Enhancing Cotton Fabrics Through Grafting of Glycine-Based Polyamidoamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of M-GLY Oligomers
2.3. M-GLY Grafting onto Cotton Fabrics
2.4. Water Uptake
2.5. Solid-State Nuclear Magnetic Resonance
2.6. Scanning Electron Microscopy
2.7. Thermogravimetric Analysis
2.8. Flame Spread Test
2.9. Accelerated Photoaging Test
3. Results
3.1. Synthesis of M-GLY-Grafted Cotton—COT-g-M-GLY
| COT-g-M-GLY0.9 | COT-g-M-GLY0.85 | COT-g-M-GLY0.8 | ||||
|---|---|---|---|---|---|---|
| Solution concentration (wt %) | 10 | 20 | 10 | 20 | 10 | 20 |
| Add-on (%) (b) | 2 | 11 | 5.4 | 11 | 4.5 | 15 |
| Water uptake (%) (c) | 2.4 | 2.7 | 2.7 | 2.4 | 2.0 | 2.4 |
3.2. Morphological Analysis
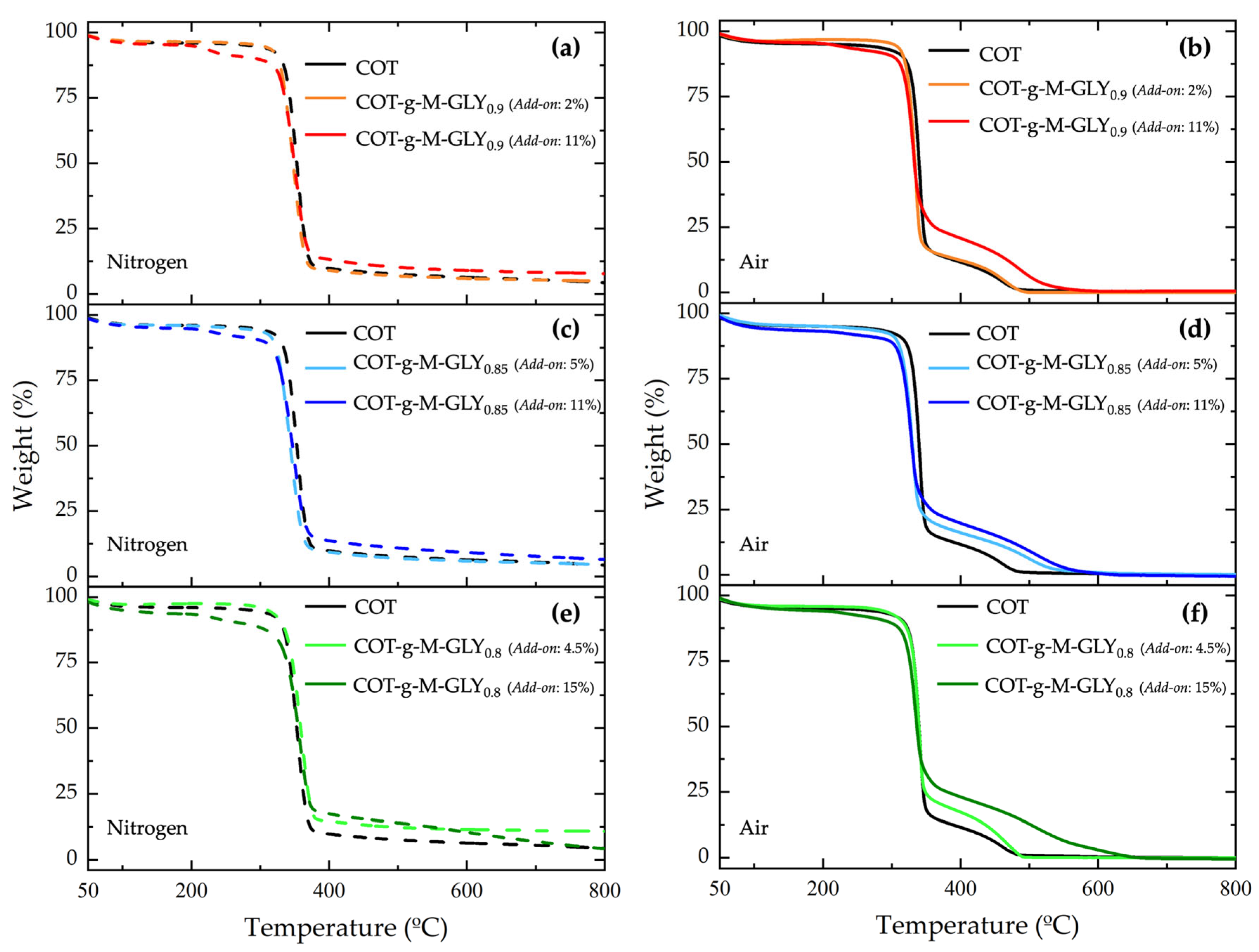
3.3. Thermal Analysis
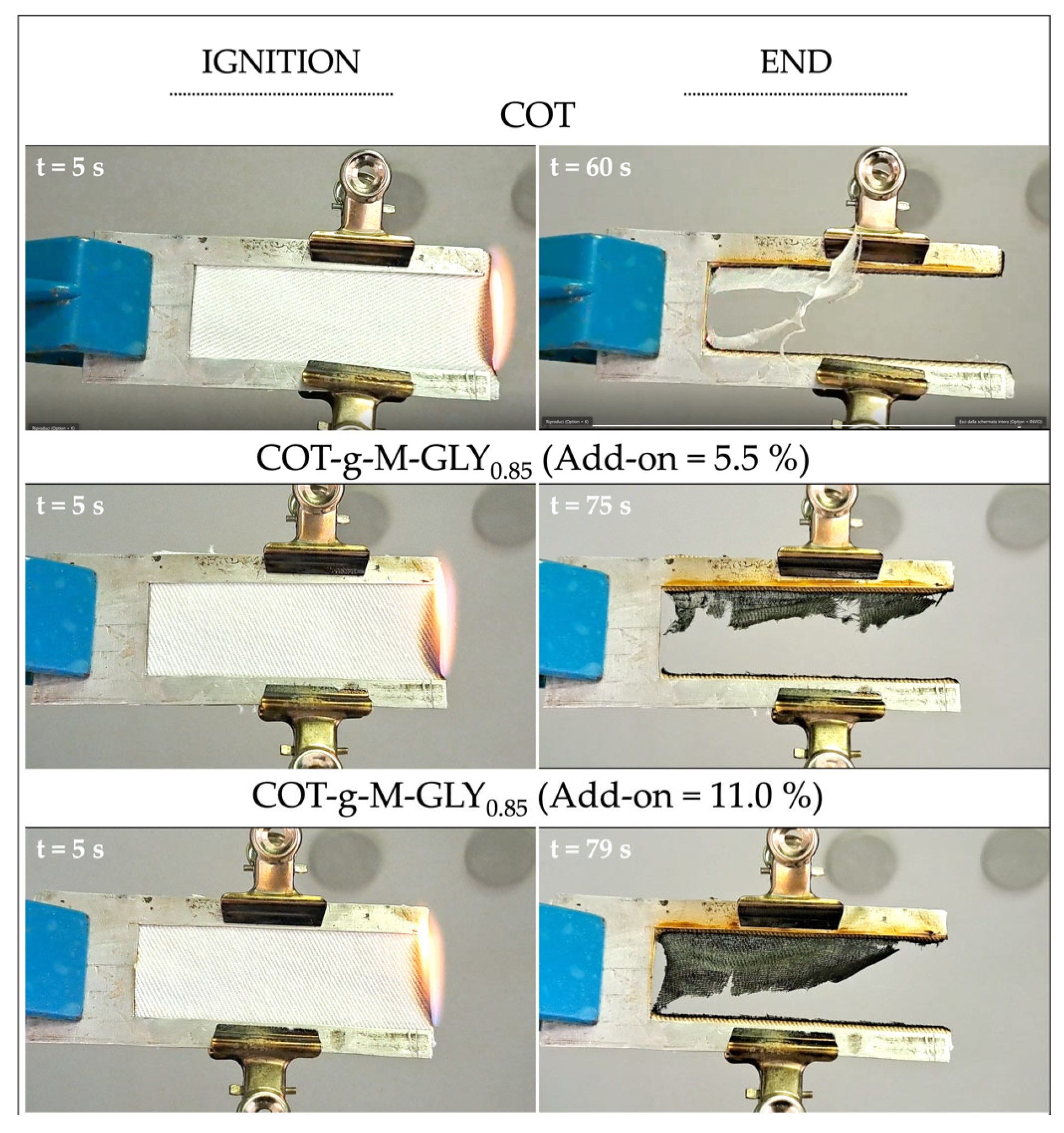
3.4. Combustion Tests
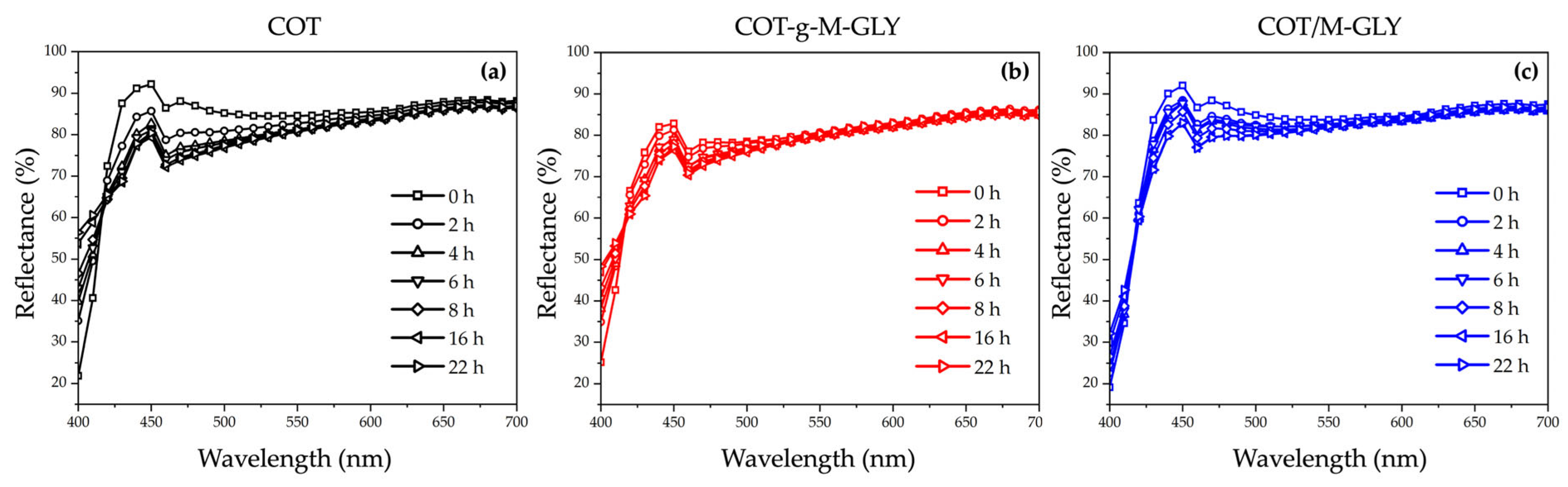
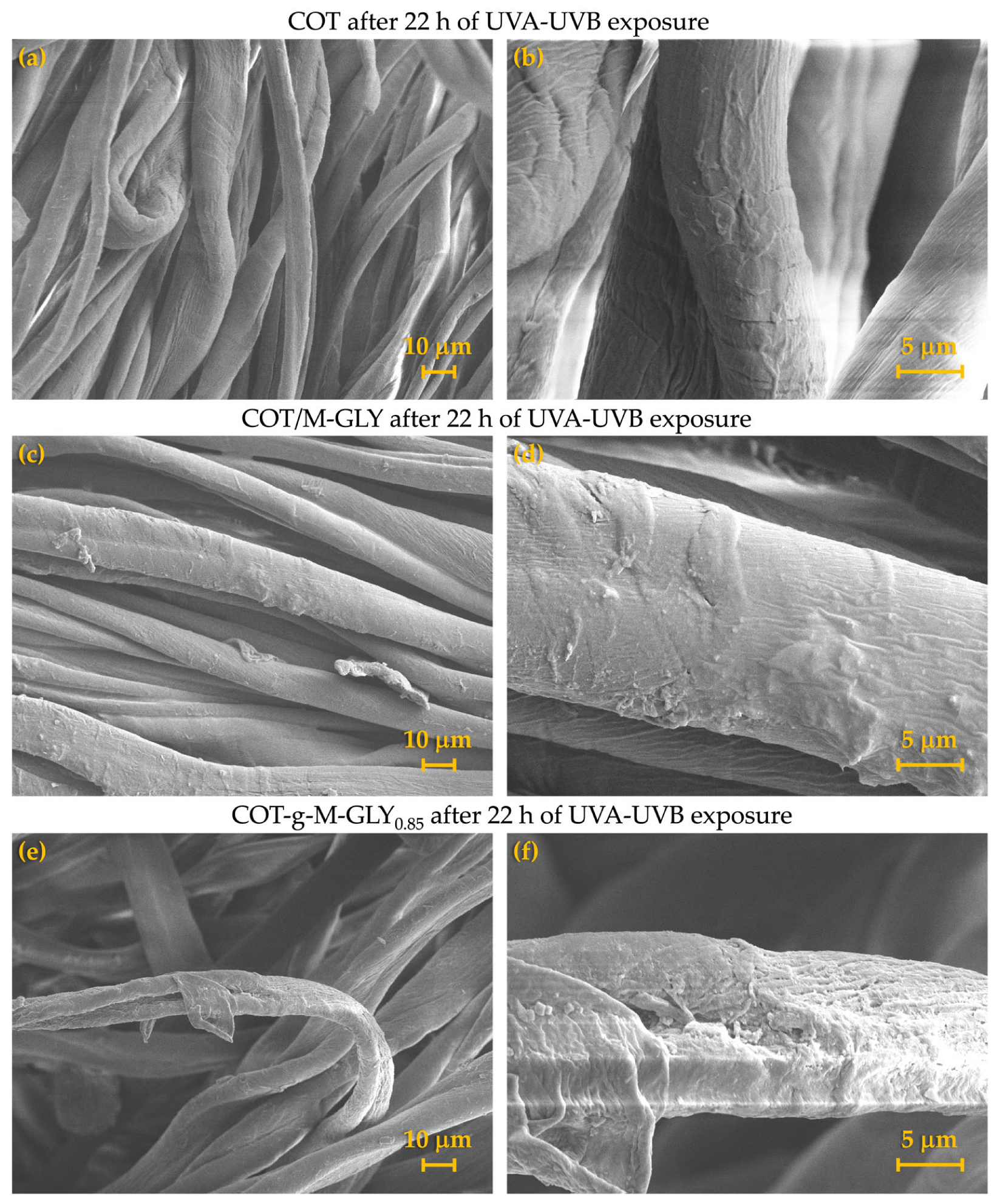
3.5. Accelerated Photoaging Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Cavallo, E.; Cerrutti, P.; Foresti, M.L.; Peltzer, M.A. Reinforcement of yeast biomass films with bacterial cellulose and rice husk cellulose nanofibers. J. Polym. Environ. 2021, 29, 3242–3251. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Tosh, B.; Routray, C.R. Grafting of Cellulose Based Materials: A Review. Chem. Sci. Rev. Lett. 2014, 3, 74–92. [Google Scholar]
- Mondal, I.H.; Uraki, Y.; Ubukata, M.; Itoyama, K. Graft polymerization of vinyl monomers onto cotton fibres pretreated with amines. Cellulose 2008, 15, 581–592. [Google Scholar] [CrossRef]
