Polyacrylonitrile/Silver Nanoparticles Composite for Catalytic Dye Reduction and Real-Time Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
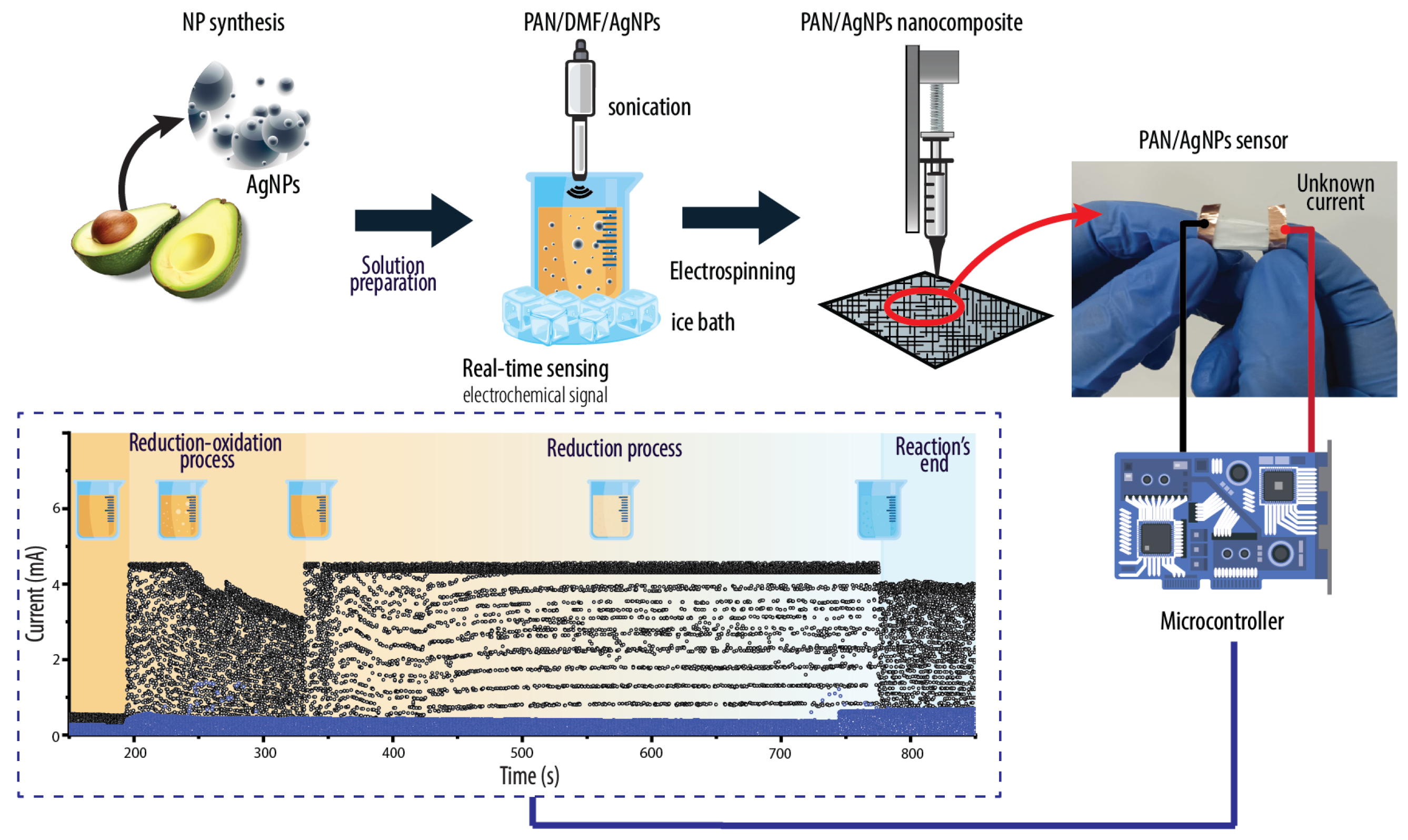
2.2. Preparation of AgNPs
2.3. Preparation of Electrospun PAN/AgNPs Membranes
2.4. Characterization
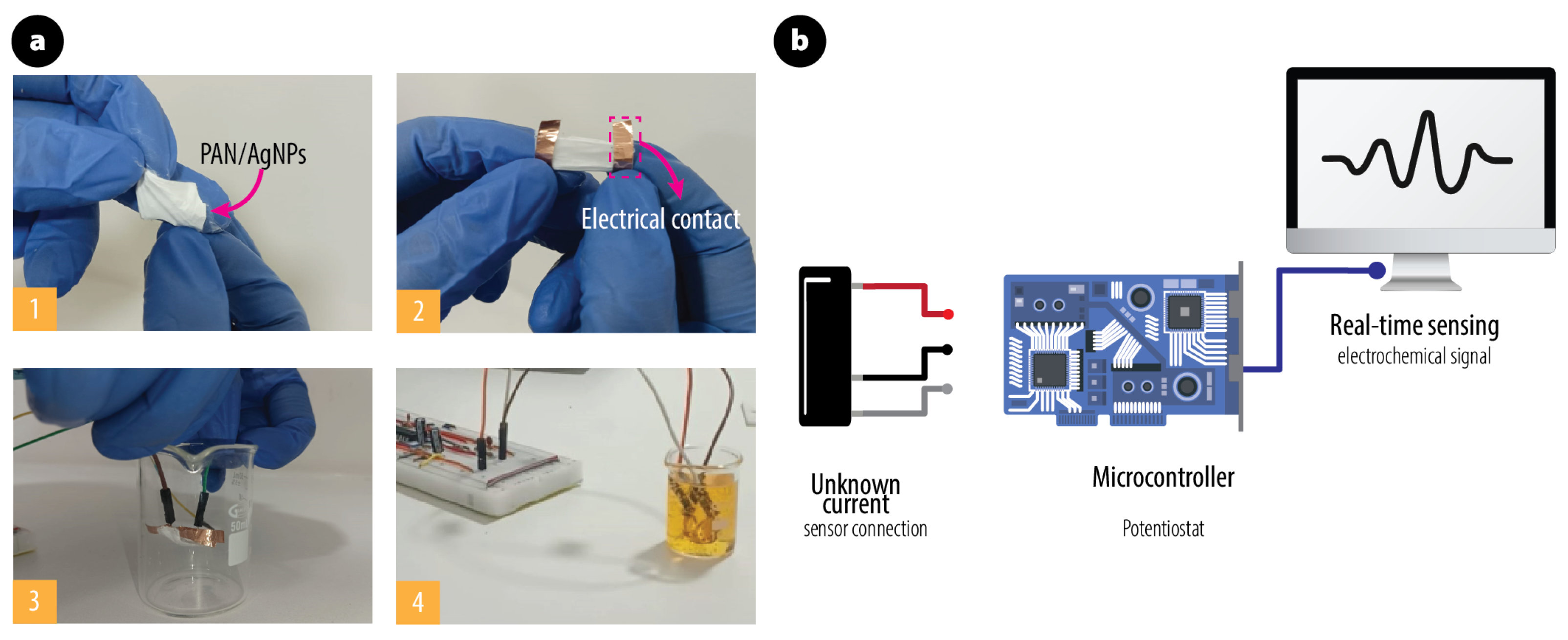
2.5. Evaluation of Catalytic Activity and Real-Time Electrochemical Sensing
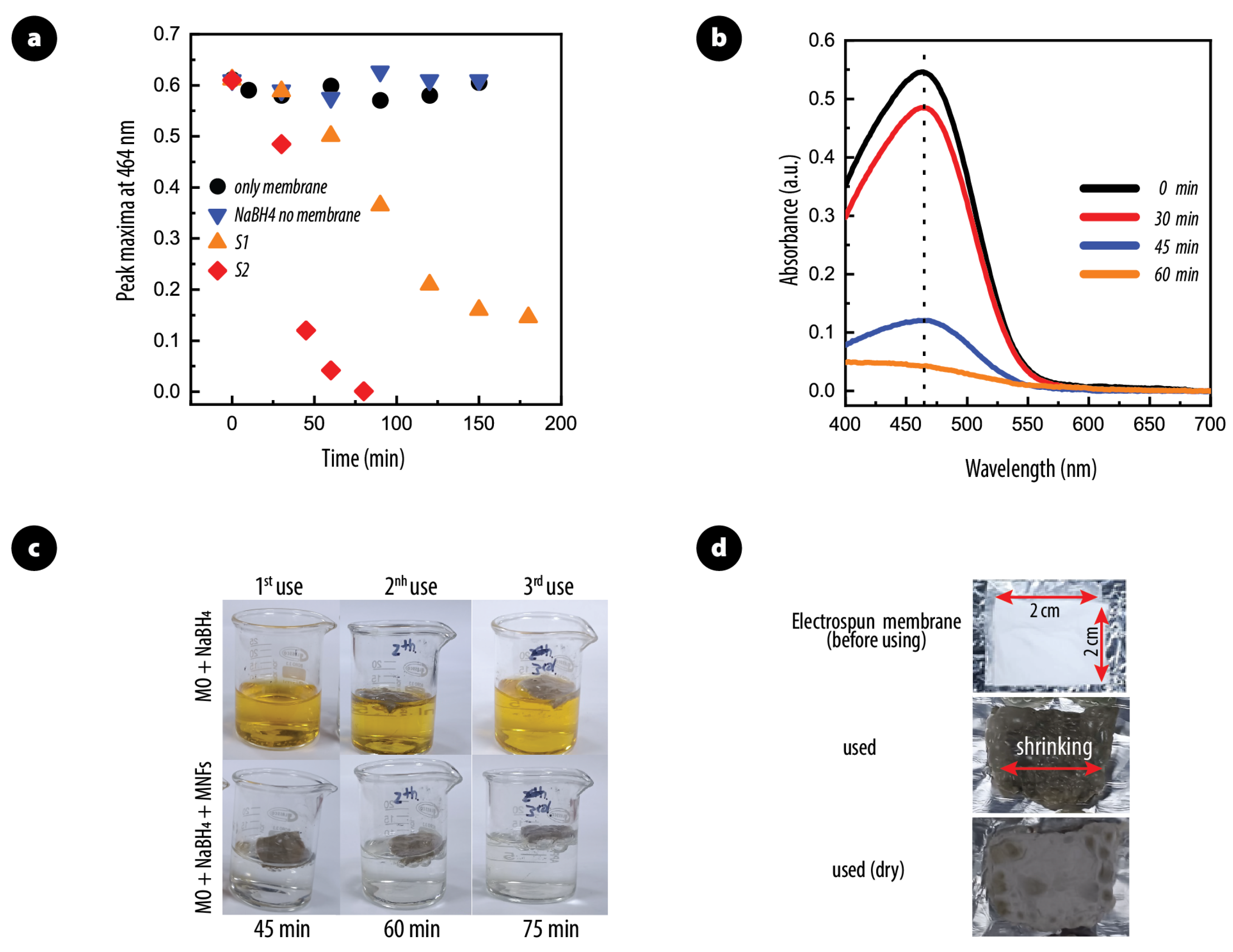
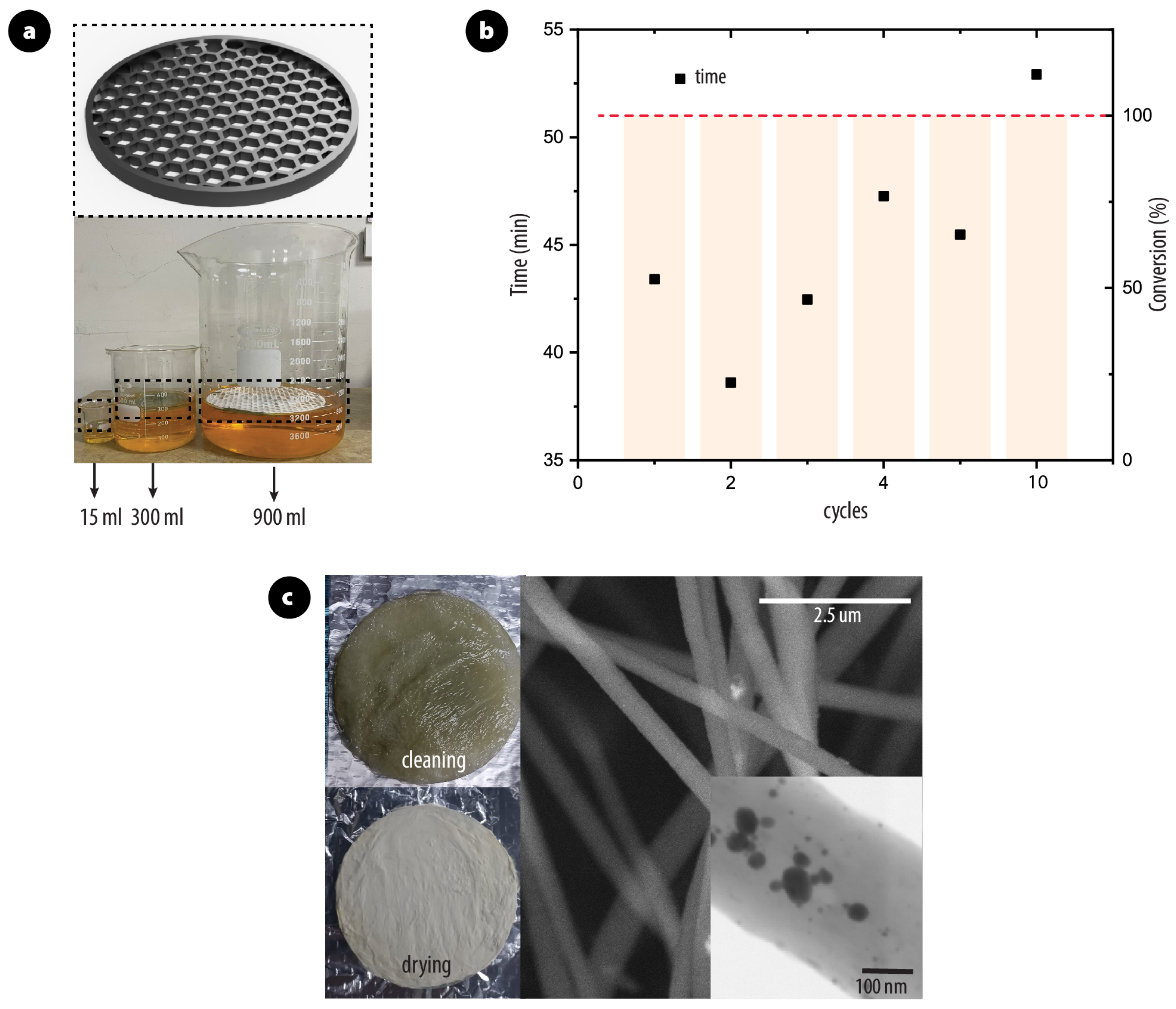
2.6. Scalability and Reusability
3. Results and Discussion
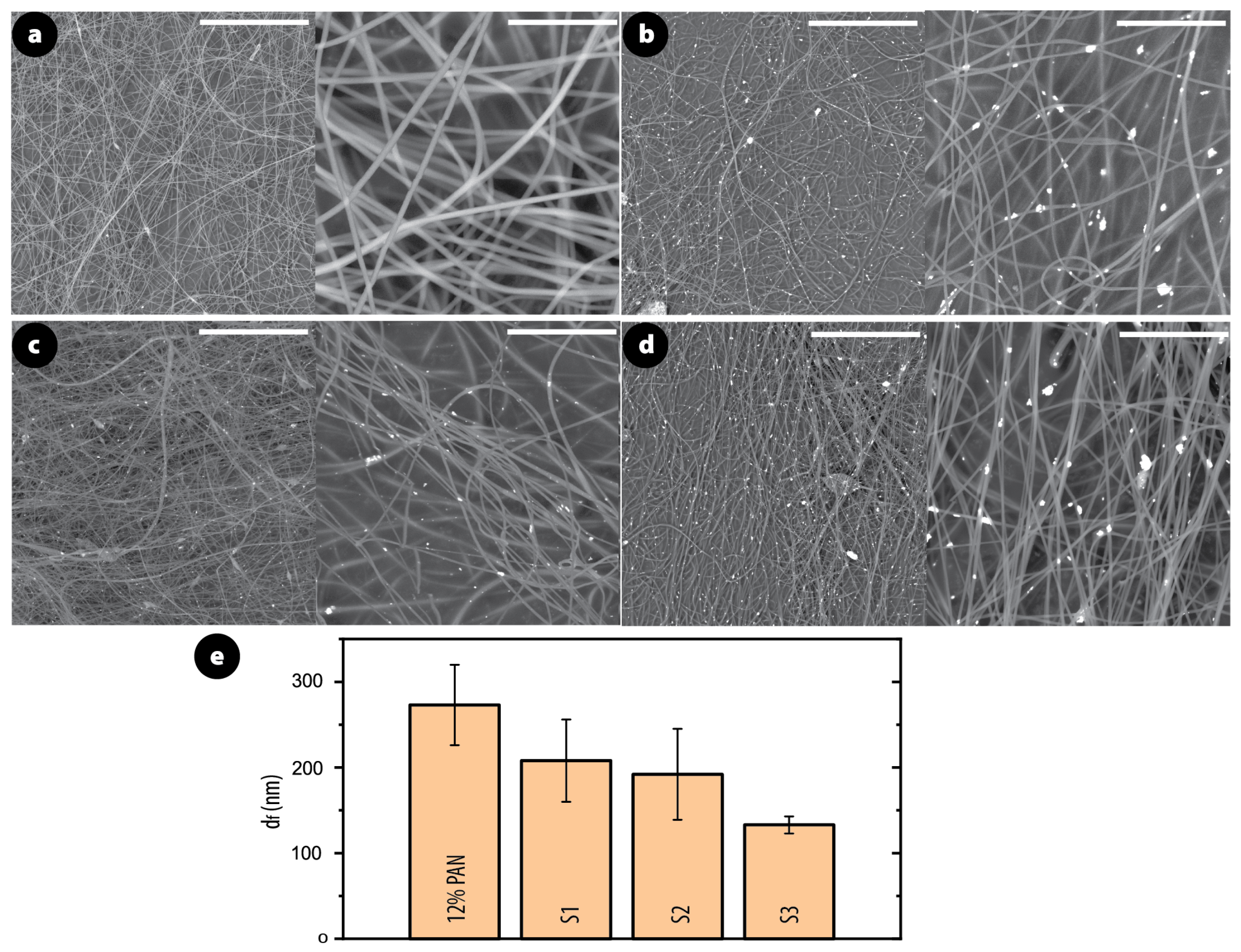
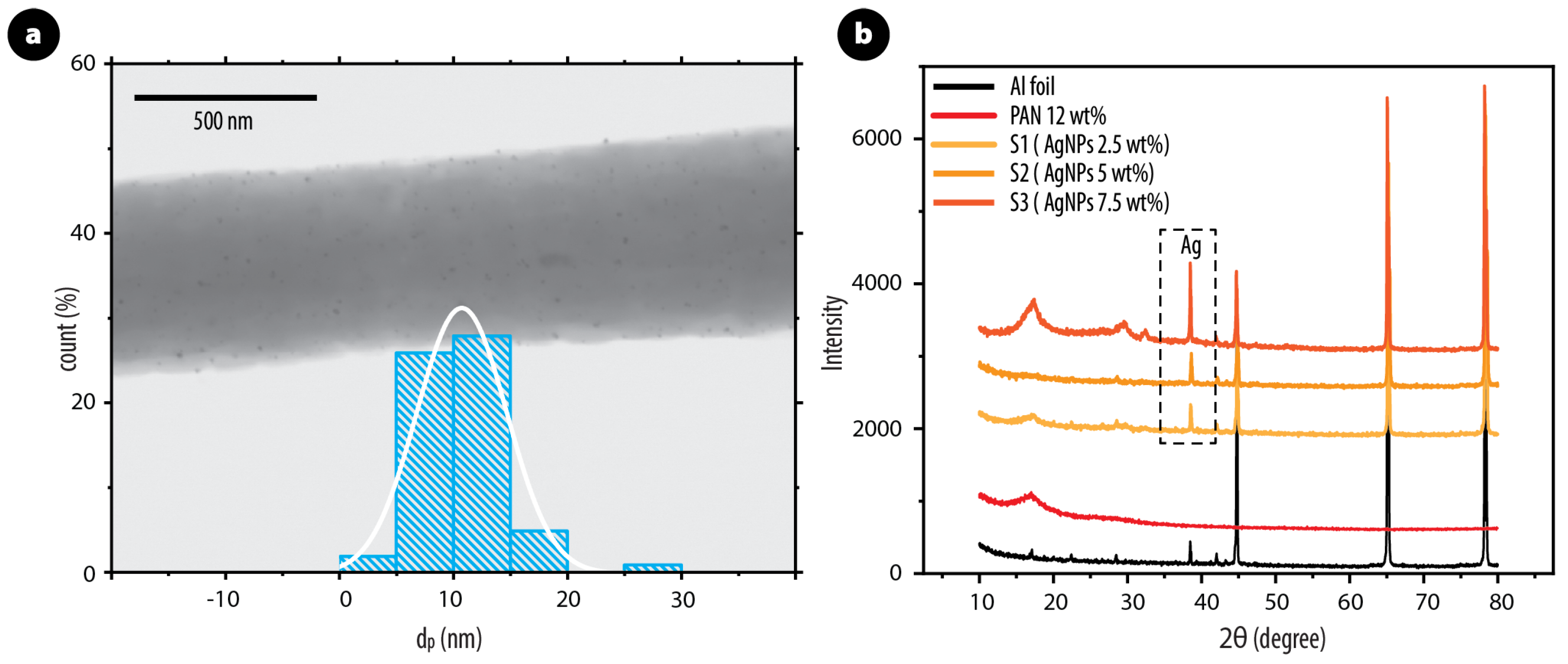
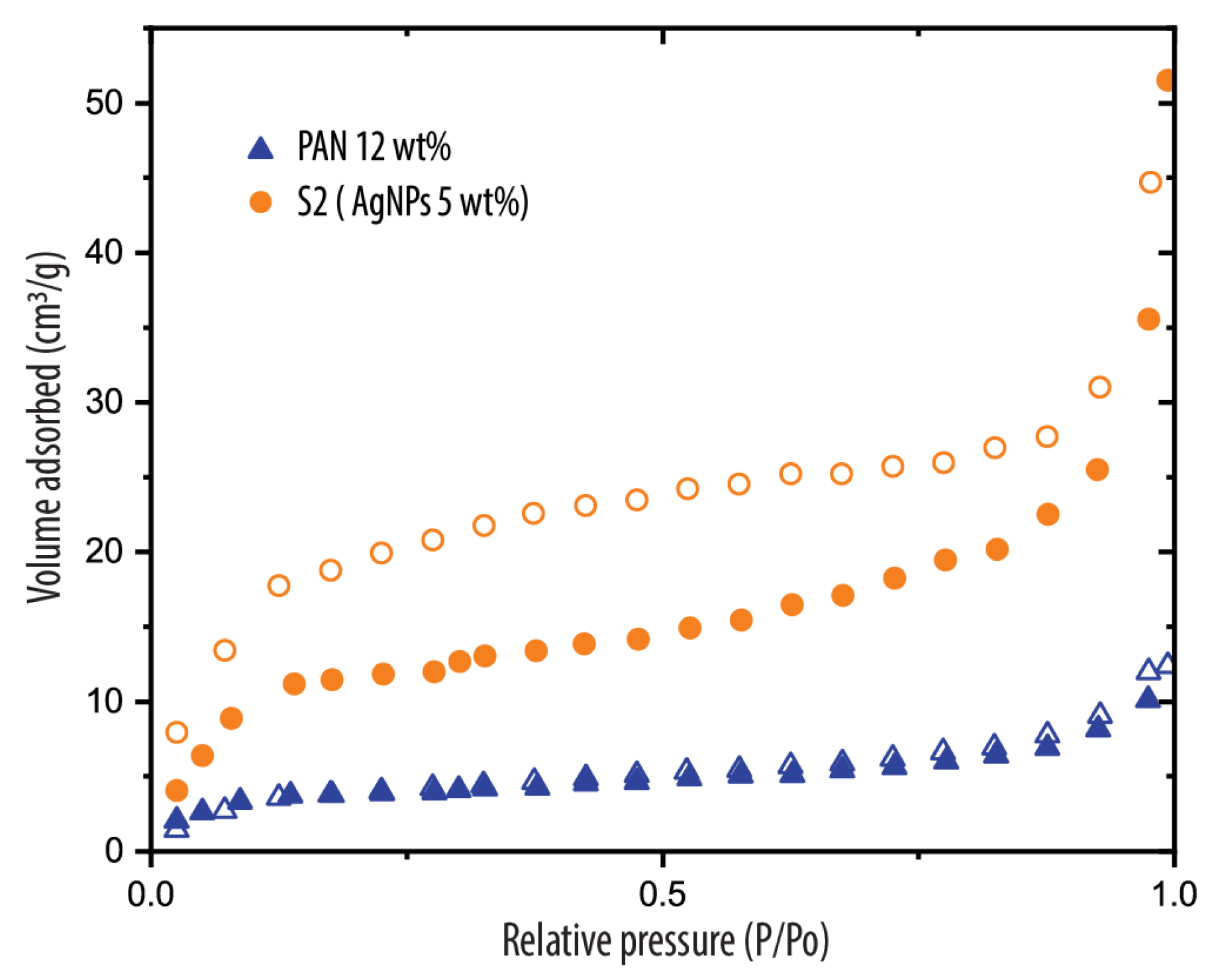
3.1. Morphology and Structure of PAN/AgNPs Nanofiber Membranes
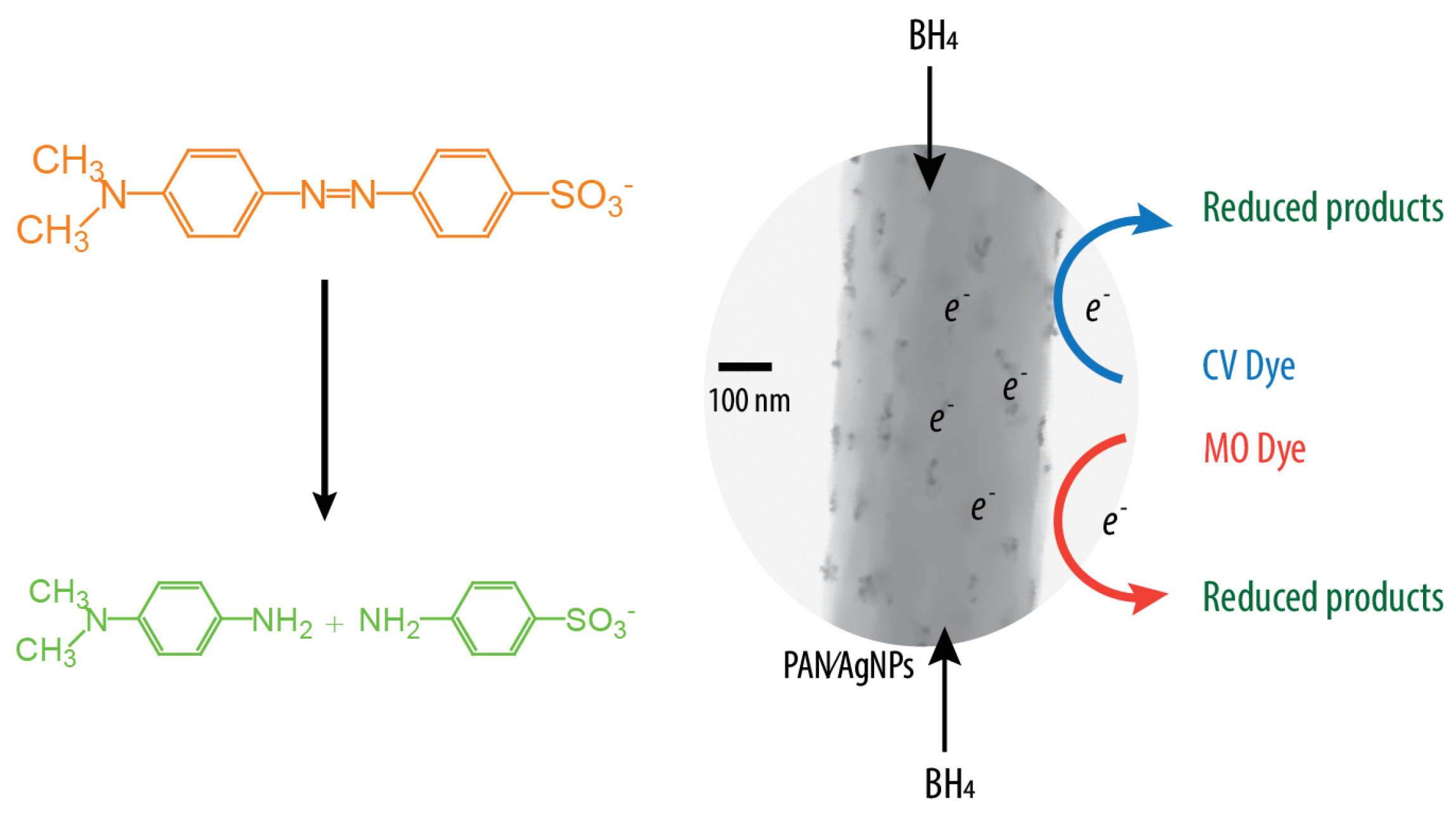
3.2. Catalytic Activity of PAN/AgNPs Nanofiber Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rashid, T.U.; Kabir, S.F.; Biswas, M.C.; Bhuiyan, M.R. Sustainable wastewater treatment via dye–surfactant interaction: A critical review. Ind. Eng. Chem. Res. 2020, 59, 9719–9745. [Google Scholar] [CrossRef]
- Khan, M.D.; Singh, A.; Khan, M.Z.; Tabraiz, S.; Sheikh, J. Current perspectives, recent advancements, and efficiencies of various dye-containing wastewater treatment technologies. J. Water Process. Eng. 2023, 53, 103579. [Google Scholar] [CrossRef]
- Negrete-Bolagay, D.; Zamora-Ledezma, C.; Chuya-Sumba, C.; De Sousa, F.B.; Whitehead, D.; Alexis, F.; Guerrero, V.H. Persistent organic pollutants: The trade-off between potential risks and sustainable remediation methods. J. Environ. Manag. 2021, 300, 113737. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Recent advances in biopolymer-based dye removal technologies. Molecules 2021, 26, 4697. [Google Scholar] [CrossRef]
