Coconut Residue-Derived Nanoporous Carbon via Hydrothermal Carbonization for Nanoporous Carbon-Based Supercapacitor Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
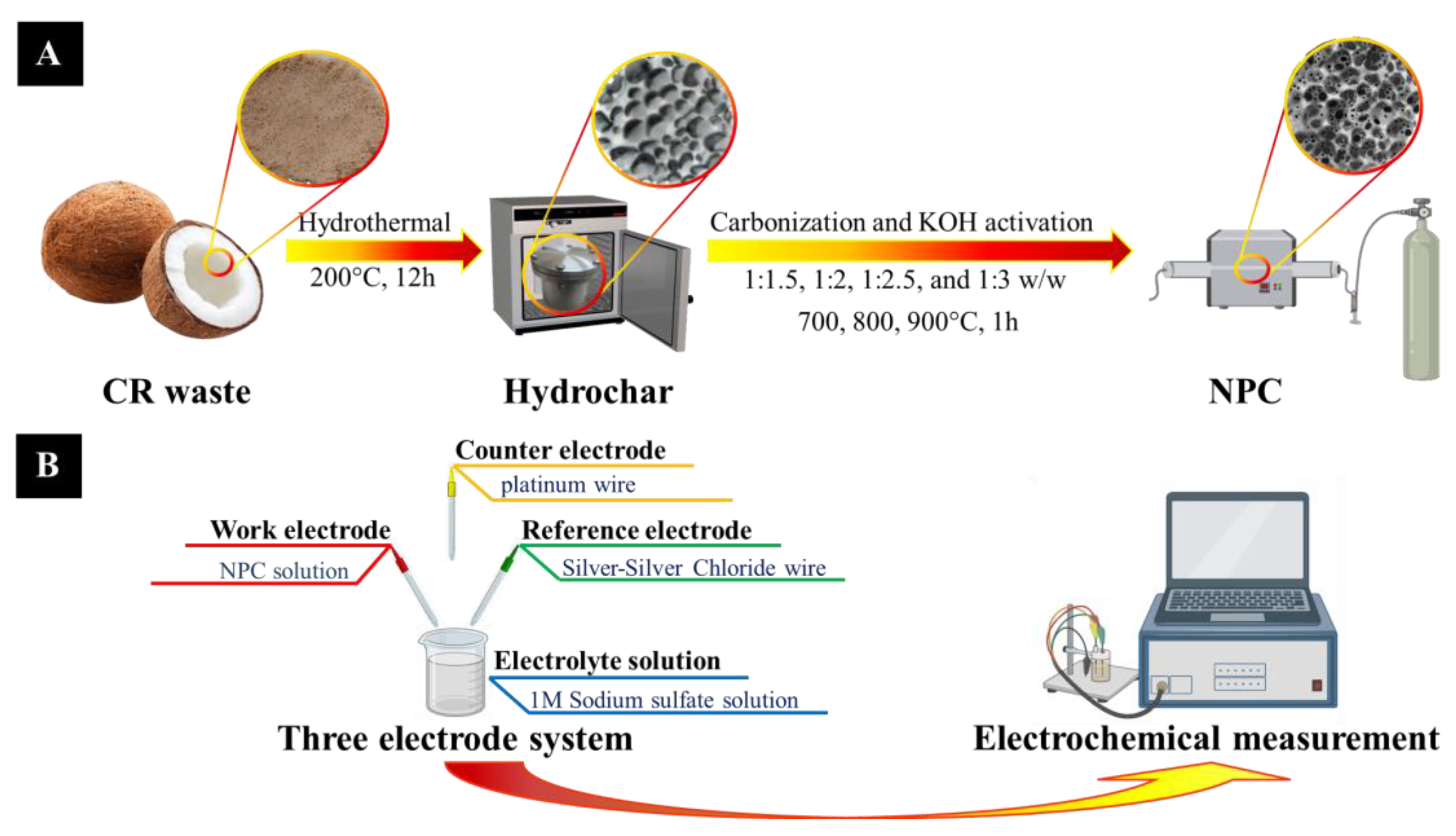
2.2. Synthesis of Nanoporous Carbon (NPC)
2.3. Characterization
2.4. Preparation of the Three-Electrode System
3. Results and Discussions
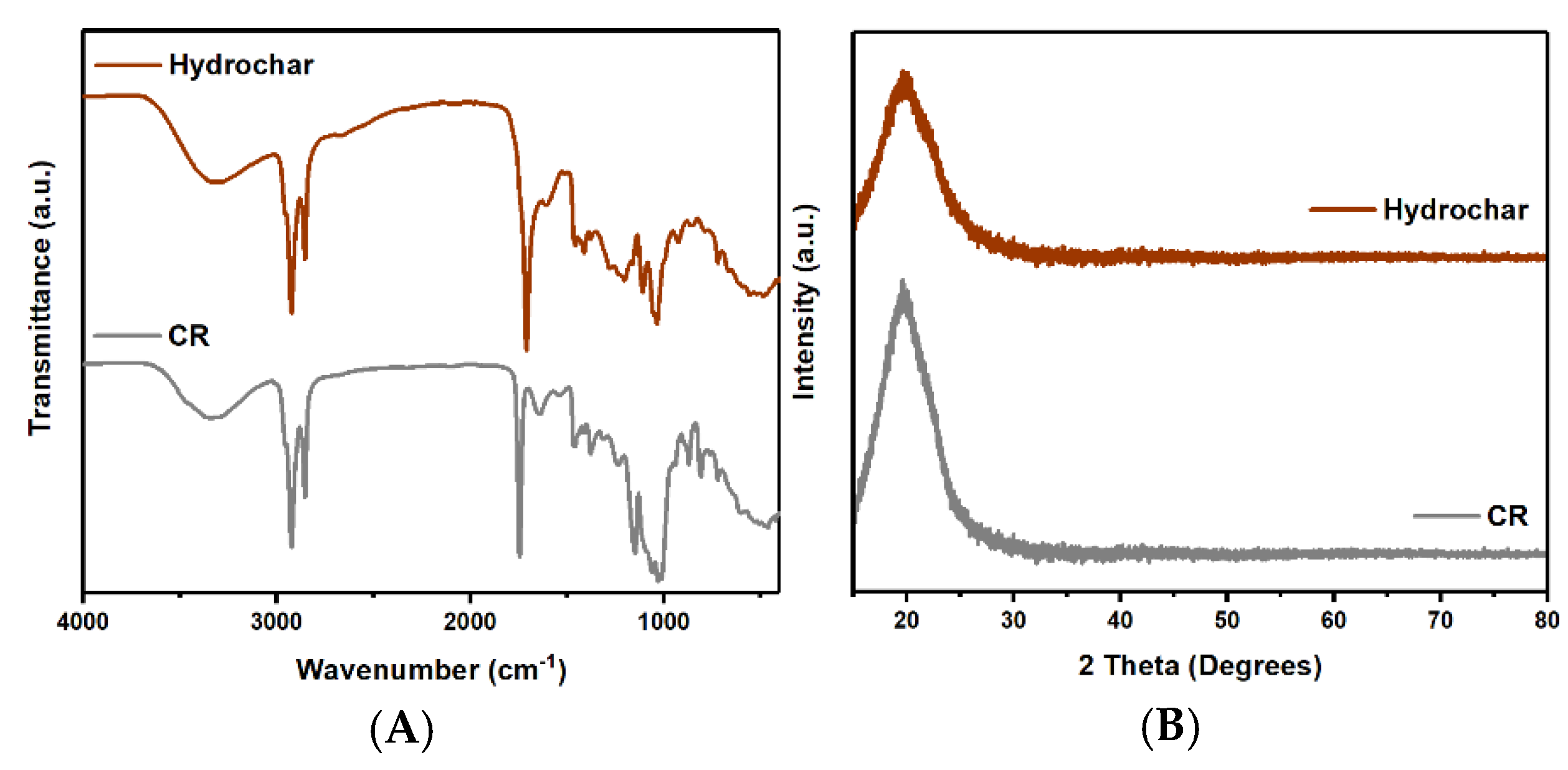
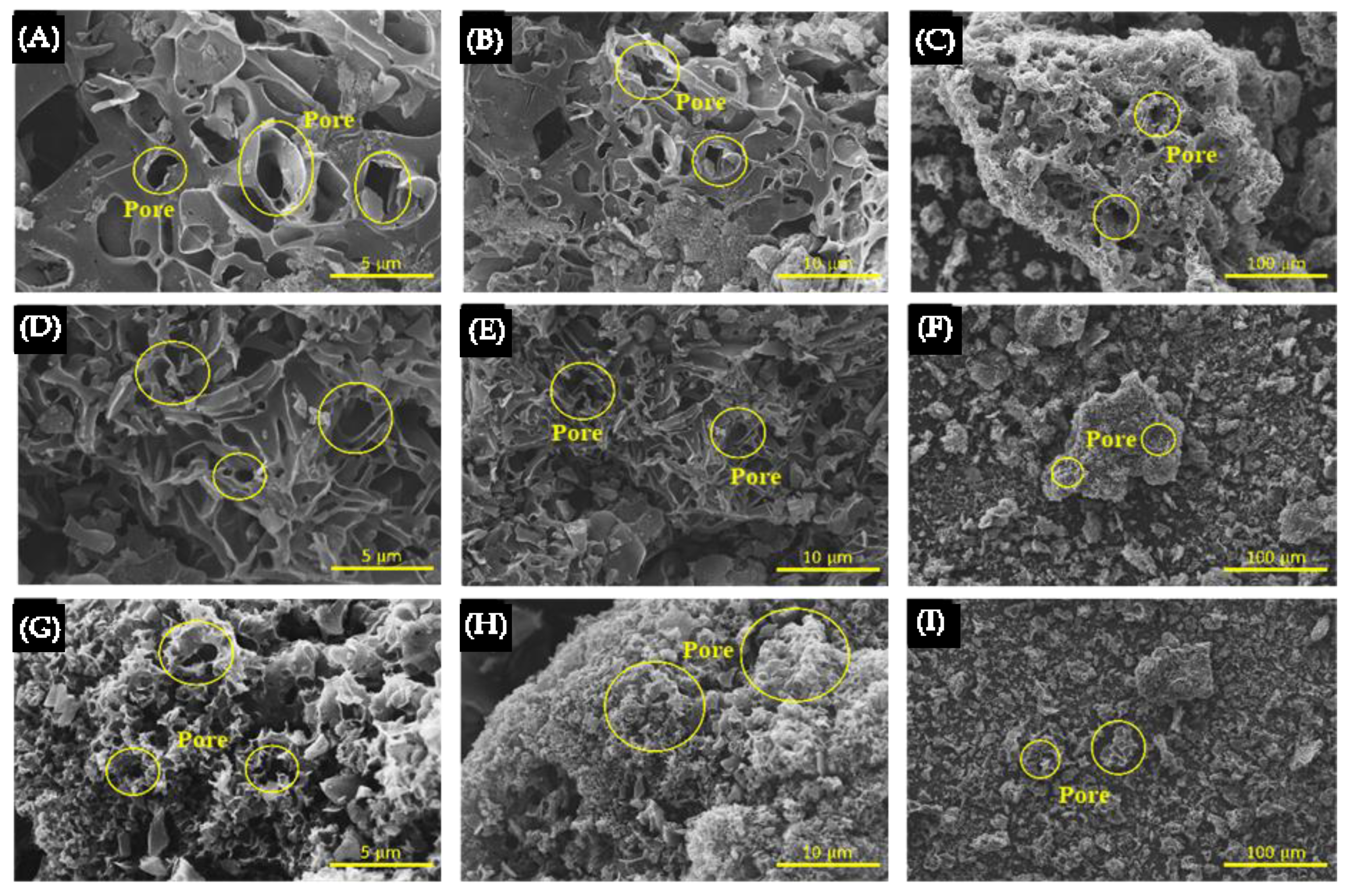
3.1. The Effect of HT
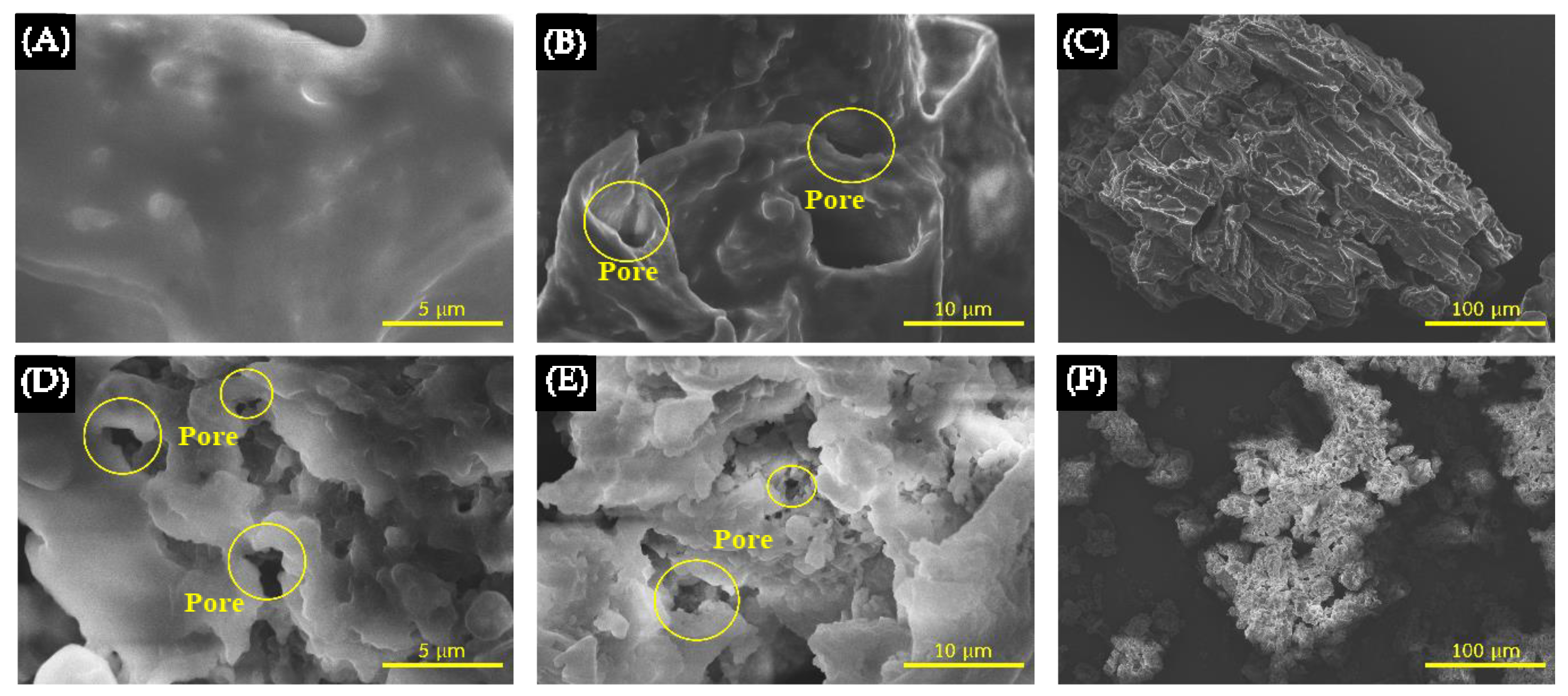
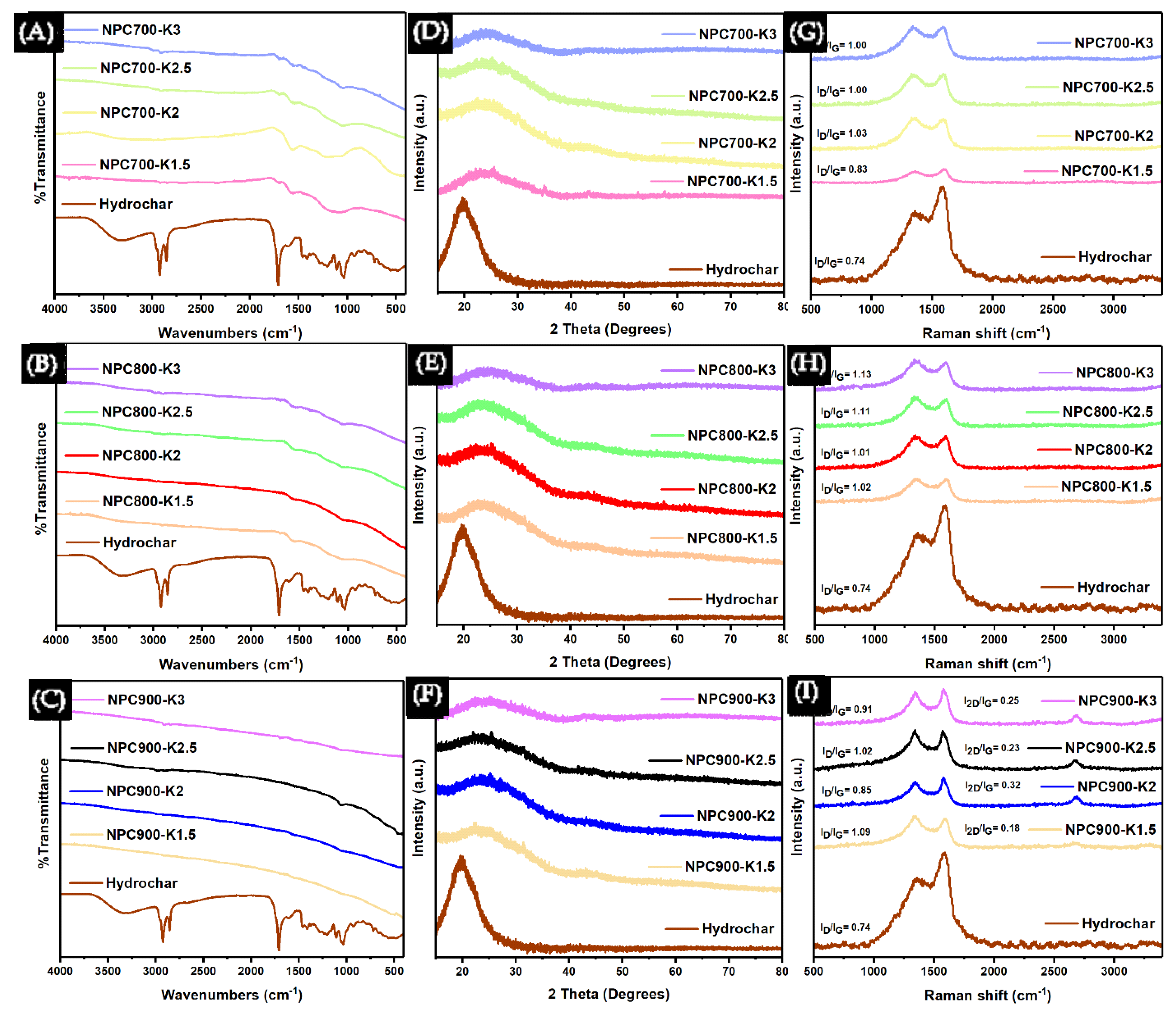
3.2. The Effect of HTC Combined with KOH Activation
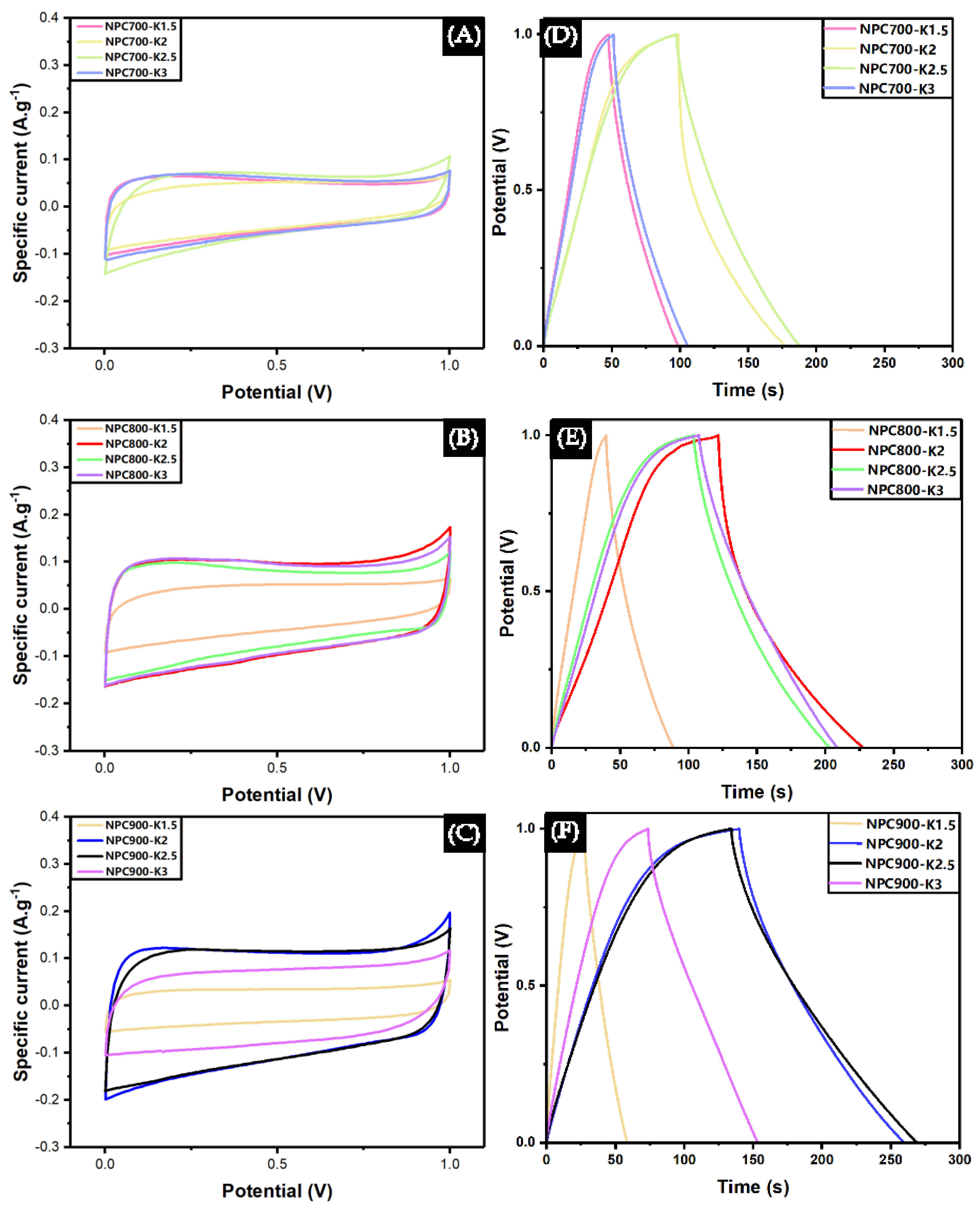
3.3. The Effect of NPC on Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, M.; Park, J.; Lee, K.; Attia, N.; Oh, H. Effective synthesis route of renewable nanoporous carbon adsorbent for high energy gas storage and CO2/N2 selectivity. Renew. Energy 2020, 161, 30–42. [Google Scholar] [CrossRef]
- Salehizadeh, S.; Zandi Lak, S.; Rahimpour, M.R. Energy Storage Technologies for Renewable Energy Sources. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Cui, J.; Zhang, Y.; Cao, Z.; Yu, D.; Wang, Y.; Liu, J.; Zhang, J.; Zhang, Y.; Wu, Y. Nanoporous carbon nanowires derived from one-dimensional metal-organic framework core-shell hybrids for enhanced electrochemical energy storage. Appl. Surf. Sci. 2022, 576, 151800. [Google Scholar] [CrossRef]
- Khalifa, A.; Ebrahim, S.; ElSaid, A.; Ayad, M.M. Highly exposed active sites of MOFs-derived N-doped nanoporous carbon decorated with platinum for enhanced energy storage application. J. Energy Storage 2024, 84, 110774. [Google Scholar] [CrossRef]
- Elalfy, D.A.; Gouda, E.; Kotb, M.F.; Bureš, V.; Sedhom, B.E. Comprehensive review of energy storage systems technologies objectives challenges and future trends. Energy Strategy Rev. 2024, 54, 101482. [Google Scholar] [CrossRef]
- Yin, T.; Guo, Y.; Huang, X.; Yang, X.; Qin, L.; Ning, T.; Tan, L.; Li, L.; Zou, K. Heteroatom Doping Strategy of Advanced Carbon for Alkali Metal-Ion Capacitors. Batteries 2025, 11, 69. [Google Scholar] [CrossRef]
- Poochai, C.; Srikhaow, A.; Lohitkarn, J.; Kongthong, T.; Tuantranont, S.; Tuantranont, S.; Primpray, V.; Maeboonruan, N.; Wisitsoraat, A.; Sriprachuabwong, C. Waste coffee grounds derived nanoporous carbon incorporated with carbon nanotubes composites for electrochemical double-layer capacitors in organic electrolyte. J. Energy Storage 2021, 43, 103169. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, F.; Smith, R.L., Jr.; Qi, X. High-performance supercapacitor electrode materials from chitosan via hydrothermal carbonization and potassium hydroxide activation. Energy Technol. 2017, 5, 452–460. [Google Scholar] [CrossRef]
