Construction of Fire-Retardant PEO Composite Based on Calcium Sulfate Whiskers Fabricated from Phosphogypsum and DOPO Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
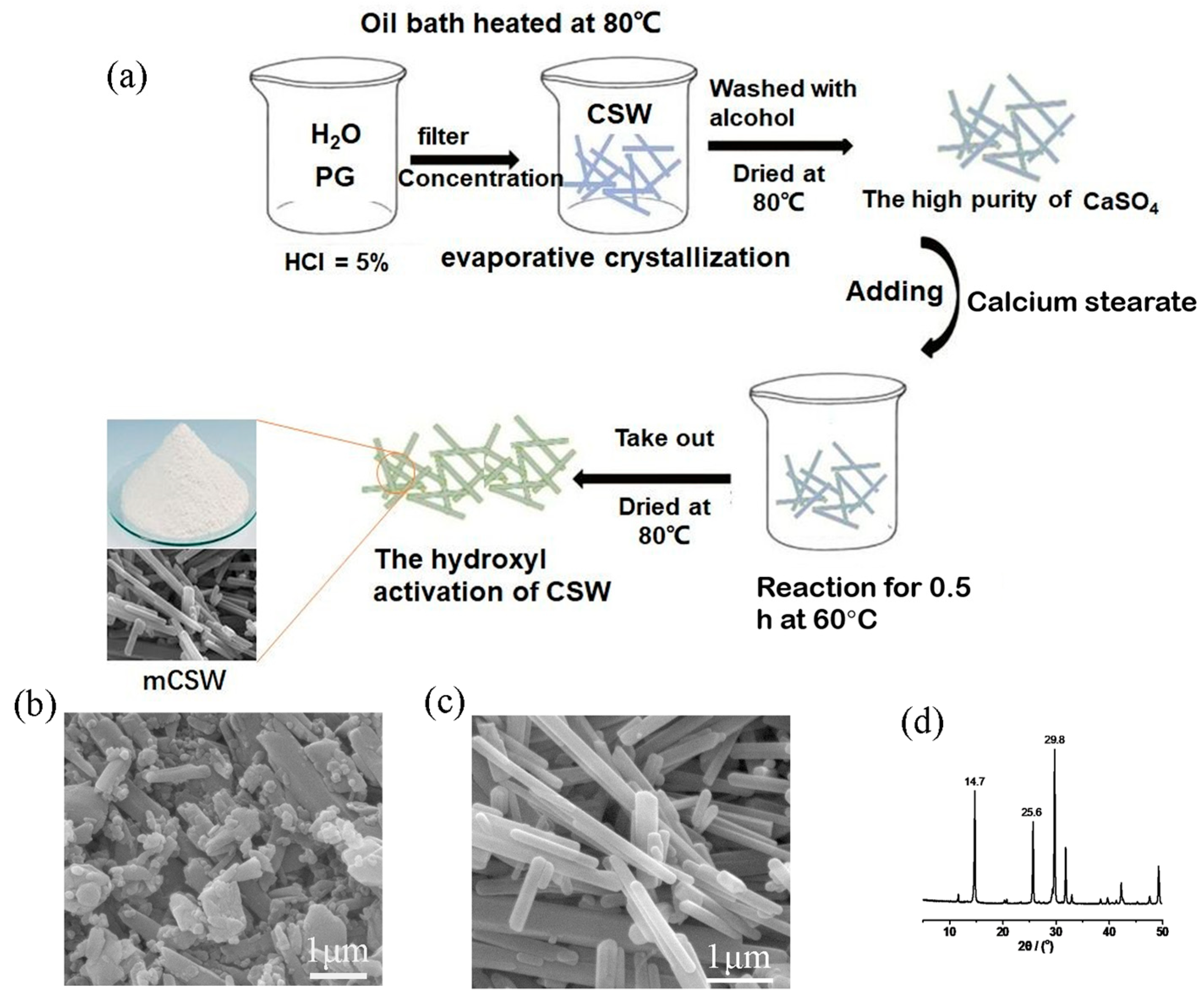
2.2. Preparation of Phosphogypsum-Extracted CSWs
