Simple Preparation of Tetrazole Chitosan Derivatives Which Exhibit High Catalytic and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemistry
3.1.1. Electrochemical Preparation and Characterization of Tetrazole Chitosan Derivatives
3.1.2. Catalytic Activity of Tetrazole Chitosan Derivatives
3.2. Biological Activity
3.2.1. In Vitro Antibacterial Activity Assessment
3.2.2. Permeabilization of Bacterial Cell Membranes
3.2.3. In Vitro Toxicity Assessment
3.2.4. In Vivo Antibacterial Activity and Toxicity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hoogenboom, R. Click chemistry in polymer science. Chem 2023, 9, 2416–2424. [Google Scholar] [CrossRef]
- Pasieka, A.; Diamanti, E.; Uliassi, E.; Laura Bolognesi, M. Click Chemistry and Targeted Degradation: A Winning Combination for Medicinal Chemists? ChemMedChem 2023, 18, e202300422. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Finn, M.G. Introduction: Click Chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Click polymerization. Chem. Soc. Rev. 2010, 39, 2522–2544. [Google Scholar] [CrossRef]
- Mondal, P.; Behera, P.K.; Singha, N.K. Macromolecular engineering in functional polymers via ‘click chemistry’ using triazolinedione derivatives. Prog. Polym. Sci. 2021, 113, 101343. [Google Scholar] [CrossRef]
- Geng, Z.; Shin, J.J.; Xi, Y.; Hawker, C.J. Click chemistry strategies for the accelerated synthesis of functional macromolecules. J. Polym. Sci. 2021, 59, 963–1042. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Karavana, S.Y.; Yilmaz, O. Functionalization of chitosan by click chemistry. AIP Conf. Proc. 2017, 1918, 020009. [Google Scholar]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Skorik, Y.A. Click reactions in chitosan chemistry. Russ. Chem. Bull. 2017, 66, 769–781. [Google Scholar] [CrossRef]
- Lunkov, A.; Shagdarova, B.; Lyalina, T.; Dubinnyi, M.A.; Karpova, N.; Lopatin, S.; Il’ina, A.; Varlamov, V. Simple method for ultrasound assisted «click» modification of azido-chitosan derivatives by CuAAC. Carbohydr. Polym. 2022, 282, 119109. [Google Scholar] [CrossRef]