- Sarkodie, B.; Amesimeku, J.; Frimpong, C.; Howard, E.K.; Feng, Q.; Xu, Z. Photocatalytic degradation of dyes by novel electrospun nanofibers: A review. Chemosphere 2023, 313, 137654. [Google Scholar] [CrossRef]
- Yang, X.; Cui, H.; Li, Y.; Qin, J.; Zhang, R.; Tang, H. Fabrication of Ag3PO4-graphene composites with highly efficient and stable visible light photocatalytic performance. ACS Catal. 2013, 3, 363–369. [Google Scholar] [CrossRef]
- Wang, W.; Hao, X.; Yan, Y.; Sun, R.; Petit, E.; Moderne, M.; Li, J.; Liu, J.; Wu, H.; Qi, K.; et al. 2D vermiculite nanolaminated membranes for efficient organic solvent nanofiltration. Adv. Funct. Mater. 2025, 35, 2410635. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, H.P.; Sharma, R.K. Metal nanoparticles with high catalytic activity in degradation of methyl orange: An electron relay effect. J. Mol. Catal. A Chem. 2011, 335, 248–252. [Google Scholar] [CrossRef]
- Sana, S.S.; Haldhar, R.; Parameswaranpillai, J.; Chavali, M.; Kim, S.C. Silver nanoparticles-based composite for dye removal: A comprehensive review. Clean. Mater. 2022, 6, 100161. [Google Scholar] [CrossRef]
- Pant, H.R.; Pandeya, D.R.; Nam, K.T.; Baek, W.i.; Hong, S.T.; Kim, H.Y. Photocatalytic and antibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J. Hazard. Mater. 2011, 189, 465–471. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. A green method to prepare nanosilica modified graphene oxide to inhibit nanoparticles re-aggregation during melt processing. Chem. Eng. J. 2017, 308, 1034–1047. [Google Scholar] [CrossRef]
