Biocompatible Glues: Recent Progress and Emerging Frontiers in Surgical Adhesion
Abstract
1. Introduction
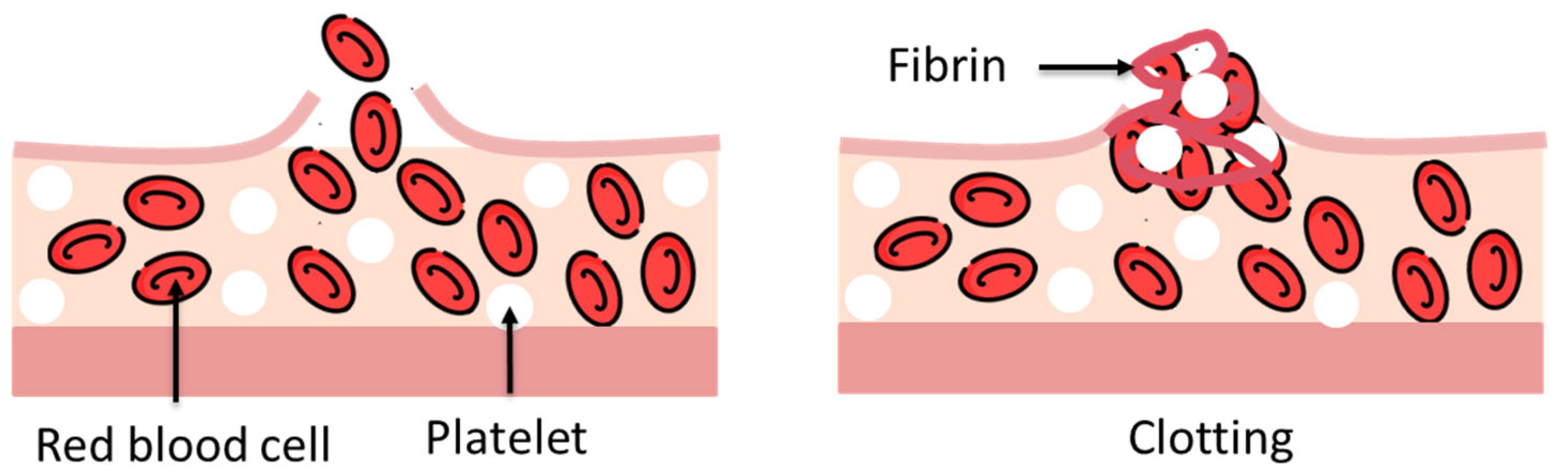
- Hemostats: require the presence of blood to initiate clot formation, accelerating hemostasis via mechanical means. They are especially useful in cases of non-suturable hemorrhages.
- Sealants: designed to prevent fluid leakage, they are effective against a variety of bodily fluids beyond blood and are available in both active and flowable formulations.
- Bioadhesives: biocompatible glues used to bond biological materials—either native tissues or synthetic/biological implants—to biological surfaces such as skin, blood vessels, or organs [10].
- Biocompatibility: both the adhesive and its degradation products must be safe, non-toxic, and non-immunogenic.
- Ease of application and repositionability: the material should be user-friendly and allow repositioning shortly after application.
- Fast gelation: the adhesive must gel or cure rapidly, even in moist environments containing biological fluids.
- Strong bonding under wet conditions: after crosslinking, the material should exhibit good flexibility and maintain robust adhesion in physiological environments.
- Physiological degradation: ideally, the adhesive should undergo enzymatic or hydrolytic degradation into non-toxic byproducts, which are then safely excreted via the kidneys or liver [24].
- Safety: no toxicity, no disease transmission, and biocompatibility of degradation products.
- Efficacy: high enough bonding strength to perform reliably in the intended surgical environment.
- Usability: the material should be easy and quick to apply.
- Affordability: cost must remain reasonable (ideally under $100 per application) to be competitive in surgical settings.
- Regulatory Approval: products must obtain formal approval (e.g., from the FDA) for clinical use [28].
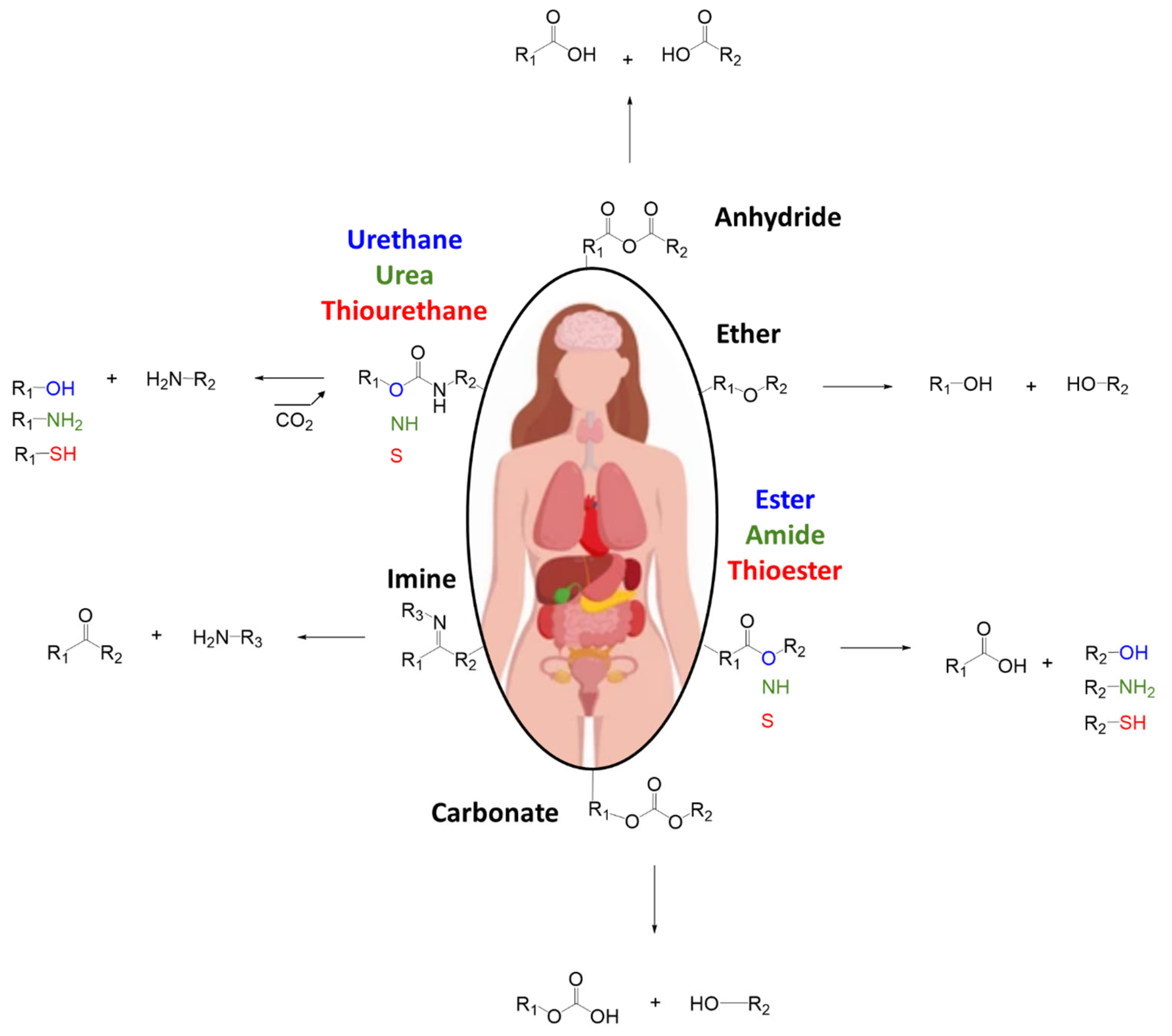
2. Mechanisms of Adhesion
2.1. Physiological Mechanisms
2.2. Physical Bonds
2.2.1. Physical Interactions
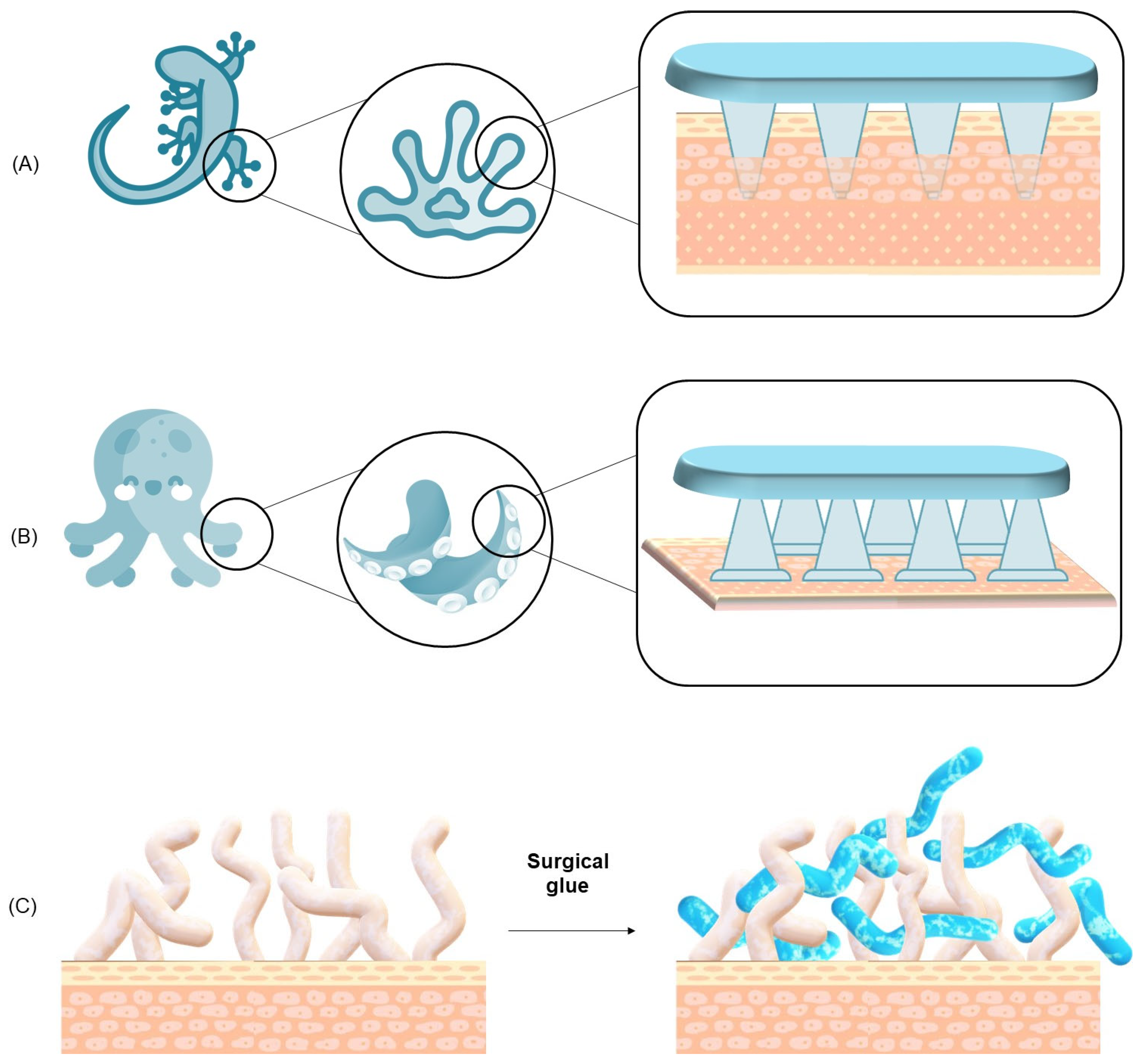
- Van der Waals Forces: these are fundamental, short-range interactions that arise from induced or permanent dipoles between molecules. Although individually weak, they become collectively significant when two surfaces are brought into nanometer-scale proximity, enabling effective adhesion. These forces are critical in the adhesion mechanisms of organisms such as geckos, which utilize microscopic fibrillar structures on their feet to maximize contact area and thus enhance van der Waals interactions across the substrate interface.
- Hydrogen Bonds: strong directional interactions formed between hydrogen atoms covalently bound to electronegative atoms (typically oxygen or nitrogen) and lone pairs on other electronegative atoms. These bonds are critical in protein folding and many natural adhesives.
- Electrostatic Interactions: result from attractions between oppositely charged ions or dipoles. The interaction strength depends on ionic concentration, pH, and the dielectric properties of the surrounding medium.
- Hydrophobic Interactions: nonpolar surfaces aggregate in aqueous environments to minimize their exposure to water, leading to adhesion through entropy-driven processes. These interactions are particularly relevant in lipid-rich tissues.
- Mechanical Interlocking: adhesive penetration into micro- or nano-scale roughness or pores on the tissue surface provides anchoring through physical constraint.
- Polymer Chain Entanglement: long polymer chains from the adhesive can diffuse into the tissue matrix, leading to entanglement and increased interfacial cohesion. This interpenetration enhances load transfer and improves the durability of the adhesive bond.
2.2.2. Mechanical Interlocking in Surgical Adhesives
2.3. Chemical Bonds
- Methacrylates: often used in photopolymerizable adhesives, these groups undergo radical polymerization upon exposure to light. Although effective, their need for external initiation (e.g., UV light) can limit intraoperative utility.
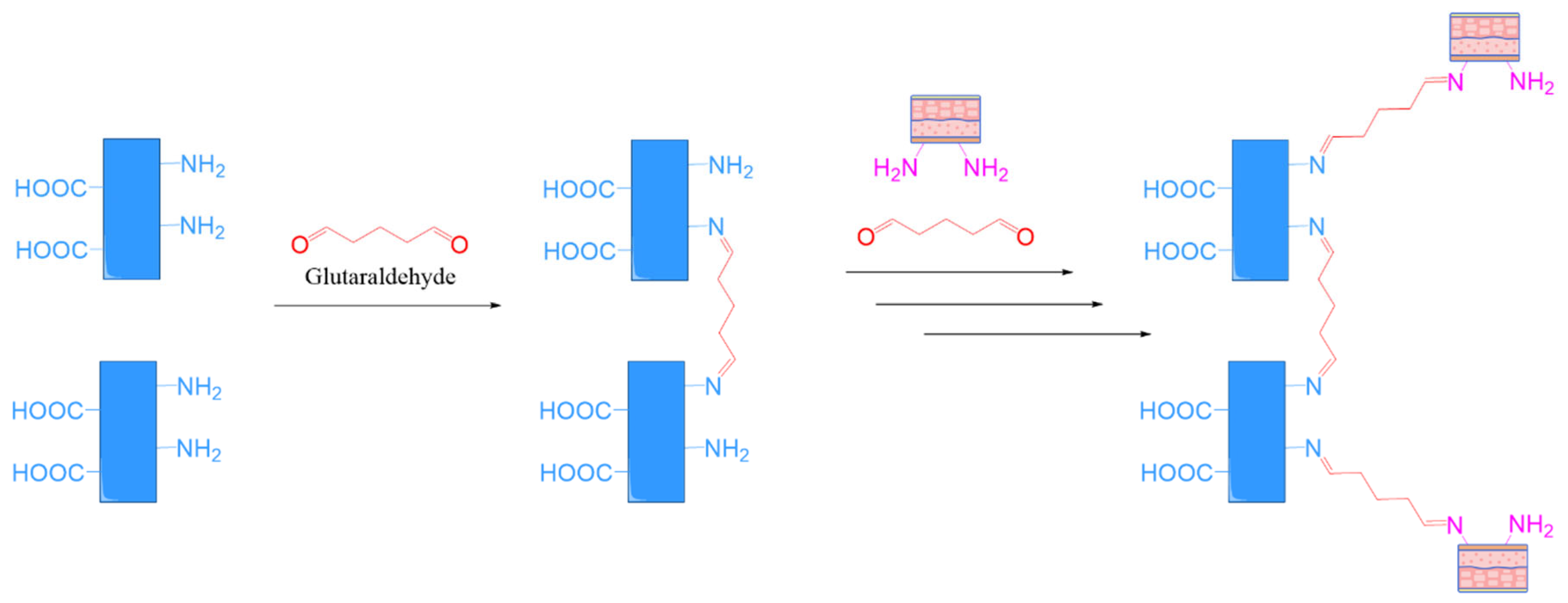
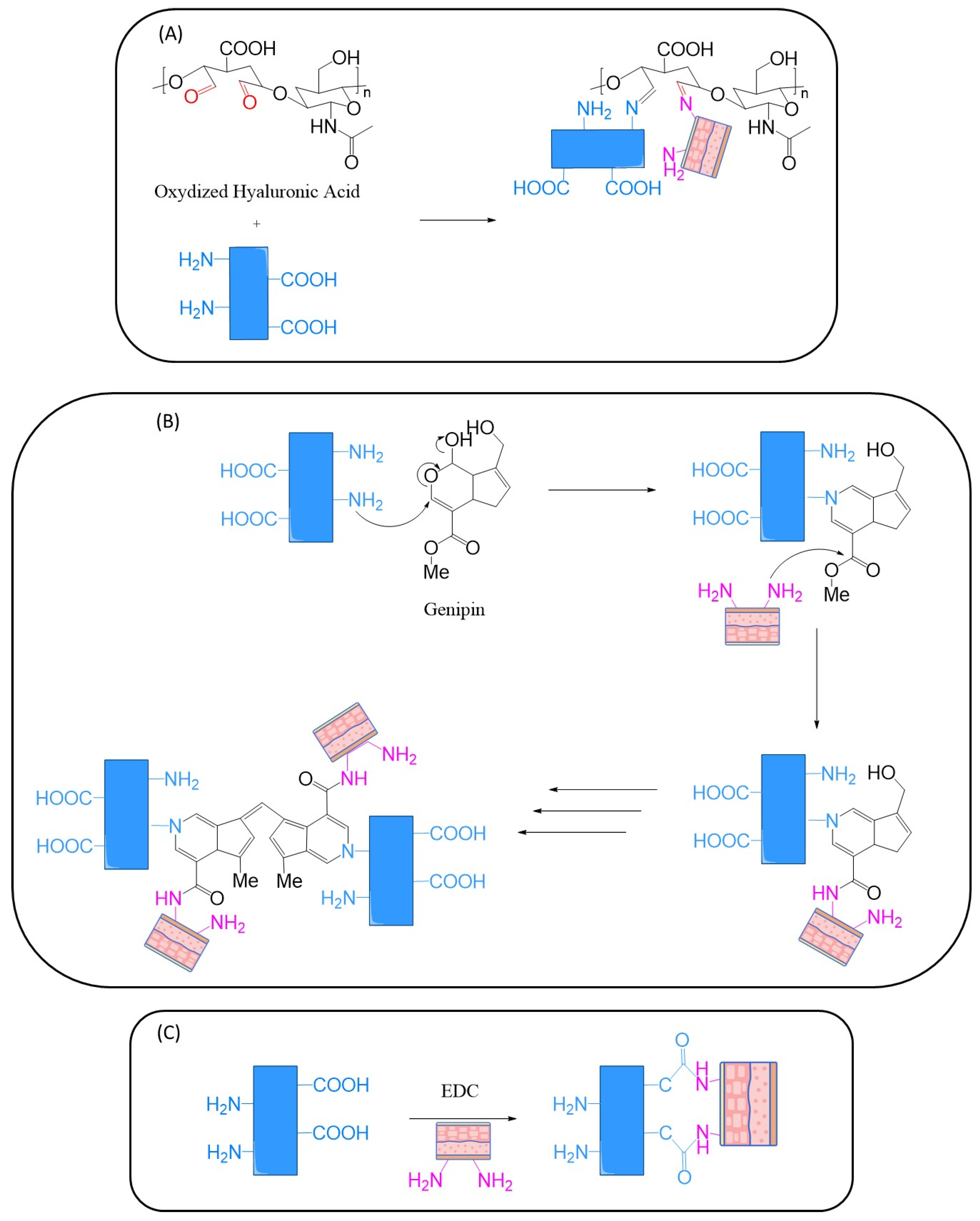
- Aldehyde Chemistry: aldehydes can react with primary amines in tissues to form Schiff bases (imines), which may further stabilize via crosslinking. However, some aldehydes (e.g., glutaraldehyde) are associated with cytotoxicity and must be used with caution.
