1. Introduction
The global energy crisis has intensified in recent decades due to the growing demand for energy and the progressive depletion of fossil fuels, such as oil, coal, and natural gas [
1]. As the global population increases and economies develop, the demand for electricity has increased exponentially, leading to increased exploitation of these non-renewable resources [
2]. However, this exploitation has resulted in significant environmental consequences, including climate change and pollution, which highlights the urgency of adopting more sustainable energy sources [
3]. In response to this challenge, renewable energies, including solar, wind, hydro, and biomass, have emerged as viable alternatives to meet global energy demand while reducing greenhouse gas emissions [
4].
Among renewable energy sources, biomass is distinguished by its capacity to provide sustainable energy and to reduce dependence on fossil fuels [
5]. In Spain, biomass has become a particularly salient topic due to the abundance of agricultural, forestry, and urban waste that can be utilised as a raw material. In 2020, energy production from municipal solid waste (MSW) incineration in Spain constituted a significant proportion of the country’s renewable energy mix [
6]. This process not only contributes to electricity generation, but also facilitates waste management by transforming materials that would otherwise be destined for landfill into a useful energy source [
7,
8].
However, the generation of solid wastes, such as fly ash and bottom ash, represents a significant environmental challenge [
9]. The ashes in question contain heavy metals and other pollutants that, in the absence of appropriate management, have the potential to leach into the soil and subsequently contaminate surrounding water bodies [
10,
11]. Nevertheless, recent research has investigated the potential for the reuse of these wastes in the manufacture of new materials, such as additives in cements and concretes, which could mitigate their environmental impact and provide added value [
12,
13,
14]. A number of studies have demonstrated that the use of biomass bottom ash can enhance the mechanical properties and durability of building materials [
15,
16,
17,
18], opening up new opportunities for the circular economy in the energy and construction sectors. However, fly ash generated by municipal solid waste incineration is currently disposed of in landfills, without any economically viable recovery options and at an environmental cost.
On the other hand, the construction sector is one of the world’s largest consumers of raw materials, which has a significant impact on the environment. The extraction, processing, and transportation of materials such as cement, steel, and aggregates not only require large amounts of energy, but also contribute to environmental degradation and greenhouse gas emissions. In fact, cement production, which is fundamental to construction, is responsible for around 8% of global CO
2 emissions [
19]. In addition, the sector consumes around 50% of extracted natural resources, making it one of the largest contributors to resource depletion and waste generation [
20]. This situation has highlighted the need to look for sustainable alternatives that minimise the environmental impacts associated with construction [
21,
22,
23].
In response to growing concerns about sustainability, new building materials have been developed that incorporate industrial and agricultural waste, helping to reduce the carbon footprint and conserve natural resources. For example, fly ash, blast furnace slag, glass waste, recycled tyres, and demolition waste have been used to create more sustainable building materials [
24,
25,
26,
27]. These materials not only help to reduce the amount of waste sent to landfill, but also improve the technical properties of the end products, such as durability, strength, and thermal insulation [
28,
29,
30]. In addition, the use of waste in the production of building materials can reduce the demand for virgin raw materials, which in turn reduces the pressure on ecosystems and the energy required for production [
31,
32,
33].
Along with the most innovative sustainable materials are geopolymers, which were discovered by the French scientist Joseph Davidovits in the 1970s [
34]. Geopolymers are inorganic materials produced by alkaline activation of aluminosilicates, such as coal fly ash, metakaolin, and blast furnace slag [
35,
36]. This chemical process, which takes place at low temperatures, results in a material with similar or superior properties to those of traditional Portland cement, but with a significantly lower carbon footprint [
37]. Success stories in the use of geopolymers include the production of concretes and mortars with improved properties, such as high compressive strength, resistance to aggressive conditions, and better thermal performance [
38,
39]. Furthermore, by using industrial waste as a raw material, geopolymers contribute to waste reduction and by-product valorisation, which strengthens their position as an environmentally favourable solution compared with other conventional materials [
40,
41].
Based on this, this research proposes the development of a geopolymeric material using a mining residue as a source of aluminosilicates. This geopolymer was added with fly ash from the incineration of municipal solid waste, considered hazardous waste and currently of no use. More specifically, the fly ash used in this research came from the gas neutralisation stage (hereafter FAN).
To this end, the highest-quality geopolymer made from the mining residue and various NaOH solutions was initially studied and then the ashes under study were added to it. The samples thus manufactured were physically and mechanically evaluated to determine whether this material could become a substitute for the ceramic material. At the same time, and in order to determine that there was no environmental impact with this new material, leachate tests were carried out.
3. Results and Discussion
The results obtained from the tests described in the methodology are presented in this section in a structured manner. Partial conclusions derived from the experimental data are included and the decisions made during the research process to achieve the stated objectives are detailed. This approach allows for a clear and systematic evaluation of the findings and facilitates the interpretation of their impact on the research.
