Design of Polysaccharide-Based Nanocomposites for Eco-Friendly Flexible Electronics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Details
2.3. Synthesis of Inorganic Filler and Composites Preparations
3. Results and Discussion
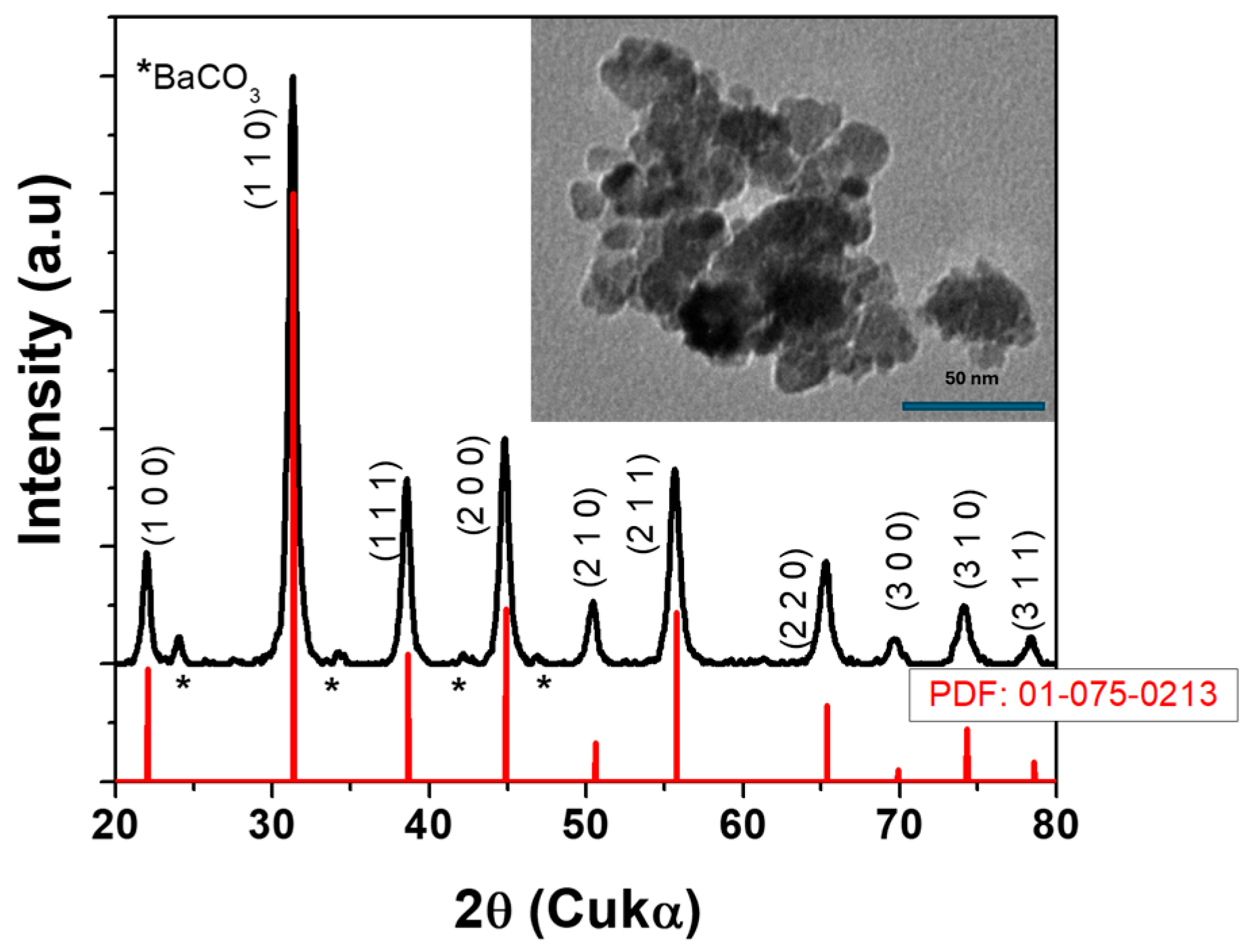
3.1. Characterisation of the BT Powders
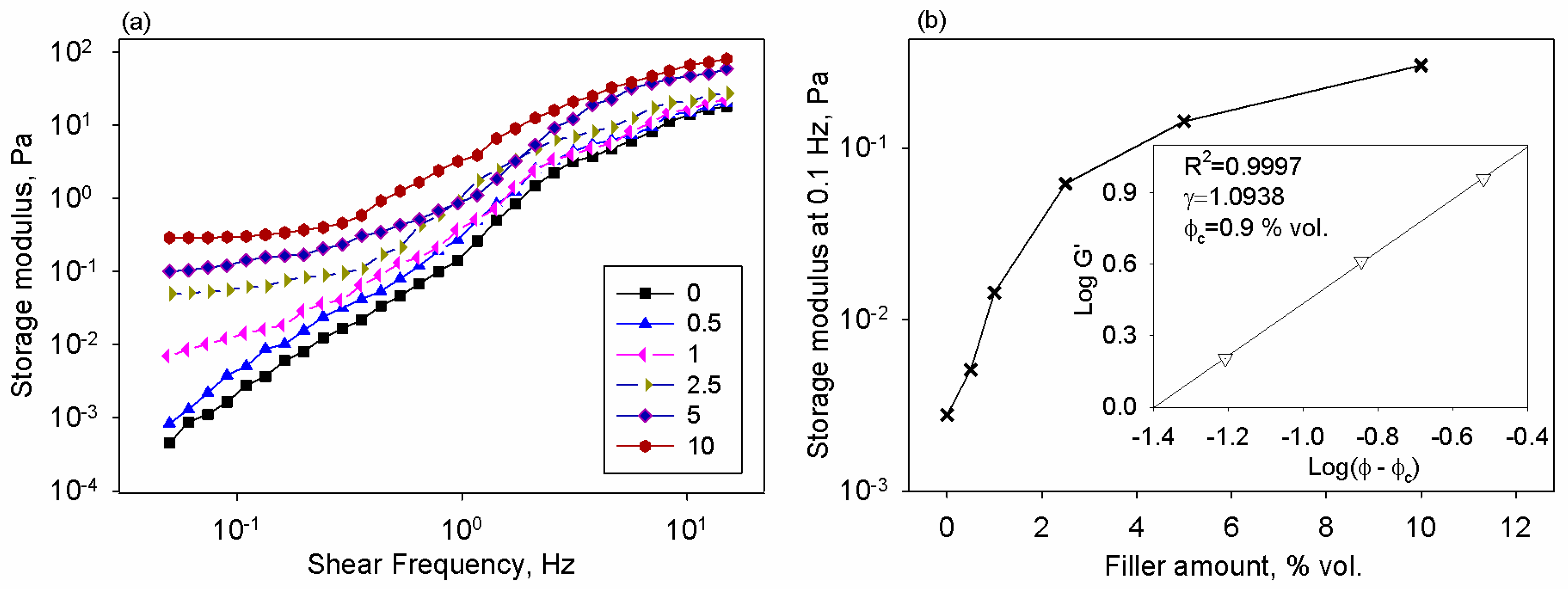
3.2. Shear Oscillatory Testing of the Matrix and Composite Solutions
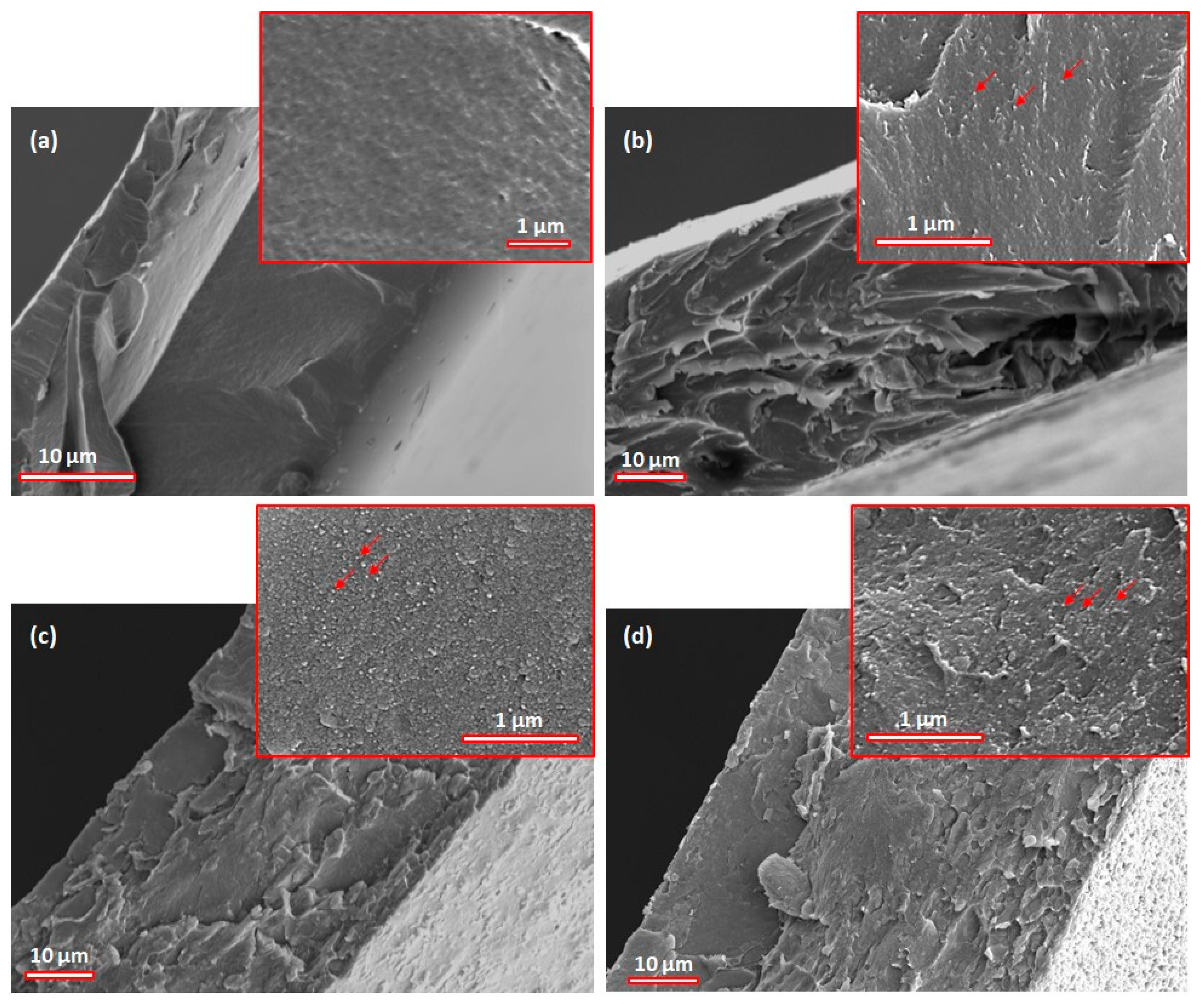
3.3. Morphological Analysis of the Films