- Barbosa, M.; Vale, N.; Costa, F.M.T.A.; Martins, M.C.L.; Gomes, P. Tethering antimicrobial peptides onto chitosan: Optimization of azide-alkyne “click” reaction conditions. Carbohydr. Polym. 2017, 165, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Efficient synthesis of chitosan derivatives as clickable tools. Eur. Polym. J. 2022, 166, 111039. [Google Scholar] [CrossRef]
- Berezin, A.S.; Ishmetova, R.I.; Rusinov, G.L.; Skorik, Y.A. Tetrazole derivatives of chitosan: Synthetic approaches and evaluation of toxicity. Russ. Chem. Bull. 2014, 63, 1624–1632. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Gadipelly, C.; Mannepalli, L.K. Advances in Tetrazole Synthesis—An Overview. ChemistrySelect 2022, 7, e202200706. [Google Scholar] [CrossRef]
- Summa, A.; Scafato, P.; Belviso, S.; Monaco, G.; Zanasi, R.; Longhi, G.; Abbate, S.; Superchi, S. Synthesis and Stereochemical Characterization of a Novel Chiral α-Tetrazole Binaphthylazepine Organocatalyst. Molecules 2022, 27, 5113. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A.; Keskinov, V.A.; Kritchenkov, A.S.; Murin, I.V. Fullerenol-d Solubility in Fullerenol-d–Inorganic Salt–Water Ternary Systems at 25 °C. Ind. Eng. Chem. Res. 2013, 52, 16095–16100. [Google Scholar] [CrossRef]
- Prieto, A.; Halland, N.; Jørgensen, K.A. Novel imidazolidine-tetrazole organocatalyst for asymmetric conjugate addition of nitroalkanes. Org. Lett. 2005, 7, 3897–3900. [Google Scholar] [CrossRef]
- Mulwad, V.V.; Pawar, R.B.; Chaskar, A.C. Synthesis and antibacterial activity of new tetrazole derivatives. J. Korean Chem. Soc. 2008, 52, 249–256. [Google Scholar]
- Gao, F.; Xiao, J.; Huang, G. Current scenario of tetrazole hybrids for antibacterial activity. Eur. J. Med. Chem. 2019, 184, 111744. [Google Scholar] [CrossRef]
