Study of Total Ammoniacal Nitrogen Recovery Using Polymeric Thin-Film Composite Membranes for Continuous Operation of a Hybrid Membrane System
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Solutions and Membranes
2.2. Experimental Setup
2.3. Filtration Procedure
3. Results and Discussion
3.1. Membrane Performance
3.2. Filtration of Ammonium Salts with RO and NF Membranes
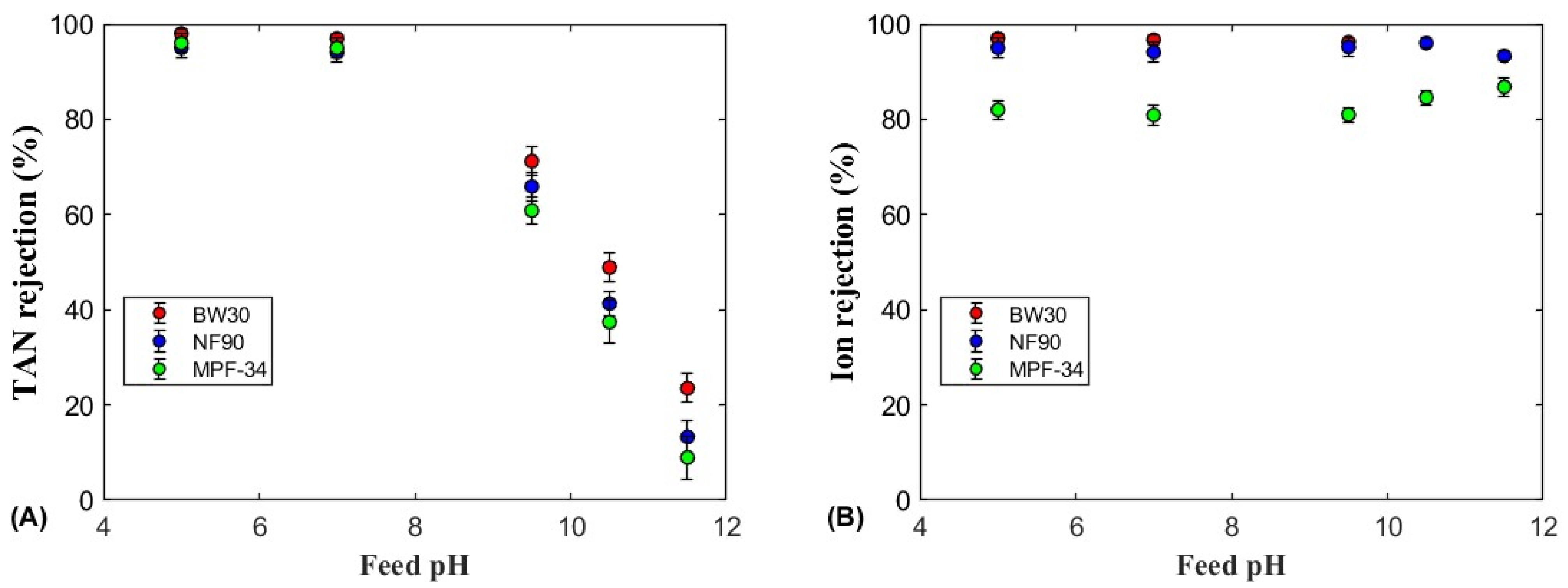
3.2.1. TAN Rejection
3.2.2. Ion Rejection
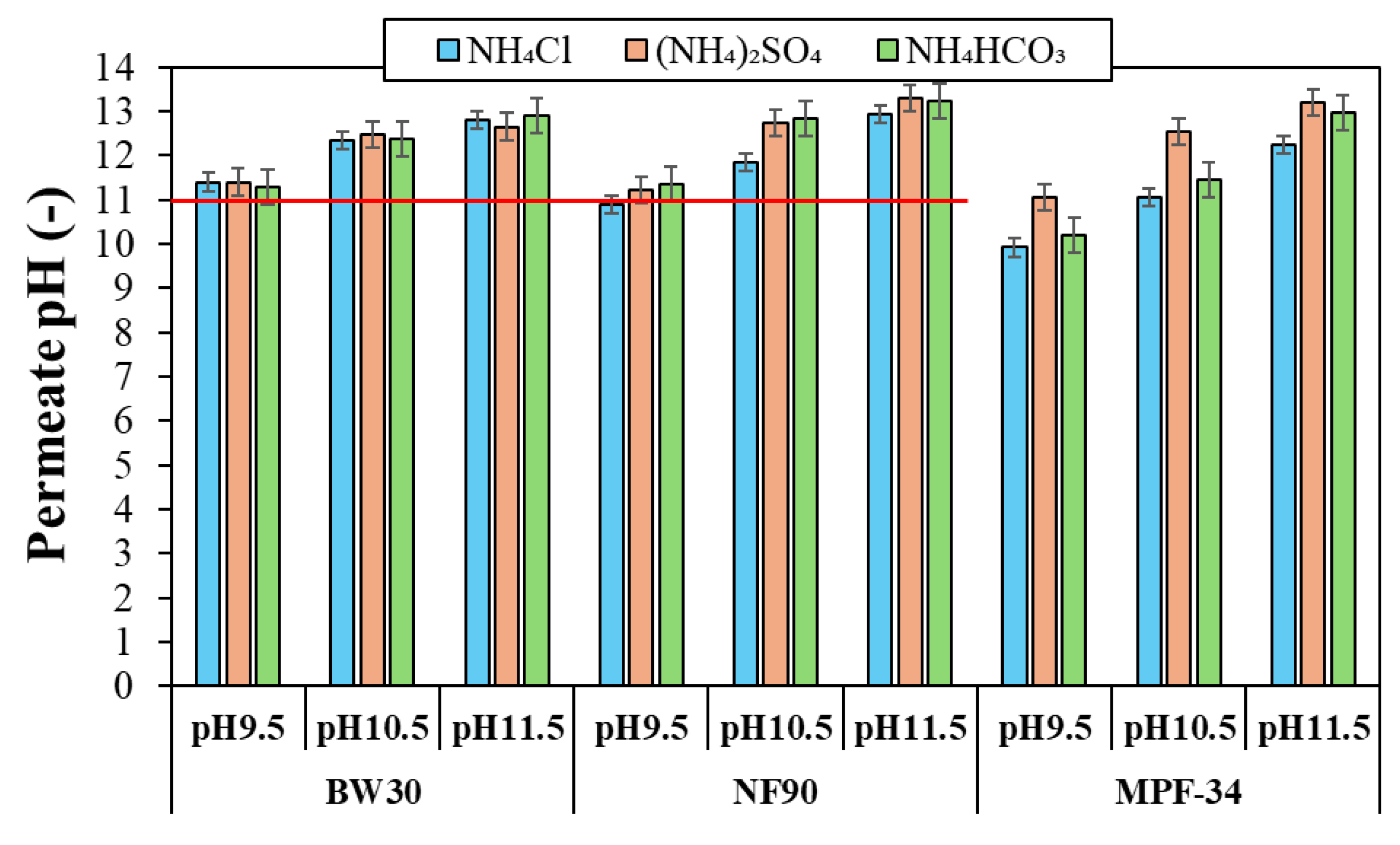
3.2.3. The Role of Feed pH on TAN Rejection
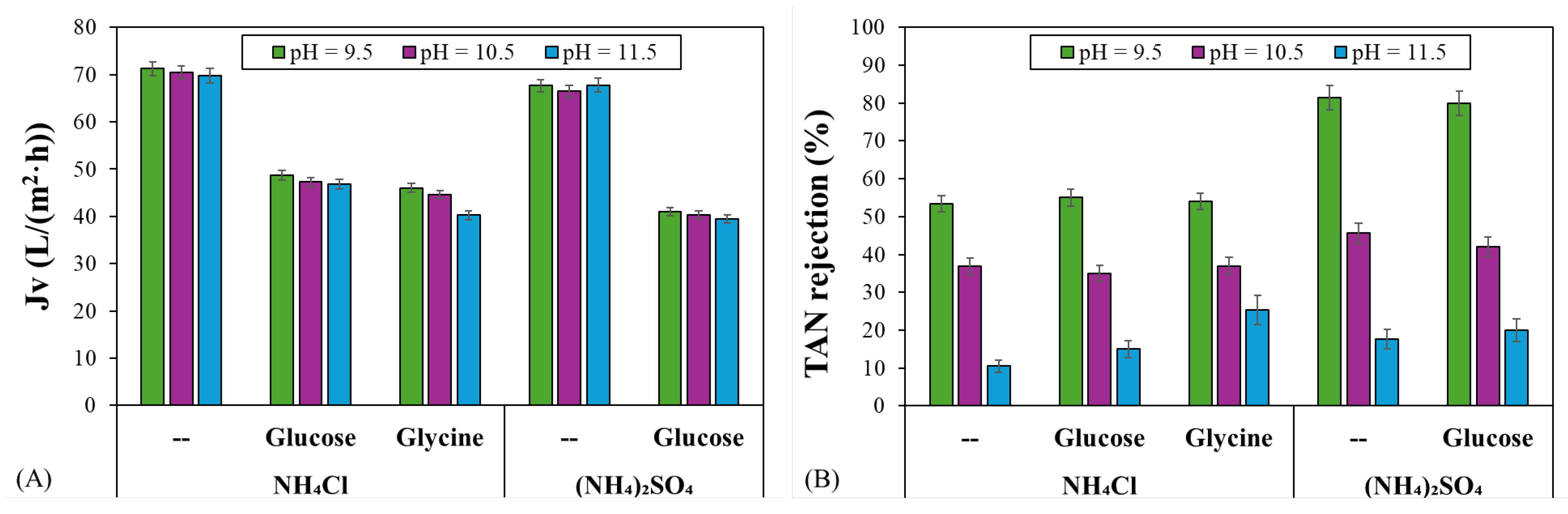
3.3. Effect of Organic Matter on TAN Rejection
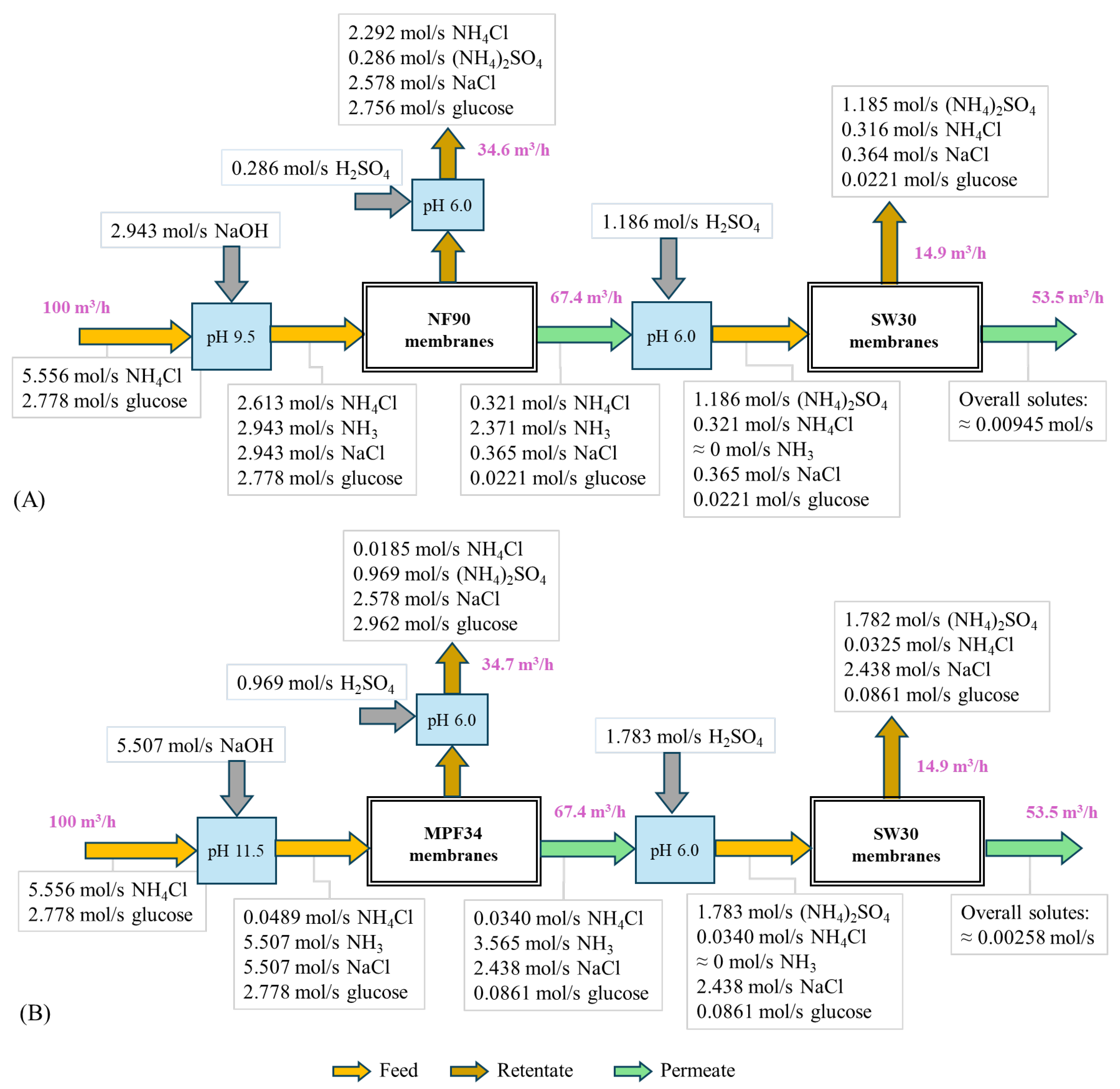
3.4. Evaluation of Hybrid Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abou-Elanwar, A.M.; Lee, S.; Jang, I.; Lee, S.; Hong, S.; Kim, S.; Hwang, M.H.; Kim, J.; Kim, Y. Evaluation of a Novel Fertilizer-Drawn Forward Osmosis and Membrane Contactor Hybrid System for CO2 Capture Using Ammonia-Rich Wastewater. J. Memb. Sci. 2024, 700, 122654. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Kumar, S.M. Microalgae Biomass from Swine Wastewater and Its Conversion to Bioenergy. Bioresour. Technol. 2019, 275, 109–122. [Google Scholar] [CrossRef]
- Robles, Á.; Aguado, D.; Barat, R.; Borrás, L.; Bouzas, A.; Giménez, J.B.; Martí, N.; Ribes, J.; Ruano, M.V.; Serralta, J.; et al. New Frontiers from Removal to Recycling of Nitrogen and Phosphorus from Wastewater in the Circular Economy. Bioresour. Technol. 2020, 300, 122673. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T.; Wang, Z.; Wang, Y.; Zhong, H.; Shen, P.; Wei, Y. Effects of Magnetite on Anaerobic Digestion of Swine Manure: Attention to Methane Production and Fate of Antibiotic Resistance Genes. Bioresour. Technol. 2019, 291, 121847. [Google Scholar] [CrossRef]
- Livolsi, S.; Franz, S.; Costa, A.; Buoio, E.; Bazzocchi, C.; Bestetti, M.; Selli, E.; Chiarello, G.L. Innovative Photoelectrocatalytic Water Remediation System for Ammonia Abatement. Catal. Today 2023, 413–415, 113996. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Blatchley, E.R.; Wang, X.; Ren, P. UV/Chlorine Process for Ammonia Removal and Disinfection by-Product Reduction: Comparison with Chlorination. Water Res. 2015, 68, 804–811. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Verstraete, W. Resource Recovery from Used Water: The Manufacturing Abilities of Hydrogen-Oxidizing Bacteria. Water Res. 2015, 68, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Larriba, O.; Rovira-Cal, E.; Juznic-Zonta, Z.; Guisasola, A.; Baeza, J.A. Evaluation of the Integration of P Recovery, Polyhydroxyalkanoate Production and Short Cut Nitrogen Removal in a Mainstream Wastewater Treatment Process. Water Res. 2020, 172, 115474. [Google Scholar] [CrossRef]
- Putra, R.N.; Lee, Y.H. Entrapment of Micro-Sized Zeolites in Porous Hydrogels: Strategy to Overcome Drawbacks of Zeolite Particles and Beads for Adsorption of Ammonium Ions. Sep. Purif. Technol. 2020, 237, 116351. [Google Scholar] [CrossRef]
- Shin, C.; Szczuka, A.; Jiang, R.; Mitch, W.A.; Criddle, C.S. Optimization of Reverse Osmosis Operational Conditions to Maximize Ammonia Removal from the Effluent of an Anaerobic Membrane Bioreactor. Environ. Sci. 2021, 7, 739–747. [Google Scholar] [CrossRef]
- Wu, H.; Vaneeckhaute, C. Nutrient Recovery from Wastewater: A Review on the Integrated Physicochemical Technologies of Ammonia Stripping, Adsorption and Struvite Precipitation. Chem. Eng. J. 2022, 433, 133664. [Google Scholar] [CrossRef]
- Cancino-Madariaga, B.; Hurtado, C.F.; Ruby, R. Effect of Pressure and PH in Ammonium Retention for Nanofiltration and Reverse Osmosis Membranes to Be Used in Recirculation Aquaculture Systems (RAS). Aquac. Eng. 2011, 45, 103–108. [Google Scholar] [CrossRef]
- van Voorthuizen, E.M.; Zwijnenburg, A.; Wessling, M. Nutrient Removal by NF and RO Membranes in a Decentralized Sanitation System. Water Res. 2005, 39, 3657–3667. [Google Scholar] [CrossRef]
- Tarek, S.; Jamil, I.D.; Sayed, S. Usage of Permeate Water for Treated Domestic Wastewater by Direct Capillary Nanofiltration Membrane in Agriculture Reuse. Desalination Water Treat 2013, 51, 2584–2591. [Google Scholar] [CrossRef]
- Jia, X.; Lan, L.; Zhang, X.; Wang, T.; Wang, Y.; Ye, C.; Lin, J. Pilot-Scale Vacuum Membrane Distillation for Decontamination of Simulated Radioactive Wastewater: System Design and Performance Evaluation. Sep. Purif. Technol. 2021, 275, 119129. [Google Scholar] [CrossRef]
- He, Q.; Tu, T.; Yan, S.; Yang, X.; Duke, M.; Zhang, Y.; Zhao, S. Relating Water Vapor Transfer to Ammonia Recovery from Biogas Slurry by Vacuum Membrane Distillation. Sep. Purif. Technol. 2018, 191, 182–191. [Google Scholar] [CrossRef]