- Chawla, J.; Singh, D.; Sundaram, B.; Kumar, A. Identifying challenges in assessing risks of exposures of silver nanoparticles. Expo. Health 2018, 10, 61–75. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, V.; Singh, P.P.; Matharu, A.S.; Zhang, W.; Kim, K.H.; Singh, J.; Rawat, M. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020, 143, 105924. [Google Scholar] [CrossRef]
- Sher Shah, M.S.A.; Park, A.R.; Zhang, K.; Park, J.H.; Yoo, P.J. Green synthesis of biphasic TiO2–reduced graphene oxide nanocomposites with highly enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2012, 4, 3893–3901. [Google Scholar] [CrossRef]
- Cañadas, A.; Gualle, A.; Vizuete, K.; Debut, A.; Rojas-Silva, P.; Ponce, S.; Orejuela-Escobar, L.M. Green Synthesis of Antibacterial Silver Nanocolloids with Agroindustrial Waste Extracts, Assisted by LED Light. Colloids Interfaces 2022, 6, 74. [Google Scholar] [CrossRef]
- Xiao, S.; Wu, S.; Shen, M.; Guo, R.; Huang, Q.; Wang, S.; Shi, X. Polyelectrolyte multilayer-assisted immobilization of zero-valent iron nanoparticles onto polymer nanofibers for potential environmental applications. ACS Appl. Mater. Interfaces 2009, 1, 2848–2855. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Z.; Kang, L.; Chen, X.; Tang, Y.; Liang, Q.; Tang, C.; Zhao, K. Piezoelectric plasma synergistic catalytic degradation of organic pollutants on BNBT/Ag composite nanofibers. Desalination 2024, 587, 117932. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Zamora-Ledezma, C.; Almeida, F.; Debut, A.; Vizuete, K.; Alexis, F. The impact of electric fields on rheology and fiber formation in ElectroHydroDynamics-based manufacturing. Eur. Phys. J. Spec. Top. 2025, 1–10. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Hashemi, A.R.; Hashemi, M.R.; Segura, L.J.; Ryzhakov, P.B. Computational ElectroHydroDynamics in microsystems: A Review of Challenges and Applications. Arch. Comput. Methods Eng. 2024, 32, 535–569. [Google Scholar] [CrossRef]