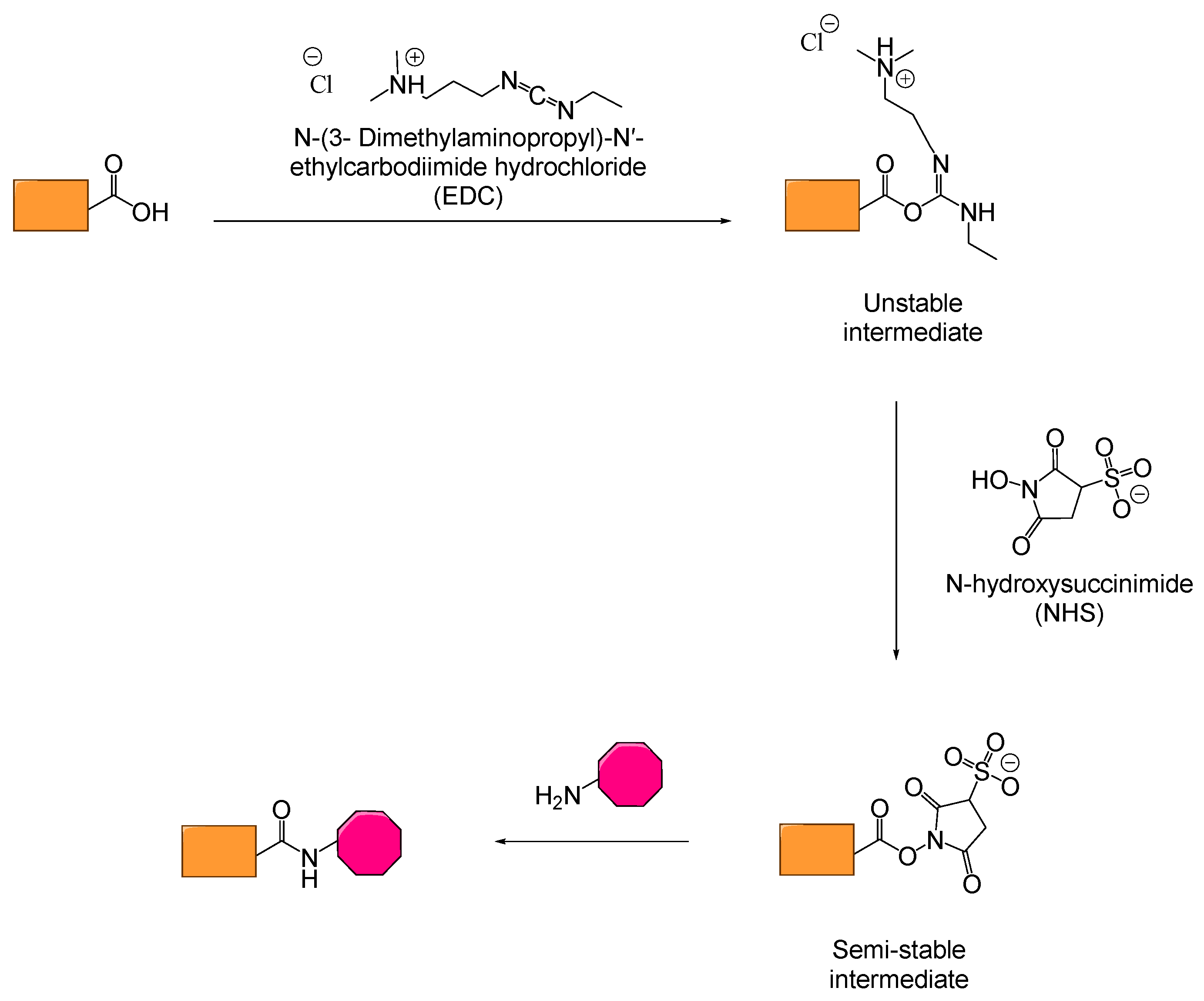
- NHS-Esters: N-hydroxysuccinimide esters react rapidly and selectively with primary amines, forming stable amide bonds. Their high reactivity and relatively low toxicity make them attractive for bioadhesive development.
- Epoxides and Isocyanates: these groups can form covalent bonds with various nucleophilic tissue groups. However, while epoxides are generally less reactive than isocyanates, both groups can pose biocompatibility challenges. Isocyanates, in particular, are highly reactive and potentially toxic, which severely limits their use in medical products. To improve biocompatibility, their application often requires chemical modifications, such as the addition of blocking groups.
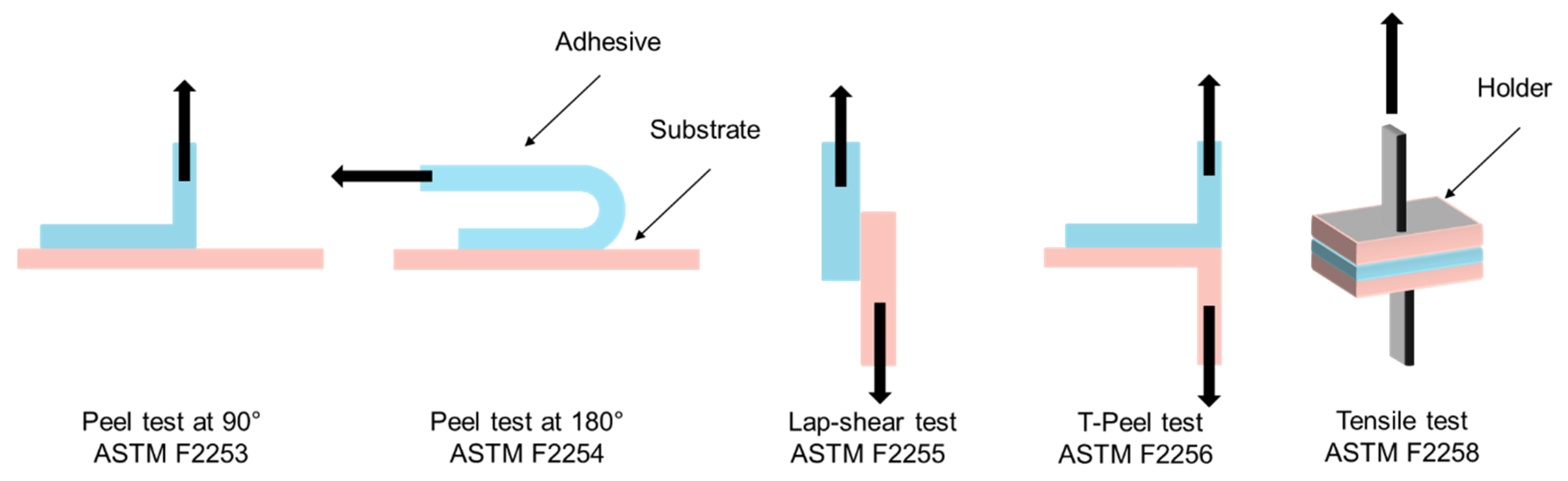
3. Adhesion Tests
3.1. Overview of Adhesion Testing Methods
- Peel Tests (90° and 180°): the peel test measures the force required to detach an adhesive from a substrate at a specified angle, typically 90° or 180°. This method is especially relevant for adhesives applied to flexible substrates such as skin or thin membranes. In the 90° peel test, the adhesive is peeled perpendicularly from the backing, making it particularly sensitive to the cohesive strength of the adhesive. In contrast, the 180° peel test involves peeling the adhesive parallel to the substrate, more accurately replicating the forces that surgical adhesives might experience on curved or expansive body surfaces.
- Lap Shear Test: the lap shear test evaluates the shear strength of an adhesive by applying a force parallel to the bonded surfaces. This test is particularly suited for adhesives used in anatomical regions subject to lateral stresses, such as joints or internal tissues under tension. It is especially informative for characterizing gel-based polymer adhesives and viscoelastic glues, where the ability to withstand shear stress without rupturing is critical.
- T-Peel Test: the T-peel test is a variation of the peel test designed to assess the peel strength of adhesives applied to soft, deformable substrates such as skin or membranes. It provides important data on adhesive performance in dynamic environments where bonded surfaces undergo frequent movement or mechanical deformation.
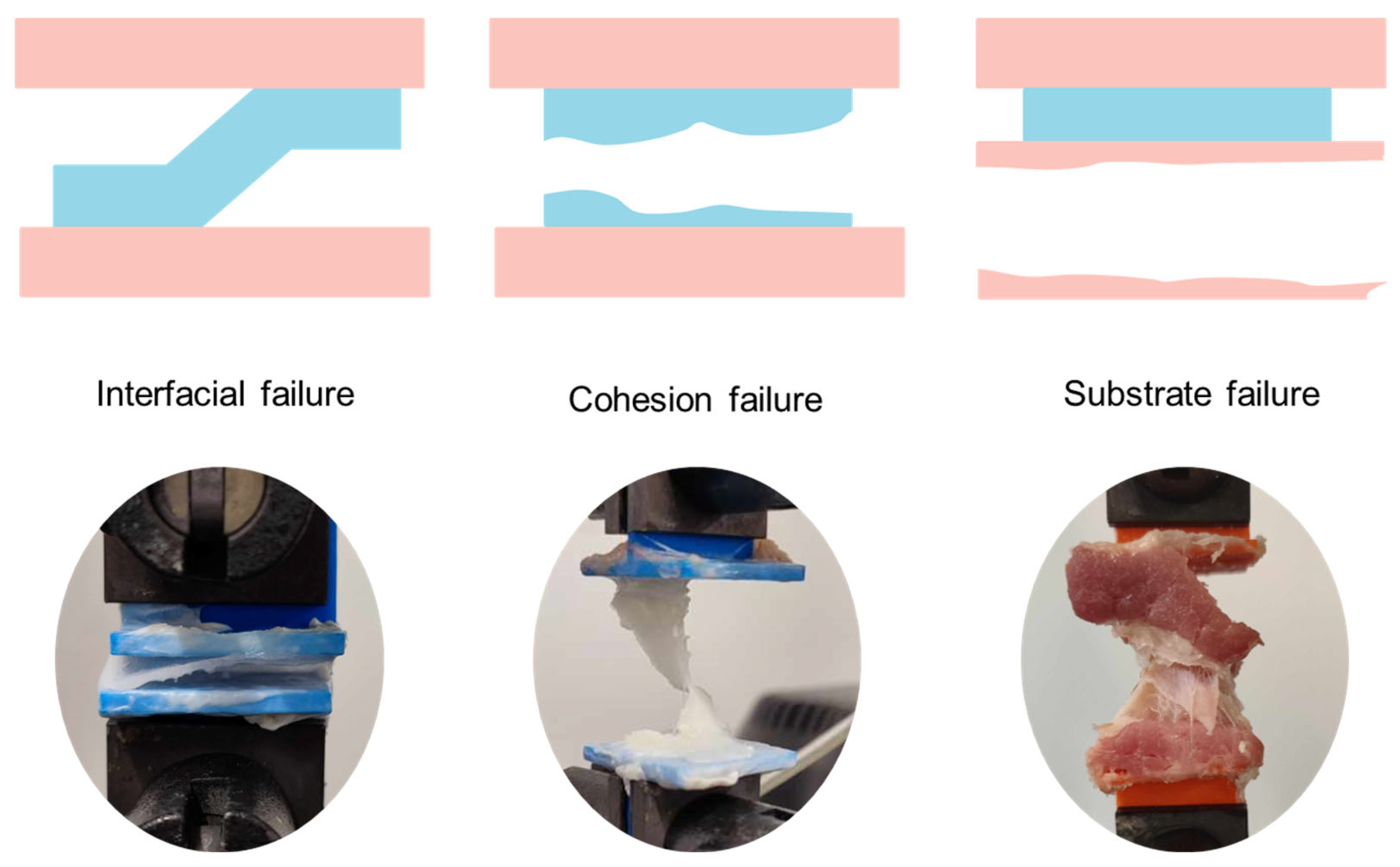
- Tensile Test: the tensile test measures the resistance of an adhesive bond to a force applied perpendicularly to the bonded interface, up to the point of complete failure. This test is particularly relevant for assessing adhesives used in wound closure under tension. It provides key parameters such as tensile strength and ultimate bond strength. A cohesive failure indicates that the adhesive itself has ruptured, while an interfacial failure points to a breakdown in adhesion to the tissue.
3.2. Failure Modes
- Adhesive Failure: occurs when the bond between the adhesive and the substrate fails, indicating poor interfacial interaction.
- Cohesive Failure: the failure occurs within the adhesive material itself, suggesting strong adhesion to the substrate but insufficient cohesive strength.
- Substrate Failure: the tissue or test material fails before the adhesive, indicating superior bonding strength.
- Mixed Failure: a combination of the above, typically considered acceptable if the adhesive bond is maintained under stress.
