Catechol-Modified Alkali Lignin for Cr (VI) Removal from Synthetic Wastewater
Abstract
1. Introductory
2. Materials and Methods
2.1. Material
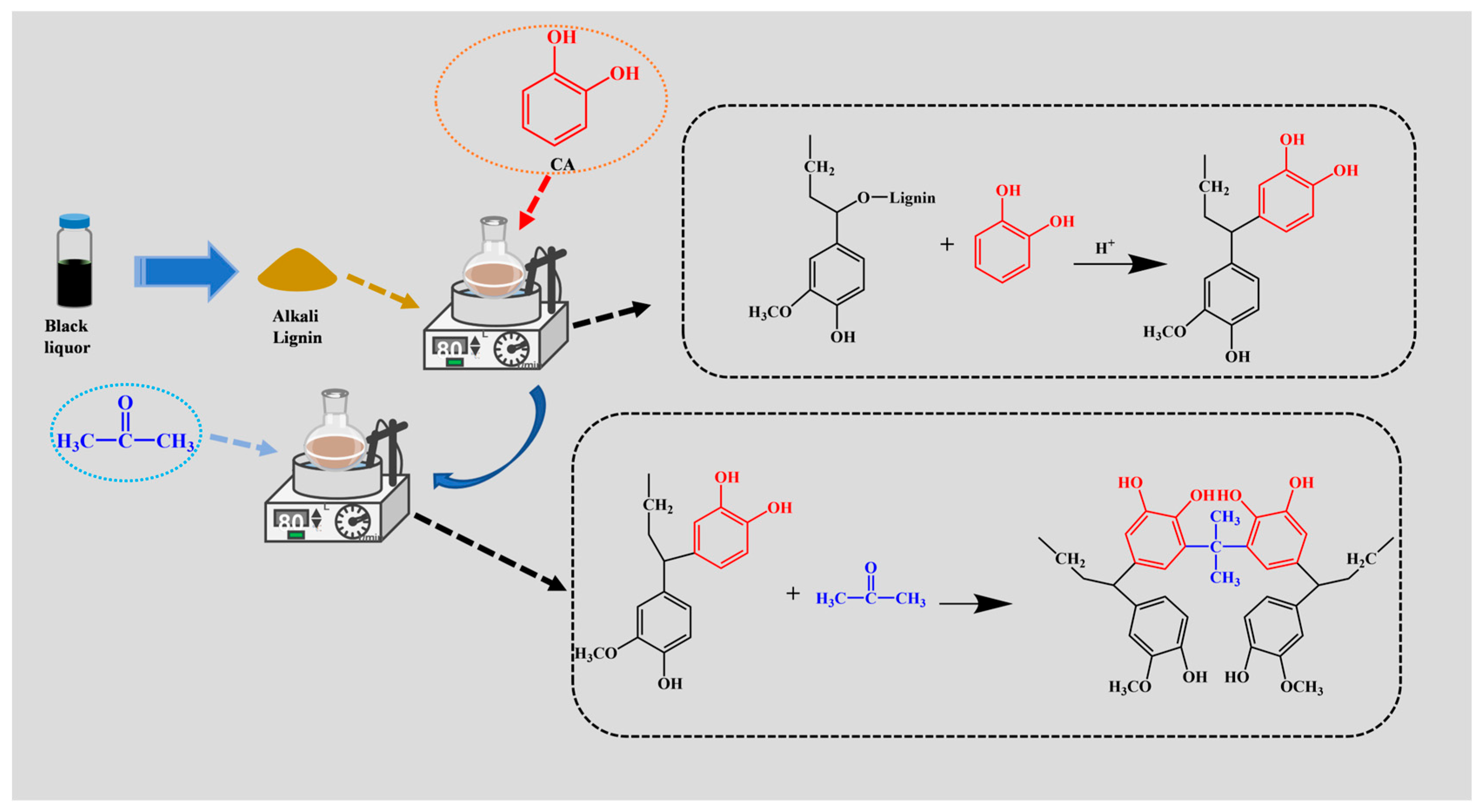
2.2. Preparation of CAL
2.3. Physicochemical Characteristics
2.4. Batch Adsorption Experiments
3. Results and Discussion
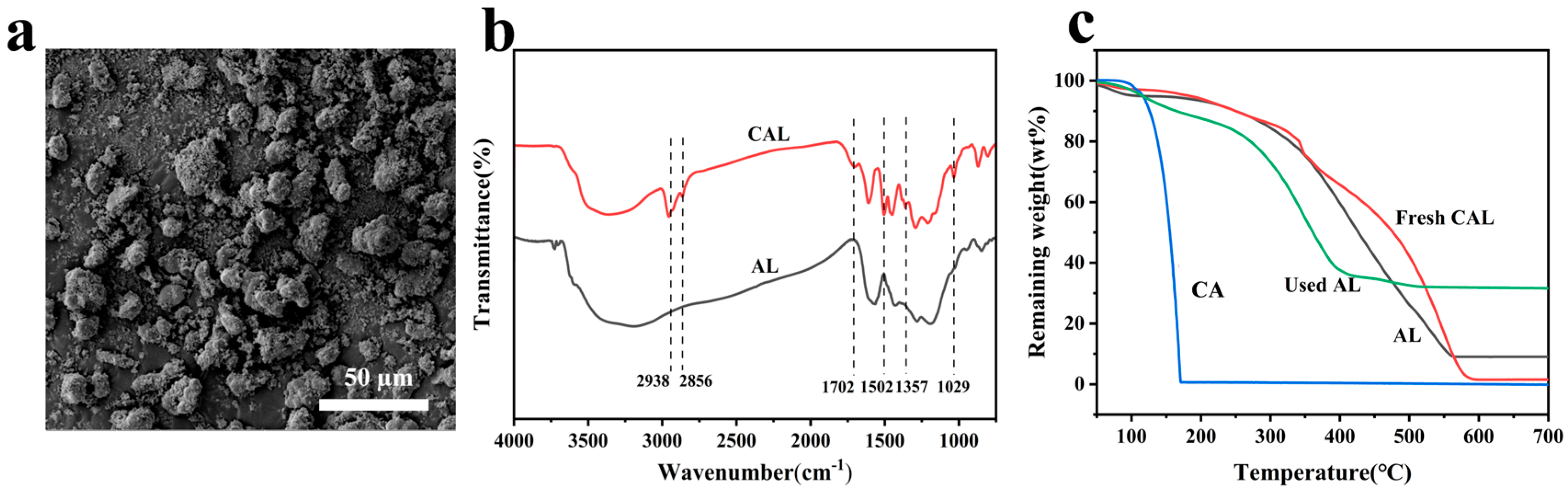
3.1. Characterization of the CAL Structure
3.2. Effect of the Monomer and Ratio Composition on the Adsorption Amount
3.3. Effect of Time
3.4. Effect of Initial Target Ion Concentration and Temperature
3.5. Effect of pH
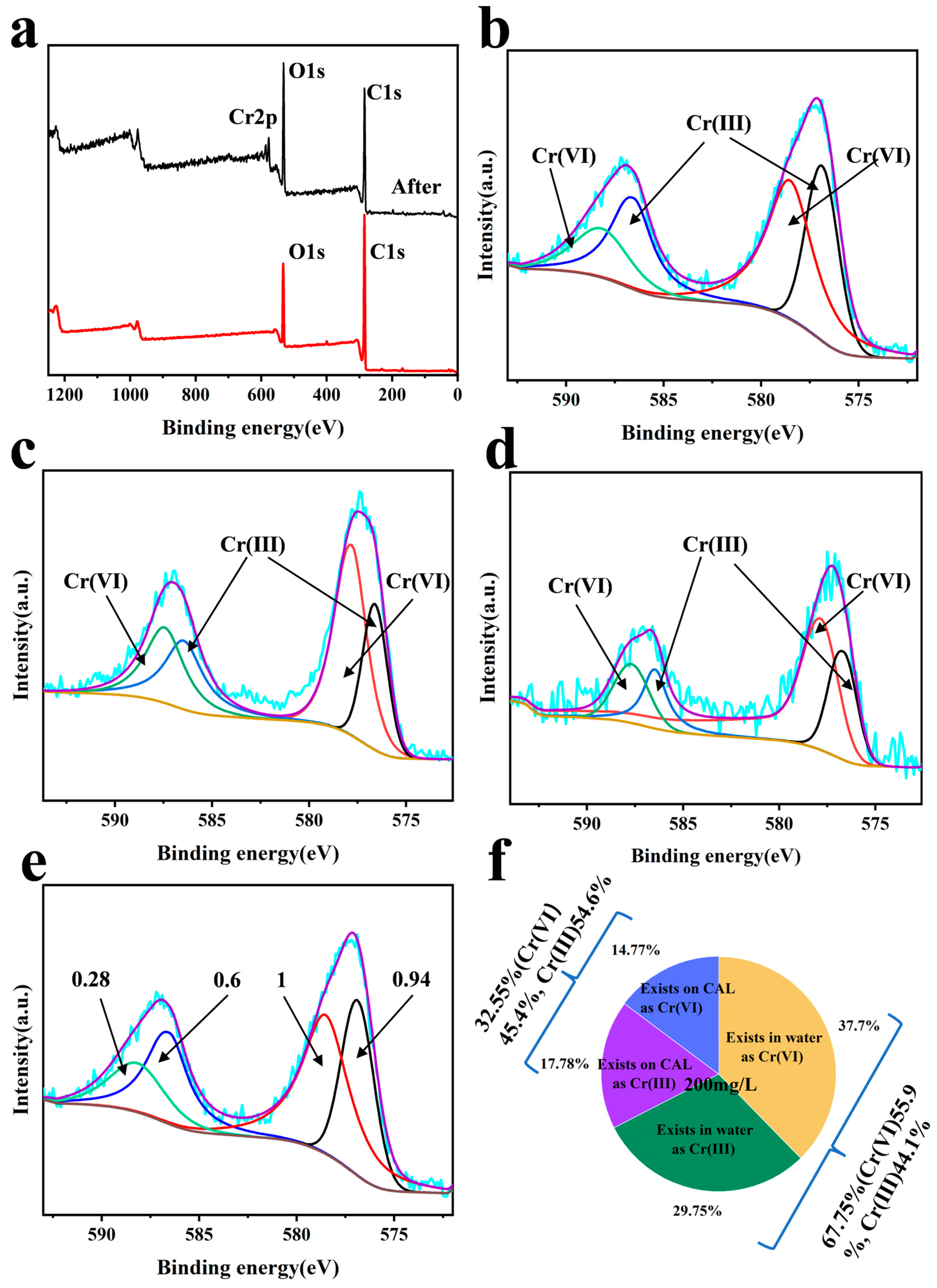
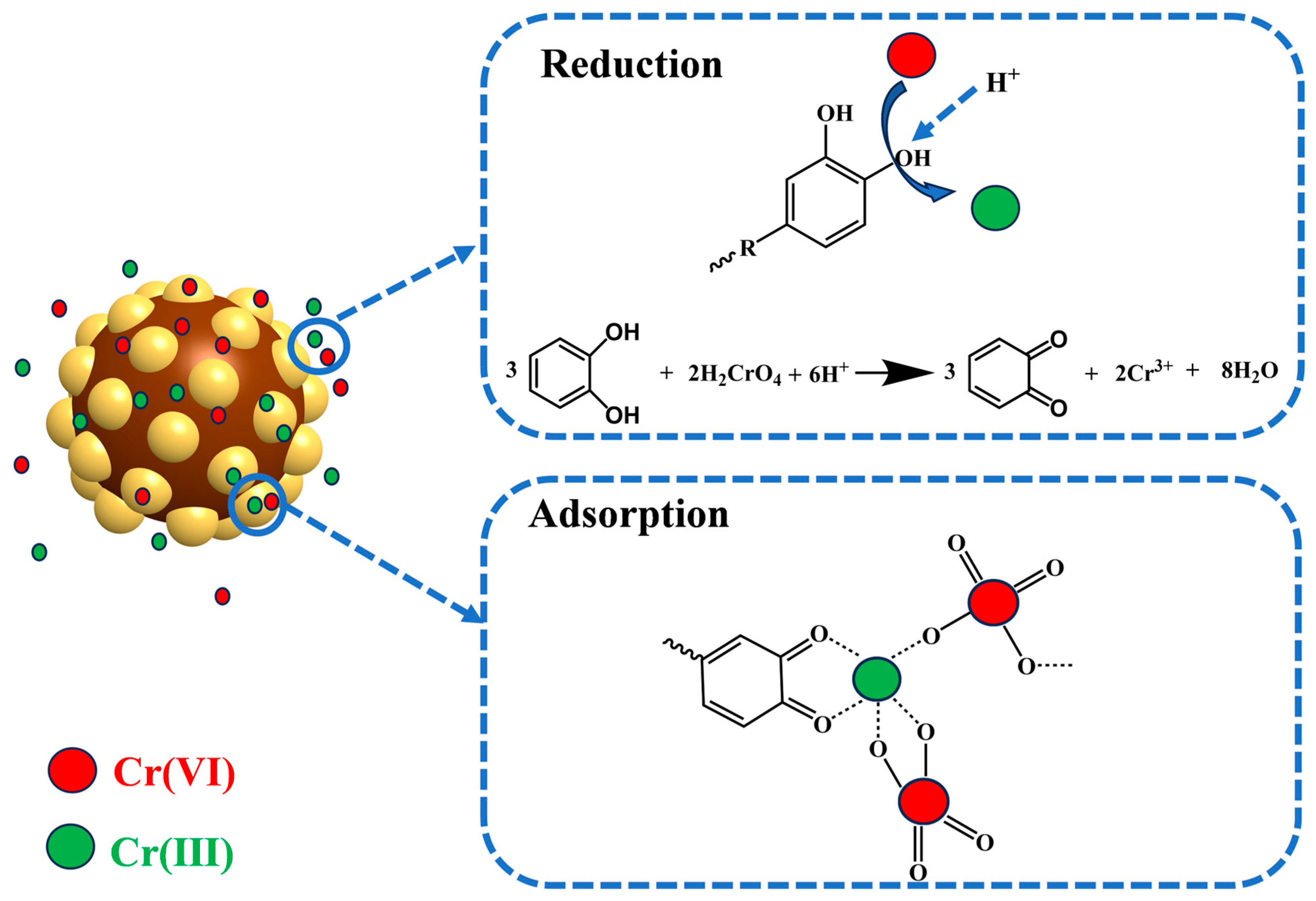
3.6. XPS Analysis
3.7. Comparison of Cr (VI) Absorptivity with Others
3.8. Isothermal and Adsorption Kinetic Modeling
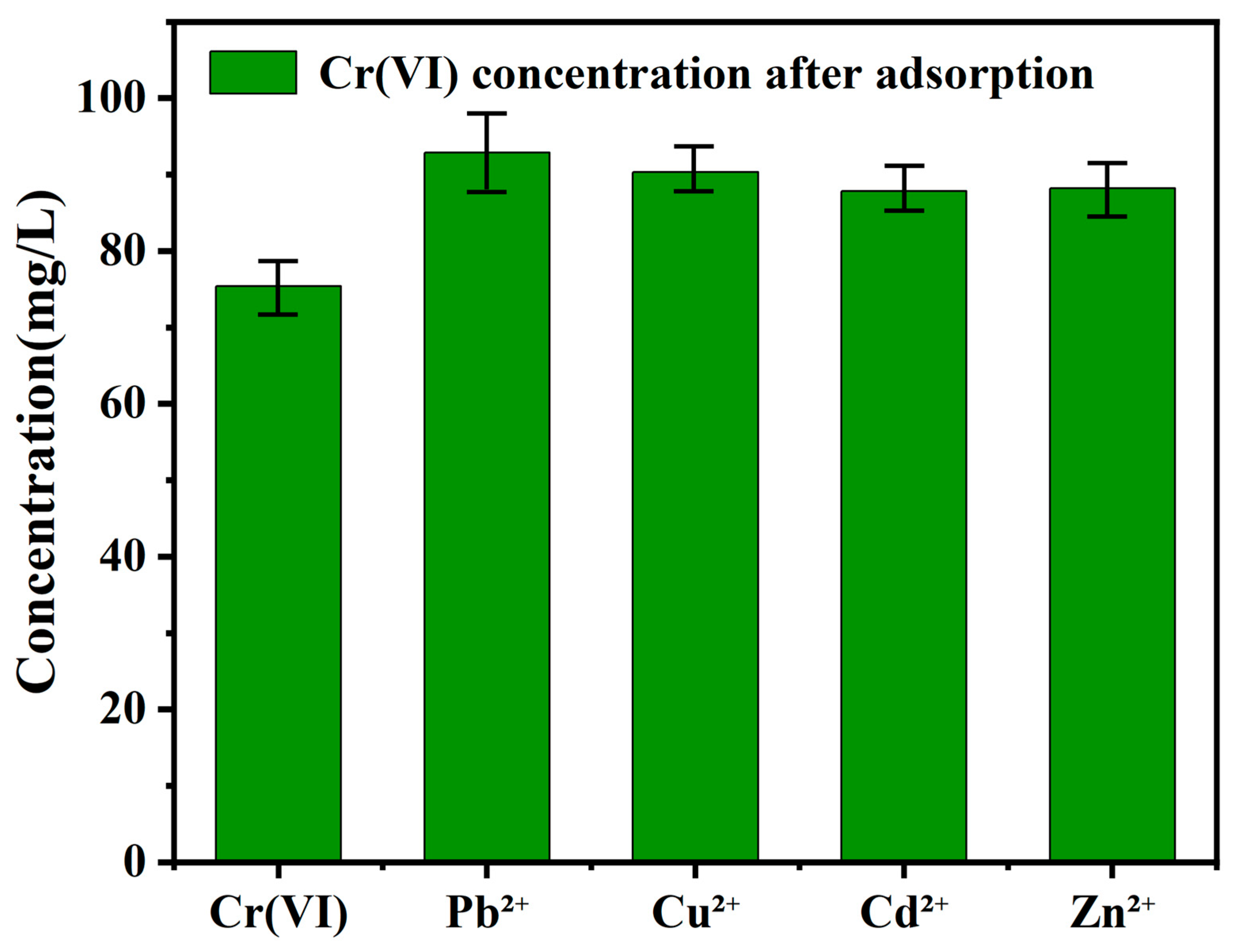
3.9. Effects of Competing Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Koleli, N.; Demir, A. Chapter 11—Chromite. In Environmental Materials and Waste; Prasad, M.N.V., Shih, K., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 245–263. [Google Scholar] [CrossRef]
- du Preez, S.P.; van Kaam, T.P.M.; Ringdalen, E.; Tangstad, M.; Morita, K.; Bessarabov, D.G.; van Zyl, P.G.; Beukes, J.P. An Overview of Currently Applied Ferrochrome Production Processes and Their Waste Management Practices. Minerals 2023, 13, 809. [Google Scholar] [CrossRef]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Health 2018, 12, 51–62. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Wang, Y.; Tan, C.; Zhou, M.; Tian, Y.; Li, L.; Qiu, F. Research progress on hazardous chromite ore processing residue treatment and utilization: A critical review. Chem. Eng. Commun. 2024, 211, 1445–1468. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef]
