Investigation of Surface Properties and Antibacterial Activity of 3D-Printed Polyamide 12-Based Samples Coated by a Plasma SiOxCyHz Amorphous Thin Film Approved for Food Contact
Abstract
1. Introduction
2. Materials and Methods
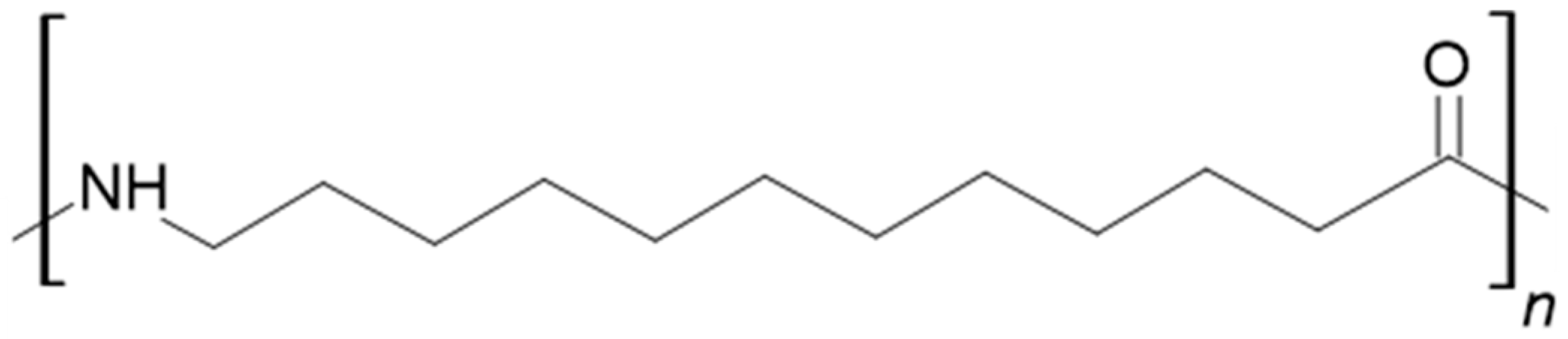
2.1. Sample Preparation
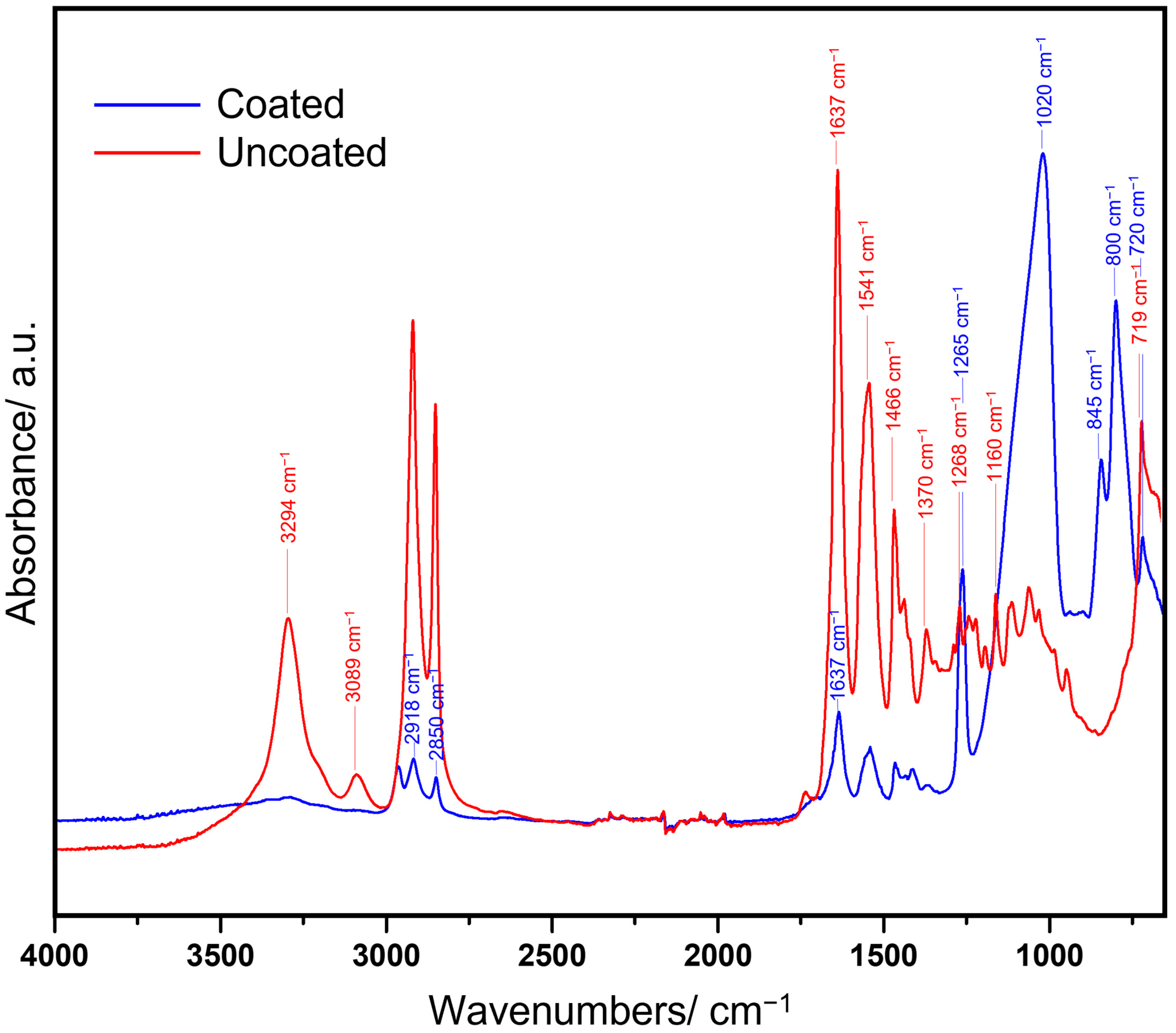
2.2. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.3. Raman Spectroscopy Analysis
2.4. Thermogravimetric Analysis (TGA) and Derivative Thermogravimetric Analysis (DTG)
2.5. Contact Angle (CA) Analysis
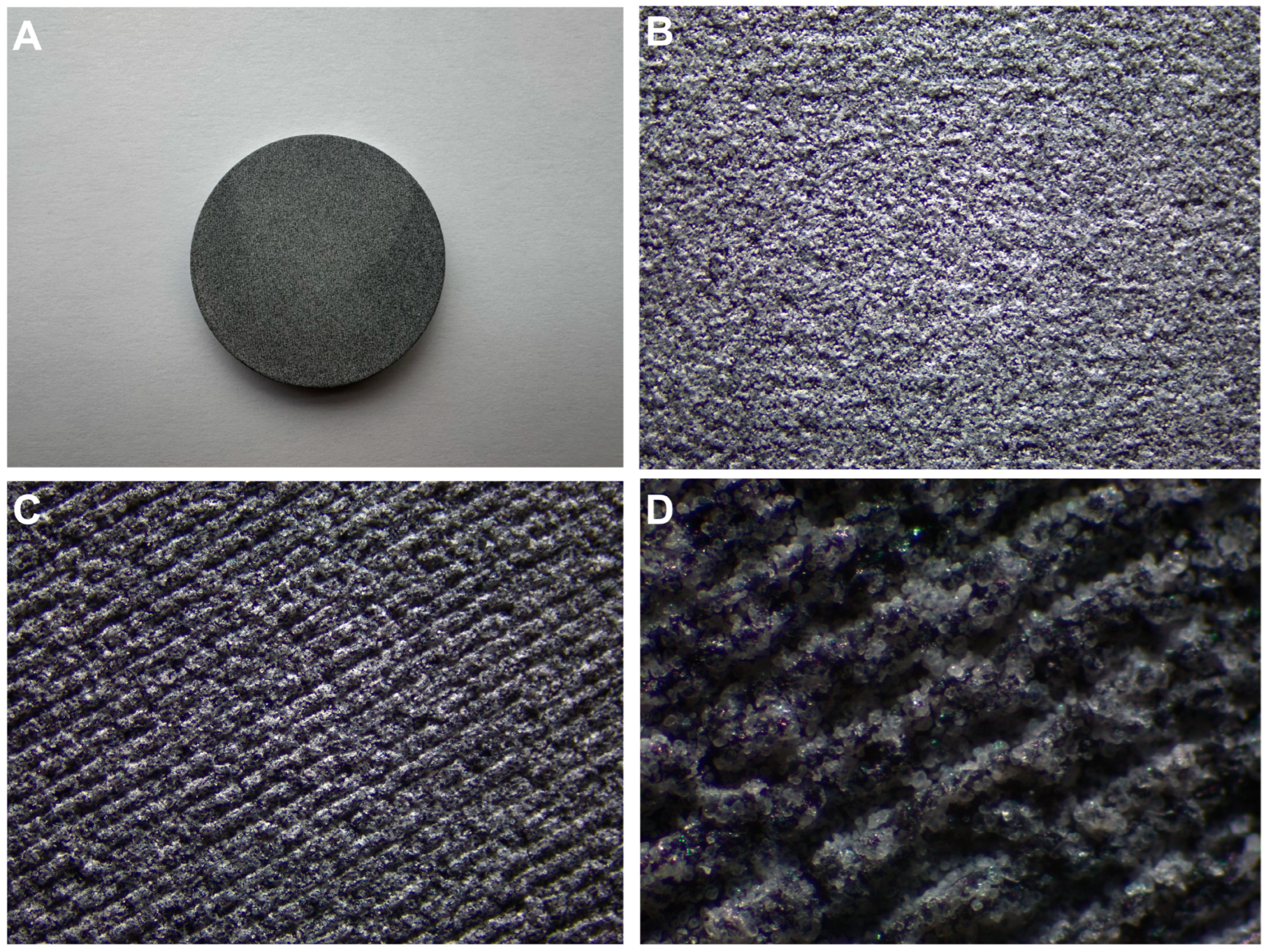
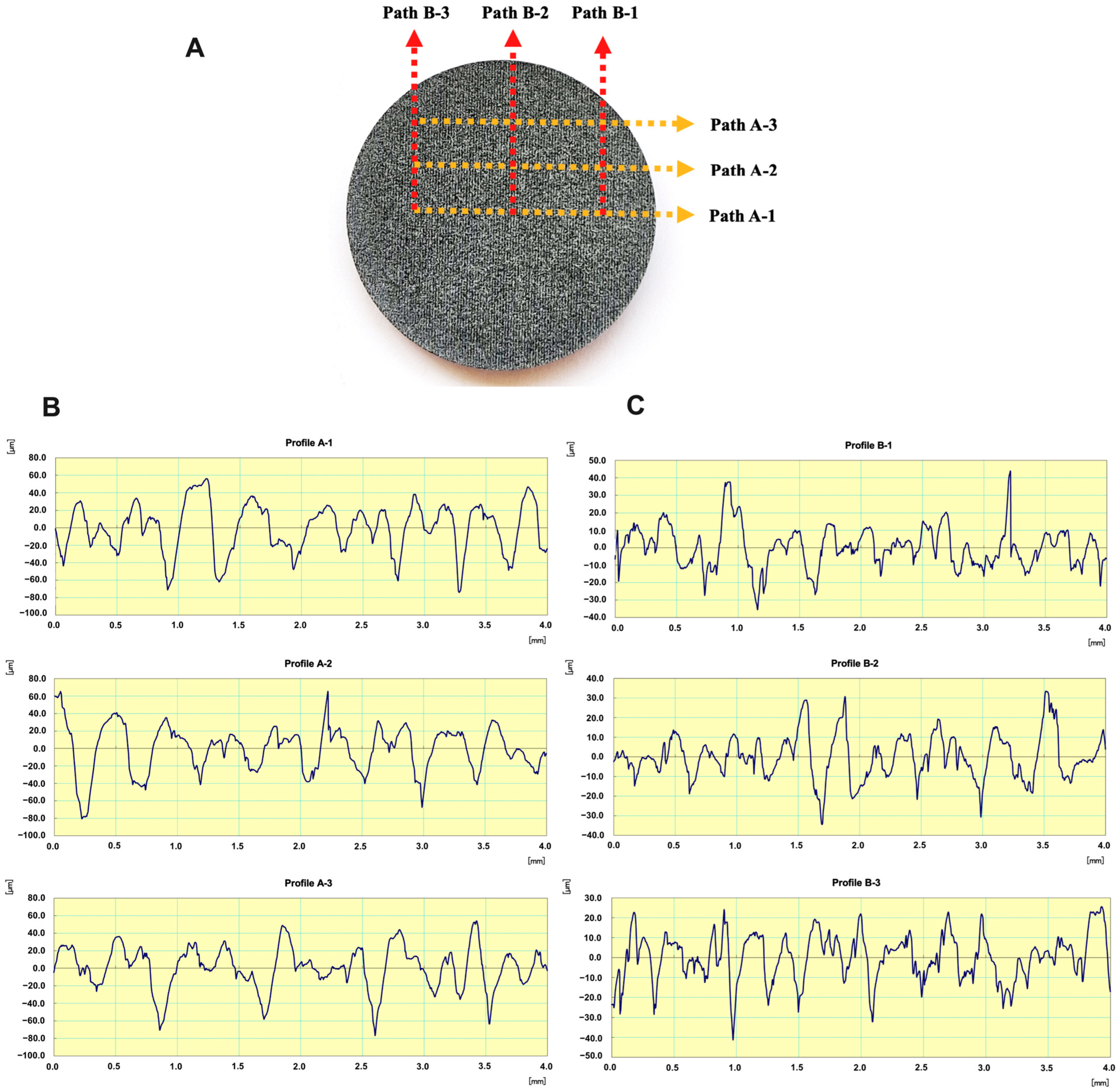
2.6. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS) Analysis
2.7. The Bacterial Strains
2.8. Microbiological Analysis
2.9. Time-Course Assay and Sanitizing Procedures
2.10. Statistical Analysis
3. Results
3.1. FTIR Analysis
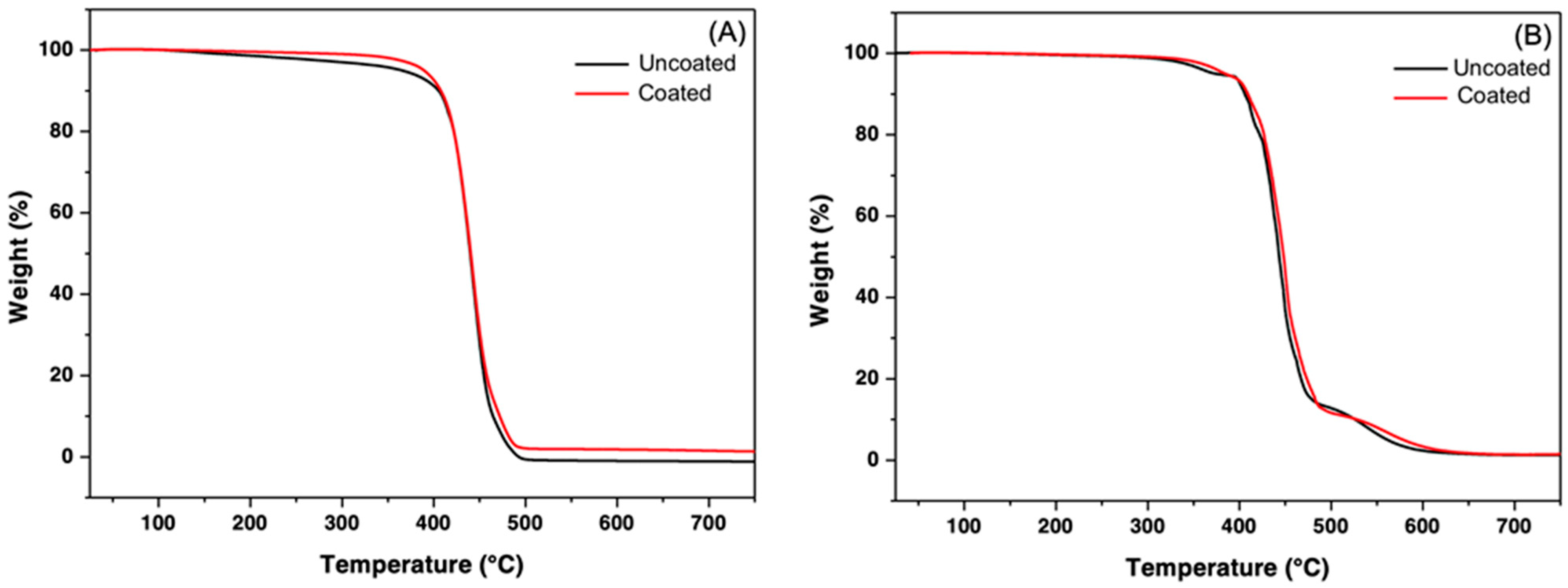
3.2. TGA Analysis