- Takahashi, A.; Takahashi, S. Graft Copolymerization onto Cellulose Derivatives. IV. Graft Copolymerization of Styrene and Styrene-Methyl Methacrylate onto Cellulosic Materials Containing Carbonyl and Carboxyl Groups. Polym. J. 1974, 6, 201–206. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Dong, W.; Xu, Y. Synthesis and characterization of hydroxypropyl methylcellulose and ethyl acrylate graft copolymers. Carbohydr. Polym. 2007, 68, 626–636. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G. A Review on Grafting of Biofibers for Biocomposites. Materials 2016, 9, 303. [Google Scholar] [CrossRef]
- Kang, H.; Liu, R.; Huang, Y. Graft modification of cellulose: Methods, properties and applications. Polymer 2015, 70, A1–A16. [Google Scholar] [CrossRef]
- Daly, W.H.; Evenson, T.S.; Iacono, S.T.; Jones, R.W. Recent developments in cellulose grafting chemistry utilizing Barton ester intermediates and nitroxide mediation. Macromol. Symp. 2001, 174, 155–163. [Google Scholar] [CrossRef]
- Carlmark, A.; Malmstrom, E. Atom Transfer Radical Polymerization from Cellulose Fibers at Ambient Temperature. J. Am. Chem. Soc. 2002, 124, 900–901. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, H.; Haque, A.; Rahman, A.; Rana, M.; Parvez, M.; Nur Alam, S.M. Grafting of Cellulose and Microcrystalline Cellulose with Oligo(L-lactic acid) by Polycondensation Reaction. Reactions 2022, 3, 213–223. [Google Scholar] [CrossRef]
- Carlmark, A.; Larsson, E.; Malmström, E. Grafting of cellulose by ring-opening polymerisation—A review. Eur. Polym. J. 2012, 48, 1646–1659. [Google Scholar] [CrossRef]
