Corn Waste Arabinoxylans with Zinc and Thymol Nanohydroxides Coating for Salmonella enterica Survival on Cherry Tomato (Solanum lycopersicum var. cerasiforme)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Zinc Hydroxide Salt and Hybrid Synthesis
2.3. Filmogenic Solutions
2.4. Filmogenic Solution and Edible Coating Characterization
2.5. Antioxidant Activity of Filmogenic Solutions
2.6. Antibacterial Activity of Coatings
2.7. Physicochemical Characteristics of Coated Tomatoes
2.8. Statistical Analysis
3. Results and Discussion
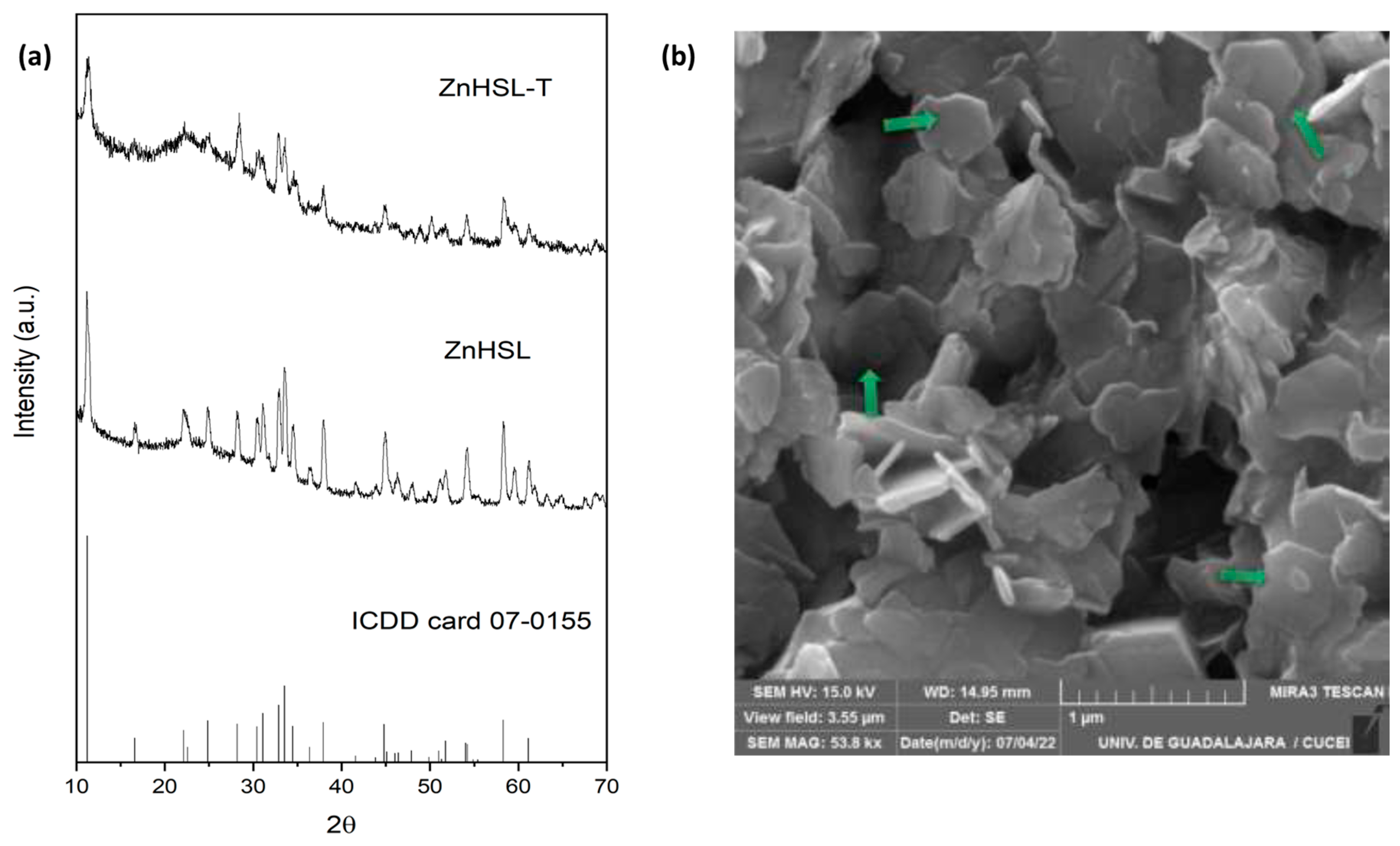
3.1. Nanomaterials Characterization
3.2. Characterization of Filmogenic Solution and Edible Coating
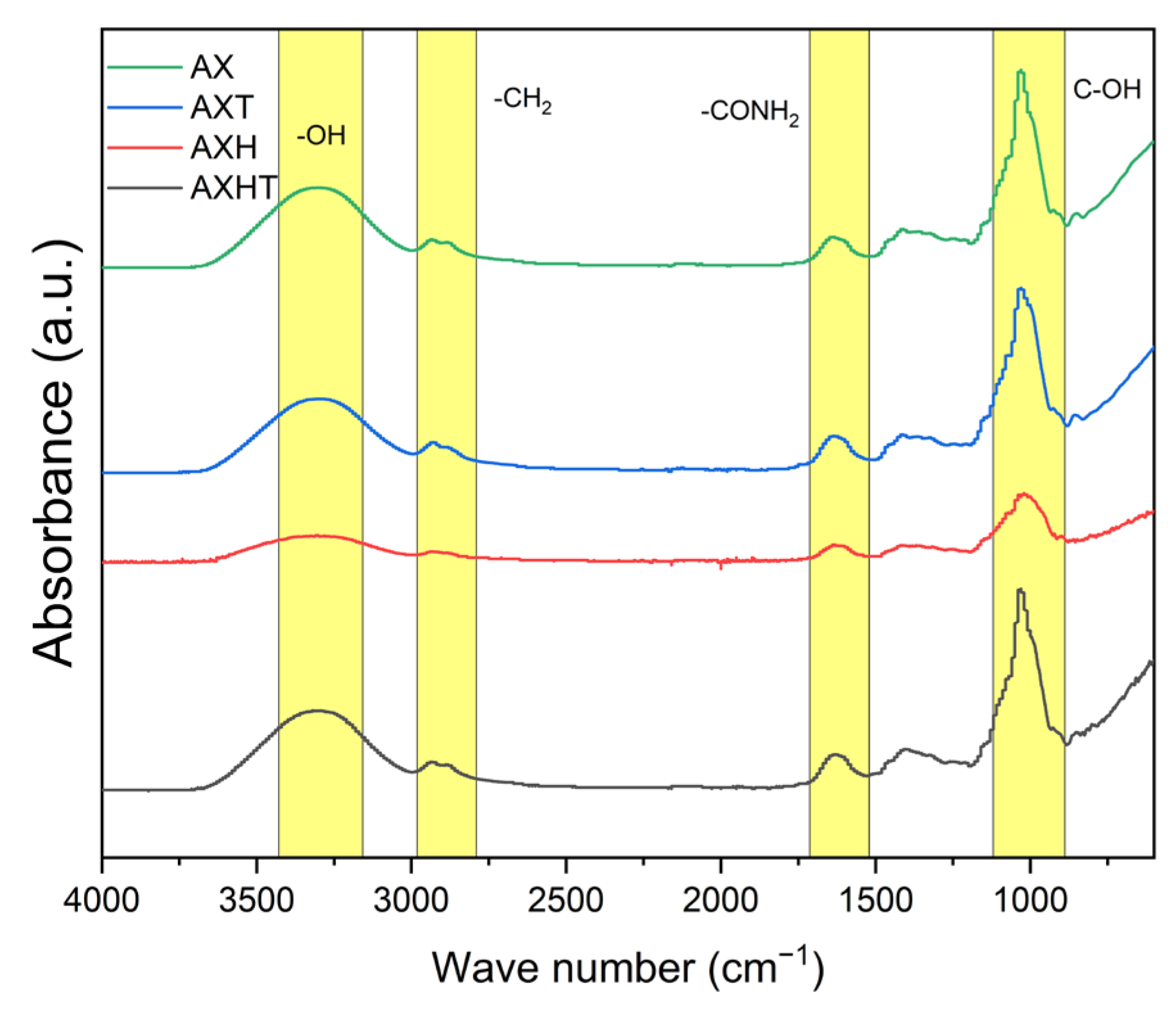
3.2.1. Fourier Transform Infrared Spectroscopy
3.2.2. Physicochemical Properties of Filmogenic Solutions
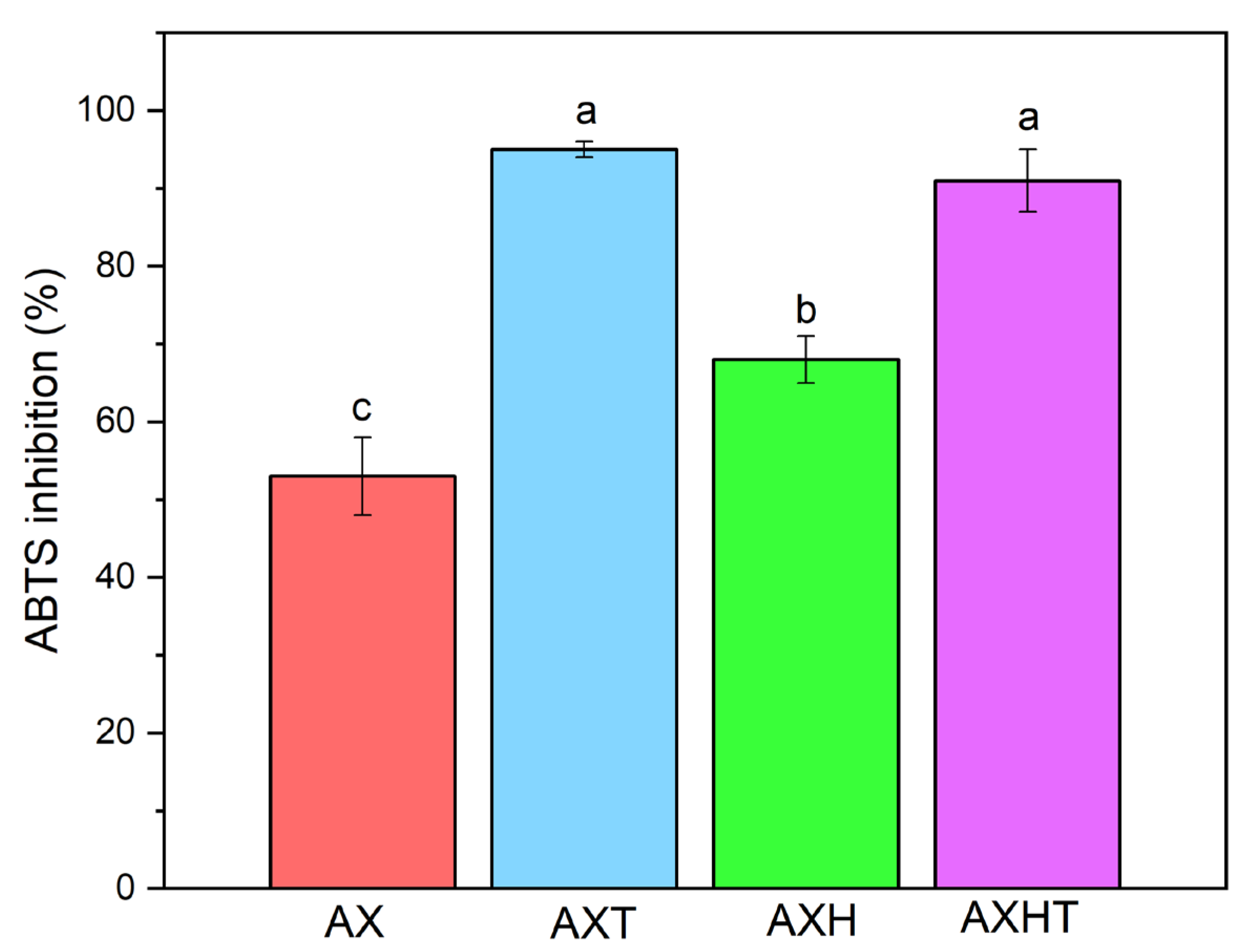
3.3. Antioxidant Capacity of Filmogenic Solutions
3.4. Efficiency of Edible Coatings on Salmonella Adherence
3.5. Physicochemical Properties of Coated Tomatoes
3.5.1. Weight Loss
3.5.2. Color Parameters
3.5.3. Lycopene, pH, Soluble Solids, and Titratable Acidity
3.5.4. Titratable Acidity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AX | Arabinoxylan edible coat |
| AXT | Arabinoxylan edible coat with thymol |
| AXH | Arabinoxylan edible coat with zinc hydroxide salt |
| AXHT | Arabinoxylan edible coat with hybrid of zinc hydroxide and thymol |
| ZnHSL | Zinc layered hydroxide salt |
| ZnHSL-T | Hybrid of zinc layered hydroxide and thymol |
| TCD | Total color difference |
| TSS | Total soluble solids |
| TA | Titratable acidity |
References
- Aviña-Padilla, K.; Zamora-Macorra, E.J.; Ochoa-Martínez, D.L.; Alcántar-Aguirre, F.C.; Hernández-Rosales, M.; Calderón-Zamora, L.; Hammond, R.W. Mexico: A Landscape of Viroid Origin and Epidemiological Relevance of Endemic Species. Cells 2022, 11, 3487. [Google Scholar] [CrossRef]
- Luna-Fletes, J.A.; Cruz-Crespo, E.; Can-Chulim, Á. Pumice, tezontle and nutritive solutions in cherry tomato crop. Terra Latinoam. 2021, 39, 1–12. [Google Scholar] [CrossRef]
- Renuka, D.M.; Sadashiva, A.T.; Kavita, B.T.; Vijendrakumar, R.C.; Hanumanthiah, M.R. Evaluation of cherry tomato lines (Solanum lycopersicum var. cerasiforme) for growth, yield and quality traits. Plant Arch. 2014, 14, 151–154. [Google Scholar]
- Delices, G.; Leyva-Ovalle, O.R.; Mota-Vargas, C.; Nunez-Pastrana, R.; Andres-Meza, P.; Herrera-Corredor, J.A. Morphological characterization of wild populations of Solanum lycopersicum var. cerasiforme in the tomato domestication area. Emir. J. Food Agric. 2021, 33, 303–313. [Google Scholar] [CrossRef]
- Ramírez-Ojeda, G.; Rodríguez-Pérez, J.E.; Rodríguez-Guzmán, E.; Sahagún-Castellanos, J.; Chávez-Servia, J.L.; Peralta, I.E.; Barrera-Guzmán, L.Á. Distribution and climatic adaptation of wild tomato (Solanum lycopersicum L.) populations in Mexico. Plants 2022, 11, 2007. [Google Scholar] [CrossRef] [PubMed]
