Synthesis and Assessment of Novel Sustainable Antioxidants with Different Polymer Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Antioxidants Synthesis
2.3. Formulations Based on TPO and TPU Matrices
2.4. Characterization Techniques and Procedures
3. Results
3.1. Antioxidants’ Synthesis and Characterizations
3.2. Evaluation of Antioxidant Activity
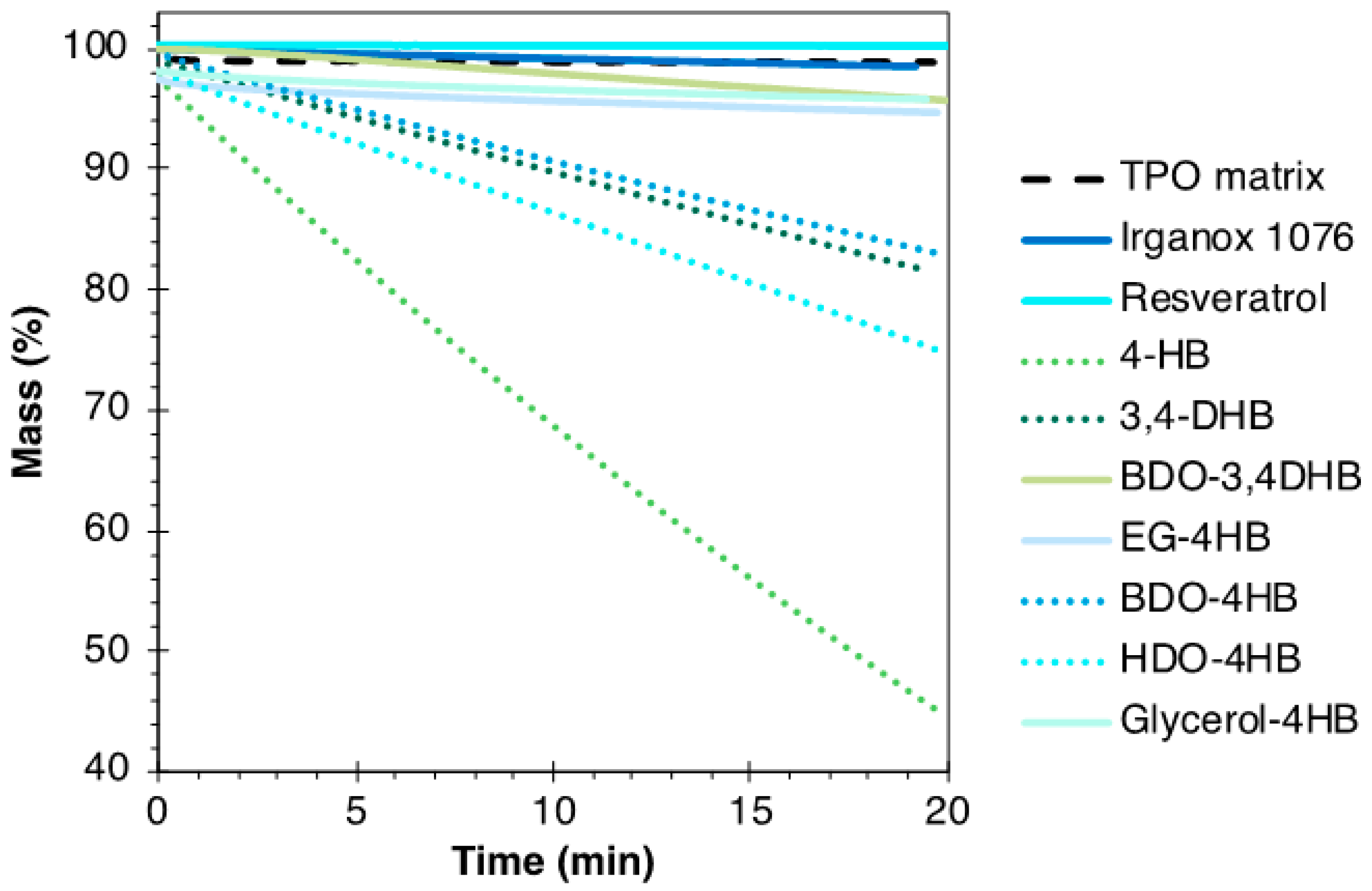