3.1. Physical and Chemical Characterisation of Fly Ash Produced by Municipal Solid Waste Incineration After the Neutralisation Stage (FAN)
The physical and chemical properties of MSW incineration ashes were first determined to evaluate their characteristics and the possible problems with incorporating them into geopolymers. The particle density of the FAN was found to be 2.70 Tn/m3. This is similar to the particle density of normal aggregates and materials, which is usually around 2.65 Tn/m3. The similar density means there should be no problems mixing the FAN and mine waste.
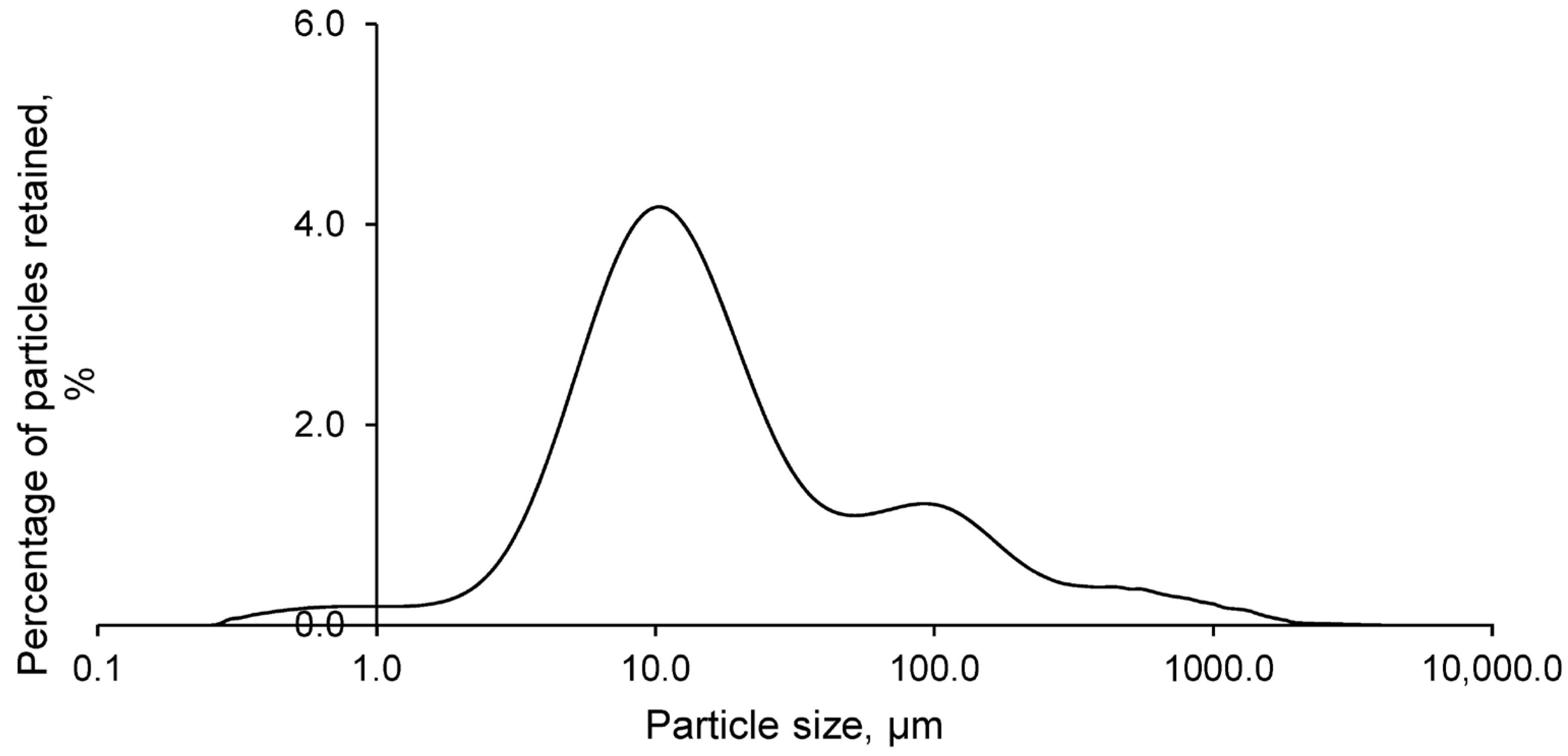
The particle size analysis of the FANs, as shown in
Figure 3, revealed that the particle size varied between 5 and 500 μm. However, most of the particles were concentrated around 10 μm. This small particle size contributed to the hazardousness of HABs, as it facilitated their dispersion and transport into the environment. However, this characteristic also favoured the retention of the residue in the geopolymer matrix, benefiting the material forming process.
Additionally, an elemental analysis was performed to determine the percentages of carbon, nitrogen, and hydrogen present in the FAN. The results of this test are presented in
Table 5. This analysis is fundamental for the chemical characterisation of the FANs and provides key information on their chemical composition and potential environmental impact.