- Parsafar, B.; Ahmadi, M.; Jahed Khaniki, G.R.; Shariatifar, N.; Rahimi Foroushani, A. The impact of fruit and vegetable waste on economic loss estimation. Glob. J. Environ. Sci. Manag. 2023, 9, 871–884. [Google Scholar] [CrossRef]
- Ariahu, E.; Aasogwa, I.S.; Ajoke, A.P.; Agbo, A.C. Microorganisms associated with the spoilage of tomatoes (Lycopersicon esculentum), sold in Nigerian market. J. Educ. Res. Pract. 2024, 4, 111–120. [Google Scholar]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R. Chemical and nutritional quality changes of tomato during postharvest transportation and storage. J. Saudi Soc. Agric. Sci. 2021, 20, 401–408. [Google Scholar] [CrossRef]
- Pathare, P.B.; Al Dairi, M.; Al-Mahdouri, A. Effect of storage conditions on postharvest quality of tomatoes: A case study at market-level. J. Agric. Mar. Sci. 2021, 26, 13–20. [Google Scholar] [CrossRef]
- Godínez, A.; Tamplin, L.; Bowman, P.; Hernández, M. Salmonella entérica in Mexico 2000–2017: Epidemiology, Antimicrobial Resistance, and Prevalence in Food. Foodborne Pathog. Dis. 2020, 17, 98–118. [Google Scholar] [CrossRef]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, food safety and food handling practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef]
- Lenzi, A.; Marvasi, M.; Baldi, A. Agronomic practices to limit pre- and post-harvest contamination and proliferation of human pathogenic Enterobacteriaceae in vegetable produce. Food Control 2021, 119, 107486. [Google Scholar] [CrossRef]
- Meneses-Espinosa, E.; Gálvez-López, D.; Rosas-Quijano, R.; Adriano-Anaya, L.; Vázquez-Ovando, A. Advantages and Disadvantages of Using Emerging Technologies to Increase Postharvest Life of Fruits and Vegetables. Food Rev. Int. 2023, 40, 1348–1373. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Liu, X.; Du, M.; Tian, Y. Effect of polysaccharide derived from Osmunda japonica Thunb-incorporated carboxymethyl cellulose coatings on preservation of tomatoes. J. Food Process. Preserv. 2019, 43, e14239. [Google Scholar] [CrossRef]
- Algarni, E.H.; Elnaggar, I.A.; Abd El-wahed, A.E.W.N.; Taha, I.M.; Al-Jumayi, H.A.; Elhamamsy, S.M.; Mahmoud, S.F.; Fahmy, A. Effect of chitosan nanoparticles as edible coating on the storability and quality of apricot fruits. Polymers 2022, 14, 2227. [Google Scholar] [CrossRef]
- Tauferová, A.; Javůrková, Z.; Pospiech, M.; Koudelková Mikulášková, H.; Těšíková, K.; Dordevic, D.; Dordevic, S.; Tremlová, B. Nanoparticles and Plant By-Products for Edible Coatings Production: A Case Study with Zinc, Titanium, and Silver. Polymers 2022, 14, 2837. [Google Scholar] [CrossRef]
- Leite, A.C.C.O.; Cerqueira, M.A.; Michelin, M.; Fuciños, P.; Pastrana, L. Antiviral edible coatings and films: A strategy to ensure food safety. Trends Food Sci. Technol. 2023, 138, 551–563. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Cruz-Monterrosa, R.G.; Rayas-Amor, A.A.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Aguilar-Toalá, J.E.; Liceaga, A.M. Application of polysaccharide-based edible coatings on fruits and vegetables: Improvement of food quality and bioactivities. Polysaccharides 2023, 4, 99–115. [Google Scholar] [CrossRef]
