Abstract
Global concerns about environmental pollution, poor waste management, and the rise in antimicrobial resistance due to uncontrolled antibiotic use have driven researchers to seek alternative, multifaceted solutions. Plants, animals, microorganisms, and their processing wastes serve as valuable sources of natural biopolymers and bioactive compounds. Through nanotechnology, these can be assembled into formulations with enhanced antimicrobial properties, high safety, and low toxicity. This review explores polysaccharides, including chitosan, alginate, starch, pectin, cellulose, hemicellulose, gums, carrageenan, dextran, pullulan, and hyaluronic acid, used in nanotechnology, highlighting their advantages and limitations as nanocarriers. Addressing the global urgency for alternative antimicrobials, we examined natural compounds derived from plants, microorganisms, and animals, such as phytochemicals, bacteriocins, animal antimicrobial peptides, and proteins. Focusing on their protection and retained activity, this review discusses polysaccharide-based nanoformulations with natural antimicrobials, including nanoparticles, nanoemulsions, nanocapsules, nanoplexes, and nanogels. Special emphasis is placed on strategies and formulations for the encapsulation, entrapment, and conjugation of natural compounds using polysaccharides as protective carriers and delivery systems, including a brief discussion on their future applications, prospects, and challenges in scaling up.
Keywords:
chitosan; alginate; cellulose; gum; dextran; complexation; conjugation; essential oil; bacteriocins; AMP 1. Introduction
Antimicrobial resistance (AMR) has emerged as a critical challenge to modern healthcare systems worldwide. The uncontrolled use of antibiotics has been identified as a principal causative factor in the emergence of this critical public health challenge. Innovative antibacterial agents with unique modes of action are urgently needed to overcome current resistance challenges. Nanomaterials, natural antimicrobial compounds, and particularly their synergistic combinations offer promising solutions to address antimicrobial resistance [1,2,3,4].
Nanomaterials can be categorized into three primary classes based on composition: organic, carbon-based, and inorganic [5]. The key advantages of nanoparticles as carriers include their ultra-small and tunable size, protective encapsulation of payloads, controllable release kinetics, reduced systemic side effects, and capacity for combinatorial loading of therapeutic agents [6]. The utilization of nanocarriers as delivery systems enhances bioactive molecules’ therapeutic potential by improving their absorption, bioavailability, and stability while reducing toxicity and optimizing pharmacokinetic profiles [4].
Organic nanoparticles (NPs) fabricated from biopolymers exhibit favorable biocompatibility, biodegradability, and flexible loading capacity for various bioactive molecules [7,8]. Biopolymers are classified by origin as (1) synthetic polymers from bio-derived monomers (e.g., polylactic acid [PLA] and other polyesters), (2) natural animal- and plant-derived polymers (e.g., chitin, cellulose, collagen, and zein), and (3) microbial polymers (e.g., polyhydroxyalkanoates [PHA], bacterial cellulose, xanthan, and pullulan) [9]. Biopolymers directly extracted from biomass represent the most abundant category of natural polymers. This group encompasses polysaccharides (starch, cellulose derivatives, gums, alginates, pectins, and chitin/chitosan), animal proteins (casein, collagen, gelatin, and whey), and plant proteins (soy, pumpkin, wheat, and zein) [9,10,11,12,13], demonstrating significant potential for developing organic-based delivery systems [14]. Synthetic polymers, including those fabricated from bio-derived monomers, demonstrate several challenges in their application. The main limitations include potential toxicity, a complicated and expensive synthesis process, hydrophobicity, as well as poor biocompatibility and biodegradability of some synthetic polymers [15]. However, a group of synthetic polymers such as PLA, poly(lactide-co-glycolide) (PLGA), poly(caprolactone) (PCL), etc., are biocompatible and biodegradable [15,16]. PLA-based carriers have low drug loading capacity and encapsulation efficiency [17], while PLGA may develop biocompatibility problems [18].
The combined factors of potential toxicity and immunogenicity aligned with the limited biocompatibility and accumulation risks of synthetic polymers combined with commercial interests have shifted focus toward biomass-derived biopolymers, which offer natural abundance and sustainable sourcing. Polysaccharides offer low immunogenicity and toxicity and excellent biocompatibility and biodegradability [19,20,21], and they are extensively utilized in biopolymeric nanoparticle fabrication [22,23], exhibiting not only superior mechanical and physicochemical properties but also intrinsic biological activity [24] that is often enhanced in nanoform. Bioactive compounds could be simply trapped, encapsulated, or conjugated with polysaccharides (Figure 1) [25]. Their nanoformulations as polysaccharide-based NPs can extend drug half-life, reduce cytotoxicity, and control release [26,27]. Polysaccharide-based nanoformulations serve as versatile delivery systems, material-reinforcing agents, and Pickering emulsion stabilizers for diverse applications including drug and gene delivery; antibacterial platforms; tissue engineering; cancer therapy; cosmetics; food fortification, preservation, and packaging; etc. [22,28,29,30,31].
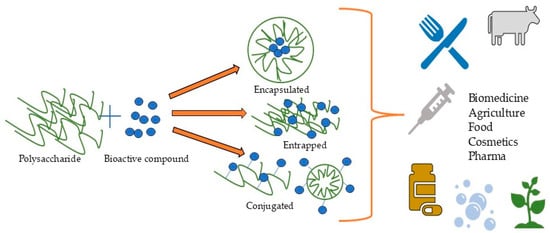
Figure 1.
Formation of polysaccharide-based nanoformulations and their applications.
Natural antimicrobials represent a diverse group of compounds derived from various sources, including plants, animals, and microorganisms [14]. These encompass phytochemicals (in either isolated forms or complex mixtures such as essential oils and extracts), peptides, proteins, polymers, etc. [32], and exhibit low toxicity, targeted antimicrobial activity (including against antibiotic-resistant strains), and high bioavailability [4,33,34]. Natural antimicrobial compounds face significant application challenges due to rapid degradation and volatility—issues that can be effectively addressed through encapsulation strategies [14].
This review systematically consolidates current knowledge on prevalent polysaccharides employed in nanocarrier synthesis, fabrication methodologies, and established nanomaterial technologies incorporating plant-, microbial-, and animal-derived antimicrobial compounds.
2. Natural Polysaccharides for Nanocarrier Fabrication
Polysaccharides are widely utilized in nanocarrier fabrication, either individually or in complex/conjugated forms [22,35,36,37,38,39,40]. Among natural polysaccharides, chitosan, alginate, and cellulose have been most extensively studied and implemented in nanoformulations [8,41,42]. Polysaccharide-based nanocarriers synergistically combine the advantageous properties of biopolymers, including low immunogenicity and toxicity, biodegradability, biocompatibility, and bioavailability, with the benefits of nanoformulations, such as enhanced drug bioavailability, prolonged drug half-life, reduced systemic toxicity, and controlled release kinetics [19,20,21,26,27,43]. Figure 2 illustrates the main common benefits of polysaccharides.
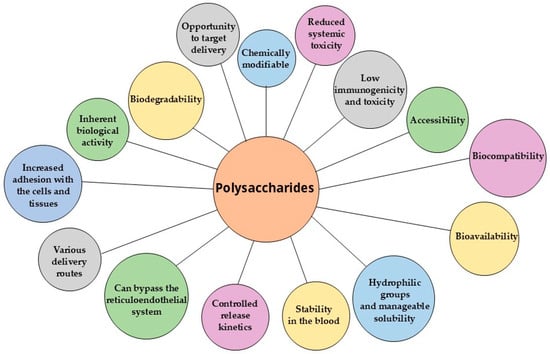
Figure 2.
Benefits of polysaccharides as nanocarriers.
However, the toxicity and side effects of polysaccharide-based nanoformulations remain insufficiently characterized, necessitating further systematic studies to evaluate their potential adverse effects. Moreover, their properties could be influenced by a heterogeneous chemical structure, molecular weight, and modifications [44]. Table 1 presents the chemical structures of polysaccharides, and Table 2 systematically summarizes the non-common advantages and limitations of certain polysaccharides as nanocarriers.

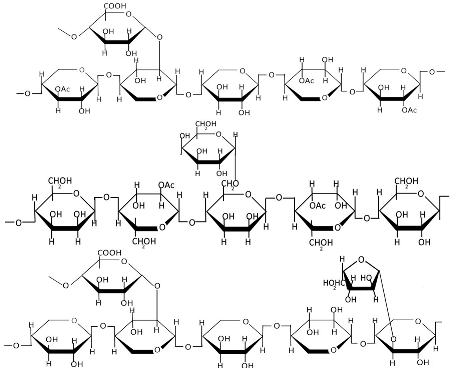
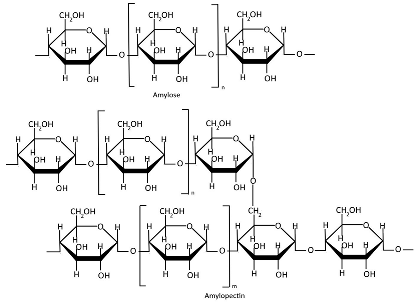
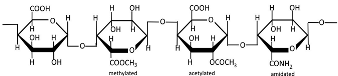
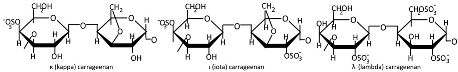
Table 1.
Chemical structures of polysaccharides.
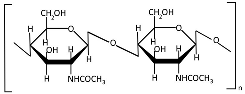
Chitin (Table 1), a linear polysaccharide of poly(β-(1→4)-N-acetyl-D-glucosamine, is abundantly distributed in nature as crystalline microfibrils that constitute the structural framework of arthropod exoskeletons and fungal cell walls [45]. Industrially, chitin is sourced primarily from exoskeletons obtained as byproducts of global seafood processing [46,47]. Its extraction involves sequential treatments: (1) acid hydrolysis for demineralization (removing calcium carbonate), (2) alkaline solution for deproteinization, and (3) oxidative decolorization to yield a purified, colorless polymer [48,49].
Chitosan (Table 1), the principal chitin derivative, is produced via alkaline deacetylation [50]. Its physicochemical and biological properties are critically dependent on the molecular weight (50–2000 kDa), degree of deacetylation (54–100%), and functional group modifications (including nitration, sulfonation, graft copolymerization, and cross-linking) [51]. The deacetylation process constitutes a two-stage nucleophilic substitution reaction, typically achieved through thermal treatment in alkali at elevated temperatures [52,53]. Chitosan processing employs chemical, physical, and enzymatic techniques to achieve lower molecular weights, with physical methods being increasingly favored due to their ability to mitigate key limitations of alternative approaches—including random chain cleavage, high costs, and environmental concerns [54]. The most prevalent chitosan NP morphologies include nanospheres, nanocapsules, and nanofibers [55]. In recent decades, chitosan and its nanoforms have garnered significant research interest owing to their exceptional properties, particularly their biological activities: mucoadhesion, anti-inflammatory effects, antioxidant capacity, antimicrobial/antifungal action, antihyperglycemic activity, antitumor potential, and wound-healing promotion [55,56,57,58]. However, these materials may also exhibit cytotoxicity [51], which correlates with both acetylation degree and molecular mass [59].
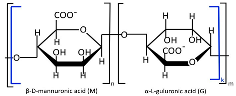
Alginate, a highly abundant natural polysaccharide, exhibits unique cation-binding properties that enable the formation of stable and tunable hydrogels [60]. Alginate is primarily extracted from brown seaweed species and can also be produced through bacterial biosynthesis [61]. Alginate (Table 1) is composed of linear copolymer chains containing β-D-mannuronate (M) and α-L-guluronate (G) residues interconnected via 1→4 glycosidic linkages [62,63]. These monomers assemble into three distinct block configurations, poly-M homopolymers, poly-G homopolymers, and alternating MG heteropolymers, whose relative proportions dictate the polymer’s structural diversity, molecular weight distribution, and ultimately its functional physicochemical properties [64]. The molecular weight (32–400 kDa) and M/G composition of alginates are source-dependent, showing distinct variations across harvest locations and seasons [64,65]. Commercial alginate production employs both acidic and non-acidic extraction methods to isolate polysaccharides from brown seaweed biomass [65]. The classical acidic extraction method sequentially involves mineral acid treatment, alkaline conversion to sodium alginate, precipitation (CaCl2 or ethanol), and purification (acidification/Ca2⁺/ethanol) [64]. Novel extraction techniques are now being actively researched and implemented, including microwave-assisted extraction, ultrasound treatment, high-pressure processing, pressurized fluid extraction, and enzymatic hydrolysis methods [66]. Alginate-based nanocarriers (e.g., nanoparticles, nanofibers, nanoemulsions, nanocomplexes, and nanohydrogels) are extensively fabricated to encapsulate diverse bioactive payloads such as therapeutic drugs, proteins, and even whole cells [67,68]. Alginate nanomaterials exhibit advantageous physicochemical and biological properties, biocompatibility, pH sensitivity, mucoadhesiveness, and controlled biodegradability along with excellent safety profiles, though their performance is critically dependent on structural parameters (molecular weight, M/G ratio, and chemical modifications) [69,70].
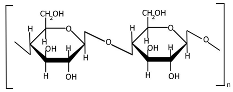
Cellulose (Table 1) is a linear homopolysaccharide composed of D-glucoses connected by β(1→4) glycosidic bonds [71,72]. Cellulose is the most abundant renewable biopolymer on Earth, biosynthesized across multiple biological kingdoms including bacteria, plants, and even some animals [72]. The molecular weight of cellulose and its derivatives varies significantly with the degree of polymerization [73], which directly affects polymer solubility. Native cellulose is insoluble in conventional solvents due to its robust network of intermolecular and intramolecular hydrogen bonds, coupled with hydrophobic interactions [74]. Cellulose extraction can be performed across multiple scales (nano, micro, and macro) using chemical, mechanical, chemo-mechanical, or green techniques [75,76,77]. Both the mechanical and physicochemical properties of cellulose can be tailored through chemical modifications [78], though these processes typically require reagent application and subsequent purification steps to remove residual chemicals. Nanocellulose is broadly categorized into three principal types: cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), and bacterial nanocellulose (BNC) [79]. The mechanical properties of cellulose-based materials critically determine their suitability for diverse applications [80]. Despite possessing unique advantages (high surface area, tunable surface chemistry, and exceptional mechanical strength), recent studies indicate that nanocellulose may exhibit complex toxicity profiles, necessitating further investigation into its biological safety [42].
Alongside cellulose, lignin and hemicellulose constitute fundamental components of plant cell walls, exhibiting remarkable properties. However, their inherent structural heterogeneity profoundly impacts all downstream processes, from extraction and purification to the final characteristics of derived nanomaterials [81,82]. Hemicellulose (Table 1) is a branched heteropolysaccharide composed of a sugar backbone with substituted side chains [83,84]. Hemicellulose comprises pentoses, hexoses, and uronic acids, including arabinose, xylose, glucose, mannose, galactose, glucuronic acid, and galacturonic acid, with minor constituents like fucose and rhamnose [85,86,87]. These form relatively short, branched chains of 500–3000 sugar units. In contrast, cellulose consists exclusively of linear β(1→4)-linked glucose polymers with higher degrees of polymerization (7000–15,000 units) [88,89]. The composition of this biopolymer varies significantly by species, with mannose-rich hemicellulose dominating softwoods and xylose-rich forms prevalent in hardwoods [90]. Hemicellulose is extracted from various plant sources and, while water-insoluble, can be isolated through alkaline treatment or mild acid hydrolysis [83,91]. Thus, conventional methods for hemicellulose extraction involve chemical treatments using alkali and organic solvents, while newer approaches employing H2O2, steam explosion, microwave, and ultrasonic techniques are also being investigated [92]. The mechanical, physical, chemical, and biological properties of hemicellulose vary significantly due to its structural heterogeneity and can be precisely manipulated through targeted modifications [93,94]. Current research indicates that nano-hemicellulose exhibits low toxicity and immunogenicity, though these properties remain insufficiently characterized in the literature [95].
Starch (Table 1), a plant-derived polysaccharide composed of amylose and amylopectin, is extracted from various agricultural crops [96]. Amylose is a linear α-glucan connected by α(1→4) glycosidic linkages, typically comprising 1000–20,000 glucose units with an average molecular weight of ~100 kDa [39,97,98]. Amylopectin is a highly branched macromolecule with a molecular weight of 1000–10,000 kDa, significantly larger than amylose. Its structure consists of α(1→4)-linked glucose chains with 5% α(1→6) branch points, creating a dendritic architecture [39,97,98]. Amylose typically comprises 5–35% of native starch, whereas genetically modified starches can achieve elevated amylose contents of 50–80% [99,100]. The amylose-to-amylopectin ratio significantly influences the physicochemical and functional properties of starch, with variations dependent on botanical origin [100,101,102]. Starch is stored in various plant organs, including seeds, fruits, roots, tubers, stems, and leaves [103,104]. Starch is primarily extracted through traditional methods involving washing, grinding, filtration, and sedimentation, typically via wet or dry processing pathways. Mechanical extraction methods additionally employ crushing, pressing, and centrifugation for higher yields [105]. Starch extraction is also accomplished through chemical methods (alkaline or surfactant-based treatments) and enzymatic approaches [106,107,108], with emerging green techniques gaining increasing application [109,110]. Native starch typically exhibits limited mechanical strength and poor water barrier properties [111,112]. Starch modification is crucial for enhancing functional properties and overcoming the inherent limitations of native starches [113]. Starch modification techniques include physical, enzymatic, and genetic methods [114]. Nanostarches are primarily categorized into two types: starch nanocrystals (SNCs) and starch nanoparticles (SNPs), which differ in their crystallinity and preparation methods [115,116]. Although starch nanoforms demonstrate enhanced mechanical strength, barrier performance, and thermal stability, these properties are strongly influenced by amylose/amylopectin ratios and often require modification to achieve optimal functionality [115,116,117,118,119].

Table 2.
Benefits and drawbacks of polysaccharides as nanocarriers.
Table 2.
Benefits and drawbacks of polysaccharides as nanocarriers.
| Biopolymer | Advantages | Disadvantages | Ref. and Pub. Year |
|---|---|---|---|
| Chitosan |
|
| [51,55,58,59,120,121] 2023, 2023, 2023, 2024, 2022, 2025 |
| Alginate |
|
| [60,62,67,69,122,123] 2021, 2012, 2022, 2024, 2022, 2022 |
| Cellulose |
|
| [42,78,79,80,124,125] 2020, 2021, 2015, 2023, 2022, 2022 |
| Hemicellulose and xylan |
|
| [86,92,93,95,126,127] 2024, 2020, 2021, 2023, 2021, 2024 |
| Starch |
|
| [115,116,117,118,119] 2020, 2023, 2022, 2015, 2024 |
| Pectin |
|
| [128,129,130,131,132,133] 2023, 2015, 2008, 2021, 2023, 2022 |
| Gums |
|
| [134,135,136,137] 2020, 2023, 2019, 2025 |
| Carrageenan |
|
| [138,139,140,141,142,143,144,145,146] 2021, 2022, 2021, 2017, 2017 2001, 2023, 2013, 2017 |
| Dextran |
|
| [43,147,148,149,150,151] 2023, 2022, 2016, 2022, 2023, 2020 |
| Pullulan |
|
| [152,153,154,155,156,157,158,159,160] 2022, 2021, 2021, 2016, 2016, 2013, 2023, 2023, 2025 |
| Hyaluronic acid |
|
| [161,162,163,164,165,166] 2020, 2022, 2024, 2024, 2021, 2022 |
Notes: MW—molecular weight; DA—deacetylation degree; DE/DA—degree of esterification and amidation (DA).
Pectins are hydrophilic polymers naturally present in plant cell walls [167]. Their functional properties are determined by the degree of esterification (DE) and degree of amidation (DA) [130]. Pectins consist of a covalently linked, galacturonic acid-rich polysaccharide backbone (up to 70%, α-1,4-linked) and are classified into the following main types: homogalacturonan (HG, Table 1) as the major component, and rhamnogalacturonan I (RGI), rhamnogalacturonan II (RGII), xylogalacturonan (XGA), and apiogalacturonan (AGA) as the minor components [168,169,170]. The backbone of RGI contains L-rhamnose, while the branched structures of pectins (RGI, RGII, and XG) include L-rhamnose, L-arabinose, D-apiose, D-glucuronic acid, D- and L-galactose, D-xylose, L-fucose, and L-aceric acid [171]. Pectin is commercially extracted from sugar beet pulp, citrus peel, and apple pomace through conditional extraction with mild acids, as well as enzyme-, microwave-, and ultrasound-assisted extraction, subcritical water extraction, pressurized CO2 and deionized water methods, deep eutectic solvents, and ohmic heating [171,172]. Pectin exhibits poor mechanical properties and moisture resistance [133]. Pectin-based nanostructures, including nanoparticles, nanoemulsions, nanocapsules, and nanogels, have attracted significant interest due to their outstanding properties, such as physical sensitivity (to light, temperature, and electricity), chemical sensitivity (to pH, redox reactions, and glucose), and biological sensitivity (to enzymes) [173,174].
Plant gums are polysaccharides composed of covalently bound sugar monomers [134]. The most extensively studied varieties include arabic gum, carrageenan, xanthan gum, and tragacanth [136]. Their structural backbones typically contain galactose, arabinose, rhamnose, uronic acids, galacturonic acid, proteins, calcium, and magnesium [175]. These biopolymers exhibit unique structural and biological properties—including water retention capacity, thermal stability, and hydrophilicity, along with antibacterial, antioxidant, and immunomodulatory activities—making them promising candidates for drug delivery applications [137]. While less studied than other biopolymers, gum-based nanostructures such as nanoemulsions, nanoparticles, nanocomplexes, and nanofibers have shown particular promise for colorectal therapy due to their selective digestibility properties [134,135,136]. Carrageenan (Table 1) is extracted from specific red seaweed species through alkaline processing [176]. This sulfated polysaccharide consists of alternating galactose and anhydrogalactose units linked by glycosidic bonds [177], with an average molecular mass exceeding 100 kDa. The major types of carrageenans are ι (iota), κ (kappa), and λ (lambda), which differ in their sulfate group content [178,179]. Carrageenan exhibits significant antioxidant, immunomodulatory, and disease-preventive properties, making it a promising candidate for pharmaceutical development [144]. Its chain conformation and gelation behavior are strongly influenced by temperature and the electrolyte concentration [139], but when carrageenan is used as an individual matrix material, zero-order kinetics and pH-independent release profiles cannot be achieved [146]. Toxicological studies indicate excellent biocompatibility with no observed teratogenic effects [145].
Dextran (Table 1) is a neutral bacterial exopolysaccharide produced by lactic acid bacteria or their enzymes in sucrose-rich environments, featuring α-(1→6)-linked glucose backbones with potential α-(1→2), α-(1→3), or α-(1→4) branches, and exhibiting molecular weights ranging from <40 kDa (low) to >40 kDa (high) [148,180,181]. The solubility and rheological properties of dextran depend on its molecular weight (up to 440 MDa) and branching structure [180]. Dextran’s alcohol insolubility enables ethanol/methanol precipitation (the conventional isolation method), with contemporary approaches employing membrane filtration or deep eutectic solvents [182,183]. The polymer’s molecular weight determines the functionality of dextran-coated NPs and bioconjugates [184,185]. While comprehensive toxicity profiles remain scarce [149], dextran’s optimal biocompatibility and tunable properties [150] have established it as a premier nanocarrier material [186], adaptable to nanoparticle, nanogel, microsphere, and micelle formulations [151].
Pullulan (Table 1) is a natural, neutral exopolysaccharide primarily composed of maltotriose units, produced through aerobic fermentation by black yeasts [152,187]. Maltotriose units consist of three glucose molecules linked by α-1,4 glycosidic bonds, which are further connected to each other via α-1,6 glycosidic bonds [188,189]. The molecular weight of pullulan ranges from 45 to 600 kDa depending on the culture conditions and the strain of A. pullulans [190], and it significantly influences its rheological and mechanical properties [191]. The production of pullulan involves microbe harvesting, the elimination of unwanted substances (mainly melanin), precipitation, membrane purification, and freeze drying [164]. Pullulan is virtually insoluble in organic solvents; thus, ethanol and isopropanol are the most commonly used solvents for its purification. However, chromatographic techniques or aqueous two-phase systems may also be employed [192]. Pullulan exhibits high chemical versatility, enabling facile modification through carboxymethylation, oxidation, amination, sulfation, acetylation, and esterification [153,158]. Hydrophobic modifications, in particular, facilitate the spontaneous self-assembly of nanocarriers, which exhibit high drug-loading capacity and a low critical micellar concentration, making them promising for targeted delivery systems [153,158]. Pullulan-based nanostructures can be engineered into diverse forms, including nanoparticles, nanogels, and nanoplexes, leveraging the enhanced permeability and retention effect [154]. Despite its advantages, pullulan’s poor mechanical strength, extreme hydrophilicity, and lack of inherent antibacterial properties restrict its applications [193].