Analysis of fly ash (FAN) revealed very low contents of carbon, nitrogen, and hydrogen, indicating a low percentage of organic matter and hydrated compounds in the ash. The virtual absence of carbon and hydrogen suggests that the FAN does not contain carbonate or hydrated compounds, although other volatile components may be present.
To determine the composition of the elements present in the higher atomic weight FAN, an X-ray fluorescence (XRF) assay was performed. The results of the analysis are presented in
Table 6.
The X-ray fluorescence assay showed a significantly high ignition loss of 77.34%, indicating an unstable nature of the ash, with the presence of volatile elements and unstable chemical compounds that were transformed during the analysis. This high instability suggests a potential environmental risk due to the ability of FAN to disperse in aquatic environments.
Among the chemical elements detected, chlorine is present in the highest proportion. This element may negatively affect the quality of the geopolymer and requires further evaluation. Sodium, potassium, sulphur, calcium and silicon were also detected, which is expected given the role of the neutralisation system and the injection of sodium bicarbonate in the incineration process. On the other hand, metals such as zinc, tin and lead are present in smaller proportions, derived from the incinerated materials. The presence of these potentially contaminating metals in FAN underscores the need to carefully evaluate the geopolymeric material incorporated in FAN to ensure that hazardous leachates are not produced.
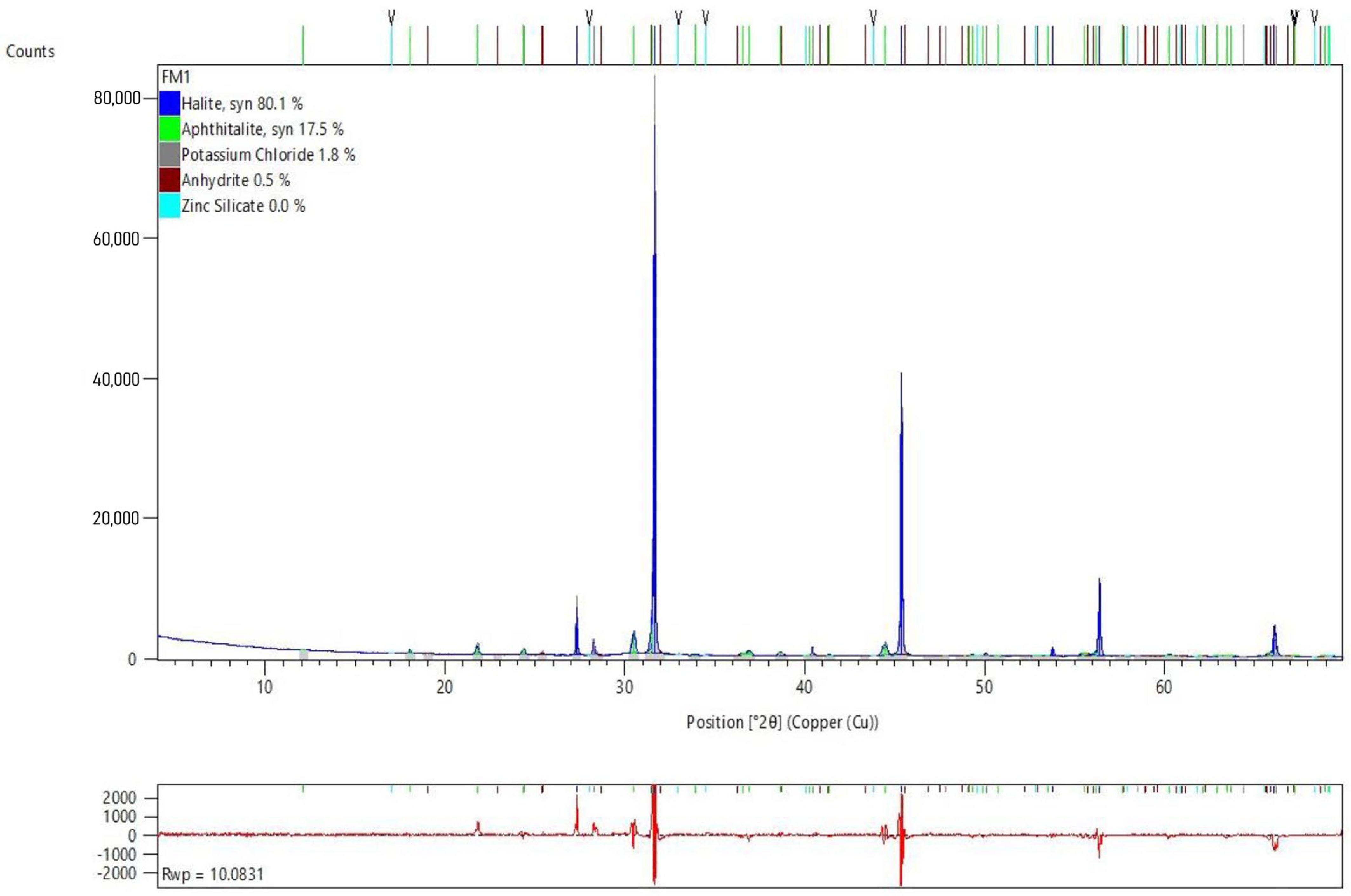
In order to determine the chemical compounds in which the above chemical elements are combined, as well as to evaluate their greater or lesser chemical instability, the X-ray diffraction test was performed. The results of this test are shown in
Figure 4.