- Miranda, M.; Marilene De Mori, M.R.; Spricigo, P.C.; Pilon, L.; Mitsuyuki, M.C.; Correa, D.S.; Ferreira, M.D. Carnauba wax anoemulsion applied as an edible coating on fresh tomato for postharvest quality evaluation. Heliyon 2022, 8, e09803. [Google Scholar] [CrossRef]
- Salvada, J.; Alke, B.; Brazinha, C.; Alves, V.D.; Coelhoso, I.M. Development and characterisation of arabinoxylan-based composite films. Coatings 2022, 12, 813. [Google Scholar] [CrossRef]
- Ali, U.; Kanwar, S.; Yadav, K.; Basu, S.; Mazumder, K. Effect of arabinoxylan and β-glucan stearic acid ester coatings on post-harvest quality of apple (Royal Delicious). Carbohydr. Polym. 2019, 209, 338–349. [Google Scholar] [CrossRef]
- Guimarães, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible Films and Coatings as Carriers of Living Microorganisms: A New Strategy Towards Biopreservation and Healthier Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 594–614. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Zinno, P.; Guantario, B.; Lombardi, G.; Ranaldi, G.; Finamore, A.; Allegra, S.; Mammano, M.M.; Fascella, G.; Raffo, A.; Roselli, M. Chemical composition and biological activities of essential oils from Origanum vulgare genotypes belonging to the carvacrol and thymol chemotypes. Plants 2023, 12, 1344. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Roccaro, A.S.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Valero, D. Bioactive compounds in tomato fruit and its antioxidant activity as affected by incorporation of Aloe, eugenol, and thymol in fruit package during storage. Int. J. Food Prop. 2017, 20 (Suppl. 2), 1798–1806. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, J.; Wang, P.; Feng, G. The environmental fate of thymol, a novel botanical pesticide, in tropical agricultural soil and water. Toxicol. Environ. Chem. 2017, 99, 223–232. [Google Scholar] [CrossRef]
- Bizymis, A.P.; Tzia, C. Edible films and coatings: Properties for the selection of the components, evolution through composites and nanomaterials, and safety issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 8777–8792. [Google Scholar] [CrossRef]
- Velázquez-Carriles, C.; Macías-Rodríguez, M.E.; Ramírez-Alvarado, O.; Corona-González, R.I.; Macías-Lamas, A.; García-Vera, I.; Cavazos-Garduño, A.; Villagrán, Z.; Silva-Jara, J.M. Nanohybrid of thymol and 2D simonkolleite enhances inhibition of bacterial growth, biofilm formation, and free radicals. Molecules 2022, 27, 6161. [Google Scholar] [CrossRef]