- Shukla, S.R.; Athalye, A.R. Ultraviolet-radiation induced graft-copolymerization of hydroxyethyl methacrylate onto cotton cellulose. J. Appl. Polym. Sci. 1992, 44, 435–442. [Google Scholar] [CrossRef]
- Takacs, E.; Wojnarovits, L.; Borsa, J.; Papp, J.; Hargittai, P.; Korecz, L. Modification of cotton-cellulose by preirradiation grafting. Nucl. Instrum. Methods B 2005, 236, 259–265. [Google Scholar] [CrossRef]
- Li, J.F.; Jiang, W.; Liu, M.L. Durable phosphorus/nitrogen flame retardant for cotton fabric. Cellulose 2022, 29, 4725–4751. [Google Scholar] [CrossRef]
- Trovato, V.; Sfameni, S.; Ben Debabis, R.; Rando, G.; Rosace, G.; Malucelli, G.; Plutino, M.R. How to Address Flame-Retardant Technology on Cotton Fabrics by Using Functional Inorganic Sol–Gel Precursors and Nanofillers: Flammability Insights, Research Advances, and Sustainability Challenges. Inorganics 2023, 11, 306. [Google Scholar] [CrossRef]
- Horrocks, A.R. An introduction to the burning behaviour of cellulosic fibres. J. Soc. Dye. Colour. 1983, 99, 191–197. [Google Scholar] [CrossRef]
- Qi, P.; Li, Y.; Yao, Y.; Sun, J.; Li, L.; Liu, J.; Gu, X.; Li, H.; Zhang, S. Ultra washing durable flame retardant coating for cotton fabric by the covalent bonding and interface polymerization. Chem. Eng. J. 2023, 452, 139453. [Google Scholar] [CrossRef]
- Horrocks, R.A. Flame retardant challenges for textiles and fibres: New chemistry versus innovatory solutions. Polym. Degrad. Stabil. 2011, 96, 377–392. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S.; Malucelli, G. Recent advances for flame retardancy of textiles based on phosphorous chemistry. Polymers 2017, 8, 319. [Google Scholar] [CrossRef]