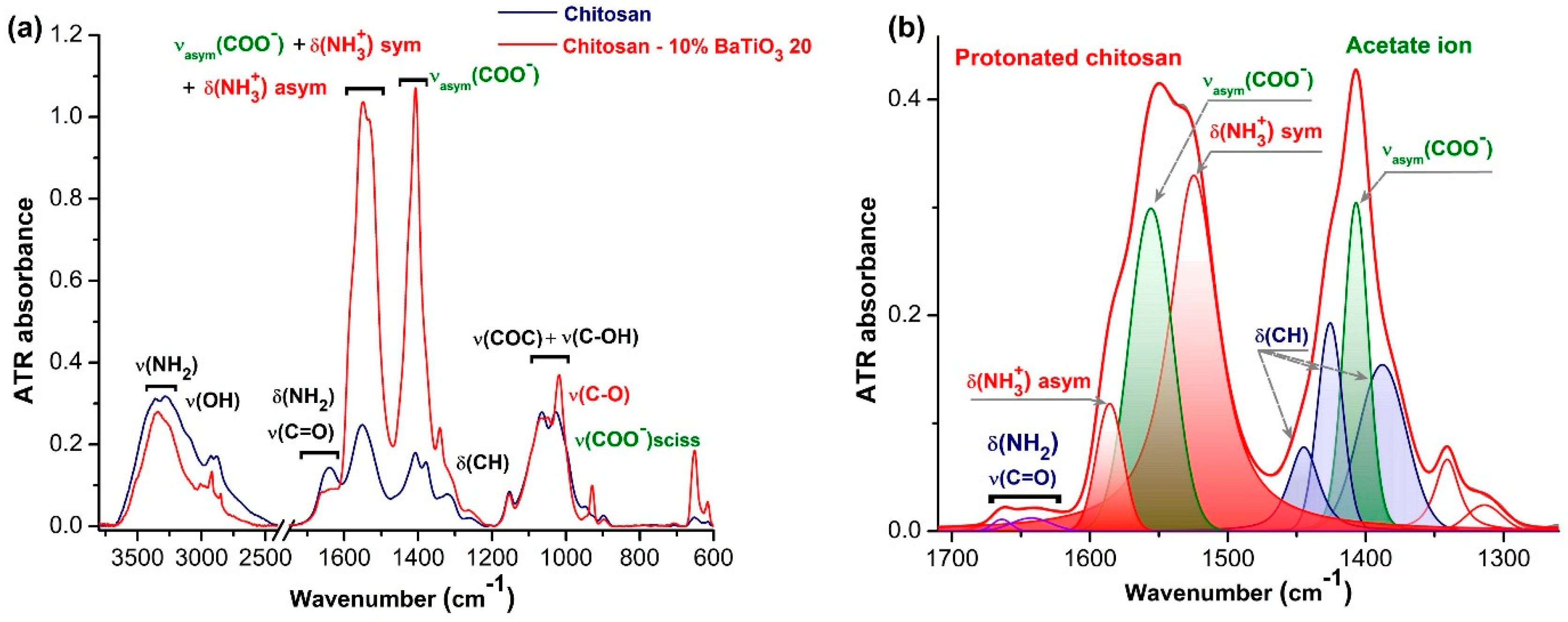
3.4. FTIR Characterisation
3.5. Thermal Behavior
3.6. Optical Properties
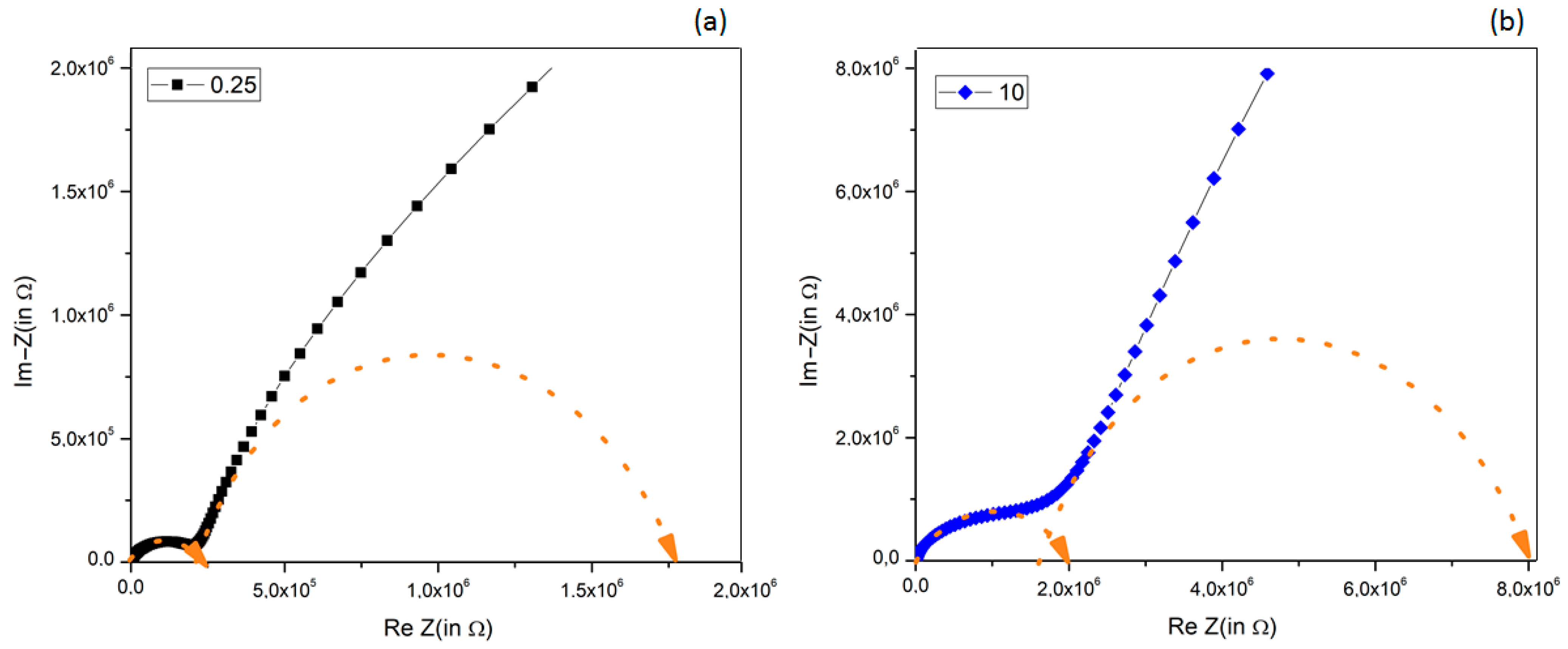
3.7. Impedance Spectroscopy Analysis
- A tendency to reduce the series resistance Rs (i.e., to reduce the overall composite dc-resistivity) when increasing the BT filler amount, due to the good dielectric properties of BT powders, which strongly limit the dc-conduction paths in chitosan; these results agree with previously observed ones [78,79,80].
