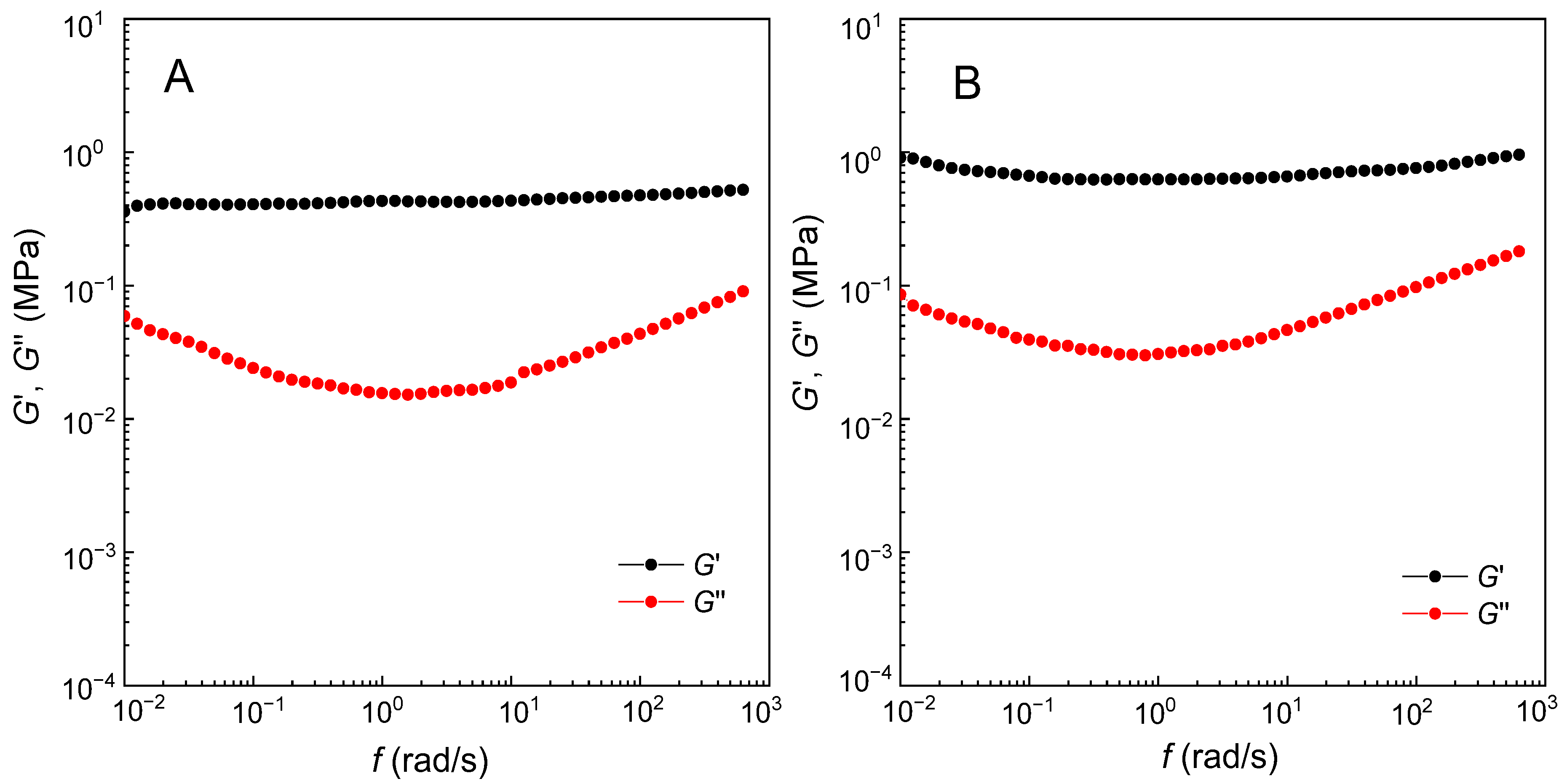
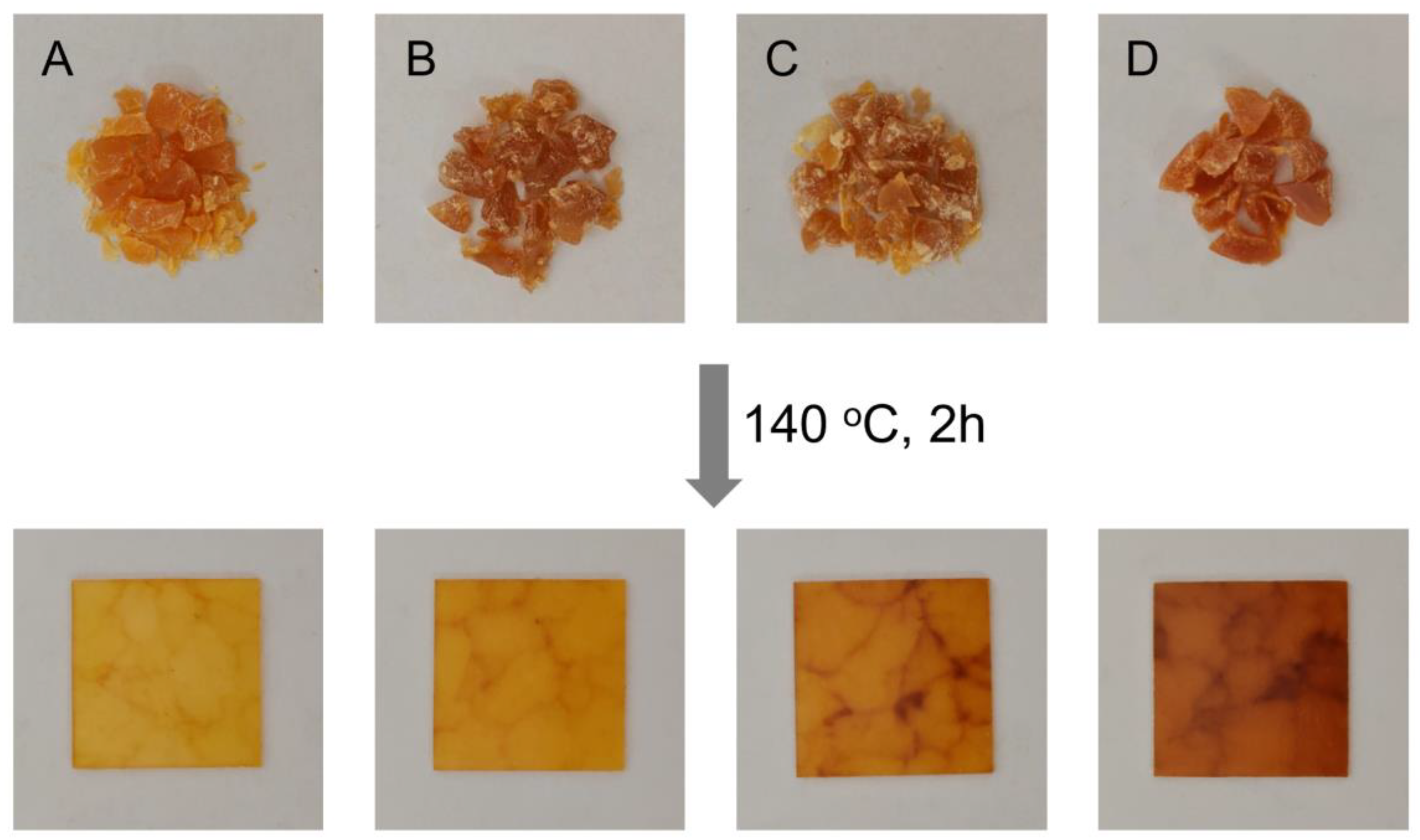
3.4. Reprocessing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2022, 7, 83–103. [Google Scholar] [CrossRef]
- Yoshimura, T.; Shimasaki, T.; Teramoto, N.; Shibata, M. Bio-based polymer networks by thiol-ene photopolymerizations of allyl-etherified eugenol derivatives. Eur. Polym. J. 2015, 67, 397–408. [Google Scholar] [CrossRef]
- Imbert, E.; Ladu, L.; Tani, A.; Morone, P. The transition towards a bio-based economy: A comparative study based on social network analysis. J. Environ. Manag. 2019, 230, 255–265. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; B, A.K.; H, R.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Taher, M.A.; Wang, X.; Faridul Hasan, K.M.; Miah, M.R.; Zhu, J.; Chen, J. Lignin modification for enhanced performance of polymer composites. ACS Appl. Bio Mater. 2023, 6, 5169–5192. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Zheng, L.; Zeng, J. Dynamic crosslinking: An efficient approach to fabricate epoxy vitrimer. Materials 2021, 14, 919. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Li, K.; Lin, H.; Wang, M.; Zheng, L.; Wu, C.; Zhang, X. Recycling of epoxy resins with degradable structures or dynamic cross-linking networks: A review. Ind. Eng. Chem. Res. 2024, 63, 5005–5027. [Google Scholar] [CrossRef]
- Türel, T.; Dağlar, Ö.; Eisenreich, F.; Tomović, Ž. Epoxy thermosets designed for chemical recycling. Chem. Asian J. 2023, 18, e202300373. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, F.; Chen, X.; Shen, L.; Chen, Y.; Lin, Y. Recyclable, degradable, and fully bio-based covalent adaptable polymer networks enabled by a dynamic diacetal motif. ACS Sustain. Chem. Eng. 2023, 11, 3065–3073. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Wang, Z.; Tang, C. Chemical syntheses of bioinspired and biomimetic polymers toward biobased materials. Nat. Rev. Chem. 2021, 5, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Long, Y.; Xu, S.; Liu, X.; Chen, L.; Wang, Y.-Z. Recovery of epoxy thermosets and their composites. Mater. Today 2023, 64, 72–97. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, L.; Xu, X.; Shang, S.; Liu, H. A fully bio-based epoxy vitrimer: Self-healing, triple-shape memory and reprocessing triggered by dynamic covalent bond exchange. Mater. Des. 2020, 186, 108248. [Google Scholar] [CrossRef]