- Roszkowski, P.; Szymańska-majchrzak, J.; Koliński, M.; Kmiecik, S.; Wrzosek, M.; Struga, M.; Szulczyk, D. Novel tetrazole-based antimicrobial agents targeting clinical bacteria strains: Exploring the inhibition of staphylococcus aureus dna topoisomerase iv and gyrase. Int. J. Mol. Sci. 2022, 23, 378. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Krytchankou, I.S.; Dubashynskaya, N.V.; Volkova, O.V.; Shakola, T.V.; Kurliuk, A.V.; Skorik, Y.A. Synthesis of novel 1H-tetrazole derivatives of chitosan via metal-catalyzed 1,3-dipolar cycloaddition. Catalytic and antibacterial properties of [3-(1H-tetrazole-5-yl)ethyl]chitosan and its nanoparticles. Int. J. Biol. Macromol. 2019, 132, 340–350. [Google Scholar] [CrossRef]
- Zhu, C.; Ang, N.W.J.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Organic Electrochemistry: Molecular Syntheses with Potential. ACS Cent. Sci. 2021, 7, 415–431. [Google Scholar] [CrossRef]
- Shakola, T.V.; Rubanik, V.V.; Rubanik, V.V., Jr.; Kurliuk, A.V.; Kirichuk, A.A.; Tskhovrebov, A.G.; Egorov, A.R.; Kritchenkov, A.S. The first electrochemical N-arylation of chitosan. Antibacterial effect of novel cationic chitosan derivatives. Eur. Polym. J. 2023, 198, 112418. [Google Scholar] [CrossRef]
- Shakola, T.V.; Rubanik, V.V.; Kurliuk, A.V.; Kirichuk, A.A.; Tskhovrebov, A.G.; Egorov, A.R.; Kritchenkov, A.S. Benzothiazole Derivatives of Chitosan and Their Derived Nanoparticles: Synthesis and In Vitro and In Vivo Antibacterial Effects. Polymers 2023, 15, 3469. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, F.; Li, Y.; Zhang, F. Electrochemical synthesis of tetrazoles: Via metal- and oxidant-free [3 + 2] cycloaddition of azides with hydrazones. Green Chem. 2018, 20, 5271–5275. [Google Scholar] [CrossRef]
- Pogodina, N.V.; Pavlov, G.M.; Bushin, S.V.; Mel’nikov, A.B.; Lysenko, Y.B.; Nud’ga, L.A.; Marsheva, V.N.; Marchenko, G.N.; Tsvetkov, V.N. Conformational characteristics of chitosan molecules as demonstrated by diffusion-sedimentation analysis and viscometry. Polym. Sci. (USSR) 1987, 28, 251–259. [Google Scholar] [CrossRef]