- Velázquez-Carriles, C.A.; Carbajal-Arizaga, G.G.; Silva-Jara, J.M.; Reyes-Becerril, M.C.; Aguilar-Uscanga, B.R.; Macías-Rodríguez, M.E. Chemical and biological protection of food grade nisin through their partial intercalation in laminar hydroxide salts. J. Food Sci. Technol. 2020, 57, 3252–3258. [Google Scholar] [CrossRef]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An Overview of the Applications of Nanomaterials and Nanodevices in the Food Industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Lee, C.J.; Nah, C.S.; Teng, C.S.; Jun, W.W.; Saravanan, M. Spray dried calcium gelled arabinoxylan microspheres: A novel carrier for extended drug delivery. Chem. Pap. 2015, 69, 1325–1330. [Google Scholar] [CrossRef]
- Gennadios, A.; Weller, C.L.; Hanna, M.A.; Froning, G.W. Mechanical and Barrier Properties of Egg Albumen Films. J. Food Sci. 1996, 61, 585–589. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plas. Film. Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Li, P.; Li, Y.; Wang, H. Comparative study of antioxidant activity of grape (Vitis vinifera) seed powder assessed by different methods. J. Food Drug Anal. 2020, 16, 67–73. [Google Scholar] [CrossRef]
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- de Jesús Salas-Méndez, E.; Vicente, A.; Pinheiro, A.C.; Ballesteros, L.F.; Silva, P.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez, M.L.V.; Flores-López, M.L.; Villarea-Quintanilla, J.A.; et al. Application of edible nanolaminate coatings with antimicrobial extract of Flourensia cernua to extend the shelf-life of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2019, 150, 19–27. [Google Scholar] [CrossRef]
- Mattson, T.E.; Johny, A.K.; Amalaradjou, M.A.R.; More, K.; Schreiber, D.T.; Patel, J.; Venkitanarayanan, K. Inactivation of Salmonella spp. on tomatoes by plant molecules. Int. J. Food Microbiol. 2011, 144, 464–468. [Google Scholar] [CrossRef]
- López-Palestina, C.U.; López-Duran, M.C.; Gutiérrez-Tlahque, J.; Arenales-Sierra, I.M.; Laureano-López, B.; Vargas-Torres, A.; Hernández-Fuentes, A.D. Efecto del uso de biopeliculas sobre calidad poscosecha de tomate nativo ‘ojo de venado’ (Solanum lycopersicum L var. cerasiforme). Investig. Desarro. Cienc. Tecnol. Aliment. 2016, 1, 405–411. [Google Scholar]
- Khan, M.; Raza, M.; Razak, S.; Kadir, M.; Haider, M.; Shah, S.; Yusof, A.; Haider, S.; Shakir, I.; Aftab, S. Novel functional antimicrobial and biocompatible arabinoxylan/guar gum hydrogel for skin wound dressing applications. J. Tissue Eng. Regen. Med. 2020, 14, 1488–1501. [Google Scholar] [CrossRef]
- Jahromi, M.; Niakousari, M.; Golmakani, M.T.; Mohammadifar, M.A. Physicochemical and structural characterization of sodium caseinate based film-forming solutions and edible films as affected by high methoxyl pectin. Int. J.Biol. Macromol. 2020, 165, 1949–1959. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Ahmed, J.; Vahora, A.; Pathania, S. Effect of pectin incorporation on characteristics of chitosan based edible films. J. Food Meas. Charact. 2023, 17, 5569–5581. [Google Scholar] [CrossRef]