- Sitinjak, E.M.; Masmur, I.; Marbun, N.V.M.D.; Hutajulu, P.E.; Gultom, G.; Sitanggang, Y. Direct Z-scheme of n-type CuS/p-type ZnS@ electrospun PVP nanofiber for the highly efficient catalytic reduction of 4-nitrophenol and mixed dyes. RSC Adv. 2022, 12, 16165–16173. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S. Photocatalytic degradation of malachite green dye from aqueous solution using environmentally compatible Ag/ZnO polymeric nanofibers. Polymers 2021, 13, 2033. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical sensors and their applications: A review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Hsu, P.C.; Wang, S.; Wu, H.; Narasimhan, V.K.; Kong, D.; Ryoung Lee, H.; Cui, Y. Performance enhancement of metal nanowire transparent conducting electrodes by mesoscale metal wires. NAture Commun. 2013, 4, 2522. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, J.; Miao, Y.E.; Huang, Y.; Liu, T. Catalytic and antibacterial activities of green-synthesized silver nanoparticles on electrospun polystyrene nanofiber membranes using tea polyphenols. Compos. Part B Eng. 2015, 79, 217–223. [Google Scholar] [CrossRef]
- Ajitha, B.; Ahn, C.W.; Yadav, P.K.; Reddy, Y.A.K. Silver nanoparticle embedded polymethacrylic acid/polyvinylpyrrolidone nanofibers for catalytic application. J. Environ. Chem. Eng. 2021, 9, 106291. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Yang, S.; Du, J.; Wang, S.; Zhang, C.; Yang, Q.; Chen, X. Synthesis of AgCl/PAN composite nanofibres using an electrospinning method. Nanotechnology 2007, 18, 305601. [Google Scholar] [CrossRef]