- Sahm, D.H. Antibacterial susceptibility tests: Dilution methods. In Manual of Clinical Microbiology; Murray, P.R., Ed.; ASM Press: Washington, DC, USA, 1991; pp. 1105–1116. [Google Scholar]
- Nikš, M.; Otto, M. Towards an optimized MTT assay. J. Immunol. Methods 1990, 130, 149–151. [Google Scholar] [CrossRef]
- Christ, H.A.; Bourgat, Y.; Menzel, H. Optimization of critical parameters for carbodiimide mediated production of highly modified chitosan. Polymers 2021, 13, 2702. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Dzolkifle, N.A.N.; Wan Nawawi, W.M.F. A review on chitin dissolution as preparation for electrospinning application. Int. J. Biol. Macromol. 2024, 265, 130858. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, T.; Zhou, M.; Xue, J.; Luo, Y. Formation of redispersible polyelectrolyte complex nanoparticles from gallic acid-chitosan conjugate and gum arabic. Int. J. Biol. Macromol. 2016, 92, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Mayanovic, R.A. A Review of the Preparation, Characterization, and Applications of Chitosan Nanoparticles in Nanomedicine. Nanomaterials 2023, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, T.; Guo, Z.; Zhou, L.; Liu, G.; He, Y.; Ma, L.; Gao, J.; Bai, J.; Hollmann, F.; et al. Asymmetric α-benzylation of cyclic ketones enabled by concurrent chemical aldol condensation and biocatalytic reduction. Nat. Commun. 2024, 15, 71. [Google Scholar] [CrossRef]
- Zárate-Roldán, S.; Trujillo-Rodríguez, M.J.; Gimeno, M.C.; Herrera, R.P. L-proline-based deep eutectic solvents as green and enantioselective organocatalyst/media for aldol reaction. J. Mol. Liq. 2024, 396, 123971. [Google Scholar] [CrossRef]
- Heathcock, C.H. 1.5—The Aldol Reaction: Acid and General Base Catalysis. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; pp. 133–179. [Google Scholar]
- Ghosh, A.K.; Dawson, Z.L. Synthesis of bioactive natural products by asymmetric syn-and anti-aldol reactions. Synthesis 2009, 2009, 2992–3002. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Caciolli, L.; Zaghi, A.; Pandoli, O.; Bortolini, O.; Cavazzini, A.; De Risi, C.; Massi, A. A monolithic 5-(pyrrolidin-2-yl)tetrazole flow microreactor for the asymmetric aldol reaction in water-ethanol solvent. React. Chem. Eng. 2016, 1, 183–193. [Google Scholar] [CrossRef]