- Moon, S.; Kim, D.H.; Kwak, J.H.; Lee, S.M.; Lim, H.D.; Kang, K.; Jin, H.J.; Yun, Y.S. Unveiling the pseudocapacitive effects of ultramesopores on nanoporous carbon. Appl. Surf. Sci. 2021, 537, 148037. [Google Scholar] [CrossRef]
- Kiniman, V.; Kanokwhale, C.; Boonto, P.; Pholauyphon, W.; Nantasaksiri, K.; Charoen-amornkitt, P.; Suzuki, T.; Tsushima, S. Modeling cyclic voltammetry responses of porous electrodes: An approach incorporating faradaic and non-faradaic contributions through porous model and constant phase element. J. Energy Storage 2024, 83, 110804. [Google Scholar] [CrossRef]
- Lee, S.; An, G.-H. Reversible faradaic reactions involving redox mediators and oxygen-containing groups on carbon fiber electrode for high-performance flexible fibrous supercapacitors. J. Energy Chem. 2022, 68, 1–11. [Google Scholar] [CrossRef]
- Han, B.; Cheng, G.; Wang, Y.; Wang, X. Structure and functionality design of novel carbon and faradaic electrode materials for high-performance capacitive deionization. Chem. Eng. J. 2019, 360, 364–384. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, C.; Hu, Y.; Zhong, H.; Zhao, Y.; Xu, X.; Liu, H. Preparation of hierarchically porous carbon spheres by hydrothermal carbonization process for high-performance electrochemical capacitors. J. Appl. Electrochem. 2018, 48, 233–241. [Google Scholar] [CrossRef]
- Boonraksa, N.; Swatsitang, E.; Wongsaprom, K. Biomass nanoarchitectonics with activated rice husk char for nanoporous carbon as electrode material: Enhancing supercapacitor electrochemical performance. J. Non-Cryst. Solids 2024, 637, 123064. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Deng, X.Q.; Zhang, S.; Shen, Y.F.; Shan, Y.Q.; Zhang, Z.M.; Luque, R.; Duan, P.G.; Hu, X. Benign-by-design N-doped carbonaceous materials obtained from the hydrothermal carbonization of sewage sludge for supercapacitor applications. Green Chem. 2020, 22, 3885–3895. [Google Scholar] [CrossRef]
- Tong, X.; Chen, Z.; Zhuo, H.; Hu, Y.; Jing, S.; Liu, J.; Zhong, L. Tailoring the physicochemical properties of chitosan-derived N-doped carbon by controlling hydrothermal carbonization time for high-performance supercapacitor application. Carbohydr. Polym. 2019, 207, 764–774. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Song, Y.; Yang, H.; Yang, L.; Bai, L.; Wei, D.; Wang, W.; Liang, Y.; Chen, H. Apple residues derived porous carbon nanosheets synthesized with FeCl3 assisted hydrothermal carbonization for supercapacitors with high rate performance. Carbon Lett. 2023, 33, 549–560. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.C.; Lin, Z.; Taberna, P.L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef] [PubMed]