X-ray diffraction (XRD) analysis revealed that the predominant chemical composition of the fly ash (FAN) consists mainly of halite (NaCl) and aphthalite (K, Na)3Na(SO4)2. This composition is consistent with the origin of the ashes, coming from the neutralisation of gases generated during municipal solid waste incineration, in which large amounts of sulphur and chlorine are produced. Both sodium chloride (NaCl) and potassium sulphate (K2SO4) are soluble in water, and in high concentrations could modify soil and water chemistry, negatively affecting local ecosystems.
In addition to these major compounds, XRD analysis identified other compounds in smaller proportions, such as potassium chloride (KCl), anhydrite (CaSO4), and zinc silicate (Zn2(SiO4)). These compounds also present potential environmental problems similar to those of the main compounds detected. The presence of zinc silicate, while not extremely toxic to humans, can easily transfer to soil and surface or groundwater, causing significant environmental impacts and affecting organisms and microorganisms in these environments.
Consequently, the small particle size of FAN, together with its unstable chemical composition and the presence of potentially toxic metallic elements, classifies this ash as a hazardous waste. The main motivation of this research is to avoid the deposition of these ashes in landfills, where they could be transferred to the environment, and to ensure their safe retention in the geopolymer matrix, as detailed in later sections.
3.2. Conforming of Geopolymer Samples with the Mining Residue and FAN—Physical and Mechanical Tests
As detailed in the methodology, the first step in the development of the geopolymer consisted of using the mining residue as a source of aluminosilicate, which was alkaline-activated by means of sodium hydroxide solutions at different concentrations (0%, 2.5%, 5%, 7.5%, and 10%) in relation to the mass of the mining residue. To these solutions, 20% of water was added in relation to the mass of the mining residue.
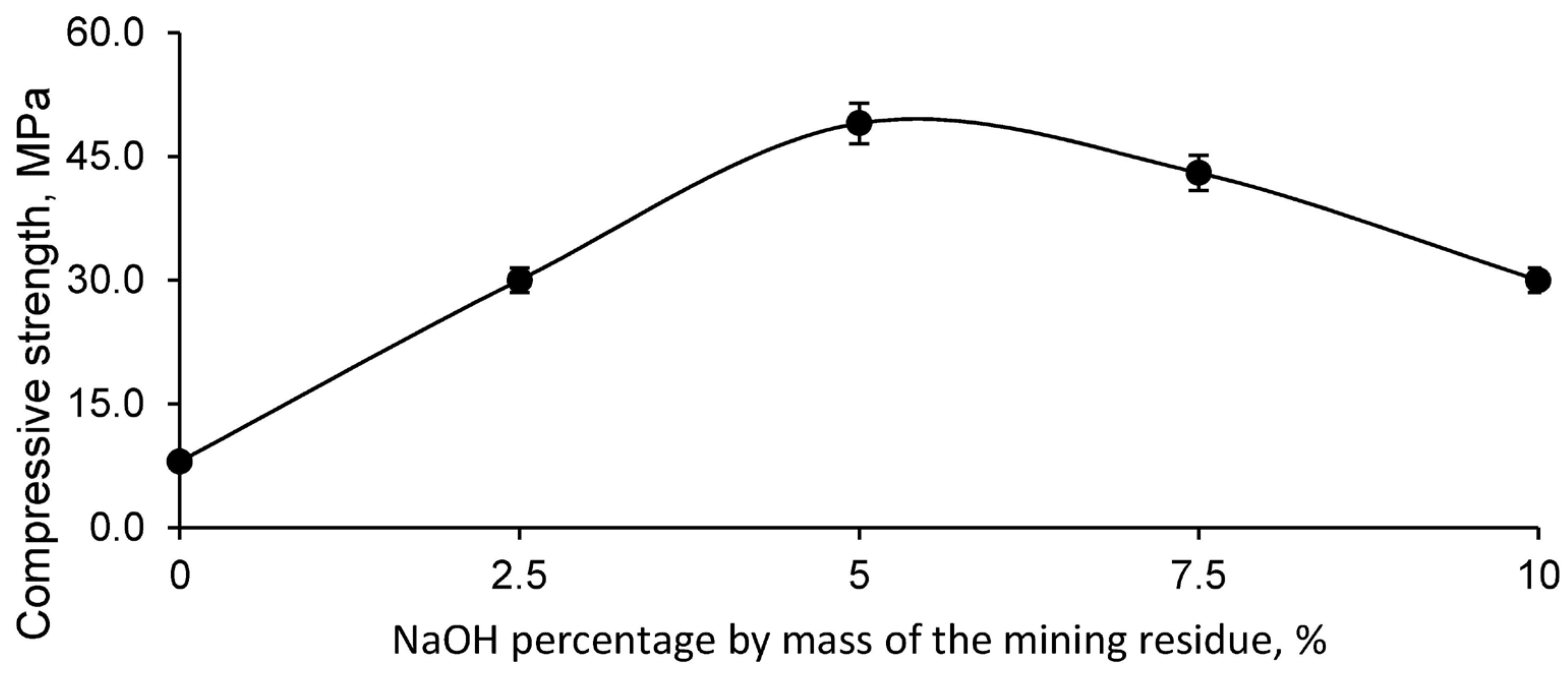
The main criterion for determining the optimum geopolymer was the compressive strength, since the aim was to obtain a geopolymeric material with the highest possible strength for construction applications. The results obtained from the compressive strength test for the different geopolymer mixtures, formed with the mining residue and different concentrations of NaOH, are shown in
Figure 5.