- Iyer, M.; Anand, U.; Thiruvenkataswamy, S.; Babu, H.W.S.; Narayanasamy, A.; Prajapati, V.K.; Tiwari, C.K.; Gopalakrishnan, A.V.; Bontempi, E.; Sonne, C.; et al. A review of chromium (Cr) epigenetic toxicity and health hazards. Sci. Total Environ. 2023, 882, 163483. [Google Scholar] [CrossRef]
- Khoo, P.S.; Ilyas, R.A.; Aiman, A.; Wei, J.S.; Yousef, A.; Anis, N.; Zuhri, M.Y.M.; Abral, H.; Sari, N.H.; Syafri, E.; et al. Revolutionizing wastewater treatment: A review on the role of advanced functional bio-based hydrogels. Int. J. Biol. Macromol. 2024, 278, 135088. [Google Scholar] [CrossRef]
- Xing, R.F.; Song, Y.X.; Gao, T.T.; Cai, X.X.; Yao, J.S.; Liu, Q.Z.; Zhang, C.B. High capacity and fast removal of Cr(vi) by alkali lignin-based poly(tetraethylene pentamine-pyrogallol) sorbent. Rsc Adv. 2023, 13, 1627–1639. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Tripathi, R.D.; Tong, Y.W. Chromium toxicity and tolerance mechanisms in plants through cross-talk of secondary messengers: An overview of pathways and mechanisms. Environ. Pollut. 2023, 320, 121049. [Google Scholar] [CrossRef]
- Yan, G.; Gao, Y.; Xue, K.; Qi, Y.; Fan, Y.; Tian, X.; Wang, J.; Zhao, R.; Zhang, P.; Liu, Y.; et al. Toxicity mechanisms and remediation strategies for chromium exposure in the environment. Front. Environ. Sci. 2023, 11, 1131204. [Google Scholar] [CrossRef]
- Swaroop, A.; Bagchi, M.; Preuss, H.G.; Zafra-Stone, S.; Ahmad, T.; Bagchi, D. Chapter 8—Benefits of chromium(III) complexes in animal and human health. In The Nutritional Biochemistry of Chromium (III), 2nd ed.; Vincent, J.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 251–278. [Google Scholar] [CrossRef]
- Cojocaru, C.; Clima, L. Polymer assisted ultrafiltration of AO7 anionic dye from aqueous solutions: Experimental design, multivariate optimization, and molecular docking insights. J. Membr. Sci. 2020, 604, 118054. [Google Scholar] [CrossRef]