3.3. Parameters Affecting Adhesion Performance
3.4. Regulatory Considerations
4. Skin Pressure-Sensitive Adhesives
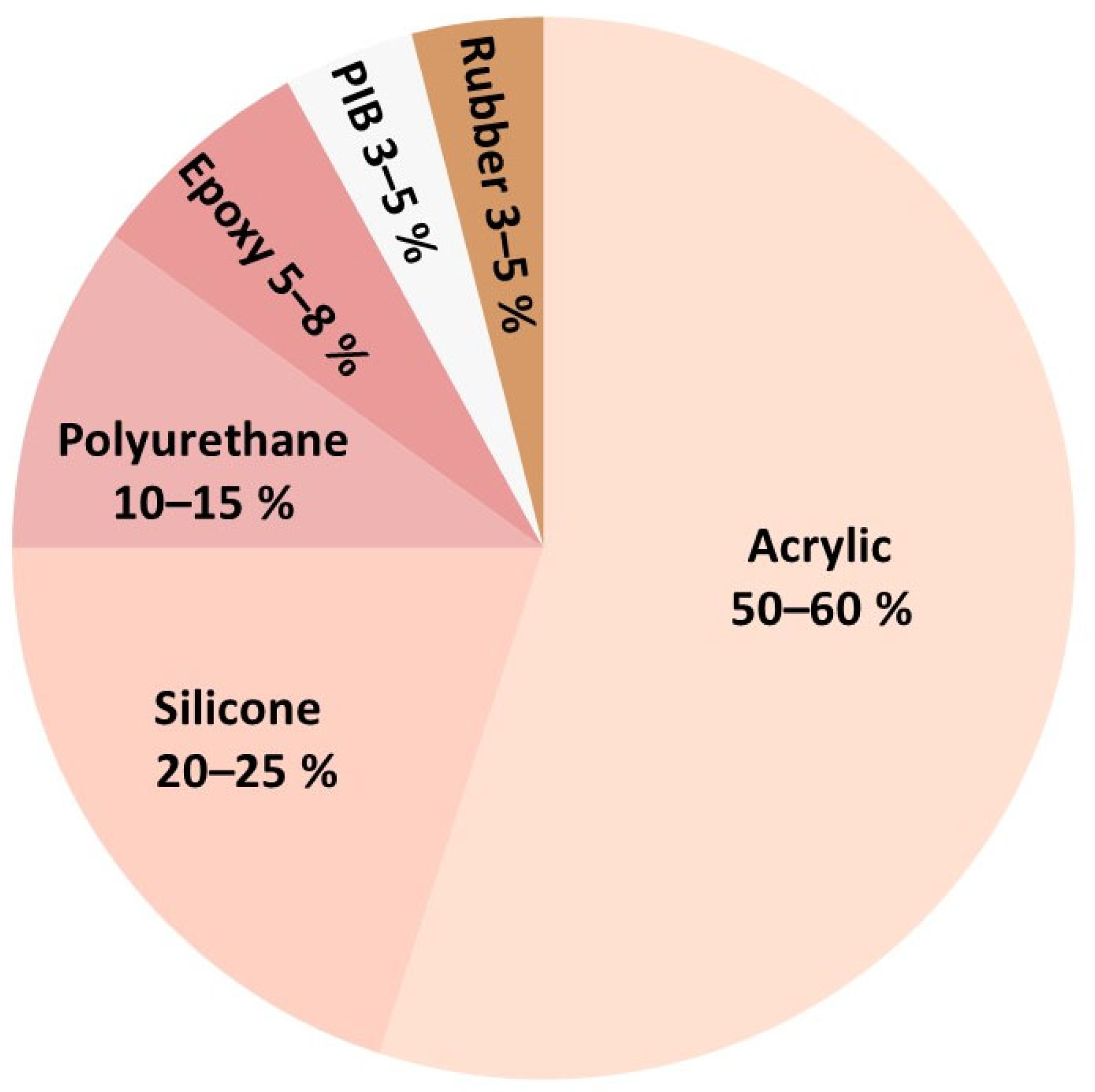
4.1. Polyacrylates
4.2. Silicone
4.3. Polyurethanes
4.4. Polyisobutylene
4.5. Rubber
4.6. Smart Adhesives
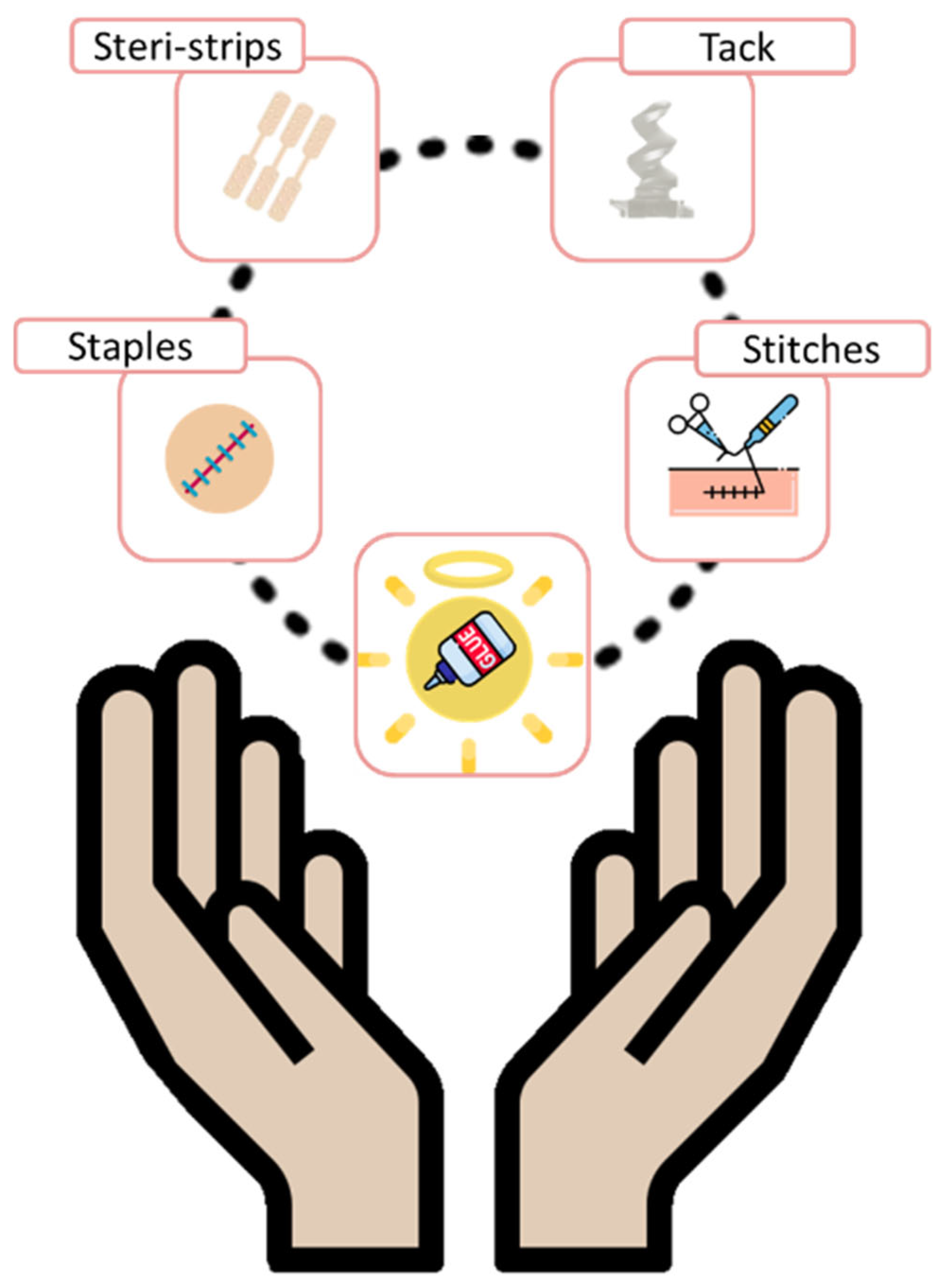
5. Surgical Use Adhesives
5.1. Wound Closure Adhesives
5.2. Implantable Surgical Adhesives
5.2.1. Biological and Biochemical Adhesives
A. Based on Proteins
- (a)
- Fibrin
- (b)
- Collagen and gelatin
- (c)
- Albumin
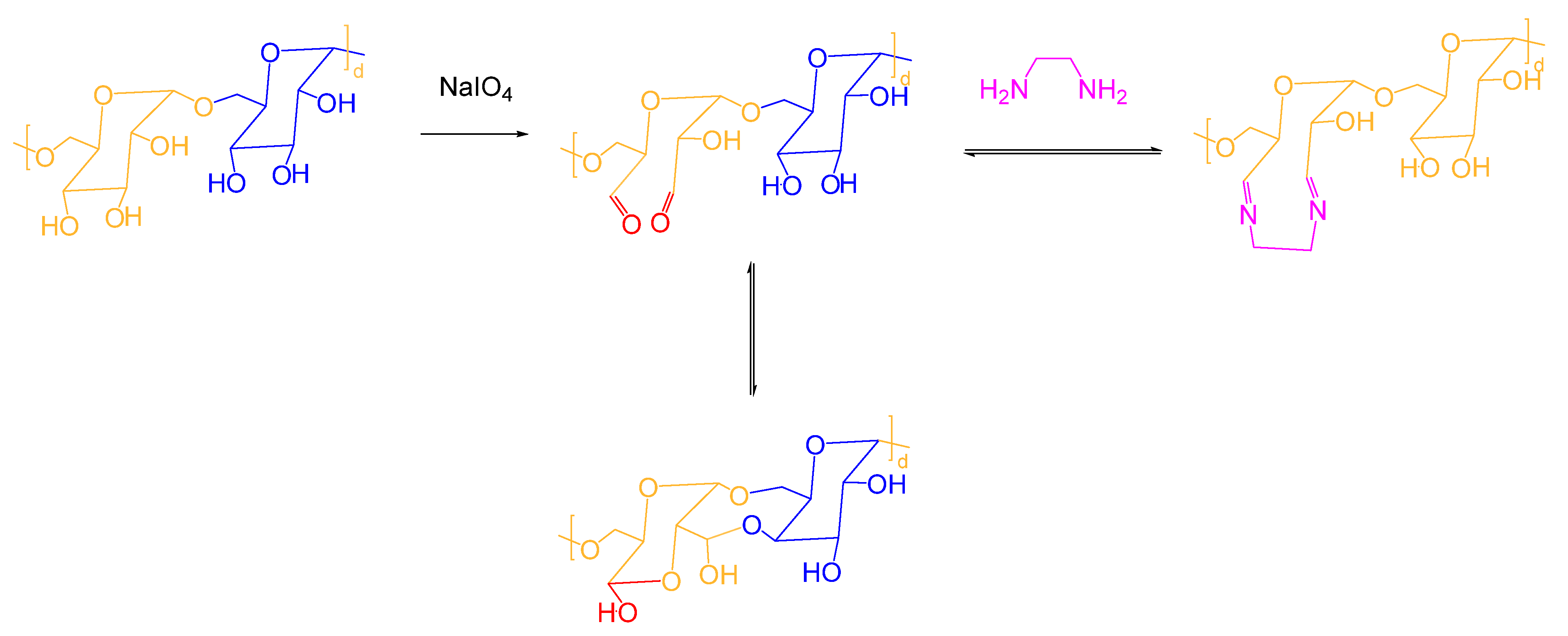
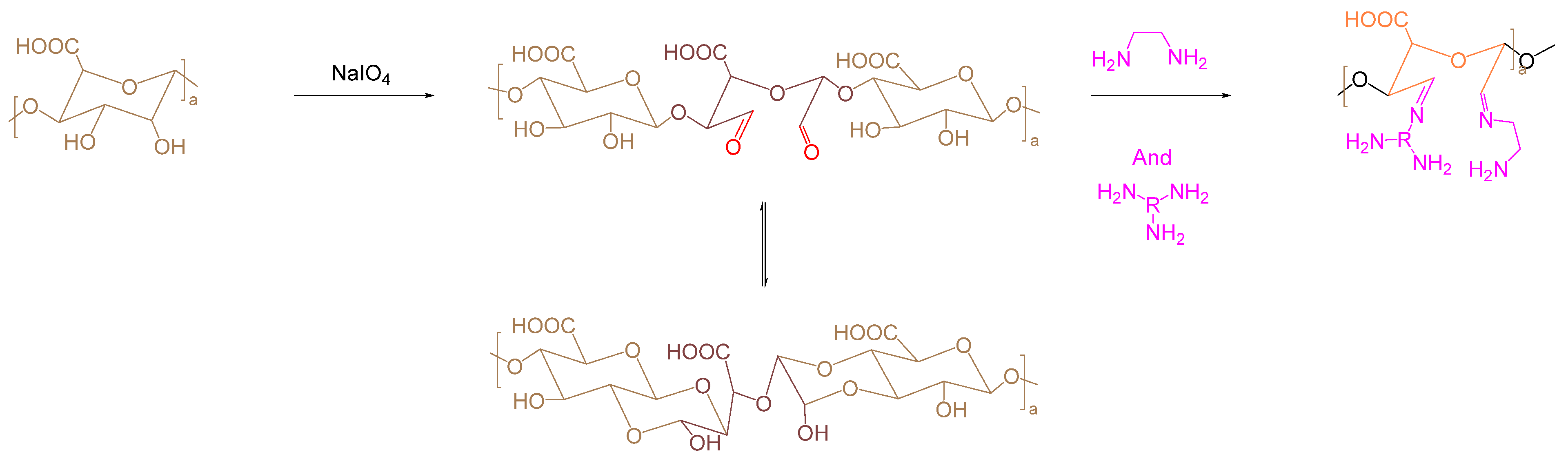
B. Based on Polysaccharides
- (a)
- Dextran
- (b)
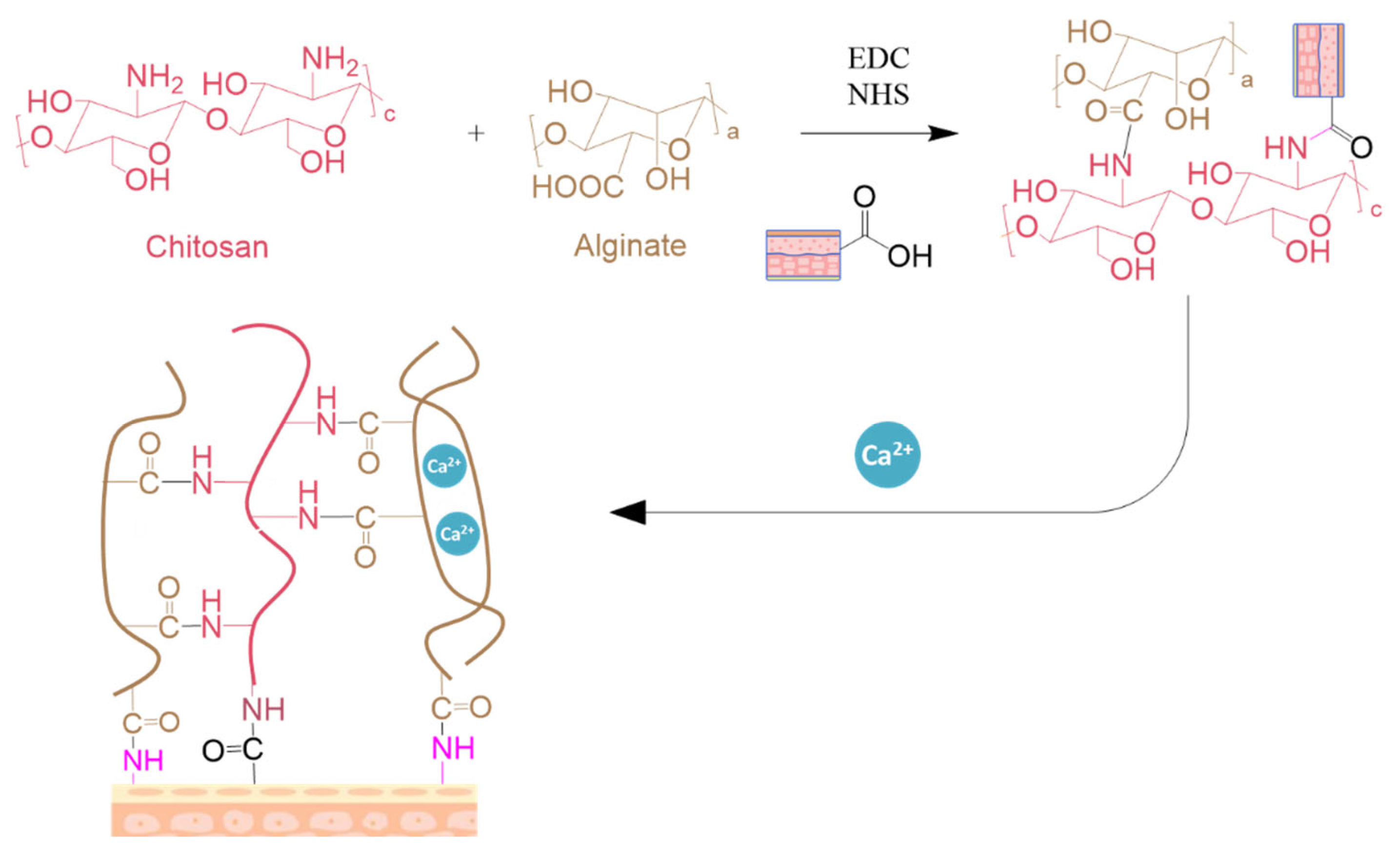
- Chitosan
- (c)
- Alginate
C. Conclusion
5.2.2. Biomimetic
A. Mussel/Clams
B. Gecko
C. Slug/Snail
D. Other Naturally Occurring Adhesives
E. Conclusions
5.2.3. Synthetic Surgical Glues
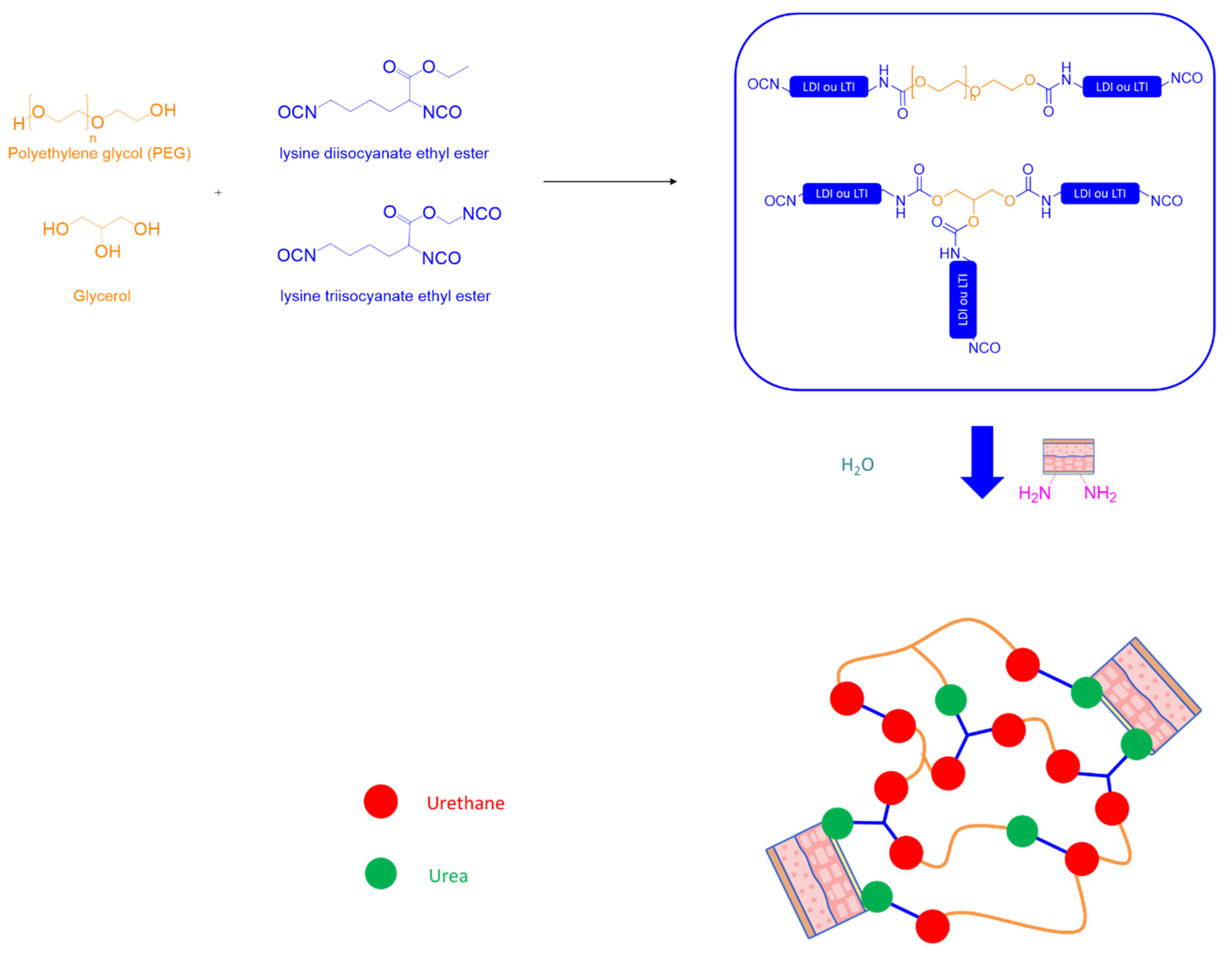
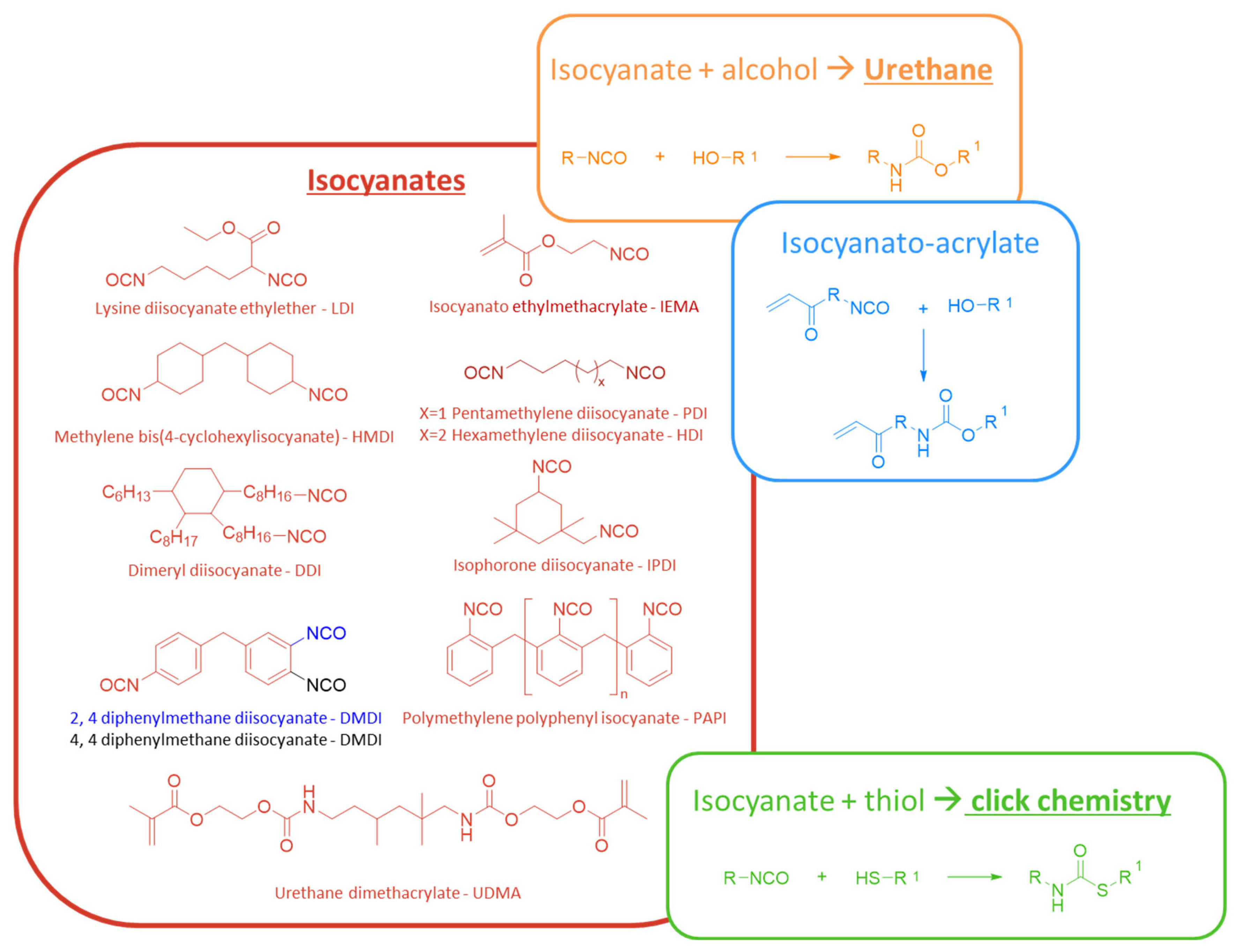
A. Polyurethanes—Polyurea
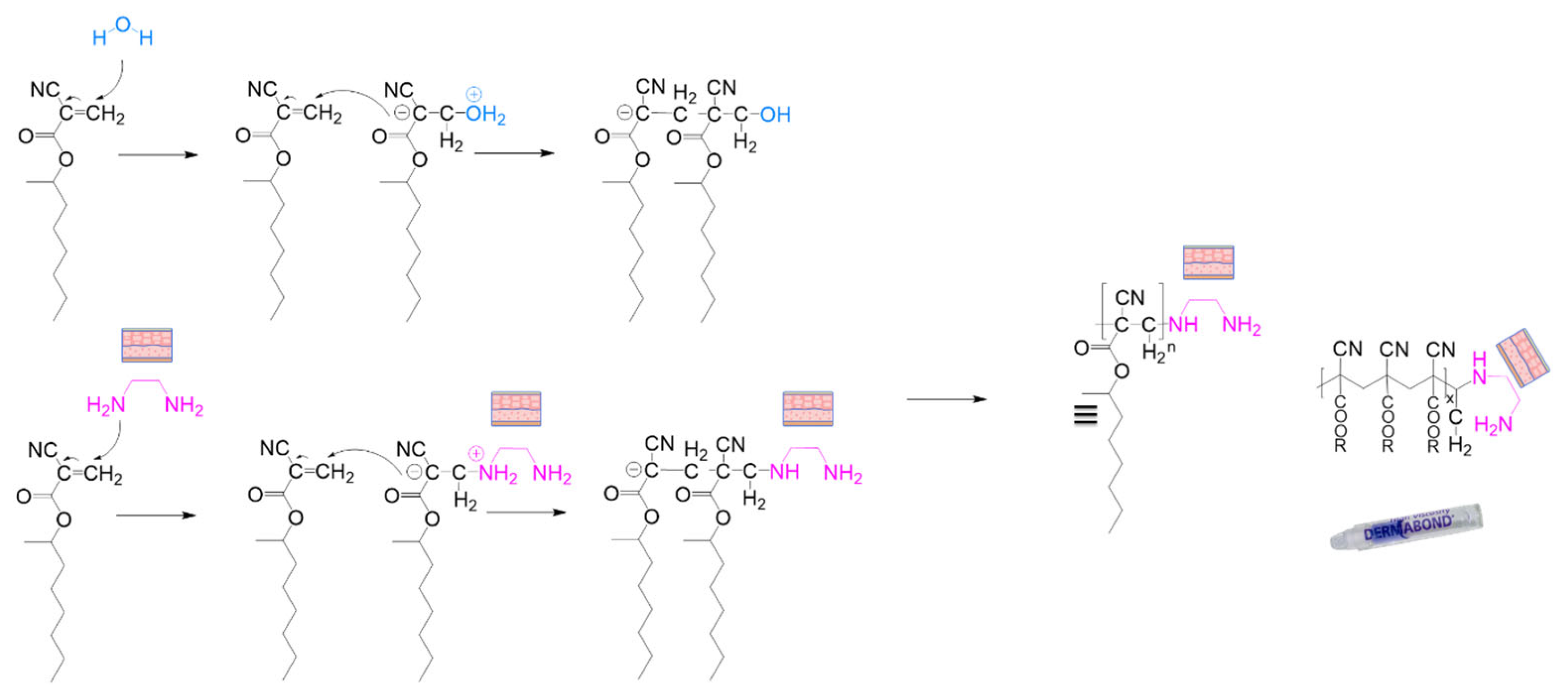
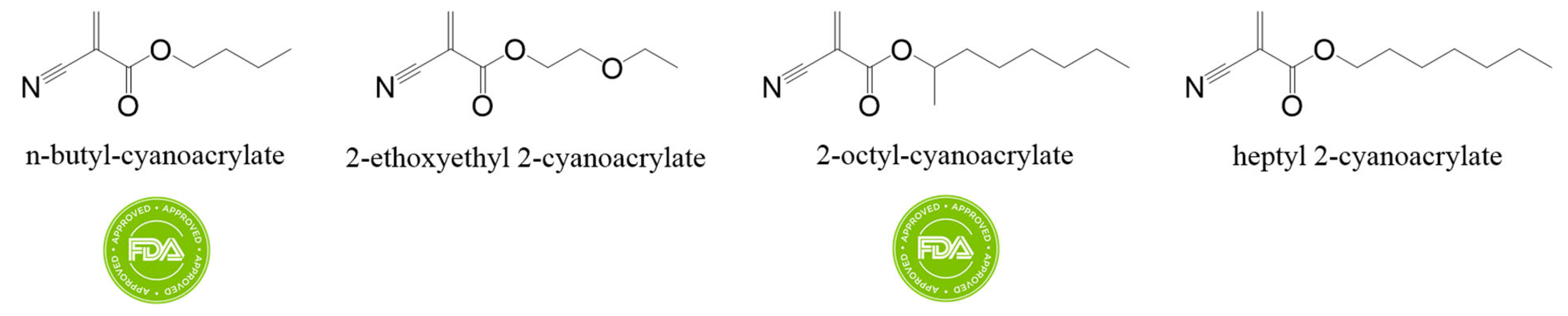
B. Cyanoacrylates
C. Polyethylene Glycol Hydrogels
D. Conclusion
6. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Mazur, M.; Zakrzewski, W.; Szymonowicz, M.; Rybak, Z. Medical Adhesives and Their Role in Laparoscopic Surgery—A Review of Literature. Materials 2022, 15, 5215. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; De Manzini, N.; Paoletti, S. Adhesive and Sealant Interfaces for General Surgery Applications. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Yepremyan, M.; Yepremyan, A. Surgical Glue: A Brief Overview. Austin J. Biomed. Eng. 2014, 1, 1020. [Google Scholar]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical Adhesives: Systematic Review of the Main Types and Development Forecast. Prog. Polym. Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Zaokari, Y.; Persaud, A.; Ibrahim, A. Biomaterials for Adhesion in Orthopedic Applications: A Review. Eng. Regen. 2020, 1, 51–63. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef]
- Larousse—Définition Colle. Available online: https://www.larousse.fr/dictionnaires/francais/colle/17164 (accessed on 7 November 2024).
- Centre National de Resources Textuelles et Lexicales. Définition Colle. Available online: https://cnrtl.fr/definition/colle (accessed on 8 February 2025).
- Yang, J.; Yu, H.; Wang, L.; Liu, J.; Liu, X.; Hong, Y.; Huang, Y.; Ren, S. Advances in Adhesive Hydrogels for Tissue Engineering. Eur. Polym. J. 2022, 172, 111241. [Google Scholar] [CrossRef]
- Dhandapani, V.; Saseedharan, P.; Groleau, D.; Vermette, P. Overview of Approval Procedures for Bioadhesives in the United States of America and Canada. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2022, 110, 950–966. [Google Scholar] [CrossRef]
- Shokrani, H.; Shokrani, A.; Seidi, F.; Munir, M.T.; Rabiee, N.; Fatahi, Y.; Kucinska-Lipka, J.; Saeb, M.R. Biomedical Engineering of Polysaccharide-Based Tissue Adhesives: Recent Advances and Future Direction. Carbohydr. Polym. 2022, 295, 119787. [Google Scholar] [CrossRef]
- Naureen, B.; Haseeb, A.S.M.A.; Basirun, W.J.; Muhamad, F. Recent Advances in Tissue Engineering Scaffolds Based on Polyurethane and Modified Polyurethane. Mater. Sci. Eng. C 2021, 118, 111228. [Google Scholar] [CrossRef]
- Sougrati, L.; Wendels, S.; Dinescu, S.; Balahura, L.-R.; Sleiman, L.; Avérous, L. Renewable Adhesives Based on Oleo-Chemistry: From Green Synthesis to Biomedical Applications. Sustain. Mater. Technol. 2023, 37, e00656. [Google Scholar] [CrossRef]
- Wendels, S.; Balahura, R.; Dinescu, S.; Costache, M.; Avérous, L. Synthesis and Properties of Biobased Polyurethane Tissue Adhesives from Bacterial Polyester. Sustain. Mater. Technol. 2022, 34, e00515. [Google Scholar] [CrossRef]