As illustrated in
Figure 5, the geopolymer made from the mining residue showed the highest compressive strength when using a 5% NaOH dilution in the mass of the mining residue, with an addition of 20% water over the mass of the residue. This combination proved to be the most effective in terms of mechanical strength. Consequently, the geopolymer formulated for the incorporation of different percentages of fly ash (FAN) was based on this optimum geopolymer formulation, as detailed in
Table 3.
The different geopolymer formulations with various percentages of FAN, presented in
Table 3, were prepared following the procedure described in the methodology. Subsequently, physical tests were carried out on the samples obtained, the results of which are shown in
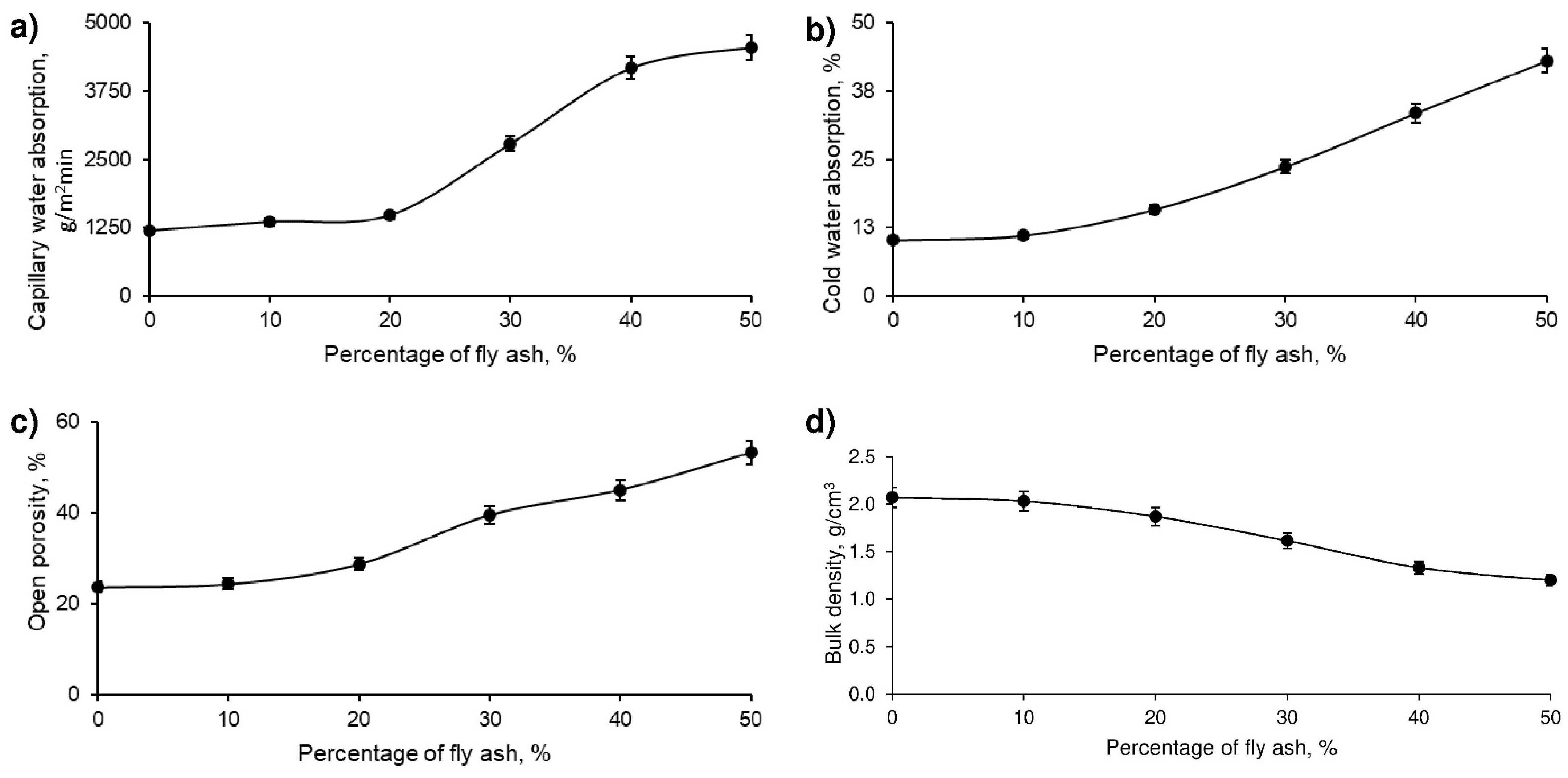
Figure 6. These tests made it possible to objectively evaluate the impact of the addition of FAN on the physical properties of the geopolymer.