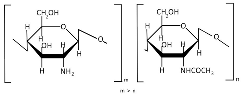
Hyaluronic acid (HA, Table 1) is an anionic polysaccharide consisting of D-glucuronic acid and N-acetyl-D-glucosamine linked by β-1,3- or β-1,4-glycosidic bonds [37]. It is produced by membrane-bound HA synthases and can be obtained through extraction from animal tissues, microbial production, or cell-free enzymatic synthesis [166], with membrane technologies being the preferred purification technique [194]. The molecular weight of HA, which can exceed 500 kDa, significantly influences its structural, biological, physical, physicochemical, and degradation characteristics [163,195]. HA functions as an endogenous ligand for cell surface receptors, with high affinity for CD44. This receptor-specific binding enables HA-based systems to selectively target and deliver therapeutic agents to pathological tissues [165]. Despite its outstanding properties, HA has several substantial limitations. These include rapid biodegradation, poor stability and mechanical strength, extreme hydrophilicity, limited ability to encapsulate and deliver hydrophobic drugs, molecular weight-dependent tissue penetration, and potential immunogenicity, particularly for chemically modified forms. Many of these limitations can be addressed through strategic chemical modifications [161,164].
Polysaccharides are renewable, widely accessible, biodegradable, and biocompatible materials that confer low toxicity and allergenicity to polysaccharide-based nanomaterials. Due to their unique physical and chemical properties, polysaccharide-based nanocarriers exhibit all advantageous characteristics of organic nanocarriers, including an extended drug half-life, enhanced solubility, and controlled release profiles. Furthermore, these nanocarriers can be readily modified to achieve tailored properties while inherently possessing various beneficial biological activities. The main limitation in applying polysaccharides for nanoformulation is their variable chemical structure, which affects physicochemical properties and water absorption capacity, along with poorly characterized cytotoxic effects that may arise from structural variations, modifications, impurities, or NP characteristics (size, shape, and biological barrier penetration ability) [37].
3. Methods of Synthesis for Polysaccharide-Based Nanocarriers
Polysaccharide nanocarriers can be synthesized through various methods including self-assembly, ionic gelation, cross-linking, complex coacervation, emulsification, ultrasonication, desolvation, supercritical fluid technology, and nanoprecipitation or solvent displacement, with the choice of method depending on the desired nanoparticle characteristics and polysaccharide properties [36,196].
Self-assembly (Figure 3a) is one of the most promising techniques for nanoparticle synthesis due to its environmental friendliness, biocompatibility, process simplicity, and low toxicity [40]. This method involves the spontaneous organization of amphiphilic polysaccharides into ordered nanostructures with a hydrophobic core (for drug encapsulation) and a hydrophilic shell (for aqueous stability). The driving forces include non-covalent interactions such as hydrophobic effects, electrostatic forces, van der Waals interactions, and π–π stacking (in aromatic-modified systems) [36,40,197]. Chemical modification (e.g., grafting hydrophobic chains) can convert naturally hydrophilic polysaccharides into amphiphilic polymers, enabling controlled self-assembly [36]. Conversely, chemical modification involves the application of harmful reagents and could be inefficient and wasteful [198], therefore requiring subsequent purification steps, and it limits the potential of self-assembly for the industrial scale.
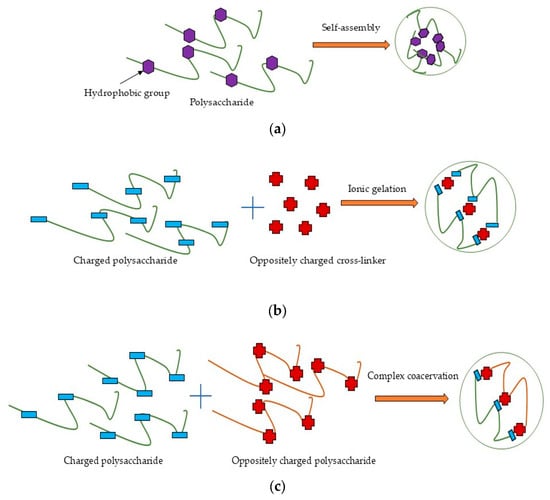
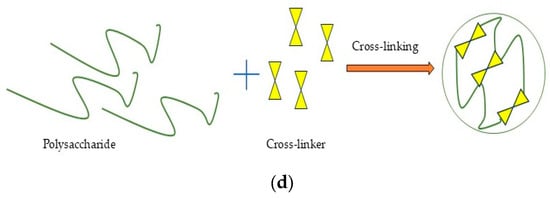
Figure 3.
Schematic illustration of preparation process for polysaccharide nanoformulations: (a) self-assembly; (b) ionic gelation; (c) complex coacervation; (d) cross-linking.
Ionic gelation (Figure 3b) is a widely used method to form nanostructures from charged polysaccharides through electrostatic interactions with oppositely charged cross-linkers in dilute aqueous solutions [22]. Chitosan and other cationic polysaccharides can be cross-linked with polyanions like tripolyphosphate (TPP), whereas anionic polysaccharides such as alginate are typically cross-linked with cations like Ca2⁺ from CaCl2 [199,200,201]. Ionic gelation is a widely used nanoparticle synthesis method characterized by its simplicity and convenience. Furthermore, this controllable process avoids organic solvents and exhibits no toxicity [36] and can thus be used for industrial scale-up by optimizing the polymer and cross-linker concentration and mixing speed or by using droplet-producing devices [202,203].
Complex coacervation (Figure 3c) occurs through electrostatic interactions between oppositely charged species, typically involving two oppositely charged polysaccharides [36,204]. Electrostatic complexation induces phase separation, forming a biopolymer-rich coacervate phase and a solvent-rich phase [205]. Ionic strength, pH, temperature, charge density, and polyelectrolyte molar mass critically influence coacervate particle stability and formation, while these systems also exhibit excellent biocompatibility and low toxicity [22]. The simplicity and safety of this method made it attractive for scaling up, but it requires careful consideration of numerous factors like mixing systems, polymer ratios and concentrations, temperature, pH, and balance in ionic strength [206].
Covalent cross-linking (Figure 3d) in the nanoformulations created irreversible cross-linking points, yielding highly stable nanostructures [22]. Glutaraldehyde, compounds with PO43− groups, enzymes, carbodiimide, genipin, epoxy, acrylamide, citric acid, formaldehyde, etc., are the most common synthetic and natural cross-linkers applied for polysaccharides [207]. The application of toxic reagents in covalent cross-linking has limited its industrial scalability. To address this challenge, green alternatives including citric acid, tannic acid, vanillin, gallic acid, ferulic acid, proanthocyanidins, phytic acid, squaric acid, and epigallocatechin have been investigated for developing polysaccharide-based nanoformulations [208].
Emulsification (Figure 4) can be performed in either oil-in-water or water-in-oil systems through mechanical stirring or ultrasonication [36]. More complex systems, such as double emulsions (oil-in-water-in-oil or water-in-oil-in-water), may also be employed for specialized applications [22]. Ultrasound treatment can depolymerize biopolymers, modify their physical properties (e.g., viscosity and molecular weight), and promote structural reorganization of polysaccharide molecules [209,210]. Nanoparticles can be obtained from emulsions through various methods including internal/external gelation, solvent evaporation/diffusion, and reverse salting out [22]. Despite enabling the production of controllable, spherical nanoparticles, emulsification remains a more complex technique that requires significantly greater energy input and larger quantities of organic solvents compared to alternative methods [25,36,56]. These requirements make scale-up more complicated and energy-intensive, and consequently more expensive. Additionally, the technique requires the optimization of numerous parameters, including ones for internal/external gelation, solvent evaporation/diffusion, and reverse salting out [25].
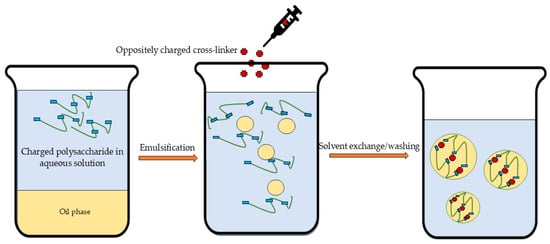
Figure 4.
Schematic illustration of emulsification followed by ionic gelation.
The desolvation technique (Figure 5) involves the coacervation or precipitation of dissolved polysaccharides through the gradual addition of desolvating agents (e.g., salts and alcohols). This process is typically followed by cross-linking to stabilize the formed nanoparticles [38,211]. The selection of desolvating agents depends on the encapsulated substance’s properties [22] but may require a subsequent purification step due to the potential toxicity of residual molecules [36], which could limit its scaling-up ability. The desolvation method is typically used for protein nanoparticle preparation, but there is a lack of systematic studies on this approach for polysaccharides, particularly regarding physical stability [212]. However, the desolvation technique followed by cross-linking could be successfully applied for the fabrication of polysaccharide NPs [213].
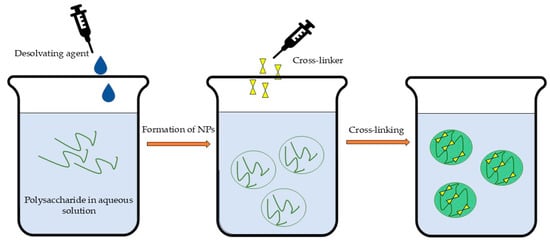
Figure 5.
Schematic illustration of desolvation followed by cross-linking.
Nanoprecipitation or solvent displacement (Figure 6) is an easy, less complex, less energy-consuming, widely applicable method that involve the following steps: (1) the dissolution of the polymer in a fully or partly water-miscible solvent, (2) the transfer of the solution to another non-solvent which may contain a surfactant, and (3) nanoprecipitation due to the rapid diffusion of solvent provided that aggregation is limited [214,215,216]. The excess solvent is typically eliminated through evaporation, dialysis, or lyophilization [22]. However, this method’s primary limitations involve both the requirement for organic solvents and their substantial dilution to prevent particle aggregation during precipitation [36]. Furthermore, studies indicate that nanoprecipitation demonstrates optimal efficacy for hydrophobic drugs [217]. These limitations, coupled with the demanding optimization requirements, present significant challenges in scaling up nanoprecipitation and solvent displacement methods for industrial applications.
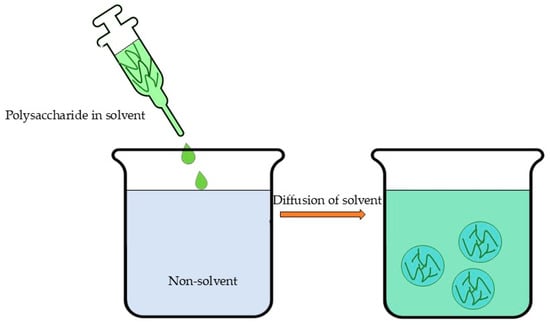
Figure 6.
Schematic illustration of nanoprecipitation or solvent displacement.
Equipment-based methods such as electrospinning enable the fabrication of polysaccharide nanomaterials with enhanced specific surface area and porosity [218]. However, challenges in electrospinning polysaccharides include poor solubility (e.g., cellulose), high viscosity, and elevated surface tension [219]. These limitations can be addressed through optimized solvent selection, material modification, and tailored structural design [220].
Target substances can be incorporated into nanostructures either through covalent conjugation via surface functional groups or via physical encapsulation during fabrication, mediated by electrostatic interactions, hydrogen bonding, or other entrapment mechanisms [196,221,222].
Safety concerns associated with toxic or potentially toxic reagents and challenges in process parameter optimization represent major barriers to industrial-scale implementation. The primary obstacles in scaling up nanoformulation production include (1) developing safe and effective nanoformulations through green nanotechnology approaches, (2) ensuring batch-to-batch reproducibility and controlled manufacturing processes, (3) achieving target-specific delivery while maintaining functionality, (4) guaranteeing long-term stability and structural properties, and (5) understanding nanoparticle exposure effects, including systemic toxicity, and characterizing in vivo behavior [25].
4. Polysaccharide-Based Nanocarriers for Plant-Derived Antimicrobials
Plant antimicrobials comprise a broad group of bioactive compounds demonstrating antioxidant, antibacterial, and antifungal activities [223]. Essential oils (EOs) and plant extracts (PEs) represent complex mixtures containing diverse phytochemicals, including monoterpenes, phenylpropanoids, monoterpenoids, phenolic compounds, and other major substance classes that serve as effective antimicrobial agents [4,35,224,225]. Notable examples of these plant-derived antimicrobials include curcumin, quercetin, saponin, resveratrol, gallic acid, magnoflorin, sulforaphane, naringenin, honokiol, glycyrrhetinic acid, rutin, luteolin, carotene, α-tocopherol, zoledronic acid, kaempferol, carvacrol, thymol, linalool, and menthol, among others—all exhibiting superior antimicrobial and antifungal properties [4,14,221,222].
Chitosan is extensively utilized in nanoparticle (NP) formulation (Table 3) due to its intrinsic antimicrobial properties [121,226,227]. The formulation of nanoparticles (NPs) with plant essential oils (EOs) typically requires emulsification [228], followed by biopolymer-specific techniques such as ionic gelation or coacervation for chitosan and alginate [229,230,231,232]. Chitosan-based NPs incorporating tea water extract effectively inhibited P. grisea and X. oryzae [233], while those with grape pomace extract demonstrated significant antimicrobial efficacy: a 6-log reduction in C. albicans, 5-log reduction in methicillin-susceptible S. aureus (MSSA), 3-log reduction in L. monocytogenes and P. aeruginosa, and 1-log reduction in E. coli and S. enteritidis [234]. Similarly, chitosan NPs containing Lavandula angustifolia water extract suppressed biofilm formation in P. aeruginosa, S. aureus, and C. albicans [235], and those with Martynia annua leaf ethanol extract showed strong antibacterial activity (in descending order): B. fragilis > S. oralis > P. acnes > P. aeruginosa > S. aureus > E. coli > B. cereus > S. mutans > A. hydrophila > S. faecalis [236]. Clove and guava leaf essential oils (EOs) were successfully incorporated into chitosan NPs through oil-in-water emulsification followed by ionic gelation. The resulting NPs exhibited significant antimicrobial activity against L. monocytogenes, S. aureus, S. typhi, E. coli [229], and K. pneumoniae [230]. Resveratrol, a potent polyphenolic antioxidant, demonstrates broad-spectrum antimicrobial properties against diverse bacteria, viruses, and fungi [237]. When encapsulated in chitosan NPs, resveratrol showed enhanced activity against H. pylori [238].
Chitosan is synergistically combined with alginate, pectin, or gums through electrostatic interactions to form complex nanostructures with enhanced stability and broad-spectrum antimicrobial activity [239,240,241,242]. These hybrid systems employ techniques like pre-ionic gelation (for chitosan/alginate or chitosan/pectin NPs) and complex coacervation [240,243,244], as demonstrated by oregano EO- and Ocimum sanctum-loaded chitosan/alginate NPs effective against S. aureus [243], E. coli, P. aeruginosa, and B. cereus [244]. Anthocyanins, a class of bioactive water-soluble flavonoid pigments [245], exhibit broad-spectrum antimicrobial activity with particularly pronounced efficacy against Gram-positive bacteria [246]. These compounds have been successfully encapsulated into hybrid chitosan/pectin NPs [241].

Table 3.
Representative polysaccharide-based nanoformulations incorporating plant-derived antimicrobial compounds.
Table 3.
Representative polysaccharide-based nanoformulations incorporating plant-derived antimicrobial compounds.
| Active Component | Nanocarrier | Composition | Formulation Method | Antimicrobial Activity | Ref. and Pub. Year |
|---|---|---|---|---|---|
| Tea water extract (TPS) | Chitosan | Chitosan (85% DA, MW 27 kDa, 250 mg/100 mL) TPS (2.5 mg/mL) | Complex coacervation | Against P. grisea, X. oryzae | [233] 2024 |
| Lavendula angustifolia water extract | Chitosan (1% w/v) Ratio to extract (1:1) | Self-assembling | Suppressed P. aeruginosa, S. aureus, and C. albicans biofilm formation | [235] 2023 | |
| Martynia annua leaves ethanol extract | Chitosan (1% w/v) Ratio to extract (1:1) | Considerable antibacterial activity in order of B. fragilis > S. oralis > P. acnes > P. aeruginosa > S. aureus > E. coli > B. cereus > S. mutans > A. hydrophila > S. faecalis | [236] 2022 | ||
| Clove essential oil (CEO) | Chitosan (75–85% DA, MW 50–190 kDa, 1% w/v) Ratios to CEO (1:0, 1:0.25, 1:0.5, and 1:1) Sodium tripolyphosphate (TPP) | Oil-in-water emulsification followed by ionic gelation | Against L. monocytogenes, S. aureus, S. typhi, and E. coli | [229] 2020 | |
| Guava leaves essential oil (GLEO) | Chitosan (75% DA, MW 50 kDa, 1% w/v) Ratio to GLEO (1:1) TPP | Against K. pneumoniae | [230] 2020 | ||
| Curcumin | Chitosan (1 mg/mL) Curcumin stock (1 mg/mL) in ethanol TPP (1 mg/mL) | Ionic gelation | Against S. aureus and P. aeruginosa | [247] 2014 | |
| Resveratrol | Chitosan (75–85% DA, MW 50–190 kDa, 2 mg/mL) Resveratrol stock (5 mg/mL) in ethanol Ratio (1:5) TPP (1 mg/mL) | Against H. pylori | [238] 2024 | ||
| Grape pomace extract | Chitosan (low molecular weight) Grape pomace extract TPP (1 mg/mL) | 6-log reduction in C. albicans, 5-log reduction in MSSA, a 3-log reduction in L. monocytogenes and P. aeruginosa, and a 1-log reduction in E. coli and S. enteritidis | [234] 2021 | ||
| Grape pomace extract | Alginate | Sodium alginate Grape pomace extract Calcium chloride (2 mg/mL) | Ionic gelation | 6-log reduction in C. albicans, 3-log reduction in MSSA, a 2-log reduction in L. monocytogenes, P. aeruginosa, and S. enteritidis, and 1-log reduction in E. coli | [234] 2021 |
| Cuminum cyminum and Zataria multiflora EOs | Alginate solution (0.25% w/v) EO (0.25% w/v) Calcium chloride (0.04–0.05%) | Oil-in-water emulsification followed by ionic gelation | Superior efficacy for NPs containing Z. multiflora EO against E.coli, P.aeruginosa, and S. aureus | [231] 2024 | |
| Lemon EO | Alginate Myristic acid Ethylene carbo-di-imide hydrochloride (EDC) N-hydroxysuccinimide (NHS) Addition of EO in drop-wise manner | Emulsification and cross-linking | Inhibit multidrug-resistant (MDR) isolates of Acinetobacter baumannii | [232] 2025 | |
| Oregano EO | Chitosan Alginate | Chitosan (MW 110–150 kDa) Alginate (very low viscosity) Calcium chloride Oregano EO | Oil-in-water emulsification followed by pre-ionic gelation of alginate and coacervation with chitosan | Against S. aureus | [243] 2022 |
| O. sanctum methanolic extract | Sodium alginate solution (0.06%, w/v) Calcium chloride (18 mM) Chitosan solution (0.05%, w/v) Methanolic extract of O. sanctum | Pre-ionic gelation of alginate followed by chitosan coacervation | Against E. coli, P. aeruginosa, B. cereus, and S. aureus | [244] 2013 | |
| Curcumin | Sodium alginate (3%) Chitosan (75–85% DA, low MW) Ratio (5:4) Curcumin dissolved in ethanol | Coacervation | Mild activity against S. aureus, B. subtilis, and E. aerogenes | [248] 2024 | |
| Terminalia arjuna (arjuna), Azadirachta indica (neem), Withania somnifera (ashwagandha), Tinospora cordifolia (giloy), Murraya koenigii (curry leaves) extracts | Bacterial Nanocellulose (BNC) | BNC Extracts (20% w/v in water) | Ex situ modification of BNC by simple dipping method | Against E. coli and A. viridans | [249] 2020 |
| Curcumin | BNC Curcumin and curcumin degradation products (0.05, 0.1, and 0.5 mg/mL) | Loaded from aqueous solution during autoclaving | Against S. epidermidis and E. coli | [250] 2020 | |
| Cell cultures of Chelidonium majus | BNC C. majus cells | C. majus cells were cultured in vitro on BNC matrices in liquid media, followed by enzymatic digestion and purification | Against S.aureus, P. aeruginosa, and C. albicans | [251] 2022 | |
| Peppermint (PM), Cinnamon (CN) and lemongrass (LG) EOs | Cellulose Acetate (CA) | CA (acetyl content of 39.8%, MW 30 kDa, 1% w/v) EO in acetone (0.5% v/v) | Nanoprecipitation by solvent/anti-solvent technique EOs were grafted on cellulose acetate molecules | CA/CN significantly inhibited growth of S. aureus, E. coli, P. aeruginosa, and C. albicans | [252] 2018 |
| Thymol | Cellulose Nanofibrils (CNFs) | CNFs Thymol (200 mg) | Impregnation with thymol in scCO2 | Against E. coli, S. epidermidis, and C. albicans | [253] 2020 |
| Curcumin | Xylan | Xylan (0.132 g, 1 mmol) Curc-monosuccinate (0.864 g, 2 mmol) DMSO N, N’-dicyclohexylcarbodiimide (DCC, 0.412 g, 2 mmol) 4-Dimethylaminopyridine (DMAP, 0.116 g, 1 mmol) Precipitation in ethanol/ethyl ether (1:1 v/v) | Conjugation followed by precipitation | No data | [254] 2018 |
| Menthone, oregano, cinnamon, lavender, and citral EOs | Starch | Debranched starch (1% w/v in water) EOs (250 µL dissolved in 20 mL hot ethanol) | Precipitation and freeze drying | Better antimicrobial activity against S. aureus than E. coli | [255] 2017 |
| Triphala Churna (TC) extract | Heating soluble starch (5 g) in 0.4 M NaOH Addition of 0.3% of TC and acetone | Precipitation and graft copolymerization | Antibacterial activity against S. typhi and S. dysenteriae; antibiofilm activity against ATCC MRSA 33591 and clinical strain N7 | [256] 2020 | |
| Linalyl acetate | Corn starch (2 wt%) Mixture of 1 M sodium hydroxide and 1 M urea, volume ratio 1:2 Linalyl acetate (1.5 wt%) Tween 80 Ethanol was used as anti-solvent (1:15 ratio to solvent) | Nanoprecipitation by solvent/anti-solvent technique | Promote wound healing | [257] 2025 | |
| Curcumin | Cinnamic acid-esterified debranched starch Curcumin | Additional π-π interactions provided from cinnamic acid | Biofilm scavenging ability, superior antibacterial effects | [258] 2022 | |
| Quercetin | Pea, corn and potato starches (20 mg/mL) and quercetin (2 mg/mL) dissolved in NaOH/urea/H2O Ratio (0.8:1:98.2 by weight) 0.1 M HCl | Nanoprecipitation | No data | [259] 2018 | |
| Rutin | Quinoa and maize starch suspensions (1.5%) preheated (80 °C) in 0.1 M NaOH solution Rutin (1.5%) dissolved in ethanol Ratio (1:10) | Ultrasonication | [260] 2021 | ||
| Flavonoids of citrus peel extracts (CPE) | Pectin | Pectin water solution CPE ethanol extract Calcium chloride | Ionic gelation | No data | [261] 2017 |
| Quercetin | Pectin Chitosan | Chitosan (80% DA, MW 190–300 kDa, 1% w/v) TPP Quercetin dehydrate Pectin Calcium chloride | [240] 2022 | ||
| Anthocyanins (ANCs) | Chitosan (95% DA, MW 300 kDa, 1% w/v) ANCs (1–4% w/v) Pectin (MD 30%, 5% w/v) Mass ratio of chitosan/pectin/anthocyanin (1:1:3) | Coacervation | [241] 2020 | ||
| Lippia sidoides EO | Chitosan Cashew Gum | Gum (MW 110 kDa, 5%) Chitosan (75% DA, MW 180 kDa) Ratio (1:1) Polymer matrix/EO (10:2) | Complex coacervation | Against St. Aegypti larvae | [242] 2012 |
| Saffron extract | Chitosan Arabic Gum | Chitosan (DA > 75%, MW 50–180 kDa, 1–10 mg/mL) Gum arabic (MW 295–1860 kDa, 1–5 mg/mL) Saffron (5–15 mg/mL) | No data | [262] 2019 | |
| L. sidoide EO | Alginic Acid Sodium Salt Cashew Gum | Alginate (low viscosity, MW 54 kDa) Cashew gum (MW 110 kDa) Relative ratios of alginate/gum (1:3, 1:1, and 3:1) Blend/oil ratio (10:1, w/w) Calcium chloride (0.5%, w/w) | Ionic gelation followed by spray drying | [263] 2014 | |
| Curcumin | Prunus armeniaca Gum (PAGE) | PAGE solution in water Ethanolic solution of curcumin (400 μL, 10 mg/mL) Calcium chloride-to-mixture ratio (1:1) | Ionic gelation | Against S. aureus and E. coli | [264] 2021 |
| Quercetin | PAGE Quercetin Calcium chloride | Significant decline in bacterial load | [265] 2024 | ||
| D-limonene (DL, R-(+)-Limonene) | Carrageenan | Carrageenan (sulfate content around 27% w/w, MW 672 ± 32 kDa) DL | Electrospray | No data | [266] 2022 |
| Curcumin | Carrageenan (0.15% and 0.44% in 0.5 mL/L NaCl aqueous solution) Curcumin ethanolic solution (100 mg/mL and 10 mg/mL) | Self-assembling | [139] 2022 | ||
| Grapefruit seed extract and cinnamon oil (GCN) | Chitosan Carrageenan | Chitosan Carrageenan GCN | Complex coacervation | Against Streptococcus mutans and sobrinus | [267] 2023 |
| Quercetin | Modified Dextran | Grafted dextran with L-cysteine and octadecylamine onto carboxymethyl dextran Quercetin | Self-assembling | No data | [268] 2023 |
| Eucalyptus staigeriana EO | Dextran Sulfate Chitosan | Dextran sulfate Chitosan Aloe Vera Eucalyptus staigeriana EO | Formation of hydrogel | Inhibited bacteria growth | [269] 2023 |
| α-Tocopherol | Dextran sulfate (MW 15 kDa) Chitosan (95%DA) α-Tocopherol Lecithin | Multi-layer nanoemulsions | No data | [270] 2024 | |
| Curcumin | Dextran sulfate (MW > 500 kDa, 0.1 wt%) Chitosan (DA > 75%, low MW, 0.1 wt%) Volume ratio of 3:2 Curcumin (2 mg) was loaded into NPs in 5 wt% of polymer | Complex coacervation | [271] 2011 | ||
| Naringenin | Dextran sulfate (MW 500 kDa, 0.1 wt%) Chitosan (DA > 75%, 0.1 wt%) Volume ratio (3:2) Naringenin (2 mg/mL) was equal to 5% weight of polymers | [272] 2021 | |||
| Clove extract | Pullulan Whey | Pullulan (20% w/w) Whey (20% w/w) Pullulan: whey protein ratios (100:0 w/w, 50:50 w/w, and 25:75 w/w) Clove extract (5% w/w) | Electrospinning | Against S. aureus and M. luteus | [273] 2022 |
| Resveratrol | Pullulan | Pullulan Resveratrol | Surface-functionalized with the ligand n-acetyl glucosamine | No data | [274] 2025 |
| Tannic acid | Pullulan Chitosan | Pullulan (18 wt%) Chitosan (DA 75–85%, MW 50–190 kDa, 3 wt%) Tannic acid (1 wt%) | Force-spinning | Against E.coli | [275] 2015 |
| Curcumin | Pullulan Hyaluronic Acid (HA) | Succinylated pullulan (SPu, 200 kDa, 400 mg) HA (5.4 kDa, 0.528 mmol disaccharide repeat unit) DMAP (0.106 mmol) EDC (0.528 mmol) Formamide Curcumin Mass ratios Cur/HA-SPu (1/5, 1/10, 1/15) | Conjugation | Against E. coli and S. aureus | [276] 2020 |
| Olive leaf extract (OLE) | Hyaluronic Acid Silk Fibroin (SF) | SF (15% w/v in formic acid) HA (0.5% w/v in distilled water) OLE (12 and 15% w/v) | Electrospinning | Perfect antibacterial activities against both Gram-negative and Gram-positive bacteria, while antifungal activity against C. albicans was rather poor | [277] 2016 |
| Curcumin | Pluronic Chitosan Hyaluronic Acid | Pluronic Chitosan (DA 97%, MW 1–3 kDa) HA Triethanolamine (TEA) DMAP | Conjugation and nebulisation | No data | [278] 2021 |
| Curcumin (CUR) and resveratrol (REV) | Hyaluronic Acid Chitosan | Chitosan (0.1% w/v) 1 mg of each CUR and REV is dissolved into 70:30 ratio of ethanol and water TPP (0.1% v/v) HA (0.01–0.05% w/v) | Ionic gelation followed coacervation | [279] 2020 | |
| Quercetin | Hyaluronic Acid | HA sodium salt solution (0.5% w/v, MW 200 kDa) DMSO (1:1 v/v ratio) Glutaraldehyde (5% v/v) HCl Quercetin (34 µmol) in phosphate buffer containing 10% v/v of ethanol | Nanoprecipitation with solvent–non solvent method and cross-linking | [280,281] 2017 2018 | |
| Tannic acid (TA) | Sodium Hyaluronate (HA) | HA (MW 44 KDa (low), 375 kDa (medium) and 737 kDa (high), 10 mg/mL) 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM, 404 μmol) 3-aminophenylboronic acid hydrochloride (3-APBA∙HCl, 91 μmol) HA-APBA (2 mg/mL) TA water solution (28, 56, 112, 224, or 448 μg/mL) | Cross-linking (Catechol/Boronate complexation) | Against E. coli, MSSA, and MRSA | [282] 2016 |
Notes: TPS—tea water extract; DA—deacetylation degree; MW—molecular weight; CEO—cove essential oil; GLEO—guava leaf essential oil; TPP—sodium tripolyphosphate; MSSA—methicillin-susceptible S. aureus; EDC—ethylene carbo-di-imide hydrochloride; NHS—N-hydroxysuccinimide; MDR—multidrug-resistant; EO—essential oil; BNC—bacterial nanocellulose; CA—cellulose acetate; PM—peppermint; CN—cinnamon; LG—lemongrass; CNFs—cellulose nanofibrils; DCC—N, N’-dicyclohexylcarbodiimide; DMAP—4-Dimethylaminopyridine; TC—Triphala Churna; MRSA—methicillin-resistant S. aureus; CPE—citrus peel extract; ANCs—anthocyanins; MD—methylation degree; PAGE—Prunus armeniaca gum exudate; DL—D-limonene; GCN—grapefruit seed extract and cinnamon oil; SPu—succinylated pullulan; HA—hyaluronic acid; OLE—olive leaf extract; SF—silk fibroin; TEA—triethanolamine; DMTMM—4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride; 3-APBA∙HCl—3-aminophenylboronic acid hydrochloride.