- Hejazi, S.; Carpentieri, A.; Marotta, A.; Restaino, O.F.; AntonellaGiarra; Solimeno, I.; Zannini, D.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/Poly-γ-Glutamic Acid Crosslinked Hydrogels: Characterization and Application as Bio-Glues. Int. J. Biol. Macromol. 2024, 277 Pt 1, 133653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Peng, X.; Li, Z.; Bai, W.; Wang, T.; Gu, Z.; Li, Y. Smart Internal Bio-Glues. Adv. Sci. 2022, 9, e2203587. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, G.; He, Y.; Mao, H.; Kong, D.; Luo, K.; Tang, W.; Liu, R.; Gu, Z. A Dual-Bioinspired Tissue Adhesive Based on Peptide Dendrimer with Fast and Strong Wet Adhesion. Adv. Healthc. Mater. 2022, 11, e2200874. [Google Scholar] [CrossRef]
- Merlin, D.L.; Sivasankar, B. Synthesis and Characterization of Semi-Interpenetrating Polymer Networks Using Biocompatible Polyurethane and Acrylamide Monomer. Eur. Polym. J. 2009, 45, 165–170. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Sun, F.; Dou, X.; Liu, J.; Wang, Y.; Li, M.; Gao, J.; Liu, X.; Wang, X.; et al. A Super Tough, Rapidly Biodegradable, Ultrafast Hemostatic Bioglue. Adv. Mater. 2023, 35, e2208622. [Google Scholar] [CrossRef]
- Santos, M.; Cernadas, T.; Martins, P.; Miguel, S.P.; Correia, I.J.; Alves, P.; Ferreira, P. Polyester-Based Photocrosslinkable Bioadhesives for Wound Closure and Tissue Regeneration Support. React. Funct. Polym. 2021, 158, 104798. [Google Scholar] [CrossRef]
- Zhu, H.; Tian, J.; Mao, H.; Gu, Z. Bioadhesives: Current Hotspots and Emerging Challenges. Curr. Opin. Biomed. Eng. 2021, 18, 100271. [Google Scholar] [CrossRef]
- Palacio, M.L.B.; Bhushan, B. Research Article: Bioadhesion: A Review of Concepts and Applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 2321–2347. [Google Scholar] [CrossRef]
- Jain, R.; Wairkar, S. Recent Developments and Clinical Applications of Surgical Glues: An Overview. Int. J. Biol. Macromol. 2019, 137, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; Van Goor, H.; Van Hest, J.C.M.; Hoogenboom, R. The Chemistry of Tissue Adhesive Materials. Prog. Polym. Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Wang, X.; Zhang, M.; Li, C.; Zhou, J. Recent Advances on Synthetic and Polysaccharide Adhesives for Biological Hemostatic Applications. Front. Bioeng. Biotechnol. 2020, 8, 926. [Google Scholar] [CrossRef]
- Smeets, R.; Tauer, N.; Vollkommer, T.; Gosau, M.; Henningsen, A.; Hartjen, P.; Früh, L.; Beikler, T.; Stürmer, E.K.; Rutkowski, R.; et al. Tissue Adhesives in Reconstructive and Aesthetic Surgery—Application of Silk Fibroin-Based Biomaterials. Int. J. Mol. Sci. 2022, 23, 7687. [Google Scholar] [CrossRef]
- Dey, A.; Bhattacharya, P.; Neogi, S. Bioadhesives in Biomedical Applications: A Critical Review. Rev. Adhes. Adhes. 2020, 8, 130–152. [Google Scholar] [CrossRef]
- Spotnitz, W.D.; Burks, S. Hemostats, Sealants, and Adhesives: Components of the Surgical Toolbox. Transfusion 2008, 48, 1502–1516. [Google Scholar] [CrossRef]
- Polyurethane Market Size, Share & Trends Analysis Report by Product (Rigid Foam, Flexible Foam), by Application (Construction, Furniture & Interiors), by Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/polyurethane-pu-market# (accessed on 11 January 2025).
- Medical Adhesives Market Size, Share Forecast Report 2032. Available online: https://www.fortunebusinessinsights.com/medical-adhesives-market-102354 (accessed on 31 July 2024).
- Medical Adhesives Market Size, Industry Share Forecast. Available online: https://www.marketsandmarkets.com/Market-Reports/medical-adhesive-market-41880473.html?gad_source=1&gclid=Cj0KCQjwwae1BhC_ARIsAK4JfrywvihdlVyIZrIX6_3AYk0ZXAtkdCNm2rAYhRFhVcGZd57rY7_ube0aAgyDEALw_wcB (accessed on 31 July 2024).