- The high-frequency components R1 and C1, assigned to the intrinsic material’s properties (resistivity and conductivity), present a strong composition-induced effect: (i) up to 2.5% vol. BT the resistance R1 remained almost constant at 0.2 MΩ, while its capacitance increased almost two times; (ii) for higher BT concentration (>2.5% vol. BT), the resistance R1 strongly increased together with a reduction in capacitance.
- At low-frequency response (R2, C2), circuit elements were affected both by the presence of BT filler and electrode effect: thus, for BT addition ≤ 2.5%, a decrease in R2 and an increase in C2 was observed, while for larger BT amount, an increase in R2 and a decrease in C2 was obtained.
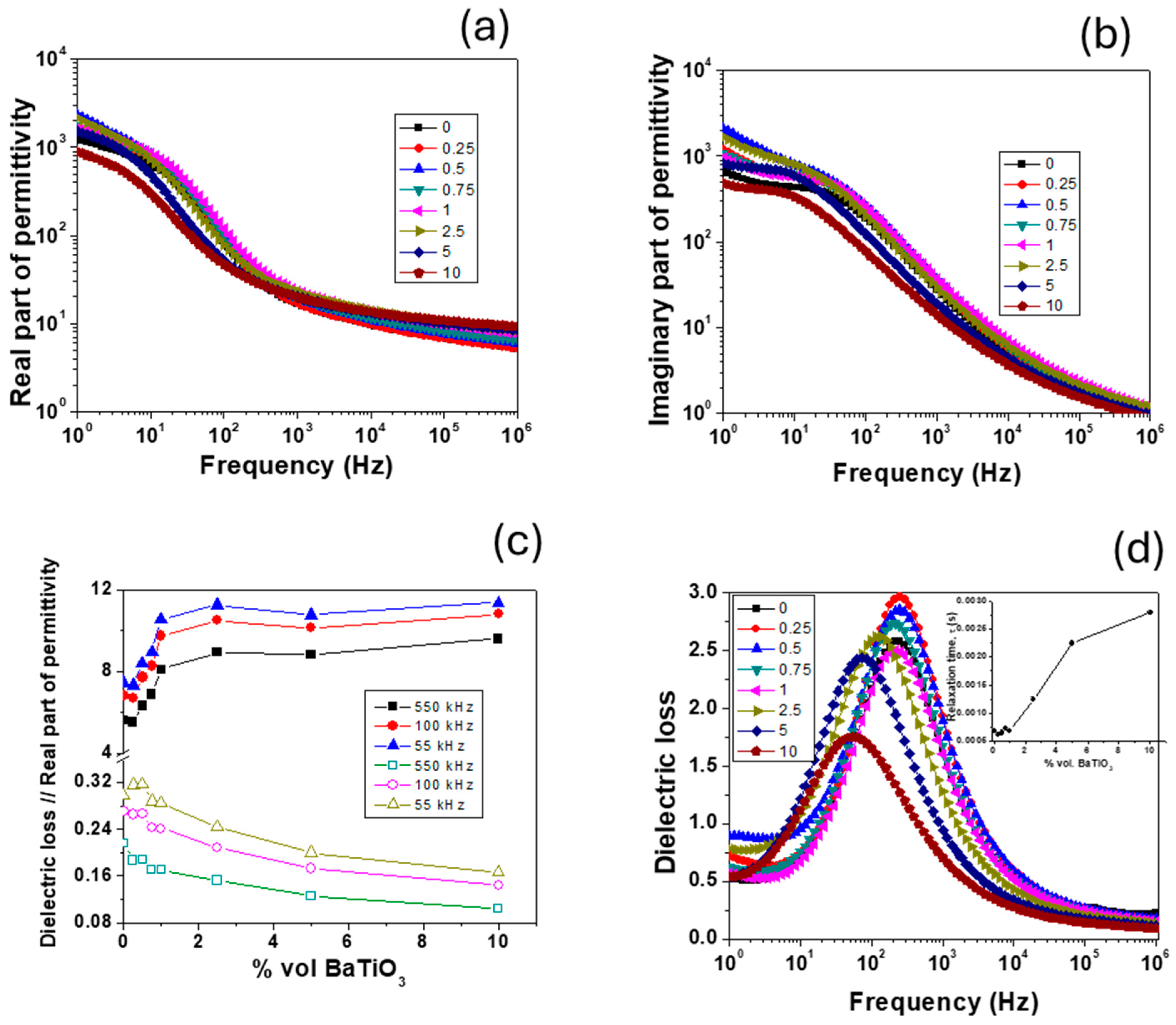
3.8. Frequency Dependence of Dielectric Properties
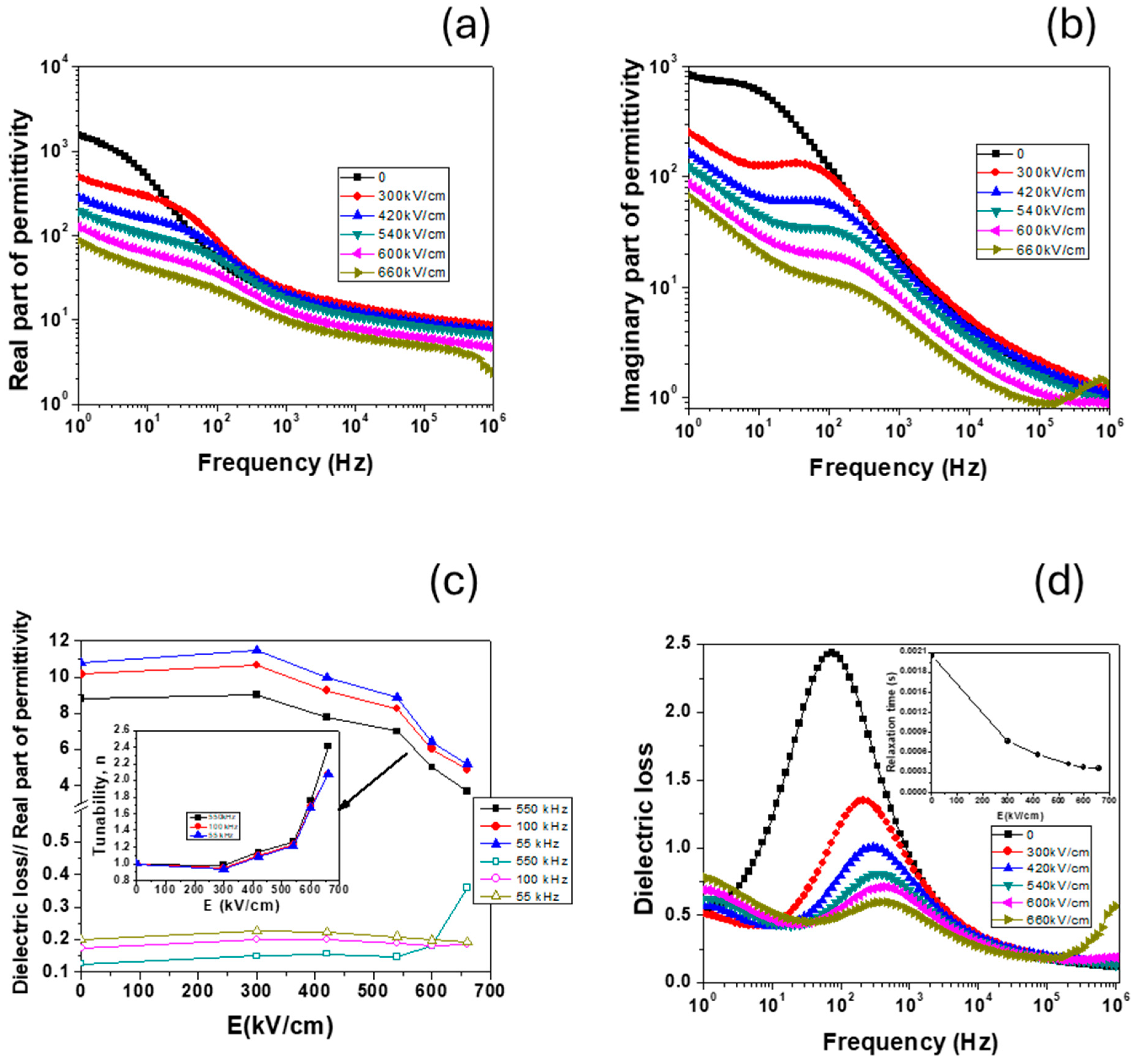
3.9. High Field Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BT | Barium titanate |
| PT | Percolation threshold |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscope |
| SEM | Scanning electron microscope |
| UV-VIS | Ultraviolet-visible |
| TGA | Thermogravimetric analysis |
| DSC | Differential scanning calorimetry |
| Powder diffraction file | |
| LVR | Linear viscoelastic regime |
| DTG | Differential thermogravimetry curve |
References
- Wang, T.; Liu, Y.; Hernandez, J. Strategies for Cost Reduction in Electronics Manufacturing: A Comprehensive Analysis. J. Manuf. Syst. 2020, 54, 167–175. [Google Scholar]
- Song, J.; Liu, H.; Zhao, Z.; Lin, P.; Yan, F. Flexible organic transistors for biosensing: Devices and applications. Advanced Materials 2024, 36, 2300034. [Google Scholar] [CrossRef] [PubMed]
- Altay, B.N.; Bolduc, M.; Cloutier, S.G. Sustainable advanced manufacturing of printed electronics: An environmental consideration. In Green Energy and Environment; IntechOpen: London, UK, 2020. [Google Scholar]
- European Commission. Directive 2011/65/EU on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment. 2021. Available online: https://eur-lex.europa.eu/eli/dir/2011/65/oj/eng (accessed on 14 May 2025).
- Ndayishimiye, A.; Sengul, M.Y.; Sada, T.; Dursun, S.; Bang, S.H.; Grady, Z.A.; Tsuji, K.; Funahashi, S.; van Duin, A.C.T.; Randall, C.A. Roadmap for densification in cold sintering: Chemical pathway. Open Ceram 2020, 2, 100019. [Google Scholar] [CrossRef]
- Ding, L.; Yu, Z.D.; Wang, X.Y.; Yao, Z.F.; Lu, Y.; Yang, C.Y.; Wang, J.Y.; Pei, J. Polymer Semiconductors: Synthesis, Processing, and Applications. Chem. Rev. 2023, 123, 7421–7497. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Tan, Y.R.; Lao, J.Z.; Gao, H.J.; Yu, J. Hydrogels for Flexible Electronics. ACS Nano 2023, 17, 9681–9693. [Google Scholar] [CrossRef]
- Lan, L.Y.; Ping, J.F.; Xiong, J.Q.; Ying, Y.B. Sustainable Natural Bio-Origin Materials for Future Flexible Devices. Adv. Sci. 2022, 9, 15. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.J.; Liu, J.P.; Ye, D.; Duan, Y.Q.; Li, K.; Yin, Z.P.; Huang, Y.A. Flexible metamaterial electronics. Adv. Mater. 2022, 34, e2200070. [Google Scholar] [CrossRef]
- Espera, A.; Dizon, J.; Valino, A.; Advíncula, R. Advancing flexible electronics and additive manufacturing. Jpn. J. Appl. Phys. 2022, 61, SE0803. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Prihandini, W.W.; Kusrini, E.; Prasetyo, A.B.; Prasetyarni, N.A.; Mawarni, D.P.; Sufyan, M.; Usman, A. New physical and chemical properties of chitosan-samarium composite: Synthesis and characterization. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 882, p. 012013. [Google Scholar]