3.3. Thermal Stability of the Formulations
4. Analysis of the Matrices and the Formulations
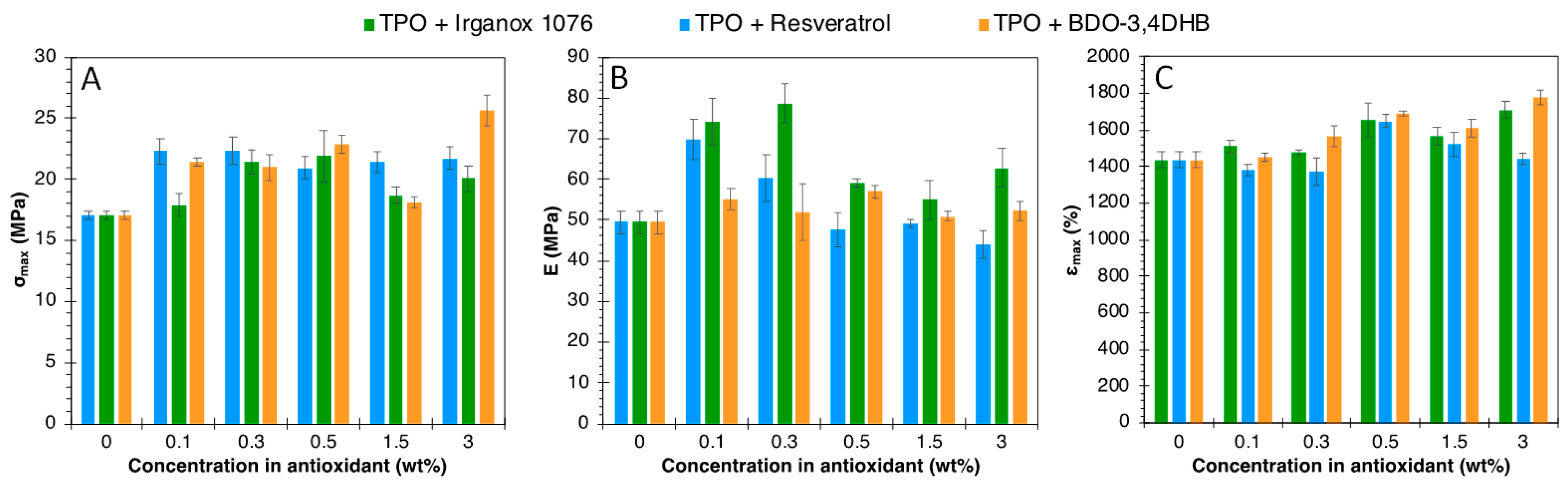
4.1. TPO Case
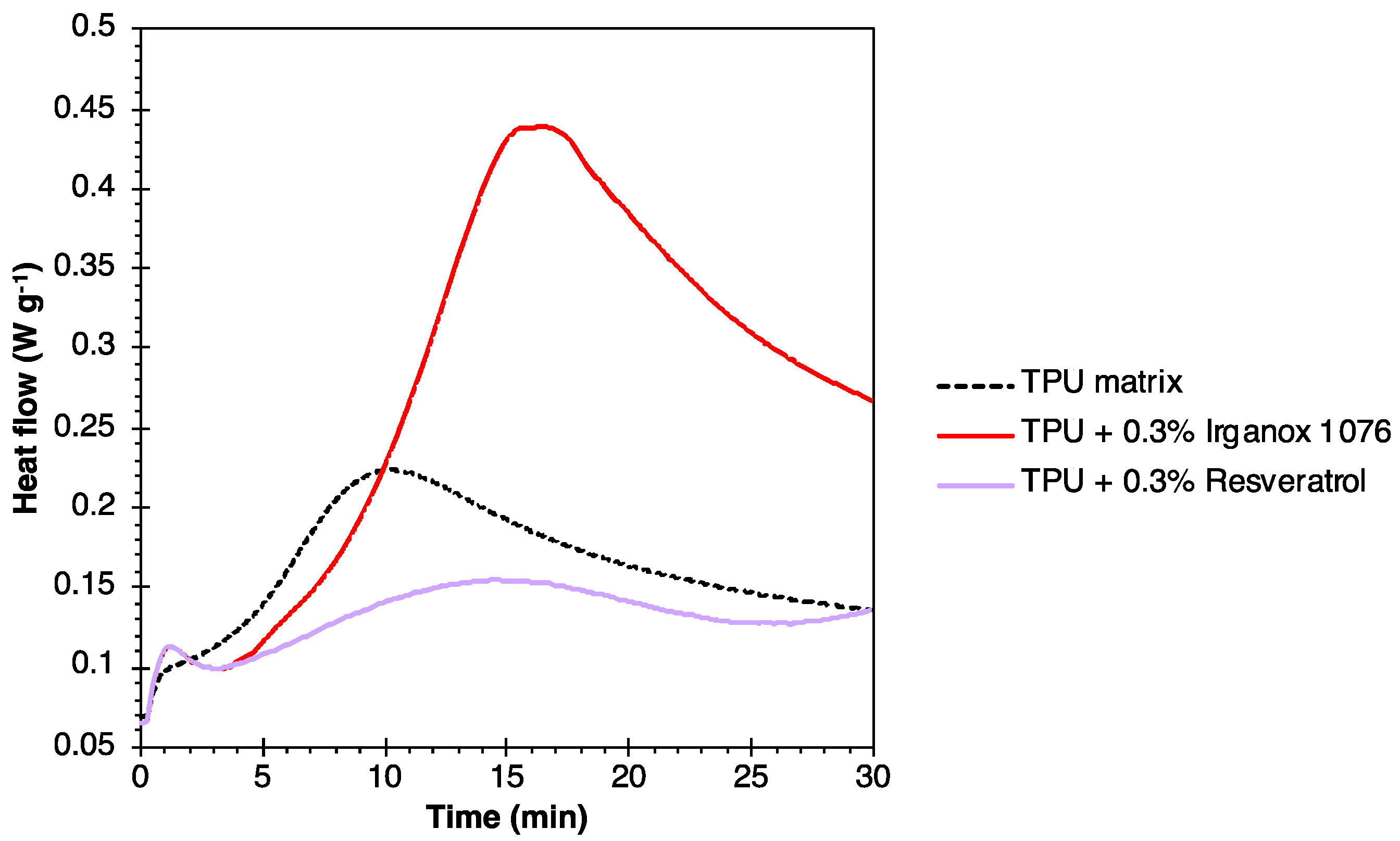
4.2. TPU Case
4.3. Analysis of an Accelerated Aging Test on TPO-Based Formulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- International Union of Pure and Applied Chemistry. Compendium of Polymer Terminology and Nomenclature: IUPAC Recommendations, 2008; RSC Publishing: Cambridge, UK, 2009. [Google Scholar]
- Plastic Additives Market Share, Size & Forecast 2024–2032|Industry Report. 2023. Global Market Insights Inc. Available online: https://www.gminsights.com/industry-analysis/plastic-additives-market (accessed on 5 September 2023).