- Chanpee, S.; Kaewtrakulchai, N.; Khemasiri, N.; Eiad-Ua, A.; Assawasaengrat, P. Nanoporous carbon from oil palm leaves via hydrothermal carbonization-combined KOH activation for paraquat removal. Molecules 2022, 27, 5309. [Google Scholar] [CrossRef]
- Apinyakul, N.; Chanpee, S.; Kaewtrakulchai, N.; Khemasiri, N.; Eiad-Ua, A.; Assawasaengrat, P. Synthesis of nanoporous carbon from brewer waste by hydrothermal carbonization assisted chemical activation for carbamazepine adsorption. Case Stud. Chem. Environ. Eng. 2024, 9, 100716. [Google Scholar] [CrossRef]
- Gnawali, C.L.; Shrestha, L.K.; Hill, J.P.; Ma, R.; Ariga, K.; Adhikari, M.P.; Rajbhandari, R.; Pokharel, B.P. Nanoporous activated carbon material from terminalia chebula seed for supercapacitor application. C—J. Carbon Res. 2023, 9, 109. [Google Scholar] [CrossRef]
- Siraorarnroj, S.; Kaewsichan, L.; Naranart, T.; Kongparakul, S.; Kidkhunthod, P.; Seangwang, W.; Phumying, S.; La-on, T.; Rachtanapun, P.; Kongnual, P. High performance nanoporous carbon from mulberry leaves (Morus alba L.) residues via microwave treatment assisted hydrothermal-carbonization for methyl orange adsorption: Kinetic equilibrium and thermodynamic studies. Materialia 2022, 21, 101288. [Google Scholar] [CrossRef]
- Li, N.; Yuan, K.; Gao, T.; Li, S.; Qin, J.; Zhu, Y.; Du, J.; Xu, L.; Xu, J. Controllable synthesis of hierarchical nanoporous carbon@Ni(OH)2 rambutan-like composite microspheres for high-performance hybrid supercapacitor. Arab. J. Chem. 2022, 15, 103580. [Google Scholar] [CrossRef]
- Rawat, S.; Mishra, R.K.; Bhaskar, T. Biomass derived functional carbon materials for supercapacitor applications. Chemosphere 2022, 286, 131961. [Google Scholar] [CrossRef]
- Celiktas, M.S.; Alptekin, F.M. Conversion of model biomass to carbon-based material with high conductivity by using carbonization. Energy 2019, 188, 116089. [Google Scholar] [CrossRef]
- Abe, H.; Nakayasu, Y.; Haga, K.; Watanabe, M. Progress on separation and hydrothermal carbonization of rice husk toward environmental applications. Glob. Chall. 2023, 7, 2300112. [Google Scholar] [CrossRef]
- Hamid, N.; You, J. Mangosteen peel-derived hydrochar prepared via hydrothermal carbonization for methylene blue removal. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Devianto, H.; Susanti, R.F.; Winata, A.S. Synthesis of activated carbon from salacca peel with hydrothermal carbonization for supercapacitor application. Mater. Today Proc. 2021, 44, 3268–3272. [Google Scholar]
- Tu, W.; Liu, Y.; Xie, Z.; Chen, M.; Ma, L.; Du, G.; Zhu, M. A novel activation-hydrochar via hydrothermal carbonization and KOH activation of sewage sludge and coconut shell for biomass wastes: Preparation characterization and adsorption properties. J. Colloid Interface Sci. 2021, 593, 390–407. [Google Scholar] [CrossRef]