- Aziz, S.B.; Aziz, D.M.; Muhammad, D.S.; Hama, P.O.; Abdullah, O.G. Enhancing the optical properties of chitosan-based biopolymer for optoelectronic applications using natural dye extracted from hollyhock waste flowers. Opt. Mater. 2024, 159, 116596. [Google Scholar] [CrossRef]
- Salavati, M. Mechanical properties of α-chitin and chitosan biocomposite: A molecular dynamic study. J. Compos. Sci. 2023, 7, 464. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.B.; Ni, Y.H. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2023, 7, 85–98. [Google Scholar] [CrossRef]
- Kumar, R.; Ranwa, S.; Kumar, G. Biodegradable flexible substrate based on chitosan/pvp blend polymer for disposable electronics device applications. J. Phys. Chem. B 2019, 124, 149–155. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Labeeba Abdulla, A.C.; Kalladi, A.J.; Sunojkumar, P. Biopolymer blend composite films based on polyvinyl alcohol/chitosan/grape seed extract via green approach for flexible optoelectronic devices. J. Thermoplast. Compos. Mater. 2024, 37, 3697–3714. [Google Scholar] [CrossRef]
- Khankhuean, A.; Morimura, Y.; Ajiro, H. Improving the mechanical properties of chitosan through blending with poly (trimethylene carbonate) copolymer. Int. J. Biol. Macromol. 2024, 283, 137830. [Google Scholar] [CrossRef]
- Li, A.; Ma, B.; Hua, S.; Ping, R.; Ding, L.; Tian, B.; Zhang, X. Chitosan-based injectable hydrogel with multifunction for wound healing: A critical review. Carbohydr. Polym. 2024, 333, 121952. [Google Scholar] [CrossRef]
- Khan, Y.; Thielens, A.; Muin, S.; Ting, J.; Baumbauer, C.; Arias, A. A new frontier of printed electronics: Flexible hybrid electronics. Adv. Mater. 2019, 32, e1905279. [Google Scholar] [CrossRef]
- Wu, K.; Wang, W.; Deng, Y.; Hou, B.; Wang, H.; Duan, J.; Ding, D.; Fan, H.; Liu, H. Bifunctional chitosan/tannin aerogel for gold recovery via electrostatic attraction and in-situ reduction. J. Hazard. Mater. 2025, 490, 137839. [Google Scholar] [CrossRef]
- Aziz Shujahadeen, B.; Hamsan, M.H.; Kadir, M.F.Z.; Woo, H.J. Design of Polymer Blends Based on Chitosan:POZ with Improved Dielectric Constant for Application in Polymer Electrolytes and Flexible Electronics. Adv. Polym. Technol. 2020, 2020, 8586136. [Google Scholar]
- Johns, J.; Nakason, C. Dielectric properties of natural rubber/chitosan blends: Effects of blend ratio and compatibilization. J. Non-Cryst. Solids 2011, 357, 1816–1821. [Google Scholar] [CrossRef]
- Bonardd, S.; Robles, E.; Barandiaran, I.; Saldías, C.; Leiva, Á.; Kortaberria, G. Biocomposites with increased dielectric constant based on chitosan and nitrile-modified cellulose nanocrystals. Carbohydr. Polym. 2018, 199, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, S.S. Structural, morphological and electrical properties of chitosan/methylcellulose blend polymer doped with different concentrations of NH4NO3. Mater. Res. Express 2021, 8, 086301. [Google Scholar] [CrossRef]
- Sierra, D.L.G.; Bdikin, I.; Tkach, A.; Vilarinho, P.M.; Nunes, C.; Ferreira, P. Flexible Piezoelectric Chitosan and Barium Titanate Biocomposite Films for Sensor Applications. Eur. J. Inorg. Chem. 2021, 2021, 792. [Google Scholar] [CrossRef]
- Mattos, R.I.; Raphael, E.; Majid, S.R.; Arof, A.K.; Pawlicka, A. Enhancement of Electrical Conductivity in Plasticized Chitosan Based Membranes. Mol. Cryst. Liq. Cryst. 2012, 554, 150–159. [Google Scholar] [CrossRef]
- Klaytae, T.; Thountom, S. Microstructure and dielectric properties of ST ceramics prepared by the sol–gel combustion technique with chitosan addition. Ceram. Int. 2015, 41 (Suppl. 1), S117–S122. [Google Scholar] [CrossRef]