- Maji, B.; Yamamoto, H. Proline-tetrazole-catalyzed enantioselective N-nitroso aldol reaction of aldehydes with in situ generated nitrosocarbonyl compounds. Angew. Chem. Int. Ed. 2014, 53, 8714–8717. [Google Scholar] [CrossRef]
- Odedra, A.; Seeberger, P.H. 5-(Pyrrolidin-2-yl)tetrazole-catalyzed aldol and mannich reactions: Acceleration and lower catalyst loading in a continuous-flow reactor. Angew. Chem. Int. Ed. 2009, 48, 2699–2702. [Google Scholar] [CrossRef]
- Ricci, A.; Bernardi, L.; Gioia, C.; Vierucci, S.; Robitzer, M.; Quignard, F. Chitosan aerogel: A recyclable, heterogeneous organocatalyst for the asymmetric direct aldol reaction in water. Chem. Commun. 2010, 46, 6288–6290. [Google Scholar] [CrossRef]
- Devi, M.; Jaiswal, S.; Yaduvanshi, N.; Kaur, N.; Kishore, D.; Dwivedi, J.; Sharma, S. Design, Synthesis, Antibacterial Evaluation and Docking Studies of Triazole and Tetrazole Linked 1,4-benzodiazepine Nucleus via Click Approach. ChemistrySelect 2023, 8, e202204710. [Google Scholar] [CrossRef]
- Bourhou, C.; Benouda, H.; Bellaouchi, R.; Merzouki, M.; Fraj, E.; Harit, T.; Challioui, A.; Asehraou, A.; Touzani, R.; Ozdemir, I.; et al. Synthesis of novel tetrazolic derivatives and evaluation of their antimicrobial activity. J. Mol. Struct. 2023, 1278, 134913. [Google Scholar] [CrossRef]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S., Jr.; Dutta, P.K.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833–1852. [Google Scholar]
- Yilmaz Atay, H. Antibacterial activity of chitosan-based systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Springer: Singapore, 2020; pp. 457–489. [Google Scholar]
- Shai, Y.; Oren, Z. From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides 2001, 22, 1629–1641. [Google Scholar] [CrossRef]