- Hirschler, M.M. Fire Retardancy of Polymer Materials. In Regulations, Codes, and Standards Relevant to Fire Issues in the United States; Wilkie, C.A., Morgan, A.B., Eds.; CRC Press: Boca Raton, FL, USA, 2010; Chapter 21; pp. 587–598. [Google Scholar]
- Chen, Y.; Li, J.; Liu, L.; Zhao, N. Polybrominated diphenylethersfate in China: A review with an emphasis on environmental contamination levels, human exposure and regulation. J. Environ. Manag. 2012, 113, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Wazedi Ali, S. Sustainable flame retardancy of textiles using bio-macromolecules. Polym. Degrad. Stabil. 2016, 133, 47–64. [Google Scholar] [CrossRef]
- Botti, S.; Di Lazzaro, P.; Flora, F.; Mezi, L.; Murra, D. Raman spectral mapping reveal molecular changes in cellulose aging induced by ultraviolet and extreme ultraviolet radiation. Cellulose 2024, 31, 749–758. [Google Scholar] [CrossRef]
- Malesic, J.; Kolar, J.; Strlic, M.; Kocar, D.; Fromageot, D.; Lemaire, J.; Haillant, O. Photo-induced degradation of cellulose. Polym. Degrad. Stabil. 2005, 89, 64–69. [Google Scholar] [CrossRef]
- Merlin, A.; Fouassier, J. Photochemical investigations into cellulosic materials I. Free radical generation in cellulose by photosensitized excitation. Angew. Makromol. Chem. 1980, 86, 109–121. [Google Scholar] [CrossRef]
- Muasher, M.; Sain, M. The efficacy of photostabilizers on the color change of wood filled plastic composites. Polym. Degrad. Stabil. 2006, 91, 1156–1165. [Google Scholar] [CrossRef]
- Forte, C.; Alongi, J.; Beduini, A.; Borsacchi, S.; Calucci, L.; Carosio, F.; Ferruti, P.; Ranucci, E. The Thermo-Oxidative Behavior of Cotton Coated with an Intumescent Flame Retardant Glycine-Derived Polyamidoamine: A Multi-Technique Study. Polymers 2021, 13, 4382. [Google Scholar] [CrossRef]