- Hong, K.H.; Park, J.L.; Sul, I.H.; Youk, J.H.; Kang, T.J. Preparation of antimicrobial poly (vinyl alcohol) nanofibers containing silver nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- Zhuang, X.; Cheng, B.; Kang, W.; Xu, X. Electrospun chitosan/gelatin nanofibers containing silver nanoparticles. Carbohydr. Polym. 2010, 82, 524–527. [Google Scholar] [CrossRef]
- Li, C.; Fu, R.; Yu, C.; Li, Z.; Guan, H.; Hu, D.; Zhao, D.; Lu, L. Silver nanoparticle/chitosan oligosaccharide/poly (vinyl alcohol) nanofibers as wound dressings: A preclinical study. Int. J. Nanomed. 2013, 8, 4131–4145. [Google Scholar]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Destaye, A.G.; Lin, C.K.; Lee, C.K. Glutaraldehyde vapor cross-linked nanofibrous PVA mat with in situ formed silver nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4745–4752. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Hernández, A.B.; López-González, I.; Elango, J.; Paindépice, J.; Alexis, F.; González-Sánchez, M.; Morales-Flórez, V.; Mowbray, D.J.; Meseguer-Olmo, L. Fabrication, physical–chemical and biological characterization of retinol-loaded poly (vinyl alcohol) electrospun fiber mats for wound healing applications. Polymers 2023, 15, 2705. [Google Scholar] [CrossRef]
- Saquing, C.D.; Manasco, J.L.; Khan, S.A. Electrospun nanoparticle–nanofiber composites via a one-step synthesis. Small 2009, 5, 944–951. [Google Scholar] [CrossRef]