Lippia sidoides essential oil and saffron extract were effectively loaded into chitosan/gum NPs via complex coacervation [242,262], while chitosan/carrageenan NPs containing grapefruit seed extract and cinnamon oil demonstrated strong activity against St. mutans and S. sobrinus [267]. Alginate NPs with grape pomace extract exhibited dose-dependent antimicrobial effects, achieving a 6-log reduction in C. albicans; 3-log reduction in MSSA; 2-log reduction in L. monocytogenes, P. aeruginosa, and S. enteritidis; and 1-log reduction in E. coli [234]. Furthermore, alginate NPs encapsulating Cuminum cyminum, Zataria multiflora, or lemon essential oils (prepared by oil-in-water emulsification with ionic gelation/cross-linking) showed species-specific activity: Z. multiflora-loaded NPs were effective against E. coli, P. aeruginosa, and S. aureus [231], whereas lemon oil NPs targeted multidrug-resistant A. baumannii [232]. Additionally, citrus peel flavonoids were successfully incorporated into pectin NPs through ionic gelation [261], further expanding the repertoire of plant-polysaccharide antimicrobial delivery systems.
Curcumin and quercetin exhibit strong antimicrobial potential, though their therapeutic applications are limited by poor water solubility and low bioavailability [283,284]. These challenges have been addressed through advanced nanoformulations: (1) curcumin-loaded NPs using Prunus armeniaca gum, chitosan, and chitosan/alginate composites demonstrated efficacy against S. aureus [247,248,264], E. coli [264], P. aeruginosa [247], B. subtilis, and E. aerogenes [248]; (2) quercetin was successfully encapsulated in Prunus armeniaca gum and chitosan/pectin NPs via ionic gelation [240,265], as well as in hyaluronic acid nanostructures through nanoprecipitation with solvent–non-solvent methods and cross-linking [280,281]. Additional curcumin delivery systems include BNC loading during autoclaving, carrageenan encapsulation, and xylan conjugation [139,250,254]. Furthermore, formulations like succinylated pullulan/hyaluronic acid and pluronic/chitosan/hyaluronic acid NPs have enhanced curcumin’s activity against E. coli and S. aureus [276,278], demonstrating the versatility of polysaccharide-based nanocarriers for these bioactive compounds.
Nanocellulose possesses exceptional physicochemical properties, including high surface area, porosity, and modifiable surface chemistry, making it a suitable platform for antimicrobial agent encapsulation and immobilization [285]. BNC can incorporate natural antimicrobials or living cell cultures through various approaches: physical adsorption, in situ incorporation, immersion techniques, chemical fixation, or electrostatic self-assembly [251,286]. Hemicelluloses (xylans, mannans, and β-glucans) demonstrate synergistic effects with polyphenolic compounds and can be functionalized through either physical adsorption or covalent conjugation, while nanoparticles are typically prepared via precipitation or dialysis methods [127,287]. BNC composites incorporating traditional medicinal plant extracts (Terminalia arjuna, Azadirachta indica, Withania somnifera, Tinospora cordifolia, and Murraya koenigii) or Chelidonium majus cell cultures was prepared by dipping or cultivation with subsequent enzymatic digestion and exhibited broad-spectrum antimicrobial activity against E. coli, A. viridans [249], S.aureus, P. aeruginosa, and C. albicans [251]. Notably, cinnamon essential oil encapsulated in cellulose acetate nanoparticles shows potent inhibitory effects against clinically relevant pathogens including S. aureus, E. coli, P. aeruginosa, and C. albicans [252]. Thymol, a bioactive monoterpenoid phenol abundant in essential oils, demonstrates particularly strong antimicrobial properties [288] when impregnated into cellulose nanofibrils using supercritical CO2 technology, showing efficacy against E. coli, S. epidermidis, and C. albicans [253].
Starch NPs are typically prepared via precipitation [115] and effectively encapsulate diverse bioactive compounds including phytochemicals, essential oils (EOs), and plant extracts [255,256,257,258,259,260], often requiring debranching pretreatment for optimal formulation [255,258]. These NPs with EO-loaded systems (menthone, oregano, cinnamon, lavender, and citral) showed greater efficacy against S. aureus than E. coli [255]. Triphala Churna extract-loaded starch NPs exhibit dual activity: antibacterial action against S. typhi and S. dysenteriae, plus antibiofilm effects against methicillin-resistant S. aureus (MRSA) ATCC 33591 and clinical strain N7 [256]. The platform also successfully encapsulates linalyl acetate, curcumin, quercetin, and rutin [257,258,259,260].
Dextran derivatives and their NPs demonstrate antimicrobial properties [289,290]. Quercetin has been successfully encapsulated into self-assembled NPs prepared from dextran grafted with L-cysteine and octadecylamine [268]. Dextran sulfate and chitosan were combined to incorporate Eucalyptus staigeriana essential oil (EO), α-tocopherol, curcumin, and naringenin through three fabrication methods: complex coacervation [271,272], multilayer nanoemulsions [270], and antibacterial hydrogels [269]. Resveratrol, a bioactive polyphenol with demonstrated antibacterial and antibiofilm properties [291], has been effectively encapsulated in both pullulan-based structures [274] and hybrid hyaluronic acid/chitosan systems via ionic gelation–coacervation, with the latter showing synergistic effects when combined with curcumin [279].
Equipment-based spinning techniques were employed to fabricate nanoformulations of pullulan/whey, pullulan/chitosan, and hyaluronic acid/silk fibroin. These systems incorporated clove extract, olive leaf extract, and tannic acid, demonstrating antimicrobial activity against S. aureus and M. luteus [273], E. coli [275,277], and MRSA [277]. Tannic acid, a natural polyphenolic tannin, exhibits broad-spectrum antimicrobial and antiviral properties [292]. Sodium hyaluronate NPs containing tannic acid showed significant efficacy against E. coli, MSSA, and MRSA [282].
Plants represent a rich source of diverse secondary metabolites, such as tannins, terpenoids, alkaloids, and flavonoids, which exhibit broad-spectrum bioactive properties, particularly antimicrobial activity [293]. These phytochemicals can be effectively encapsulated within polysaccharide nanomatrices in various forms (essential oils, crude extracts, or purified compounds), serving as potent antimicrobial agents with sustained release profiles [294,295].
5. Polysaccharide-Based Nanocarriers for Microbial-Derived Antimicrobials
Microorganisms produce diverse antimicrobial agents, including peptides, spirotetronates, polyketides, alkaloids, organic acids, and sesquiterpene derivatives [296]. Among these, bacteriocins represent one of the most promising and intensively studied groups of microbial-derived antimicrobials [4,297,298,299,300]. Bacteriocins are classified into three main categories based on their chemical structure, molecular weight, biochemical properties, spectrum of antimicrobial activity, and mechanism of antimicrobial action: (1) Class I—heat-stable lanthionine-containing peptides (lantibiotics, thiopeptides, sactibiotics, lasso peptides, and cyclic bacteriocins); (2) Class II—small heat-stable non-lanthionine peptides; and (3) Class III—large heat-labile proteins [224,301,302,303]. Class I bacteriocins include lantibiotics, lipolantins, thiopeptides, botromycins, linear azole-containing peptides, sactibiotics (sactipeptides), lasso peptides, and cyclic bacteriocins [303]. Class II bacteriocins are divided into three subclasses and include pediocin, enterocin, sakacins, leucocin, carnobacteriocins, etc. [304]. Class III bacteriocins include megacins, klebicin, helveticin I, and enterolysin [305].
While bacteriocins show promise as antimicrobial agents, they face several application challenges that limit their clinical and industrial utilization. Thus, bacteriocins have a limited spectrum of activity against closely related bacterial strains [306,307]. The intensive application of bacteriocins could lead to the potential development of bacterial resistance. Thus, some species of B. cereus and P. polymyxa produce nisinase, while Listeria species demonstrate alterations in the surface charge of their cell walls due to gene mutations that disrupt bacteriocin binding, and some C. botulinum strains also show cross-resistance to Class II bacteriocins [308]. Low production yield, purification challenges, and formulation stability issues hinder the widespread adoption of bacteriocins as biopreservatives [309,310]. Due to their peptide nature, bacteriocins are sensitive to proteases, possess poor pharmacokinetic profiles, and may act as host sensitizers or allergens [311]. These limitations may be partially overcome through nanoencapsulation strategies.
Nisin is a widely recognized Class I bacteriocin with Generally Recognized as Safe (GRAS) status [312,313]. Composed of 34 amino acids, it is produced by Lactococcus lactis ssp. [314] and exhibits potent inhibitory activity against spore-forming bacteria such as Bacillus and Clostridium. Additionally, nisin demonstrates efficacy against Listeria, Micrococcus, Staphylococcus, Streptococcus, Lactobacillus, Lactococcus, Leuconostoc, Mycobacterium, and Pediococcus. However, it shows minimal to no activity against Gram-negative bacteria [315]. Nisin-incorporated nanoparticles (NPs) were successfully developed using various biopolymers, each demonstrating distinct antimicrobial properties. Nisin was encapsulated into chitosan NPs via ionic gelation (Table 4), exhibiting antimicrobial activity against S. aureus, L. monocytogenes, and E. coli [316,317]. Additionally, nisin-loaded NPs were fabricated using chitosan combined with alginate [318,319], pectin [320], or carrageenan [321] through electrostatic coacervation. These NPs effectively suppressed the growth of L. monocytogenes, S. aureus, E. coli, S. enterica, M. luteus, P. aeruginosa, and E. aerogenes. Bacterial cellulose nanocrystals, carboxymethylcellulose, and nanofibrillated cellulose can form nanocomposites with nisin through complexation. These nanocomposites exhibit microbial inactivation and demonstrate activity against S. aureus and B. subtilis [322,323,324]. Co-culturing Enterobacter sp. FY-07 (a bacterial nanocellulose producer) with Lactococcus lactis N8 (a nisin producer) resulted in the formation of a nanomaterial with strong inhibitory effects against Gram-positive bacteria [325]. Nisin-functionalized cellulose nanofibers exhibit inhibitory activity against B. thermosphacta and L. innocua [326], while holocellulose nanofibrils conjugated with nisin demonstrate antimicrobial efficacy, particularly against Gram-positive bacteria including S. aureus and L. monocytogenes [327].

Table 4.
Polysaccharide-based nanostructures incorporating microbial-derived antimicrobial agents.
Alginate–nisin and alginate–starch–nisin NPs, prepared by emulsification followed by ionic gelation showed effective activity against L.monocytogenes [328]. Nisin-incorporated pectin NPs exhibited spectrum-specific antimicrobial activity that varied with the esterification degree, showing efficacy against Arthrobacter sp., B. subtilis, E. coli, and Klebsiella sp. [329]. Notably, high-methoxyl pectin nanoparticles demonstrated inhibition against S. aureus and E. coli [330]. Similarly, gellan gum- and dextran-based nisin nanoparticles showed potent activity against S. aureus [295]. Similarly, gellan gum- and dextran-based NPs with nisin were active against S. aureus [331], and radiation-synthesized dextran-nisin conjugates showed broader spectrum activity against E. coli, P. fluorescence, S. aureus, and B. cereus [332]. Electrospun NPs fabricated from pullulan, amaranth protein isolate, and nisin demonstrated antimicrobial effects against L. mesenteroides, L. monocytogenes, and S. Typhimurium [333]. Hyaluronic acid-based formulations showed promising results: HA–nisin nanoformulations prepared by electrostatic complexation exhibited superior inhibition against hyaluronidase-producing S. aureus compared to B. cereus [334], while HA–nisin conjugates were also effective against S. epidermidis, S. aureus, and P. aeruginosa [335].
Pediocin-like bacteriocins are small (<5 kDa) peptides characterized by the conserved sequence -Y-G-N-G-V-X1-C-X2-K/N-X3-X4-C- [349], produced primarily by some Pediococcus spp. [350]. They exhibit broad-spectrum activity against Gram-positive bacteria, with particularly strong inhibition of L. monocytogenes, as well as efficacy against E. faecalis, S. aureus, and C. perfringens [350,351,352]. When encapsulated in alginate–guar gum via complex coacervation, pediocin demonstrated significant activity against L. innocua [336]. Plantaricins, bacteriocins derived from L. plantarum [353], include variants such as plantaricin E/F, which inhibit Gram-positive bacteria. Alginate-encapsulated plantaricin E/F showed antimicrobial effects against the sensitive indicator strain L. plantarum NCIMB 700965 (LP965) [337]. Enterocins, produced by Enterococcus spp. [354,355], display potent activity against foodborne pathogens, including S. aureus, L. monocytogenes, and S. enterica et al. [356]. Alginate and bacterial cellulose nanomaterials were successfully fabricated through simple ball milling [338] or soaking methods [339], demonstrating effective antimicrobial activity against C. perfringens and L. monocytogenes. In parallel, sakacins—a group of bacteriocins produced by specific L. sakei strains with a narrow antibacterial spectrum [357]—showed activity when conjugated with bacterial cellulose nanocrystals, particularly against L. innocua [340]. Further developments in bacteriocin delivery systems include chitosan nanoparticles produced by ionic gelation encapsulation of Levilactobacillus brevis and Lactococcus lactis subsp lactis bacteriocins. These nanoparticles exhibited superior antibacterial effects against Gram-positive pathogens (especially under acidic conditions) compared to Gram-negative bacteria [341] and demonstrated activity against S. typhimurium, E. coli, B. cereus, and S. aureus [342]. Similarly, cellulose nanocrystals functionalized with bacteriocins from P. acidilactici and E. faecium effectively inhibited the growth of multiple pathogens including S. aureus, L. monocytogenes, E. coli, E. herbicola, B. subtilis, B. cereus, and P. aeruginosa [343].
Natamycin, a natural antifungal compound produced by Streptomyces species, is widely approved as a food preservative [358,359]. While ineffective against bacteria, it demonstrates broad-spectrum activity against fungi and yeasts including Candida spp., Aspergillus spp., Cephalosporium spp., Fusarium spp., and Penicillium spp. [360]. Various nanoformulations have been developed to enhance its efficacy: chitosan-based nanoparticles (either alone or combined with zein or lecithin) showed strong activity against C. albicans [347], completely inhibiting spore germination and suppressing mycelial growth by 64.4% [344], with additional activity against A. fumigates [345]. Similarly, carboxymethylcellulose-gliadin NPs inhibited P. expansum [346], and alginate nanoparticles prepared by emulsification–ion gelation achieved a 2-log reduction in A. flavus populations [348].
Bacteriocins exhibit low toxicity to eukaryotic cells and demonstrate minimal inhibitory concentrations against numerous bacterial strains, along with high-temperature stability. However, they are sensitive to proteases, possess poor pharmacokinetic profiles, and may act as host sensitizers or allergens [311]. These limitations can be partially mitigated through nanoencapsulation, which enhances their stability and antimicrobial functionality [361].
6. Polysaccharide-Based Nanocarriers for Animal-Derived Antimicrobial Proteins and Peptides
Animal-derived antimicrobials include enzymes (e.g., lysozyme and lactoperoxidase), glycoproteins (lactoferrin, ovotransferrin, and avidin), histones, and antimicrobial peptides (arenicins, magainins, seroins, pleurocidins, cecropins, cathelicidins, protegrins, and defensins) [4,224,303,362].
Despite their potential as antimicrobial agents, animal-derived peptides and proteins face significant translational challenges that restrict their widespread clinical and industrial application, including a lack of selectivity, off-target effects, proteolytic instability, potential toxicity, and immunogenicity [363]. Low production yields and purification difficulties could also limit the application of animal-derived antimicrobial peptides and proteins [364]. Thus, studies have demonstrated that bacteria can develop resistance to AMPs under in vitro conditions [365]. Furthermore, certain antimicrobial peptides (AMPs) exhibit low specificity, targeting both pathogenic microbes and host cells. This non-selective activity can induce cytotoxic effects in human cells, resulting in adverse side effects that limit their therapeutic application [363,366]. Their proteinaceous nature renders animal-derived peptides and proteins susceptible to proteolytic degradation and results in suboptimal pharmacokinetic properties [367]. These limitations can be partially mitigated through nanoencapsulation.
Lysozyme, a 14.3 kDa secretory enzyme composed of 129 amino acids [368,369,370], is most abundant in egg white but also present in milk, cauliflower, cabbage, papaya juice, spleen, thymus, pancreas, and mucus [370], or it can be produced recombinantly [371]. Its antimicrobial activity primarily targets Gram-positive bacteria through the cleavage of β-(1,4)-glycosidic bonds in peptidoglycan, while it exhibits limited or negligible effects against Gram-negative bacteria [370,372]. Chitosan-based NPs incorporating lysozyme demonstrate broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria through the synergistic action of chitosan and lysozyme (Table 5). Ionic gelation-fabricated chitosan-lysozyme NPs effectively inhibited the growth of E. coli and B. subtilis [373], while nanoprecipitated formulations significantly reduced A. parasiticus viability and strongly suppressed spore germination [374]. Lysozyme-conjugated chitosan NPs showed potent activity against S. aureus, E. coli, P. aeruginosa, and K. pneumoniae [375]. Nanogels fabricated from carboxymethyl chitosan, lysozyme, and amorphous calcium phosphate demonstrated activity against S. mutans [376]. Researchers have developed various innovative delivery systems for lysozyme, including chitosan/alginate NPs prepared via alginate pre-ionic gelation followed by chitosan coacervation [377], and complexes combining chitosan, CNCs, and lysozyme that exhibited antimicrobial effects against E. coli and L. innocua [378]. Additionally, lysozyme has been successfully encapsulated in depolymerized chitosan/dextran sulfate NPs through polyelectrolyte complexation [379] and in alginate formulations via ionic gelation [380], further expanding its potential applications in antimicrobial therapies. CNCs enable both nonspecific and covalent immobilization of lysozyme. The resulting nanomaterials exhibited antimicrobial activity against M. deykticus [381], Corynebacterium sp., E. coli, and Ps. mendocina [382].

Table 5.
Polysaccharide-based nanostructures incorporating animal-derived antimicrobial agents.