- Li, X.; Xiang, X.-y.; Wu, Y.-x.; Sun, Y.-m.; Wei, Y.-f. Separation of V(V) and Cr(VI) from vanadium-containing solutions based on E-pH diagrams of V-Cr-S-H2O system. Sep. Purif. Technol. 2024, 350, 127890. [Google Scholar] [CrossRef]
- Jiang, Y.; Tian, Q.; Xu, J.; Qiu, F.; Zhang, T. Enhanced separation of dual pollutants from wastewater containing Cr(VI) and oil via Fe-doped sludge derived membrane. Chem. Eng. Sci. 2024, 292, 120020. [Google Scholar] [CrossRef]
- Kohila, N.; Subramaniam, P. Removal of Cr(VI) using polyaniline based Sn(IV), Ce(IV) and Bi(III) iodomolybdate hybrid ion exchangers: Mechanistic and comparative study. J. Environ. Chem. Eng. 2020, 8, 104376. [Google Scholar] [CrossRef]
- Luo, Y.; Lan, Y.; Liu, X.; Xue, M.; Zhang, L.; Yin, Z.; He, X.; Li, X.; Yang, J.; Hong, Z.; et al. Hydrochar effectively removes aqueous Cr(VI) through synergistic adsorption and photoreduction. Sep. Purif. Technol. 2023, 317, 123926. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Zhou, S.; Gao, X. Adsorption mechanism of Cr(VI) on woody-activated carbons. Heliyon 2023, 9, e13267. [Google Scholar] [CrossRef]
- Tu, W.; Cai, W. Selective Adsorption of Hazardous Substances from Wastewater by Hierarchical Oxide Composites: A Review. Toxics 2024, 12, 447. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018, 17, 195–213. [Google Scholar] [CrossRef]
- Vara, D.; Jha, S.; Bisht, S.; Shahabuddin, S.; Gaur, R.; Suhas; Tyagi, I. Sustainable Bio-Based Adsorbents for Simultaneous and Efficient Removal of Hazardous Dyes from Aqueous Solutions. Toxics 2024, 12, 266. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Bio-Based Polymeric Flocculants and Adsorbents for Wastewater Treatment. Sustainability 2023, 15, 9844. [Google Scholar] [CrossRef]
- Zhou, H.R.; Huang, J.; Chen, M.; Li, Y.; Yuan, M.; Yang, H. Effect of metal ions with reducing properties on hydrogels containing catechol groups. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127657. [Google Scholar] [CrossRef]