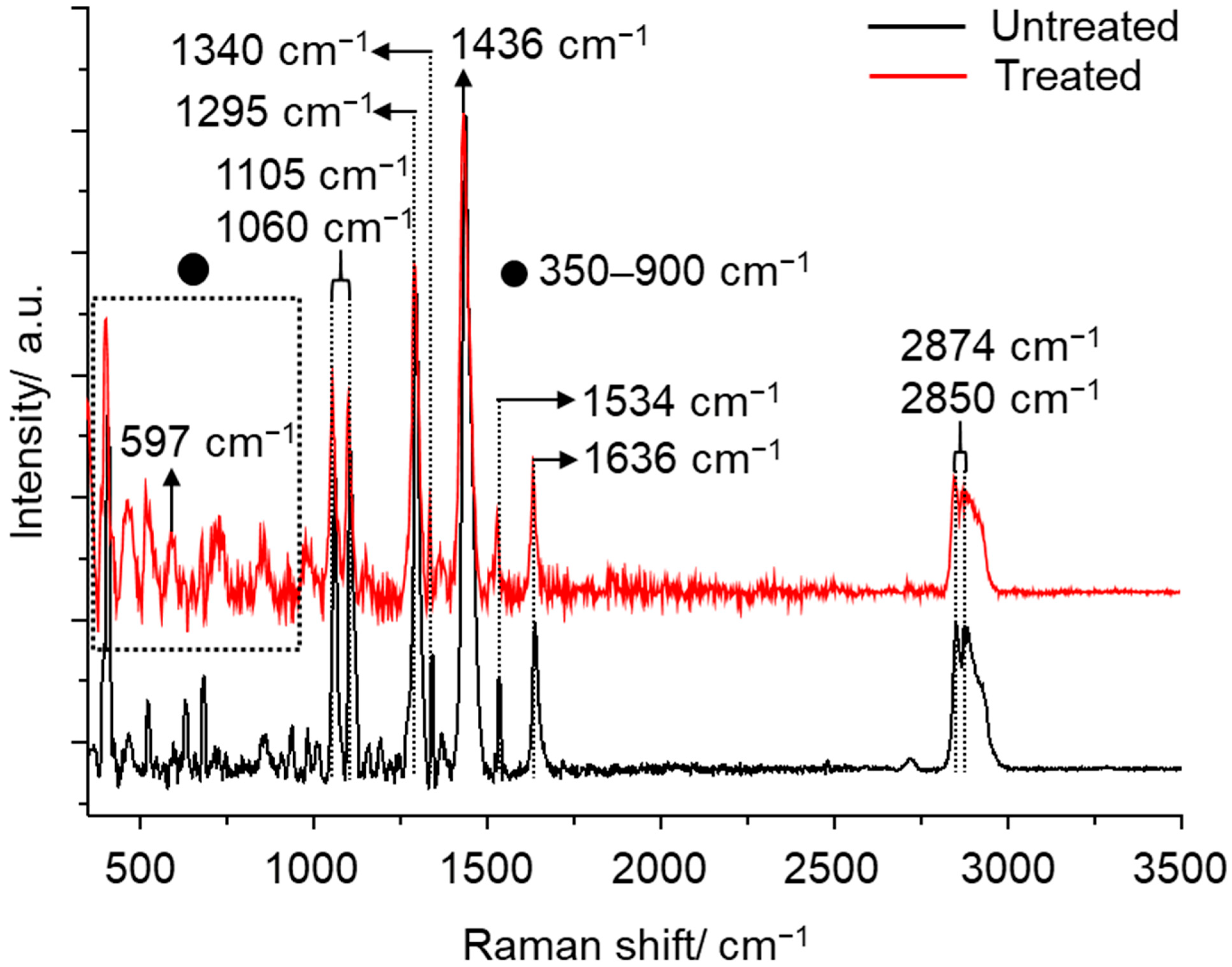
3.3. Raman Characterization
3.4. CA Analysis
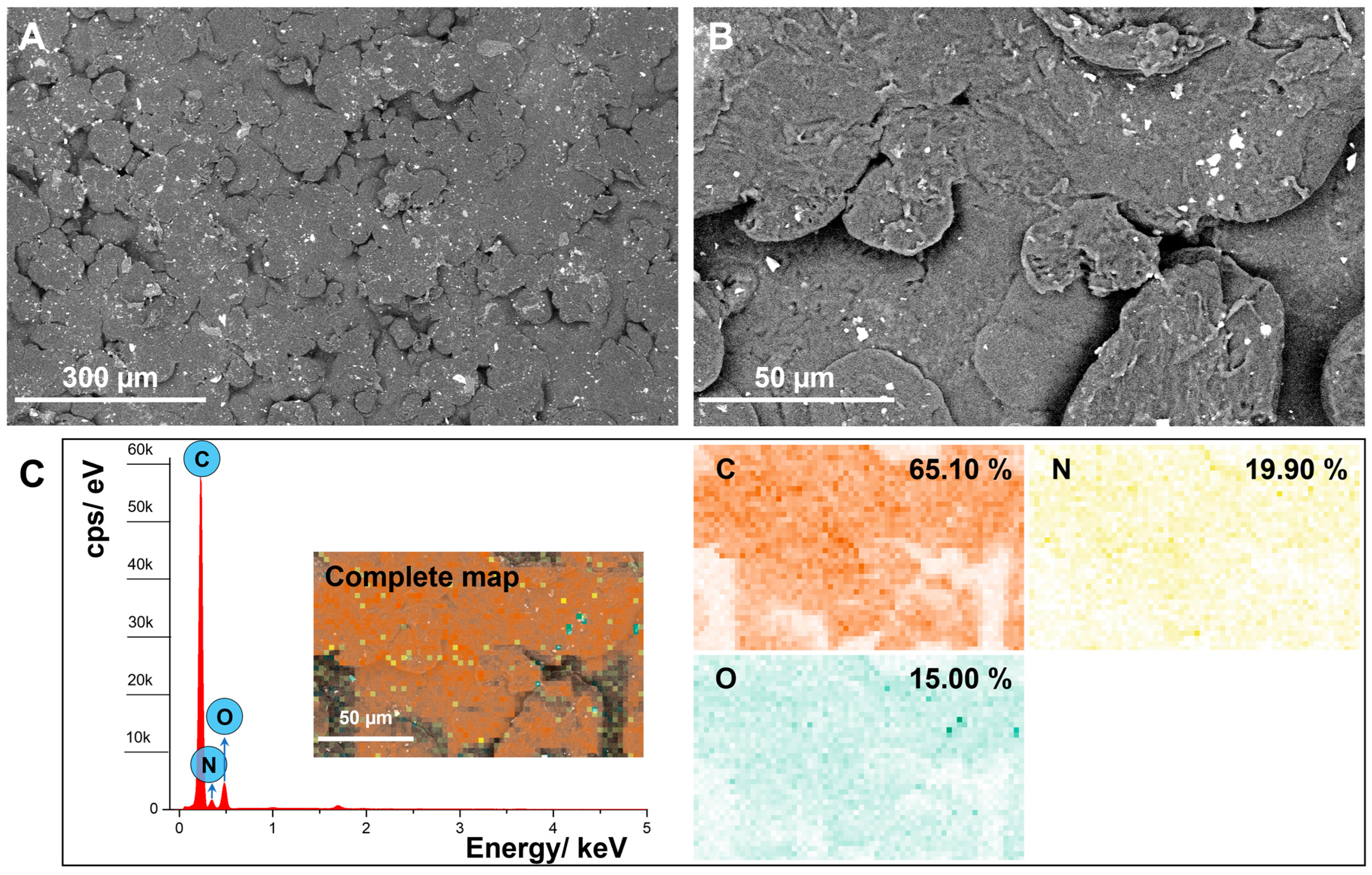
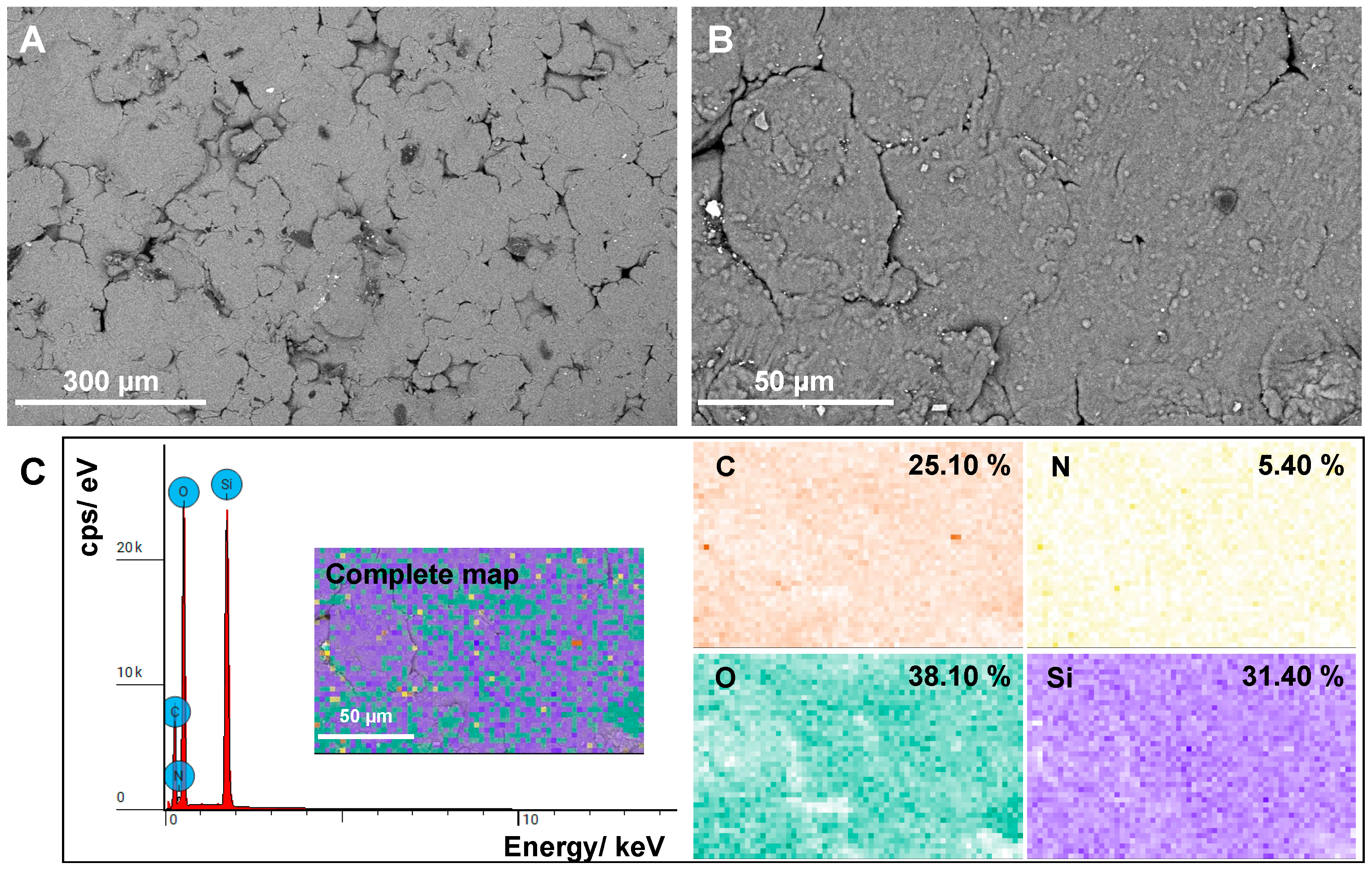
3.5. SEM and EDS Analysis
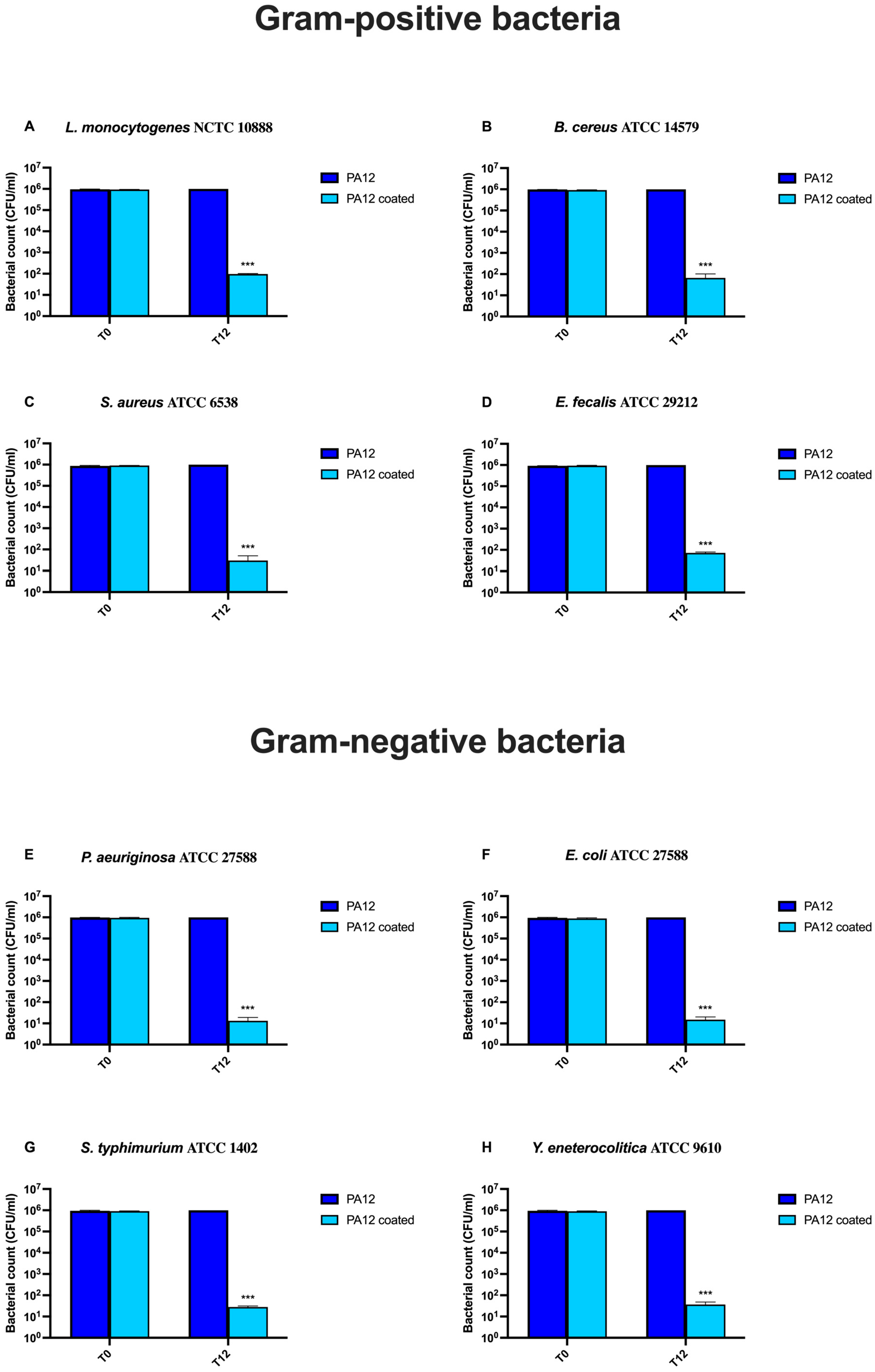
3.6. Microbiological Analysis
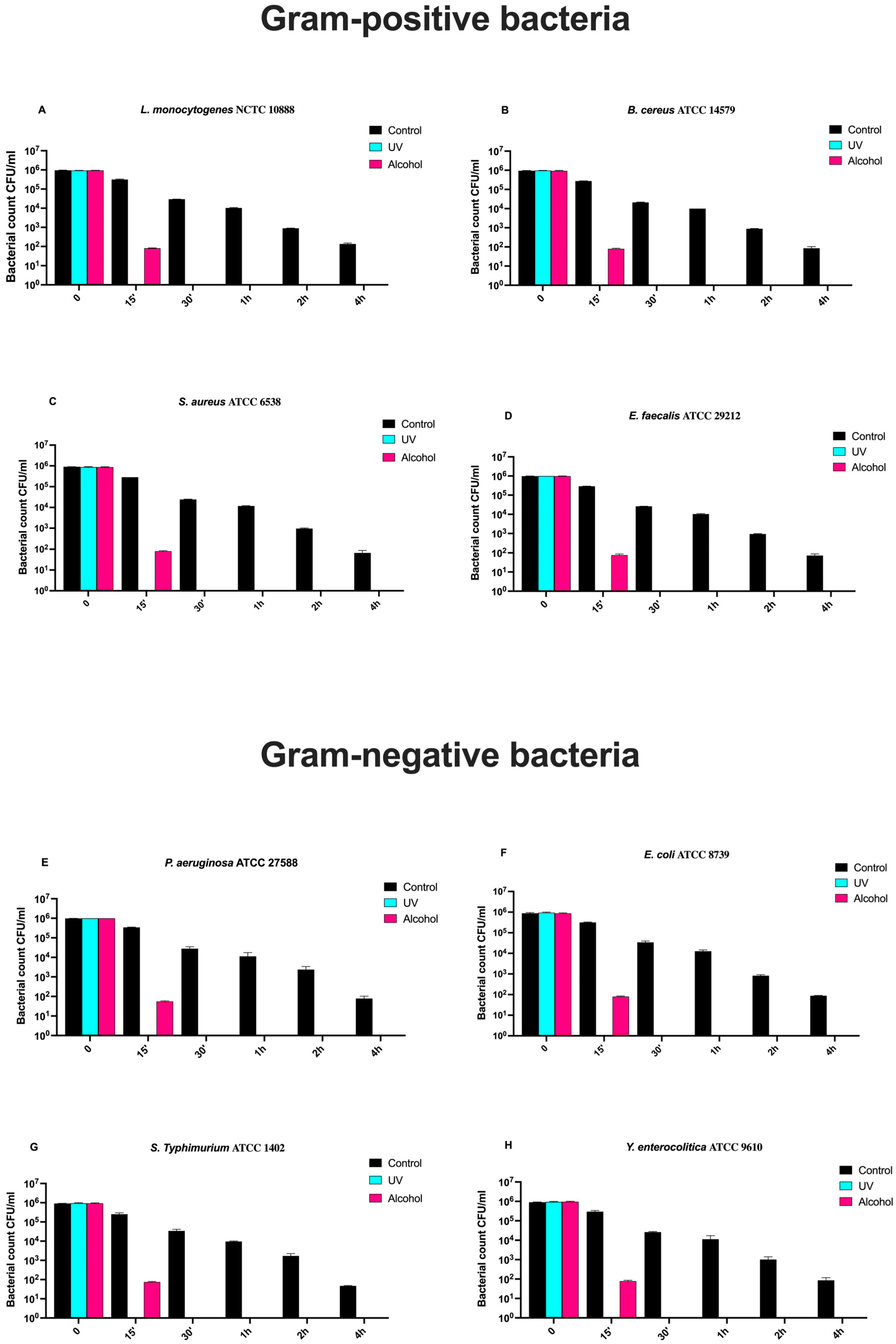
3.7. Time-Course Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PA12 | Polyamide 12 |
| PECVD | Plasma-enhanced chemical vapor deposition |
| FTIR | Fourier-transform infrared spectroscopy |
| TGA | Thermogravimetric analysis |
| SEM | Scanning electron microscopy |
| FSC | Food supply chain |
| SDGs | Sustainable Development Goals |
| FCMs | Food contact materials |
| AR | Antibiotic resistance |
| ATR | Attenuated total reflection |
| CA | Contact angle |
| EDS | Energy dispersive spectroscopy |
| ATCC | American Type Culture Collection |
| CFU | Colony-forming units |
References