- Lee, W.; An, S.; Choi, Y. Ammonia Harvesting via Membrane Gas Extraction at Moderately Alkaline PH: A Step toward Net-Profitable Nitrogen Recovery from Domestic Wastewater. Chem. Eng. J. 2021, 405, 126662. [Google Scholar] [CrossRef]
- Shahgodari, S.; Llorens, J.; Labanda, J. Viability of Total Ammoniacal Nitrogen Recovery Using a Polymeric Thin-Film Composite Forward Osmosis Membrane: Determination of Ammonia Permeability Coefficient. Polymers 2024, 16, 1834. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad, S. Forward Osmosis Membrane Technology for Nutrient Removal/Recovery from Wastewater: Recent Advances, Proposed Designs, and Future Directions. Chemosphere 2021, 263, 128116. [Google Scholar] [CrossRef]
- Thörneby, L.; Persson, K.; Trägårdh, G. Treatment of Liquid Effluents from Dairy Cattle and Pigs Using Reverse Osmosis. J. Agric. Eng. Res. 1999, 73, 159–170. [Google Scholar] [CrossRef]
- Escoda, A.; Fievet, P.; Lakard, S.; Szymczyk, A.; Déon, S. Influence of Salts on the Rejection of Polyethyleneglycol by an NF Organic Membrane: Pore Swelling and Salting-out Effects. J. Memb. Sci. 2010, 347, 174–182. [Google Scholar] [CrossRef]
- Labanda, J.; Shahgodari, S.; Llorens, J. Influence of pH and NaCl on the Rejection of Glycine and Triglycine in Binary Solutions for Desalination with Diananofiltration. Heliyon 2023, 9, e16797. [Google Scholar] [CrossRef] [PubMed]
- Masse, L.; Massé, D.I.; Pellerin, Y. The Effect of PH on the Separation of Manure Nutrients with Reverse Osmosis Membranes. J. Memb. Sci. 2008, 325, 914–919. [Google Scholar] [CrossRef]
- Popova, A.; Rattanakom, R.; Yu, Z.-Q.; Li, Z.; Nakagawa, K.; Fujioka, T. Evaluating the Potential of Nanofiltration Membranes for Removing Ammonium, Nitrate, and Nitrite in Drinking Water Sources. Water Res. 2023, 244, 120484. [Google Scholar] [CrossRef]
- Ray, H.; Perreault, F.; Boyer, T.H. Rejection of Nitrogen Species in Real Fresh and Hydrolyzed Human Urine by Reverse Osmosis and Nanofiltration. J. Envrion. Chem. Eng. 2020, 8, 103993. [Google Scholar] [CrossRef]
- Ray, H.; Perreault, F.; Boyer, T.H. Ammonia Recovery and Fouling Mitigation of Hydrolyzed Human Urine Treated by Nanofiltration and Reverse Osmosis. Environ. Sci. Water Res. Technol. 2022, 8, 429–442. [Google Scholar] [CrossRef]