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.S.; Edge, M. Perspectives on Additives for Polymers. 1. Aspects of Stabilization. J. Vinyl Addit. Technol. 2021, 27, 5–27. [Google Scholar] [CrossRef]
- Ambrogi, V.; Carfagna, C.; Cerruti, P.; Marturano, V. Additives in Polymers. In Modification of Polymer Properties; Jasso-Gastinel, C.F., Kenny, J.M., Eds.; William Andrew Publishing: New York, NY, USA, 2017; pp. 87–108. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Ambrogi, V. Polymer Additives. Phys. Sci. Rev. 2017, 2, 20160130. [Google Scholar] [CrossRef]
- Gol’dberg, V.M.; Zaikov, G.E. Kinetics of Mechanical Degradation in Melts under Model Conditions and during Processing of Polymers—A Review. Polym. Degrad. Stab. 1987, 19, 221–250. [Google Scholar] [CrossRef]
- Moss, S.; Zweifel, H. Degradation and Stabilization of High Density Polyethylene during Multiple Extrusions. Polym. Degrad. Stab. 1989, 25, 217–245. [Google Scholar] [CrossRef]
- Klemchuk, P.P.; Horng, P.-L. Perspectives on the Stabilization of Hydrocarbon Polymers against Thermo-Oxidative Degradation. Polym. Degrad. Stab. 1984, 7, 131–151. [Google Scholar] [CrossRef]
- Singh, N.; O’Malley, P.J.; Popelier, P.L.A. Mechanistic Aspects of Hydrogen Abstraction for Phenolic Antioxidants. Electronic Structure and Topological Electron Density Analysis. Phys. Chem. Chem. Phys. 2005, 7, 614–619. [Google Scholar] [CrossRef]
- Földes, E. Transport of Small Molecules in Polyolefins. II. Diffusion and Solubility of Irganox 1076 in Ethylene Polymers. J. Appl. Polym. Sci. 1993, 48, 1905–1913. [Google Scholar] [CrossRef]
- Brocca, D.; Arvin, E.; Mosbæk, H. Identification of Organic Compounds Migrating from Polyethylene Pipelines into Drinking Water. Water Res. 2002, 36, 3675–3680. [Google Scholar] [CrossRef]
- Cherif Lahimer, M.; Ayed, N.; Horriche, J.; Belgaied, S. Characterization of Plastic Packaging Additives: Food Contact, Stability and Toxicity. Arab. J. Chem. 2017, 10, S1938–S1954. [Google Scholar] [CrossRef]
- Galotto, M.J.; Torres, A.; Guarda, A.; Moraga, N.; Romero, J. Experimental and Theoretical Study of LDPE versus Different Concentrations of Irganox 1076 and Different Thickness. Food Res. Int. 2011, 44, 566–574. [Google Scholar] [CrossRef]
- den Braver-Sewradj, S.P.; van Spronsen, R.; Hessel, E.V.S. Substitution of Bisphenol A: A Review of the Carcinogenicity, Reproductive Toxicity, and Endocrine Disruption Potential of Alternative Substances. Crit. Rev. Toxicol. 2020, 50, 128–147. [Google Scholar] [CrossRef]
- Holaas, E.; Bohne, V.B.; Hamre, K.; Arukwe, A. Hepatic Retention and Toxicological Responses during Feeding and Depuration Periods in Atlantic Salmon (Salmo salar) Fed Graded Levels of the Synthetic Antioxidant, Butylated Hydroxytoluene. J. Agric. Food Chem. 2008, 56, 11540–11549. [Google Scholar] [CrossRef]
- Soto-Vaca, A.; Gutierrez, A.; Losso, J.N.; Xu, Z.; Finley, J.W. Evolution of Phenolic Compounds from Color and Flavor Problems to Health Benefits. J. Agric. Food Chem. 2012, 60, 6658–6677. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Averous, L.; Boquillon, N. Antioxidant Properties of Lignin in Polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural Antioxidants as Stabilizers for Polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Antioxidant Polyethylene Films Based on a Resveratrol Containing Clay of Interest in Food Packaging Applications. Food Packag. Shelf Life 2015, 6, 30–41. [Google Scholar] [CrossRef]