- Haessner, P.; Haessner, J.; McMurtrey, M. Trends & Challenges in the Food Supply Chain. J. Strateg. Innov. Sustain. 2024, 19, 115–124. [Google Scholar] [CrossRef]
- Su, I.H.; Wu, L.; Tan, K.H. The future of the food supply chain: A systematic literature review and research directions towards sustainability, resilience, and technology adoption. J. Digit. Econ. 2023, 2, 303–316. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Mescola, A.; Iseppi, R.; Canton, R.; Rossi, G.; Stocchi, R.; Loschi, A.R.; Alessandrini, A.; Rea, S.; Sabia, C. Antibacterial Effect of Aluminum Surfaces Untreated and Treated with a Special Anodizing Based on Titanium Oxide Approved for Food Contact. Biology 2020, 9, 456. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Rosace, G.; Rea, S.; Stocchi, R.; Morales-Medina, J.C.; Canton, R.; Mescola, A.; Condo, C.; Loschi, A.R.; Sabia, C. Time-Course Study of the Antibacterial Activity of an Amorphous SiO(x)C(y)H(z) Coating Certified for Food Contact. Antibiotics 2021, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Mescola, A.; Rosace, G.; Trovato, V.; Canton, R.; Iseppi, R.; Stocchi, R.; Ghazanfar, S.; Rea, S.; Loschi, A.R.; et al. A Time-Course Study on a Food Contact Material (FCM)-Certified Coating Based on Titanium Oxide Deposited onto Aluminum. Biology 2022, 11, 97. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Mescola, A.; Rosace, G.; Stocchi, R.; Rossi, G.; Alessandrini, A.; Preziuso, S.; Scarano, A.; Rea, S.; Loschi, A.R.; et al. Antibacterial Effect of Stainless Steel Surfaces Treated with a Nanotechnological Coating Approved for Food Contact. Microorganisms 2021, 9, 248. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Canton, R.; Stocchi, R.; Rea, S.; Loschi, A.R. Antibacterial Activity of Nanotechnologically-Coated Materials Approved for Food Contact. Adv. Mater. Sci. Res. 2022, 52, 145–185. [Google Scholar]