- Gillman, N.; Lloyd, D.; Bindra, R.; Ruan, R.; Zheng, M. Surgical Applications of Intracorporal Tissue Adhesive Agents: Current Evidence and Future Development. Expert Rev. Med. Devices 2020, 17, 443–460. [Google Scholar] [CrossRef]
- Uma, K. Bioadhesives for Clinical Applications—A Mini Review. Mater. Adv. 2023, 4, 2062–2069. [Google Scholar] [CrossRef]
- Zheng, K.; Gu, Q.; Zhou, D.; Zhou, M.; Zhang, L. Recent Progress in Surgical Adhesives for Biomedical Applications. Smart Mater. Med. 2021, 3, 41–65. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A Review of the Properties and Applications of Bioadhesive Hydrogels. Polym. Chem. 2021, 12, 3721–3739. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Z.; Chen, X.; He, C. Stimuli-Responsive Hydrogel Adhesives for Wound Closure and Tissue Regeneration. Macromol. Biosci. 2024, 24, e2300379. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Chun, B.; Seo, J. Rational Engineering and Applications of Functional Bioadhesives in Biomedical Engineering. Biotechnol. J. 2021, 16, 2100231. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, C.; Wang, C.; Xiao, Y.; Lin, W. An Overview on Collagen and Gelatin-Based Cryogels: Fabrication, Classification, Properties and Biomedical Applications. Polymers 2021, 13, 2299. [Google Scholar] [CrossRef] [PubMed]
- Ratnoff, O.D. Physiological Pharmacology—Blood—The Physiology of Blood Clotting, 1st ed.; Root, W.S., Berlin, N.I., Eds.; Elsevier: New York, NY, USA; Academic Press: San Francisco, CA, USA; London, UK, 1974. [Google Scholar]
- Ge, L.; Chen, S. Recent Advances in Tissue Adhesives for Clinical Medicine. Polymers 2020, 12, 939. [Google Scholar] [CrossRef]
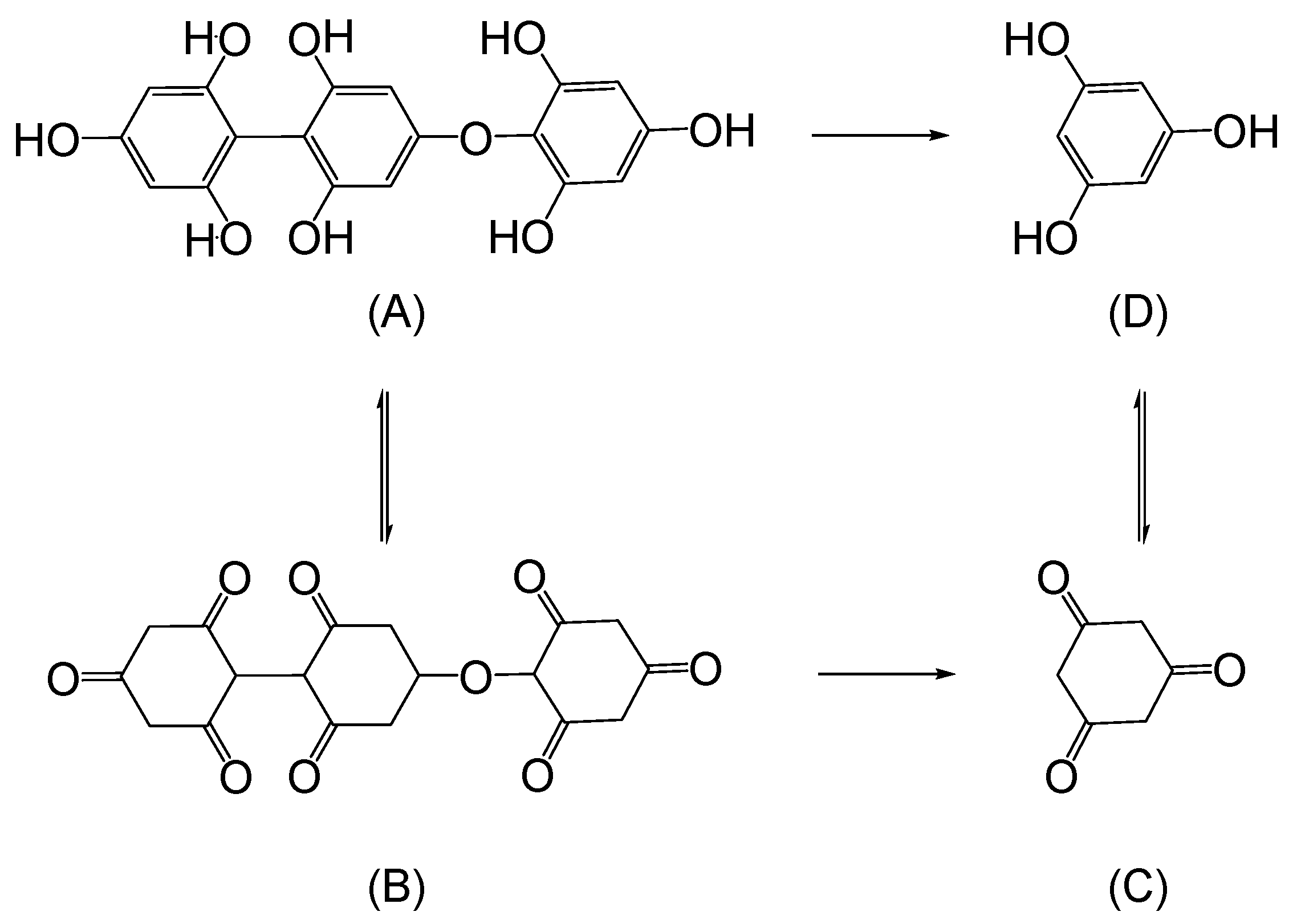
- Sacramento, M.M.A.; Oliveira, M.B.; Gomes, J.R.B.; Borges, J.; Freedman, B.R.; Mooney, D.J.; Rodrigues, J.M.M.; Mano, J.F. Natural Polymer-Polyphenol Bioadhesive Coacervate with Stable Wet Adhesion, Antibacterial Activity, and On-Demand Detachment. Adv. Healthc. Mater. 2024, 13, e2304587. [Google Scholar] [CrossRef]
- Xu, R.; Yu, F.; Huang, L.; Zhou, W.; Wang, Y.; Wang, F.; Sun, X.; Chang, G.; Fang, M.; Zhang, L.; et al. Isocyanate-Terminated Urethane-Based Dental Adhesive Bridges Dentinal Matrix Collagen with Adhesive Resin. Acta Biomater. 2019, 83, 140–152. [Google Scholar] [CrossRef]
- Yuen, H.Y.; Bei, H.P.; Zhao, X. Underwater and Wet Adhesion Strategies for Hydrogels in Biomedical Applications. Chem. Eng. J. 2022, 431, 133372. [Google Scholar] [CrossRef]
- Li, W.; Zheng, Y.; Pang, W.; Lai, P. Bio-Inspired Adhesive Hydrogel for Wound Healing. Biomed. Technol. 2023, 1, 65–72. [Google Scholar] [CrossRef]
- Ma, Z.; Bao, G.; Li, J. Multifaceted Design and Emerging Applications of Tissue Adhesives. Adv. Mater. 2021, 33, 2007663. [Google Scholar] [CrossRef]
- Pandey, N.; Soto-Garcia, L.F.; Liao, J.; Zimmern, P.; Nguyen, K.T.; Hong, Y. Mussel-Inspired Bioadhesives in Healthcare: Design Parameters, Current Trends, and Future Perspectives. Biomater. Sci. 2020, 8, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wang, J.; Cong, Y.; Fu, J. Recent Progress in Polymer Hydrogel Bioadhesives. J. Polym. Sci. 2021, 59, 1312–1337. [Google Scholar] [CrossRef]
- Zhu, S.; Dou, W.; Zeng, X.; Chen, X.; Gao, Y.; Liu, H.; Li, S. Recent Advances in the Degradability and Applications of Tissue Adhesives Based on Biodegradable Polymers. Int. J. Mol. Sci. 2024, 25, 5249. [Google Scholar] [CrossRef]
- Montazerian, H.; Davoodi, E.; Baidya, A.; Badv, M.; Haghniaz, R.; Dalili, A.; Milani, A.S.; Hoorfar, M.; Annabi, N.; Khademhosseini, A.; et al. Bio-Macromolecular Design Roadmap towards Tough Bioadhesives. Chem. Soc. Rev. 2022, 51, 9127–9173. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Pandit, A. Cohesion Mechanisms for Bioadhesives. Bioact. Mater. 2022, 13, 105–118. [Google Scholar] [CrossRef]
- Han, G.Y.; Hwang, S.K.; Cho, K.H.; Kim, H.J.; Cho, C.S. Progress of Tissue Adhesives Based on Proteins and Synthetic Polymers. Biomater. Res. 2023, 27, 57. [Google Scholar] [CrossRef]
- Chen, S.; Gil, C.J.; Ning, L.; Jin, L.; Perez, L.; Kabboul, G.; Tomov, M.L.; Serpooshan, V. Adhesive Tissue Engineered Scaffolds: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 683079. [Google Scholar] [CrossRef]
- Wu, S.J.; Zhao, X. Bioadhesive Technology Platforms. Chem. Rev. 2023, 123, 14084–14118. [Google Scholar] [CrossRef]
- Yadav, D.; Giri, P.; Das, C. Polymer-Based Biomaterials and Their Applications in Tissue Adhesives. J. Adhes. Sci. Technol. 2024, 38, 2019–2046. [Google Scholar] [CrossRef]
- Mondal, P.; Chakraborty, I.; Chatterjee, K. Injectable Adhesive Hydrogels for Soft Tissue Reconstruction: A Materials Chemistry Perspective. Chem. Rec. 2022, 22, e202200155. [Google Scholar] [CrossRef]
- Venkatraman, S.; Gale, R. Skin Adhesives and Skin Adhesion. Biomaterials 1998, 19, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Kim, H.N.; Seong, M.; Lee, S.H.; Kang, M.; Yi, H.; Bae, W.G.; Kwak, M.K.; Jeong, H.E. Multifunctional Smart Skin Adhesive Patches for Advanced Health Care. Adv. Healthc. Mater. 2018, 7, e1800275. [Google Scholar] [CrossRef] [PubMed]
- Tighe, B.J.; Mann, A. Adhesives and Interfacial Phenomena in Wound Healing. Adv. Wound Repair Ther. 2011, 11, 247–283. [Google Scholar] [CrossRef]
- Czech, Z.; Kowalczyk, A.; Swiderska, J. Pressure-Sensitive Adhesives for Medical Applications. In Wide Spectra of Quality Control; IntechOpen: London, UK, 2011. [Google Scholar][Green Version]
- Xin, H.; Al Maruf, D.S.A.; Akin-Ige, F. Stimuli-responsive hydrogels for skin wound healing and regeneration. Emergent Mater. 2024. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, J.; Wu, H.; Lin, X.; Cai, L. Stimuli-responsive hydrogel dressing for wound healing. APL Mater. 2025, 13, 10601. [Google Scholar] [CrossRef]
- Serpico, L.; Dello Iacono, S.; Cammarano, A.; De Stefano, L. Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing. Gels 2023, 9, 451. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Chen, M.; Wu, Y.; Chen, B.; Tucker, A.M.; Jagota, A.; Yang, S. Fast, strong, and reversible adhesives with dynamic covalent bonds for potential use in wound dressing. Proc. Natl. Acad. Sci. USA 2022, 119, e2203074119. [Google Scholar] [CrossRef]
- Huang, C.; Wu, Q.; Li, X.; Pan, P.; Gu, S.; Tang, T.; Wu, J. Silicone Bioadhesive with Shear-Stiffening Effect: Rate-Responsive Adhesion Behavior and Wound Dressing Application. Biomacromolecules 2024, 25, 4510–4522. [Google Scholar] [CrossRef]
- An, S.; Jeon, E.J.; Han, S.Y.; Jeon, J.; Lee, M.J.; Kim, S.; Shin, M.; Cho, S. pH-Universal Catechol-Amine Chemistry for Versatile Hyaluronic Acid Bioadhesives. Small 2022, 18, 2202729. [Google Scholar] [CrossRef]
- Zarur, M.; Seijo-Rabina, A.; Goyanes, A.; Concheiro, A.; Alvarez-Lorenzo, C. pH-responsive scaffolds for tissue regeneration: In vivo performance. Acta Biomater. 2023, 168, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Shin, J.; DeFrates, K.; Arslan, M.; Dale, K.; Chen, H.; Ramirez, D.; Messersmith, P.B. Recyclable surgical, consumer, and industrial adhesives of poly(α-lipoic acid). Science 2024, 385, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Dai, C.; Fan, L.; Jiang, Y.; Liu, C.; Zhou, Z.; Guan, P.; Tian, Y.; Xing, J.; Li, X.; et al. Injectable Self-Healing Natural Biopolymer-Based Hydrogel Adhesive with Thermoresponsive Reversible Adhesion for Minimally Invasive Surgery. Adv. Funct. Mater. 2021, 31, 2007457. [Google Scholar] [CrossRef]
- Xu, Y.; Ji, Y.; Ma, J. Temperature-sensitive mussel-inspired citrate-based tissue adhesives with low-swelling. J. Adhes. 2023, 99, 1244–1261. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Z.; Sun, Y.; Li, B.; Wu, B.; Ma, C.; Petrovskii, V.S.; Gu, X.; Chen, D.; Potemkin, I.I.; et al. An Artificial Phase-Transitional Underwater Bioglue with Robust and Switchable Adhesion Performance. Angew. Chem. Int. Ed. 2021, 60, 12082–12089. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Jumelle, C.; Yung, A.; Sani, E.S.; Taketani, Y.; Gantin, F.; Bourel, L.; Wang, S.; Yüksel, E.; Seneca, S.; Annabi, N.; et al. Development and characterization of a hydrogel-based adhesive patch for sealing open-globe injuries. Acta Biomater. 2022, 137, 53–63. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Zhu, J.; Du, J.; Han, J.; Lv, W.; Cheng, Y. Recent advances on in situ tissue adhesives. Fundam. Res. 2025; in press. [Google Scholar] [CrossRef]
- Ulijn, R.V. Enzyme-responsive materials: A new class of smart biomaterials. J. Mater. Chem. 2006, 16, 2217–2225. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, H.; Gerhard, E.M.; Zhang, S.; Rodríguez, F.I.P.; Pan, T.; Yang, H.; Lin, Y.; Yang, J.; Cheng, H. Smart bioadhesives for wound healing and closure. Bioact. Mater. 2023, 19, 360–375. [Google Scholar] [CrossRef]
- Han, Z.; Yuan, M.; Liu, L.; Zhang, K.; Zhao, B.; He, B.; Liang, Y.; Li, F. pH-Responsive wound dressings: Advances and prospects. Nanoscale Horiz. 2023, 8, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Cintron-Cruz, J.A.; Freedman, B.R.; Lee, M.; Johnson, C.; Ijaz, H.; Mooney, D.J. Rapid Ultratough Topological Tissue Adhesives. Adv. Mater. 2022, 34, 2205567. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, R.; Wang, Y.; Wei, C.; Li, F.; Qing, N.; Tang, L. Highly adhesive chitosan/poly(vinyl alcohol) hydrogels via the synergy of phytic acid and boric acid and their application as highly sensitive and widely linear strain sensors. Mater. Horiz. 2023, 10, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Ananth, V.; Velayutham, J.; Manickam, P.; Veerapandian, M. Bioadhesive Gauze Embedded with Chitosan-Butein Bioconjugate: A Redox-Active pH Sensor Platform. Biosensors 2023, 13, 6. [Google Scholar] [CrossRef]
- Jiang, J.; Ding, J.; Wu, X.; Zeng, M.; Tian, Y.; Wu, K.; Wei, D.; Sun, J.; Guo, Z.; Fan, H. Flexible and temperature-responsive hydrogel dressing for real-time and remote wound healing monitoring. J. Mater. Chem. B 2023, 11, 4934–4945. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Zhao, J.; Chai, P.; Ma, G.; Dong, Y.; He, X.; Jiang, Y.; Wu, Q.; Hu, Z.; et al. Body temperature-induced adhesive hyaluronate/gelatin-based hybrid hydrogel dressing for promoting skin regeneration. Int. J. Biol. Macromol. 2023, 253, 126848. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Moreira, A.F.; Correia, I.J. Application of near-infrared light responsive biomaterials for improving the wound healing process: A review. J. Drug Deliv. Sci. Technol. 2024, 93, 105409. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; He, Y.; Lu, Q.; Chen, R.; Zhao, C.; Dong, D.; Sun, Y.; He, H. Dual light-responsive cellulose nanofibril-based in situ hydrogel for drug-resistant bacteria infected wound healing. Carbohydr. Polym. 2022, 297, 120042. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, Z.; Li, Q.; Tang, X.; Chen, X.; Ge, Y.; Ling, J. Light-Triggered Adhesive Silk-Based Film for Effective Photodynamic Antibacterial Therapy and Rapid Hemostasis. Front. Bioeng. Biotechnol. 2022, 9, 820434. [Google Scholar] [CrossRef]
- Uzun, M. A review of wound management materials. J. Text. Eng. Fash. Technol. 2018, 4, 1. [Google Scholar] [CrossRef]
- O’Callaghan, S.; Galvin, P.; O’Mahony, C.; Moore, Z.; Derwin, R. ‘Smart’ wound dressings for advanced wound care: A review. J. Wound Care 2020, 29, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Taboada, G.M.; Yang, K.; Pereira, M.J.N.; Liu, S.S.; Hu, Y.; Karp, J.M.; Artzi, N.; Lee, Y. Overcoming the translational barriers of tissue adhesives. Nat. Rev. Mater. 2020, 5, 310–329. [Google Scholar] [CrossRef]
- Sanders, L.; Nagatomi, J. Clinical applications of surgical adhesives and sealants. Crit. Rev. Biomed. Eng. 2014, 42, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Leggat, P.A.; Smith, D.R.; Kedjarune, U. Surgical applications of cyanoacrylate adhesives: A review of toxicity. ANZ J. Surg. 2007, 77, 209–213. [Google Scholar] [CrossRef]
- Bhamidipati, C.M.; Coselli, J.S.; LeMaire, S.A. BioGlue® in 2011: What is its role in cardiac surgery? J. Extra-Corpor. Technol. 2012, 44, P6–P12. [Google Scholar]
- Waldner, F.A.; Sadeghi, P.; Grimaldi, L.; Nisi, G.; Calomino, N.; Cuomo, R. Tissue Adhesive: Current Uses and Strengths. J. Investig. Surg. 2022, 35, 1415–1416. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Shadden, Z.T.; Lau, L.W.; Ayyub, O.B.; Djouini, M.; Srinivasan, P.; Erdi, M.; Sandler, A.D.; Kofinas, P. Pressure-Sensitive Tissue Adhesion and Biodegradation of Viscoelastic Polymer Blends. ACS Appl. Mater. Interfaces 2020, 12, 16050–16057. [Google Scholar] [CrossRef]
- Zou, Y.; Yan, F.; Tong, R.; Mo, M.; Li, Z. Progress in the Design Principle of Biomedical Tissue Adhesive Hydrogel with Naturally Derived Substance. J. Polym. Sci. 2024, 62, 4341–4359. [Google Scholar] [CrossRef]
- Demirci, G.; Niedźwiedź, M.J.; Kantor-Malujdy, N.; El Fray, M. Elastomer–Hydrogel Systems: From Bio-Inspired Interfaces to Medical Applications. Polymers 2022, 14, 1822. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Martinez, E.E.; Zhu, J.; Wang, T.; Shi, S.; Shin, S.R.; Hassan, S.; Guo, C. Emerging Biopolymer-Based Bioadhesives. Macromol. Biosci. 2022, 22, 2100340. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Han, L.; Lu, X. Biodegradable Polymer Hydrogel-Based Tissue Adhesives: A Review. Biosurf. Biotribol. 2021, 7, 163–179. [Google Scholar] [CrossRef]
- Saha, N.; Saha, N.; Sáha, T.; Öner, E.T.; Brodnjak, U.V.; Redl, H.; von Byern, J.; Sáha, P. Polymer Based Bioadhesive Biomaterials for Medical Application—A Perspective of Redefining Healthcare System Management. Polymers 2020, 12, 3015. [Google Scholar] [CrossRef]
- Hushmand Shahrifi, B.; Mohammadi, M.; Manoochehri, M.; Atashi, A. Mechanical and Biological Properties of Polycaprolactone/Fibrin Nanocomposite Adhesive Produced by Electrospinning Method. Bull. Mater. Sci. 2020, 43, 135. [Google Scholar] [CrossRef]
- Atrah, H.I. Fibrin Glue. BMJ 1994, 308, 933. [Google Scholar] [CrossRef]