In a separate formulation, cellulose acetate (CA) nanofibers were functionalized with pectin and lysozyme via electrostatic layer-by-layer assembly, yielding a nanomaterial with inhibitory effects against E. coli and S. aureus [383]. Starch-based formulations incorporating lysozyme have been successfully developed through cross-linking techniques [384,385], demonstrating potent antimicrobial activity against various bacterial strains including B. licheniformis 7558, B. licheniformis 6993, B. subtilis 168, L. monocytogenes LR991, and L. monocytogenes 001 [384]. Lysozyme-loaded NPs of pectin, κ-carrageenan, and xanthan gum were prepared through ionic gelation (retaining activity against M. lysodeikticus) [386] and complex coacervation via electrostatic polysaccharide–protein interactions [387,389,390], followed by either alkaline gelatinization [391,392] or high-pressure homogenization-assisted electrostatic complexation [393]. Gum arabic NPs incorporating lysozyme were fabricated through complex coacervation via electrostatic interactions, followed by Maillard reaction-induced conjugation, demonstrating antimicrobial activity against E. coli and S. aureus [388]. Similarly, lysozyme–dextran and lysozyme–pullulan conjugates prepared via Maillard dry heat processing [394,395,396,397] exhibited broad-spectrum inhibition against multiple pathogens, including M. Lysodeikticus, V. parahaemolyticus IFO 13286, E. coli IFO 12713, A. hydrophila IFO 13286, P. mirabilis IFO 12668, K. pneumoniae IFO 14438, B. cereus IFO 13690, S. aureus IFO 14462 [395], E. coli, S. enterica, and S. aureus [396]. Electrospun pullulan fibers cross-linked with lysozyme also showed efficacy against E. coli and S. aureus [397]. Additionally, hyaluronic acid–lysozyme complex coacervates [398,399] were developed with demonstrated wound-healing properties [399].
Lactoperoxidase (LPO) is an 80 kDa calcium- and iron-containing enzyme [432] predominantly found in mammalian secretions, particularly milk, where it constitutes ~1% (w/w) of whey proteins [433,434]. The LPO antimicrobial system, composed of lactoperoxidase (LPO), thiocyanate, and hydrogen peroxide, is naturally occurring and exhibits both bacteriostatic and bactericidal activity against diverse Gram-positive and Gram-negative microorganisms [435]. Lactoferrin (LF), a cationic glycosylated protein [436], is similarly abundant in milk (~1% of whey proteins) and colostrum, and it is also present in tears, saliva, gastric mucosa, spleen, lymph nodes, skin, and white blood cells [437]. While its primary role is iron binding, LF demonstrates broad-spectrum antibacterial activity [438], with reported efficacy against E. coli, S. typhi, Streptococcus, L. pneumophila, and S. aureus [439]. The LPO can be incorporated into NPs through complex coacervation, either with chitosan and gum tragacanth or in combination with LF, chitosan, and dextran [400,401,402,403]. Chitosan–LF NPs prepared by ionic gelation demonstrated antimicrobial activity against S. aureus [404]. Similarly, LF was successfully encapsulated in alginate formulations using ionic gelation [407,408], while pectin–LF NPs fabricated through complex coacervation [411,412] inhibited P. aeruginosa growth [411]. LF has also been conjugated with hyaluronic acid (either non-covalently or covalently) [413] or BNC, exhibiting antimicrobial effects against S. aureus and E. coli [409]. Additionally, the electrostatic complexation of LF with gellan gum formed NPs active against both S. aureus and E. coli [410]. Ovotransferrin (OVT), a 76 kDa glycoprotein constituting approximately 12% of total egg white protein [440], exhibits broad-spectrum antimicrobial activity against pathogens including S. aureus, B. cereus, L. monocytogenes, E. coli, and H. pylori [441]. Research has demonstrated OVT’s ability to form nanoformulations through polysaccharide–protein complexation with carboxymethyl chitosan, pectin, and various gums [414,415,416,417,418].
Antimicrobial peptides (AMPs), conserved across nearly all species, serve as components of innate host defense systems [362]. The Antimicrobial Peptide Database (APD) catalogs 2580 animal-derived AMPs, including 154 human host defense peptides, 397 from mammals, and 1110 active peptides from amphibians [442]. Their activity is largely attributed to cationic and amphipathic structural features [443], which enable broad-spectrum antimicrobial effects [444] through cell membrane disruption, the inhibition of cell wall synthesis, and interference with nucleic acid and protein production [445]. Chitosan is widely employed for encapsulating AMPs through ionic gelation, leveraging both the technique’s simplicity and chitosan’s inherent antimicrobial properties. This approach was proven successful with various AMPs. Cryptdin-2 from Paneth cells, when encapsulated in chitosan nanoparticles, reduced Salmonella Typhimurium loads in murine tissues by 2 log units [419]. Similarly, chitosan NPs incorporating frog skin-derived temporin B demonstrated efficacy against S. epidermidis [420], while those containing insect-derived cecropin-B showed activity against multidrug-resistant K. pneumoniae [421]. The encapsulation of human neutrophil defensin (HNP-1) in chitosan yielded NPs with broad-spectrum antibacterial activity against S. aureus ATCC 25923, E. coli NCTC 9001, P. aeruginosa ATCC 10145, K. aerogenes NCTC 10006, and MRSA [422]. Human cathelicidin peptide LL-37 encapsulated in chitosan NPs exhibited potent effects against MRSA [423]. Pleurocidin-like AMPs, identified across multiple flounder species, include NRC-07, which was successfully complexed with chitosan to form NPs exhibiting antimicrobial activity against P. aeruginosa [424].
AMPs have been successfully conjugated and immobilized into CNFs and modified starch matrices. These fabricated materials, incorporating either cecropin CA(1–7)M(2–9) or the LL-37 antimicrobial motif (KR-12), demonstrated growth inhibition against B. subtilis [425], E. coli, S. aureus [426,428], and even MRSA [428]. Electrospun pullulan fibers functionalized with the minimal bovine lactoferricin motif (LfcinB) (20–25)Pal showed potent activity against E. coli [427]. Microfluidic chip technology enabled the fabrication of nanogels from octenyl succinic anhydride-modified hyaluronic acid conjugated with snake cathelicidin Ab-Cath. These nanogels demonstrated antimicrobial activity, inhibiting the growth S. aureus, A. baumannii, and E. coli in biological fluids while significantly reducing S. aureus and A. baumannii biofilms [429]. Cecropin B exhibited electrostatic interactions with hyaluronic acid in aqueous solutions [431]. Hyaluronic acid/PLGA complex coacervates incorporating insect thanatin from Podisus maculiventri thanatin [446] effectively mitigated sepsis caused by metallo-β-lactamases-1 (NDM-1) producing E. coli [430].
Synthetic AMPs are designed based on either known AMP structures or chemical composition–structure–activity relationships to achieve desired biological properties [447,448]. These engineered peptides address the key limitations of natural AMPs, including toxicity concerns, their short half-life, and their restricted antibacterial efficacy [449]. Synthetic AMPs have been successfully incorporated into various nanomaterial systems, including hyaluronic acid-based carriers [450], chitosan/alginate composites [451], alginate matrices [452], BNC platforms [453], and dextran formulations [454].
7. Conclusions
Natural antimicrobial compounds derived from plants, animals, and bacteria demonstrate significant potential while facing key application challenges, particularly their susceptibility to rapid degradation, poor pharmacokinetic profiles, short biological half-lives, potential allergenicity or toxicity, volatility, and hydrophobicity. Nanoencapsulation presents a promising solution to these challenges by improving compound stability, extending the circulation time, and enhancing antimicrobial functionality while minimizing adverse effects. Polysaccharides are renewable, widely accessible, biodegradable, and biocompatible, which makes polysaccharide-based NPs less toxic and allergenic. They are commonly used in polymer-based nanoformulations due to their excellent mechanical and physicochemical properties and their inherent biological activities, which can be further enhanced in nanoform.
Due to their advantageous properties, polysaccharide-based nanocarriers loaded with natural antimicrobials demonstrate high potential for applications in the food, cosmetic, and medical industries. In the food industry, these systems may be integrated both into packaging materials and directly into food products. A particularly promising application is their use for targeted delivery to specific regions of the gastrointestinal tract owing to the well-documented ability of certain polysaccharides to be selectively degraded in these regions by local enzymatic activity or microbiota. In addition, tissue engineering represents a highly relevant field for such systems as many polysaccharides inherently possess antimicrobial and/or regenerative activities. When combined with natural antimicrobial agents, these properties may result in a synergistic effect, enhancing the overall therapeutic efficacy of the nanocarriers.
However, the main challenges in using polysaccharides for nanoformulations include their variable chemical structure, which affects their physical and chemical properties; water absorption capacity; and poorly understood cytotoxic effects of their nano-forms. Moreover, challenges in process parameter optimization for their fabrication represent major barriers to industrial-scale implementation, as well as safety concerns. In addition, the issue of antimicrobial resistance remains a critical concern. The remarkable adaptability of microorganisms may limit the long-term effectiveness of such systems. Therefore, any practical implementation must undergo rigorous scientific and regulatory evaluation and will likely face usage restrictions to mitigate the risk of resistance development.
Author Contributions
Conceptualization, M.N. and E.K.; data curation, A.K. and E.K.; formal analysis, A.K. and E.K.; writing—original draft preparation, A.K. and E.K.; writing—review and editing, M.N. and E.K.; supervision, M.N.; project administration, M.N.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Higher Education of the Russian Federation, agreement 075-03-2025-662 projects FSMG-2023-0017 (Section 6—Nanocarriers for animal-derived antimicrobials), FSMG-2025-0082 (Section 3—Nanocarrier synthesis methods), FSMG-2025-0023 (Sections 1, 4 and 7—Nanocarriers for plant-derived antimicrobials, Introduction, Conclusion) and agreement 075-10-2021-093 (Section 5—Nanocarriers for Microbial-derived antimicrobials), and the Russian Science Foundation, project no. 24-14-00120, https://rscf.ru/en/project/24-14-00120/ (accessed on 17 June 2025) (Section 2—Polysaccharides review).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PLA | Polylactic acid |
| PHA | Polyhydroxyalkanoates |
| PLGA | Poly(lactide-co-glycolide) |
| PCL | Poly(caprolactone) |
| TPS | Tea water extract |
| DA | Deacetylation degree |
| MW | Molecular weight |
| CEO | Clove essential oil |
| GLEO | Guava leaves essential oil |
| TPP | Sodium tripolyphosphate |
| MSSA | Methicillin-susceptible S. aureus |
| EDC | Ethylene carbo-di-imide hydrochloride |
| NHS | N-hydroxysuccinimide |
| MDR | Multidrug-resistant |
| EO | Essential oil |
| BNC | Bacterial nanocellulose |
| CA | Cellulose acetate |
| PM | Peppermint |
| CN | Cinnamon |
| LG | Lemongrass |
| CNFs | Cellulose nanofibrils |
| DCC | N, N’-dicyclohexylcarbodiimide |
| DMAP | 4-Dimethylaminopyridine |
| TC | Triphala Churna |
| MRSA | Methicillin-resistant S. aureus |
| CPE | Citrus peel extracts |
| ANCs | Anthocyanins |
| MD | Methylation degree |
| PAGE | Prunus armeniaca gum exudates |
| DL | D-limonene |
| GCN | Grapefruit seed extract and cinnamon oil |
| SPu | Succinylated pullulan |
| HA | Hyaluronic acid |
| OLE | Olive leaf extract |
| SF | Silk fibroin |
| TEA | Triethanolamine |
| DMTMM | 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride |
| 3-APBA∙HCl | 3-aminophenylboronic acid hydrochloride |
| DS | Degree of substitution |
| TONFC | 2,2,6,6-tetramethyl-1-piperidinyloxyl-oxidized nanofibrillated cellulose |
| HCNF | Holocellulose nanofibrils |
| DAC | Dialdehyde HCNF |
| HMP | High-methoxyl pectin |
| LMP | Low-methoxyl pectin |
| DE | Degree of esterification |
| HMPOS | High methoxy pectin oligo-saccharide |
| CMCS | Carboxymethyl chitosan |
| CMC | Carboxymethylcellulose |
| AMP | Antimicrobial peptide |
| CNCs | Cellulose nanocrystals |
| LNFs | Lysozyme nanofibers |
| TEMPO | 2,2,6,6-tetramethyl1-piperidinyloxy |
| STMP | Sodium trimetaphosphate |
| XG | Xanthan gum |
| GA | Gum arabic |
| CRG | κ-carrageenan |
| Ly | Lysozyme |
| LPO | Lactoperoxidase |
| LF | Lactoferrin |
| SBE-β-CD | Sulfobutylether-β-cyclodextrin |
| OVT | Ovotransferrin |
| OVTFs | Ovotransferrin fibrils |
| CP | Citrus pectin |
| SBP | Sugar beet pectin |
| HS-PEG-SH | Dithiol-functionalized poly (ethylene glycol) |
| OSA-HA | Octenyl succinic anhdride-modified hyaluronic acid |
References
- Guedes, B.N.; Krambeck, K.; Durazzo, A.; Lucarini, M.; Santini, A.; Oliveira, M.B.P.P.; Fathi, F.; Souto, E.B. Natural Antibiotics against Antimicrobial Resistance: Sources and Bioinspired Delivery Systems. Braz. J. Microbiol. 2024, 55, 2753–2766. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, S.; Jia, W.; Guo, T.; Wang, F.; Li, J.; Yao, Z. Natural Antimicrobials from Plants: Recent Advances and Future Prospects. Food Chem. 2024, 432, 137231. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.K. Nanobiotics against Antimicrobial Resistance: Harnessing the Power of Nanoscale Materials and Technologies. J. Nanobiotechnol. 2022, 20, 1–25. [Google Scholar] [CrossRef]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds With Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and Inorganic Nanomaterials: Fabrication, Properties and Applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial Nanoparticles: Current Landscape and Future Challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Popović, S.Z.; Lazić, V.L.; Hromiš, N.M.; Šuput, D.Z.; Bulut, S.N. Biopolymer Packaging Materials for Food Shelf-Life Prolongation. In Handbook of Food Bioengineering, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 20, pp. 223–277. [Google Scholar] [CrossRef]
- Râpă, M.; Popa, E.E. Biopolymers for Edible Films and Coatings in Food Applications. In Handbook of Biopolymers; Thomas, S., AR, A., Jose Chirayil, C., Thomas, B., Eds.; Springer: Singapore, 2023; pp. 1085–1115. [Google Scholar] [CrossRef]
- Zhen, N.; Wang, X.; Li, X.; Xue, J.; Zhao, Y.; Wu, M.; Zhou, D.; Liu, J.; Guo, J.; Zhang, H. Protein-Based Natural Antibacterial Materials and Their Applications in Food Preservation. Microb. Biotechnol. 2022, 15, 1324–1338. [Google Scholar] [CrossRef]
- Nunes, C.; Silva, M.; Farinha, D.; Sales, H.; Pontes, R.; Nunes, J. Edible Coatings and Future Trends in Active Food Packaging–Fruits’ and Traditional Sausages’ Shelf Life Increasing. Foods 2023, 12, 3308. [Google Scholar] [CrossRef]
- Devi, L.S.; Jaiswal, A.K.; Jaiswal, S. Lipid Incorporated Biopolymer Based Edible Films and Coatings in Food Packaging: A Review. Curr. Res. Food Sci. 2024, 8, 100720. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, P.R.; Oliveira-Fernandes, J.; Pereira-Dias, L.; Sousa, R.M.O.F.; Santos, C. Organic Nanoparticles as Delivery Tools for Bio-Based Antimicrobials. In Nanoparticles in Plant Biotic Stress Management; Khan, M., Chen, J.T., Eds.; Springer: Singapore, 2024; pp. 107–179. [Google Scholar] [CrossRef]
- Farzi, G.; Gheysipour, M. Encapsulation with Polymers. In Principles of Biomaterials Encapsulation; Sefat, F., Farzi, G., Mozafari, M., Eds.; Woodhead Publishing: Cambridge, UK, 2023; Volume 2, pp. 3–38. [Google Scholar] [CrossRef]
- Amgoth, C.; Phan, C.; Banavoth, M.; Rompivalasa, S.; Tang, G.; Amgoth, C.; Phan, C.; Banavoth, M.; Rompivalasa, S.; Tang, G. Polymer Properties: Functionalization and Surface Modified Nanoparticles. In Role of Novel Drug Delivery Vehicles in Nanobiomedicine; Tyagi, R.K., Garg, N., Shukla, R., Bisen, P.S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.; Park, K. PLA Micro- and Nano-Particles. Adv. Drug Deliv. Rev. 2016, 107, 176. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Gul, I.; Basharat, A.; Qamar, S.A. Polysaccharides-Based Bio-Nanostructures and Their Potential Food Applications. Int. J. Biol. Macromol. 2021, 176, 540–557. [Google Scholar] [CrossRef]
- Durán-Lara, E.F.; Marican, A.; Rafael, D.; Vijayakumar, S. Perspectives toward the Development of Advanced Materials Based on Bacterial Polysaccharides. Curr. Med. Chem. 2022, 30, 1963–1970. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of Polysaccharide Particle-Based Functional Drug Delivery. Carbohydr. Polym. 2019, 221, 94. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide Nanoparticles: From Fabrication to Applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- De Frates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef]
- Wang, X.F.; Chen, X.; Tang, Y.; Wu, J.M.; Qin, D.L.; Yu, L.; Yu, C.L.; Zhou, X.G.; Wu, A.G. The Therapeutic Potential of Plant Polysaccharides in Metabolic Diseases. Pharmaceuticals 2022, 15, 1329. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up Polymeric-Based Nanoparticles Drug Delivery Systems: Development and Challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-Status and Applications of Polysaccharides in Drug Delivery Systems. Colloid. Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-Based Nanoparticles as Drug Delivery Systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Gulraiz, Y.; Ilyas, S.; Bashir, S. Polysaccharide Based Nano Materials: Health Implications. Food Hydrocoll. Health 2022, 2, 100075. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Fabi, J.P. Polysaccharide-Based Nanotechnology Approaches to Deliver Bioactive Compounds for Food Applications. In Advances in Chemical Engineering; Mauri, E., Zhang, Z.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 62, pp. 215–256. [Google Scholar] [CrossRef]
- Młynek, M.; Trzciński, J.W.; Ciach, T. Recent Advances in the Polish Research on Polysaccharide-Based Nanoparticles in the Context of Various Administration Routes. Biomedicines 2023, 11, 1307. [Google Scholar] [CrossRef]
- Shokrani, H.; Shokrani, A.; Sajadi, S.M.; Khodadadi Yazdi, M.; Seidi, F.; Jouyandeh, M.; Zarrintaj, P.; Kar, S.; Kim, S.J.; Kuang, T.; et al. Polysaccharide-Based Nanocomposites for Biomedical Applications: A Critical Review. Nanoscale Horiz. 2022, 7, 1136–1160. [Google Scholar] [CrossRef]
- Fan, X.; Ngo, H.; Wu, C. Natural and Bio-Based Antimicrobials: A Review. ACS Symp. Ser. 2018, 1287, 1–24. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Temane, L.T.; Kesavan Pillai, S.; Ray, S.S. Advancements in Antimicrobial Textiles: Fabrication, Mechanisms of Action, and Applications. ACS Omega 2025, 10, 12772–12816. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-Loaded Nanocarriers for Food Packaging Applications. Adv. Colloid. Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Singh, V.; Malviya, T.; Shehala; Gupta, S.; Dwivedi, L.M.; Baranwal, K.; Prabha, M.; Aayushee. Polysaccharide-Based Nanoparticles: Nanocarriers for Sustained Delivery of Drugs. In Advanced Biopolymeric Systems for Drug Delivery. Advances in Material Research and Technology; Nayak, A., Hasnain, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 151–181. [Google Scholar] [CrossRef]
- Bushra, R.; Ahmad, M.; Seidi, F.; Qurtulen; Song, J.; Jin, Y.; Xiao, H. Polysaccharide-Based Nanoassemblies: From Synthesis Methodologies and Industrial Applications to Future Prospects. Adv. Colloid. Interface Sci. 2023, 318, 102953. [Google Scholar] [CrossRef]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef]
- Pawar, R.; Jadhav, W.; Bhusare, S.; Borade, R.; Farber, S.; Itzkowitz, D.; Domb, A. Polysaccharides as Carriers of Bioactive Agents for Medical Applications. In Natural-Based Polymers for Biomedical Applications, 1st ed.; Reis, R.L., Neves, N.M., Mano, J.F., Gomes, M.E., Marques, A.P., Azevedo, H.S., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 3–53. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Wu, Y.; Dai, F.; Yuan, M.; Wang, F.; Bai, Y.; Deng, H. Natural Polysaccharides Based Self-Assembled Nanoparticles for Biomedical Applications—A Review. Int. J. Biol. Macromol. 2021, 192, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 535734. [Google Scholar] [CrossRef]
- Visan, A.I.; Cristescu, R. Polysaccharide-Based Coatings as Drug Delivery Systems. Pharmaceutics 2023, 15, 2227. [Google Scholar] [CrossRef]
- Zhang, J.; Zhan, P.; Tian, H. Recent Updates in the Polysaccharides-Based Nano-Biocarriers for Drugs Delivery and Its Application in Diseases Treatment: A Review. Int. J. Biol. Macromol. 2021, 182, 115–128. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2021, 10, 1097–1123. [Google Scholar] [CrossRef]
- Peniche Covas, C.A.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133. [Google Scholar] [CrossRef]
- Zannat, A.; Shamshina, J.L. Chitin Isolation from Crustaceans and Mushrooms: The Need for Quantitative Assessment. Carbohydr. Polym. 2025, 348, 122882. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and Challenges in the Use of Chitosan and Its Derivatives in Biomedical Fields: A Review. Carbohydr. Polym. Technol. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Namli, S.; Guven, O.; Simsek, F.N.; Gradišek, A.; Sumnu, G.; Yener, M.E.; Oztop, M. Effects of Deacetylation Degree of Chitosan on the Structure of Aerogels. Int. J. Biol. Macromol. 2023, 250, 126123. [Google Scholar] [CrossRef] [PubMed]
- Mania, S.; Banach-Kopeć, A.; Staszczyk, K.; Kulesza, J.; Augustin, E.; Tylingo, R. An Influence of Molecular Weight, Deacetylation Degree of Chitosan Xerogels on Their Antimicrobial Activity and Cytotoxicity. Comparison of Chitosan Materials Obtained Using Lactic Acid and CO2 Saturation. Carbohydr. Res. 2023, 534, 108973. [Google Scholar] [CrossRef]