- Alongi, J.; Treccani, S.; Comite, V.; Fermo, P.; Ferruti, P.; Ranucci, E. Polyamidoamine-based photostabilizers for cotton fabrics. Polym. Degrad. Stabil. 2024, 228, 110938. [Google Scholar] [CrossRef]
- Ranucci, E.; Manfredi, A. Polyamidoamines: Versatile bioactive polymers with potential for biotechnological applications. Chem. Afr. 2019, 2, 167–193. [Google Scholar] [CrossRef]
- Argenziano, M.; Dianzani, C.; Ferrara, B.; Swaminathan, S.; Manfredi, A.; Ranucci, E.; Cavalli, R.; Ferruti, P. Cyclodextrin-Based Nanohydrogels Containing Polyamidoamine Units: A New Dexamethasone Delivery System for Inflammatory Diseases. Gels 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Primo, L.; Sessa, R.; Chiaverina, G.; di Blasio, L.; Alongi, J.; Manfredi, A.; Ranucci, E.; Ferruti, P. The AGMA1 Polyamidoamine Mediates the Efficient Delivery of SiRNA. J. Drug Target. 2017, 25, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Costantini, A.; Ferruti, P.; Ranucci, E. Evaluation of the eco-compatibility of polyamidoamines by means of seed germination test. Polym. Degrad. Stabil. 2022, 197, 109854. [Google Scholar] [CrossRef]
- Ranucci, E.; Treccani, S.; Ferruti, P.; Alongi, J. The Seed Germination Test as a Valuable Tool for the Short-Term Phytotoxicity Screening of Water-Soluble Polyamidoamines. Polymers 2024, 16, 1744. [Google Scholar] [CrossRef]
- Treccani, S.; Ferruti, P.; Alongi, J.; Monti, E.; Zizioli, D.; Ranucci, E. Ecotoxicity Assessment of α-Amino Acid-Derived Polyamidoamines Using Zebrafish as a Vertebrate Model. Polymers 2024, 16, 2087. [Google Scholar] [CrossRef]
- Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Polyamidoamines Derived from Natural α-Amino Acids as Effective Flame Retardants for Cotton. Polymers 2021, 13, 3714. [Google Scholar] [CrossRef]
- Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Sulfur-Based Copolymeric Polyamidoamines as Efficient Flame-Retardants for Cotton. Polymers 2019, 11, 1904. [Google Scholar] [CrossRef]
- ISO 11664; Colorimetry, Part 4: CIE 1976 L*a*b* Colour Space. International Organization for Standardization: Geneve, Switzerland, 2019.
- GZ. Schuessler 2024. Available online: https://zschuessler.github.io/DeltaE/learn/ (accessed on 27 May 2025).
- Maggi, F.; Manfredi, A.; Carosio, F.; Maddalena, L.; Alongi, J.; Ferruti, P.; Ranucci, E. Toughening Polyamidoamine Hydrogels through Covalent Grafting of Short Silk Fibers. Molecules 2022, 27, 7808. [Google Scholar] [CrossRef]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Linear polyamidoamines as novel biocompatible phosphorus-free surface confined intumescent flame retardants for cotton fabrics. Polym. Degrad. Stabil. 2018, 151, 52. [Google Scholar] [CrossRef]
- Newman, R.H.; Hemmingson, J.A. Determination of the Degree of Cellulose Crystallinity in Wood by Carbon-13. Prog. Nucl. Magn. Reson. Spectrosc. 1990, 44, 351–355. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.H.; Zhang, Z.C.; Brown, H.M.; Arey, B.W.; Holladay, J.E. Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis. Carbohydr. Polym. 2007, 68, 235–241. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef] [PubMed]