- Awitdrus, A.; Agustino, A.; Nopriansyah, R.; Farma, R.; Iwantono, I.; Deraman, M. Fabrication of nanoporous carbon constructions derived from young coconut husk fibers for symmetrical supercapacitor applications. Bioresour. Technol. Rep. 2024, 25, 101765. [Google Scholar] [CrossRef]
- Hassan, S.H.; Velayutham, T.S.; Chen, Y.W.; Lee, H.V. TEMPO-oxidized nanocellulose films derived from coconut residues: Physicochemical mechanical and electrical properties. Int. J. Biol. Macromol. 2021, 180, 392–402. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Zheng, M.; Xiao, Y.; Hu, H.; Liang, Y.; Liu, Y.; Dong, H. Preparation of High-Performance Porous Carbon Materials by Citric Acid-Assisted Hydrothermal Carbonization of Bamboo and Their Application in Electrode Materials. Energy Fuels 2022, 36, 9303–9312. [Google Scholar] [CrossRef]
- Li, H.; Shi, F.; An, Q.; Zhai, S.; Wang, K.; Tong, Y. Three-dimensional hierarchical porous carbon derived from lignin for supercapacitors: Insight into the hydrothermal carbonization and activation. Int. J. Biol. Macromol. 2021, 166, 923–933. [Google Scholar] [CrossRef]
- Eniola, J.O.; Sizirici, B.; Stephen, S.; Yildiz, I.; Khaleel, A.; El Fadel, M. Anew synthesis route of hydrothermally carbonized Na2CO3 activated bentonite-clay as a novel adsorbent for cadmium removal from wastewater. Sep. Purif. Technol. 2024, 350, 127960. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Benavente, V.; Weidemann, E.; Gentili, F.G.; Jansson, S. Influence of hydrothermal carbonization conditions on the porosity, functionality, and sorption properties of microalgae hydrochars. Sci. Rep. 2023, 13, 8562. [Google Scholar] [CrossRef] [PubMed]
- Petrović, J.; Ercegović, M.; Simić, M.; Koprivica, M.; Dimitrijević, J.; Jovanović, A.; Janković Pantić, J. Hydrothermal Carbonization of Waste Biomass: AReview of Hydrochar Preparation and Environmental Application. Processes 2024, 12, 207. [Google Scholar] [CrossRef]
- Amelia, S.T.W.; Nurtono, T.; Setyawan, H.; Widiyastuti, W. Electrocapacitive and electrocatalytic performances of hydrochar prepared by one-step hydrothermal carbonization without further activation. Mater. Res. Express 2023, 10, 075602. [Google Scholar] [CrossRef]
- Hasan, R.O.; Ercan, B.; Acikkapi, A.N.; Ucar, S.; Karagoz, S. Effects of metal chlorides on the hydrothermal carbonization of grape seeds. Energy Fuels 2021, 35, 8834–8843. [Google Scholar] [CrossRef]
- Thu, M.M.; Chaiammart, N.; Jongprateep, O.; Techapiesancharoenkij, R.; Thant, A.A.; Saito, N.; Panomsuwan, G. Introducing micropores into carbon nanoparticles synthesized via a solution plasma process by thermal treatment and their charge storage properties in supercapacitors. RSC Adv. 2023, 13, 16136–16144. [Google Scholar] [CrossRef]