- Pandit, A.; Indurkar, A.; Deshpande, C.; Jain, R.; Dandekar, P. A Systematic Review of Physical Techniques for Chitosan Degradation. Carbohydr. Polym. Technol. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation Methods and Applications of Chitosan Nanoparticles; with an Outlook toward Reinforcement of Biodegradable Packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Alemu, D.; Getachew, E.; Mondal, A.K. Study on the Physicochemical Properties of Chitosan and Their Applications in the Biomedical Sector. Int. J. Polym. Sci. 2023, 2023, 5025341. [Google Scholar] [CrossRef]
- Goyal, S.; Thirumal, D.; Rana, J.; Gupta, A.K.; Kumar, A.; Babu, M.A.; Kumar, P.; Sindhu, R.K. Chitosan Based Nanocarriers as a Promising Tool in Treatment and Management of Inflammatory Diseases. Carbohydr. Polym. Technol. 2024, 7, 100442. [Google Scholar] [CrossRef]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Jameel, E.; Mohanta, B.; Dhara, A.K.; Alkahtani, S.; Nayak, A.K. Alginates: Sources, Structure, and Properties. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–17. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106. [Google Scholar] [CrossRef] [PubMed]
- Qosim, N.; Dai, Y.; Williams, G.R.; Edirisinghe, M. Structure, Properties, Forming, and Applications of Alginate Fibers: A Review. Int. Mater. Rev. 2024, 69, 309–333. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Kumar, B.; Singh, N.; Kumar, P. A Review on Sources, Modification Techniques, Properties and Potential Applications of Alginate-Based Modified Polymers. Eur. Polym. J. 2024, 213, 113078. [Google Scholar] [CrossRef]
- Bojorges, H.; López-Rubio, A.; Martínez-Abad, A.; Fabra, M.J. Overview of Alginate Extraction Processes: Impact on Alginate Molecular Structure and Techno-Functional Properties. Trends Food Sci. Technol. 2023, 140, 104142. [Google Scholar] [CrossRef]
- Karim, A.; Rehman, A.; Feng, J.; Noreen, A.; Assadpour, E.; Kharazmi, M.S.; Lianfu, Z.; Jafari, S.M. Alginate-Based Nanocarriers for the Delivery and Controlled-Release of Bioactive Compounds. Adv. Colloid. Interface Sci. 2022, 307, 102744. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, Physical and Biological Properties of Alginates and Their Biomedical Implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Lai, J.; Azad, A.K.; Sulaiman, W.M.A.W.; Kumarasamy, V.; Subramaniyan, V.; Alshehade, S.A. Alginate-Based Encapsulation Fabrication Technique for Drug Delivery: An Updated Review of Particle Type, Formulation Technique, Pharmaceutical Ingredient, and Targeted Delivery System. Pharmaceutics 2024, 16, 370. [Google Scholar] [CrossRef]
- Lakkakula, J.; Roy, A.; Krishnamoorthy, K.; Alghamdi, S.; Almehmadi, M.; Gujarathi, P.; Pansare, P.; Allahyani, M.; Abdulaziz, O.; Velhal, K.; et al. Alginate-Based Nanosystems for Therapeutic Applications. J. Nanomater. 2022, 2022, 6182815. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.T.; Morgan, J.L.W.; Zimmer, J. A Molecular Description of Cellulose Biosynthesis. Annu. Rev. Biochem. 2015, 84, 895. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, E.O. Molecular Weights of Celluloses. Ind. Eng. Chem. 1938, 30, 1200–1203. [Google Scholar] [CrossRef]
- McNeice, P.; ten Brink, G.H.; Gran, U.; Karlson, L.; Edvinsson, R.; Feringa, B.L. Cellulose Modification for Sustainable Polymers: Overcoming Problems of Solubility and Processing. RSC Sustain. 2024, 2, 369–376. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Rani, M.S.A.; Mohammad, M.; Sainorudin, M.H.; Asim, N.; Yaakob, Z.; Razali, H.; Emdadi, Z. Nanocellulose from Agricultural Waste as an Emerging Material for Nanotechnology Applications—An Overview. Polimery 2021, 66, 157–168. [Google Scholar] [CrossRef]
- Chopra, L. Manikanika Extraction of Cellulosic Fibers from the Natural Resources: A Short Review. Mater. Today Proc. 2022, 48, 1265–1270. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, H.; Vignolini, S. Recent Progress in Production Methods for Cellulose Nanocrystals: Leading to More Sustainable Processes. Adv. Sustain. Syst. 2022, 6, 2100100. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, N.; Munagala, M.; Rajkhowa, R.; Aallardyce, B.; Shastri, Y.; Agrawal, R. Nanocellulose: Resources, Physio-Chemical Properties, Current Uses and Future Applications. Front. Nanotechnol. 2021, 3, 747329. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kamalesh, T.; Kumar, P.S.; Hemavathy, R.V.; Rangasamy, G. A Critical Review on Sustainable Cellulose Materials and Its Multifaceted Applications. Ind. Crops Prod. 2023, 203, 117221. [Google Scholar] [CrossRef]
- Pompêu, G.C.S.; Pasquini, D. Extraction of Lignin and Modifications. In Handbook of Biomass; Thomas, S., Hosur, M., Pasquini, D., Jose Chirayil, C., Eds.; Springer: Singapore, 2024; pp. 575–609. [Google Scholar] [CrossRef]
- Jincy, E.M.; Femina, K.S. Heteropolymer in Biomass: Hemicellulose Extraction and Modifications. In Handbook of Biomass; Thomas, S., Hosur, M., Pasquini, D., Jose Chirayil, C., Eds.; Springer: Singapore, 2024; pp. 665–696. [Google Scholar] [CrossRef]
- Spiridon, I.; Popa, V.I. Hemicelluloses: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 289–304. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M.N. The State of the Art of Polymers from Renewable Resources. In Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; Ebnesajjad, S., Ed.; William Andrew: Norwich, NY, USA, 2013; pp. 71–85. [Google Scholar] [CrossRef]
- Kobetičová, K.; Nábělková, J. Effect of Wood Hemicellulose Composition on Binding Interactions with Caffeine. Buildings 2021, 11, 515. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic Liquid Based Pretreatment of Lignocellulosic Biomass for Enhanced Bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant Celluloses, Hemicelluloses, Lignins, and Volatile Oils for the Synthesis of Nanoparticles and Nanostructured Materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J. The Hierarchical Structure and Mechanics of Plant Materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Bueno Moron, J.; van Klink, G.P.M.; Gruter, G.J.M. Lignocellulose Saccharification: Historical Insights and Recent Industrial Advancements towards 2nd Generation Sugars. RSC Sustain. 2025, 3, 1170–1211. [Google Scholar] [CrossRef]
- Huang, L.Z.; Ma, M.G.; Ji, X.X.; Choi, S.E.; Si, C. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9, 690773. [Google Scholar] [CrossRef]
- Ma, M.; Ji, X. Extraction, Purification, and Applications of Hemicellulose. Pap. Biomater. 2021, 6, 47–60. [Google Scholar]
- Mathura, S.R.; Landázuri, A.C.; Mathura, F.; Andrade Sosa, A.G.; Orejuela-Escobar, L.M. Hemicelluloses from Bioresidues and Their Applications in the Food Industry—Towards an Advanced Bioeconomy and a Sustainable Global Value Chain of Chemicals and Materials. Sustain. Food Technol. 2024, 2, 1183–1205. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, M.; Wang, D.; Zhao, D.; Wang, M. Advances in Extraction, Purification, Structural Characteristics and Biological Activities of Hemicelluloses: A Review. Int. J. Biol. Macromol. 2023, 225, 467–483. [Google Scholar] [CrossRef]
- Sandhya, P.V. Nano-Hemicellulose. In Handbook of Biomass; Thomas, S., Hosur, M., Pasquini, D., Jose Chirayil, C., Eds.; Springer: Singapore, 2024; pp. 729–772. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A Review of Starch, a Unique Biopolymer—Structure, Metabolism and in Planta Modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding Starch Structure: Recent Progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Seung, D. Amylose in Starch: Towards an Understanding of Biosynthesis, Structure and Function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.C.; Åman, P. The Influence of Amylose and Amylopectin Characteristics on Gelatinization and Retrogradation Properties of Different Starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Varghese, S.; Awana, M.; Mondal, D.; Rubiya, M.H.; Melethil, K.; Singh, A.; Krishnan, V.; Thomas, B. Amylose–Amylopectin Ratio. In Handbook of Biopolymers; Thomas, S., AR, A., Jose Chirayil, C., Thomas, B., Eds.; Springer: Singapore, 2022; pp. 1–30. [Google Scholar] [CrossRef]
- Hermiati, E.; Sondari, D.; Sunarti, T.C. Extraction and Classification of Starch from Different Sources: Structure, Properties, and Characterization. In Handbook of Natural Polymers, Volume 1: Sources, Synthesis, and Characterization; Sreekala, M.S., Goda, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 19–60. [Google Scholar] [CrossRef]
- Burrell, M.M. Starch: The Need for Improved Quality or Quantity—An Overview. J. Exp. Bot. 2003, 54, 451–456. [Google Scholar] [CrossRef]
- Dorantes-Fuertes, M.G.; López-Méndez, M.C.; Martínez-Castellanos, G.; Meléndez-Armenta, R.Á.; Jiménez-Martínez, H.E. Starch Extraction Methods in Tubers and Roots: A Systematic Review. Agronomy 2024, 14, 865. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Qian, Y.; Wang, X.; Zhang, S.; Chang, J.; Ruan, Y.; Ma, B. Influences of Extraction Methods on Physicochemical and Functional Characteristics of Three New Bulbil Starches from Dioscorea opposita Thunb. cv. Tiegun. Molecules 2019, 24, 2232. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Ahmad, S.W.; Ahmad, S.; Qutab, H.G.; Dasih, M.; Imran, M. Enzymatic Extraction of Potato Starch: A Parametric Optimization Study Using Response Surface Methodology. Pol. J. Chem. Technol. 2020, 22, 48–54. [Google Scholar] [CrossRef]
- Li, M.; Tian, Y.; Dhital, S. Starch Extraction: Principles and Techniques. In Starch and Starchy Food Products; Bello-Pérez, L., Alvarez-Ramírez, J., Dhital, S., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 17–40. [Google Scholar] [CrossRef]
- Mgoma, S.T.; Basitere, M.; Mshayisa, V.V.; Jager, D. De A Systematic Review on Sustainable Extraction, Preservation, and Enhancement in Food Processing: The Advancement from Conventional to Green Technology Through Ultrasound. Processes 2025, 13, 965. [Google Scholar] [CrossRef]
- Pasumarthi, P.; Sindhu, S.; Manickavasagan, A. Ultrasound-Assisted Extraction and Modification of Pulse Starches: A Review. Cereal Chem. 2025, 102, 266–289. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ferreira, M.S.; Magalhães, M.T.; Santos, W.J.; Oliveira, J.C.; Silva, J.B.A.; José, N.M. Mechanical, Thermal and Barrier Properties of Starch-Based Films Plasticized with Glycerol and Lignin and Reinforced with Cellulose Nanocrystals. Mater. Today Proc. 2015, 2, 63–69. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M. Mechanical, Physical and Thermal Properties of Sugar Palm Nanocellulose Reinforced Thermoplastic Starch (TPS)/Poly (Lactic Acid) (PLA) Blend Bionanocomposites. Polymers 2020, 12, 2216. [Google Scholar] [CrossRef]
- Compart, J.; Singh, A.; Fettke, J.; Apriyanto, A. Customizing Starch Properties: A Review of Starch Modifications and Their Applications. Polymers 2023, 15, 3491. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Younis, H.A.; Abdelshafy, A.M.; Osman, A.I.; Eletmany, M.R.; Hafouda, M.A.; Chen, W. Plant Starch Extraction, Modification, and Green Applications: A Review. Environ. Chem. Lett. 2024, 22, 2483–2530. [Google Scholar] [CrossRef]
- Marta, H.; Rizki, D.I.; Mardawati, E.; Djali, M.; Mohammad, M.; Cahyana, Y. Starch Nanoparticles: Preparation, Properties and Applications. Polymers 2023, 15, 1167. [Google Scholar] [CrossRef]
- Hassan, N.A.; Darwesh, O.M.; Smuda, S.S.; Altemimi, A.B.; Hu, A.; Cacciola, F.; Haoujar, I.; Abedelmaksoud, T.G. Recent Trends in the Preparation of Nano-Starch Particles. Molecules 2022, 27, 5497. [Google Scholar] [CrossRef]
- Campelo, P.H.; Sant’Ana, A.S.; Pedrosa Silva Clerici, M.T. Starch Nanoparticles: Production Methods, Structure, and Properties for Food Applications. Curr. Opin. Food Sci. 2020, 33, 136–140. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, S.S.; Lim, S.T. Preparation, Characterization and Utilization of Starch Nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef]
- Sreya, E.S.; Kumar, D.P.; Sreya, P.S.; Baby, A.; Balakrishnan, P.; Gopi, S.; Antony, N. Nano Starch: A Review. In Handbook of Biomass; Thomas, S., Hosur, M., Pasquini, D., Jose Chirayil, C., Eds.; Springer: Singapore, 2024; pp. 855–874. [Google Scholar] [CrossRef]
- Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.u.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef] [PubMed]
- Akdaşçi, E.; Duman, H.; Eker, F.; Bechelany, M.; Karav, S. Chitosan and Its Nanoparticles: A Multifaceted Approach to Antibacterial Applications. Nanomaterials 2025, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Alginate-Based Micro- and Nanosystems for Targeted Cancer Therapy. Mar. Drugs 2022, 20, 598. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.I.; Alshaer, W.; Hatmal, M.M.; Lambuk, L.; Ahmed, N.; Mustafa, M.Z.; Low, S.C.; Jaafar, J.; Ferji, K.; Six, J.L.; et al. Applications of Alginate-Based Nanomaterials in Enhancing the Therapeutic Effects of Bee Products. Front. Mol. Biosci. 2022, 9, 865833. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef]
- Phuong, H.T.; Thoa, N.K.; Tuyet, P.T.A.; Van, Q.N.; Hai, Y.D. Cellulose Nanomaterials as a Future, Sustainable and Renewable Material. Crystals 2022, 12, 106. [Google Scholar] [CrossRef]
- Hamawand, I.; Seneweera, S.; Kumarasinghe, P.; Bundschuh, J. Nanoparticle Technology for Separation of Cellulose, Hemicellulose and Lignin Nanoparticles from Lignocellulose Biomass: A Short Review. Nano-Struct. Nano-Obj. 2020, 24, 100601. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Ismadji, S.; Gunawan, S. A Review of Lignocellulosic-Derived Nanoparticles for Drug Delivery Applications: Lignin Nanoparticles, Xylan Nanoparticles, and Cellulose Nanocrystals. Molecules 2021, 26, 676. [Google Scholar] [CrossRef]
- Hassan, N.H.; El-Hawary, S.S.; Emam, M.; Rabeh, M.A.; Tantawy, M.A.; Seif, M.; Abd-Elal, R.M.A.; Bringmann, G.; Abdelmohsen, U.R.; Selim, N.M. Pectin Nanoparticle-Loaded Soft Coral Nephthea Sp. Extract as In Situ Gel Enhances Chronic Wound Healing: In Vitro, In Vivo, and In Silico Studies. Pharmaceuticals 2023, 16, 957. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhou, Z.Q. Synthesis and Applications of Pectin-Based Nanomaterials. Curr. Nanosci. 2015, 12, 103–109. [Google Scholar] [CrossRef]
- Opanasopit, P.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Ruktanonchai, U. Development and Characterization of Pectinate Micro/Nanoparticles for Gene Delivery. AAPS PharmSciTech 2008, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Wadhwa, A.; Kumar, V. Pectin-Based Nanomaterials: Synthesis, Toxicity and Applications: A Review. Asian J. Chem. 2021, 33, 2579–2588. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Wang, L.; Goksen, G.; Shao, P. Multifunctional Pectin Films Based on Mussel-Inspired Modified 2D Ag Nanosheets for Long-Lasting Antibacterial and Enhanced Barrier Properties. Food Hydrocoll. 2023, 137, 108331. [Google Scholar] [CrossRef]
- Ren, W.; Qiang, T.; Chen, L. Recyclable and Biodegradable Pectin-Based Film with High Mechanical Strength. Food Hydrocoll. 2022, 129, 107643. [Google Scholar] [CrossRef]
- Iravani, S. Plant Gums for Sustainable and Eco-Friendly Synthesis of Nanoparticles: Recent Advances. Inor Nano-Met. Chem. 2020, 50, 469–488. [Google Scholar] [CrossRef]
- Shakya, S.; Tiwari, R.K.; Gupta, A. Gum-Based Nanoparticles Targeting for Colon Rectal Cancer: Latest Research and Patents. Recent. Adv. Drug Deliv. Formul. 2023, 17, 255–263. [Google Scholar] [CrossRef]
- Taheri, A.; Jafari, S.M. Gum-Based Nanocarriers for the Protection and Delivery of Food Bioactive Compounds. Adv. Colloid. Interface Sci. 2019, 269, 277–295. [Google Scholar] [CrossRef]
- Shiam, M.A.H.; Islam, M.S.; Ahmad, I.; Haque, S.S. A Review of Plant-Derived Gums and Mucilages: Structural Chemistry, Film Forming Properties and Application. J. Plast. Film Sheeting 2025, 41, 195–237. [Google Scholar] [CrossRef]
- Manna, S.; Jana, S. Carrageenan-Based Nanomaterials in Drug Delivery Applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Bera, H., Hossain, C.M., Saha, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 365–382. [Google Scholar] [CrossRef]
- Nogueira, L.F.B.; Cruz, M.A.E.; Tovani, C.B.; Lopes, H.B.; Beloti, M.M.; Ciancaglini, P.; Bottini, M.; Ramos, A.P. Curcumin-Loaded Carrageenan Nanoparticles: Fabrication, Characterization, and Assessment of the Effects on Osteoblasts Mineralization. Colloids Surf. B Biointerfaces 2022, 217, 112622. [Google Scholar] [CrossRef]
- Das, N.; Kumar, A.; Rayavarapu, R.G. The Role of Deep Eutectic Solvents and Carrageenan in Synthesizing Biocompatible Anisotropic Metal Nanoparticles. Beilstein J. Nanotechnol. 2021, 12, 924. [Google Scholar] [CrossRef]
- Khan, A.K.; Saba, A.U.; Nawazish, S.; Akhtar, F.; Rashid, R.; Mir, S.; Nasir, B.; Iqbal, F.; Afzal, S.; Pervaiz, F.; et al. Carrageenan Based Bionanocomposites as Drug Delivery Tool with Special Emphasis on the Influence of Ferromagnetic Nanoparticles. Oxid. Med. Cell Longev. 2017, 2017, 8158315. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A Review on Synthesis, Properties and Applications of Natural Polymer Based Carrageenan Blends and Composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Tobacman, J.K. Review of Harmful Gastrointestinal Effects of Carrageenan in Animal Experiments. Environ. Health Perspect. 2001, 109, 983. [Google Scholar] [CrossRef]
- Pradhan, B.; Ki, J.S. Biological Activity of Algal Derived Carrageenan: A Comprehensive Review in Light of Human Health and Disease. Int. J. Biol. Macromol. 2023, 238, 124085. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A Review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Guan, J.; Li, L.; Mao, S. Applications of Carrageenan in Advanced Drug Delivery. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications; Venkatesan, J., Anil, S., Kim, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 283–303. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Haghi, M.; MacLoughlin, R.; Chellappan, D.K.; Gupta, G.; Paudel, K.R.; Hansbro, P.M.; George Oliver, B.G.; et al. Advances and Applications of Dextran-Based Nanomaterials Targeting Inflammatory Respiratory Diseases. J. Drug Deliv. Sci. Technol. 2022, 74, 103598. [Google Scholar] [CrossRef]
- Banerjee, A.; Bandopadhyay, R. Use of Dextran Nanoparticle: A Paradigm Shift in Bacterial Exopolysaccharide Based Biomedical Applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef]
- Ukkund, S.J.; Alke, B.; Taqui, S.N.; Syed, U.T. Dextran Nanoparticles: Preparation and Applications. In Polysaccharide Nanoparticles: Preparation and Biomedical Applications; Venkatesan, J., Kim, S., Anil, S., D, R.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–31. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Pinteala, M.; Simionescu, N. Dextran Formulations as Effective Delivery Systems of Therapeutic Agents. Molecules 2023, 28, 1086. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.; Huang, H. Preparation and Application of Dextran and Its Derivatives as Carriers. Int. J. Biol. Macromol. 2020, 145, 827–834. [Google Scholar] [CrossRef]
- Ahmed, H.B.; Emam, H.E. Pullulan in the Delivery of Therapeutics. In Polysaccharide-Based Biomaterials: Delivery of Therapeutics and Biomedical Applications; Jana, S., Jana, S., Domb, A.J., Eds.; RSC Publishing: Cambridge, UK, 2022; pp. 299–330. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Barbu, E.; Liew, K. Bin Hydrophobically Grafted Pullulan Nanocarriers for Percutaneous Delivery: Preparation and Preliminary in Vitro Characterisation. Polymers 2021, 13, 2852. [Google Scholar] [CrossRef]
- Ganie, S.A.; Rather, L.J.; Li, Q. A Review on Anticancer Applications of Pullulan and Pullulan Derivative Nanoparticles. Carbohydr. Polym. Technol. 2021, 2, 100115. [Google Scholar] [CrossRef]
- Dionísio, M.; Braz, L.; Corvo, M.; Lourenço, J.P.; Grenha, A.; Rosa da Costa, A.M. Charged Pullulan Derivatives for the Development of Nanocarriers by Polyelectrolyte Complexation. Int. J. Biol. Macromol. 2016, 86, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.J.; Xie, Y.C.; Zhang, Q.F.; Qiu, X.M.; Yuan, L.M.; Wen, Y.; Li, M.; Yang, X.P.; Tao, T.; Xie, M.H.; et al. Cholesterol-Modified Amino-Pullulan Nanoparticles as a Drug Carrier: Comparative Study of Cholesterol-Modified Carboxyethyl Pullulan and Pullulan Nanoparticles. Nanomaterials 2016, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Rodrigues, S. Pullulan-Based Nanoparticles: Future Therapeutic Applications in Transmucosal Protein Delivery. Ther. Deliv. 2013, 4, 1339–1341. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Shen, Z.; Hongtao, Z.; Xiaobei, Z. Arginine-Carboxylated Pullulan, a Potential Antibacterial Material for Food Packaging. Biomater. Adv. 2023, 154, 213584. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, S.; Naman, S.; Baldi, A. Pullulan Based Polymeric Novel Drug Delivery Systems: A Review on Current State of Art and Prospects. J. Drug Deliv. Sci. Technol. 2023, 90, 105117. [Google Scholar] [CrossRef]
- Castle, L.; Andreassen, M.; Aquilina, G.; Bastos, M.L.; Boon, P.; Fallico, B.; Fitzgerald, R.; Frutos Fernandez, M.J.; Grasl-Kraupp, B.; Gundert-Remy, U.; et al. Re-evaluation of Pullulan (E 1204) as a Food Additive and New Application for Its Extension of Use. EFSA J. 2025, 23, e9267. [Google Scholar] [CrossRef]
- Rao, N.V.; Rho, J.G.; Um, W.; Ek, P.K.; Nguyen, V.Q.; Oh, B.H.; Kim, W.; Park, J.H. Hyaluronic Acid Nanoparticles as Nanomedicine for Treatment of Inflammatory Diseases. Pharmaceutics 2020, 12, 931. [Google Scholar] [CrossRef]
- Lee, W.H.; Rho, J.G.; Yang, Y.; Lee, S.; Kweon, S.; Kim, H.M.; Yoon, J.; Choi, H.; Lee, E.; Kim, S.H.; et al. Hyaluronic Acid Nanoparticles as a Topical Agent for Treating Psoriasis. ACS Nano 2022, 16, 20057–20074. [Google Scholar] [CrossRef]
- Matějková, N.; Korecká, L.; Šálek, P.; Kočková, O.; Pavlova, E.; Kašparová, J.; Obořilová, R.; Farka, Z.; Frolich, K.; Adam, M.; et al. Hyaluronic Acid Nanoparticles with Parameters Required for In Vivo Applications: From Synthesis to Parametrization. Biomacromolecules 2024, 25, 4934–4945. [Google Scholar] [CrossRef]