- Beduini, A.; Ferruti, P.; Carosio, F.; Ranucci, E.; Alongi, J. Synergism between α-amino acid-derived polyamidoamines and sodium montmorillonite for enhancing the flame retardancy of cotton fabrics. Polym. Degrad. Stabil. 2024, 225, 110764. [Google Scholar] [CrossRef]
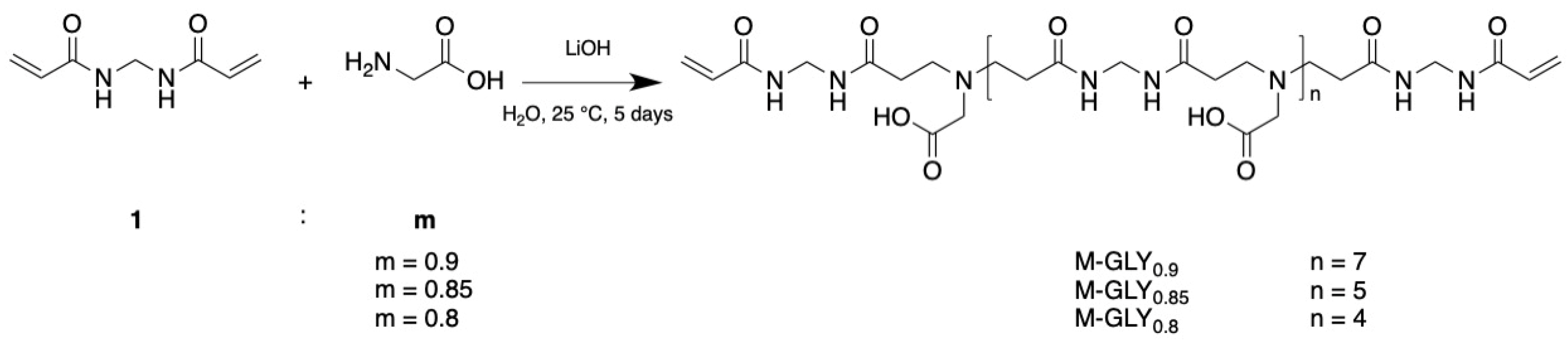

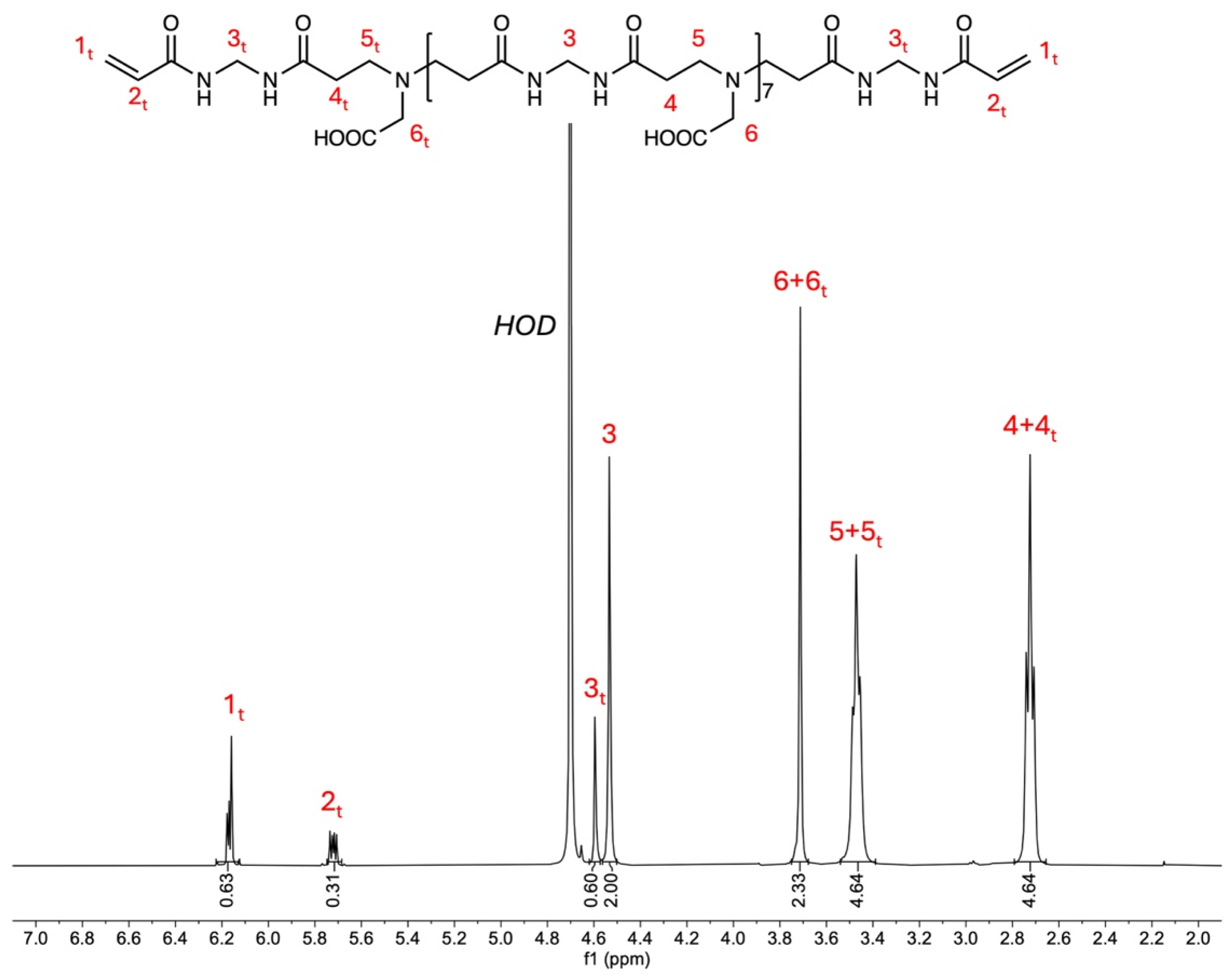
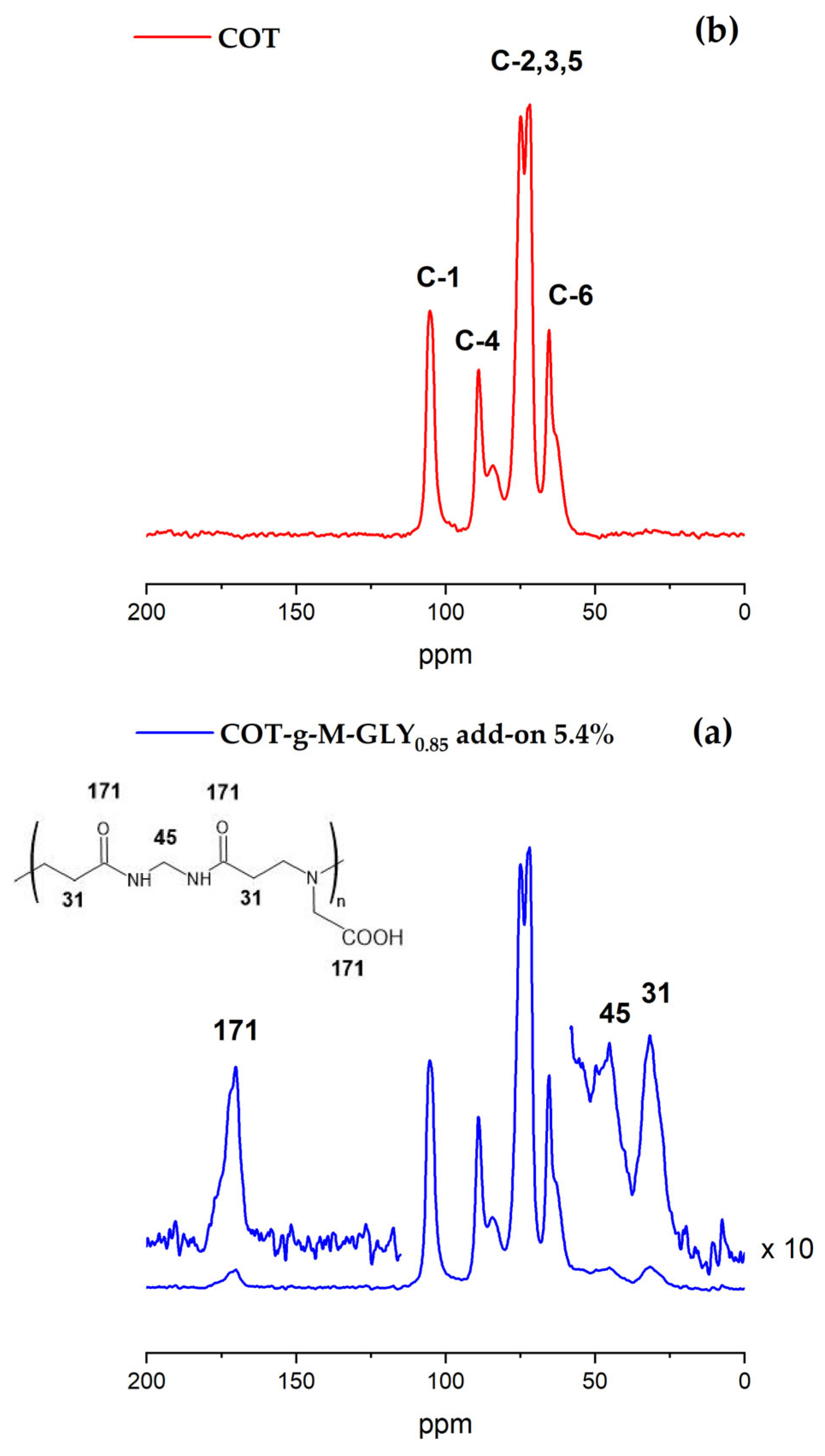
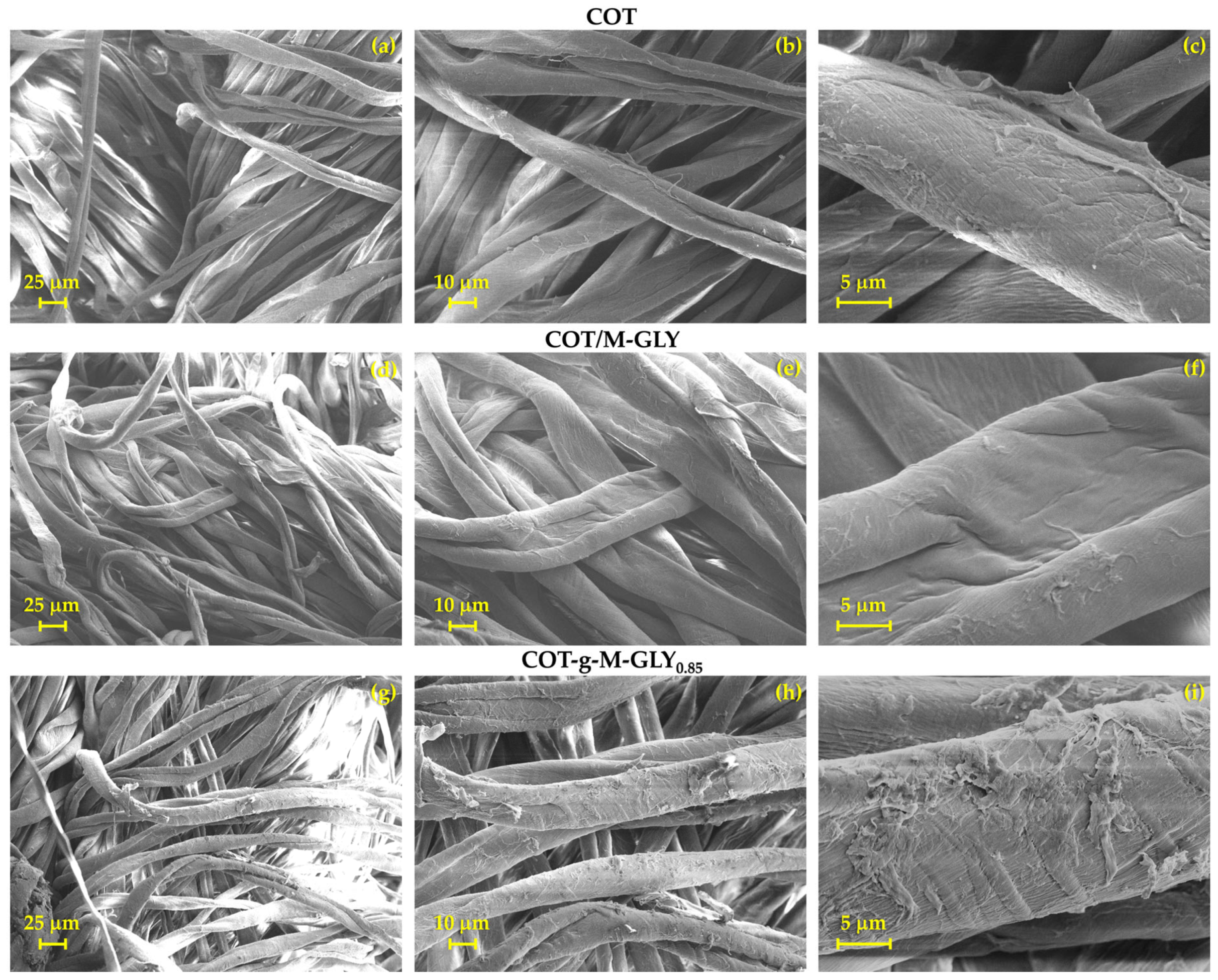


| MBA | GLY | LiOH·H2O | H2O | ||||
|---|---|---|---|---|---|---|---|
| (g) | (mmol) | (g) | (mmol) | (g) | (mmol) | (mL) | |
| M-GLY0.9 | 9.34 | 60 | 4.10 | 54 | 2.32 | 54 | 25 |
| M-GLY0.85 | 9.34 | 60 | 3.87 | 51 | 2.19 | 51 | 25 |
| M-GLY0.8 | 9.34 | 60 | 3.64 | 48 | 2.06 | 48 | 25 |
| Sample | Theoretical r (b) Value | Experimental r Value | n (c) | (d) | (e) |
|---|---|---|---|---|---|
| M-GLY0.9 | 0.90 | 0.896 | 7 | 17 | 1990 |
| M-GLY0.85 | 0.85 | 0.860 | 5 | 13 | 1530 |
| M-GLY0.8 | 0.80 | 0.823 | 4 | 10 | 1300 |
| Sample | Add-On (%) | T onset10% (a) (°C) | Tmax1 (b) (°C) | Tmax2 (b) (°C) | RMF (c) (%) |
|---|---|---|---|---|---|
| Nitrogen | |||||
| COT | - | 330 | 352 | - | 4.5 |
| COT-g-M-GLY0.9 | 2 | 325 | 349 | - | 5 |
| COT-g-M-GLY0.9 | 11 | 293 | 351 | - | 7 |
| COT-g-M-GLY0.85 | 5 | 319 | 344 | - | 4.5 |
| COT-g-M-GLY0.85 | 11 | 303 | 347 | - | 6.5 |
| COT-g-M-GLY0.8 | 4.5 | 331 | 358 | - | 11 |
| COT-g-M-GLY0.8 | 15 | 270 | 360 | - | 10 |
| COT/M-GLY [47] | 7 | 270 | 325 | - | 20 |
| COT/M-GLY [42] | 14 | 247 | 310 | - | 23 |
| Air | |||||
| COT | - | 310 | 327 | 523 | 0 |
| COT-g-M-GLY0.9 | 2 | 315 | 333 | 460 | 0 |
| COT-g-M-GLY0.9 | 11 | 303 | 330 | 486 | 0 |