- Li, B.; Zhu, G.; Hao, Y.; Ren, T. An investigation on the performance of epoxy vitrimers based on disulfide bond. J. Appl. Polym. Sci. 2022, 139, 51589. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Solera, G.; Azcarate-Ascasua, I.; Boucher, V.; Grande, H.J.; Rekondo, A. Chemical control of the aromatic disulfide exchange kinetics for tailor-made epoxy vitrimers. Polymer 2022, 239, 124457. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Epoxy vitrimers: The effect of transesterification reactions on the network structure. Polymers 2018, 10, 43. [Google Scholar] [CrossRef]
- Lu, L.; Pan, J.; Li, G. Recyclable high-performance epoxy based on transesterification reaction. J. Mater. Chem. A 2017, 5, 21505–21513. [Google Scholar] [CrossRef]
- Tratnik, N.; Tanguy, N.R.; Yan, N. Recyclable, self-strengthening starch-based epoxy vitrimer facilitated by exchangeable disulfide bonds. Chem. Eng. J. 2023, 451, 138610. [Google Scholar] [CrossRef]
- Gao, N.; Zheng, Y.; ShangGuan, J.; Sun, H.; Jiang, J.; Xiang, S.; Zhao, S.; Fu, F.; Liu, X. Superior epoxy vitrimer containing acetal and disulfide bonds for achieving high mechanical properties, reprocessability, and degradability. Macromolecules 2024, 57, 5450–5460. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; Liu, Y.; Jiang, Y.; Li, X.; Bai, Y. Rapid repair and degradation: A study of high-performance recyclable vitrimer epoxy resin based on disulfide bonds. Polym. Test. 2024, 137, 108528. [Google Scholar] [CrossRef]
- Azcune, I.; Huegun, A.; Ruiz de Luzuriaga, A.; Saiz, E.; Rekondo, A. The effect of matrix on shape properties of aromatic disulfide based epoxy vitrimers. Eur. Polym. J. 2021, 148, 110362. [Google Scholar] [CrossRef]