- Feng, C.; Chen, J.; Ye, W.; Liao, K.; Wang, Z.; Song, X.; Qiao, M. Synthetic Biology-Driven Microbial Production of Resveratrol: Advances and Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 833920. [Google Scholar] [CrossRef]
- Tiso, T.; Winter, B.; Wei, R.; Hee, J.; de Witt, J.; Wierckx, N.; Quicker, P.; Bornscheuer, U.T.; Bardow, A.; Nogales, J.; et al. The Metabolic Potential of Plastics as Biotechnological Carbon Sources—Review and Targets for the Future. Metab. Eng. 2022, 71, 77–98. [Google Scholar] [CrossRef]
- Antony, F.M.; Wasewar, K. Reactive Extraction: A Promising Approach to Separate Protocatechuic Acid. Environ. Sci. Pollut. Res. 2020, 27, 27345–27357. [Google Scholar] [CrossRef]
- Johnston, B.; Adamus, G.; Ekere, A.I.; Kowalczuk, M.; Tchuenbou-Magaia, F.; Radecka, I. Bioconversion of Plastic Waste Based on Mass Full Carbon Backbone Polymeric Materials to Value-Added Polyhydroxyalkanoates (PHAs). Bioengineering 2022, 9, 432. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation. Prog. Lipid Res. 1980, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Ho, C.-T. Antioxidant Activities of Caffeic Acid and Its Related Hydroxycinnamic Acid Compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure–Antioxidant Activity Relationships of o-Hydroxyl, o-Methoxy, and Alkyl Ester Derivatives of p-Hydroxybenzoic Acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Torres de Pinedo, A.; Peñalver, P.; Morales, J.C. Synthesis and Evaluation of New Phenolic-Based Antioxidants: Structure–Activity Relationship. Food Chem. 2007, 103, 55–61. [Google Scholar] [CrossRef]
- Tiso, T.; Narancic, T.; Wei, R.; Pollet, E.; Beagan, N.; Schröder, K.; Honak, A.; Jiang, M.; Kenny, S.T.; Wierckx, N.; et al. Towards Bio-Upcycling of Polyethylene Terephthalate. Metab. Eng. 2021, 66, 167–178. [Google Scholar] [CrossRef]
- Westhues, S.; Idel, J.; Klankermayer, J. Molecular Catalyst Systems as Key Enablers for Tailored Polyesters and Polycarbonate Recycling Concepts. Sci. Adv. 2018, 4, eaat9669. [Google Scholar] [CrossRef]
- Guo, H.; Liu, H.; Jin, Y.; Zhang, R.; Yu, Y.; Deng, L.; Wang, F. Advances in Research on the Bio-Production of 1,4-Butanediol by the Engineered Microbes. Biochem. Eng. J. 2022, 185, 108478. [Google Scholar] [CrossRef]
- Salusjärvi, L.; Havukainen, S.; Koivistoinen, O.; Toivari, M. Biotechnological Production of Glycolic Acid and Ethylene Glycol: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Q.; Wang, F.; Li, R.; Yu, X.; Kang, L.; Zhao, J.; Li, A. One-Pot Biosynthesis of 1,6-Hexanediol from Cyclohexane by de Novo Designed Cascade Biocatalysis. Green Chem. 2020, 22, 7476–7483. [Google Scholar] [CrossRef]
- Doudin, K.; Al-Malaika, S.; Sheena, H.H.; Tverezovskiy, V.; Fowler, P. New Genre of Antioxidants from Renewable Natural Resources: Synthesis and Characterisation of Rosemary Plant-Derived Antioxidants and Their Performance in Polyolefins. Polym. Degrad. Stab. 2016, 130, 126–134. [Google Scholar] [CrossRef]
- Francis, F.J.; Clydesdale, F.M. Food Colorimetry: Theory and Applications; The Avi Publishing Company: Westport, CT, USA, 1975. [Google Scholar]
- EN 14575:2005; Geosynthetic Barriers—Screening Test Method for Determining the Resistance to Oxidation. European Committee for Standardization: Brussels, Belgium, 2005.