| COT-g-M-GLY0.85 | 5 | 306 | 328 | 498 | 0 |
| COT-g-M-GLY0.85 | 11 | 291 | 330 | 510 | 0 |
| COT-g-M-GLY0.8 | 4.5 | 315 | 339 | 470 | 0 |
| COT-g-M-GLY0.8 | 15 | 293 | 334 | 509 | 0 |
| COT/M-GLY | 7 | 270 | 316 | 427 | 1 |
| COT/M-GLY | 14 | 256 | 304 | 433/481 | 0 |
| Add-On (a) (%) | Combustion Time in the Presence of Flame (s) (b) | Combustion Time in the Presence of Incandescence (s) (b) | Burning Rate (c) (mm s−1) | RMF (d) (%) | |
|---|---|---|---|---|---|
| Cotton | - | 55 | 2 | 1.0 | 0.5 |
| COT-g-M-GLY0.9 | 2 | 62 | 3 | 0.9 | 2 |
| 12 | 73 | 2 | 0.8 | 6 | |
| COT-g-M-GLY0.85 | 5.5 | 66 | 5 | 0.8 | 2 |
| 11 | 80 | 7 | 0.7 | 7 | |
| COT-g-M-GLY0.8 | 4.5 | 69 | 3 | 0.8 | 1.5 |
| 15 | 77 | 4 | 0.7 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arioli, M.; Alongi, J.; Forte, C.; Pizzanelli, S.; Ranucci, E. Enhancing Cotton Fabrics Through Grafting of Glycine-Based Polyamidoamine. Polymers 2025, 17, 1676. https://doi.org/10.3390/polym17121676
Arioli M, Alongi J, Forte C, Pizzanelli S, Ranucci E. Enhancing Cotton Fabrics Through Grafting of Glycine-Based Polyamidoamine. Polymers. 2025; 17(12):1676. https://doi.org/10.3390/polym17121676
Chicago/Turabian StyleArioli, Matteo, Jenny Alongi, Claudia Forte, Silvia Pizzanelli, and Elisabetta Ranucci. 2025. "Enhancing Cotton Fabrics Through Grafting of Glycine-Based Polyamidoamine" Polymers 17, no. 12: 1676. https://doi.org/10.3390/polym17121676
APA StyleArioli, M., Alongi, J., Forte, C., Pizzanelli, S., & Ranucci, E. (2025). Enhancing Cotton Fabrics Through Grafting of Glycine-Based Polyamidoamine. Polymers, 17(12), 1676. https://doi.org/10.3390/polym17121676