- Bao, Z.; Gao, M.; Sun, Y.; Nian, R.; Xian, M. The Recent Progress of Tissue Adhesives in Design Strategies, Adhesive Mechanism and Applications. Mater. Sci. Eng. C 2020, 111, 110796. [Google Scholar] [CrossRef]
- Young, J.Z.; Medawar, P.B. Fibrin Suture of Peripheral Nerves. Br. Med. Bull. 1943, 1, 77. [Google Scholar] [CrossRef]
- Silver, F.H.; Wang, M.C.; Pins, G.D. Preparation and Use of Fibrin Glue in Surgery. Biomaterials 1995, 16, 891–903. [Google Scholar] [CrossRef]
- Mintz, P.; Mayers, L.; Flanagan, H.; Burks, S.; Spotnitz, W. Fibrin Sealant: Clinical Use and the Development of the University of Virginia Tissue Adhesive Center—PubMed. Ann. Clin. Lab. Sci. 2001, 1, 108–118. [Google Scholar]
- Spotnitz, W.D. Fibrin Sealant: The Only Approved Hemostat, Sealant, and Adhesive-a Laboratory and Clinical Perspective. ISRN Surg. 2014, 2014, 203943. [Google Scholar] [CrossRef]
- Fang, H.; Peng, S.; Chen, A.; Li, F.; Ren, K.; Hu, N. Biocompatibility Studies on Fibrin Glue Cultured with Bone Marrow Mesenchymal Stem Cells in Vitro. J. Huazhong Univ. Sci. Technol.—Med. Sci. 2004, 24, 272–274. [Google Scholar] [CrossRef]
- Bernie, J.E.; Ng, J.; Bargman, V.; Gardner, T.; Cheng, L.; Sundaram, C.P. Evaluation of Hydrogel Tissue Sealant in Porcine Laparoscopic Partial-Nephrectomy Model. J. Endourol. 2005, 19, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Alsarraf, J.; Ammar, Y.A.; Robert, F.; Cloutet, E.; Cramail, H.; Landais, Y. Cyclic Guanidines as Efficient Organocatalysts for the Synthesis of Polyurethanes. Macromolecules 2012, 45, 2249–2256. [Google Scholar] [CrossRef]
- Thomas, V.; Jose, M.V.; Chowdhury, S.; Sullivan, J.F.; Dean, D.R.; Vohra, Y.K. Mechano-Morphological Studies of Aligned Nanofibrous Scaffolds of Polycaprolactone Fabricated by Electrospinning. J. Biomater. Sci. Polym. Ed. 2006, 17, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Karmaker, A.; Hasan, M.; Sharifuzzaman, M.; Ahmed, S. Development of Natural Fiber-Supported High-Strength Autologous Fibrin Glue from Human Plasma. J. Adhes. Sci. Technol. 2020, 34, 1898–1911. [Google Scholar] [CrossRef]
- Karmaker, A.; Hasan, M.; Ali, S.; Asif, K.M.; Ahmed, S. A Novel High-Strength Autologous Fibrin Glue Augmented with Biocompatible Polymers. J. Adhes. 2023, 99, 632–647. [Google Scholar] [CrossRef]
- Rajabi, N.; Kharaziha, M.; Emadi, R.; Zarrabi, A.; Mokhtari, H.; Salehi, S. An Adhesive and Injectable Nanocomposite Hydrogel of Thiolated Gelatin/Gelatin Methacrylate/Laponite® as a Potential Surgical Sealant; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 564. [Google Scholar] [CrossRef]
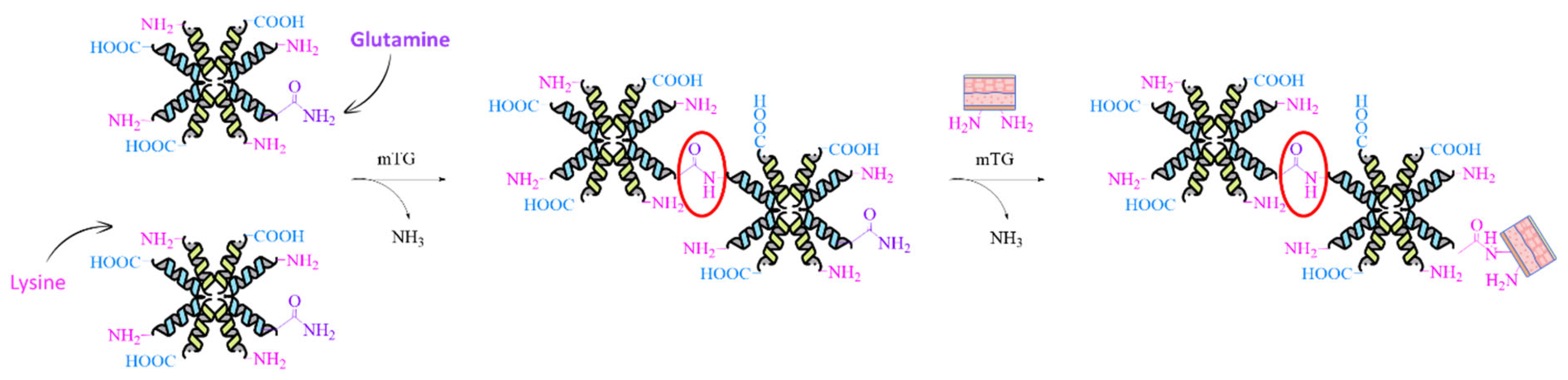
- McDermott, M.K.; Chen, T.; Williams, C.M.; Markley, K.M.; Payne, G.F. Mechanical Properties of Biomimetic Tissue Adhesive Based on the Microbial Transglutaminase-Catalyzed Crosslinking of Gelatin. Biomacromolecules 2004, 5, 1270–1279. [Google Scholar] [CrossRef]
- Ahmady, A.; Abu Samah, N.H. A Review: Gelatine as a Bioadhesive Material for Medical and Pharmaceutical Applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef]
- Sharifi, S.; Islam, M.M.; Sharifi, H.; Islam, R.; Koza, D.; Reyes-Ortega, F.; Alba-Molina, D.; Nilsson, P.H.; Dohlman, C.H.; Mollnes, T.E.; et al. Tuning Gelatin-Based Hydrogel towards Bioadhesive Ocular Tissue Engineering Applications. Bioact. Mater. 2021, 6, 3947–3961. [Google Scholar] [CrossRef]
- Wu, X.; Deng, J.; Jian, W.; Yang, Y.; Shao, H.; Zhou, X.; Xiao, Y.; Ma, J.; Zhou, Y.; Wang, R.; et al. A Bioinspired Switchable Adhesive Patch with Adhesion and Suction Mechanisms for Laparoscopic Surgeries. Mater. Today Bio 2024, 27, 101142. [Google Scholar] [CrossRef]
- Zhu, Z.; Ye, H.; Zhang, K.; He, G.; Pan, Z.; Xian, Y.; Yang, Y.; Zhang, C.; Wu, D. Naturally Derived Injectable Dual-Cross-Linked Adhesive Hydrogel for Acute Hemorrhage Control and Wound Healing. Biomacromolecules 2024, 25, 2574–2586. [Google Scholar] [CrossRef]
- Gowda, A.H.J.; Bu, Y.; Kudina, O.; Krishna, K.V.; Bohara, R.A.; Eglin, D.; Pandit, A. Design of Tunable Gelatin-Dopamine Based Bioadhesives. Int. J. Biol. Macromol. 2020, 164, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tang, L.; Guo, C.; Deng, F.; Wu, H.; Chen, L.; Huang, L.; Lu, P.; Ding, C.; Ni, Y.; et al. A Bioinspired Gallol-Functionalized Collagen as Wet-Tissue Adhesive for Biomedical Applications. Chem. Eng. J. 2021, 417, 127962. [Google Scholar] [CrossRef]
- Tang, Y.; Gong, G.; He, X.; Dai, M.; Chen, M.; Wang, B.; Wang, Y.; Wang, X.; Guo, J. Multifunctional Dual Cross-Linked Bioadhesive Patch with Low Immunogenic Response and Wet Tissues Adhesion. Adv. Healthc. Mater. 2023, 12, e2201578. [Google Scholar] [CrossRef]
- Eshkol-Yogev, I.; Tobias, T.; Keren, A.; Gilhar, A.; Gilboa, E.; Furer, A.; Ullmann, Y.; Zilberman, M. Dual Composite Bioadhesives for Wound Closure Applications: An in Vitro and in Vivo Study. Polym. Adv. Technol. 2022, 33, 3862–3877. [Google Scholar] [CrossRef]
- Wei, S.M.; Pei, M.Y.; Pan, W.L.; Thissen, H.; Tsai, S.W. Gelatin Hydrogels Reinforced by Absorbable Nanoparticles and Fibrils Cured in Situ by Visible Light for Tissue Adhesive Applications. Polymers 2020, 12, 1113. [Google Scholar] [CrossRef]
- Tian, Y.; Guan, P.; Wen, C.; Lu, M.; Li, T.; Fan, L.; Yang, Q.; Guan, Y.; Kang, X.; Jiang, Y.; et al. Strong Biopolymer-Based Nanocomposite Hydrogel Adhesives with Removability and Reusability for Damaged Tissue Closure and Healing. ACS Appl. Mater. Interfaces 2022, 14, 54488–54499. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Du, X.; Li, W.; Wang, Q.; He, D.; Yuan, J. Natural Polymer-Derived Photocurable Bioadhesive Hydrogels for Sutureless Keratoplasty. Bioact. Mater. 2022, 8, 196–209. [Google Scholar] [CrossRef]
- Kong, B.; Liu, R.; Cheng, Y.; Cai, X.; Liu, J.; Zhang, D.; Tan, H.; Zhao, Y. Natural biopolymers derived hydrogels with injectable, self-healing, and tissue adhesive abilities for wound healing. Nano Res. 2023, 16, 2798–2807. [Google Scholar] [CrossRef]
- He, X.Y.; Sun, A.; Li, T.; Qian, Y.J.; Qian, H.; Ling, Y.F.; Zhang, L.H.; Liu, Q.Y.; Peng, T.; Qian, Z. Mussel-Inspired Antimicrobial Gelatin/Chitosan Tissue Adhesive Rapidly Activated in Situ by H2O2/Ascorbic Acid for Infected Wound Closure. Carbohydr. Polym. 2020, 247, 116692. [Google Scholar] [CrossRef]
- Kang, J., II; Park, K.M. Advances in Gelatin-Based Hydrogels for Wound Management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef]
- Montazerian, H.; Baidya, A.; Haghniaz, R.; Davoodi, E.; Ahadian, S.; Annabi, N.; Khademhosseini, A.; Weiss, P.S. Stretchable and Bioadhesive Gelatin Methacryloyl-Based Hydrogels Enabled by in Situ Dopamine Polymerization. ACS Appl. Mater. Interfaces 2021, 13, 40290–40301. [Google Scholar] [CrossRef] [PubMed]
- Pirmoradian, M.; Hooshmand, T.; Najafi, F.; Haghbin Nazarpak, M.; Davaie, S. Design, Synthesis, and Characterization of a Novel Dual Cross-Linked Gelatin-Based Bioadhesive for Hard and Soft Tissues Adhesion Capability. Biomed. Mater. 2022, 17, 065010. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wang, X.; Yue, O.; Zheng, M.; Zhang, H.; Liu, X. Development of a Multifunctional Injectable Temperature-Sensitive Gelatin-Based Adhesive Double-Network Hydrogel. Biomater. Adv. 2022, 134, 112556. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, H.; Huang, Y.; Zhong, H.; Qin, P.; Cheng, S.; Wang, Y.; Yang, C. Tough and Biocompatible Hydrogel Tissue Adhesives Entirely Based on Naturally Derived Ingredients. ACS Appl. Polym. Mater. 2024, 6, 1141–1151. [Google Scholar] [CrossRef]
- Rizky, S.; Budhijanto; Wintoko, J. Modification of Bioadhesive Based on Crosslinked Alginate and Gelatin. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Kowanko, N. Adhesive Composition and Method. US,538,5606A, 22 March 1993. [Google Scholar]
- FDA. US Food and Drug Administration—Premarket Approval (PMA) for Bioglue®. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P010003 (accessed on 6 August 2024).
- Bhagat, V.; Becker, M.L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef]
- Barrows, T.; Lewis, T.; Truong, M. Adhesive Sealant Composition. US5583114A, 10 December 1996. Available online: https://patents.google.com/patent/US5583114A/en (accessed on 6 August 2024).
- FDA. US Food and Drug Administration—Premarket Approval (PMA) for ProgelTM. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P010047 (accessed on 6 August 2024).
- Zhao, K.; Li, B.; Sun, Y.; Jia, B.; Chen, J.; Cheng, W.; Zhao, L.; Li, J.; Wang, F.; Su, J.; et al. Engineered Bicomponent Adhesives with Instantaneous and Superior Adhesion Performance for Wound Sealing and Healing Applications. Adv. Funct. Mater. 2023, 33, 2303509. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Li, B.; Li, D.; Meng, Z.; Sun, S.K. Biocompatible Therapeutic Albumin/Genipin Bioglue for Postoperative Wound Adhesion and Residual Tumor Ablation. Biomaterials 2021, 279, 121179. [Google Scholar] [CrossRef]
- Della Sala, F.; Malle, B.M.; Ambrosio, L.; Borzacchiello, A. Fermentation-Derived Albumin-Based Hydrogels for Tissue Adhesion Applications. Polymers 2023, 15, 2530. [Google Scholar] [CrossRef]
- Park, M.K.; Li, M.X.; Yeo, I.; Jung, J.; Yoon, B.I.L.; Joung, Y.K. Balanced Adhesion and Cohesion of Chitosan Matrices by Conjugation and Oxidation of Catechol for High-Performance Surgical Adhesives. Carbohydr. Polym. 2020, 248, 116760. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Liang, Y.; He, J.; Guo, B. Multifunctional Tissue-Adhesive Cryogel Wound Dressing for Rapid Nonpressing Surface Hemorrhage and Wound Repair. ACS Appl. Mater. Interfaces 2020, 12, 35856–35872. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, C.; Xu, X.; Ren, L.; Zhou, X.; Xu, H.; Zhao, L.; Xin, J.; Wang, S.; Wang, Z. Chitosan-Based Multifunctional Hydrogel with Bio-Adhesion and Antioxidant Properties for Efficient Wound Hemostasis. Colloids Surf. B Biointerfaces 2024, 234, 113697. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, J.; Liu, Q.; Han, T.; Zhao, J.; Ma, X.; Tong, Y.; Jin, G.; Qu, K.; Li, B.; et al. Liquid Bandage Harvests Robust Adhesive, Hemostatic, and Antibacterial Performances as a First-Aid Tissue Adhesive. Adv. Funct. Mater. 2020, 30, 2001820. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Payanam, U.; Laurent, A.; Wassef, M.; Jayakrishnan, A. Efficacy Evaluation of an in Situ Forming Tissue Adhesive Hydrogel as Sealant for Lung and Vascular Injury. Biomed. Mater. 2021, 16, 044106. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chandel, A.K.S.; Mitsuhashi, K.; Fujiyabu, T.; Inagaki, N.F.; Ito, T. Injectable, Shear-Thinning, Self-Healing, and Self-Cross-Linkable Benzaldehyde-Conjugated Chitosan Hydrogels as a Tissue Adhesive. Biomacromolecules 2024, 25, 1084–1095. [Google Scholar] [CrossRef]
- Cao, B.; Wang, C.; Guo, P.; Zhang, Q.; Wang, C.; Sun, H.; Wen, H.; Chen, X.; Wang, Y.; Wang, Y.; et al. Photo-Crosslinked Enhanced Double-Network Hydrogels Based on Modified Gelatin and Oxidized Sodium Alginate for Diabetic Wound Healing. Int. J. Biol. Macromol. 2023, 245, 125528. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Liu, C.; Lu, Z.; Li, M.; Hurren, C.; Wang, D. Photopolymerized Multifunctional Sodium Alginate-Based Hydrogel for Antibacterial and Coagulation Dressings. Int. J. Biol. Macromol. 2024, 260, 129428. [Google Scholar] [CrossRef]
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A Novel Bovine Serum Albumin and Sodium Alginate Hydrogel Scaffold Doped with Hydroxyapatite Nanowires for Cartilage Defects Repair. Colloids Surf. B Biointerfaces 2020, 192, 111041. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, L.; Farhadi, F.; Xia, W.; Chang, J.; He, Y.; Li, H. An Injectable Continuous Stratified Structurally and Functionally Biomimetic Construct for Enhancing Osteochondral Regeneration. Biomaterials 2019, 192, 149–158. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An Engineered Cell-Laden Adhesive Hydrogel Promotes Craniofacial Bone Tissue Regeneration in Rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Ao, Q.; Zhang, H.; Cheng, J.; Ao, Q.; Rodríguez-Argüelles, C.; Simón-Vázquez, R. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhou, Y.; Peng, J.; Xu, C.; Xu, Q.; Xing, M.; Chang, J. A Novel Dual-Adhesive and Bioactive Hydrogel Activated by Bioglass for Wound Healing. NPG Asia Mater. 2019, 11, 66. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R. Mechanisms of Bioadhesion of Macrophytic Algae. Russ. J. Plant Physiol. 2013, 61, 19–25. [Google Scholar] [CrossRef]
- Bitton, R.; Ben-Yehuda, M.; Davidovich, M.; Balazs, Y.; Potin, P.; Delage, L.; Colin, C.; Bianco-Peled, H. Structure of Algal-Born Phenolic Polymeric Adhesives. Macromol. Biosci. 2006, 6, 737–746. [Google Scholar] [CrossRef]
- Bitton, R.; Bianco-Peled, H. Novel Biomimetic Adhesives Based on Algae Glue. Macromol. Biosci. 2008, 8, 393–400. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, T.; Yin, R.T.; Wu, M.; Xu, Y.; Koo, J.; Choi, Y.S.; Xie, Z.; Chen, S.W.; Kandela, I.; et al. Photocurable Bioresorbable Adhesives as Functional Interfaces between Flexible Bioelectronic Devices and Soft Biological Tissues. Nat. Mater. 2021, 20, 1559–1570. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Li, L.; Huo, R.; Ghezelbash, F.; Ma, Z.; Bao, G.; Liu, S.; Yang, Z.; Weber, M.H.; et al. Tissue-Mimetic Hybrid Bioadhesives for Intervertebral Disc Repair. Mater. Horiz. 2023, 10, 1705–1718. [Google Scholar] [CrossRef]
- Ma, X.; Bian, Q.; Hu, J.; Gao, J. Stem from Nature: Bioinspired Adhesive Formulations for Wound Healing. J. Control. Release 2022, 345, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Inamuddin, I.; Boddula, R.; Ahamed, M.I.; Asiri, A.M. Green Adhesives. Preparation, Properties and Applications; Scrivener Publishing: Beverly, MA, USA, 2020; ISBN 978-1-119-65509-1. [Google Scholar]
- Shi, C.; Chen, X.; Zhang, Z.; Chen, Q.; Shi, D.; Kaneko, D. Mussel Inspired Bio-Adhesive with Multi-Interactions for Tissue Repair. J. Biomater. Sci. Polym. Ed. 2020, 31, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Colombani, T.; Saleh, B.; Mohammed, H.S.; Annabi, N.; Bencherif, S.A. Engineering Injectable, Biocompatible, and Highly Elastic Bioadhesive Cryogels. Mater. Today Bio 2023, 19, 100572. [Google Scholar] [CrossRef]