- Hussain, M.; Saeed, F.; Niaz, B.; Imran, A.; Tufail, T. Biochemical and structural characterization of ferulated arabinoxylans extracted from nixtamalized and non-nixtamalized maize bran. Foods 2022, 11, 3374. [Google Scholar] [CrossRef]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R. Quality changes kinetic of tomato during transportation and storage. J. Food Process Eng. 2021, 13, 21–42. [Google Scholar] [CrossRef]
- Castorena-Sánchez, A.M.; Velázquez-Carriles, C.A.; López-Álvarez, M.A.; Serrano-Niño, J.C.; Cavazos-Garduño, A.; Garay-Martínez, L.E.; Silva-Jara, J.M. Magnesium anohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens. Green. Process. Synth. 2023, 12, 20230145. [Google Scholar] [CrossRef]
- Khitous, M.; Salem, Z.; Halliche, D. Removal of phosphate from industrial wastewater using uncalcined MgAl-NO3 layered double hydroxide: Batch study and modeling. Desalin. Water Treat. 2016, 57, 15920–15931. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Chen, Z.; Huang, X.; Chen, H.; Zeng, Z.; Li, C. Cross-linking treatment of arabinoxylan improves its antioxidant and hypoglycemic activities after simulated in vitro digestion. LWT 2021, 145, 111386. [Google Scholar] [CrossRef]
- Weng, V.; Brazinha, C.; Coelhoso, I.M.; Alves, V.D. Decolorization of a Corn Fiber Arabinoxylan Extract and Formulation of Biodegradable Films for Food Packaging. Membranes 2021, 11, 321. [Google Scholar] [CrossRef]
- Alzarea, A.I.; Alruwaili, N.K.; Ahmad, M.M.; Munir, M.U.; Butt, A.M.; Alrowaili, Z.A.; Shahari, M.S.B.; Almalki, Z.S.; Alqahtani, S.S.; Dolzhenko, A.V.; et al. Development and Characterization of Gentamicin-Loaded Arabinoxylan-Sodium Alginate Films as Antibacterial Wound Dressing. Int. J. Mol. Sci. 2022, 23, 2899. [Google Scholar] [CrossRef] [PubMed]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as edible films and coatings: Characteristics and influence on fruit and vegetable quality—A review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Sukhavattanakul, P.; Thanyacharoen, T.; Chuysinuan, P.; Techasakul, S.; Ummartyotin, S. Influence of a transparent and edible coating of encapsulated cannabidiol nanoparticles on the quality and shelf life of strawberries. ACS Appl. Mater. Interfaces 2023, 15, 23834–23843. [Google Scholar] [CrossRef]
- Bahrami, A.; Mokarram, R.R.; Khiabani, M.S.; Ghanbarzadeh, B.; Salehi, R. Physico-mechanical and antimicrobial properties of tragacanth/hydroxypropyl methylcellulose/beeswax edible films reinforced with silver nanoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. [Google Scholar] [CrossRef]
- Mateus, A.R.S.; Serrano, C.; Almeida, C.; Soares, A.; Rolim Lopes, V.; Sanches-Silva, A. Beyond Thymol and Carvacrol: Characterizing the Phenolic Profiles and Antioxidant Capacity of Portuguese Oregano and Thyme for Food Applications. Appl. Sci. 2024, 14, 8924. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, W.; Wang, T.; Pei, F.; Yue, M.; Li, F.; Liu, X.; Wang, X.; Li, H. Proteomic analysis of the antimicrobial effects of sublethal concentrations of thymol on Salmonella enterica serovar Typhimurium. Appl. Microbiol. Biotechnol. 2020, 104, 3493–3505. [Google Scholar] [CrossRef]