- Wang, S.; Bai, J.; Li, C.; Zhang, J. Functionalization of electrospun β-cyclodextrin/polyacrylonitrile (PAN) with silver nanoparticles: Broad-spectrum antibacterial property. Appl. Surf. Sci. 2012, 261, 499–503. [Google Scholar] [CrossRef]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-step green synthesis of antibacterial silver nanoparticles embedded in electrospun cyclodextrin nanofibers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, S.H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 43, 4423–4448. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Zamora-Ledezma, C.; Carrión-Matamoros, L.M.; Guerrero, I.E.; Debut, A.; Vizuete, K.; Haro, E.E.; López López, A.; Zamora-Ledezma, E. Influence of Ultrasonication on the Properties of Hybrid Electrospun Polyacrylonitrile and Silver Nanoparticles Fibers and Their Potential Use in Water Decontamination. In Applied Technologies, ICAT 2021; Springer: Cham, Switzerland, 2021; pp. 176–188. [Google Scholar]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Noar, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.; McCord, M.; Zhang, X. One-step synthesis of silver nanoparticle-filled nylon 6 nanofibers and their antibacterial properties. J. Mater. Chem. 2011, 21, 10330–10335. [Google Scholar] [CrossRef]
- He, M.; Chen, M.; Dou, Y.; Ding, J.; Yue, H.; Yin, G.; Chen, X.; Cui, Y. Electrospun silver nanoparticles-embedded feather keratin/poly (vinyl alcohol)/poly (ethylene oxide) antibacterial composite nanofibers. Polymers 2020, 12, 305. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Cornejo-Bravo, J.M.; Vera-Graziano, R.; Grande, D. Electrospinning as a powerful technique for biomedical applications: A critically selected survey. J. Biomater. Sci. Polym. Ed. 2016, 27, 157–176. [Google Scholar] [CrossRef]
- Hartman, T.; Geitenbeek, R.G.; Wondergem, C.S.; Van Der Stam, W.; Weckhuysen, B.M. Operando nanoscale sensors in catalysis: All eyes on catalyst particles. ACS Nano 2020, 14, 3725–3735. [Google Scholar] [CrossRef] [PubMed]