- Torres de Pinedo, A.; Peñalver, P.; Pérez-Victoria, I.; Rondón, D.; Morales, J.C. Synthesis of New Phenolic Fatty Acid Esters and Their Evaluation as Lipophilic Antioxidants in an Oil Matrix. Food Chem. 2007, 105, 657–665. [Google Scholar] [CrossRef]
- Lecomte, J.; Giraldo, L.J.L.; Laguerre, M.; Baréa, B.; Villeneuve, P. Synthesis, Characterization and Free Radical Scavenging Properties of Rosmarinic Acid Fatty Esters. J. Am. Oil Chem. Soc. 2010, 87, 615–620. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Xu, L.; Lian, X.; Xu, K.; Chen, M. Synthesis of 3,5-Ditert-Butyl-4-Hydroxybenzoates and Their Thermal Antioxidation Behavior for Polypropylene. Polym. Degrad. Stab. 2009, 94, 1906–1913. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Gomes, B.A.Q.; Moraes, W.M.; Borges, R.S. A Theoretical Antioxidant Pharmacophore for Resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef]
- Földes, E.; Lohmeijer, J. Relationship between Chemical Structure and Performance of Primary Antioxidants in PBD. Polym. Degrad. Stab. 1999, 66, 31–39. [Google Scholar] [CrossRef]
- Laguerre, M.; Bayrasy, C.; Lecomte, J.; Chabi, B.; Decker, E.A.; Wrutniak-Cabello, C.; Cabello, G.; Villeneuve, P. How to Boost Antioxidants by Lipophilization? Biochimie 2013, 95, 20–26. [Google Scholar] [CrossRef]
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the Antioxidant Features of Polyphenols by Spectroscopic and Electrochemical Methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef]
- Calle, M.; Chan, Y.; Jo, H.J.; Lee, Y.M. The Relationship between the Chemical Structure and Thermal Conversion Temperatures of Thermally Rearranged (TR) Polymers. Polymer 2012, 53, 2783–2791. [Google Scholar] [CrossRef]
- Reano, A.F.; Domenek, S.; Pernes, M.; Beaugrand, J.; Allais, F. Ferulic Acid-Based Bis/Trisphenols as Renewable Antioxidants for Polypropylene and Poly(Butylene Succinate). ACS Sustain. Chem. Eng. 2016, 4, 6562–6571. [Google Scholar] [CrossRef]
- Kabir, A.S.; Yuan, Z.; Kuboki, T.; Xu, C.C. De-Polymerization of Industrial Lignins to Improve the Thermo-Oxidative Stability of Polyolefins. Ind. Crops Prod. 2018, 120, 238–249. [Google Scholar] [CrossRef]