- Nabipour, H.; Hu, Y. Layered zinc hydroxide as vehicle for drug delivery systems: A critical review. J. Porous Mater. 2022, 29, 341–356. [Google Scholar] [CrossRef]
- Romero-García, D.M.; Velázquez-Carriles, C.A.; Gomez, C.; Velázquez-Juárez, G.; Silva-Jara, J.M. Tannic acid-layered hydroxide salt hybrid: Assessment of antibiofilm formation and foodborne pathogen growth inhibition. J. Food Sci. Technol. 2023, 60, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Takala, P.N.; Vu, K.D.; Salmieri, S.; Khan, R.A.; Lacroix, M. Antibacterial effect of biodegradable active packaging on the growth of Escherichia coli, Salmonella typhimurium and Listeria monocytogenes in fresh broccoli stored at 4 °C. Food Sci. Technol. 2013, 53, 499–506. [Google Scholar] [CrossRef]
- Won, J.S.; Lee, S.J.; Park, H.H.; Song, K.B.; Min, S.C. Edible Coating Using a Chitosan-Based Colloid Incorporating Grapefruit Seed Extract for Cherry Tomato Safety and Preservation. J. Food Sci. 2017, 83, 138–146. [Google Scholar] [CrossRef]
- Salehi, F. Edible Coating of Fruits and Vegetables Using Natural Gums: A Review. Int. J. Fruit Sci. 2020, 20, 570–589. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdelrahman, S.Z.; Megahed, M.M.A.; Abdeldaym, E.A.; El-Mogy, M.M.; Abdelgawad, K.F. Extending Shelf Life and Maintaining Quality of Tomato Fruit by Calcium Chloride, Hydrogen Peroxide, Chitosan, and Ozonated Water. Horticulturae 2021, 7, 309. [Google Scholar] [CrossRef]
- Taşdelen, Ö.; Bayindirli, L. Controlled atmosphere storage and edible coating effects on storage life and quality of tomatoes. J. Food Process. Preserv. 1998, 22, 303–320. [Google Scholar] [CrossRef]
- Al-Juhaimi, F.Y. Physicochemical and Sensory Characteristics of Arabic Gum-Coated Tomato (Solanum lycopersicum L.) Fruits during Storage. J. Food Process. Preserv. 2014, 38, 971–979. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ribeiro, C.P.P.; Uliana, N.R.; Rodrigues, M.B.C.; da Rosa, C.G.; Ferrareze, J.P.; de Lima Veeck, A.P.; Nunes, M.R. Bioactive and pH-sensitive films based on carboxymethyl cellulose and blackberry (Morus nigra L.) anthocyanin-rich extract: A perspective coating material to improve the shelf life of cherry tomato (Solanum lycopersicum L. var. cerasiforme). Biocatal. Agric. Biotechnol. 2021, 33, 101989. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Sábio, R.M.; Chorilli, M. An Overview of Properties and Analytical Methods for Lycopene in Organic Nanocarriers. Crit. Rev. Anal. Chem. 2021, 51, 674–686. [Google Scholar] [CrossRef]