- Memon, H.; Wei, Y.; Zhang, L.; Jiang, Q.; Liu, W. An imine-containing epoxy vitrimer with versatile recyclability and its application in fully recyclable carbon fiber reinforced composites. Compos. Sci. Techno. 2020, 199, 108314. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Cui, X.; Qin, J.; Shi, M.; Wang, D.; Liang, L.; Yang, C. Imine-containing epoxy vitrimer cured by active ester: Properties and theoretical analysis. ACS Appl. Polym. Mater. 2023, 5, 10042–10052. [Google Scholar] [CrossRef]
- Rashid, M.A.; Zhu, S.; Jiang, Q.; Wei, Y.; Liu, W. Developing easy processable, recyclable, and self-healable biobased epoxy resin through dynamic covalent imine bonds. ACS Appl. Polym. Mater. 2023, 5, 279–289. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, M.; Wen, Q.; Zhang, L.; Jiang, Q.; Wang, J.; Liu, W. Recyclable epoxy resins with different silyl ether structures: Structure-property relationships and applications in diverse functional composites. Compos. Part A Appl. Sci. Manuf. 2024, 179, 108017. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Z.; Fang, S.; Tang, Z.; Liu, F.; Guo, B. Malleable organic/inorganic thermosetting hybrids enabled by exchangeable silyl ether interfaces. J. Mater. Chem. A 2019, 7, 1459–1467. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, L.; Zhao, Y.; Wang, Y.; Lei, J. Reprocessable and shape memory thermosetting epoxy resins based on silyl ether equilibration. Macromol. Chem. Phys. 2019, 220, 1900149. [Google Scholar] [CrossRef]
- Wu, W.; Feng, H.; Xie, L.; Zhang, A.; Liu, F.; Liu, Z.; Zheng, N.; Xie, T. Reprocessable and ultratough epoxy thermosetting plastic. Nat. Sustain. 2024, 7, 804–811. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, S.; Xu, X.; Chen, Y.; Zhang, F. Fabrication and curing properties of o-cresol formaldehyde epoxy resin with reversible cross-links by dynamic boronic ester bonds. Polymer 2020, 211, 123116. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Gao, Y.; Hu, J.; Zheng, S. Curing of epoxy with aromatic amine bearing boronic ester bonds enables reprocessing and configurable shape recovery. React. Funct. Polym. 2023, 191, 105689. [Google Scholar] [CrossRef]