- Helgeson, H.C.; Kirkham, D.H.; Flowers, G.C. Theoretical Prediction of the Thermodynamic Behavior of Aqueous Electrolytes by High Pressures and Temperatures; IV, Calculation of Activity Coefficients, Osmotic Coefficients, and Apparent Molal and Standard and Relative Partial Molal Properties to 600 Degrees C and 5kb. Am. J. Sci. 1981, 281, 1249–1516. [Google Scholar] [CrossRef]
- García-González, M.C.; Vanotti, M.B.; Szogi, A.A. Recovery of Ammonia from Swine Manure Using Gas-Permeable Membranes: Effect of Aeration. J. Envrion. Manag. 2015, 152, 19–26. [Google Scholar] [CrossRef]
- Idil Mouhoumed, E.; Szymczyk, A.; Schäfer, A.; Paugam, L.; La, Y.H. Physico-Chemical Characterization of Polyamide NF/RO Membranes: Insight from Streaming Current Measurements. J. Memb. Sci. 2014, 461, 130–138. [Google Scholar] [CrossRef]
- Koutsou, C.P.; Yiantsios, S.G.; Karabelas, A.J. A Numerical and Experimental Study of Mass Transfer in Spacer-Filled Channels: Effects of Spacer Geometrical Characteristics and Schmidt Number. J. Memb. Sci. 2009, 326, 234–251. [Google Scholar] [CrossRef]
- Dražević, E.; Košutić, K.; Dananić, V. Mass Transfer of Differently Sized Organic Solutes at Spacer Covered and Permeable Nanofiltration Wall. Chem. Eng. J. 2014, 244, 152–159. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Vecino, X.; Gibert, O.; Cortina, J.L. Comparison of Acid-Resistant Ceramic and Polymeric Nanofiltration Membranes for Acid Mine Waters Treatment. Chem. Eng. J. 2020, 382, 122786. [Google Scholar] [CrossRef]
- Sharma, U.; Shalini, S.; Basu, S.; Saravanan, P.; Jang, M. Active Layer Modification of Commercial Nanofiltration Membrane Using CuBTC/PVA Matrix for Improved Surface and Separation Characteristics. J. Appl. Polym. Sci. 2021, 138, app50508. [Google Scholar] [CrossRef]
- Ricci, B.C.; Ferreira, C.D.; Marques, L.S.; Martins, S.S.; Reis, B.G.; Amaral, M.C.S. Assessment of the Chemical Stability of Nanofiltration and Reverse Osmosis Membranes Employed in Treatment of Acid Gold Mining Effluent. Sep. Purif. Technol. 2017, 174, 301–311. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration Membranes Review: Recent Advances and Future Prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Hilal, N.; Kochkodan, V.; Al Abdulgader, H.; Johnson, D. A Combined Ion Exchange–Nanofiltration Process for Water Desalination: II. Membr. Sel. Desalination 2015, 363, 51–57. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Mohammad, A.W.; Darwish, N.A. Nanofiltration of Highly Concentrated Salt Solutions up to Seawater Salinity. Desalination 2005, 184, 315–326. [Google Scholar] [CrossRef]