- Saini, R.K.; Bekhit, A.E.D.A.; Roohinejad, S.; Rengasamy, K.R.R.; Keum, Y.S. Chemical Stability of Lycopene in Processed Products: A Review of the Effects of Processing Methods and Modern Preservation Strategies. J. Agric. Food Chem. 2020, 68, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Aviña, J.E.; Villa-Rodríguez, J.A.; Villegas-Ochoa, M.A.; Tortoledo-Ortiz, O.; Olivas, G.I.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of edible coatings on bioactive compounds and antioxidant capacity of tomatoes at different maturity stages. J. Food Sci. Technol. 2014, 51, 2706–2712. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Food Analysis; Food Science Texts Series; Springer Science+ Business Media, LLC.: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Rodrigo, M.J.; Alquézar, B.; Alférez, F.; Zacarías, L. Biochemistry of fruits and fruit products. In Handbook of Fruits and Fruit Processing; Wiley: Hoboken, NJ, USA, 2012; Volume 2, pp. 13–34. [Google Scholar]
- Ruelas-Chacon, X.; Contreras-Esquivel, J.C.; Montañez, J.; Aguilera-Carbo, A.F.; Reyes-Vega, M.L.; Peralta-Rodriguez, R.D.; Sanchéz-Brambila, G. Guar gum as an edible coating for enhancing shelf-life and improving postharvest quality of roma tomato (Solanum lycopersicum L.). J. Food Qual. 2017, 2017, 8608304. [Google Scholar] [CrossRef]


| Filmogenic Formulation | TSS (°Brix) | Viscosity (cP) | Turbidity (NTU) |
|---|---|---|---|
| AX | 3.2 ± 0.5 a | 20.0 ± 10.0 a | 1220 ± 144 a |
| AXT | 3.3 ± 0.2 a | 10.0 ± 0.0 a | 1376 ± 51 a |
| AXH | 3.3 ± 0.3 a | 20.0 ± 10.0 a | 2233 ± 124 b |
| AXHT | 3.3 ± 0.3 a | 10.0 ± 0.0 a | 3613 ± 65 c |
| Parameter | Edible Coating Formulation | |||
|---|---|---|---|---|
| AX | AXT | AXH | AXHT | |
| L* | 37.57 ± 1.14 a | 38.30 ± 2.59 a | 39.56 ± 0.11 a | 47.35 ± 0.60 b |
| a* | 4.04 ± 0.71 a | 4.19 ± 0.86 a | 4.05 ± 0.78 a | 4.33 ± 1.23 a |
| b* | 15.05 ± 0.03 a | 14.42 ± 1.14 a | 18.19 ± 2.68 ab | 21.32 ± 3.78 b |
| ΔE* | NA | 2.99 ± 0.66 a | 2.79 ± 2.31 a | 10.52 ± 1.57 b |
| C* | 15.69 ± 0.47 a | 15.03 ± 1.32 a | 18.67 ± 2.42 ab | 21.82 ± 3.40 b |
| H° | 73.76 ± 2.22 a | 73.90 ± 2.00 a | 77.09 ± 4.00 a | 77.92 ± 5.75 a |
| Thickness (mm) | 0.199 ± 0.053 a | 0.196 ± 0.029 a | 0.152 ± 0.022 b | 0.163 ± 0.018 b |
| Transparency (%) | 2.343 ± 0.015 a | 2.307 ± 0.052 a | 1.834 ± 0.187 b | 1.681 ± 0.138 b |
| Moisture (%) | 15.5 ± 0.2 a | 15.0 ± 0.3 a | 15.0 ± 0.1 a | 15.0 ± 0.4 a |
| Parameter | Control | AX | AXT | AXH | AXHT |
|---|---|---|---|---|---|
| TCD | 2.02 ± 1.45 | 2.14 ± 1.34 | 1.84 ± 0.76 | 1.85 ± 1.23 | |
| C* | 18.71 ± 1.16 | 17.80 ± 1.09 | 17.62 ± 1.00 | 18.43 ± 1.22 | 18.55 ± 1.10 |
| H° | 55.69 ± 2.36 | 59.38 ± 2.84 | 58.54 ± 2.90 | 55.73 ± 2.87 | 55.87 ± 2.35 |
| Parameter | Day | Control | AX | AXT | AXH | AXHT |
|---|---|---|---|---|---|---|
| Lycopene content (mg/100 g) | 1 | 10.14 ± 0.14 aA | 8.92 ± 0.67 aB | 8.15 ± 0.76 aB | 9.15 ± 0.64 aB | 9.68 ± 0.50 aAB |
| 3 | 10.01 ± 1.10 abA | 9.25 ± 0.93 aA | 9.16 ± 0.49 aA | 9.44 ± 0.64 aA | 8.93 ± 0.37 aAB | |
| 6 | 9.23 ± 0.53 bA | 8.26 ± 0.83 aAB | 8.59 ± 0.02 aB | 9.86 ± 0.65 aA | 9.83 ± 0.96 aA | |
| 9 | 9.21 ± 0.68 bA | 8.46 ± 0.63 aAB | 9.24 ± 0.63 aA | 9.21 ± 1.21 aA | 9.64 ± 1.09 aAB | |