- Dean, R.E.; O’Brien, L.M.; Thwaite, J.E.; Fox, M.A.; Atkins, H.; Ulaeto, D.O. A carpet-based mechanism for direct antimicrobial peptide activity against vaccinia virus membranes. Peptides 2010, 31, 1966–1972. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial activity of chitosan nanoparticles: A review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Alqahtani, F.; Aleanizy, F.; Tahir, E.E.; Alhabib, H.; Alsaif, R.; Shazly, G.; Alqahtani, H.; Alsarra, I.; Mahdavi, J. Antibacterial activity of chitosan nanoparticles against pathogenic N. Gonorrhoea. Int. J. Nanomed. 2020, 15, 7877–7887. [Google Scholar] [CrossRef]
- Kulawik, P.; Jamróz, E.; Özogul, F. Chitosan role for shelf-life extension of seafood. Environ. Chem. Lett. 2020, 18, 61–74. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Artemjev, A.A.; Kurliuk, A.V.; Anh Le, T.; Hieu Truong, H.; Le-Nhat-Thuy, G.; Van Tran Thi, T.; Van Tuyen, N.; et al. Novel biopolymer-based nanocomposite food coatings that exhibit active and smart properties due to a single type of nanoparticles. Food Chem. 2021, 343, 128676. [Google Scholar] [CrossRef]
- Mirbagheri, V.S.; Alishahi, A.; Ahmadian, G.; Hashemi Petroudi, S.H.; Ojagh, S.M.; Romanazzi, G. Toward understanding the antibacterial mechanism of chitosan: Experimental approach and in silico analysis. Food Hydrocoll. 2024, 147, 109382. [Google Scholar] [CrossRef]
- Egorov, A.R.; Khubiev, O.; Rubanik, V.V.; Lobanov, N.N.; Savilov, S.V.; Kirichuk, A.A.; Kritchenkov, I.S.; Tskhovrebov, A.G.; Kritchenkov, A.S. The first selenium containing chitin and chitosan derivatives: Combined synthetic, catalytic and biological studies. Int. J. Biol. Macromol. 2022, 209, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The mtt assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W. Peritonitis. In Small Animal Critical Care Medicine, 2nd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; pp. 643–648. [Google Scholar]