- López-de-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active Antioxidant Packaging Films: Development and Effect on Lipid Stability of Brined Sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- Takács, K.; Pregi, E.; Vági, E.; Renkecz, T.; Tátraaljai, D.; Pukánszky, B. Processing Stabilization of Polyethylene with Grape Peel Extract: Effect of Extraction Technology and Composition. Molecules 2023, 28, 1011. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen Peroxide Scavenging, Antioxidant and Anti-Radical Activity of Some Phenolic Acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Adhikari, B. Thermal Stability of Lignin–Hydroxy-Terminated Polybutadiene Copolyurethanes. Polym. Degrad. Stab. 2001, 73, 169–175. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and Stabilization of Polyurethane Elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Motokucho, S.; Nakayama, Y.; Morikawa, H.; Nakatani, H. Environment-Friendly Chemical Recycling of Aliphatic Polyurethanes by Hydrolysis in a CO2-Water System. J. Appl. Polym. Sci. 2018, 135, 45897. [Google Scholar] [CrossRef]
- Bachmann, M.; Marxen, A.; Schomäcker, R.; Bardow, A. High Performance, but Low Cost and Environmental Impact? Integrated Techno-Economic and Life Cycle Assessment of Polyoxazolidinone as a Novel High-Performance Polymer. Green Chem. 2022, 24, 9143–9156. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal Stability and Flame Retardancy of Polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Gray, R.L.; Lee, R.E. Scorch Inhibitors for Flexible Polyurethanes. In Plastics Additives: An A-Z Reference; Pritchard, G., Ed.; Polymer Science and Technology Series; Springer: Dordrecht, The Netherlands, 1998; pp. 567–575. [Google Scholar] [CrossRef]
- Griffiths, P.J.F.; Hughes, J.G.; Park, G.S. The Autoxidation of Poly(Propylene Oxide)s. Eur. Polym. J. 1993, 29, 437–442. [Google Scholar] [CrossRef]
- Servay, T.; Voelkel, R.; Schmiedberger, H.; Lehmann, S. Thermal Oxidation of the Methylene Diphenylene Unit in MDI-TPU. Polymer 2000, 41, 5247–5256. [Google Scholar] [CrossRef]
- Yildiz, B.; Seydibeyoğlu, M.Ö.; Güner, F.S. Polyurethane–Zinc Borate Composites with High Oxidative Stability and Flame Retardancy. Polym. Degrad. Stab. 2009, 94, 1072–1075. [Google Scholar] [CrossRef]
- Gama, N.; Evtyugin, D.D.; Lourenço, A.; Lopes, C.; Evtuguin, D.V. Ellagic Acid as Stabilizer in the Thermo-Oxidative Degradation of Thermoplastic Polyurethane. Polym. Degrad. Stab. 2023, 215, 110456. [Google Scholar] [CrossRef]