- Prakash, B.; Jothirajan, M.A.; Umapathy, S.; Amala, V. Synthesis and Characterization of Biodegradable Ultrasonicated Films made from Chitosan/al2o3 Polymer Nanocomposites. Phys. Procedia 2013, 49, 84–91. [Google Scholar] [CrossRef][Green Version]
- Tu, H.; Li, X.; Xie, K.; Zhang, J.; Liu, Y.; Lin, X.; Zhang, R.; Duan, B. High strength and biodegradable dielectric film with synergistic alignment of chitosan nanofibrous networks and BNNSs. Carbohydr. Polym. 2023, 299, 120234. [Google Scholar] [CrossRef]
- Golda, R.A.; Marikani, A.; Alex, E.J. Effect of Ceramic Fillers on the Dielectric, Ferroelectric and Magnetic Properties of Polymer Nanocomposites for Flexible Electronics. J. Electron. Mater. 2021, 50, 3652–3667. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, D.; Xiao, Y.; Wang, K.; Zhang, Q.; Wu, K.; Fu, Q. The effect of filler permittivity on the dielectric properties of polymer-based composites. Compos. Sci. Technol. 2022, 222, 109342. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Wang, C.; Xiao, Y. The effect of interfacial interaction-induced soft percolation regime on dielectric properties in Ba(Fe0.5Nb0.5)O3/P(VDF-TrFE) nanocomposites. J. Mater. Sci. 2017, 52, 11496–11505. [Google Scholar] [CrossRef]
- Münstedt, H.; Starý, Z. Is electrical percolation in carbon-filled polymers reflected by rheological properties? Polymer 2016, 98, 51–60. [Google Scholar] [CrossRef]
- Khastgir, D.; Adachi, K. Rheological and dielectric studies of aggregation of barium titanate particles suspended in polydimethylsiloxane. Polymer 2000, 41, 6403–6413. [Google Scholar] [CrossRef]
- Ribeiro, B.; Pipes, R.B.; Costa, M.L.; Botelho, E.C. Electrical and rheological percolation behavior of multiwalled carbon nanotube-reinforced poly (phenylene sulfide) composites. J. Compos. Mater. 2017, 51, 199–208. [Google Scholar] [CrossRef]
- Zohrevand, A.; Ajji, A.; Mighri, F. Relationship between rheological and electrical percolation in a polymer nanocomposite with semiconductor inclusions. Rheol. Acta 2014, 53, 235–254. [Google Scholar] [CrossRef]
- Liu, Y.; Wilkinson, A. Rheological percolation behaviour and fracture properties of nanocomposites of MWCNTs and a highly crosslinked aerospace-grade epoxy resin system. Compos. Part A Appl. Sci. Manuf. 2018, 105, 97–107. [Google Scholar] [CrossRef]
- Silva, S.; Barbosa, J.M.; Sousa, J.D.; Paiva, M.C.; Teixeira, P.F. High-Performance PEEK/MWCNT nanocomposites: Combining enhanced electrical conductivity and nanotube dispersion. Polymers 2024, 16, 583. [Google Scholar] [CrossRef]
- Lage-Rivera, S.; Ares-Pernas, A.; Becerra Permuy, J.C.; Gosset, A.; Abad, M.J. Enhancement of 3D printability by FDM and electrical conductivity of PLA/MWCNT filaments using lignin as bio-dispersant. Polymers 2023, 15, 999. [Google Scholar] [CrossRef]
- Chrissafis, K.; Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochim. Acta 2011, 523, 1–24. [Google Scholar] [CrossRef]
- Wang, Z.; Yalagala, B.P.; Heidari, H.; Feeney, A. A Flexible and Self-Powered Chitosan-BaTiO3 Composite Pressure Sensor for E-Skin Applications. In Proceedings of the 2024 IEEE SENSORS, Kobe, Japan, 20–23 October 2024; pp. 1–4. [Google Scholar]
- Prokhorov, E.; Bárcenas, G.L.; España Sánchez, B.L.; Franco, B.; Padilla-Vaca, F.; Hernández Landaverde, M.A.; Yáñez Limón, J.M.; López, R.A. Chitosan-BaTiO3 nanostructured piezopolymer for tissue engineering. Colloids Surf. B Biointerfaces 2020, 196, 111296. [Google Scholar] [CrossRef]
- Curecheriu, L.P.; Barzic, A.I.; Stoica, I.; Albu, R.M.; Zaltariov, M.F.; Turcanu, G.; Gheorghiu, F.; Stagnaro, P.; Buscaglia, M.T. Tailoring the optical and dielectric properties of polysaccharide-based composites through synthesis methods of inorganic filler. Polym. Compos. 2025, submitted.