- Ghovvati, M.; Baghdasarian, S.; Baidya, A.; Dhal, J.; Annabi, N. Engineering a Highly Elastic Bioadhesive for Sealing Soft and Dynamic Tissues. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2022, 110, 1511–1522. [Google Scholar] [CrossRef]
- Menon, A.V.; Putnam-Neeb, A.A.; Brown, C.E.; Crain, C.J.; Breur, G.J.; Narayanan, S.K.; Wilker, J.J.; Liu, J.C. Biocompatibility of Mussel-Inspired Water-Soluble Tissue Adhesives. J. Biomed. Mater. Res.—Part A 2024, 12, 2243–2256. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Wu, X.; Han, M.; Peng, R.; Zhao, R.; Qin, M.; Li, T.; Yin, J.; Yu, L.; et al. A Sprayable Janus Hydrogel as an Effective Bioadhesive for Gastrointestinal Perforation Repair. Adv. Funct. Mater. 2024, 34, 2408479. [Google Scholar] [CrossRef]
- Ashrafi, A.; Khadem, E.; Kharaziha, M. Engineering a Mussel-Inspired Hemostatic Sealant with a Strong Tissue Anchor as a First-Aid Tissue Adhesive. Mater. Today Chem. 2024, 35, 101864. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Song, C.; Shen, X.; Wang, F.; Zhang, Y.; Ma, Y.; Ding, X. A Mussel Inspired Polyvinyl Alcohol/Collagen/Tannic Acid Bioadhesive for Wet Adhesion and Hemostasis. Colloids Surf. B Biointerfaces 2024, 235, 113766. [Google Scholar] [CrossRef]
- Bilalis, P.; Alrashoudi, A.I.; Susapto, H.H.; Moretti, M.; Alshehri, S.; Abdelrahman, S.; Elsakran, A.; Hauser, C.A.E. Dipeptide-Based Photoreactive Instant Glue for Environmental and Biomedical Applications. ACS Appl. Mater. Interfaces 2023, 15, 46710–46720. [Google Scholar] [CrossRef]
- Guo, Z.; Xiong, Y.; Zhang, S.; Yuan, T.; Xia, J.; Wei, R.; Chen, L.; Sun, W. Naturally Derived Highly Resilient and Adhesive Hydrogels with Application as Surgical Adhesive. Int. J. Biol. Macromol. 2023, 253 Pt 5, 127192. [Google Scholar] [CrossRef]
- Hauser, D.; Septiadi, D.; Turner, J.; Petri-Fink, A.; Rothen-Rutishauser, B. From Bioinspired Glue to Medicine: Polydopamine as a Biomedical Material. Materials 2020, 13, 1730. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-Modified Hyaluronic Acid Hydrogel Adhesives with Fast-Forming and High Tissue Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yuan, M.; Sonamuthu, J.; Yan, S.; Huang, W.; Cai, Y.; Yao, J. A Dopamine-Functionalized Aqueous-Based Silk Protein Hydrogel Bioadhesive for Biomedical Wound Closure. New J. Chem. 2020, 44, 884–891. [Google Scholar] [CrossRef]
- Oz, Y.; Roy, A.; Jain, S.; Zheng, Y.; Mahmood, E.; Baidya, A.; Annabi, N. Designing a Naturally Inspired Conductive Copolymer to Engineer Wearable Bioadhesives for Sensing Applications. ACS Appl. Mater. Interfaces 2024, 16, 36002–36016. [Google Scholar] [CrossRef]
- Nijst, C.L.E.; Bruggeman, J.P.; Karp, J.M.; Ferreira, L.; Zumbuehl, A.; Bettinger, C.J.; Langer, R. Synthesis and Characterization of Photocurable Elastomers from Poly(Glycerol-Co-Sebacate). Biomacromolecules 2007, 8, 3067–3073. [Google Scholar] [CrossRef]
- Mahdavi, A.; Ferreira, L.; Sundback, C.; Nichol, J.W.; Chan, E.P.; Carter, D.J.D.; Bettinger, C.J.; Patanavanich, S.; Chignozha, L.; Ben-joseph, E.; et al. A Biodegradable and Biocompatible Gecko-Inspired Tissue Adhesive. Proc. Natl. Acad. Sci. USA 2008, 105, 2307–2312. [Google Scholar] [CrossRef]
- Lang, N.; Pereira, M.J.; Lee, Y.; Friehs, I.; Vasilyev, N.V.; Feins, E.N.; Ablasser, K.; O’Cearbhaill, E.D.; Xu, C.; Fabozzo, A.; et al. A Blood-Resistant Surgical Glue for Minimally Invasive Repair of Vessels and Heart Defects. Sci. Transl. Med. 2014, 6, 218ra6. [Google Scholar] [CrossRef]
- Pellenc, Q.; Touma, J.; Coscas, R.; Edorh, G.; Pereira, M.; Karp, J.; Castier, Y.; Desgranges, P.; Alsac, J.M. Preclinical and Clinical Evaluation of a Novel Synthetic Bioresorbable, on-Demand, Light-Activated Sealant in Vascular Reconstruction. J. Cardiovasc. Surg. 2019, 60, 599–611. [Google Scholar] [CrossRef]
- Wilks, A.M.; Rabice, S.R.; Garbacz, H.S.; Harro, C.C.; Smith, A.M. Double-Network Gels and the Toughness of Terrestrial Slug Glue. J. Exp. Biol. 2015, 218 Pt. 19, 3128–3137. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Fan, Y.; Yu, S.; Liu, M.; Feng, L.; Sun, Q.; Pan, P. Principles and Design of Bionic Hydrogel Adhesives for Skin Wound Treatment. Polymers 2024, 16, 1937. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough Adhesives for Diverse Wet Surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef]
- Hu, S.; Wang, S.; He, Q.; Li, D.; Xin, L.; Xu, C.; Zhu, X.; Mei, L.; Cannon, R.D.; Ji, P.; et al. A Mechanically Reinforced Super Bone Glue Makes a Leap in Hard Tissue Strong Adhesion and Augmented Bone Regeneration. Adv. Sci. 2023, 10, 2206450. [Google Scholar] [CrossRef]
- Pawlicki, J.M.; Pease, L.B.; Pierce, C.M.; Startz, T.P.; Zhang, Y.; Smith, A.M. The Effect of Molluscan Glue Proteins on Gel Mechanics. J. Exp. Biol. 2004, 207 Pt 7, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, Y.; Bu, S.; Wu, T.; Chang, Q.; Singh, G.; Cao, X.; Deng, C.; Li, B.; Luo, G.; et al. Egg Albumen as a Fast and Strong Medical Adhesive Glue. Adv. Healthc. Mater. 2017, 6, 1700132. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry Double-Sided Tape for Adhesion of Wet Tissues and Devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Tan, L.; Sun, L.; Petrosino, J.; Cui, M.-Z.; Hao, F.; Zhang, M. Nanospherical Arabinogalactan Proteins Are a Key Component of the High-Strength Adhesive Secreted by English Ivy. Proc. Natl. Acad. Sci. USA 2016, 113, E3193–E3202. [Google Scholar] [CrossRef]
- Li, Q.; Song, W.; Li, J.; Ma, C.; Zhao, X.; Jiao, J.; Mrowczynski, O.; Webb, B.S.; Rizk, E.B.; Lu, D.; et al. Bioinspired Super-Strong Aqueous Synthetic Tissue Adhesives. Matter 2022, 5, 933–956. [Google Scholar] [CrossRef]
- Bal-Ozturk, A.; Cecen, B.; Avci-Adali, M.; Topkaya, S.N.; Alarcin, E.; Yasayan, G.; Li, Y.C.E.; Bulkurcuoglu, B.; Akpek, A.; Avci, H.; et al. Tissue Adhesives: From Research to Clinical Translation. Nano Today 2021, 36, 101049. [Google Scholar] [CrossRef]
- Bayer, O. Das Di-Lsocganat-Poluadditionsverfahren (Polyurethane). Angew. Chem. 2006, 59, 257–272. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Doll, B.A.; Beckman, E.J.; Hollinger, J.O. Three-Dimensional Biocompatible Ascorbic Acid-Containing Scaffold for Bone Tissue Engineering. Tissue Eng. 2003, 9, 1143–1157. [Google Scholar] [CrossRef]
- Schulten, L.; Spillner, J.; Kanzler, S.; Teubner, A.; Jockenhoevel, S.; Apel, C. A Polyurethane-Based Surgical Adhesive for Sealing Blood Vessel Anastomoses—A Feasibility Study in Pigs. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2022, 110, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Bremer, L.; Hagemeister, K.; Moss, M.; Ernst, L.; Tolba, R.H.; Jockenhoevel, S.; Apel, C. Long-Term Degradation Assessment of a Polyurethane-Based Surgical Adhesive—Assessment and Critical Consideration of Preclinical In Vitro and In Vivo Testing. J. Funct. Biomater. 2023, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mahanwar, P. A Brief Discussion on Advances in Polyurethane Applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- FDA. US Food and Drug Administration—Premarket Approval (PMA) for Tissuglu®. Available online: https://www.accessdata.fda.gov/scrIpts/cdrh/cfdocs/cfpma/pma.cfm?id=P130023 (accessed on 6 August 2024).
- Beckman, E.J. One Part Moisture Curable Tissue Sealant. WO,150,199,A2, 26 May 2011. [Google Scholar]
- Beckman, E.J. Biodegradable Compositions Having Pressure Sensitive Adhesive Properties. US2015079151A1, 24 November 2015. [Google Scholar]
- Smith, E.; Beckman, E.J. Hydrophilic Biodegradable Adhesives. US,2009,0028,812,A1, 22 May 2012. Available online: https://patents.google.com/patent/US20090028812A1/en?oq=US2009028812A1 (accessed on 6 August 2024).
- Beckman, E.J.; Buckley, M.; Agarwal, S.; Zhang, J. Medical Adhesive and Methods of Tissue Adhesion. US20040170597A1, 4 September 2007. Available online: https://patents.google.com/patent/US20040170597A1/en?oq=US2004170597A1 (accessed on 6 August 2024).
- Smith, J.; Beckman, E.J. Hydrophilic Biodegradable Adhesives. PL2176370T3, 7 July 2008. Available online: https://patents.google.com/patent/PL2176370T3/en?oq=PL2176370T3_cohera+2007 (accessed on 6 August 2024).
- Su, Q.; Wei, D.; Dai, W.; Zhang, Y.; Xia, Z. Designing a Castor Oil-Based Polyurethane as Bioadhesive. Colloids Surf. B Biointerfaces 2019, 181, 740–748. [Google Scholar] [CrossRef]
- Zhao, X.; Ming, H.; Wang, Y.; Luo, F.; Li, Z.; Li, J.; Tan, H.; Fu, Q. Mussel-Inspired, Injectable Polyurethane Tissue Adhesives Demonstrate in Situ Gel Formation under Mild Conditions. ACS Appl. Bio Mater. 2021, 4, 5352–5361. [Google Scholar] [CrossRef]
- Wendels, S.; Balahura, R.; Dinescu, S.; Ignat, S.; Costache, M.; Avérous, L. Influence of the Macromolecular Architecture on the Properties of Biobased Polyurethane Tissue Adhesives. Eur. Polym. J. 2022, 164, 110968. [Google Scholar] [CrossRef]
- Agnol, L.D.; Dias, F.T.G.; Nicoletti, N.F.; Marinowic, D.; Moura e Silva, S.; Marcos-Fernandez, A.; Falavigna, A.; Bianchi, O. Polyurethane Tissue Adhesives for Annulus Fibrosus Repair: Mechanical Restoration and Cytotoxicity. J. Biomater. Appl. 2019, 34, 673–686. [Google Scholar] [CrossRef]
- Ferreira, P.; Silva, A.F.M.; Pinto, M.I.; Gil, M.H. Development of a Biodegradable Bioadhesive Containing Urethane Groups. J. Mater. Sci. Mater. Med. 2008, 19, 111–120. [Google Scholar] [CrossRef]
- Eling, B.; Tomović, Ž.; Schädler, V. Current and Future Trends in Polyurethanes: An Industrial Perspective. Macromol. Chem. Phys. 2020, 221, 2000114. [Google Scholar] [CrossRef]
- Wegener, G.; Brandt, M.; Duda, L.; Hofmann, J.; Klesczewski, B.; Koch, D.; Kumpf, R.J.; Orzesek, H.; Pirkl, H.G.; Six, C.; et al. Trends in Industrial Catalysis in the Polyurethane Industry. Appl. Catal. A Gen. 2001, 221, 303–335. [Google Scholar] [CrossRef]
- Lei, K.; Zhu, Q.; Wang, X.; Xiao, H.; Zheng, Z. In Vitro and in Vivo Characterization of a Foam-Like Polyurethane Bone Adhesive for Promoting Bone Tissue Growth. ACS Biomater. Sci. Eng. 2019, 5, 5489–5497. [Google Scholar] [CrossRef] [PubMed]
- Upson, S.J.; Benning, M.J.; Fulton, D.A.; Corbett, I.P.; Dalgarno, K.W.; German, M.J. Bond Strength and Adhesion Mechanisms of Novel Bone Adhesives. Bioengineering 2023, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Cernadas, T.; Morgado, S.; Alves, P.; Gonçalves, F.A.M.M.; Correia, T.R.; Correia, I.J.; Ferreira, P. Preparation of Functionalized Poly(Caprolactone Diol)/Castor Oils Blends to Be Applied as Photocrosslinkable Tissue Adhesives. J. Appl. Polym. Sci. 2020, 137, 49092. [Google Scholar] [CrossRef]
- Balcioglu, S.; Noma, S.A.A.; Ulu, A.; Karaaslan-Tunc, M.G.; Ozhan, O.; Koytepe, S.; Parlakpinar, H.; Vardi, N.; Colak, M.C.; Ates, B. Fast Curing Multifunctional Tissue Adhesives of Sericin-Based Polyurethane-Acrylates for Sternal Closure. ACS Appl. Mater. Interfaces 2022, 14, 41819–41833. [Google Scholar] [CrossRef]
- Balcioglu, S.; Gurses, C.; Ozcan, I.; Yildiz, A.; Koytepe, S.; Parlakpinar, H.; Vardi, N.; Ates, B. Photocrosslinkable Gelatin/Collagen Based Bioinspired Polyurethane-Acrylate Bone Adhesives with Biocompatibility and Biodegradability. Int. J. Biol. Macromol. 2021, 192, 1344–1356. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wei, W.; Liu, X. Strong Dual-Components Bioadhesive Based on Solventless Thiolisocyanate Click Chemistry. ACS Biomater. Sci. Eng. 2021, 7, 3389–3398. [Google Scholar] [CrossRef]
- Santerre, P.; Sone, E.; Floros, M. Water Soluble Bioadhesives. WO,119,855, 21 December 2020. [Google Scholar]
- Santerre, P.; Sone, E.; Floros, M. Biocompatible Adhesives and Methods of Manufacture Thereof. WO,2021,119,854, 21 December 2020. [Google Scholar]
- Burmaster, D.; Azzi, J.; McDonald, R. Clear Polypropylene Composition for Thermoforming. WO,006,196, 30 June 2021. [Google Scholar]
- Heilig, M.L. United States Patent Office. ACM SIGGRAPH Comput. Graph. 1994, 28, 131–134. [Google Scholar] [CrossRef]
- Shantha, K.L.; Thennarasu, S.; Krishnamurti, N. Developments and Applications of Cyanoacrylate Adhesives. J. Adhes. Sci. Technol. 1989, 3, 237–260. [Google Scholar] [CrossRef]
- Lins, R.D.A.U.; Gomes, R.C.B.; dos Santos, K.S.A.; da Silva, P.V.; da Silva, R.T.M.; Ramos, I.A. Use of Cyanoacrylate in the Coaptation of Edges of Surgical. An. Bras. Dermatol. 2012, 87, 871. [Google Scholar] [CrossRef]
- Tarafder, S.; Park, G.Y.; Felix, J.; Lee, C.H. Bioadhesives for Musculoskeletal Tissue Regeneration. Acta Biomater. 2020, 117, 77–92. [Google Scholar] [CrossRef]
- FDA. US Food and Drug Administration—Premarket Approval (PMA) for Dermabond®. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052 (accessed on 6 August 2024).
- Singer, A.J.; Quinn, J.V.; Hollander, J.E. The Cyanoacrylate Topical Skin Adhesives. Am. J. Emerg. Med. 2008, 26, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Leonard, F.; Kulkarni, R.K.; Brandes, G.; Nelson, J.; Cameron, J.J. Synthesis and Degradation of Poly (Alkyl A-cyanoacrylates). J. Appl. Polym. Sci. 1966, 10, 259–272. [Google Scholar] [CrossRef]
- FDA. US Food and Drug Administration—Premarket Approval (PMA) for OmnexTM. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P060029 (accessed on 6 August 2024).
- Patil, N.A.; Kandasubramanian, B. Functionalized Polylysine Biomaterials for Advanced Medical Applications: A Review. Eur. Polym. J. 2021, 146, 110248. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Bioerodible Hydrogels Based on Photopolymerized Poly(Ethylene Glycol)-Co-Poly (α-Hydroxy Acid) Diacrylate Macromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- Holowka, E.P.; Bhatia, S.K. Drug Delivery: Materials Design and Clinical Perspective; Springer: New York, NY, USA, 2014; pp. 1–355. [Google Scholar] [CrossRef]
- FDA. US Food and Drug Administration—Premarket Approval (PMA) for Duraseal®. Available online: https://www.accessdata.fda.gov/scrIpts/cdrh/cfdocs/cfpma/pma.cfm?id=P040034 (accessed on 6 August 2024).