- Frimodt-Møller, N. The mouse peritonitis model: Present and future use. J. Antimicrob. Chemother. 1993, 31, 55–60. [Google Scholar] [CrossRef]
- Deng, Y.; Weng, X.; Li, Y.; Su, M.; Wen, Z.; Ji, X.; Ren, N.; Shen, B.; Duan, Y.; Huang, Y. Late-stage functionalization of platensimycin leading to multiple analogues with improved antibacterial activity in vitro and in vivo. J. Med. Chem. 2019, 62, 6682–6693. [Google Scholar] [CrossRef]


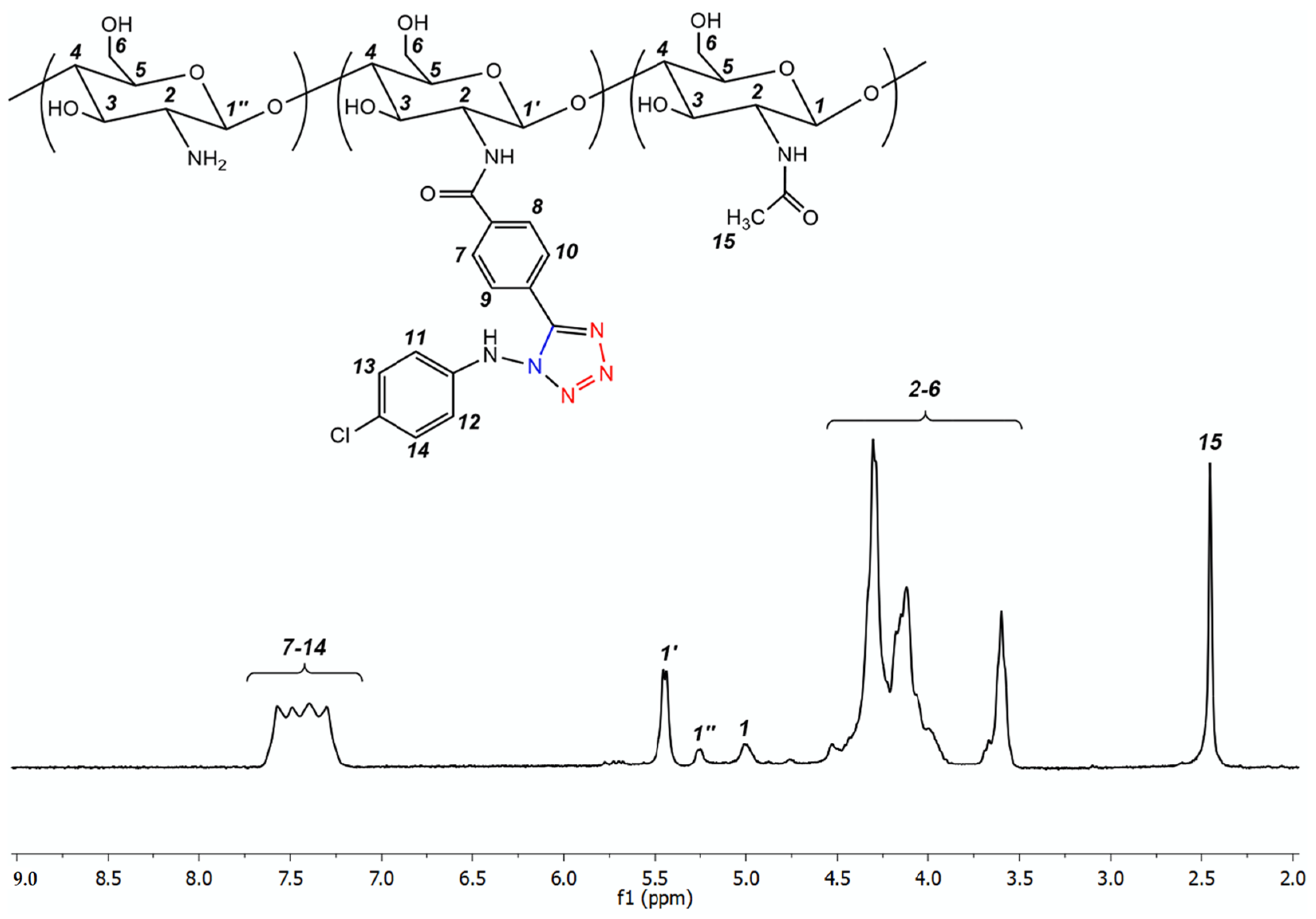
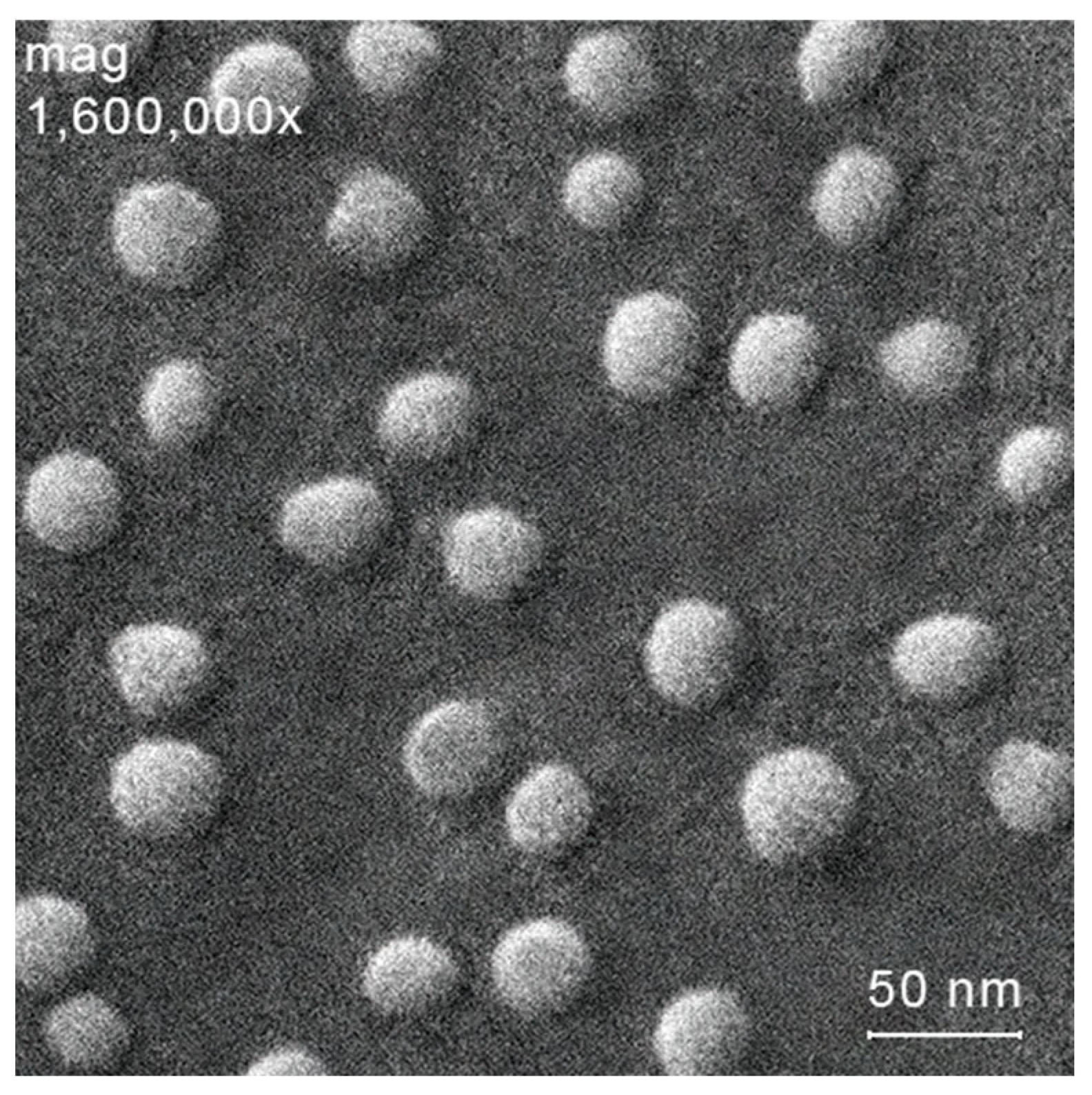

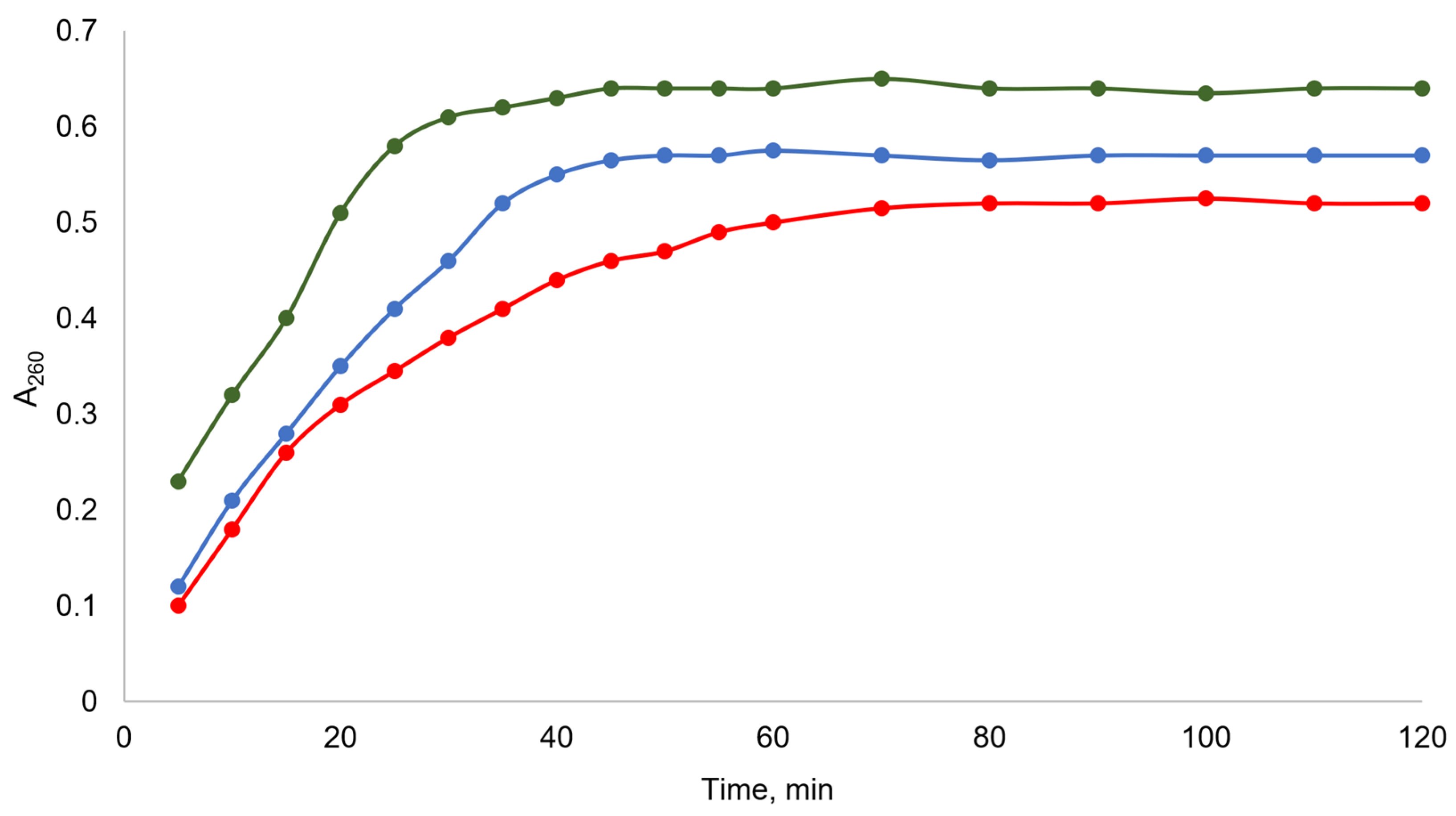
| Hydrazone Chitosan Derivatives/Solubility | Tetrazole Chitosan Derivatives/Solubility | Molecular Weight of the Starting Chitosan, kDa | Degree of Substitution |
|---|---|---|---|
| HCs-0.10-L | TCs-0.10-L | 15 | 0.10 |
| HCs-0.10-M | 60 | 0.10 | |
| HCs-0.10-H | 160 | 0.10 | |
| HCs-0.30-L | TCs-0.30-L | 15 | 0.30 |
| HCs-0.30-M | TCs-0.30-M | 60 | 0.30 |
| HCs-0.30-H | TCs-0.35-H | 160 | 0.30 |
| HCs-0.65-L | TCs-0.65-L | 15 | 0.60 |
| HCs-0.65-M | TCs-0.65-M | 60 | 0.60 |
| HCs-0.65-H | TCs-0.65-H | 160 | 0.60 |
| Tested Catalyst | Conversion, % | ||||
|---|---|---|---|---|---|
| 5 min | 10 min | 15 min | 30 min | 60 min | |
| Chitosan | 0 | 0 | 0 | 0 | 0 |
| TCs-0.10-L | 0 | 0 | 5 | 17 | 26 |
| TCs-0.30-L | 8 | 28 | 57 | 85 | 100 |
| TCs-0.30-M | 10 | 28 | 58 | 85 | 100 |
| TCs-0.35-H | 9 | 28 | 58 | 85 | 100 |
| TCs-0.65-L | 61 | 78 | 100 | ||
| TCs-0.65-M | 58 | 80 | 100 | ||
| TCs-0.65-H | 61 | 77 | 100 | ||
| A | 70 | 85 | 100 | ||
| Tested Sample | Inhibition zone, mm * | |
|---|---|---|
| Staphylococcus aureus | Escherichia coli | |
| Chitosan 15 kDa | 12.7 ± 0.1 | 8.5 ± 0.1 |
| Chitosan 60 kDa | 10.3 ± 0.3 | 7.1 ± 0.1 |
| Chitosan 160 kDa | 10.2 ± 0.2 | 7.1 ± 0.2 |
| TCs-0.10-L | 27.8 ± 0.1 | 22.6 ± 0.3 |
| TCs-0.30-L | 14.6 ± 0.2 | 11.1 ± 0.2 |
| TCs-0.30-M | 13.2 ± 0.1 | 9.4 ± 0.3 |
| TCs-0.35-H | 13.2 ± 0.1 | 9.3 ± 0.1 |
| TCs-0.65-L | 13.5 ± 0.1 | 9.1 ± 0.2 |
| TCs-0.65-M | 13.7 ± 0.3 | 9.2 ± 0.2 |
| TCs-0.65-H | 13.7 ± 0.3 | 9.3 ± 0.2 |
| A | 14.4 ± 0.3 | 10.0 ± 0.3 |