- Xu, C.; Kong, L.; Gao, H.; Cheng, X.; Wang, X. A Review of Current Bacterial Resistance to Antibiotics in Food Animals. Front. Microbiol. 2022, 13, 822689. [Google Scholar] [CrossRef]
- CDC. Antimicrobial Resistance, Food, and Food Animals. 2024. Available online: https://www.cdc.gov/food-safety/foods/antimicrobial-resistance.html#:~:text=When%20animals%20are%20slaughtered%20and,get%20into%20the%20surrounding%20environment (accessed on 6 February 2025).
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; de Schaetzen, M.A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef]
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Myszka, K.; Czaczyk, K. Bacterial Biofilms on Food Contact Surfaces—A Review. Pol. J. Food Nutr. Sci. 2011, 61, 173–180. [Google Scholar] [CrossRef]
- Schelin, J.; Susilo, Y.B.; Johler, S. Expression of Staphylococcal Enterotoxins under Stress Encountered during Food Production and Preservation. Toxins 2017, 9, 401. [Google Scholar] [CrossRef]
- Hsu, L.C.; Fang, J.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Effect of micro- and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef]
- Poole, K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92, 55S–64S. [Google Scholar] [CrossRef]
- Verran, J.; Rowe, D.L.; Boyd, R.D. The Effect of Nanometer Dimension Topographical Features on the Hygienic Status of Stainless Steel. J. Food Prot. 2001, 64, 1183–1187. [Google Scholar] [CrossRef]
- McMillin, K.W. Where is MAP Going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 2008, 80, 43–65. [Google Scholar] [CrossRef] [PubMed]
- The Commission of the European Communities. Commission Regulation (EC) No 450/2009 on Active and Intelligent Materials and Articles Intended to Come Into Contact with Food; Official Journal of the European Union: Luxembourg, 2009; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R0450 (accessed on 19 March 2025).