- Testino, A.; Buscaglia, M.T.; Buscaglia, V.; Viviani, M.; Bottino, C.; Nanni, P. Kinetics and Mechanism of Aqueous Chemical Synthesis of BaTiO3 Particles. Chem. Mater. 2004, 16, 1536–1543. [Google Scholar] [CrossRef]
- Curecheriu, L.; Balmus, S.B.; Buscaglia, M.T.; Buscaglia, V.; Ianculescu, A.; Mitoseriu, L. Grain size-dependent properties of dense nanocrystalline barium titanate ceramics. J. Am. Ceram. Soc. 2012, 95, 3912–3921. [Google Scholar] [CrossRef]
- Buscaglia, V.; Randall, C.A. Size and scaling effects in barium titanate. An overview. J. Eur. Ceram. Soc. 2020, 40, 3744–3758. [Google Scholar] [CrossRef]
- Jiang, B.; Iocozzia, J.; Zhao, L.; Zhang, H.; Harn, Y.W.; Chen, Y.; Lin, Z. Barium titanate at the nanoscale: Controlled synthesis and dielectric and ferroelectric properties. Chem. Soc. Rev. 2019, 48, 1194–1228. [Google Scholar] [CrossRef]
- Yashima, M.; Hoshina, T.; Ishimura, D.; Kobayashi, S.; Nakamura, W.; Tsurumi, T.; Wada, S. Size effect on the crystal structure of barium titanate nanoparticles. J. Appl. Phys. 2005, 98, 014313. [Google Scholar] [CrossRef]
- Rueda, M.M.; Auscher, M.C.; Fulchiron, R.; Périé, T.; Martin, G.; Sonntag, P.; Cassagnau, P. Rheology and applications of highly filled polymers: A review of current understanding. Prog. Polym. Sci. 2017, 66, 22–53. [Google Scholar] [CrossRef]
- Hausnerova, B.; Honkova, N.; Kitano, T.; Saha, P. Superposed flow properties of ceramic powder-filled polymer melts. Polym. Compos. 2009, 30, 1027–1034. [Google Scholar] [CrossRef]
- Bek, M.; Gonzalez-Gutierrez, J.; Kukla, C.; Pušnik Črešnar, K.; Maroh, B.; Slemenik Perše, L. Rheological behaviour of highly filled materials for injection moulding and additive manufacturing: Effect of particle material and loading. Appl. Sci. 2020, 10, 7993. [Google Scholar] [CrossRef]
- Havet, G.; Isayev, A.I. Rheology of silica-filled polystyrene: From microcomposites to nanocomposites. Polym. Sci. Ser. A 2012, 54, 476–492. [Google Scholar] [CrossRef]
- Borchani, K.E.; Carrot, C.; Jaziri, M. Rheological behavior of short Alfa fibers reinforced Mater-Bi® biocomposites. Polym. Test. 2019, 77, 105895. [Google Scholar] [CrossRef]
- Abraham, J.; Sharika, T.; George, S.C.; Thomas, S. Rheological percolation in thermoplastic polymer nanocomposites. Rheol. Open Access 2017, 1, 1–15. [Google Scholar]
- Pascariu, V.; Padurariu, L.; Avadanei, O.; Mitoseriu, L. Dielectric properties of PZT–epoxy composite thick films. J. Alloys Compounds 2013, 574, 591–599. [Google Scholar] [CrossRef]
- Tan, W.; Dong, F.; Zhang, J.; Zhao, X.; Li, Q.; Guo, Z. Physical and Antioxidant Properties of Edible Chitosan Ascorbate Films. J. Agric. Food Chem. 2019, 67, 2530–2539. [Google Scholar] [CrossRef]
- Osman, Z.; Arof, A.K. FTIR studies of chitosan acetate based polymer electrolytes. Electrochim. Acta 2003, 48, 993–999. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons Ltd.: Chichester, UK, 2001; Chapter 10. [Google Scholar]
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Costa, C.M.; Lanceros-Méndez, S.; Maciavello, M.N.T.; Ribelles, J.L.G.; Sentanin, F.; Pawlicka, A.; Silva, M.M. Thermal–mechanical behaviour of chitosan–cellulose derivative thermoreversible hydrogel films. Cellulose 2015, 22, 1911–1929. [Google Scholar] [CrossRef]
- Mafirad, S.; Mehrnia, M.R.; Zahedi, P.; Hosseini, S.N. Chitosan-based nanocomposite membranes with improved properties: Effect of cellulose acetate blending and TiO2 nanoparticles incorporation. Polym. Compos. 2018, 39, 4452–4466. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, R.; Fu, Y.; Guan, Y.; Yao, J.; Xiao, L.; Zeng, G. Effective photocatalytic decolorization of methyl orange utilizing TiO2/ZnO/chitosan nanocomposite films under simulated solar irradiation. Desalination 2012, 286, 41–48. [Google Scholar] [CrossRef]