- Liu, Z.; Song, Z.; Lv, B.; Qiu, Z. Re-assemblable, recyclable, and self-healing epoxy resin adhesive based on dynamic boronic esters. Polymers 2023, 15, 3488. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Miao, R.; Liu, J.; Xin, Z.; Bao, C. Covalent adaptable networks containing nitrogen-coordinated boronic ester and imine bonds. ACS Appl. Polym. Mater. 2024, 6, 9008–9016. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, H.; Chai, M.; Yang, R.; Xu, G.; Sun, H.; Wang, Q. The carbonate exchange reaction strategy for chemical recycling of poly(bisphenol A carbonate) into an epoxy-curing agent. Polym. Chem. 2025, 16, 465–474. [Google Scholar] [CrossRef]
- Du, S.; Yang, S.; Wang, B.; Li, P.; Zhu, J.; Ma, S. Acetal-thiol click-like reaction: Facile and efficient synthesis of dynamic dithioacetals and recyclable polydithioacetals. Angew. Chem. Int. Ed. 2024, 63, e202405653. [Google Scholar] [CrossRef]
- Orrillo, A.G.; La-Venia, A.; Escalante, A.M.; Furlan, R.L.E. Rewiring chemical networks based on dynamic dithioacetal and disulfide bonds. Chem. Eur. J. 2018, 24, 3141–3146. [Google Scholar] [CrossRef]
- Orrillo, A.G.; Escalante, A.M.; Furlan, R.L.E. Dithioacetal exchange: A new reversible reaction for dynamic combinatorial chemistry. Chem. Eur. J. 2016, 22, 6746–6749. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, D.; Guo, D.; Wang, P.; Han, M. Chemoselective synthesis of unsymmetrical dithioacetals through sequential carbene insertion and acetal exchange of acylsilanes and thiols under visible light irradiation. Org. Lett. 2024, 26, 1282–1286. [Google Scholar] [CrossRef]
- Kariyawasam, L.S.; Highmoore, J.F.; Yang, Y. Chemically recyclable dithioacetal polymers via reversible entropy-driven ring-opening polymerization. Angew. Chem. Int. Ed. 2023, 62, e202303039. [Google Scholar] [CrossRef]
- Lei, J.; Wang, Q.; Du, F.; Li, Z. Dynamic ring-chain equilibrium of nucleophilic thiol-yne “Click” polyaddition for recyclable poly(dithioacetal)s. Chin. J. Polym. Sci. 2021, 39, 1146–1154. [Google Scholar] [CrossRef]
- Kassim, A.O.; Kariyawasam, L.S.; Yang, Y. Degradable polydithioacetals with adjustable mechanical properties and insights into entropy-driven ring-opening polymerization. Macromolecules 2025, 58, 3395–3406. [Google Scholar] [CrossRef]