- Matalqah, S.; Lafi, Z.; Asha, S.Y. Hyaluronic Acid in Nanopharmaceuticals: An Overview. Curr. Issues Mol. Biol. 2024, 46, 10444–10461. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V. Hyaluronic Acid Nanoparticles. In Biopolymeric Nanomaterials: Fundamentals and Applications; Kanwar, S., Kumar, A., Nguyen, T.A., Sharma, S., Slimani, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 155–171. [Google Scholar] [CrossRef]
- Alipoor, R.; Ayan, M.; Hamblin, M.R.; Ranjbar, R.; Rashki, S. Hyaluronic Acid-Based Nanomaterials as a New Approach to the Treatment and Prevention of Bacterial Infections. Front. Bioeng. Biotechnol. 2022, 10, 913912. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, N.I.; Al-Mahallawi, A.M.; Ahmed, I.S.; Shamma, R.N. Pectin Nanoparticles Loaded with Nitric Oxide Donor Drug: A Potential Approach for Tissue Regeneration. Int. J. Pharm. X 2024, 7, 100244. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Xu, Y.; Gao, S.; Xu, Q.; Gong, H.; Yao, L.; Shi, S.; Wang, S.; Wang, H.; et al. Structural Characterization of a Pectic Polysaccharide from Rubus Chingii Hu. Unripe Fruits and Its Efficacy in Inhibiting Intestinal Lipid Absorption In Vivo. Carbohydr. Polym. 2025, 363, 123728. [Google Scholar] [CrossRef]
- Roman-Benn, A.; Contador, C.A.; Li, M.W.; Lam, H.M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An Overview of Sources, Extraction and Applications in Food Products, Biomedical, Pharmaceutical and Environmental Issues. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Riyamol, N.; Gada Chengaiyan, J.; Rana, S.S.; Ahmad, F.; Haque, S.; Capanoglu, E. Recent Advances in the Extraction of Pectin from Various Sources and Industrial Applications. ACS Omega 2023, 8, 46309–46324. [Google Scholar] [CrossRef]
- Sultana, N. Biological Properties and Biomedical Applications of Pectin and Pectin-Based Composites: A Review. Molecules 2023, 28, 7974. [Google Scholar] [CrossRef]
- Rajabzadeh-Khosroshahi, M.; Khoshfetrat, A.B.; Salami-Kalajahi, M. A Review on Pectin-Based Nanostructures for Drug and Gene Delivery Systems. Int. J. Biol. Macromol. 2025, 304, 140932. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Amid, B.T. A Review Study on Chemical Composition and Molecular Structure of Newly Plant Gum Exudates and Seed Gums. Food Res. Int. 2012, 46, 387–398. [Google Scholar] [CrossRef]
- Chee, B.S.; Nugent, M. Electrospun Natural Polysaccharide for Biomedical Application. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 589–615. [Google Scholar] [CrossRef]
- Nandi, G.; Hasnain, M.S.; Nayak, A.K. Polysaccharide-Based Polyelectrolyte Complex Systems in Drug Delivery. In Tailor-Made Polysaccharides in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 177–210. [Google Scholar] [CrossRef]
- Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dilillo, D.; Bosetti, A.; Zuccotti, G.V.; D’auria, E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients 2021, 13, 3402. [Google Scholar] [CrossRef] [PubMed]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan From Kappaphycus Alvarezii (Rhodophyta, Solieriaceae): Metabolism, Structure, Production, and Application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E.; Lukova, P.; Pierre, G. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Walter, R.H. Classifications. In Polysaccharide Dispersions; Walter, R.H., Ed.; Academic Press: Cambridge, MA, USA, 1998; pp. 157–180. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Yáñez-Fernández, J.; Castro-Muñoz, R. Microfiltration-Mediated Extraction of Dextran Produced by Leuconostoc mesenteroides SF3. Food Bioprod. Process 2020, 119, 317–328. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Pasupathi, S.; Karuppiah, S. Influence of Deep-Eutectic and Organic Solvents on the Recovery, Molecular Mass, and Functional Properties of Dextran: Application Using Dextran Film. Int. J. Biol. Macromol. 2025, 293, 139202. [Google Scholar] [CrossRef]
- Strbak, O.; Antal, I.; Khmara, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Molcan, M.; Jurikova, A.; Hnilicova, P.; Gombos, J.; et al. Influence of Dextran Molecular Weight on the Physical Properties of Magnetic Nanoparticles for Hyperthermia and MRI Applications. Nanomaterials 2020, 10, 2468. [Google Scholar] [CrossRef]
- Yan, C.; Ding, C.; Cheng, C.; Gong, Y.; Han, S.; Xia, Z.; Kang, D. A Functional Analysis of the Effects of the Molecular Weight of Dextran on ε-Polylysine-Dextran Conjugate Created through the Maillard Reaction. Food Chem. 2022, 390, 133212. [Google Scholar] [CrossRef]
- Wasiak, I.; Kulikowska, A.; Janczewska, M.; Michalak, M.; Cymerman, I.A.; Nagalski, A.; Kallinger, P.; Szymanski, W.W.; Ciach, T. Dextran Nanoparticle Synthesis and Properties. PLoS ONE 2016, 11, e0146237. [Google Scholar] [CrossRef]
- Kabil, M.F.; Abo Dena, A.S.; El-Sherbiny, I.M. Pullulan-Based Nanocarriers for Therapeutic Applications. In Polymeric Nanosystems: Theranostic Nanosystems: Volume 1; Hasnain, M.S., Nayak, A.K., Aminabhavi, T.M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 309–331. [Google Scholar] [CrossRef]
- Lochhead, R.Y. The Use of Polymers in Cosmetic Products. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–221. [Google Scholar] [CrossRef]
- Jiang, T.; Singh, B.; Choi, Y.J.; Akaike, T.; Cho, C.S. Liver Tissue Engineering Using Functional Marine Biomaterials. In Functional Marine Biomaterials: Properties and Applications; Kim, S., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 91–106. [Google Scholar] [CrossRef]
- Aquinas, N.; Chithra, C.H.; Bhat, M.R. Progress in Bioproduction, Characterization and Applications of Pullulan: A Review. Polym. Bull. 2024, 81, 12347–12382. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G.; Kontogiorgos, V. Molecular Weight Effects on Solution Rheology of Pullulan and Mechanical Properties of Its Films. Carbohydr. Polym. 2003, 52, 151–166. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Singh, D.; Bajaj, B.K.; Kennedy, J.F. Downstream Processing and Structural Confirmation of Pullulan—A Comprehensive Review. Int. J. Biol. Macromol. 2022, 208, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, M.; Nathan, S.S.; Sasikumar, P.; Ramkumar, L.; Navaneethan, D.; Prabu, P.; Anjalin, F.M.; Dharamarj, N.; Alqahtani, M.S.; Abbas, M. Review on Applications of Pullulan in Bone Tissue Engineering: Blends and Composites with Natural and Synthetic Polymers. Polym. Polym. Compos. 2023, 31, 1–13. [Google Scholar] [CrossRef]
- Rodriguez-marquez, C.D.; Arteaga-marin, S.; Rivas-sánchez, A.; Autrique-hernández, R.; Castro-muñoz, R. A Review on Current Strategies for Extraction and Purification of Hyaluronic Acid. Int. J. Mol. Sci. 2022, 23, 6038. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable Nanoparticles Are Excellent Vehicle for Site Directed In-Vivo Delivery of Drugs and Vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef]
- Myrick, J.M.; Vendra, V.K.; Krishnan, S. Self-Assembled Polysaccharide Nanostructures for Controlled-Release Applications. Nanotechnol. Rev. 2014, 3, 319–346. [Google Scholar] [CrossRef]
- Tomasik, P. Chemical Modification of Polysaccharides. Int. Sch. Res. Notices 2013, 2013, 417672. [Google Scholar] [CrossRef]
- Elzayat, A.M.; Adam-Cervera, I.; Albus, M.; Cháfer, A.; Badia, J.D.; Pérez-Pla, F.F.; Muñoz-Espí, R. Polysaccharide/Silica Microcapsules Prepared via Ionic Gelation Combined with Spray Drying: Application in the Release of Hydrophilic Substances and Catalysis. Polymers 2023, 15, 4116. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Seaweed Polysaccharide-Based Nanoparticles: Preparation and Applications for Drug Delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef]
- Wang, H.; Deng, H.; Gao, M.; Zhang, W. Self-Assembled Nanogels Based on Ionic Gelation of Natural Polysaccharides for Drug Delivery. Front. Bioeng. Biotechnol. 2021, 9, 703559. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ng, W.K.; Shen, S.; Kim, S.; Tan, R.B.H. Scalable Ionic Gelation Synthesis of Chitosan Nanoparticles for Drug Delivery in Static Mixers. Carbohydr. Polym. 2013, 94, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.S.; Nascimento, J.A.d.A.; Britto, D. de Scale-up in the Synthesis of Nanoparticles for Encapsulation of Agroindustrial Active Principles. Ciência e Agrotecnologia 2020, 43, e023819. [Google Scholar] [CrossRef]
- Zheng, J.; Van der Meeren, P.; Sun, W. New Insights into Protein–Polysaccharide Complex Coacervation: Dynamics, Molecular Parameters, and Applications. Aggregate 2024, 5, e449. [Google Scholar] [CrossRef]
- Klemmer, K.J.; Waldner, L.; Stone, A.; Low, N.H.; Nickerson, M.T. Complex Coacervation of Pea Protein Isolate and Alginate Polysaccharides. Food Chem. 2012, 130, 710–715. [Google Scholar] [CrossRef]
- Eghbal, N.; Choudhary, R. Complex Coacervation: Encapsulation and Controlled Release of Active Agents in Food Systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for Polysaccharides and Proteins: Synthesis Conditions, Mechanisms, and Crosslinking Efficiency, a Review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Maiti, S.; Maji, B.; Yadav, H. Progress on Green Crosslinking of Polysaccharide Hydrogels for Drug Delivery and Tissue Engineering Applications. Carbohydr. Polym. 2024, 326, 121584. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic Emulsification: An Overview on the Preparation of Different Emulsifiers-Stabilized Emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound Modified Polysaccharides: A Review of Structure, Physicochemical Properties, Biological Activities and Food Applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Akram, M.; Owuamanam, I.C.; Ogueke, C.C.; Awuchi, C.G.; Twinomhwezi, H. Nanotechnological Application of Peptide- and Protein-Based Therapeutics. In Applications of Nanotechnology in Drug Discovery and Delivery; Egbuna, C., Găman, M.A., Jeevanandam, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 205–238. [Google Scholar] [CrossRef]
- Jiang, L.; Duan, H.; Ji, X.; Wang, T.; Wang, Y.; Qiu, J. Application of a Simple Desolvation Method to Increase the Formation Yield, Physical Stability and Hydrophobic Drug Encapsulation Capacity of Chitosan-Based Nanoparticles. Int. J. Pharm. 2018, 545, 117–127. [Google Scholar] [CrossRef]
- Ammanage, A.S.; Mastiholimath, V.S. Quality by Design Approach Assisted Development and Optimization of Chitosan–Vildagliptin Nanoparticles Using a Simple Desolvation Technique. J. Appl. Pharm. Sci. 2025, 15, 174–182. [Google Scholar] [CrossRef]
- Gavory, C.; Durand, A.; Six, J.L.; Nouvel, C.; Marie, E.; Leonard, M. Polysaccharide-Covered Nanoparticles Prepared by Nanoprecipitation. Carbohydr. Polym. 2011, 84, 133–140. [Google Scholar] [CrossRef]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Kiafar, F.; Jelvehgari, M. Development of a Nanoprecipitation Method for the Entrapment of a Very Water Soluble Drug into Eudragit RL Nanoparticles. Res. Pharm. Sci. 2017, 12, 1. [Google Scholar] [CrossRef]
- Gericke, M.; Schulze, P.; Heinze, T. Nanoparticles Based on Hydrophobic Polysaccharide Derivatives—Formation Principles, Characterization Techniques, and Biomedical Applications. Macromol. Biosci. 2020, 20, 1900415. [Google Scholar] [CrossRef]
- Kuddushi, M.; Kanike, C.; Xu, B.B.; Zhang, X. Recent Advances in Nanoprecipitation: From Mechanistic Insights to Applications in Nanomaterial Synthesis. Soft Matter 2025, 21, 2759–2781. [Google Scholar] [CrossRef]
- Su, W.; Chang, Z.; E, Y.; Feng, Y.; Yao, X.; Wang, M.; Ju, Y.; Wang, K.; Jiang, J.; Li, P.; et al. Electrospinning and Electrospun Polysaccharide-Based Nanofiber Membranes: A Review. Int. J. Biol. Macromol. 2024, 263, 130335. [Google Scholar] [CrossRef]
- Arash, A.; Dehgan, F.; Zamanlui Benisi, S.; Jafari-Nodoushan, M.; Pezeshki-Modaress, M. Polysaccharide Base Electrospun Nanofibrous Scaffolds for Cartilage Tissue Engineering: Challenges and Opportunities. Int. J. Biol. Macromol. 2024, 277, 134054. [Google Scholar] [CrossRef]
- Tan, G.; Wang, L.; Pan, W.; Chen, K. Polysaccharide Electrospun Nanofibers for Wound Healing Applications. Int. J. Nanomed. 2022, 17, 3913. [Google Scholar] [CrossRef]
- Cao, J.; Gao, M.; Wang, J.; Liu, Y.; Zhang, X.; Ping, Y.; Liu, J.; Chen, G.; Xu, D.; Huang, X.; et al. Construction of Nano Slow-Release Systems for Antibacterial Active Substances and Its Applications: A Comprehensive Review. Front. Nutr. 2023, 10, 1109204. [Google Scholar] [CrossRef]
- Petrovic, S.M.; Barbinta-Patrascu, M.E. Organic and Biogenic Nanocarriers as Bio-Friendly Systems for Bioactive Compounds’ Delivery: State-of-the Art and Challenges. Materials 2023, 16, 7550. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Assadpour, E.; Williams, L.; Jafari, S.M. Nano/Microencapsulated Natural Antimicrobials to Control the Spoilage Microorganisms and Pathogens in Different Food Products. Food Control 2021, 128, 108180. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Lopes, N.A.; Brandelli, A. Lipid-Based Nanostructures for the Delivery of Natural Antimicrobials. Molecules 2021, 26, 3587. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2020; p. 457. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-Based Nanosystems and Their Exploited Antimicrobial Activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef]
- Nieto Marín, V.; Buccini, D.F.; Gomes da Silva, V.; Fernandez Soliz, I.A.; Franco, O.L. Nanoformulations of Bioactive Compounds Derived from Essential Oils with Antimicrobial Activity. Nano Trends 2025, 9, 100070. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan Nanoparticles Loaded with Clove Essential Oil: Characterization, Antioxidant and Antibacterial Activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Zhang, F.; Ramachandran, G.; Mothana, R.A.; Noman, O.M.; Alobaid, W.A.; Rajivgandhi, G.; Manoharan, N. Anti-Bacterial Activity of Chitosan Loaded Plant Essential Oil against Multi Drug Resistant K. Pneumoniae. Saudi J. Biol. Sci. 2020, 27, 3449. [Google Scholar] [CrossRef]
- Osanloo, M.; Ranjbar, R.; Zarenezhad, E. Alginate Nanoparticles Containing Cuminum Cyminum and Zataria Multiflora Essential Oils with Promising Anticancer and Antibacterial Effects. Int. J. Biomater. 2024, 2024, 5556838. [Google Scholar] [CrossRef]
- Gholamhossein Tabar Valookolaei, F.S.; Sazegar, H.; Rouhi, L. The Antibacterial Capabilities of Alginate Encapsulated Lemon Essential Oil Nanocapsules against Multi-Drug-Resistant Acinetobacter Baumannii. Sci. Rep. 2025, 15, 1679. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Boomija, R.V.; Muthukumar, S.; Gandhi, M.; Salma, S.; Prinsha, T.K.; Rengasamy, B. Green Synthesis of Chitosan Nanoparticles Using Tea Extract and Its Antimicrobial Activity against Economically Important Phytopathogens of Rice. Sci. Rep. 2024, 14, 7381. [Google Scholar] [CrossRef]
- Costa, J.R.; Xavier, M.; Amado, I.R.; Gonçalves, C.; Castro, P.M.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M.E. Polymeric Nanoparticles as Oral Delivery Systems for a Grape Pomace Extract towards the Improvement of Biological Activities. Mater. Sci. Eng. C 2021, 119, 111551. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Eltarahony, M.; Hafez, E.E.; Bashir, S.I. Green Fabrication of Chitosan Nanoparticles Using Lavendula Angustifolia, Optimization, Characterization and In-vitro Antibiofilm Activity. Sci. Rep. 2023, 13, 11127. [Google Scholar] [CrossRef]
- Duraisamy, N.; Dhayalan, S.; Shaik, M.R.; Shaik, A.H.; Shaik, J.P.; Shaik, B. Green Synthesis of Chitosan Nanoparticles Using of Martynia Annua L. Ethanol Leaf Extract and Their Antibacterial Activity. Crystals 2022, 12, 1550. [Google Scholar] [CrossRef]
- Abedini, E.; Khodadadi, E.; Zeinalzadeh, E.; Moaddab, S.R.; Asgharzadeh, M.; Mehramouz, B.; Dao, S.; Samadi Kafil, H. A Comprehensive Study on the Antimicrobial Properties of Resveratrol as an Alternative Therapy. Evid. Based Complement. Alternat Med. 2021, 2021, 8866311. [Google Scholar] [CrossRef]
- Spósito, L.; Fonseca, D.; Gonçalves Carvalho, S.; Sábio, R.M.; Marena, G.D.; Bauab, T.M.; Bagliotti Meneguin, A.; Parreira, P.; Martins, M.C.L.; Chorilli, M. Engineering Resveratrol-Loaded Chitosan Nanoparticles for Potential Use against Helicobacter pylori Infection. Eur. J. Pharm. Biopharm. 2024, 199, 114280. [Google Scholar] [CrossRef]
- Wathoni, N.; Herdiana, Y.; Suhandi, C.; Mohammed, A.F.A.; El-Rayyes, A.; Narsa, A.C. Chitosan/Alginate-Based Nanoparticles for Antibacterial Agents Delivery. Int. J. Nanomed. 2024, 19, 5021. [Google Scholar] [CrossRef]
- Nalini, T.; Basha, S.K.; Sadiq, A.M.; Kumari, V.S. Pectin/Chitosan Nanoparticle Beads as Potential Carriers for Quercetin Release. Mater. Today Commun. 2022, 33, 104172. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Tie, S.; Hou, S.; Wang, H.; Song, Y.; Rai, R.; Tan, M. Facile Synthesis of Nano-Nanocarriers from Chitosan and Pectin with Improved Stability and Biocompatibility for Anthocyanins Delivery: An in Vitro and in Vivo Study. Food Hydrocoll. 2020, 109, 106114. [Google Scholar] [CrossRef]
- Abreu, F.O.M.S.; Oliveira, E.F.; Paula, H.C.B.; De Paula, R.C.M. Chitosan/Cashew Gum Nanogels for Essential Oil Encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Kaleva, M.; Kroumov, A.; Slavkova, M.; Benbassat, N.; Yoncheva, K.; Najdenski, H. Advantageous Combinations of Nanoencapsulated Oregano Oil with Selected Antibiotics for Skin Treatment. Pharmaceutics 2022, 14, 2773. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Radhai, R.; Kotresh, T.M.; Csiszar, E. Development of Antimicrobial Cotton Fabrics Using Herb Loaded Nanoparticles. Carbohydr. Polym. 2013, 91, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Luiza Koop, B.; Nascimento da Silva, M.; Diniz da Silva, F.; Thayres dos Santos Lima, K.; Santos Soares, L.; José de Andrade, C.; Ayala Valencia, G.; Rodrigues Monteiro, A. Flavonoids, Anthocyanins, Betalains, Curcumin, and Carotenoids: Sources, Classification and Enhanced Stabilization by Encapsulation and Adsorption. Food Res. Int. 2022, 153, 110929. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Mirnejad, R.; Mofazzal Jahromi, M.A.; Al-Musawi, S.; Pirestani, M.; Fasihi Ramandi, M.; Ahmadi, K.; Rajayi, H.; Mohammad Hassan, Z.; Kamali, M. Curcumin-Loaded Chitosan Tripolyphosphate Nanoparticles as a Safe, Natural and Effective Antibiotic Inhibits the Infection of Staphylococcus Aureus and Pseudomonas Aeruginosa in Vivo. Iran. J. Biotechnol. 2014, 12, e1012. [Google Scholar] [CrossRef]
- Sarma, A.; Deka, C.; Devi, S.P.; Kakati, D.K. Synthesis of Curcumin-Loaded Complex Coacervate of Chitosan Phosphate and Sodium Alginate and Study of Their Antibacterial Activity. Indian J. Pharm. Educ. Res. 2024, 58, s420–s428. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Fabrication of Natural-Origin Antibacterial Nanocellulose Films Using Bio-Extracts for Potential Use in Biomedical Industry. Int. J. Biol. Macromol. 2020, 145, 914–925. [Google Scholar] [CrossRef]
- Zikmundova, M.; Vereshaka, M.; Kolarova, K.; Pajorova, J.; Svorcik, V.; Bacakova, L. Effects of Bacterial Nanocellulose Loaded with Curcumin and Its Degradation Products on Human Dermal Fibroblasts. Materials 2020, 13, 4759. [Google Scholar] [CrossRef]
- Zielińska, S.; Matkowski, A.; Dydak, K.; Czerwińska, M.E.; Dziągwa-Becker, M.; Kucharski, M.; Wójciak, M.; Sowa, I.; Plińska, S.; Fijałkowski, K.; et al. Bacterial Nanocellulose Fortified with Antimicrobial and Anti-Inflammatory Natural Products from Chelidonium Majus Plant Cell Cultures. Materials 2022, 15, 16. [Google Scholar] [CrossRef]
- Liakos, I.L.; Iordache, F.; Carzino, R.; Scarpellini, A.; Oneto, M.; Bianchini, P.; Grumezescu, A.M.; Holban, A.M. Cellulose Acetate—Essential Oil Nanocapsules with Antimicrobial Activity for Biomedical Applications. Colloids Surf. B Biointerfaces 2018, 172, 471–479. [Google Scholar] [CrossRef]
- Darpentigny, C.; Marcoux, P.R.; Menneteau, M.; Michel, B.; Ricoul, F.; Jean, B.; Bras, J.; Nonglaton, G. Antimicrobial Cellulose Nanofibril Porous Materials Obtained by Supercritical Impregnation of Thymol. ACS Appl. Bio Mater. 2020, 3, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Sauraj; Kumar, S.U.; Kumar, V.; Priyadarshi, R.; Gopinath, P.; Negi, Y.S. PH-Responsive Prodrug Nanoparticles Based on Xylan-Curcumin Conjugate for the Efficient Delivery of Curcumin in Cancer Therapy. Carbohydr. Polym. 2018, 188, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Chang, R.; Yang, J.; Ge, S.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q. Preparation and Characterization of Essential Oil-Loaded Starch Nanoparticles Formed by Short Glucan Chains. Food Chem. 2017, 221, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Ramalingam, T.; Nooruddin, T.; Shanmuganathan, R.; Arivalagan, P.; Natarajan, S. Polyherbal Drug Loaded Starch Nanoparticles as Promising Drug Delivery System: Antimicrobial, Antibiofilm and Neuroprotective Studies. Process Biochem. 2020, 92, 355–364. [Google Scholar] [CrossRef]
- Gholami, M.; Hosseini, M.; Shahavi, M.H.; Jahanshahi, M. Assessing Therapeutic Effects of Corn Starch Nanoparticles with Lavender Extract on Skin Wound Healing in Wistar Rats. Iranica J. Energy Environ. 2025, 16, 326–338. [Google Scholar] [CrossRef]
- Wang, R.; Qin, X.; Du, Y.; Shan, Z.; Shi, C.; Huang, K.; Wang, J.; Zhi, K. Dual-Modified Starch Nanoparticles Containing Aromatic Systems with Highly Efficient Encapsulation of Curcumin and Their Antibacterial Applications. Food Res. Int. 2022, 162, 111926. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of Starch Nanoparticles Loaded with Quercetin Using Nanoprecipitation Technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of Rutin Using Quinoa and Maize Starch Nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, W.; Ke, Z.; Li, Y.; Zhou, Z. In Vitro Release and Antioxidant Activity of Satsuma Mandarin (Citrus Reticulata Blanco Cv. Unshiu) Peel Flavonoids Encapsulated by Pectin Nanoparticles. Int. J. Food Sci. Technol. 2017, 52, 2362–2373. [Google Scholar] [CrossRef]