- Jose, D.A.; Prakash, P.; Baby Chakrapani, P.S. Containers Based on Polymers in Biomedical Devices/Medical Applications. In Micro- and Nano-Containers for Smart Applications; Parameswaranpillai, J., V. Salim, N., Pulikkalparambil, H., Mavinkere Rangappa, S., Suchart Siengchin, I.H., Eds.; Springer Nature: Singapore, 2022; pp. 179–195. [Google Scholar] [CrossRef]
- Hernandez, R.J. Food Packaging Materials, Barrier Properties, and Selection. In Handbook of Food Engineering Practice; Valentas, K.J., Rotstein, E., Singh, R.P., Eds.; CRC Press LLC: Boca Raton, FL, USA, 1997; Available online: https://muhammadsubchi.wordpress.com/wp-content/uploads/2010/04/handbook-of-food-engineering-practice.pdf (accessed on 19 March 2025).
- Martínez-Bueno, M.J.; Gómez Ramos, M.J.; Bauer, A.; Fernández-Alba, A.R. An overview of non-targeted screening strategies based on high resolution accurate mass spectrometry for the identification of migrants coming from plastic food packaging materials. TrAC Trends Anal. Chem. 2019, 110, 191–203. [Google Scholar] [CrossRef]
- Meng, W.; Chen, Q.; Zhang, Y.; Sun, H.; Li, J.; Sun, H.; Liu, C.; Fang, M.; Su, G. Tracking chemical feature releases from plastic food packaging to humans. J. Hazard. Mater. 2024, 480, 135897. [Google Scholar] [CrossRef]
- NSF/ANSI 51. Available online: https://www.nsf.org/ (accessed on 16 March 2025).
- The European Parliament and the Council of the European Union. REGULATION (EC) No 1935/2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC; Official Journal of the European Union: Luxembourg, 2004; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R1935 (accessed on 16 March 2025).
- Campobasso, A.; Battista, G.; Lo Muzio, E.; Colombo, S.; Paglia, M.; Federici Canova, F.; Gianolio, A.; Beretta, M. New 3D printed polymers in orthodontics: A scoping review. Eur. J. Paediatr. Dent. 2023, 24, 224–228. [Google Scholar] [CrossRef]
- HP Safety Data Sheet. Available online: https://h20195.www2.hp.com/v2/getpdf.aspx/c08246846.pdf (accessed on 24 May 2025).
- The European Commission. Commission Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food; Official Journal of the European Union: Luxembourg, 2011; Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32011R0010 (accessed on 16 March 2025).
- ISO/FDIS 10993-1; Requirements and General Principles for the Evaluation of Biological Safety Within a Risk Management Process. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/84512.html (accessed on 16 March 2025).
- US Pharmacopeia. Available online: https://www.usp.org/ (accessed on 16 March 2025).
- The European Commission. Commission Regulation on Plastic Materials and Articles Intended to Come into Contact with Food; Official Journal of the European Union: Luxembourg, 2011; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0010 (accessed on 16 March 2025).
- The European Union. Commission Regulation (EC) No 2023/2006 on Good Manufacturing Practice for Materials and Articles Intended to Come into Contact with Food; Official Journal of the European Union: Luxembourg, 2006; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02006R2023-20250316 (accessed on 16 March 2025).
- ISO 10993-5:2009; Test Methods to Assess the In Vitro Cytotoxicity of Medical Devices. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 16 March 2025).
- Moretti, G.; Guidi, F.; Canton, R.; Battagliarin, M.; Rossetto, G. Corrosion protection and mechanical performance of SiO2 films deposited via PECVD on OT59 brass. Anti-Corros. Methods Mater. 2005, 52, 266–275. [Google Scholar] [CrossRef]
- ISO 8296:2003; Plastics—Film and Sheeting—Determination of Wetting Tension. ISO: Geneva, Switzerland, 2003. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:8296:ed-2:v1:en (accessed on 16 March 2025).
- Kuracina, R.; Szabová, Z.; Buranská, E.; Pastierová, A.; Gogola, P.; Buranský, I. Determination of Fire Parameters of Polyamide 12 Powder for Additive Technologies. Polymers 2021, 13, 3014. [Google Scholar] [CrossRef]
- Kaur, T.; Nussbaum, J.; Lee, S.; Rodriguez, K.; Crane, N.B.; Harmon, J. Characterization of PA-12 specimens fabricated by projection sintering at various sintering parameters. Polym. Eng. Sci. 2021, 61, 221–233. [Google Scholar] [CrossRef]
- Han, J.; Cao, Z.; Gao, W. Remarkable sorption properties of polyamide 12 microspheres for a broad-spectrum antibacterial (triclosan) in water. J. Mater. Chem. A 2013, 1, 4941–4944. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Pełech, I.; Kwiatkowska, M.; Jędrzejewska, A.; Pełech, R.; Kowalczyk, I. Thermal and mechanical properties of polyamide 12/modified carbon nanotubes composites prepared via the in situ ring-opening polymerization. Polimery 2021, 62, 101–108. [Google Scholar] [CrossRef]