Figure 6 illustrates that capillary water absorption increased with the increase in the percentage of fly ash (FAN) in the developed geopolymer. This increase was due to the formation of a structure with a greater number of interconnected channels in the material, which facilitates greater water absorption. This behaviour can be explained by Jurin’s law. Considering that ceramic materials in direct contact with wet soils should limit their capillary water absorption to 4000 g/m
2-min, samples with 50% FAN exceed this threshold and would be considered unsuitable for applications in such conditions.
On the other hand, cold water absorption also increases with increasing percentage of FAN in the geopolymer. Although this property is not regulated by the standards used, it is crucial for building materials exposed to the elements, such as roof tiles, since higher water absorption can increase the weight of the material and, therefore, the structural load. It is relevant to note that the variation in cold water absorption is minimal when introducing 10% or even 20% FAN in the geopolymer.
Moreover, the porosity of the geopolymer material, as expected, increases with the percentage of FAN. This increase in porosity indicates a more open and less dense structure of the material. In construction applications for thermal and acoustic insulation, higher porosity can be advantageous, as it reduces the thermal and acoustic conductivity of the material.
Finally, in correlation with the previous results, it is observed that the bulk density decreases as the percentage of FAN in the geopolymer increases. Since the densities of the mining residue and FAN are similar, this decrease in density reflects a more porous structure of the material, which may negatively affect its strength. However, it is important to note that the variation in bulk density is reduced in geopolymer series with 10% and even 20% FAN.
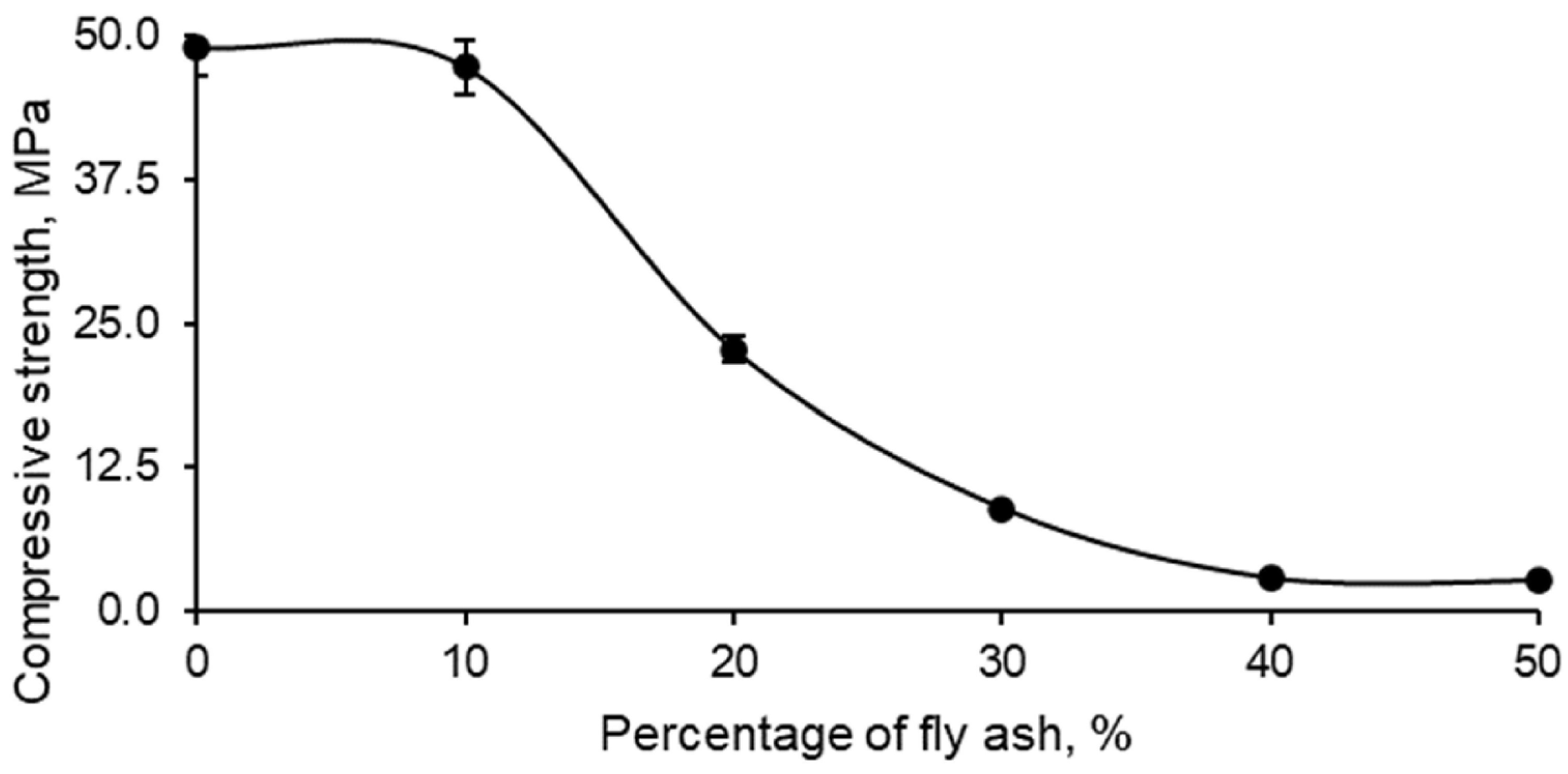
In relation to the evaluation of the strength of the different series of samples formed with geopolymer and FAN, the results obtained from the compressive strength test are shown in
Figure 7.
The compressive strength of geopolymers produced with fly ash (FAN) showed a clear tendency to decrease with increasing percentage of FAN. This behaviour can be attributed to the characteristics observed in previous tests, where it was established that greater water absorption was due to an increase in porosity, which in turn decreased the density of the material and, consequently, its compressive strength.