- Kelewou, H.; Lhassani, A.; Merzouki, M.; Drogui, P.; Sellamuthu, B. Salts Retention by Nanofiltration Membranes: Physicochemical and Hydrodynamic Approaches and Modeling. Desalination 2011, 277, 106–112. [Google Scholar] [CrossRef]
- Koter, S. Determination of the Parameters of the Spiegler–Kedem–Katchalsky Model for Nanofiltration of Single Electrolyte Solutions. Desalination 2006, 198, 335–345. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Al-Shaeli, M.; Al Aani, S.; Johnson, D.; Hilal, N. Membrane Technologies for Nitrogen Recovery from Waste Streams: Scientometrics and Technical Analysis. Membranes 2023, 13, 15. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Dube, P.J.; Szogi, A.A.; García-González, M.C. Recovery of Ammonia and Phosphate Minerals from Swine Wastewater Using Gas-Permeable Membranes. Water Res. 2017, 112, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kurama, H.; Poetzschke, J.; Haseneder, R. The Application of Membrane Filtration for the Removal of Ammonium Ions from Potable Water. Water Res. 2002, 36, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lueptow, R.M. Reverse Osmosis Filtration for Space Mission Wastewater: Membrane Properties and Operating Conditions. J. Memb. Sci. 2001, 182, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wan, Y. Effects of PH and Salt on Nanofiltration—A Critical Review. J. Memb. Sci. 2013, 438, 18–28. [Google Scholar] [CrossRef]
- Yoon, Y.; Lueptow, R.M. Reverse Osmosis Membrane Rejection for Ersatz Space Mission Wastewaters. Water Res. 2005, 39, 3298–3308. [Google Scholar] [CrossRef]
- Dalwani, M.; Benes, N.E.; Bargeman, G.; Stamatialis, D.; Wessling, M. Effect of pH on the Performance of Polyamide/Polyacrylonitrile Based Thin Film Composite Membranes. J. Memb. Sci. 2011, 372, 228–238. [Google Scholar] [CrossRef]