- Neto, C.D.T.; Giacometti, J.A.; Job, A.E.; Ferreira, F.C.; Fonseca, J.L.C.; Pereira, M.R. Thermal analysis of chitosan based networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Nieto, J.M.; Peniche-Covas, C.; Padro, G. Characterization of chitosan by pyrolysis-mass spectrometry, thermal analysis and differential scanning calorimetry. Thermochim. Acta 1991, 176, 63–68. [Google Scholar] [CrossRef]
- Luangchuang, P.; Chueangchayaphan, N.; Sulaiman, M.A.; Chueangchayaphan, W. Characterization of barium titanate reinforced acrylonitrile butadiene rubber composites for flexible electronic applications: Influences of barium titanate content. Polym. Int. 2021, 70, 154–161. [Google Scholar] [CrossRef]
- Roy, A.; Panda, S.; Gupta, J.; Singh, R.P.; Kour, P.; Sharma, M.P.; Yadav, K. Effects of interfacial interactions on structural, optical, thermal degradation properties and photocatalytic activity of low-density polyethylene/BaTiO3 nanocomposite. Polymer 2023, 276, 125932. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Preparation and characterization of chitosan/poly (vinyl alcohol) blended films: Mechanical, thermal and surface investigations. J. Chem. 2011, 8, 91–96. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Qin, A.; He, C. A comparative study on the chitosan membranes prepared from glycine hydrochloride and acetic acid. Carbohydr. Polym. 2013, 91, 477–482. [Google Scholar] [CrossRef]
- Şen, F.; Baştürk, E.; Karadoğan, B.; Madakbaş, S.; Kahraman, M.V. Effect of barium titanate on the thermal, morphology, surface, and mechanical properties of the thermoplastic polyurethane/barium titanate composites. Polym.-Plast. Technol. Eng. 2016, 55, 1325–1331. [Google Scholar] [CrossRef]
- Barzic, A.I.; Soroceanu, M.; Rotaru, R.; Doroftei, F.; Asandulesa, M.; Tugui, C.; Dascalu, I.A.; Harabagiu, V. Cellulose derivative/barium titanate composites with high refractive index, conductivity and energy density. Cellulose 2022, 29, 863–878. [Google Scholar] [CrossRef]
- Morsi, M.A.; Abdelaziz, M.; Oraby, A.H.; Mokhles, I. Structural, optical, thermal, and dielectric properties of polyethylene oxide/carboxymethyl cellulose blend filled with barium titanate. J. Phys. Chem. Solids 2019, 125, 103–114. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials; Oxford University Press: Oxford, UK, 1971; Volume 14. [Google Scholar]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical properties of chitin and chitosan biopolymers with application to structural color analysis. Opt. Mater. 2012, 35, 175–183. [Google Scholar] [CrossRef]
- Banerjee, M.; Jain, A.; Mukherjee, G.S. Microstructural and optical properties of polyvinyl alcohol/manganese chloride composite film. Polym. Compos. 2019, 40, E765–E775. [Google Scholar] [CrossRef]
- Mocanu, Z.V.; Apachitei, G.; Padurariu, L.; Tudorache, F.; Curecheriu, L.P.; Mitoseriu, L. Impedance spectroscopy method for investigation of the polycrystalline inhomogeneous ceramics. Eur. Phys. J.-Appl. Phys. 2011, 56, 10102. [Google Scholar] [CrossRef]
- Neagu, A.M.; Curecheriu, L.P.; Cazacu, A.; Mitoseriu, L. Impedance analysis and tunability of BaTiO3–chitosan composites: Towards active dielectrics for flexible electronics. Compos. Part B Eng. 2014, 66, 109–116. [Google Scholar] [CrossRef]
- González-Campos, J.B.; Prokhorov, E.; Luna-Bárcenas, G.; Fonseca-García, A.; Sanchez, I.C. Dielectric relaxations of chitosan: The effect of water on the α-relaxation and the glass transition temperature. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2259–2271. [Google Scholar] [CrossRef]
- González-Campos, J.B.; Prokhorov, E.; Luna-Bárcenas, G.; Sanchez, I.C.; Lara-Romero, J.; Mendoza-Duarte, M.E.; Villaseñor, F.; Guevara-Olvera, L. Chitosan/silver nanoparticles composite: Molecular relaxations investigation by dynamic mechanical analysis and impedance spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 739–748. [Google Scholar] [CrossRef]