| Ampicillin | 28.1 ± 0.3 | - |
| Gentamicin | - | 23.3 ± 0.3 |
| Sample | Percentage of Surviving Cells at Various Concentrations of Test Sample * | ||
|---|---|---|---|
| 10 µg/mL | 300 µg/mL | 1000 µg/mL | |
| Chitosan | 100 | 92 | 70 |
| TCs-0.10-L | 95 | 88 | 66 |
| TCs-0.30-L | 89 | 71 | 48 |
| Sample | CFU per mL of Exudate (7 h after Treatment) * |
|---|---|
| Blank experiment | 2860 |
| Chitosan | 1780 |
| TCs-0.10-L | 0 |
| Ampicillin and gentamicin (1:1) | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorov, A.R.; Nguyen, L.V.; Sikaona, N.D.; Khubiev, O.M.; Golubev, R.A.; Maharramov, A.M.; Nazarov, R.H.; Tskhovrebov, A.G.; Rubanik, V.V.; Rubanik, V.V., Jr.; et al. Simple Preparation of Tetrazole Chitosan Derivatives Which Exhibit High Catalytic and Antibacterial Activity. Polymers 2025, 17, 1657. https://doi.org/10.3390/polym17121657
Egorov AR, Nguyen LV, Sikaona ND, Khubiev OM, Golubev RA, Maharramov AM, Nazarov RH, Tskhovrebov AG, Rubanik VV, Rubanik VV Jr., et al. Simple Preparation of Tetrazole Chitosan Derivatives Which Exhibit High Catalytic and Antibacterial Activity. Polymers. 2025; 17(12):1657. https://doi.org/10.3390/polym17121657
Chicago/Turabian StyleEgorov, Anton R., Linh V. Nguyen, Nkumbu D. Sikaona, Omar M. Khubiev, Roman A. Golubev, Abel M. Maharramov, Rovshan H. Nazarov, Alexander G. Tskhovrebov, Vasili V. Rubanik, Vasili V. Rubanik, Jr., and et al. 2025. "Simple Preparation of Tetrazole Chitosan Derivatives Which Exhibit High Catalytic and Antibacterial Activity" Polymers 17, no. 12: 1657. https://doi.org/10.3390/polym17121657
APA StyleEgorov, A. R., Nguyen, L. V., Sikaona, N. D., Khubiev, O. M., Golubev, R. A., Maharramov, A. M., Nazarov, R. H., Tskhovrebov, A. G., Rubanik, V. V., Rubanik, V. V., Jr., Kurliuk, A. V., Kirichuk, A. A., Liu, W., & Kritchenkov, A. S. (2025). Simple Preparation of Tetrazole Chitosan Derivatives Which Exhibit High Catalytic and Antibacterial Activity. Polymers, 17(12), 1657. https://doi.org/10.3390/polym17121657