- Ko, S.; Baek, M.-J.; Wi, T.-U.; Kim, J.; Park, C.; Lim, D.; Yeom, S.J.; Bayramova, K.; Lim, H.Y.; Kwak, S.K.; et al. Understanding the Role of a Water-Soluble Catechol-Functionalized Binder for Silicon Anodes by Diverse In Situ Analyses. ACS Mater. Lett. 2022, 4, 831–839. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Liu, H.; Zhang, D.; Guo, Y.; Shi, Q.-S.; Xie, X. Lignin hydrogenolysis: Tuning the reaction by lignin chemistry. Int. J. Biol. Macromol. 2024, 279, 135169. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-j.; Fu, W.-k.; Ma, J.-y.; Xi, B.-j.; Wang, C.; Guan, M.-y. Efficient removal of Cr(VI) by polydopamine-modified lignin from aqueous solution: Batch and XAFS studies. Water Sci. Eng. 2024, 17, 51–61. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Li, B.-Z.; Yuan, J.S.; Yuan, Y.-J. Creative biological lignin conversion routes toward lignin valorization. Trends Biotechnol. 2022, 40, 1550–1566. [Google Scholar] [CrossRef]
- Kocaturk, E.; Salan, T.; Ozcelik, O.; Alma, M.H.; Candan, Z. Recent Advances in Lignin-Based Biofuel Production. Energies 2023, 16, 3382. [Google Scholar] [CrossRef]
- Mateo, S.; Fabbrizi, G.; Moya, A.J. Lignin from Plant-Based Agro-Industrial Biowastes: From Extraction to Sustainable Applications. Polymers 2025, 17, 952. [Google Scholar] [CrossRef]
- Zhao, C.K.; Li, S.X.; Zhang, H.; Yue, F.X.; Lu, F.C. Structural insights into the alkali lignins involving the formation and transformation of arylglycerols and enol ethers. Int. J. Biol. Macromol. 2020, 152, 411–417. [Google Scholar] [CrossRef]
- Yadav, P.; Kring, J.; Gogate, P.R. Ultrasound-assisted synthesis of carboxy-methyl lignin from sawdust based lignin as a sustainable source. Chem. Eng. Process.-Process Intensif. 2025, 210, 110211. [Google Scholar] [CrossRef]