- Behler, K.; Havel, M.; Gogotsi, Y. New solvent for polyamides and its application to the electrospinning of polyamides 11 and 12. Polymer 2007, 48, 6617–6621. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Mountakis, N.; Papadakis, V.; Argyros, A.; Charou, C. Medical grade polyamide 12 silver nanoparticle filaments fabricated with in-situ reactive reduction melt-extrusion: Rheological, thermomechanical, and bactericidal performance in MEX 3D printing. Appl. Nanosci. 2024, 14, 69–88. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Tzounis, L.; Mountakis, N.; Boura, O.; Grammatikos, S.A. Multi-functional polyamide 12 (PA12)/ multiwall carbon nanotube 3D printed nanocomposites with enhanced mechanical and electrical properties. Adv. Compos. Mater. 2022, 31, 630–654. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, P. A review of the structures of oxide glasses by Raman spectroscopy. RSC Adv. 2015, 5, 67583–67609. [Google Scholar] [CrossRef]
- Colomban, P. Polymerization degree and Raman identification of ancient glasses used for jewelry, ceramic enamels and mosaics. J. Non-Cryst. Solids 2003, 323, 180–187. [Google Scholar] [CrossRef]
- Sánchez-Santamaria, B.; Cornejo-Monroy, D.; Olivas-Armendáriz, I.; Arias-Cerón, J.S.; Villanueva-Montellano, A.; Ordoñez-Casanova, E.; Dávalos-Ramírez, J.O.; Martínez-Gómez, E.A.; Jaquez-Muñoz, J.M. Antibacterial Activity of Superhydrophobic-SiO2 Coatings to Inhibit the Growth of Escherichia coli and Staphylococcus aureus. Coatings 2024, 14, 1211. [Google Scholar] [CrossRef]
- Grivet, M.; Morrier, J.J.; Benay, G.; Barsotti, O. Effect of hydrophobicity on in vitro streptococcal adhesion to dental alloys. J. Mater. Sci. Mater. Med. 2000, 11, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.B.; Logan, B.E.; Velegol, D. Importance of Molecular Details in Predicting Bacterial Adhesion to Hydrophobic Surfaces. Langmuir 2004, 20, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Yu, L.; Shi, H. Recent advances in anti-adhesion mechanism of natural antimicrobial agents on fresh produce. Curr. Opin. Food Sci. 2021, 42, 8–14. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Fonseca, S.; Cayer, M.P.; Ahmmed, K.M.T.; Khadem-Mohtaram, N.; Charette, S.J.; Brouard, D. Characterization of the Antibacterial Activity of an SiO(2) Nanoparticular Coating to Prevent Bacterial Contamination in Blood Products. Antibiotics 2022, 11, 107. [Google Scholar] [CrossRef]
- Kumar, R.; Münstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef]
- Marini, M.; De Niederhausern, S.; Iseppi, R.; Bondi, M.; Sabia, C.; Toselli, M.; Pilati, F. Antibacterial Activity of Plastics Coated with Silver-Doped Organic-Inorganic Hybrid Coatings Prepared by Sol-Gel Processes. Biomacromolecules 2007, 8, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, M.; Varesano, A.; Tummino, M.L.; Tonetti, C.; Vineis, C. Effect of coating and testing conditions on the antibacterial efficacy of polypyrrole-treated fabrics. React. Funct. Polym. 2025, 208, 106145. [Google Scholar] [CrossRef]
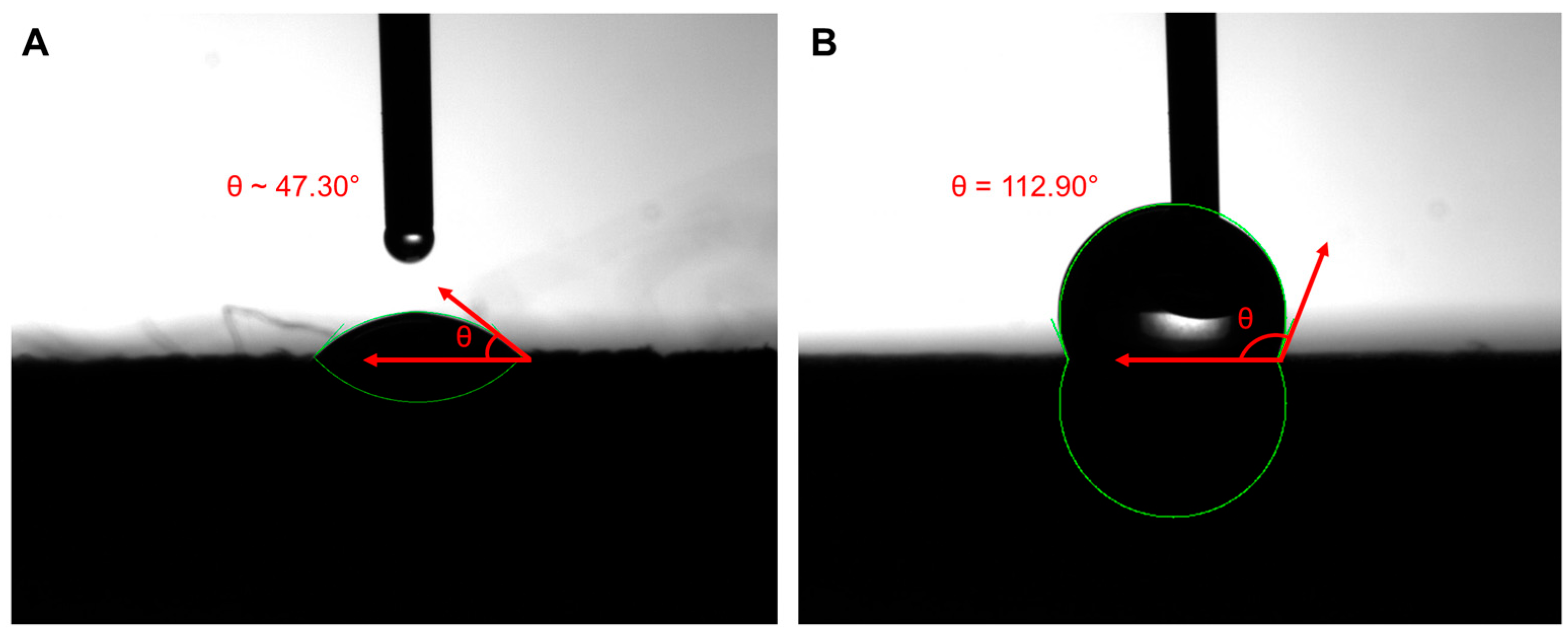
| Contact Angle (°) | Drop Volume (mm3) | Drop Surface (mm2) | Contact Surface (mm2) | |
|---|---|---|---|---|
| Sample 1 | 111.374 | 4.02 | 9.94 | 3.40 |
| Sample 2 | 114.825 | 4.31 | 10.5 | 3.32 |
| Sample 3 | 112.518 | 3.05 | 8.35 | 2.58 |
| Mean ± SD | 112.90 ± 1.76 | 3.79 ± 0.66 | 9.60 ± 1.11 | 3.10 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicotra, M.; Rosa, R.P.; Trovato, V.; Rosace, G.; Canton, R.; Loschi, A.R.; Rea, S.; Alagawany, M.; Sabia, C.; Di Cerbo, A. Investigation of Surface Properties and Antibacterial Activity of 3D-Printed Polyamide 12-Based Samples Coated by a Plasma SiOxCyHz Amorphous Thin Film Approved for Food Contact. Polymers 2025, 17, 1678. https://doi.org/10.3390/polym17121678
Nicotra M, Rosa RP, Trovato V, Rosace G, Canton R, Loschi AR, Rea S, Alagawany M, Sabia C, Di Cerbo A. Investigation of Surface Properties and Antibacterial Activity of 3D-Printed Polyamide 12-Based Samples Coated by a Plasma SiOxCyHz Amorphous Thin Film Approved for Food Contact. Polymers. 2025; 17(12):1678. https://doi.org/10.3390/polym17121678
Chicago/Turabian StyleNicotra, Mario, Raphael Palucci Rosa, Valentina Trovato, Giuseppe Rosace, Roberto Canton, Anna Rita Loschi, Stefano Rea, Mahmoud Alagawany, Carla Sabia, and Alessandro Di Cerbo. 2025. "Investigation of Surface Properties and Antibacterial Activity of 3D-Printed Polyamide 12-Based Samples Coated by a Plasma SiOxCyHz Amorphous Thin Film Approved for Food Contact" Polymers 17, no. 12: 1678. https://doi.org/10.3390/polym17121678
APA StyleNicotra, M., Rosa, R. P., Trovato, V., Rosace, G., Canton, R., Loschi, A. R., Rea, S., Alagawany, M., Sabia, C., & Di Cerbo, A. (2025). Investigation of Surface Properties and Antibacterial Activity of 3D-Printed Polyamide 12-Based Samples Coated by a Plasma SiOxCyHz Amorphous Thin Film Approved for Food Contact. Polymers, 17(12), 1678. https://doi.org/10.3390/polym17121678