- Rajabi, H.; Jafari, S.M.; Rajabzadeh, G.; Sarfarazi, M.; Sedaghati, S. Chitosan-Gum Arabic Complex Nanocarriers for Encapsulation of Saffron Bioactive Components. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123644. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Paula, H.C.B.; Paula, R.C.M. de Alginate/Cashew Gum Nanoparticles for Essential Oil Encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Salarbashi, D.; Tafaghodi, M.; Fathi, M.; Aboutorabzade, S.M.; Sabbagh, F. Development of Curcumin-Loaded Prunus Armeniaca Gum Nanoparticles: Synthesis, Characterization, Control Release Behavior, and Evaluation of Anticancer and Antimicrobial Properties. Food Sci. Nutr. 2021, 9, 6109–6119. [Google Scholar] [CrossRef] [PubMed]
- Mazyed, E.A.; Magdy, G.; Elekhnawy, E.; Yammine, M.; Rolando, C.; ElNaggar, M.H. Formulation and Characterization of Quercetin-Loaded Prunus Armeniaca Gum Nanoparticles with Enhanced Anti-Bacterial Effect. J. Drug Deliv. Sci. Technol. 2024, 94, 105485. [Google Scholar] [CrossRef]
- Fani, N.; Enayati, M.H.; Rostamabadi, H.; Falsafi, S.R. Encapsulation of Bioactives within Electrosprayed κ-Carrageenan Nanoparticles. Carbohydr. Polym. 2022, 294, 119761. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, J.S.; Lee, H.G. Nanoencapsulation of Grapefruit Seed Extract and Cinnamon Oil for Oral Health: Preparation, In Vitro, and Clinical Antimicrobial Activities. J. Agric. Food Chem. 2023, 71, 5646–5654. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Wang, H.; Li, P.; Chen, Y.; Wang, C.; Zhou, C.; Song, S.; Chen, S.; Huang, G.; et al. Dual-Grafted Dextran Based Nanomicelles: Higher Antioxidant, Anti-Inflammatory and Cellular Uptake Efficiency for Quercetin. Int. J. Biol. Macromol. 2023, 224, 1361–1372. [Google Scholar] [CrossRef]
- Darwish, M.M.; Elneklawi, M.S.; Mohamad, E.A. Aloe Vera Coated Dextran Sulfate/Chitosan Nanoparticles (Aloe Vera @ DS/CS) Encapsulating Eucalyptus Essential Oil with Antibacterial Potent Property. J. Biomater. Sci. Polym. Ed. 2023, 34, 810–827. [Google Scholar] [CrossRef]
- Hong, S.E.; Lee, J.S.; Lee, H.G. α-Tocopherol-Loaded Multi-Layer Nanoemulsion Using Chitosan, and Dextran Sulfate: Cellular Uptake, Antioxidant Activity, and in Vitro Bioaccessibility. Int. J. Biol. Macromol. 2024, 254, 127819. [Google Scholar] [CrossRef]
- Anitha, A.; Deepagan, V.G.; Divya Rani, V.V.; Menon, D.; Nair, S.V.; Jayakumar, R. Preparation, Characterization, in Vitro Drug Release and Biological Studies of Curcumin Loaded Dextran Sulphate–Chitosan Nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Muralidharan, S.; Shanmugam, K. Synthesis and Characterization of Naringenin-Loaded Chitosan-Dextran Sulfate Nanocarrier. J. Pharm. Innov. 2021, 16, 269–278. [Google Scholar] [CrossRef]
- Zambak, Ö.; Özkal, A.; Özkal, S.G. Production of Clove Extract Loaded Pullulan and Whey Protein Nanofibers as Antioxidant and Antibacterial Agent. J. Appl. Polym. Sci. 2022, 139, e53141. [Google Scholar] [CrossRef]
- Pawara, Y.; Mahajan, H. Surface-Functionalized Pullulan-Based Polymeric Nanoparticles Containing Resveratrol: A Green Approach for Lung Cancer Targeted Delivery. Part. Sci. Technol. 2025, 43, 416–432. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of Tannic Acid/Chitosan/Pullulan Composite Nanofibers from Aqueous Solution for Potential Applications as Wound Dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, K.; Wang, H.; Wu, T.; Zhao, Y.; Li, H.; Tang, H.; Yang, W. Preparation and Evaluation of Curcumin Grafted Hyaluronic Acid Modified Pullulan Polymers as a Functional Wound Dressing Material. Carbohydr. Polym. 2020, 238, 116195. [Google Scholar] [CrossRef]
- Doğan, G.; Başal, G.; Bayraktar, O.; Ozyildiz, F.; Uzel, A.; Erdoğan, I. Bioactive Sheath/Core Nanofibers Containing Olive Leaf Extract. Microsc. Res. Tech. 2016, 79, 38–49. [Google Scholar] [CrossRef][Green Version]
- Ghodke, S.S. Multifunctional Curcumin-Loaded Nanocarriers for Lung Delivery. Ph.D. Thesis, UCL (University College London), London, UK, August 2021. [Google Scholar]
- Hussain, Z.; Pandey, M.; Choudhury, H.; Ying, P.C.; Xian, T.M.; Kaur, T.; Jia, G.W.; Gorain, B. Hyaluronic Acid Functionalized Nanoparticles for Simultaneous Delivery of Curcumin and Resveratrol for Management of Chronic Diabetic Wounds: Fabrication, Characterization, Stability and in Vitro Release Kinetics. J. Drug Deliv. Sci. Technol. 2020, 57, 101747. [Google Scholar] [CrossRef]
- Quagliariello, V.; Iaffaioli, R.V.; Armenia, E.; Clemente, O.; Barbarisi, M.; Nasti, G.; Berretta, M.; Ottaiano, A.; Barbarisi, A. Hyaluronic Acid Nanohydrogel Loaded With Quercetin Alone or in Combination to a Macrolide Derivative of Rapamycin RAD001 (Everolimus) as a New Treatment for Hormone-Responsive Human Breast Cancer. J. Cell Physiol. 2017, 232, 2063–2074. [Google Scholar] [CrossRef]
- Barbarisi, M.; Iaffaioli, R.V.; Armenia, E.; Schiavo, L.; De Sena, G.; Tafuto, S.; Barbarisi, A.; Quagliariello, V. Novel Nanohydrogel of Hyaluronic Acid Loaded with Quercetin Alone and in Combination with Temozolomide as New Therapeutic Tool, CD44 Targeted Based, of Glioblastoma Multiforme. J. Cell Physiol. 2018, 233, 6550–6564. [Google Scholar] [CrossRef]
- Montanari, E.; Gennari, A.; Pelliccia, M.; Gourmel, C.; Lallana, E.; Matricardi, P.; McBain, A.J.; Tirelli, N. Hyaluronan/Tannic Acid Nanoparticles Via Catechol/Boronate Complexation as a Smart Antibacterial System. Macromol. Biosci. 2016, 16, 1815–1823. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Frenț, O.D.; Stefan, L.; Morgovan, C.M.; Duteanu, N.; Dejeu, I.L.; Marian, E.; Vicaș, L.; Manole, F. A Systematic Review: Quercetin—Secondary Metabolite of the Flavonol Class, with Multiple Health Benefits and Low Bioavailability. Int. J. Mol. Sci. 2024, 25, 12091. [Google Scholar] [CrossRef] [PubMed]
- Ong, X.R.; Chen, A.X.; Li, N.; Yang, Y.Y.; Luo, H.K. Nanocellulose: Recent Advances Toward Biomedical Applications. Small Sci. 2023, 3, 2200076. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Lewandowska, U. Encapsulation of Polyphenolic Compounds Based on Hemicelluloses to Enhance Treatment of Inflammatory Bowel Diseases and Colorectal Cancer. Molecules 2023, 28, 4189. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Tuchilus, C.G.; Nichifor, M.; Mocanu, G.; Stanciu, M.C. Antimicrobial Activity of Chemically Modified Dextran Derivatives. Carbohydr. Polym. 2017, 161, 181–186. [Google Scholar] [CrossRef]
- Madkhali, O.A.; Sivagurunathan Moni, S.; Sultan, M.H.; Bukhary, H.A.; Ghazwani, M.; Alhakamy, N.A.; Meraya, A.M.; Alshahrani, S.; Alqahtani, S.S.; Bakkari, M.A.; et al. Formulation and Evaluation of Injectable Dextran Sulfate Sodium Nanoparticles as a Potent Antibacterial Agent. Sci. Rep. 2021, 11, 9914. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564. [Google Scholar] [CrossRef]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nandi, S.; Basu, T. Nano-Antibacterials Using Medicinal Plant Components: An Overview. Front. Microbiol. 2022, 12, 768739. [Google Scholar] [CrossRef] [PubMed]
- Danquah, C.A.; Minkah, P.A.B.; Junior, I.O.D.; Amankwah, K.B.; Somuah, S.O. Antimicrobial Compounds from Microorganisms. Antibiotics 2022, 11, 285. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and Potential Use as Antimicrobials. J. Clin. Lab. Anal. 2021, 36, e24093. [Google Scholar] [CrossRef]
- Zimina, M.; Babich, O.; Prosekov, A.; Sukhikh, S.; Ivanova, S.; Shevchenko, M.; Noskova, S. Overview of Global Trends in Classification, Methods of Preparation and Application of Bacteriocins. Antibiotics 2020, 9, 553. [Google Scholar] [CrossRef]
- Hourigan, D.; Miceli de Farias, F.; O’Connor, P.M.; Hill, C.; Ross, R.P. Discovery and Synthesis of Leaderless Bacteriocins from the Actinomycetota. J. Bacteriol. 2024, 206, e0029824. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel Bacteriocins from Lactic Acid Bacteria (LAB): Various Structures and Applications. Microb. Cell Fact. 2014, 13, S3. [Google Scholar] [CrossRef]
- Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. Bacteriocins from Lactic Acid Bacteria: Purification, Properties and Use as Biopreservatives. Braz. Arch. Biol. Technol. 2007, 50, 512–542. [Google Scholar] [CrossRef]
- Grosu-Tudor, S.S.; Stancu, M.M.; Pelinescu, D.; Zamfir, M. Characterization of Some Bacteriocins Produced by Lactic Acid Bacteria Isolated from Fermented Foods. World J. Microbiol. Biotechnol. 2014, 30, 2459–2469. [Google Scholar] [CrossRef]
- Revutskaya, N.; Polishchuk, E.; Kozyrev, I.; Fedulova, L.; Krylova, V.; Pchelkina, V.; Gustova, T.; Vasilevskaya, E.; Karabanov, S.; Kibitkina, A.; et al. Application of Natural Functional Additives for Improving Bioactivity and Structure of Biopolymer-Based Films for Food Packaging: A Review. Polymers 2024, 16, 1976. [Google Scholar] [CrossRef]
- Yi, Y.; Li, P.; Zhao, F.; Zhang, T.; Shan, Y.; Wang, X.; Liu, B.; Chen, Y.; Zhao, X.; Lü, X. Current Status and Potentiality of Class II Bacteriocins from Lactic Acid Bacteria: Structure, Mode of Action and Applications in the Food Industry. Trends Food Sci. Technol. 2022, 120, 387–401. [Google Scholar] [CrossRef]
- Negash, A.W.; Tsehai, B.A. Current Applications of Bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, S.; Sathyan, A.; Balakrishnan, S.; Subramani, P.; Prahalathan, C.; Kumarasamy, A. Bactericidal and Non-Cytotoxic Activity of Bacteriocin Produced by Lacticaseibacillus Paracasei F9-02 and Evaluation of Its Tolerance to Various Physico-Chemical Conditions. Environ. Microbiol. 2023, 25, 2882–2896. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.E.; An, F.Y.; Dholpe, V.; Ramamoorthy, A.; Lopatin, D.E.; Lantz, M.S. The Spectrum of Antimicrobial Activity of the Bacteriocin Subtilosin A. J. Antimicrob. Chemother. 2007, 59, 297–300. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, Potent Antimicrobial Peptides and the Fight against Multi Drug Resistant Species: Resistance Is Futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef]
- Peng, Z.; Xiong, T.; Huang, T.; Xu, X.; Fan, P.; Qiao, B.; Xie, M. Factors Affecting Production and Effectiveness, Performance Improvement and Mechanisms of Action of Bacteriocins as Food Preservative. Crit. Rev. Food Sci. Nutr. 2023, 63, 12294–12307. [Google Scholar] [CrossRef]
- Parada Fabián, J.C.; Álvarez Contreras, A.K.; Natividad Bonifacio, I.; Hernández Robles, M.F.; Vázquez Quiñones, C.R.; Quiñones Ramírez, E.I.; Vázquez Salinas, C. Toward Safer and Sustainable Food Preservation: A Comprehensive Review of Bacteriocins in the Food Industry. Biosci. Rep. 2025, 45, 277–302. [Google Scholar] [CrossRef]
- Radaic, A.; de Jesus, M.B.; Kapila, Y.L. Bacterial Anti-Microbial Peptides and Nano-Sized Drug Delivery Systems: The State of the Art toward Improved Bacteriocins. J. Control. Release 2020, 321, 100–118. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, Synthesis, Mechanism of Action and Resistance Development in Food Spoilage Causing Bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical Applications of Nisin. J. Appl. Microbiol. 2016, 120, 1449. [Google Scholar] [CrossRef]
- van de Ven, F.J.M.; van den Hooven, H.W.; Hilbers, C.W.; Konings, R.N.H. NMR Studies of Lantibiotics: The Three-Dimensional Structure of Nisin in Aqueous Solution. In Bacteriocins, Microcins and Lantibiotics; James, R., Lazdunski, C., Pattus, F., Eds.; NATO ASI Series; Springer: Berlin/Heidelberg, Germany, 1992; Volume 65, pp. 435–447. [Google Scholar] [CrossRef]
- Tavares, T.D.; Ribeiro, A.R.M.; Silva, C.; Antunes, J.C.; Felgueiras, H.P. Combinatory Effect of Nisin Antimicrobial Peptide with Bioactive Molecules: A Review. J. Drug Deliv. Sci. Technol. 2024, 91, 105246. [Google Scholar] [CrossRef]
- Lee, E.H.; Khan, I.; Oh, D.H. Evaluation of the Efficacy of Nisin-Loaded Chitosan Nanoparticles against Foodborne Pathogens in Orange Juice. J. Food Sci. Technol. 2018, 55, 1127. [Google Scholar] [CrossRef] [PubMed]
- Alishahi, A. Antibacterial Effect of Chitosan Nanoparticle Loaded with Nisin for the Prolonged Effect. J. Food Saf. 2014, 34, 111–118. [Google Scholar] [CrossRef]
- Zimet, P.; Mombrú, Á.W.; Faccio, R.; Brugnini, G.; Miraballes, I.; Rufo, C.; Pardo, H. Optimization and Characterization of Nisin-Loaded Alginate-Chitosan Nanoparticles with Antimicrobial Activity in Lean Beef. LWT 2018, 91, 107–116. [Google Scholar] [CrossRef]
- Zohri, M.; Shafiee Alavidjeh, M.; Mirdamadi, S.S.; Behmadi, H.; Hossaini Nasr, S.M.; Eshghi Gonbaki, S.; Shafiee Ardestani, M.; Jabbari Arabzadeh, A. Nisin-Loaded Chitosan/Alginate Nanoparticles: A Hopeful Hybrid Biopreservative. J. Food Saf. 2013, 33, 40–49. [Google Scholar] [CrossRef]
- Liu, B.; Xie, Q.; Liu, Y.; Li, S.; Dong, K.; Su, Y.; Jiang, Y.; Yang, X.; Guo, L. Effects of Nisin Loaded Chitosan-Pectin Nanoparticles on Shelf Life and Storage Stability of Room Temperature Stored Processed Cheese. Food Chem. 2025, 473, 143103. [Google Scholar] [CrossRef]
- Chopra, M.; Kaur, P.; Bernela, M.; Thakur, R. Surfactant Assisted Nisin Loaded Chitosan-Carageenan Nanocapsule Synthesis for Controlling Food Pathogens. Food Control 2014, 37, 158–164. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Antimicrobial Efficacy of Nisin-Loaded Bacterial Cellulose Nanocrystals against Selected Meat Spoilage Lactic Acid Bacteria. Carbohydr. Polym. 2021, 251, 117096. [Google Scholar] [CrossRef]
- Çelen, T.; Anumudu, C.; Miri, T.; Onyeaka, H.; Fernandez-Trillo, P. Nisin:Carboxymethylcellulose Polyion Complex (PIC) Nanoparticles. Preparation and Antimicrobial Activity. Carbohydr. Polym. 2023, 317, 121032. [Google Scholar] [CrossRef]
- Weishaupt, R.; Heuberger, L.; Siqueira, G.; Gutt, B.; Zimmermann, T.; Maniura-Weber, K.; Salentinig, S.; Faccio, G. Enhanced Antimicrobial Activity and Structural Transitions of a Nanofibrillated Cellulose-Nisin Biocomposite Suspension. ACS Appl. Mater. Interfaces 2018, 10, 20170–20181. [Google Scholar] [CrossRef]
- Gao, G.; Fan, H.; Zhang, Y.; Cao, Y.; Li, T.; Qiao, W.; Wu, M.; Ma, T.; Li, G. Production of Nisin-Containing Bacterial Cellulose Nanomaterials with Antimicrobial Properties through Co-Culturing Enterobacter Sp. FY-07 and Lactococcus Lactis N8. Carbohydr. Polym. 2021, 251, 117131. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.; Tchuenbou-Magaia, F.; Maresca, D.; Mauriello, G. Development of Antimicrobial Cellulose Nanofiber-Based Films Activated with Nisin for Food Packaging Applications. Foods 2022, 11, 3051. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhang, C.; Hu, Y.; Lacroix, M.; Wang, Y. Holocellulose Nanofibrils as Effective Nisin Immobilization Substrates for Antimicrobial Food Packaging. Int. J. Biol. Macromol. 2025, 304, 140741. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; German, J.B.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Khaksar, R. Preparation and Characterization of Alginate and Alginate-Resistant Starch Microparticles Containing Nisin. Carbohydr. Polym. 2014, 103, 573–580. [Google Scholar] [CrossRef]
- Krivorotova, T.; Cirkovas, A.; Maciulyte, S.; Staneviciene, R.; Budriene, S.; Serviene, E.; Sereikaite, J. Nisin-Loaded Pectin Nanoparticles for Food Preservation. Food Hydrocoll. 2016, 54, 49–56. [Google Scholar] [CrossRef]
- Wang, X.; Qian, J.Q.; Yin, J.L.; Gong, F.; Guo, H. Preparation and Antibacterial Properties of High-Methoxy Pectin Oligosaccharide -Nisin Nanoparticles. Eur. Polym. J. 2023, 200, 112472. [Google Scholar] [CrossRef]
- Selvasudha, N.; PushpaSweety, J.; Saranya, T.V.; Ruckmani, K.; Gayathri, L. Development of Alkaline-Stable Nanoformulation of Nisin: Special Insights through Cytotoxic and Antibacterial Studies. Environ. Sci. Pollut. Res. 2024, 31, 46558–46574. [Google Scholar] [CrossRef]
- Muppalla, S.R.; Sonavale, R.; Chawla, S.P.; Sharma, A. Functional Properties of Nisin–Carbohydrate Conjugates Formed by Radiation Induced Maillard Reaction. Radiat. Phys. Chem. 2012, 81, 1917–1922. [Google Scholar] [CrossRef]
- Soto, K.M.; Hernández-Iturriaga, M.; Loarca-Piña, G.; Luna-Bárcenas, G.; Mendoza, S. Antimicrobial Effect of Nisin Electrospun Amaranth: Pullulan Nanofibers in Apple Juice and Fresh Cheese. Int. J. Food Microbiol. 2019, 295, 25–32. [Google Scholar] [CrossRef]
- Çelen, T.; Anumudu, C.; Miri, T.; Onyeaka, H.; Fernandez-Trillo, P. Pathogen-Responsive Delivery of Nisin. Food Hydrocoll. 2024, 154, 110076. [Google Scholar] [CrossRef]
- Lequeux, I.; Ducasse, E.; Jouenne, T.; Thebault, P. Addition of Antimicrobial Properties to Hyaluronic Acid by Grafting of Antimicrobial Peptide. Eur. Polym. J. 2014, 51, 182–190. [Google Scholar] [CrossRef]
- Narsaiah, K.; Jha, S.N.; Wilson, R.A.; Mandge, H.M.; Manikantan, M.R.; Malik, R.K.; Vij, S. Pediocin-Loaded Nanoliposomes and Hybrid Alginate-Nanoliposome Delivery Systems for Slow Release of Pediocin. Bionanoscience 2013, 3, 37–42. [Google Scholar] [CrossRef]
- Wei, L.; Wong, D.; Jeoh, T.; Marco, M.L. Intestinal Delivery of Encapsulated Bacteriocin Peptides in Cross-Linked Alginate Microcapsules. Food Res. Int. 2024, 188, 114473. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, H.; Belguesmia, Y.; Boukherroub, R.; Drider, D. Alginate Nanoparticles Enhance Anti-Clostridium Perfringens Activity of the Leaderless Two-Peptide Enterocin DD14 and Affect Expression of Some Virulence Factors. Probiotics Antimicrob. Proteins 2021, 13, 1213–1227. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, N.; Zhou, Z.; Han, Y. Physical and Antibacterial Properties of Bacterial Cellulose Films Supplemented with Cell-Free Supernatant Enterocin-Producing Enterococcus Faecium TJUQ1. Food Microbiol. 2021, 99, 103828. [Google Scholar] [CrossRef] [PubMed]
- Rollini, M.; Musatti, A.; Cavicchioli, D.; Bussini, D.; Farris, S.; Rovera, C.; Romano, D.; De Benedetti, S.; Barbiroli, A. From Cheese Whey Permeate to Sakacin-A/Bacterial Cellulose Nanocrystal Conjugates for Antimicrobial Food Packaging Applications: A Circular Economy Case Study. Sci. Rep. 2020, 10, 21358. [Google Scholar] [CrossRef]
- Azam, M.; Srivastava, R.; Ahmed, T. Enhanced Antibacterial Efficacy of Levilactobacillus Brevis Bacteriocin with Chitosan Nanoparticle Delivery. J. Pure Appl. Microbiol. 2024, 18, 2748–2757. [Google Scholar] [CrossRef]
- Guirguis, E.A.H. Enhancing the antibacterial effect of bacteriocin from Lactococcus lactis subsp. Lactis using chitosan nanoparticles. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e3777. [Google Scholar] [CrossRef]
- Bagde, P.; Vigneshwaran, N. Improving the Stability of Bacteriocin Extracted from Enterococcus Faecium by Immobilization onto Cellulose Nanocrystals. Carbohydr. Polym. 2019, 209, 172–180. [Google Scholar] [CrossRef]
- Lin, M.; Fang, S.; Zhao, X.; Liang, X.; Wu, D. Natamycin-Loaded Zein Nanoparticles Stabilized by Carboxymethyl Chitosan: Evaluation of Colloidal/Chemical Performance and Application in Postharvest Treatments. Food Hydrocoll. 2020, 106, 105871. [Google Scholar] [CrossRef]
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive Nanoparticles for Prolonged Ocular Delivery of Natamycin: In Vitro and Pharmacokinetics Studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ban, Z.; Chen, F.; Liu, L.; Zhang, S.; Wang, L.; Wang, H.; Wang, L.; Zhu, Y. Gliadin Nanoparticles Stabilized by Sodium Carboxymethyl Cellulose as Carriers for Improved Dispersibility, Stability and Bacteriostatic Activity of Natamycin. Food Biosci. 2023, 53, 102575. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, X.; Zhao, L.; Zhang, W.; Zhu, S.; Ma, J. Chitosan Nanoparticles to Enhance the Inhibitory Effect of Natamycin on Candida Albicans. J. Nanomater. 2021, 2021, 6644567. [Google Scholar] [CrossRef]
- Fayed, A.; Elsayed, H.; Ali, T. Packaging Fortified with Natamycin Nanoparticles for Hindering the Growth of Toxigenic Aspergillus Flavus and Aflatoxin Production in Romy Cheese. J. Adv. Vet. Anim. Res. 2021, 8, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M.; Anastasiadou, S. Pediocins: The Bacteriocins of Pediococci. Sources, Production, Properties and Applications. Microb. Cell Fact. 2009, 8, 3. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Mohammadi, M.; Mortazavian, A.M.; Yousefi, M. Antibacterial Activity of Pediocin and Pediocin-Producing Bacteria Against Listeria Monocytogenes in Meat Products. Front. Microbiol. 2021, 12, 709959. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Pediocin Applications in Antimicrobial Food Packaging Systems. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 445–454. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Gamboni, J.E.; Slavutsky, A.M.; Ibarguren, C. Novel Food Packaging Systems with Antimicrobial Agents from Microbial Source. In Food Packaging and Preservation: Antimicrobial Materials and Technologies; Jaiswal, A.K., Shankar, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 91–111. [Google Scholar] [CrossRef]
- Corsetti, A.; Valmorri, S. Lactic Acid Bacteria|Lactobacillus Spp.: Lactobacillus Plantarum. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 111–118. [Google Scholar] [CrossRef]
- Yusuf, M. Natural Antimicrobial Agents for Food Biopreservation. In Food Packaging and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 409–438. [Google Scholar] [CrossRef]
- Zdolec, N.; Kiš, M. Antimicrobial Properties of Food Enterococci. In Lactic Acid Bacteria in Food Biotechnology: Innovations and Functional Aspects; Ray, R.C., Paramithiotis, S., de Carvalho Azevedo, V.A., Montet, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–203. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, X.; Wu, Y.; Liu, X.; Zhang, X. Enterocins: Classification, Synthesis, Antibacterial Mechanisms and Food Applications. Molecules 2022, 27, 2258. [Google Scholar] [CrossRef]
- Delves-Broughton, J. Natural Antimicrobials as Additives and Ingredients for the Preservation of Foods and Beverages. In Natural Food Additives, Ingredients and Flavourings; Baines, D., Seal, R., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 127–161. [Google Scholar] [CrossRef]
- Delves-Broughton, J. Use of the Natural Food Preservatives, Nisin and Natamycin, to Reduce Detrimental Thermal Impact on Product Quality. In In-Pack Processed Foods: Improving Quality; Richardson, P., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 319–337. [Google Scholar] [CrossRef]