- Gong, L.; Wu, H.; Shan, X.; Li, Z. Facile fabrication of phosphorylated alkali lignin microparticles for efficient adsorption of antibiotics and heavy metal ions in water. J. Environ. Chem. Eng. 2021, 9, 106574. [Google Scholar] [CrossRef]
- Li, J.-F.; Li, Z.-M.; Xu, J.-B.; Guo, C.-Y.; Fang, G.-W.; Zhou, Y.; Sun, M.-S.; Tao, D.-J. Demethylation of Waste Alkali Lignin for Rapid and Efficient Ammonia Adsorption. Ind. Eng. Chem. Res. 2024, 63, 3282–3289. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Gao, T.; Qiao, C.; Yao, J.; Zhang, C. Phenolation of lignin for polycatecholamines to remove Cr (VI). J. Water Process Eng. 2022, 50, 103334. [Google Scholar] [CrossRef]
- Hagiri, M.; Fukuhara, S.; Kimura, Y.; Manaka, A. Quantitative determination of Hexavalent chromium using a microtiter plate: Analytical performance, operational efficiency, and fixation of a colorimetric reagent in the plate wells. Microchem. J. 2024, 199, 110004. [Google Scholar] [CrossRef]
- Martins, A.S.; Dantas, H.A.; Dantas, K.F. Determination of trace elements in mineral water by MIP OES using the Marin-5 nebulization system. J. Anal. At. Spectrom. 2023, 38, 1007–1015. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Khorasani, Z.; Hamzeh, Y.; Momenbeik, F.; Zadeh, Z.E.; Sun, F.; Madadi, M.; Zhang, X. Valorization of bagasse alkali lignin to water-soluble derivatives through chemical modification. Biomass Convers. Biorefinery 2022, 14, 8639–8647. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullón, P. Influence of lignin modifications on physically crosslinked lignin hydrogels for drug delivery applications. Sustain. Mater. Technol. 2022, 33, e00474. [Google Scholar] [CrossRef]
- Feng, C.-y.; Chen, H.-j.; Yang, M.-y.; Feng, Z.-s.; Wang, Y. Metallization of Polyphenylene Sulfide by Low-Cost Mussel-Inspired Catechol/Polyamine Surface Modification. ACS Appl. Polym. Mater. 2022, 4, 4445–4453. [Google Scholar] [CrossRef]