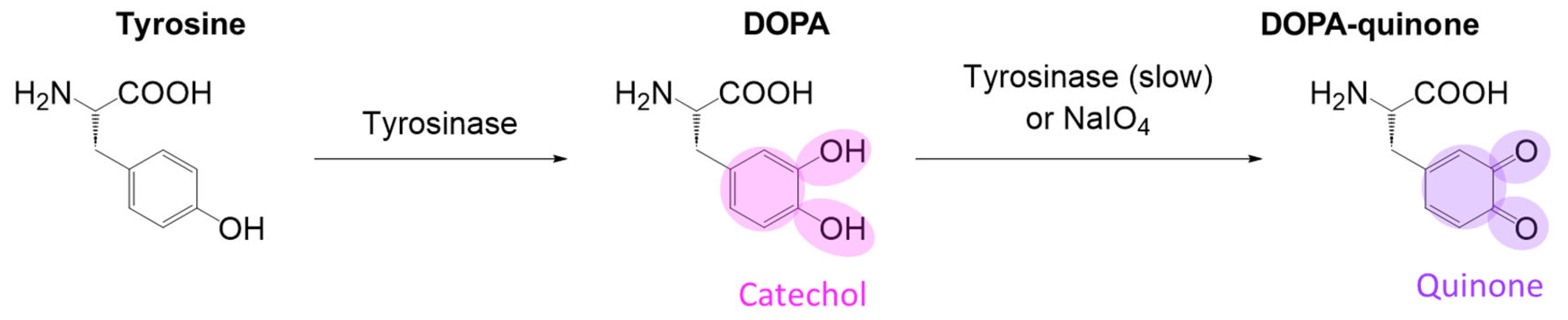
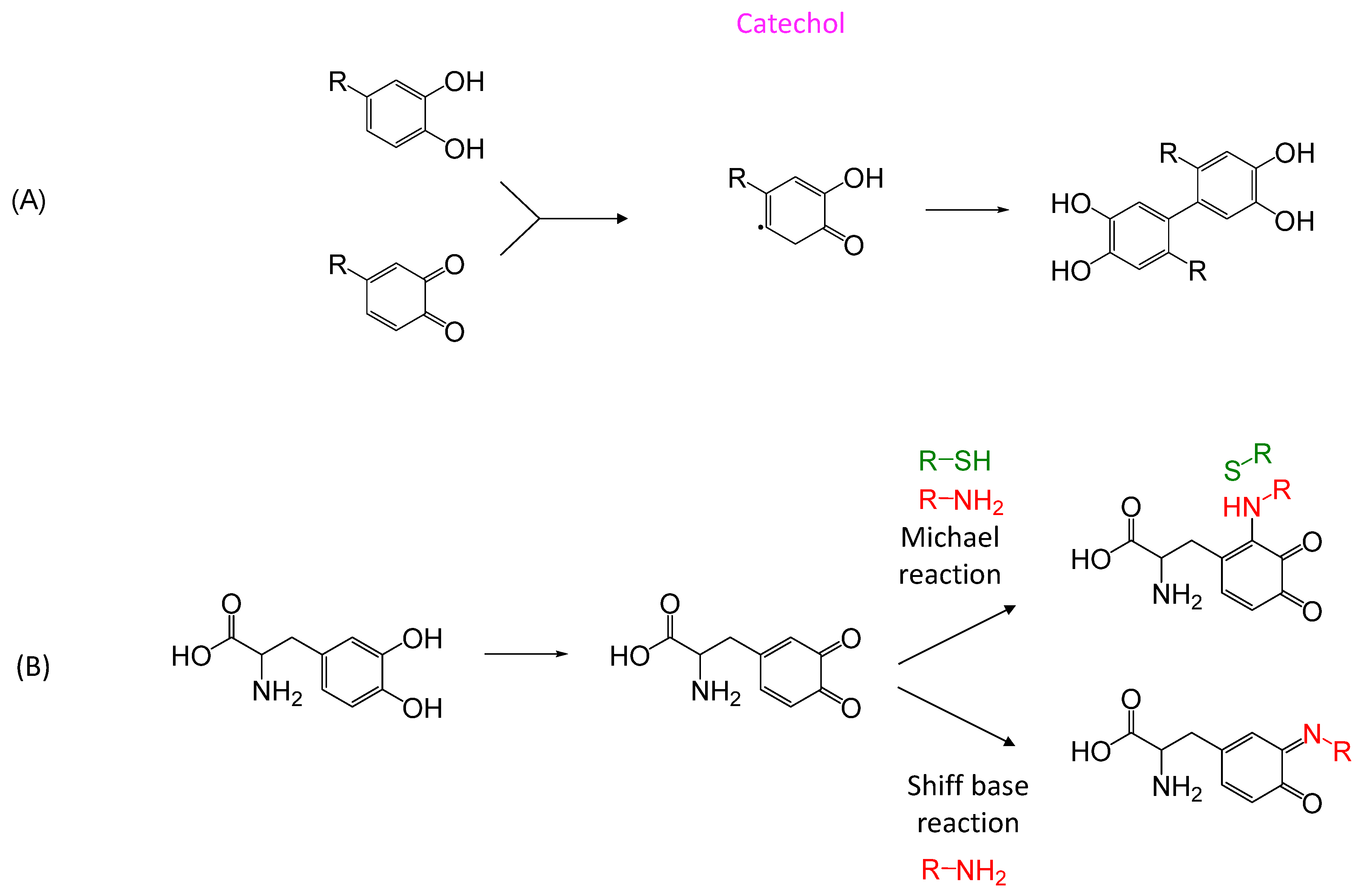

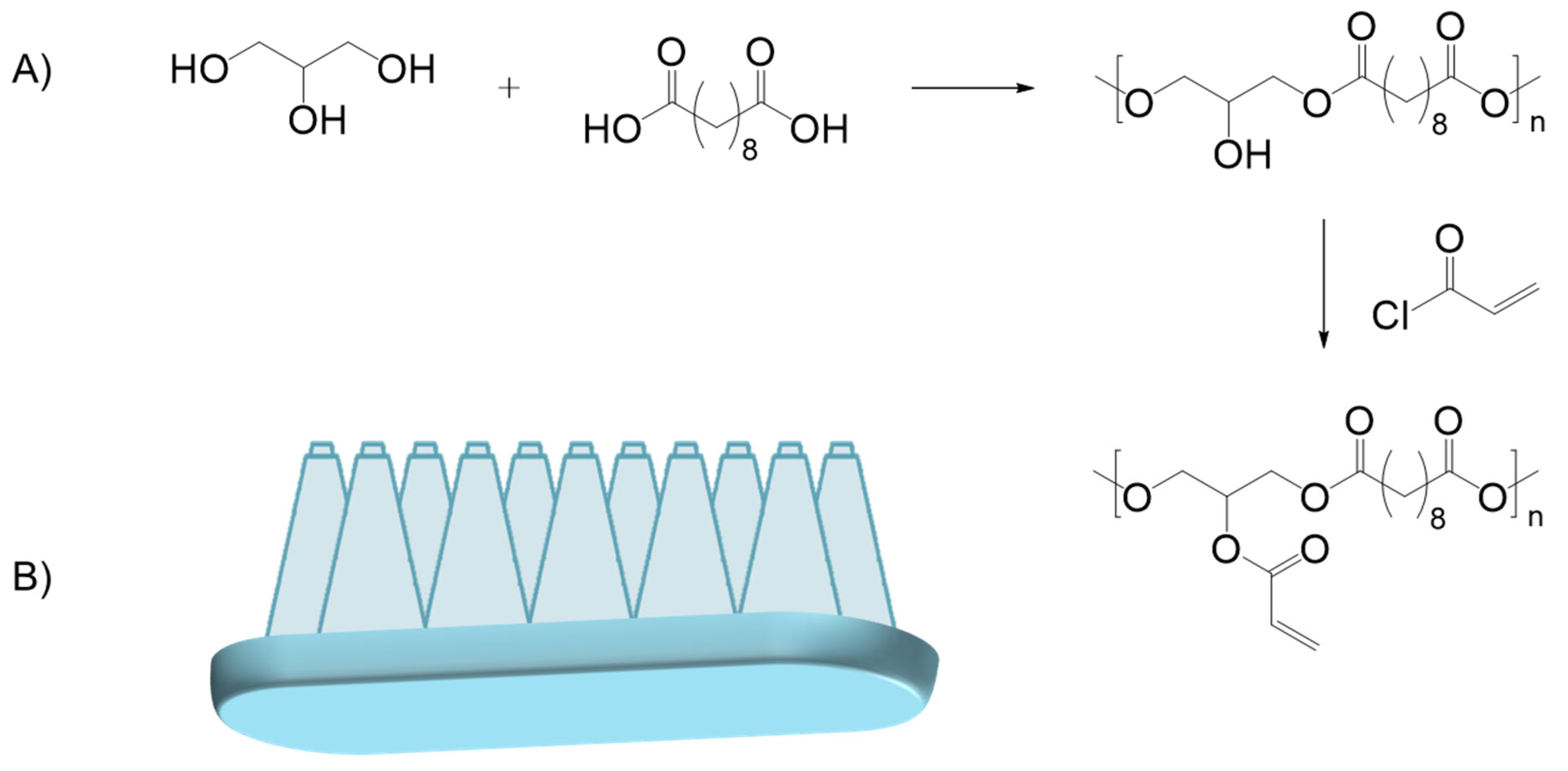


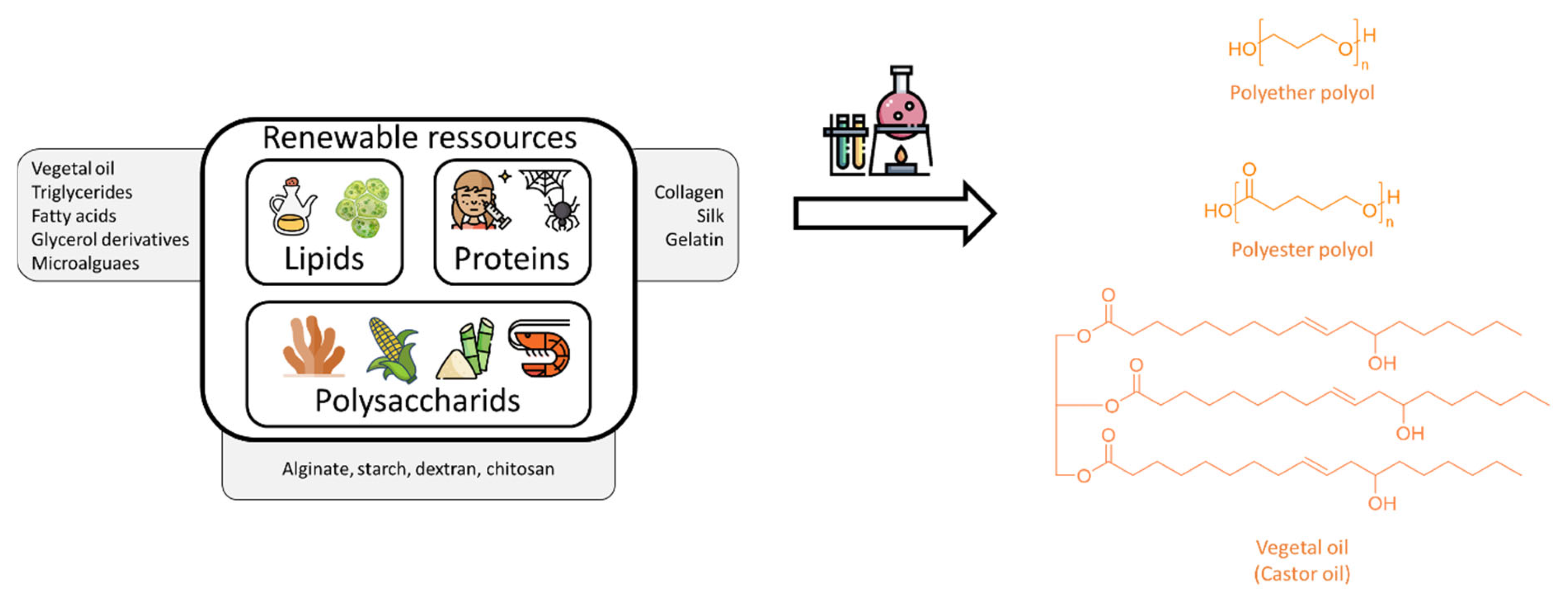
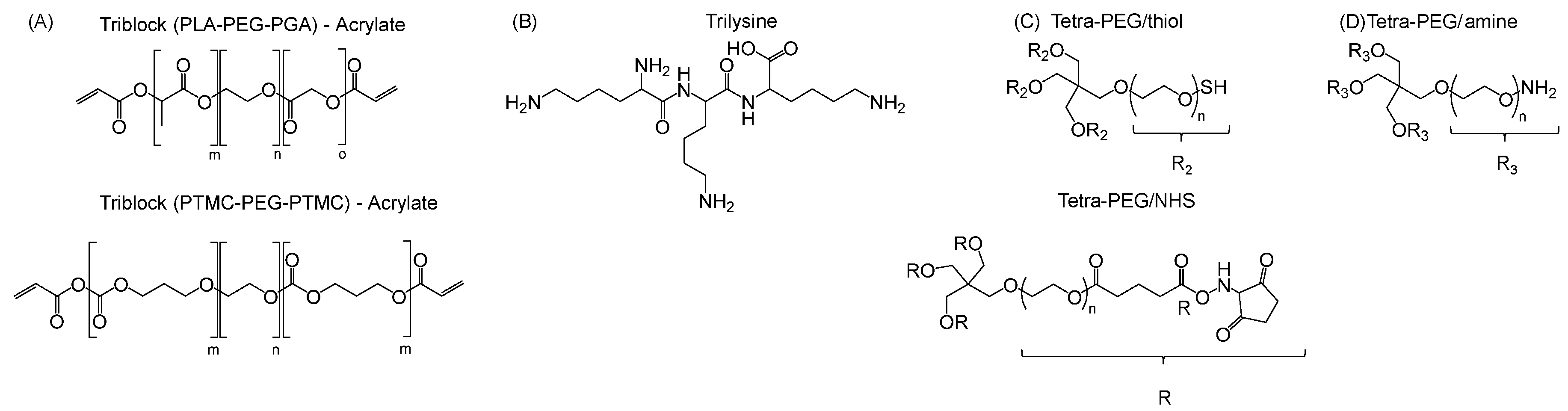
| Monomer | 2-ethylhexyl acrylate | N-octyl acrylate | N-butyl acrylate | Ethyl Acrylate | Methyl Acrylate | Vinyl Acetate | Acrylic Acid | Acrylamide |
|---|---|---|---|---|---|---|---|---|
| Formula | 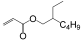 | 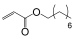 | 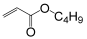 | 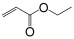 | 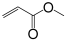 | 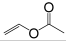 |  |  |
| Tg of homopolymer (°C) | −70 | −65 | −54 | −24 | −6 | 30 | 106 | 179 |
| Technology | Surgical Used Reported | Adhesion kPa | Price $/mL | Approval | Storage Condition (°C) | Instruction | Degradation in Physiological Condition (Body) | Commercial Name (Example) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sealant Hemostat Adhesive | Fibrine | 1970 | 5–20 | 53–159 a,b | FDA 1998 | Freezer (−20) | Warm up at 37 °C | >1 month | Tisseel (Baxter) | [70] |
| Adhesive | GRF c | 1960 | 1–3 | 60–120 | NO | 2 months | Cardial (LeMaitre vascular) | [24] | ||
| Adhesive | GRFG d | 1967 | NO | Warm up at 45 °C | >2 months | GRF (Microval) | [52] | |||
| Hemostat Adhesive | Gelatin + Thrombin | 1970 | 62–100 a,b | FDA 1999 | RT (2–25) | 4–6 weeks | Floseal (Baxter) | [28] | ||
| Adhesive | Gelatine + mTG | 2004 | 12–23 | CE 2011 | Fridge (2–8) | Warm up at 37 °C | 4–8 weeks | LifeSeal™ (Tiko-LifeBond) | [84] | |
| Sealant Adhesive | Albumin + glutaraldehyde | 1996 | 10–50 | 50–190 a,b | FDA 2001 | RT | Weeks to months | Bioglue® (Cryolife) | [71] | |
| Adhesive | Albumin + polyaldehydes | 1780 | 300 a | FDA 2017 | 6–8 weeks | PreveLeak (Baxter) | [23] | |||
| Adhesive | Albumin + PEG-NHS | 1994 | 15–25 | 100–200 | FDA 2010 | Fridge (2–8) | - | <14 days | Progel™ (Davol) | [100] |
| Species | Way of Bonding | Purpose |
|---|---|---|
| bee | chemical (propolis) | nesting |
| caddishfly | chemical (silk) | nesting/hunting |
| chameleon | chemical (saliva) | hunting |
| dandelion | chemical (nectar) | mating (pollen spreading) |
| frog | chemical (mucus) | defence |
| ivy | chemical (polysaccharides glycoproteins) | attachement |
| mussel | chemical (catechol) | attachement |
| orchid | chemical (nectar) | mating (pollen spreading) |
| sea cucumber | chemical (mucus) | defence |
| silk worm | chemical (silk) | nesting |
| slug | chemical | defence/locomotion |
| spider | chemical (silk) | hunting/locomotion |
| velvet worm | chemical (mucus) | defence/hunting |
| gellyfish | chemical (nematocytes) physical (bar; cnidocytes) | defence/hunting |
| gecko | physical (spatulae) | defence/locomotion |
| nautilus | physical (suckers) | hunting/mating |
| octopus | physical (suckers) | attachement |
| see urchin | physical | attachement/locomotion |
| star fish | physical (suckers) | attachement |
| venus flytrap | physical (trap) | hunting |
| Adhesive/Family | Structure/Inspiration | Key Mechanism/Component | Main Function/Properties | Clinical Application | Regulatory Status | Pros | Cons |
|---|---|---|---|---|---|---|---|
| Fibrine glue | Plasma protein (fibrinogen) | Enzymatic polymerization (thrombin) | Rapid hemostasis Sealant Adhesive | -Wound closure -Skin graft -Various surgeries | FDA approved 1998 | Biocompatible Biodegradable Support cell growth | High cost Low adhesion Risk of bloodborn pathogen transmition Application process |
| Gelatin–Resorcinol– Formaldehyde (±glutaraldehyde) | Collagen derivative | Aldehyde condensation | Adhesive | Various surgeries | Biocompatible biodegradable Support cell growth Strong cohesion Cost effective | Toxicity of the aldehydes | |
| Gelatin-thrombine | Gelatin + enzyme | Enzymatic activation | Hemostasis Adhesive | FDA approved 1999 | Moderate cohesion Foreign body reaction | ||
| Gelatin-mTG | Gelatin | Microbial transglutaminase | Enzymatic crosslinking adhesive | Gastrointestinal surgery | CE mark 2011 | Good tissue compatibility | Low adhesion Poor mechanical strenght |
| Albumine–Aldehydes | Protein + aldehydes | Schiff base reaction (covalent bonding) | Sealant Adhesive | Vascular and cardiac surgeries | FDA approved 2001: glutaraldehyde 2017: polyaldehyde | Enhanced adhesion (vs. fibrine) | Potential cytotoxicity Low viscosity Inflammatory |
| Albumine–PEG | Protein + polymer | Chemical crosslinking | Prevent air leakage Adhesive | Lung surgery | FDA approved 2010 | Low immunogenicity Degradable | Moderate adhesion High price Strorage condition |
| Chitosan | Polysaccharides | Hydrogen bonding Electrostatic interactions | Mucoadhesive Hemostasis | Wound healing Tissue adhesives | Biocompatible Physiodegradable Antibacterial | ||
| Dextran based adhesives | Polysaccharides | Oxidation/aldehyde formation | Hemostasis | FDA: Conditional approval | Bioresorbable Enhanced adhesion (vs. fibrine) | Toxicity of aldehydes Foreign body reactions | |
| Alginate | Polysaccharides | Ionic crosslinking | Sealant | Wound dressing Bones reconstruction Tissue regeneration | CE mark 2017 | Low cost Biocompatible Enhanced adhesion (vs. fibrine) | Not biodegradable |
| Mussel inspired | DOPA rich proteins | Catechol Strong covalent/non-covalent bonds | Wet adhesion | Not commercialized | AdhesionWater insoluble Degradable | Extraction of natural protein not scalable and economical Toxicity suspected Storage of catechol | |
| Gecko inspired | Microscopic spatulae | Van der Waals forces | Dry adhesion Hemostasis | Vascular surgery | CE mark 2017 | Reversible/reusable Biocompatible Biodegradable | Low adhesivity wich deteriorates in wet conditions |
| Slug/snail mucus inspired | Viscoelastic secretion | Physical + chemical interactions | Tough Extensible wet adhesion | Resist to humidity | |||
| Polyurethane based | Synthetic polymer | Chemical crosslinking | Adhesive | Abdominal surgery | FDA approved 2015 | Strong cohesion Biocompatible Biodegradable | Toxicity of isocyanates Long curing time |
| Cyanoacrylate | Acrylate monomer | Anionic polymerization | Adhesive Selant Hemostasis | Wound closure Dentistry Surgery | FDA approved Wound dressing: 1998 Internal surgery: 2010 | Very strong adhesion even in wet conditions Antimicrobial activity polymerize quickly | Brittle Tissue toxicity Not resorbable Exothermic |
| PEG-based hydrogel | Hydrophilic polymer | Chemical crosslinking | Sealant | Lung surgery Ophtalmic Vascular | FDA approved 2000 | Customizable Bio-inert/bioresorbable matrix | High cost Poor mechanical properties |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boursier, M.; Bayon, Y.; Negrell, C.; Pinaud, J.; Caillol, S. Biocompatible Glues: Recent Progress and Emerging Frontiers in Surgical Adhesion. Polymers 2025, 17, 1749. https://doi.org/10.3390/polym17131749
Boursier M, Bayon Y, Negrell C, Pinaud J, Caillol S. Biocompatible Glues: Recent Progress and Emerging Frontiers in Surgical Adhesion. Polymers. 2025; 17(13):1749. https://doi.org/10.3390/polym17131749
Chicago/Turabian StyleBoursier, Marine, Yves Bayon, Claire Negrell, Julien Pinaud, and Sylvain Caillol. 2025. "Biocompatible Glues: Recent Progress and Emerging Frontiers in Surgical Adhesion" Polymers 17, no. 13: 1749. https://doi.org/10.3390/polym17131749
APA StyleBoursier, M., Bayon, Y., Negrell, C., Pinaud, J., & Caillol, S. (2025). Biocompatible Glues: Recent Progress and Emerging Frontiers in Surgical Adhesion. Polymers, 17(13), 1749. https://doi.org/10.3390/polym17131749