- Luleburgaz, S.; Akar, E.; Tunca, U.; Durmaz, H. A straightforward and rapid synthesis of polydithioacetals in the presence of chlorodimethylsilane. Polym. Chem. 2024, 15, 371–383. [Google Scholar] [CrossRef]
- Hu, C.; Jin, Y.; Tian, W.; Yan, K.; Wang, J.; Yuan, L. Photodegradable dynamic polyurethane networks via dithioacetals. Macromolecules 2024, 57, 1725–1733. [Google Scholar] [CrossRef]
- Akar, E.; Tunca, U.; Durmaz, H. Polythioacetals: From old chemistry to new perspectives. Eur. Polym. J. 2024, 221, 113532. [Google Scholar] [CrossRef]
- Van Herck, N.; Maes, D.; Unal, K.; Guerre, M.; Winne, J.M.; Du Prez, F.E. Covalent adaptable networks with tunable exchange rates based on reversible thiol-yne cross-linking. Angew. Chem. Int. Ed. 2020, 59, 3609–3617. [Google Scholar] [CrossRef]
- Geetha Saraswathy, V.; Sankararaman, S. Chemoselective protection of aldehydes as dithioacetals in lithium perchlorate-diethyl ether medium. Evidence for the formation of oxocarbenium ion intermediate from acetals. J. Org. Chem. 1994, 59, 4665–4670. [Google Scholar] [CrossRef]
- Zeng, H.; Tang, Z.; Duan, Y.; Wu, S.; Guo, B. Recyclable crosslinked elastomer based on dynamic dithioacetals. Polymer 2021, 229, 124007. [Google Scholar] [CrossRef]
- Cui, C.; Zhao, X.; Wang, X.; Guo, Y.; Chen, K.; Ma, J.; Yan, X.; Cheng, Y.; Ge, Z.; Zhang, Y. Bio-based recyclable polydithioacetal covalent adaptable networks with activation-temperature-tunable shape memory properties. Polym. Chem. 2025, 16, 1595–1602. [Google Scholar] [CrossRef]
- Banerjee, G.; Chattopadhyay, P. Vanillin biotechnology: The perspectives and future. J. Sci. Food Agric. 2019, 99, 499–506. [Google Scholar] [CrossRef]
- Priefert, H.; Rabenhorst, J.; Steinbüchel, A. Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Stanzione Iii, J.F.; Sadler, J.M.; La Scala, J.J.; Reno, K.H.; Wool, R.P. Vanillin-based resin for use in composite applications. Green Chem. 2012, 14, 2346–2352. [Google Scholar] [CrossRef]