- Kaewtrakulchai, N.; Chanpee, S.; Pasee, W.; Putta, A.; Chutipaijit, S.; Kaewpanha, M.; Suriwong, T.; Puengjinda, P.; Panomsuwan, G.; Fuji, M.; et al. Valorization of horse manure conversion to magnetic carbon nanofiber for dye adsorption by hydrothermal treatment coupled with carbonization. Case Stud. Chem. Environ. Eng. 2024, 9, 100563. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, R.; Luo, L. Thermochemistry of sulfur during pyrolysis and hydrothermal carbonization of sewage sludges. Waste Manag. 2021, 121, 276–285. [Google Scholar]
- Zeng, G.; Lou, S.; Ying, H.; Wu, X.; Dou, X.; Ai, N.; Wang, J. Preparation of microporous carbon from Sargassum horneri by hydrothermal carbonization and KOH activation for CO2 capture. J. Chem. 2018, 2018, 4319149. [Google Scholar] [CrossRef]
- Lee, H.-M.; Chung, D.C.; Jung, S.C.; An, K.H.; Park, S.J.; Kim, B.J. Astudy on pore development mechanism of activated carbons from polymeric precursor: Effects of carbonization temperature nano crystallite formation. Chem. Eng. J. 2019, 377, 120836. [Google Scholar] [CrossRef]
- Mai, T.-T.; Vu, D.L.; Huynh, D.C.; Wu, N.L.; Le, A.T. Cost-effective porous carbon materials synthesized by carbonizing rice husk and K2CO3 activation and their application for lithium-sulfur batteries. J. Sci. Adv. Mater. Devices 2019, 4, 223–229. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, F.; Jia, X.; Liang, S.; Peng, K.; Qian, L. Synthesis of biomass-based porous graphitic carbon combining chemical treatment and hydrothermal carbonization as promising electrode materials for supercapacitors. Ionics 2020, 26, 3655–3668. [Google Scholar] [CrossRef]
- Si, H.; Wang, B.; Chen, H.; Li, Y.; Zhang, X.; Liang, X.; Sun, L.; Yang, S.; Hou, D. Activated carbon prepared from rose branch using H3PO4-hydrothermal carbonization and activation and its apllication for supercapacitors. Int. J. Electrochem. Sci. 2019, 14, 7899–7910. [Google Scholar] [CrossRef]
- Chaparro-Garnica, J.; Salinas-Torres, D.; Mostazo-López, M.J.; Morallon, E.; Cazorla-Amorós, D. Biomass waste conversion into low-cost carbon-based materials for supercapacitors: Asustainable approach for the energy scenario. J. Electroanal. Chem. 2021, 880, 114899. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, H.; Gao, Y.; Zhang, X. Preparation characterization of hydrochar-derived activated carbon from glucose by hydrothermal carbonization. In Biomass Conversion and Biorefinery; Spinger: Berlin/Heidelberg, Germany, 2021; pp. 1–12. [Google Scholar]
- Alfatah, T.; Mistar, E.M.; Supardan, M.D. Synthesis and characterization of activated carbon from Bambusa vulgaris striata using two-step KOH activation. J. Mater. Res. Technol. 2020, 9, 6278–6286. [Google Scholar]