- Shahgodari, S.; Labanda, J.; Llorens, J. Experimental and Modeling Study of the Nanofiltration of Alcohol-Based Molecules and Amino Acids by Commercial Membranes. Membranes 2023, 13, 631. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Relating Nanofiltration Membrane Performance to Membrane Charge (Electrokinetic) Characteristics. Envrion. Sci. Technol. 2000, 34, 3710–3716. [Google Scholar] [CrossRef]
- Boussu, K.; Zhang, Y.; Cocquyt, J.; Van der Meeren, P.; Volodin, A.; Van Haesendonck, C.; Martens, J.A.; Van der Bruggen, B. Characterization of Polymeric Nanofiltration Membranes for Systematic Analysis of Membrane Performance. J. Memb. Sci. 2006, 278, 418–427. [Google Scholar] [CrossRef]
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Degradation of Polyamide Nanofiltration and Reverse Osmosis Membranes by Hypochlorite. Envrion. Sci. Technol. 2012, 46, 852–859. [Google Scholar] [CrossRef]
- Lasisi, K.H.; Ajibade, T.F.; Zhang, K. Degradation Impact of Low PH Mineral Acids and Long Exposure Period on the Active Layer of Semi-Aromatic Polyamine-Based Nanofiltration Membrane. Polym. Degrad. Stab. 2022, 200, 109941. [Google Scholar] [CrossRef]
- Marín, N.G.-V.; López-Ramírez, J.A. Influence of Organic Fouling and Operating Conditions on Nanofiltration Membranes to Reduce Phenol Concentration in Natural Waters. Water Supply 2011, 11, 473–480. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A Mini-Review on Membrane Fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Vyas, B.B.; Ray, P. Preparation of Nanofiltration Membranes and Relating Surface Chemistry with Potential and Topography: Application in Separation and Desalting of Amino Acids. Desalination 2015, 362, 104–116. [Google Scholar] [CrossRef]
- Qi, B.; Luo, J.; Chen, X.; Hang, X.; Wan, Y. Separation of Furfural from Monosaccharides by Nanofiltration. Bioresour. Technol. 2011, 102, 7111–7118. [Google Scholar] [CrossRef]
- Bosch, L.I.; Fyles, T.M.; James, T.D. Binary and Ternary Phenylboronic Acid Complexes with Saccharides and Lewis Bases. Tetrahedron 2004, 60, 11175–11190. [Google Scholar] [CrossRef]
- Tang, C.Y.; Fu, Q.S.; Robertson, A.P.; Criddle, C.S.; Leckie, J.O. Use of Reverse Osmosis Membranes to Remove Perfluorooctane Sulfonate (PFOS) from Semiconductor Wastewater. Envrion. Sci. Technol. 2006, 40, 7343–7349. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A Review of Pressure-Driven Membrane Processes in Wastewater Treatment and Drinking Water Production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Spanish Ministry of Environment; Spanish Ministry of Agriculture, Food and Fisheries; Spanish Ministry of Health. Royal Decree 1620/2007 of 7 December, Regulating Water Reuse; Boletín Oficial del Estado: Madrid, Spain, 2007; pp. 50639–50661. [Google Scholar]