- Padurariu, L.; Horchidan, N.; Ciomaga, C.E.; Curecheriu, L.P.; Lukacs, V.A.; Stirbu, R.S.; Stoian, G.; Botea, M.; Florea, M.; Maraloiu, V.A.; et al. Influence of ferroelectric filler size and clustering on the electrical properties of (Ag–BaTiO3)–PVDF sub-percolative hybrid composites. ACS Appl. Mater. Interfaces 2023, 15, 5744–5759. [Google Scholar] [CrossRef]
- Cazacu, A.; Curecheriu, L.; Neagu, A.; Padurariu, L.; Cernescu, A.; Lisiecki, I.; Mitoseriu, L. Tunable gold-chitosan nanocomposites by local field engineering. Appl. Phys. Lett. 2013, 102, 222903. [Google Scholar] [CrossRef]
- Curecheriu, L.; Buscaglia, M.T.; Buscaglia, V.; Zhao, Z.; Mitoseriu, L. Grain size effect on the nonlinear dielectric properties of barium titanate ceramics. Appl. Phys. Lett. 2010, 97, 242909. [Google Scholar] [CrossRef]

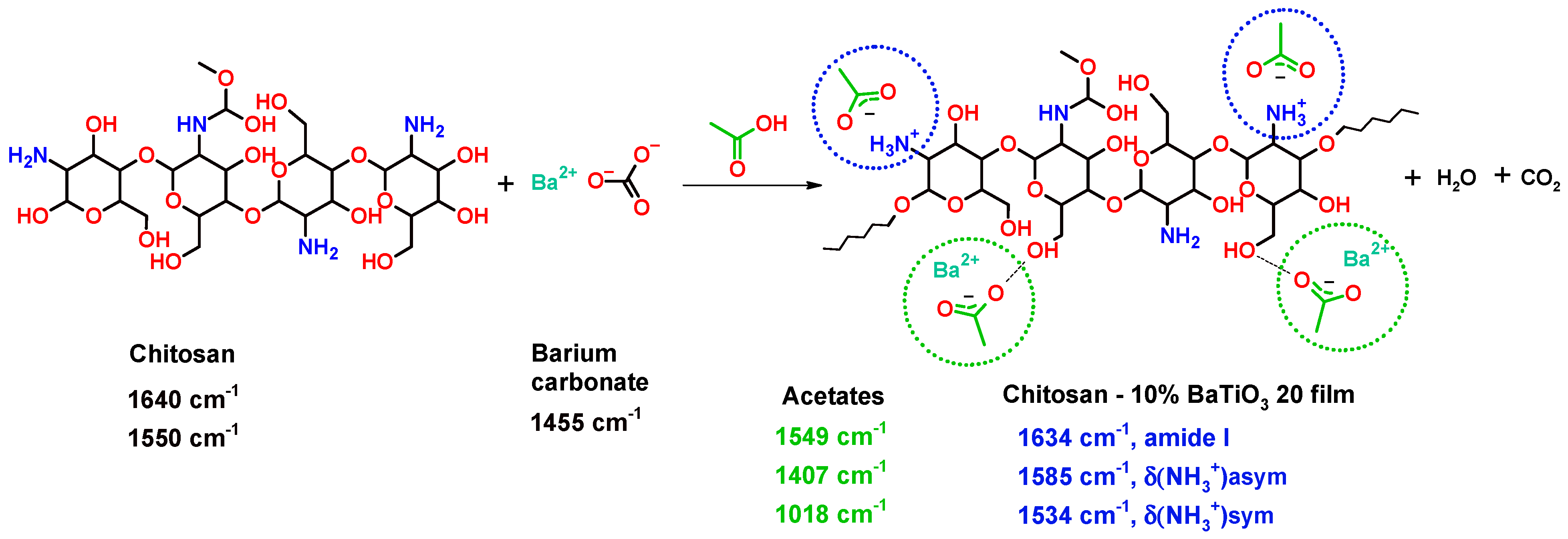

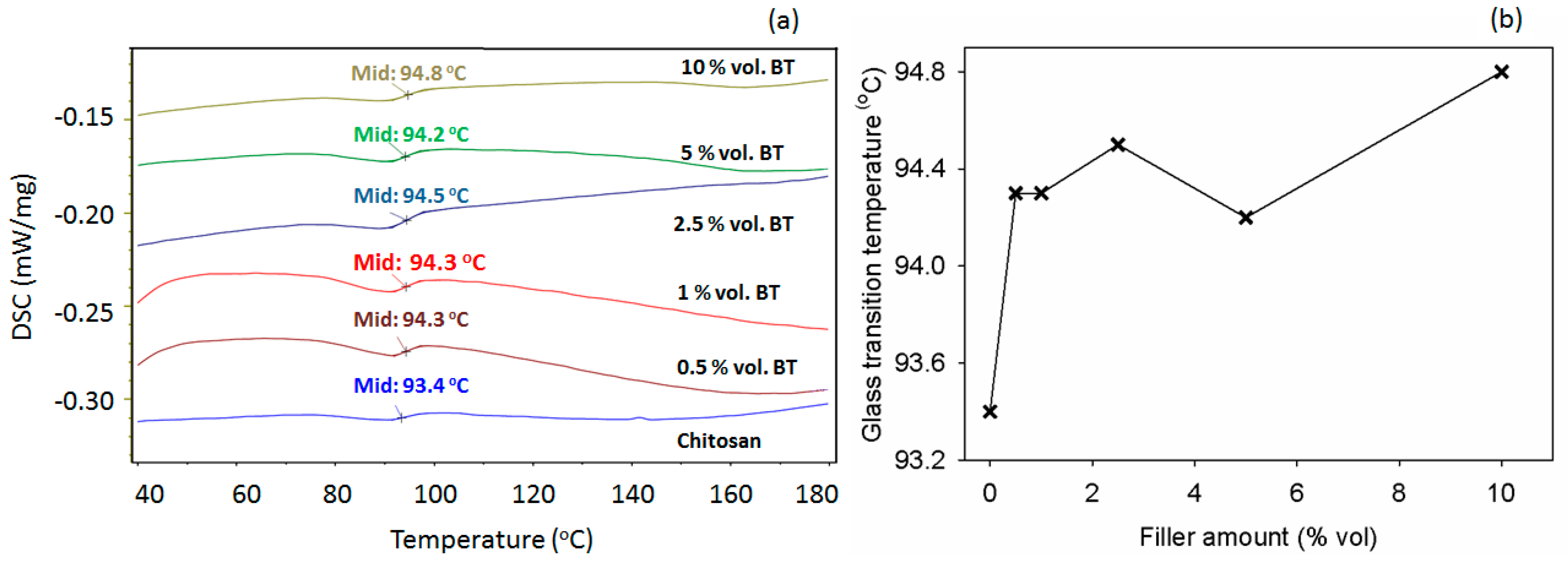
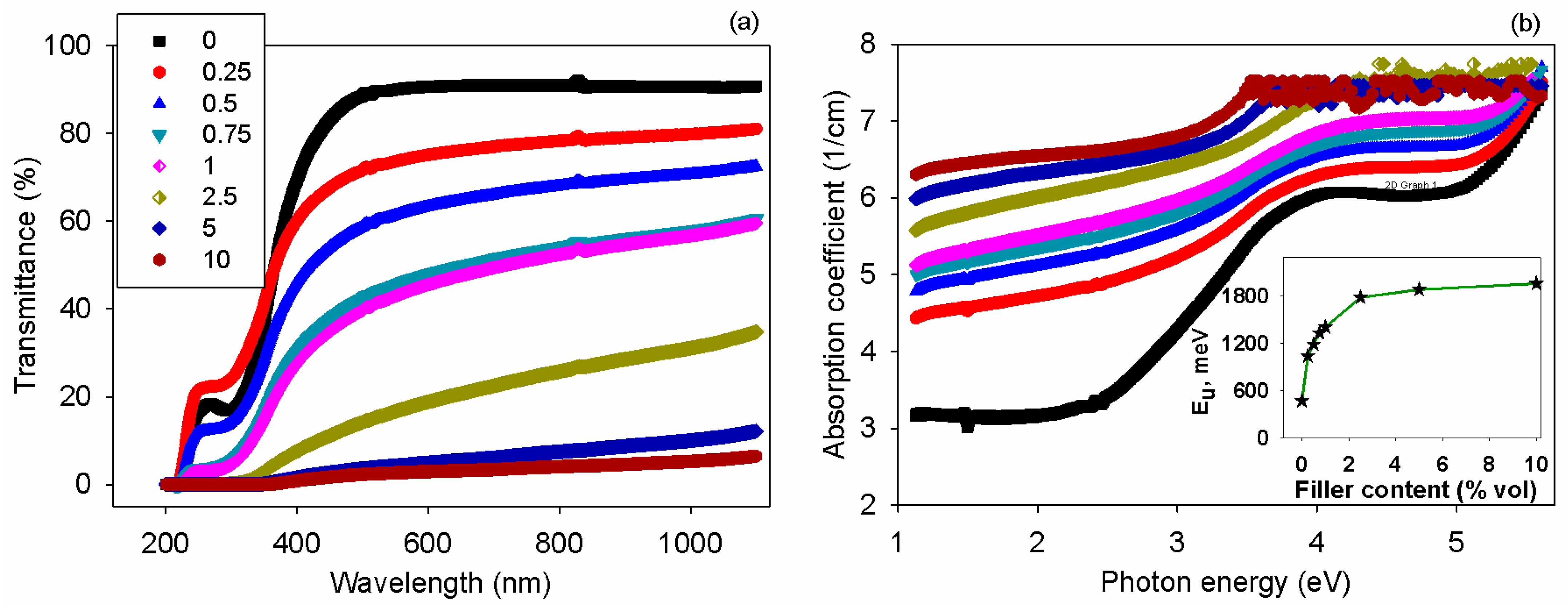
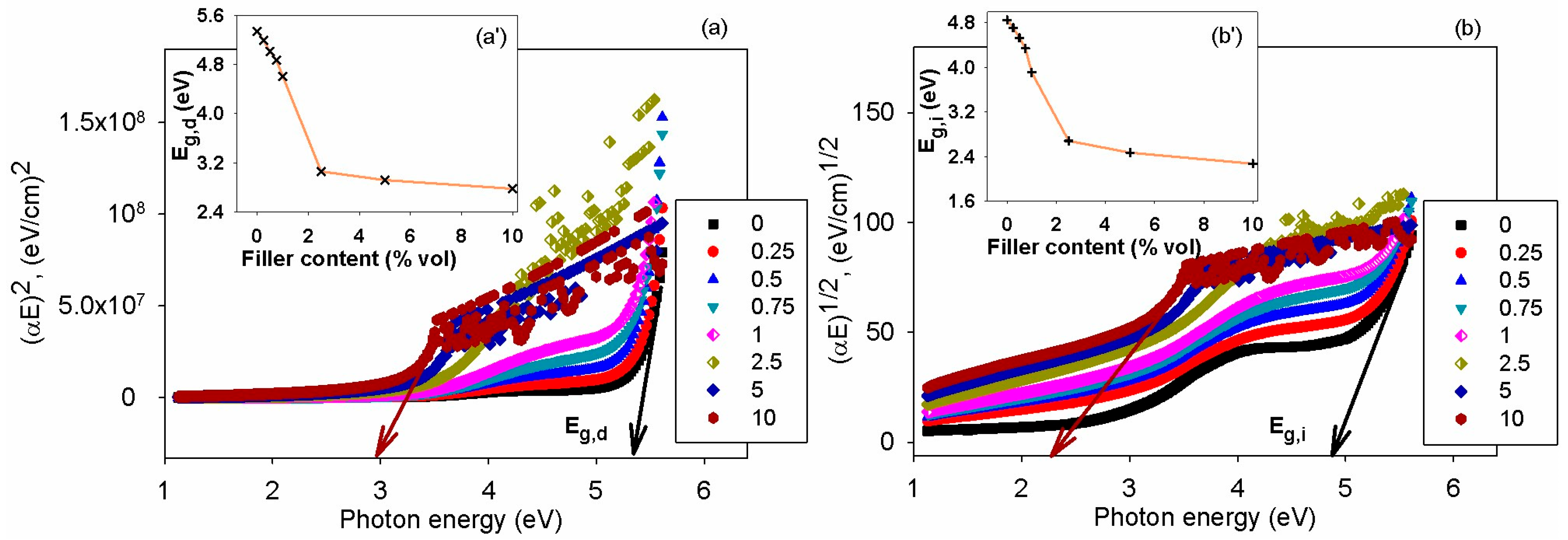

| Filler Content, % vol. | T10, °C | T50, °C | Tmax, °C | R,% |
|---|---|---|---|---|
| 0 | 112 | 321 | 275.64 | 35.15 |
| 0.5 | 127 | 340 | 278.41 | 37.76 |
| 1 | 141 | 358 | 277.82 | 40.30 |
| 2.5 | 178 | 426 | 278.50 | 45.67 |
| 5 | 202 | - | 274.28 | 51.81 |
| 10 | 211 | - | 276.32 | 58.07 |
| Filler Content, % vol. | n | α (cm−1) | kext | σopt (s−1) | εopt′ | εopt″ |
|---|---|---|---|---|---|---|
| 0 | 1.5163 | 23.9963 | 1.1253 × 10−4 | 0.87 × 1011 | 2.2992 | 3.4126 × 10−4 |
| 0.25 | 1.5221 | 115.9805 | 5.4389 × 10−4 | 4.21× 1011 | 2.3168 | 1.6557 × 10−3 |
| 0.5 | 1.5262 | 171.6537 | 8.0497 × 10−4 | 6.25 × 1011 | 2.3293 | 2.4571 × 10−3 |
| 0.75 | 1.5277 | 219.0769 | 1.0274 × 10−3 | 7.98 × 1011 | 2.3339 | 3.1390 × 10−3 |
| 1 | 1.5286 | 257.3681 | 1.2069 × 10−3 | 9.39× 1011 | 2.3366 | 3.6898 × 10−3 |
| 2.5 | 1.5347 | 423.2049 | 1.9846 × 10−3 | 1.55 × 1012 | 2.3553 | 6.0916 × 10−3 |
| 5 | 1.5533 | 573.3283 | 2.6886 × 10−3 | 2.13 × 1012 | 2.4127 | 8.3525 × 10−3 |
| 10 | 1.5704 | 706.7173 | 3.3141 × 10−3 | 2.65 × 1012 | 2.4661 | 0.0104 |
| Sample | Rs (Ω) | R1 (MΩ) | C1 (nF) | fmax1 (Hz) | R2 (MΩ) | C2 (nF) | fmax2 (Hz) |
|---|---|---|---|---|---|---|---|
| 0.25 | 230 | 0.25 | 0.35 | 1850 | 1.67 | 63.5 | 1.5 |
| 0.5 | 191 | 0.22 | 0.44 | 1585 | 1.41 | 75.2 | 1.5 |
| 0.75 | 176 | 0.22 | 0.52 | 1360 | 1.34 | 79 | 1.5 |
| 1 | 150 | 0.21 | 0.64 | 11,666 | 1.34 | 79 | 1.5 |
| 2.5 | 124 | 0.28 | 0.76 | 735 | 1.12 | 94 | 1.5 |
| 5 | 109 | 0.82 | 0.35 | 541 | 2.13 | 49 | 1.5 |
| 10 | 89 | 1.98 | 0.32 | 251 | 5.7 | 18 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turcanu, G.; Stoica, I.; Albu, R.M.; Varganici, C.-D.; Avadanei, M.I.; Barzic, A.I.; Curecheriu, L.-P.; Stagnaro, P.; Buscaglia, M.T. Design of Polysaccharide-Based Nanocomposites for Eco-Friendly Flexible Electronics. Polymers 2025, 17, 1612. https://doi.org/10.3390/polym17121612
Turcanu G, Stoica I, Albu RM, Varganici C-D, Avadanei MI, Barzic AI, Curecheriu L-P, Stagnaro P, Buscaglia MT. Design of Polysaccharide-Based Nanocomposites for Eco-Friendly Flexible Electronics. Polymers. 2025; 17(12):1612. https://doi.org/10.3390/polym17121612
Chicago/Turabian StyleTurcanu, Gabriela, Iuliana Stoica, Raluca Marinica Albu, Cristian-Dragos Varganici, Mihaela Iuliana Avadanei, Andreea Irina Barzic, Lavinia-Petronela Curecheriu, Paola Stagnaro, and Maria Teresa Buscaglia. 2025. "Design of Polysaccharide-Based Nanocomposites for Eco-Friendly Flexible Electronics" Polymers 17, no. 12: 1612. https://doi.org/10.3390/polym17121612
APA StyleTurcanu, G., Stoica, I., Albu, R. M., Varganici, C.-D., Avadanei, M. I., Barzic, A. I., Curecheriu, L.-P., Stagnaro, P., & Buscaglia, M. T. (2025). Design of Polysaccharide-Based Nanocomposites for Eco-Friendly Flexible Electronics. Polymers, 17(12), 1612. https://doi.org/10.3390/polym17121612