- El Mansouri, N.E.; Yuan, Q.L.; Huang, F. Study of chemical modification of alkaline lignin by the glyoxalation reaction. BioResources 2011, 6, 4523–4536. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Felissia, F.E.; Curvelo, A.A.S.; Zambon, M.D.; Ramos, L.; Area, M.C. Chemical and physico-chemical characterization of lignins obtained from ethanol-water fractionation of bagasse. BioResources 2011, 6, 1158–1171. [Google Scholar] [CrossRef]
- Apaydın Varol, E.; Mutlu, Ü. TGA-FTIR Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Nair, V.; Panigrahy, A.; Vinu, R. Development of novel chitosan-lignin composites for adsorption of dyes and metal ions from wastewater. Chem. Eng. J. 2014, 254, 491–502. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Ma, W.; Liu, H.; Zhu, J.; Wang, L.; Pei, H.; Liu, Q.; Yao, J. Insight into the synergistic adsorption-reduction character of chromium(VI) onto poly(pyrogallol-tetraethylene pentamine) microsphere in synthetic wastewater. J. Colloid Interface Sci. 2022, 609, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dai, Y.; Shi, Y.; Zhao, S.; Tian, H.; Zhu, K.; Jia, H. Mechanism of Cr(VI) reduction by humin: Role of environmentally persistent free radicals and reactive oxygen species. Sci. Total Environ. 2020, 725, 138413. [Google Scholar] [CrossRef] [PubMed]
- Karimova, N.V.; Luo, M.; Sit, I.; Grassian, V.H.; Gerber, R.B. Absorption Spectra and the Electronic Structure of Gallic Acid in Water at Different pH: Experimental Data and Theoretical Cluster Models. J. Phys. Chem. A 2022, 126, 190–197. [Google Scholar] [CrossRef]
- Yan, Z.; Wu, T.; Fang, G.; Ran, M.; Shen, K.; Liao, G. Self-assembly preparation of lignin–graphene oxide composite nanospheres for highly efficient Cr(vi) removal. RSC Adv. 2021, 11, 4713–4722. [Google Scholar] [CrossRef]