Figure 7 presents this decrease in compressive strength as a function of the percentage of FAN incorporated.
In particular, it is observed that the addition of 10% FAN to the geopolymer did not cause a significant variation in compressive strength. This indicates that the geopolymer material retained acceptable properties in terms of strength, water absorption, density, and porosity. In contrast, the addition of 20% FAN resulted in a drastic 50% reduction in compressive strength, which implied a notable loss of this fundamental mechanical property. The addition of higher percentages of FAN, such as 30%, 40% and 50%, led to a material with practically zero strength, making it unsuitable for structural applications. However, these percentages could be useful for alternative applications such as thermal and acoustic insulation, although these uses are not part of the scope of this research.
Finally, in order to accurately determine the colour of the different series of samples, chromatic coordinate measurements were carried out using the equipment specified in the methodology. The results of these measurements are presented in
Table 7.
As in previous properties, it can be seen how the coordinates of the primary colours varied with the additions of FAN, creating a chromatic of colours that, far from being subjectively valued, are simply presented in
Figure 8.
3.3. Leachate Testing of FAN Formed Geopolymeric Samples
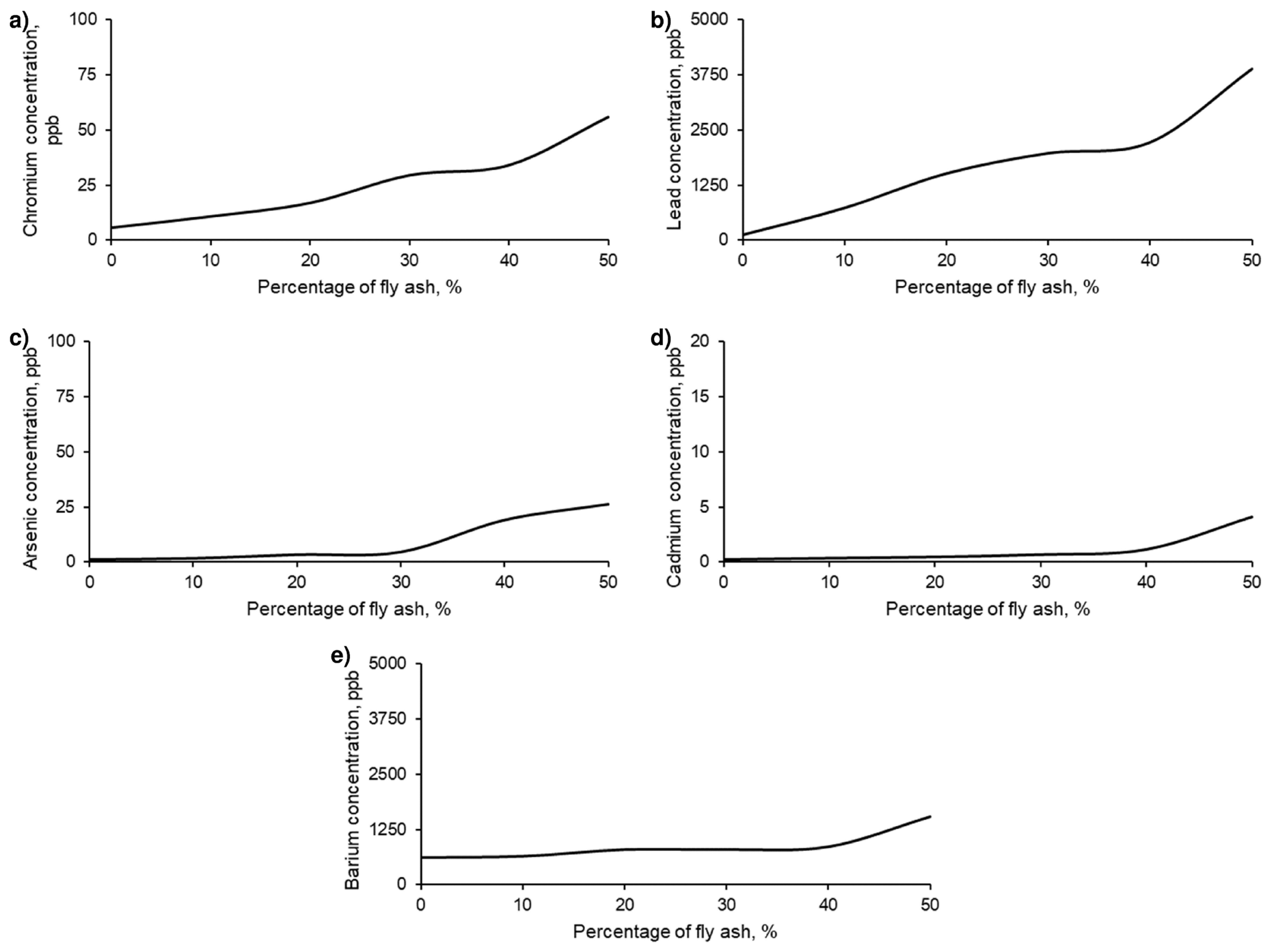
The objective of this research is the valorisation of fly ash (FAN) in the development of new sustainable materials for construction. Up to this point, the feasibility and impact of incorporating FAN in geopolymeric materials have been evaluated. However, it is essential to ensure that these ashes are adequately retained in the material matrix and to avoid leaching of contaminants that may cause adverse environmental impacts. Therefore, it is crucial to analyse leachates from geopolymeric materials made with FAN and ensure that no contaminant leachates are generated.
For all series of samples, the leachates were analysed according to the regulations and limitations specified in the methodology. The results of these analyses are presented in
Figure 9.
4. Conclusions
The results and discussion of the tests performed permit an evaluation of the fulfilment of the research objective. The principal aim was to develop a geopolymeric material utilising mining residue, which could incorporate fly ash from the incineration of municipal solid waste after its neutralisation, with physical and mechanical properties comparable to those of traditional ceramic materials. From the tests conducted, partial conclusions were derived that are fundamental for the interpretation and determination of the final conclusion of this research.