- Klemchuk, P.P.; Horng, P.-L. Transformation Products of Hindered Phenolic Antioxidants and Colour Development in Polyolefins. Polym. Degrad. Stab. 1991, 34, 333–346. [Google Scholar] [CrossRef]
- Masek, A. Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers 2015, 7, 1125–1144. [Google Scholar] [CrossRef]
- Ojeda, T.; Freitas, A.; Birck, K.; Dalmolin, E.; Jacques, R.; Bento, F.; Camargo, F. Degradability of Linear Polyolefins under Natural Weathering. Polym. Degrad. Stab. 2011, 96, 703–707. [Google Scholar] [CrossRef]





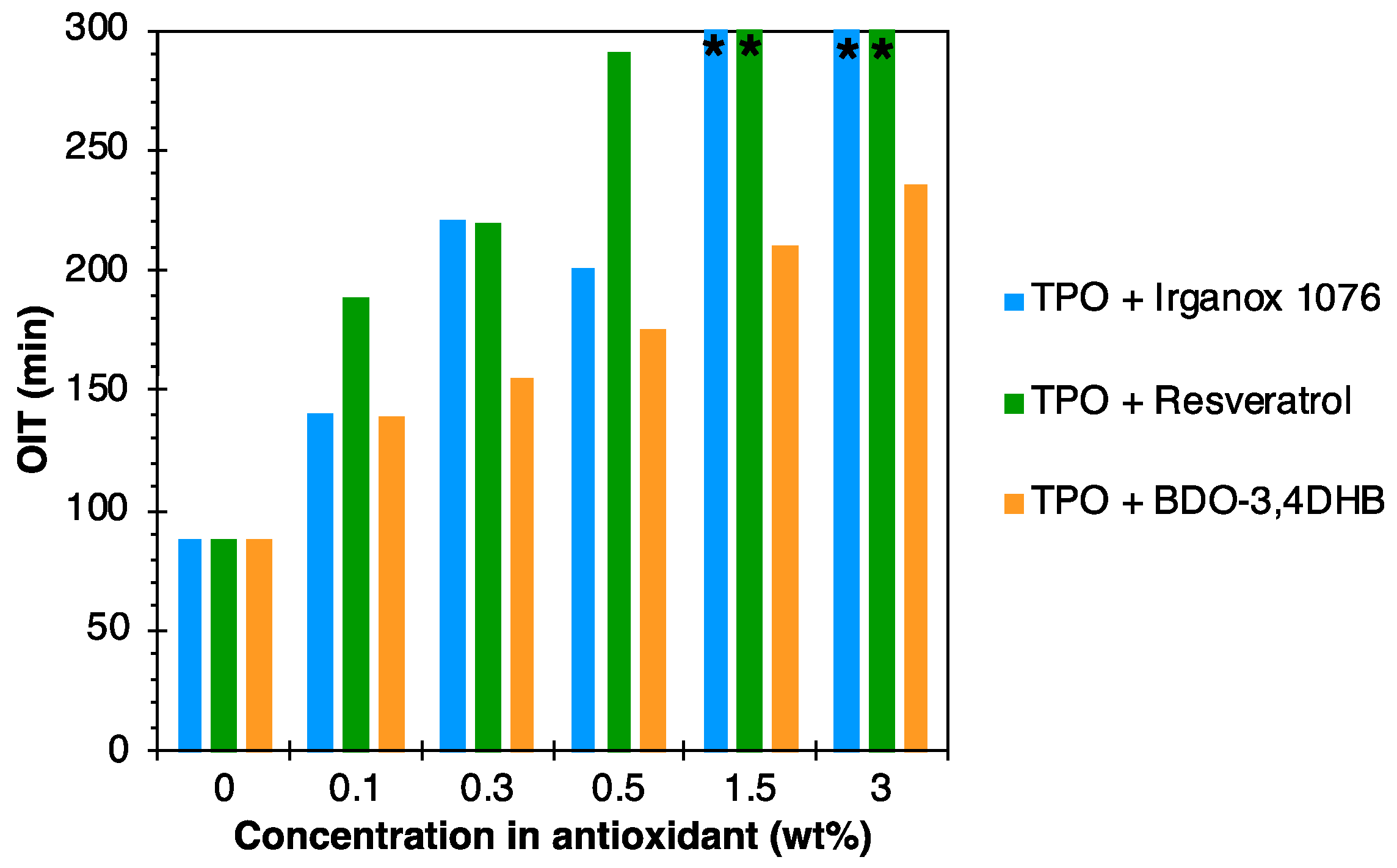




| Matrix/Antioxidant | Antioxidant Concentration in wt.% | ||||
|---|---|---|---|---|---|
| TPO + Irganox 1076 | 0.1 | 0.3 | 0.5 | 1.5 | 3 |
| TPO + Resveratrol | 0.1 | 0.3 | 0.5 | 1.5 | 3 |
| TPO + BDO-3,4DHB | 0.1 | 0.3 | 0.5 | 1.5 | 3 |
| TPU + Irganox 1076 | / | 0.3 | / | / | / |
| TPU + Resveratrol | / | 0.3 | / | / | / |
| Sample | Tg (°C) | Tm (°C) | T5% (°C) | Tdeg (°C) | σmax (MPa) | E (MPa) | εmax (%) |
|---|---|---|---|---|---|---|---|
| TPO | −30 | 143 | 291 | 330 | 17.0 ± 0.3 | 49.4 ± 2.9 | 1436 ± 46 |
| TPO + 3% Irganox 1076 | −32 | 149 | 349 | 434 | 21.7 ± 1.0 | 44.0 ± 3.3 | 1711 ± 41 |
| TPO + 3% Resveratrol | −32 | 148 | 338 | 432 | 20.3 ± 1.0 | 62.7 ± 4.8 | 1445 ± 30 |
| TPO + 3% BDO-3.4DHB | −31 | 151 | 320 | 435 | 25.6 ± 1.3 | 52.2 ± 2.4 | 1773 ± 40 |
| Formulations | T5% (°C) | Tdeg (°C) |
|---|---|---|
| TPU | 311 | 340 |
| TPU + 0.3% Irganox 1076 | 308 | 409 |
| TPU + 0.3% Resveratrol | 319 | 408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouren, A.; Pollet, E.; Avérous, L. Synthesis and Assessment of Novel Sustainable Antioxidants with Different Polymer Systems. Polymers 2024, 16, 413. https://doi.org/10.3390/polym16030413
Mouren A, Pollet E, Avérous L. Synthesis and Assessment of Novel Sustainable Antioxidants with Different Polymer Systems. Polymers. 2024; 16(3):413. https://doi.org/10.3390/polym16030413
Chicago/Turabian StyleMouren, Agathe, Eric Pollet, and Luc Avérous. 2024. "Synthesis and Assessment of Novel Sustainable Antioxidants with Different Polymer Systems" Polymers 16, no. 3: 413. https://doi.org/10.3390/polym16030413
APA StyleMouren, A., Pollet, E., & Avérous, L. (2024). Synthesis and Assessment of Novel Sustainable Antioxidants with Different Polymer Systems. Polymers, 16(3), 413. https://doi.org/10.3390/polym16030413