- Anush, S.M.; Naga Raju, S.; Gayathri, B.H.; Ajeya, K.P.; Girish, Y.R.; Darshan, S.; Naveen, Y.P.; Prashantha, K.; Narendra, B.K.; Jayaram, A. Graphitic C3N4 incorporated chitosan-poly(vinyl alcohol) blend nanocomposites for the removal of Cu(II) and Cr(VI) ions from aqueous solutions. Express Polym. Lett. 2024, 18, 102–115. [Google Scholar] [CrossRef]
- Khalil, T.E.; Abdel-Salam, A.H.; Mohamed, L.A.; El-Meligy, E.; El-Dissouky, A. Crosslinked modified chitosan biopolymer for enhanced removal of toxic Cr(VI) from aqueous solution. Int. J. Biol. Macromol. 2023, 234, 123719. [Google Scholar] [CrossRef]
- Zhang, S.; Xin, L.; Li, M.; Fan, F.; Long, H.; Gao, X. Synthesis of Amino-protected Chitosan by Tripolyphosphate and Epichlorohydrin Modification: Cr(VI) Adsorption and Reaction Mechanism. J. Polym. Environ. 2023, 32, 703–717. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Dong, T.; Liu, Z.; Liu, H. Removal of Cr(VI) from aqueous solution using amino-modified Fe3O4–SiO2–chitosan magnetic microspheres with high acid resistance and adsorption capacity. J. Appl. Polym. Sci. 2015, 133, 43078. [Google Scholar] [CrossRef]
- Xu, K.; He, T.; Li, L.; Iqbal, J.; Tong, Y.; Hua, L.; Tian, Z.; Zhao, L.; Li, H. DOTA functionalized adsorbent DOTA@Sludge@Chitosan derived from recycled shrimp shells and sludge and its application for lead and chromium removal from water. Int. J. Biol. Macromol. 2024, 255, 128263. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Liu, B.; Hu, T.; Liu, W.; Yao, J. Green synthesis of tannin-hexamethylendiamine based adsorbents for efficient removal of Cr(VI). J. Hazard. Mater. 2018, 352, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kaiblinger, N.; Hahn, R.; Beck, J.; Wang, Y.; Carta, G. Direct calculation of the equilibrium composition for multi-component Langmuir isotherms in batch adsorption. Adsorption 2023, 30, 51–56. [Google Scholar] [CrossRef]