| 12 | 9.66 ± 0.71 abA | 8.01 ± 0.77 aB | 8.44 ± 1.70 aAB | 9.87 ± 1.04 aAB | 9.91 ± 0.48 aA | |
| pH | 1 | 4.44 ± 0.14 b | 4.42 ± 0.21 a | 4.31 ± 0.11 ab | 4.41 ± 0.02 c | 4.21 ± 0.18 b |
| 3 | 4.61 ± 0.07 ab | 4.54 ± 0.19 a | 4.40 ± 0.14 a | 4.42 ± 0.34 bc | 4.54 ± 0.21 ab | |
| 6 | 4.70 ± 0.09 a | 4.62 ± 0.12 a | 4.57 ± 0.17 a | 4.58 ± 0.15 ab | 4.61 ± 0.15 ab | |
| 9 | 4.63 ± 0.08 ab | 4.58 ± 0.19 a | 4.58 ± 0.04 a | 4.72 ± 0.07 a | 4.39 ± 0.14 b | |
| 12 | 4.63 ± 0.08 ab | 4.56 ± 0.01 a | 4.49 ± 0.14 a | 4.61 ± 0.03 b | 4.63 ± 0.03 a | |
| Soluble solids (°Brix) | 1 | 6.57 ± 0.67 cB | 7.77 ± 0.55 bA | 7.30 ± 1.18 bcA | 7.73 ± 0.50 abA | 7.87 ± 0.12 bA |
| 3 | 8.37 ± 1.08 bAB | 8.30 ± 1.08 abAB | 7.40 ± 0.26 cB | 8.20 ± 2.25 aAB | 7.10 ± 1.06 bB | |
| 6 | 8.70 ± 0.20 bAB | 8.03 ± 1.37 abAB | 9.10 ± 0.20 aA | 7.25 ± 0.55 abB | 8.30 ± 1.28 abAB | |
| 9 | 9.67 ± 0.15 aAB | 9.30 ± 0.20 aB | 8.93 ± 0.75 abBC | 9.98 ± 1.56 aA | 8.43 ± 0.85 abC | |
| 12 | 9.43 ± 1.54 abAB | 8.07 ± 0.58 bB | 8.17 ± 0.25 bB | 8.77 ± 2.29 aAB | 9.53 ± 0.70 aA |
| Day | |||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 9 | 12 |
| 0.36 ± 0.08 a | 0.32 ± 0.06 a | 0.66 ± 0.23 b | 0.72 ± 0.22 b | 0.70 ± 0.17 b | 0.62 ± 0.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Jara, J.M.; García-Vera, I.; Morales-Burgos, A.M.; Hinojosa-Ventura, G.; Macías-Rodríguez, M.E.; Pérez-Montaño, J.A.; Villagrán, Z.; Anaya-Esparza, L.M.; Velázquez-Carriles, C.A. Corn Waste Arabinoxylans with Zinc and Thymol Nanohydroxides Coating for Salmonella enterica Survival on Cherry Tomato (Solanum lycopersicum var. cerasiforme). Polymers 2025, 17, 1632. https://doi.org/10.3390/polym17121632
Silva-Jara JM, García-Vera I, Morales-Burgos AM, Hinojosa-Ventura G, Macías-Rodríguez ME, Pérez-Montaño JA, Villagrán Z, Anaya-Esparza LM, Velázquez-Carriles CA. Corn Waste Arabinoxylans with Zinc and Thymol Nanohydroxides Coating for Salmonella enterica Survival on Cherry Tomato (Solanum lycopersicum var. cerasiforme). Polymers. 2025; 17(12):1632. https://doi.org/10.3390/polym17121632
Chicago/Turabian StyleSilva-Jara, Jorge Manuel, Ismael García-Vera, Ana María Morales-Burgos, Gabriela Hinojosa-Ventura, María Esther Macías-Rodríguez, Julia Aurora Pérez-Montaño, Zuami Villagrán, Luis Miguel Anaya-Esparza, and Carlos Arnulfo Velázquez-Carriles. 2025. "Corn Waste Arabinoxylans with Zinc and Thymol Nanohydroxides Coating for Salmonella enterica Survival on Cherry Tomato (Solanum lycopersicum var. cerasiforme)" Polymers 17, no. 12: 1632. https://doi.org/10.3390/polym17121632
APA StyleSilva-Jara, J. M., García-Vera, I., Morales-Burgos, A. M., Hinojosa-Ventura, G., Macías-Rodríguez, M. E., Pérez-Montaño, J. A., Villagrán, Z., Anaya-Esparza, L. M., & Velázquez-Carriles, C. A. (2025). Corn Waste Arabinoxylans with Zinc and Thymol Nanohydroxides Coating for Salmonella enterica Survival on Cherry Tomato (Solanum lycopersicum var. cerasiforme). Polymers, 17(12), 1632. https://doi.org/10.3390/polym17121632