- Lule, V.K.; Garg, S.; Gosewade, S.C.; Khedkar, C.D. Natamycin. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 56–62. [Google Scholar] [CrossRef]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A Natural Preservative for Food Applications—A Review. Food Sci. Biotechnol. 2021, 30, 1481. [Google Scholar] [CrossRef]
- Shafique, B.; Ranjha, M.M.A.N.; Murtaza, M.A.; Walayat, N.; Nawaz, A.; Khalid, W.; Mahmood, S.; Nadeem, M.; Manzoor, M.F.; Ameer, K.; et al. Recent Trends and Applications of Nanoencapsulated Bacteriocins against Microbes in Food Quality and Safety. Microorganisms 2022, 11, 85. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Wang, Y.; Song, M.; Chang, W. Antimicrobial Peptides and Proteins against Drug-Resistant Pathogens. Cell Surf. 2024, 12, 100135. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yu, H.; Lv, T.; Chen, Q.; Luo, H.; Zhang, H. Progress in the Classification, Optimization, Activity, and Application of Antimicrobial Peptides. Front. Microbiol. 2025, 16, 1582863. [Google Scholar] [CrossRef] [PubMed]
- Assoni, L.; Milani, B.; Carvalho, M.R.; Nepomuceno, L.N.; Waz, N.T.; Guerra, M.E.S.; Converso, T.R.; Darrieux, M. Resistance Mechanisms to Antimicrobial Peptides in Gram-Positive Bacteria. Front. Microbiol. 2020, 11, 593215. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tu, Y.; Li, B.; Qu, G.; Li, A.; Peng, X.; Li, S.; Shao, C. Antimicrobial Peptide Biological Activity, Delivery Systems and Clinical Translation Status and Challenges. J. Transl. Med. 2025, 23, 292. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Filippi, M.; Mohabatpour, F.; Letourneur, D.; Scherberich, A. Chicken Egg White: Hatching of a New Old Biomaterial. Mater. Today 2020, 40, 193–214. [Google Scholar] [CrossRef]
- Croguennec, T.; Tavares, G.M.; Bouhallab, S. Heteroprotein Complex Coacervation: A Generic Process. Adv. Colloid. Interface Sci. 2017, 239, 115–126. [Google Scholar] [CrossRef]
- Nawaz, N.; Wen, S.; Wang, F.; Nawaz, S.; Raza, J.; Iftikhar, M.; Usman, M. Lysozyme and Its Application as Antibacterial Agent in Food Industry. Molecules 2022, 27, 6305. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, M.; Li, Z. Exploring the Therapeutic Potential of Recombinant Human Lysozyme: A Review on Wound Management System with Antibacterial. Front. Bioeng. Biotechnol. 2023, 11, 1292149. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Liu, Y.; Jin, Y.; Ma, M. The Antimicrobial Spectrum of Lysozyme Broadened by Reductive Modification. Poult. Sci. 2018, 97, 3992–3999. [Google Scholar] [CrossRef]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of Lysozyme into Chitosan Nanoparticles for Improving Antibacterial Activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Téllez, C.N.; Rodríguez-Córdova, F.J.; Rosas-Burgos, E.C.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Lizardi-Mendoza, J.; Torres-Arreola, W.; Martínez-Higuera, A.; Plascencia-Jatomea, M. Activity of Chitosan–Lysozyme Nanoparticles on the Growth, Membrane Integrity, and β-1,3-Glucanase Production by Aspergillus Parasiticus. 3 Biotech 2017, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Jin, M.; Han, Q.; Liu, S.; Chen, X.; Han, Y. Enhancing the Thermo-Stability and Anti-Bacterium Activity of Lysozyme by Immobilization on Chitosan Nanoparticles. Int. J. Mol. Sci. 2020, 21, 1635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, L.; Chen, Z.Y.; Sun, J.; Guo, X.W.; Wang, H.R.; Zhang, X.Y.; Liu, Z.R.; Liu, J.; Zhang, K.; et al. Remineralization and Bacterial Inhibition of Early Enamel Caries Surfaces by Carboxymethyl Chitosan Lysozyme Nanogels Loaded with Antibacterial Drugs. J. Dent. 2025, 152, 105489. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Shen, N.; Yuan, C.; Hu, Y. Preparation and Characterization of Calcium Alginate-Chitosan Complexes Loaded with Lysozyme. J. Food Eng. 2018, 233, 109–116. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, M.; Chen, S.; Shi, W.; Wang, X. Incorporation of Lysozyme into Cellulose Nanocrystals Stabilized β-Chitosan Nanoparticles with Enhanced Antibacterial Activity. Carbohydr. Polym. 2020, 236, 115974. [Google Scholar] [CrossRef]
- Hadi Esfahani, T.; Azadi, A.; Joodi, N.; Cohan, R.A.; Akbarzadeh, I.; Bakhshandeh, H. Optimized Preparation of Lysozyme Loaded Dextran-Chitosan Nanoparticles Using D-Optimal Design. Health Biotechnol. Biopharma 2019, 2, 56–78. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Valente, B.F.A.; Luís, J.; Pires, L.; Oliveira, H.; Oliveira, M.; Silvestre, A.J.D.; Vilela, C.; et al. Alginate-Lysozyme Nanofibers Hydrogels with Improved Rheological Behavior, Printability and Biological Properties for 3D Bioprinting Applications. Nanomaterials 2022, 12, 2190. [Google Scholar] [CrossRef]
- Spagnuolo, L.; Micheli, L.; Dufresne, A.; Beneventi, D.; Operamolla, A. Covalent Lysozyme Immobilization on Enzymatic Cellulose Nanocrystals. Chem. Eur. J. 2024, 30, e202402171. [Google Scholar] [CrossRef]
- Abouhmad, A.; Dishisha, T.; Amin, M.A.; Hatti-Kaul, R. Immobilization to Positively Charged Cellulose Nanocrystals Enhances the Antibacterial Activity and Stability of Hen Egg White and T4 Lysozyme. Biomacromolecules 2017, 18, 1600–1608. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, P.; Zhan, Y.; Shi, X.; Lin, J.; Du, Y.; Li, X.; Deng, H. Pectin/Lysozyme Bilayers Layer-by-Layer Deposited Cellulose Nanofibrous Mats for Antibacterial Application. Carbohydr. Polym. 2015, 117, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kadam, S.; Abee, T.; Slaghek, T.M.; Timmermans, J.W.; Cohen Stuart, M.A.; Norde, W.; Kleijn, M.J. Antimicrobial Lysozyme-Containing Starch Microgel to Target and Inhibit Amylase-Producing Microorganisms. Food Hydrocoll. 2012, 28, 28–35. [Google Scholar] [CrossRef]
- Li, D.; Liu, A.; Liu, M.; Li, X.; Guo, H.; Zuo, C.; Li, Y. The Intestine-Responsive Lysozyme Nanoparticles-in-Oxidized Starch Microgels with Mucoadhesive and Penetrating Properties for Improved Epithelium Absorption of Quercetin. Food Hydrocoll. 2020, 99, 105309. [Google Scholar] [CrossRef]
- Bayarri, M.; Oulahal, N.; Degraeve, P.; Gharsallaoui, A. Properties of Lysozyme/Low Methoxyl (LM) Pectin Complexes for Antimicrobial Edible Food Packaging. J. Food Eng. 2014, 131, 18–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Cui, H.; Li, B.; Tian, J. Construction and Application of Egcg-Loaded Lysozyme/Pectin Nanoparticles for Enhancing the Resistance of Nematodes to Heat and Oxidation Stresses. Foods 2021, 10, 1127. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Aminlari, M.; Forouzan, M.M.; Moghimi, E.; Tavana, M.; Shekarforoush, S.; Mohammadifar, M.A. Production and Application of Lysozyme-Gum Arabic Conjugate in Mayonnaise as a Natural Preservative and Emulsifier. Pol. J. Food Nutr. Sci. 2018, 68, 33–43. [Google Scholar] [CrossRef]
- Huang, W.; Wang, L.; Wei, Y.; Cao, M.; Xie, H.; Wu, D. Fabrication of Lysozyme/κ-Carrageenan Complex Nanoparticles as a Novel Carrier to Enhance the Stability and in Vitro Release of Curcumin. Int. J. Biol. Macromol. 2020, 146, 444–452. [Google Scholar] [CrossRef]
- Xu, W.; Jin, W.; Li, Z.; Liang, H.; Wang, Y.; Shah, B.R.; Li, Y.; Li, B. Synthesis and Characterization of Nanoparticles Based on Negatively Charged Xanthan Gum and Lysozyme. Food Res. Int. 2015, 71, 83–90. [Google Scholar] [CrossRef]
- Xu, W.; Jin, W.; Huang, K.; Huang, L.; Lou, Y.; Li, J.; Liu, X.; Li, B. Interfacial and Emulsion Stabilized Behavior of Lysozyme/Xanthan Gum Nanoparticles. Int. J. Biol. Macromol. 2018, 117, 280–286. [Google Scholar] [CrossRef]
- Xu, W.; Li, B.; Li, J.; Huang, L.; Liu, H.; Zhu, D.; Liu, M.; Liu, X. Rheological and Spectral Analysis of Xanthan Gum/Lysozyme System during Nanoparticle Fabrication. Int. J. Food Sci. Technol. 2018, 53, 2595–2601. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, X.; Ding, J.; Fan, F.; Li, P.; Xia, J.; Luo, X.; Fang, Y. Physicochemical and Functional Properties of a Novel Xanthan Gum-Lysozyme Nanoparticle Material Prepared by High Pressure Homogenization. LWT 2021, 143, 111136. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Yao, P.; Jiang, M. Lysozyme—Dextran Core—Shell Nanogels Prepared via a Green Process. Langmuir 2008, 24, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kato, A.; Kobayashi, K. New Antimicrobial Characteristics of Lysozyme-Dextran Conjugate. J. Agric. Food Chem. 1991, 39, 647–650. [Google Scholar] [CrossRef]
- Sheng, L.; Su, P.; Han, K.; Chen, J.; Cao, A.; Zhang, Z.; Jin, Y.; Ma, M. Synthesis and Structural Characterization of Lysozyme–Pullulan Conjugates Obtained by the Maillard Reaction. Food Hydrocoll. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, R.; Song, Y.; Yin, C.; Huang, H.; Li, Y. Electrospun Nanofiber Membranes Based on Dialdehyde Pullulan Polysaccharide and Schiff Base Crosslinked Lysozyme for Antibacterial Packaging of Blueberries. Food Biosci. 2025, 67, 106348. [Google Scholar] [CrossRef]
- Water, J.J.; Schack, M.M.; Velazquez-Campoy, A.; Maltesen, M.J.; Van De Weert, M.; Jorgensen, L. Complex Coacervates of Hyaluronic Acid and Lysozyme: Effect on Protein Structure and Physical Stability. Eur. J. Pharm. Biopharm. 2014, 88, 325–331. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Gao, J.; Chen, X.; Wang, K. Hyaluronic Acid/Lysozyme Self-Assembled Coacervate to Promote Cutaneous Wound Healing. Biomater. Sci. 2020, 8, 1702–1710. [Google Scholar] [CrossRef]
- Abdel-Wahhab, K.G.; Ashry, M.; Hassan, L.K.; Gadelmawla, M.H.A.; Elqattan, G.M.; El-Fakharany, E.M.; Mannaaa, F.A. Nano-Chitosan/Bovine Lactoperoxidase and Lactoferrin Formulation Modulates the Hepatic Deterioration Induced by 7,12-Dimethylbenz[a]Anthracene. Comp. Clin. Path 2023, 32, 981–991. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Ashry, M.; Abd-Elaleem, A.E.H.; Romeih, M.H.; Morsy, F.A.; Shaban, R.A.; Abdel-Wahhab, K.G. Therapeutic Efficacy of Nano-Formulation of Lactoperoxidase and Lactoferrin via Promoting Immunomodulatory and Apoptotic Effects. Int. J. Biol. Macromol. 2022, 220, 43–55. [Google Scholar] [CrossRef]
- Abu-Serie, M.M.; El-Fakharany, E.M. Efficiency of Novel Nanocombinations of Bovine Milk Proteins (Lactoperoxidase and Lactoferrin) for Combating Different Human Cancer Cell Lines. Sci. Rep. 2017, 7, 16769. [Google Scholar] [CrossRef]
- Nayeri, H.; Fattahi, A.; Iranpoor-Mobarakeh, M.; Nori, P. Stabilization of Lactoperoxidase by Tragacanth-Chitosan Nano Biopolymer. Int. J. Biosci. 2015, 6, 418–426. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Ferreira, N.C.A.; Fiocco, A.C.T.R.; Picone, C.S.F. Lactoferrin-Chitosan-TPP Nanoparticles: Antibacterial Action and Extension of Strawberry Shelf-Life. Food Bioproc. Technol. 2023, 16, 135–148. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Lema, M.I.; Otero-Espinar, F.J. Design, Optimization, and Characterization of Lactoferrin-Loaded Chitosan/TPP and Chitosan/Sulfobutylether-β-Cyclodextrin Nanoparticles as a Pharmacological Alternative for Keratoconus Treatment. ACS Appl. Mater. Interfaces 2021, 13, 3559–3575. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Picone, C.S.F. Antimicrobial Activity of Lactoferrin-Chitosan-Gellan Nanoparticles and Their Influence on Strawberry Preservation. Food Res. Int. 2022, 159, 111586. [Google Scholar] [CrossRef]
- Raei, M.; Rajabzadeh, G.; Zibaei, S.; Jafari, S.M.; Sani, A.M. Nano-Encapsulation of Isolated Lactoferrin from Camel Milk by Calcium Alginate and Evaluation of Its Release. Int. J. Biol. Macromol. 2015, 79, 669–673. [Google Scholar] [CrossRef]
- Reyhani, V.; Zibaee, S.; Mokaberi, P.; Amiri-Tehranizadeh, Z.; Babayan-Mashhadi, F.; Chamani, J. Encapsulation of Purified Lactoferrin from Camel Milk on Calcium Alginate Nanoparticles and Its Effect on Growth of Osteoblasts Cell Line MG-63. J. Iran. Chem. Soc. 2022, 19, 131–145. [Google Scholar] [CrossRef]
- Padrão, J.; Ribeiro, S.; Lanceros-Méndez, S.; Rodrigues, L.R.; Dourado, F. Effect of Bacterial Nanocellulose Binding on the Bactericidal Activity of Bovine Lactoferrin. Heliyon 2020, 6, e04372. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Alencar, W.M.P.; Iacuzio, R.; Silva, N.C.C.; Picone, C.S.F. Synthesis, Characterization and Application of Antibacterial Lactoferrin Nanoparticles. Curr. Res. Food Sci. 2022, 5, 642–652. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Mahmoud, H.E.; Embaby, A.M.; Haroun, M.; Sabra, S.A. Lactoferrin/Pectin Nanocomplex Encapsulating Ciprofloxacin and Naringin as a Lung Targeting Antibacterial Nanoplatform with Oxidative Stress Alleviating Effect. Int. J. Biol. Macromol. 2024, 261, 129842. [Google Scholar] [CrossRef]
- Yan, J.K.; Qiu, W.Y.; Wang, Y.Y.; Wu, J.Y. Biocompatible Polyelectrolyte Complex Nanoparticles from Lactoferrin and Pectin as Potential Vehicles for Antioxidative Curcumin. J. Agric. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; McClements, D.J.; Shi, M.; Shang, Q.; Liu, X.; Liu, F. Physicochemical and Functional Properties of Lactoferrin-Hyaluronic Acid Complexes: Effect of Non-Covalent and Covalent Interactions. LWT 2021, 151, 112121. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Edible Pickering Emulsions Stabilized by Ovotransferrin–Gum Arabic Particles. Food Hydrocoll. 2019, 89, 590–601. [Google Scholar] [CrossRef]
- Wei, Z.; Dong, Y.; Si, J. Ovotransferrin Fibril—Gum Arabic Complexes as Stabilizers for Oleogel-in-Water Pickering Emulsions: Formation Mechanism, Physicochemical Properties, and Curcumin Delivery. Foods 2024, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wei, Z.; Wang, Y.; Tang, Q.; Xue, C.; Huang, Q. Oleogel-Based Pickering Emulsions Stabilized by Ovotransferrin–Carboxymethyl Chitosan Nanoparticles for Delivery of Curcumin. LWT 2022, 157, 113121. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Xue, C. Effect of Interaction between Ovotransferrin Fibrils and Pectin on Properties of Oleogel-Based Pickering Emulsions. Food Hydrocoll. 2023, 140, 108620. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, P.; Huang, Q. Investigation of Ovotransferrin Conformation and Its Complexation with Sugar Beet Pectin. Food Hydrocoll. 2019, 87, 448–458. [Google Scholar] [CrossRef]
- Rishi, P.; Bhogal, A.; Arora, S.; Pandey, S.K.; Verma, I.; Kaur, I.P. Improved Oral Therapeutic Potential of Nanoencapsulated Cryptdin Formulation against Salmonella Infection. Eur. J. Pharm. Sci. 2015, 72, 27–33. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan Nanoparticles Loaded with the Antimicrobial Peptide Temporin B Exert a Long-Term Antibacterial Activity in Vitro against Clinical Isolates of Staphylococcus Epidermidis. Front. Microbiol. 2015, 6, 135235. [Google Scholar] [CrossRef]
- Okasha, H.; Dahroug, H.; Gouda, A.E.; Shemis, M.A. A Novel Antibacterial Approach of Cecropin-B Peptide Loaded on Chitosan Nanoparticles against MDR Klebsiella Pneumoniae Isolates. Amino Acids 2023, 55, 1965–1980. [Google Scholar] [CrossRef]
- Baturin, V.A.; Bolatchiev, A.D.; Zhirov, A.M.; Kovalev, D.A. The antibacterial activity spectrum of defensin HNP-1 included in to the chitosan nanoparticles. Med. News North Cauc. 2021, 16, 413–414. [Google Scholar] [CrossRef]
- Rashki, S.; Safardoust-Hojaghan, H.; Mirzaei, H.; Abdulsahib, W.K.; Mahdi, M.A.; Salavati-Niasari, M.; Khaledi, A.; Khorshidi, A.; Mousavi, S.G.A. Delivery LL37 by Chitosan Nanoparticles for Enhanced Antibacterial and Antibiofilm Efficacy. Carbohydr. Polym. 2022, 291, 119634. [Google Scholar] [CrossRef] [PubMed]
- Turky, N.O.; Abdelmonem, N.A.; Tammam, S.N.; Gad, M.Z.; Breitinger, H.G.; Breitinger, U. Antibacterial and in Vitro Anticancer Activities of the Antimicrobial Peptide NRC-07 Encapsulated in Chitosan Nanoparticles. J. Pept. Sci. 2024, 30, e3550. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Oliver-Ortega, H.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Andreu, D. Immobilization of Antimicrobial Peptides onto Cellulose Nanopaper. Int. J. Biol. Macromol. 2017, 105, 741–748. [Google Scholar] [CrossRef]
- Blasi-Romero, A.; Ångström, M.; Franconetti, A.; Muhammad, T.; Jiménez-Barbero, J.; Göransson, U.; Palo-Nieto, C.; Ferraz, N. KR-12 Derivatives Endow Nanocellulose with Antibacterial and Anti-Inflammatory Properties: Role of Conjugation Chemistry. ACS Appl. Mater. Interfaces 2023, 15, 24186–24196. [Google Scholar] [CrossRef]
- Román, J.T.; Fuenmayor, C.A.; Dominguez, C.M.Z.; Clavijo-Grimaldo, D.; Acosta, M.; García-Castañeda, J.E.; Fierro-Medina, R.; Rivera-Monroy, Z.J. Pullulan Nanofibers Containing the Antimicrobial Palindromic Peptide LfcinB (21–25)Pal Obtained via Electrospinning. RSC Adv. 2019, 9, 20432–20438. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Xi, G.; Wang, M.; Liang, B.; Shi, Y.; Feng, Y.; Ren, X.; Shi, C. Fabricating Antimicrobial Peptide-Immobilized Starch Sponges for Hemorrhage Control and Antibacterial Treatment. Carbohydr. Polym. 2019, 222, 115012. [Google Scholar] [CrossRef]
- van Gent, M.E.; Kłodzińska, S.N.; Severin, M.; Ali, M.; van Doodewaerd, B.R.; Bos, E.; Koning, R.I.; Drijfhout, J.W.; Nielsen, H.M.; Nibbering, P.H. Encapsulation into Hyaluronic Acid-Based Nanogels Improves the Selectivity Index of the Snake Cathelicidin Ab-Cath. Nanomedicine 2023, 52, 102694. [Google Scholar] [CrossRef]
- Deng, X.; Wang, H.B.; Fang, C.; Xu, M.; Chu, Z.; Li, M.; Hou, Z.; Qin, H. Hyaluronic Acid Based Nanoparticles That Mediate Sustained Thanatin Release Protect against NDM-1–Resistant Bacterial Infections in a Murine Model. Nanomedicine 2025, 63, 102796. [Google Scholar] [CrossRef]
- Jugl, A.; Pekař, M. Hyaluronan-Cecropin B Interactions Studied by Ultrasound Velocimetry and Isothermal Titration Calorimetry. Int. J. Biol. Macromol. 2023, 227, 786–794. [Google Scholar] [CrossRef]
- Bafort, F.; Parisi, O.; Perraudin, J.P.; Jijakli, M.H. Mode of Action of Lactoperoxidase as Related to Its Antimicrobial Activity: A Review. Enzyme Res. 2014, 2014, 517164. [Google Scholar] [CrossRef]
- Barberis, S.; Quiroga, H.G.; Barcia, C.; Talia, J.M.; Debattista, N. Natural Food Preservatives Against Microorganisms. In Food Safety and Preservation; Grumezescu, A.M., Holban., A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 621–658. [Google Scholar] [CrossRef]
- Kelly, P.; Woonton, B.W.; Smithers, G.W. Improving the Sensory Quality, Shelf-Life and Functionality of Milk. In Functional and Speciality Beverage Technology; Paquin, P., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 170–231. [Google Scholar] [CrossRef]
- Wolfson, L.M.; Sumner, S.S. Antibacterial Activity of the Lactoperoxidase System: A Review. J. Food Prot. 1993, 56, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 550441. [Google Scholar] [CrossRef] [PubMed]
- Jańczuk, A.; Brodziak, A.; Czernecki, T.; Król, J. Lactoferrin—The Health-Promoting Properties and Contemporary Application with Genetic Aspects. Foods 2022, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hancock, R.E.W. Antimicrobial Properties of Lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; He, J.; Zhu, W. Antibacterial Properties of Lactoferrin: A Bibliometric Analysis from 2000 to Early 2022. Front. Microbiol. 2022, 13, 947102. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Liu, F.; Luo, Y. Recent Development of Egg Protein Fractions and Individual Proteins as Encapsulant Materials for Delivery of Bioactives. Food Chem. 2023, 403, 134353. [Google Scholar] [CrossRef]
- Rathnapala, E.C.N.; Ahn, D.U.; Abeyrathne, S. Functional Properties of Ovotransferrin from Chicken Egg White and Its Derived Peptides: A Review. Food Sci. Biotechnol. 2021, 30, 619–630. [Google Scholar] [CrossRef]
- Antimicrobial Peptide Database. Available online: https://aps.unmc.edu/ (accessed on 28 April 2025).
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Ali, M.; Garg, A.; Srivastava, A.; Arora, P.K. The Role of Antimicrobial Peptides in Overcoming Antibiotic Resistance. Microbe 2025, 7, 100337. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Dash, R.; Bhattacharjya, S. Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action. Int. J. Mol. Sci. 2021, 22, 1522. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.; Kamau, P.M.; Thuku, R.C.; Lai, R. Design Methods for Antimicrobial Peptides with Improved Performance. Zool. Res. 2023, 44, 1095. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- van der Weide, H.; Cossío, U.; Gracia, R.; te Welscher, Y.M.; ten Kate, M.T.; van der Meijden, A.; Marradi, M.; Ritsema, J.A.S.; Vermeulen-De Jongh, D.M.C.; Storm, G.; et al. Therapeutic Efficacy of Novel Antimicrobial Peptide AA139-Nanomedicines in a Multidrug-Resistant Klebsiella Pneumoniae Pneumonia-Septicemia Model in Rats. Antimicrob. Agents Chemother. 2020, 64, e00517-20. [Google Scholar] [CrossRef]
- Silva, J.P.; Gonçalves, C.; Costa, C.; Sousa, J.; Silva-Gomes, R.; Castro, A.G.; Pedrosa, J.; Appelberg, R.; Gama, F.M. Delivery of LLKKK18 Loaded into Self-Assembling Hyaluronic Acid Nanogel for Tuberculosis Treatment. J. Control. Release 2016, 235, 112–124. [Google Scholar] [CrossRef]
- Osorio-Alvarado, C.E.; Ropero-Vega, J.L.; Farfán-García, A.E.; Flórez-Castillo, J.M. Immobilization Systems of Antimicrobial Peptide Ib−M1 in Polymeric Nanoparticles Based on Alginate and Chitosan. Polymers 2022, 14, 3149. [Google Scholar] [CrossRef]
- Salvati, B.; Flórez-Castillo, J.M.; Santagapita, P.R.; Barja, B.C.; Perullini, M. One-Pot Synthesis of Alginate-Antimicrobial Peptide Nanogel. Photochem. Photobiol. Sci. 2024, 23, 665–679. [Google Scholar] [CrossRef]
- Schibeci, M.; Gaglione, R.; Russo, N.; Velotta, R.; Della Ventura, B.; Arciello, A. Enzymatically Crafted Bacterial Cellulose Nanoparticles Functionalized With Antimicrobial Peptides: Toward Sustainable Antimicrobial Formulations. Biotechnol. J. 2025, 20, e202400573. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Wang, S.; Dong, C.; Zhang, X.; Zhang, R.; Yang, L. Dextran and Peptide-Based PH-Sensitive Hydrogel Boosts Healing Process in Multidrug-Resistant Bacteria-Infected Wounds. Carbohydr. Polym. 2022, 278, 118994. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).