The mining residue from the lead mine dump in the Linares mining district has a chemical composition suitable for being a source of aluminosilicates in geopolymeric materials;
Incineration fly ash (FAN) has a small particle size, high solubility, and significant metal content, which classifies it as a hazardous waste with environmental risk if not properly treated;
It is feasible to formulate geopolymers with the mining residue alkaline-activated with a 5% NaOH solution. These geopolymers show a compressive strength of 50 MPa and physical properties suitable as a substitute for construction ceramics (bricks, tiles);
The incorporation of FAN in the geopolymeric material decreases the physical and mechanical quality, increasing the porosity and reducing the density and compressive strength. However, additions of up to 20% FAN have a lesser effect than higher concentrations;
Geopolymeric samples with 40% and 50% FAN have unacceptable compressive strengths according to standards, making them unviable for commercialisation. However, additions of 10% and 20% show acceptable values, exceeding the resistance limits;
Geopolymer leachates with different percentages of FAN show concentrations of potentially toxic elements, according to US-EPA regulations, below the maximum permitted levels.
Based on the partial conclusions obtained and the tests performed, it can be affirmed that it is feasible to manufacture geopolymers using mining residues incorporating FAN in their matrix, achieving sustainable construction materials with acceptable physical, chemical, and mechanical properties. This approach represents a clear example of circular economy, offering a viable solution for waste that would otherwise be destined for landfill, generating significant environmental and economic costs.
Looking ahead, these materials show potential for non-structural applications in the construction sector, such as blocks, tiles, pavements, or partition elements. Furthermore, this research opens the door to future lines of investigation focused on further optimising the formulation through the incorporation of additional waste streams or alternative precursors, as well as long-term durability studies, environmental exposure performance, and life-cycle assessment (LCA) to evaluate their true sustainability impact.