- Xu, L.; Liaqat, F.; Sun, J.; Khazi, M.I.; Xie, R.; Zhu, D. Advances in the vanillin synthesis and biotransformation: A review. Renew. Sustain. Energy Rev. 2024, 189, 113905. [Google Scholar] [CrossRef]
- Shi, G.; Li, T.; Zhang, D.; Zhang, J. Recyclable high-performance carbon fiber reinforced epoxy composites based on dithioacetal covalent adaptive network. Chin. J. Polym. Sci. 2024, 42, 1514–1524. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, C.; Wang, J.; Ding, Y.; Shi, J.; Wang, Z.; Xu, S.; Yuan, L. Thiol-aldehyde polycondensation for bio-based adaptable and degradable phenolic polymers. Angew. Chem. Int. Ed. 2023, 62, e202305677. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, W.; Cheng, Y.; Hu, C.; Wang, J.; Wang, B.; Yuan, L. Cross-linker-dependent fluorescence of vanillin-based dynamic epoxy networks. Macromolecules 2024, 57, 7439–7448. [Google Scholar] [CrossRef]
- Malmström, E.; Carlmark, A. Controlled grafting of cellulose fibres-an outlook beyond paper and cardboard. Polym. Chem. 2012, 3, 1702–1713. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Dupont, H.; Laurichesse, E.; Héroguez, V.; Schmitt, V. Green hydrophilic capsules from cellulose nanocrystal-stabilized pickering emulsion polymerization: Morphology control and spongelike behavior. Biomacromolecules 2021, 22, 3497–3509. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, J.; Shen, X.; Hang, G.; Li, L.; Zhang, T.; Zheng, S. Nanocomposites of polyurethane featuring cellulose nanocrystals and dynamic boronic ester bonds. ACS Appl. Polym. Mater. 2024, 6, 11320–11333. [Google Scholar] [CrossRef]
- Ge, W.; Zhao, B.; Li, L.; Nie, K.; Zheng, S. Nanocomposites of polyhydroxyurethane with nanocrystalline cellulose: Synthesis, thermomechanical and reprocessing properties. Eur. Polym. J. 2021, 149, 110287. [Google Scholar] [CrossRef]
- Ohaka, T.; Mekonnen, T.H. Recyclable and sustainable natural rubber biocomposite vitrimers induced by dynamic anhydride-epoxy bonds. ACS Appl. Polym. Mater. 2025, 7, 1347–1360. [Google Scholar] [CrossRef]
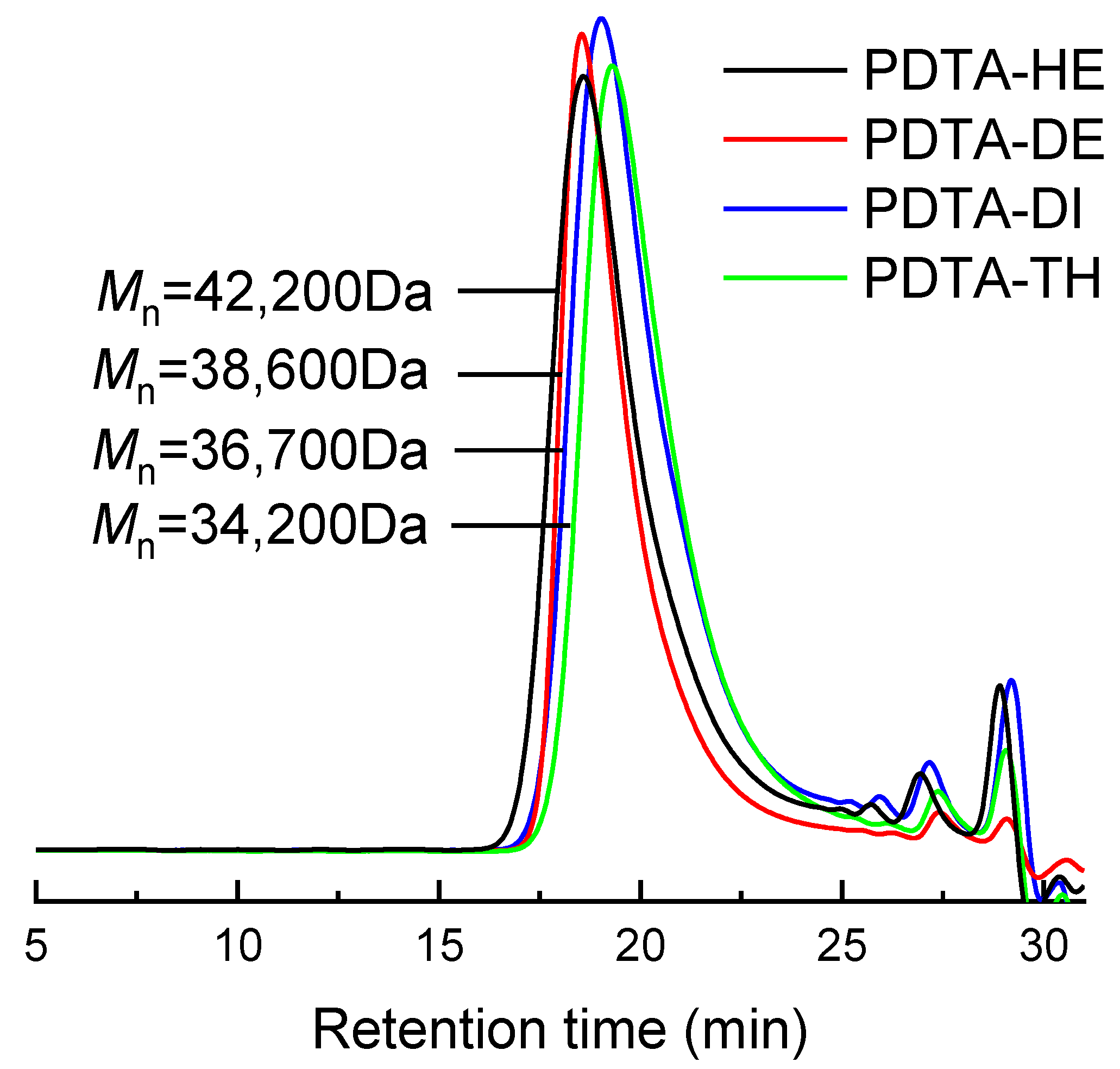





| PDTAs | Dithiols | Mn (Da) | Mw/Mn |
|---|---|---|---|