- Malaeb, L.; Ayoub, G.M. Reverse Osmosis Technology for Water Treatment: State of the Art Review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Courtney, C.; Randall, D.G. A Hybrid Nanofiltration and Reverse Osmosis Process for Urine Treatment: Effect on Urea Recovery and Purity. Water Res. 2022, 222, 118851. [Google Scholar] [CrossRef]
- Pei, K.; Xiao, K.; Hou, H.; Tao, S.; Xu, Q.; Liu, B.; Yu, Z.; Yu, W.; Wang, H.; Xue, Y.; et al. Improvement of Sludge Dewaterability by Ammonium Sulfate and the Potential Reuse of Sludge as Nitrogen Fertilizer. Envrion. Res. 2020, 191, 110050. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Huang, H.; Mao, M.; Liu, E. An Investigation of the Effect of Ammonium Sulfate Addition on Compound Fertilizer Granulation. Particuology 2017, 31, 54–58. [Google Scholar] [CrossRef]
- Rietra, R.; Van Dijk, K.; Schoumans, O. Environmental Effects of Using Ammonium Sulfate from Animal Manure Scrubbing Technology as Fertilizer. Appl. Sci. 2024, 14, 4998. [Google Scholar] [CrossRef]
- Hartz, T.K.; Smith, R.; Gaskell, M. Nitrogen Availability from Liquid Organic Fertilizers. HortTechnology Horttech 2010, 20, 169–172. [Google Scholar] [CrossRef]
- European Commission. A New Circular Economy Action Plan—For Cleaner and more Competitive Europe. Publications Office of European Commission: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Kurniawati, A.; Stankovics, P.; Hilmi, Y.S.; Toth, G.; Smol, M.; Toth, Z. Understanding the Future of Bio-Based Fertilisers: The EU’s Policy and Implementation. Sustain. Chem. Clim. Action 2023, 3, 100033. [Google Scholar] [CrossRef]
- González-García, I.; Riaño, B.; Cuéllar-Franca, R.M.; Molinuevo-Salces, B.; García-González, M.C. Environmental Sustainability Performance of a Membrane-Based Technology for Livestock Wastewater Treatment with Nutrient Recovery. J. Envrion. Chem. Eng. 2022, 10, 107246. [Google Scholar] [CrossRef]
- Lee, S.; Lueptow, R.M. Membrane Rejection of Nitrogen Compounds. Envrion. Sci. Technol. 2001, 35, 3008–3018. [Google Scholar] [CrossRef]