- Thazin, N.M.; Chaiammart, N.; Thu, M.M.; Panomsuwan, G. Effect of pre-carbonization temperature on the porous structure and electrochemical properties of activated carbon fibers derived from kapok for supercapacitor applications. J. Met. Mater. Miner. 2022, 32, 55–64. [Google Scholar] [CrossRef]
- Saba, A.; Saha, N.; Reza, M.T. Effect of hydrothermal carbonization temperature on pH, dissociation constants, and acidic functional groups on hydrochar from cellulose and wood. J. Anal. Appl. Pyrolysis 2019, 137, 138–145. [Google Scholar]
- Saha, N.; Reza, M.T. Effect of pyrolysis on basic functional groups of hydrochars. Biomass Convers. Biorefin. 2021, 11, 1117–1124. [Google Scholar] [CrossRef]
- Ahmed Khan, T.; Kim, H.J.; Gupta, A.; Jamari, S.S.; Jose, R. Synthesis and characterization of carbon microspheres from rubber wood by hydrothermal carbonization. J. Chem. Technol. Biotechnol. 2019, 94, 1374–1383. [Google Scholar] [CrossRef]
- Cheng, C.; Guo, Q.; Ding, L.; Raheem, A.; He, Q.; Lam, S.S.; Yu, G. Upgradation of coconut waste shell to value-added hydrochar via hydrothermal carbonization: Parametric optimization using response surface methodology. Appl. Energy 2022, 327, 120136. [Google Scholar] [CrossRef]
- Veltri, F.; Alessandro, F.; Scarcello, A.; Beneduci, A.; Arias Polanco, M.; Cid Perez, D.; Vacacela Gomez, C.; Tavolaro, A.; Giordano, G.; Caputi, L.S. Porous carbon materials obtained by the hydrothermal carbonization of orange juice. Nanomaterials 2020, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, Z.Z.; Krishnan, B.; Sagadevan, S.; Rafique, R.F.; Hamizi, N.A.B.; Abdul Wahab, Y.; Khan, A.A.; Johan, R.B.; Al-Douri, Y.; Kazi, S.N.; et al. Effect of temperature on the physical electro-chemical and adsorption properties of carbon micro-spheres using hydrothermal carbonization process. Nanomaterials 2018, 8, 597. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Mohammed, A.S.; Sander, G.; Wheatley, A.D.; Nyktari, E.; Usen, I.C. Production and characterisation of adsorbents synthesised by hydrothermal carbonisation of biomass wastes. SN Appl. Sci. 2021, 3, 257. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Greenway, G.M. Characterization of energy-rich hydrochars from microwave-assisted hydrothermal carbonization of coconut shell. Waste Biomass Valorization 2019, 10, 1979–1987. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Ross, A.B.; Mariner, T.; Hammerton, J.; Fitzsimmons, M. Hydrochars produced by hydrothermal carbonisation of seaweed, coconut shell and oak: Effect of pro-cessing temperature on physicochemical adsorbent characteristics. SN Appl. Sci. 2022, 4, 203. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Y.; Chen, M.; Ma, L.; Yang, B.; Li, L.; Liu, Q. Optimized preparation of activated carbon from coconut shell and municipal sludge. Mater. Chem. Phys. 2020, 241, 122327. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, X.; Wang, Y.; Sun, B.; Wu, X.; Lu, H. Effects of additives on sucrose-derived activated carbon microspheres synthesized by hydrothermal carbonization. J. Mater. Sci. 2017, 52, 10787–10799. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, W.; Yang, Y.; Chen, J.; Zhao, Y.; Mu, S. Synchronously improved graphitization and surface area in a 3D porous carbon network as a high capac-ity anode material for lithium/sodium-ion batteries. J. Mater. Chem. A 2021, 9, 1260–1268. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Jia, K.; Liu, G.; Zhang, Y.; Liu, W.; Li, K.; Zhang, B.; Wang, P. Facile synthesis 2D hierarchical structure of ultrahigh nitrogen-doped porous carbon graphene nanosheets as high-efficiency lithium-ion battery anodes. Mater. Chem. Phys. 2020, 251, 123043. [Google Scholar] [CrossRef]
- Gunasekaran, S.S.; Elumalali, S.K.; Kumaresan, T.K.; Meganathan, R.; Ashok, A.; Pawar, V.; Vediappan, K.; Ramasamy, G.; Karazhanov, S.Z.; Raman, K.; et al. Partially graphitic nanoporous activated carbon prepared from biomass for supercapacitor application. Mater. Lett. 2018, 218, 165–168. [Google Scholar] [CrossRef]
- Li, Y.; Zang, K.; Duan, X.; Luo, J.; Chen, D. Boost oxygen reduction reaction performance by tuning the active sites in Fe-NPC catalysts. J. Energy Chem. 2021, 55, 572–579. [Google Scholar] [CrossRef]
- Tian, X.-L.; Yu, J.H.; Qiu, L.; Zhu, Y.H.; Zhu, M.Q. Structural changes and electrochemical properties of mesoporous activated carbon derived from Eu-commia ulmoides wood tar by KOH activation for supercapacitor applications. Ind. Crops Prod. 2023, 197, 116628. [Google Scholar] [CrossRef]
- Chaiammart, N.; Vignesh, V.; Thu, M.M.; Eiad-ua, A.; Maiyalagan, T.; Panomsuwan, G. Chemically activated carbons derived from cashew nut shells as potential electrode materials for electrochemical supercapacitors. Carbon Resour. Convers. 2024, 8, 100267. [Google Scholar] [CrossRef]
- Luo, L.; Luo, L.; Deng, J.; Chen, T.; Du, G.; Fan, M.; Zhao, W. High performance supercapacitor electrodes based on B/N Co-doped biomass porous carbon materials by KOH activation and hydrothermal treatment. Int. J. Hydrogen Energy 2021, 46, 31927–31937. [Google Scholar] [CrossRef]
- Kalu-Uka, G.M.; Kumar, S.; Kalu-Uka, A.C.; Vikram, S.; Ihekweme, G.O.; Ranjan, N.; Anosike-Francis, E.N.; Prajapati, G.; Nduba, A.; Onwualu, A.P.; et al. Production of activated carbon electrode for energy storage application in supercapacitors via KOH activation of waste termite biomass. Waste Biomass Valorization 2022, 13, 2689–2704. [Google Scholar] [CrossRef]