- Narváez-Muñoz, C.; Zamora-Ledezma, C.; Ryzhakov, P.; Pons-Prats, J.; Elango, J.; Mena, C.; Navarrete, F.; Morales-Flórez, V.; Cano-Crespo, R.; Segura, L.J. Improving glass-fiber epoxy composites via interlayer toughening with polyacrylonitrile/multiwalled carbon nanotubes electrospun fibers. J. Appl. Polym. Sci. 2023, 140, e53400. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Ryzhakov, P.; Pons-Prats, J. Determination of the operational parameters for the manufacturing of spherical PVP particles via electrospray. Polymers 2021, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.o.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Rujitanaroj, P.o.; Pimpha, N.; Supaphol, P. Preparation, characterization, and antibacterial properties of electrospun polyacrylonitrile fibrous membranes containing silver nanoparticles. J. Appl. Polym. Sci. 2010, 116, 1967–1976. [Google Scholar] [CrossRef]
- Zhong, L.; Yang, T.; Wang, J.; Huang, C.Z. A study of the catalytic ability of in situ prepared AgNPs–PMAA–PVP electrospun nanofibers. New J. Chem. 2015, 39, 9518–9524. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Saraswathi, V.S.; Kamarudheen, N.; BhaskaraRao, K.; Santhakumar, K. Phytoremediation of dyes using Lagerstroemia speciosa mediated silver nanoparticles and its biofilm activity against clinical strains Pseudomonas aeruginosa. J. Photochem. Photobiol. B Biol. 2017, 168, 107–116. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, J.; Sood, J. Rayleigh-Bénard cell formation of green synthesized nano-particles of silver and selenium. Mater. Today Proc. 2020, 28, 1781–1787. [Google Scholar] [CrossRef]