- Petroli, G.; Brocardo de Leon, V.; Di Domenico, M.; Batista de Souza, F.; Zanini Brusamarello, C. Application of artificial neural networks and Langmuir and Freundlich isotherm models to the removal of textile dye using biosorbents: A comparative study among methodologies. Can. J. Chem. Eng. 2024, 103, 1169–1182. [Google Scholar] [CrossRef]
- Al-Arjan, W.S. Self-Assembled Nanofibrous Membranes by Electrospinning as Efficient Dye Photocatalysts for Wastewater Treatment. Polymers 2023, 15, 340. [Google Scholar] [CrossRef]
- Ali, S.I.; Lalji, S.M.; Awan, Z.; Hashmi, S.; Khan, G.; Asad, M. Comprehensive performance analysis of kinetic models used to estimate asphaltene adsorption kinetics on nanoparticles. Chem. Pap. 2023, 77, 1017–1031. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Liang, Z.; Zhou, R.; Gao, T.; Wang, Z.; Cai, X.; Lu, Q.; Li, C.; Yao, J.; Liu, Q. Catechol-Modified Alkali Lignin for Cr (VI) Removal from Synthetic Wastewater. Polymers 2025, 17, 1658. https://doi.org/10.3390/polym17121658
Yu C, Liang Z, Zhou R, Gao T, Wang Z, Cai X, Lu Q, Li C, Yao J, Liu Q. Catechol-Modified Alkali Lignin for Cr (VI) Removal from Synthetic Wastewater. Polymers. 2025; 17(12):1658. https://doi.org/10.3390/polym17121658
Chicago/Turabian StyleYu, Chenkun, Ze Liang, Ruoyao Zhou, Tingting Gao, Zhaojiang Wang, Xiaoxia Cai, Qian Lu, Cong Li, Jinshui Yao, and Qinze Liu. 2025. "Catechol-Modified Alkali Lignin for Cr (VI) Removal from Synthetic Wastewater" Polymers 17, no. 12: 1658. https://doi.org/10.3390/polym17121658
APA StyleYu, C., Liang, Z., Zhou, R., Gao, T., Wang, Z., Cai, X., Lu, Q., Li, C., Yao, J., & Liu, Q. (2025). Catechol-Modified Alkali Lignin for Cr (VI) Removal from Synthetic Wastewater. Polymers, 17(12), 1658. https://doi.org/10.3390/polym17121658