- Ye, X.; Wu, L.; Zhu, M.; Wang, Z.; Huang, Z.H.; Wang, M.X. Lotus pollen-derived hierarchically porous carbons with exceptional adsorption performance toward Re-active Black 5: Isotherms, kinetics and thermodynamics investigations. Sep. Purif. Technol. 2022, 300, 121899. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.; Shi, T.; Zha, R. Nanocasting and direct synthesis strategies for mesoporous carbons as supercapacitor electrodes. Chem. Mater. 2018, 30, 7391–7412. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Q.; Cui, L. Recent progress of mesoporous materials for high performance supercapacitors. Microporous Mesoporous Mater. 2021, 314, 110870. [Google Scholar] [CrossRef]
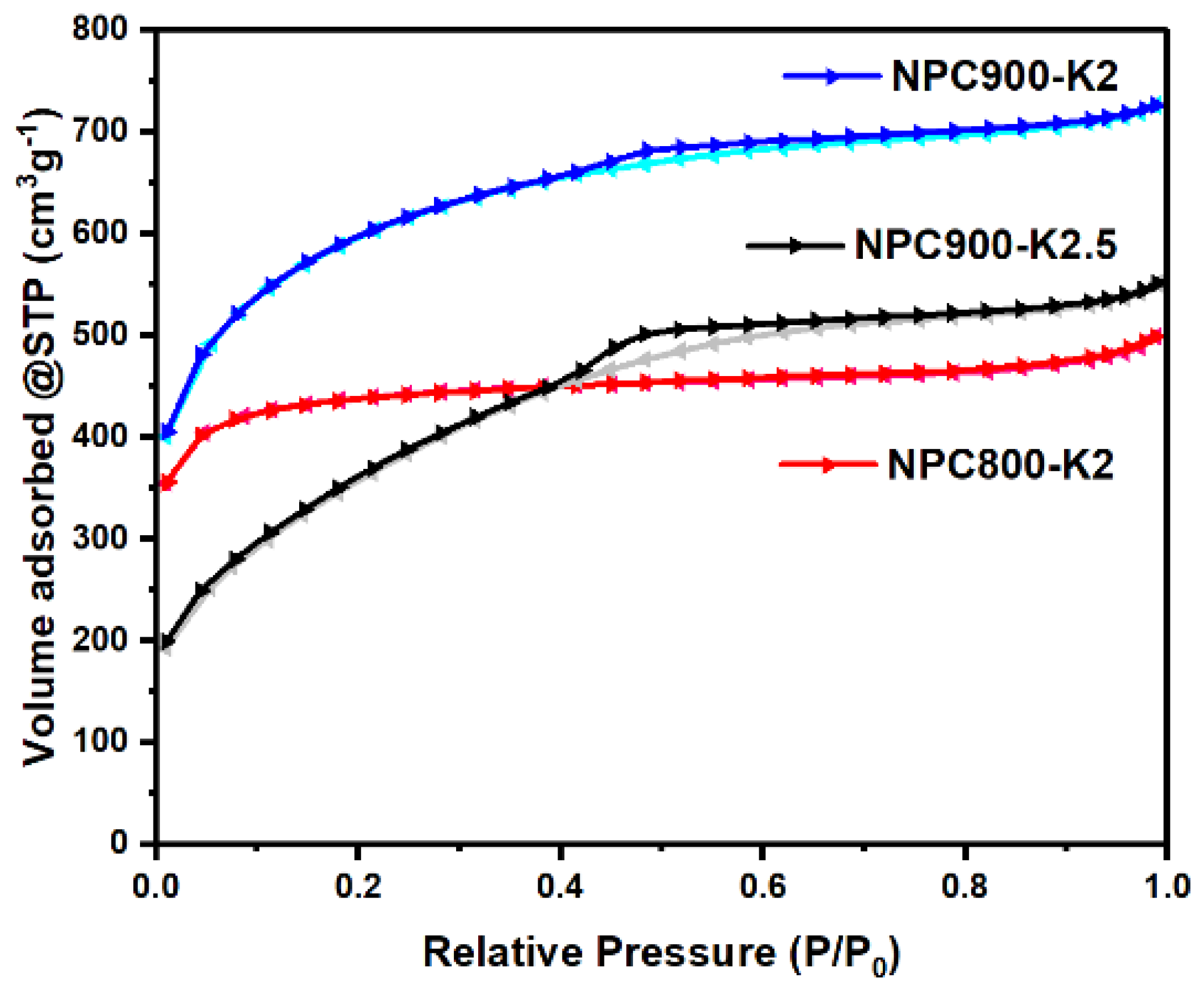
| Condition | SBET (m2/g) | Smicro (m2/g) | Smarco-meso (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Daverage (nm) |
|---|---|---|---|---|---|---|
| NPC800-K2 | 1331.67 | 1059.84 | 271.83 | 0.75 | 0.56 | 37.39 |
| NPC900-K2 | 1969.23 | 798.57 | 1170.66 | 1.11 | 0.41 | 29.32 |
| NPC900-K2.5 | 1385.51 | 73.68 | 1311.83 | 0.83 | 0.02 | 29.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruenroengrit, K.; Kunyuan, J.; Ruttanadech, N.; Kaewtrakulchai, N.; Puengjinda, P.; Chaiammart, N.; Chutipaijit, S.; Buasri, A.; Fuji, M.; Eiad-Ua, A.; et al. Coconut Residue-Derived Nanoporous Carbon via Hydrothermal Carbonization for Nanoporous Carbon-Based Supercapacitor Electrodes. Polymers 2025, 17, 1752. https://doi.org/10.3390/polym17131752
Ruenroengrit K, Kunyuan J, Ruttanadech N, Kaewtrakulchai N, Puengjinda P, Chaiammart N, Chutipaijit S, Buasri A, Fuji M, Eiad-Ua A, et al. Coconut Residue-Derived Nanoporous Carbon via Hydrothermal Carbonization for Nanoporous Carbon-Based Supercapacitor Electrodes. Polymers. 2025; 17(13):1752. https://doi.org/10.3390/polym17131752
Chicago/Turabian StyleRuenroengrit, Kemchat, Jumpon Kunyuan, Nuttapong Ruttanadech, Napat Kaewtrakulchai, Pramote Puengjinda, Nattapat Chaiammart, Sutee Chutipaijit, Achanai Buasri, Masayoshi Fuji, Apiluck Eiad-Ua, and et al. 2025. "Coconut Residue-Derived Nanoporous Carbon via Hydrothermal Carbonization for Nanoporous Carbon-Based Supercapacitor Electrodes" Polymers 17, no. 13: 1752. https://doi.org/10.3390/polym17131752
APA StyleRuenroengrit, K., Kunyuan, J., Ruttanadech, N., Kaewtrakulchai, N., Puengjinda, P., Chaiammart, N., Chutipaijit, S., Buasri, A., Fuji, M., Eiad-Ua, A., & Panomsuwan, G. (2025). Coconut Residue-Derived Nanoporous Carbon via Hydrothermal Carbonization for Nanoporous Carbon-Based Supercapacitor Electrodes. Polymers, 17(13), 1752. https://doi.org/10.3390/polym17131752