- Ahunbay, M.G.; Tantekin-Ersolmaz, S.B.; Krantz, W.B. Energy Optimization of a Multistage Reverse Osmosis Process for Seawater Desalination. Desalination 2018, 429, 1–11. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Moritz, E.; Hobisch, M.; Muster-Slawitsch, B. Recovery of Ammonia from Centrate Water in Urban Waste Water Treatment Plants via Direct Contact Membrane Distillation: Process Performance in Long-Term Pilot-Scale Operation. J. Memb. Sci. 2023, 667, 121161. [Google Scholar] [CrossRef]
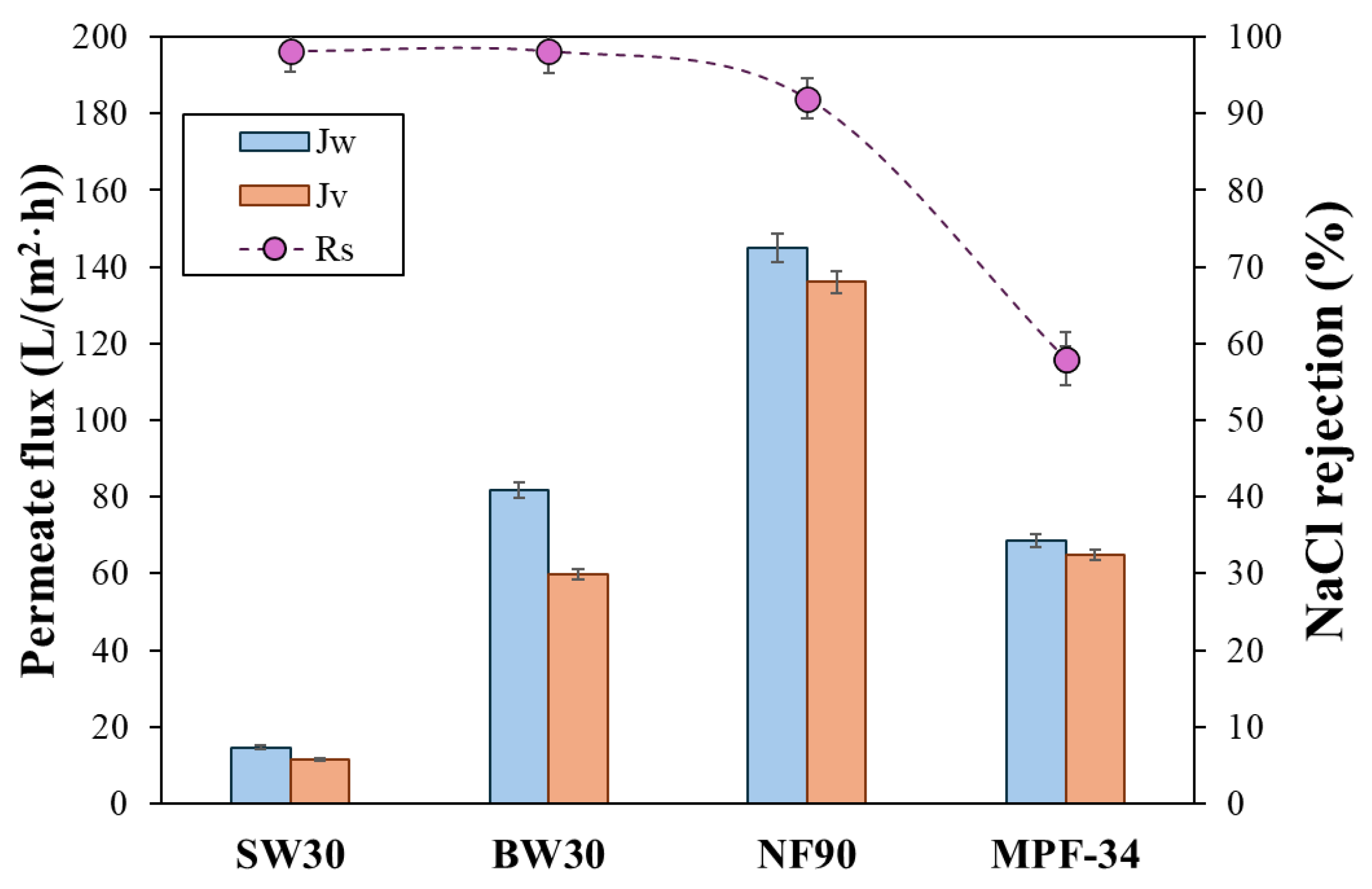
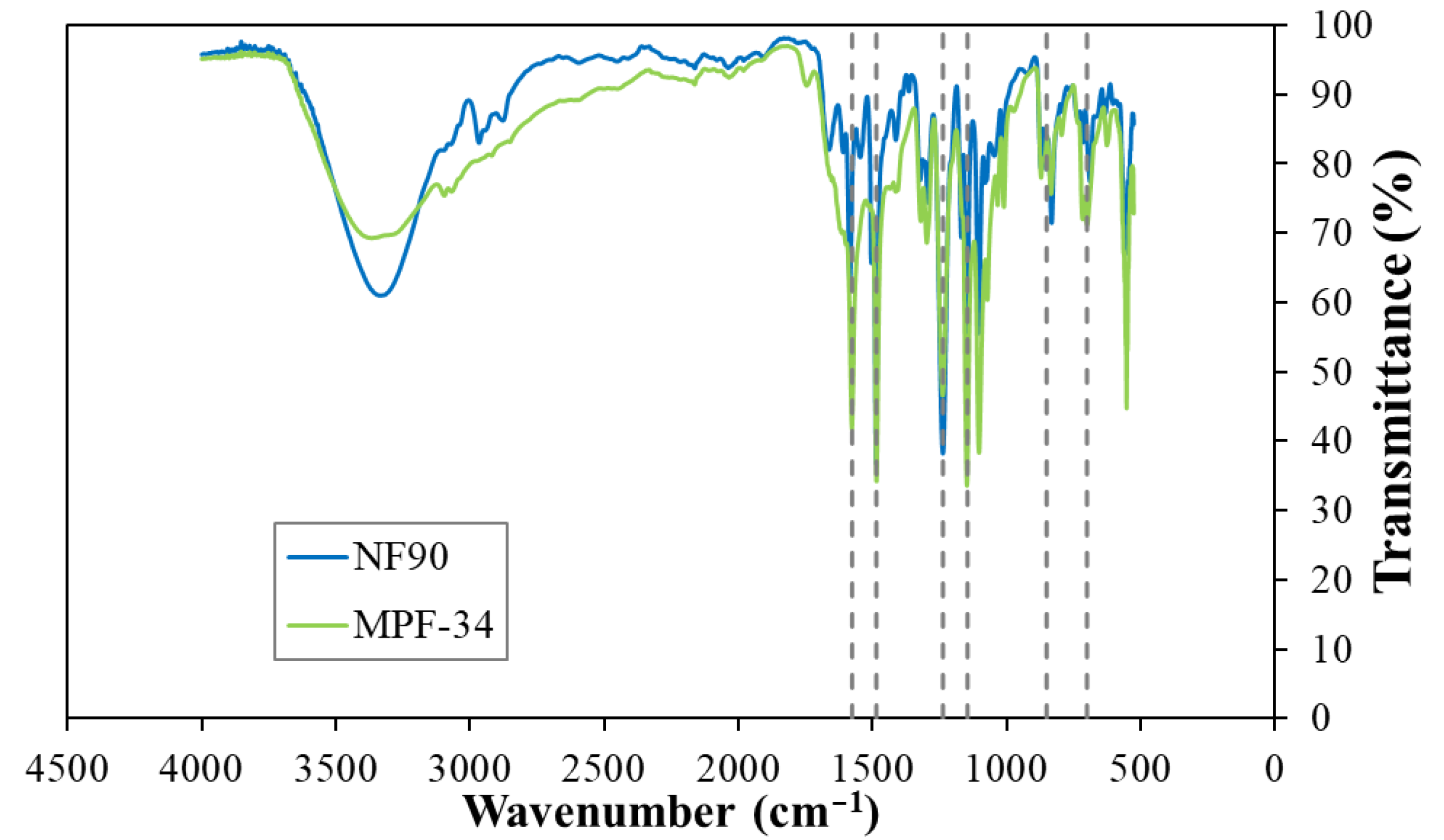
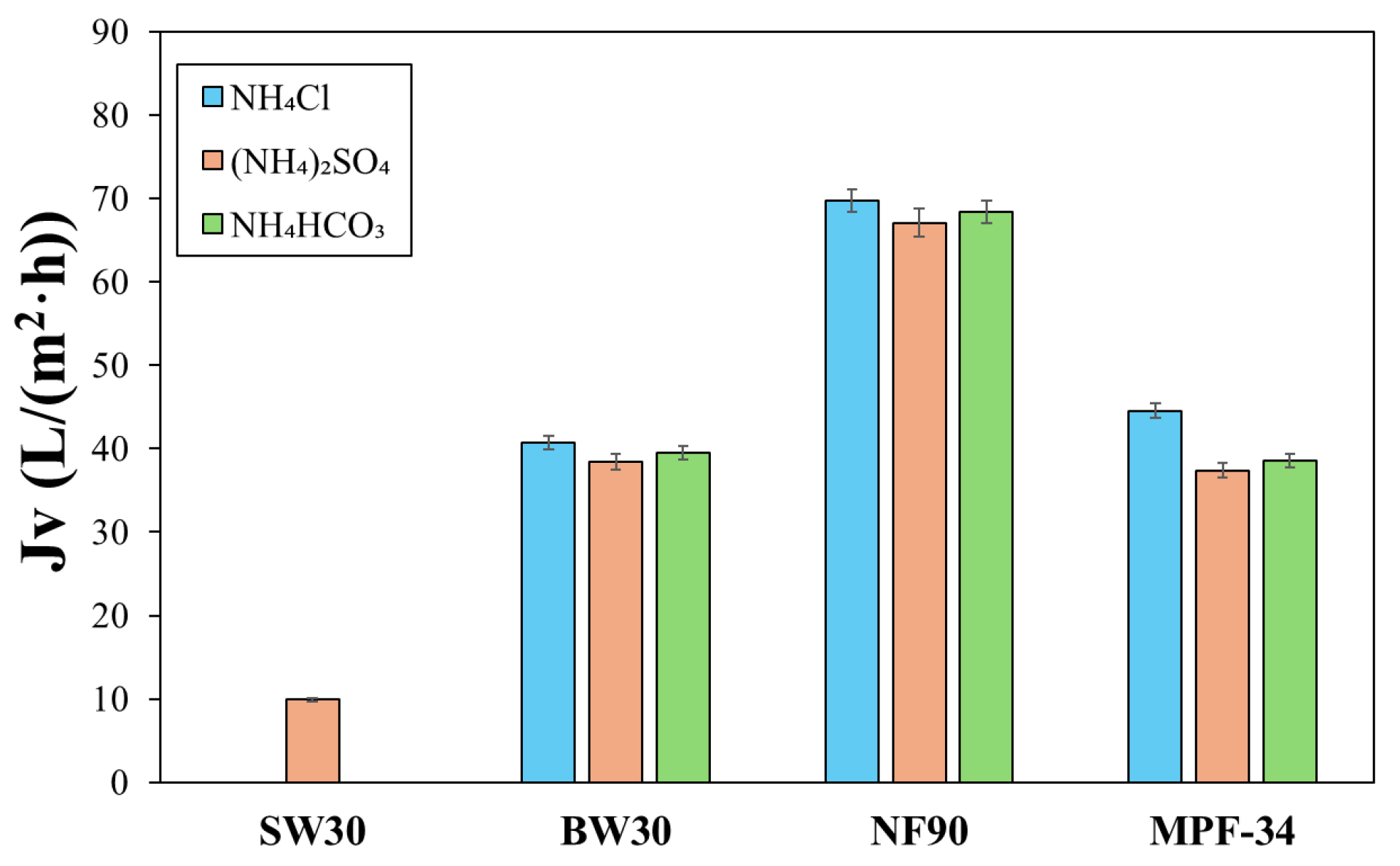
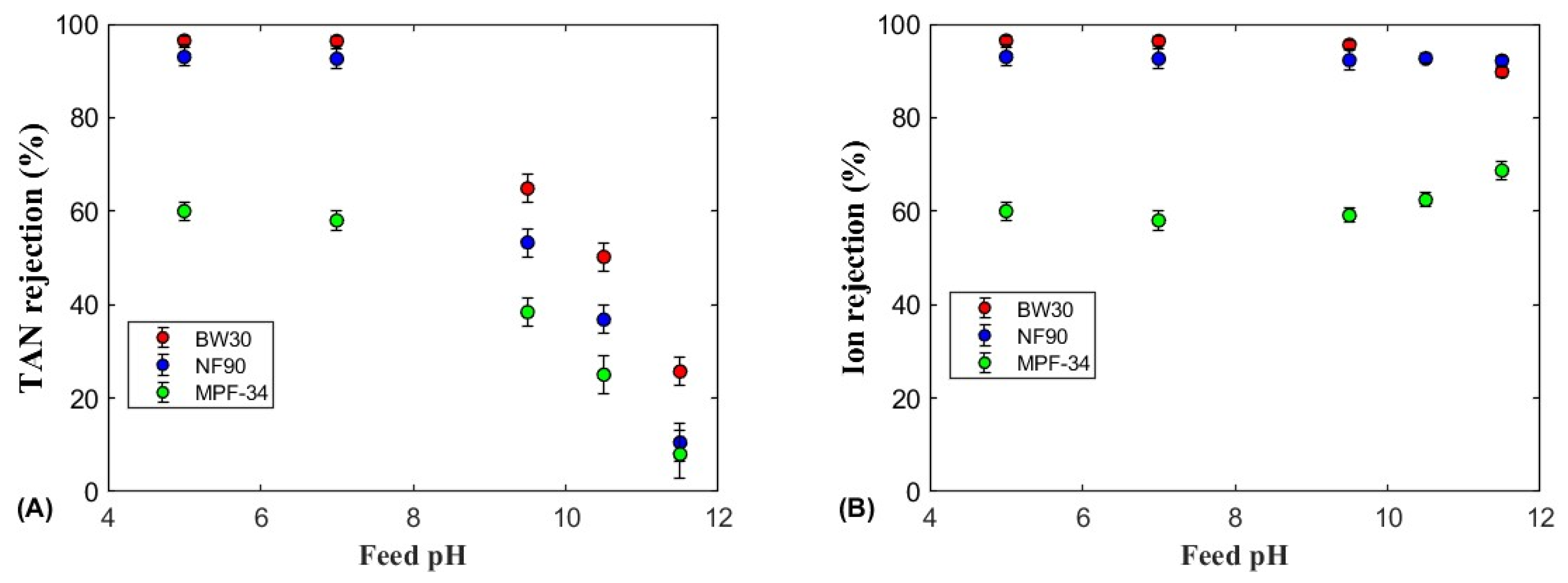

| Membrane | Material of the Skin Layer | MgSO4 Rejection (%) | NaCl Rejection (%) | Permeability Coefficient (L/(m2·h·bar)) | Molecular Weight Cut-Off (Da) | Membrane Isoelectric Point (IEP) | Operation pH |
|---|---|---|---|---|---|---|---|
| SW30 | Fully aromatic polyamide | 99.7 (a) | 0.778 (a) | N/A | ~3.8 [29] | 2–11 | |
| BW30 | Fully aromatic polyamide | 99.1 (b) | 4.79 (b) | <100 | ~4.2 [29] | 2–11 | |
| NF90 | Fully aromatic polyamide | 98.7 (c) | 8.92 (c) | ~100 | ~4.0 [29] | 2–11 | |
| MPF-34 | Proprietary | 35 (d) | 2.00 (d) | ~200 | ~6.5 [22] | 0–14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahgodari, S.; Llorens, J.; Labanda, J. Study of Total Ammoniacal Nitrogen Recovery Using Polymeric Thin-Film Composite Membranes for Continuous Operation of a Hybrid Membrane System. Polymers 2025, 17, 1696. https://doi.org/10.3390/polym17121696
Shahgodari S, Llorens J, Labanda J. Study of Total Ammoniacal Nitrogen Recovery Using Polymeric Thin-Film Composite Membranes for Continuous Operation of a Hybrid Membrane System. Polymers. 2025; 17(12):1696. https://doi.org/10.3390/polym17121696
Chicago/Turabian StyleShahgodari, Shirin, Joan Llorens, and Jordi Labanda. 2025. "Study of Total Ammoniacal Nitrogen Recovery Using Polymeric Thin-Film Composite Membranes for Continuous Operation of a Hybrid Membrane System" Polymers 17, no. 12: 1696. https://doi.org/10.3390/polym17121696
APA StyleShahgodari, S., Llorens, J., & Labanda, J. (2025). Study of Total Ammoniacal Nitrogen Recovery Using Polymeric Thin-Film Composite Membranes for Continuous Operation of a Hybrid Membrane System. Polymers, 17(12), 1696. https://doi.org/10.3390/polym17121696