| PDTA-HE | 1,6-hexanedithiol, | 42,200 | 1.34 |
| PDTA-DE | 1,10-decanedithiol, | 38,600 | 1.32 |
| PDTA-TH | 2,2′-thiodiethanethiol | 34,200 | 1.40 |
| PDTA-DI | 3,6-dioxa-1,8-octanedithiol | 36,700 | 1.36 |
| Samples | Tg (°C) | σb(MPa) | E (MPa) | εb (%) |
|---|---|---|---|---|
| c-PDTA-HE | 48.0 | 43.40 ± 4.51 | 698 ± 10.4 | 6.2 ± 0.6 |
| c-PDTA-DE | 37.8 | 30.62 ± 3.26 | 556 ± 8.9 | 7.6 ± 1.5 |
| c-PDTA-TH | 33.6 | 10.03 ± 2.08 | 360 ± 5.7 | 47.2 ± 2.7 |
| c-PDTA-DI | 27.8 | 7.63 ± 1.45 | 21.2 ± 2.5 | 122.7 ± 5.8 |
| Samples | Tg (°C) | σb (MPa) | E (MPa) | εb (%) |
|---|---|---|---|---|
| c-PDTA-DI-CNC0 | 20.9 | 6.40 ± 0.32 | 19.5 ± 2.2 | 153.6 ± 8.4 |
| c-PDTA-DI-CNC5 | 22.1 | 10.45 ± 1.03 | 21.2 ± 3.1 | 133.3 ± 6.9 |
| c-PDTA-DI-CNC10 | 25.2 | 12.92 ± 1.21 | 112.3 ± 2.3 | 106.7 ± 5.4 |
| c-PDTA-DI-CNC15 | 28.6 | 16.75 ± 1.91 | 156.5 ± 3.8 | 91.6 ± 5.1 |
| c-PDTA-DI-CNC20 | 32.7 | 22.13 ± 2.17 | 236.2 ± 5.1 | 48.2 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Shen, X.; Teng, J.; Zhao, B.; Zheng, S. Biomass-Based Nanocomposites of Polydithioacetals Derived from Vanillin with Cellulose Nanocrystals: Synthesis, Thermomechanical and Reprocessing Properties. Polymers 2025, 17, 1764. https://doi.org/10.3390/polym17131764
Li L, Shen X, Teng J, Zhao B, Zheng S. Biomass-Based Nanocomposites of Polydithioacetals Derived from Vanillin with Cellulose Nanocrystals: Synthesis, Thermomechanical and Reprocessing Properties. Polymers. 2025; 17(13):1764. https://doi.org/10.3390/polym17131764
Chicago/Turabian StyleLi, Lei, Xibin Shen, Jianglu Teng, Bo Zhao, and Sixun Zheng. 2025. "Biomass-Based Nanocomposites of Polydithioacetals Derived from Vanillin with Cellulose Nanocrystals: Synthesis, Thermomechanical and Reprocessing Properties" Polymers 17, no. 13: 1764. https://doi.org/10.3390/polym17131764
APA StyleLi, L., Shen, X., Teng, J., Zhao, B., & Zheng, S. (2025). Biomass-Based Nanocomposites of Polydithioacetals Derived from Vanillin with Cellulose Nanocrystals: Synthesis, Thermomechanical and Reprocessing Properties. Polymers, 17(13), 1764. https://doi.org/10.3390/polym17131764