- Li, Y.C.; Melenbrink, E.L.; Cordonier, G.J.; Boggs, C.; Khan, A.; Isaac, M.K.; Nkhonjera, L.K.; Bahati, D.; Billinge, S.J.; Haile, S.M.; et al. An easily fabricated low-cost potentiostat coupled with user-friendly software for introducing students to electrochemical reactions and electroanalytical techniques. J. Chem. Educ. 2018, 95, 1443–1684. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narváez-Muñoz, C.; Ponce, S.; Durán, C.; Aguayo, C.; Portero, C.; Guamán, J.; Debut, A.; Granda, M.; Alexis, F.; Zamora-Ledezma, E.; et al. Polyacrylonitrile/Silver Nanoparticles Composite for Catalytic Dye Reduction and Real-Time Monitoring. Polymers 2025, 17, 1762. https://doi.org/10.3390/polym17131762
Narváez-Muñoz C, Ponce S, Durán C, Aguayo C, Portero C, Guamán J, Debut A, Granda M, Alexis F, Zamora-Ledezma E, et al. Polyacrylonitrile/Silver Nanoparticles Composite for Catalytic Dye Reduction and Real-Time Monitoring. Polymers. 2025; 17(13):1762. https://doi.org/10.3390/polym17131762
Chicago/Turabian StyleNarváez-Muñoz, Christian, Sebastián Ponce, Carlos Durán, Cristina Aguayo, Cesar Portero, Joseph Guamán, Alexis Debut, Magaly Granda, Frank Alexis, Ezequiel Zamora-Ledezma, and et al. 2025. "Polyacrylonitrile/Silver Nanoparticles Composite for Catalytic Dye Reduction and Real-Time Monitoring" Polymers 17, no. 13: 1762. https://doi.org/10.3390/polym17131762
APA StyleNarváez-Muñoz, C., Ponce, S., Durán, C., Aguayo, C., Portero, C., Guamán, J., Debut, A., Granda, M., Alexis, F., Zamora-Ledezma, E., & Zamora-Ledezma, C. (2025). Polyacrylonitrile/Silver Nanoparticles Composite for Catalytic Dye Reduction and Real-Time Monitoring. Polymers, 17(13), 1762. https://doi.org/10.3390/polym17131762