2.3. Preparation of MCSW
2.4. Preparation of PEO/DIDOPO/MCSW
2.5. Characterization
3. Results and Discussion
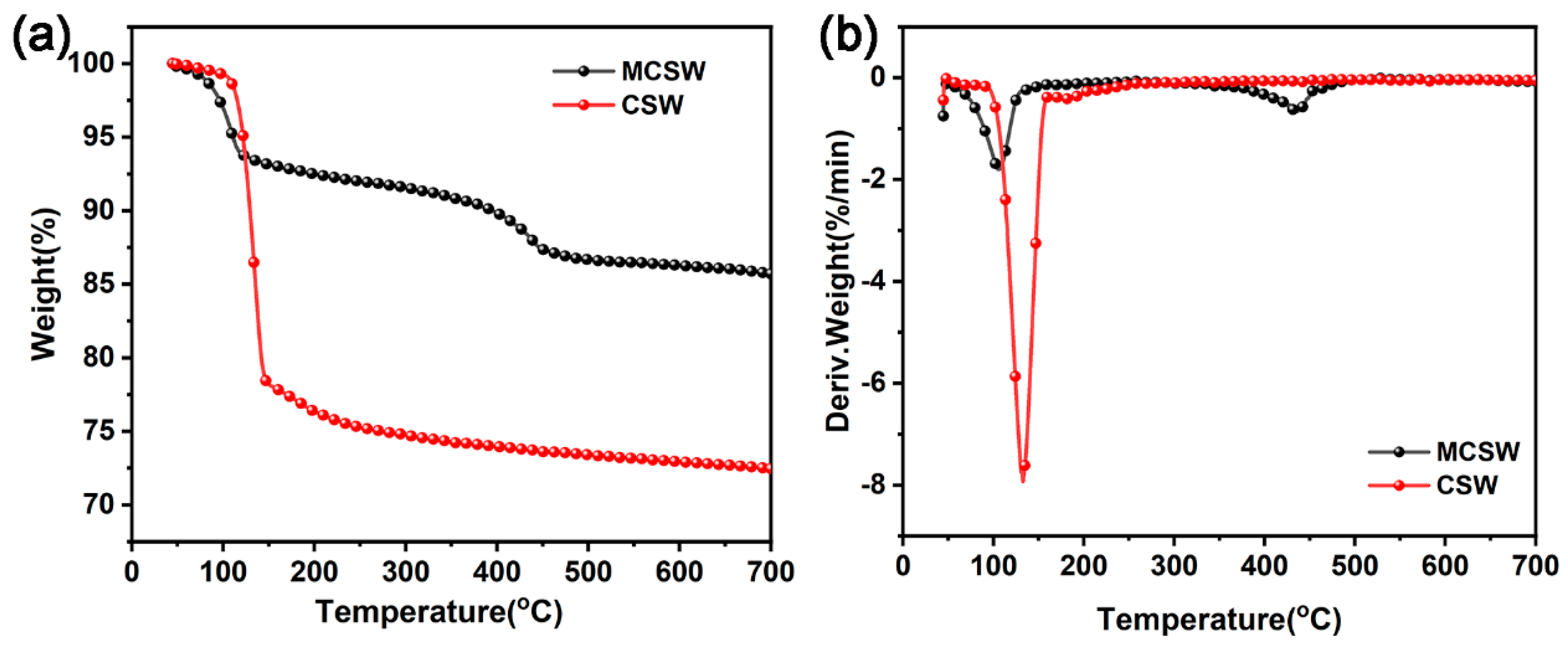
3.1. Characterization of MCSW
3.2. Flame Retardancies of the PEOs
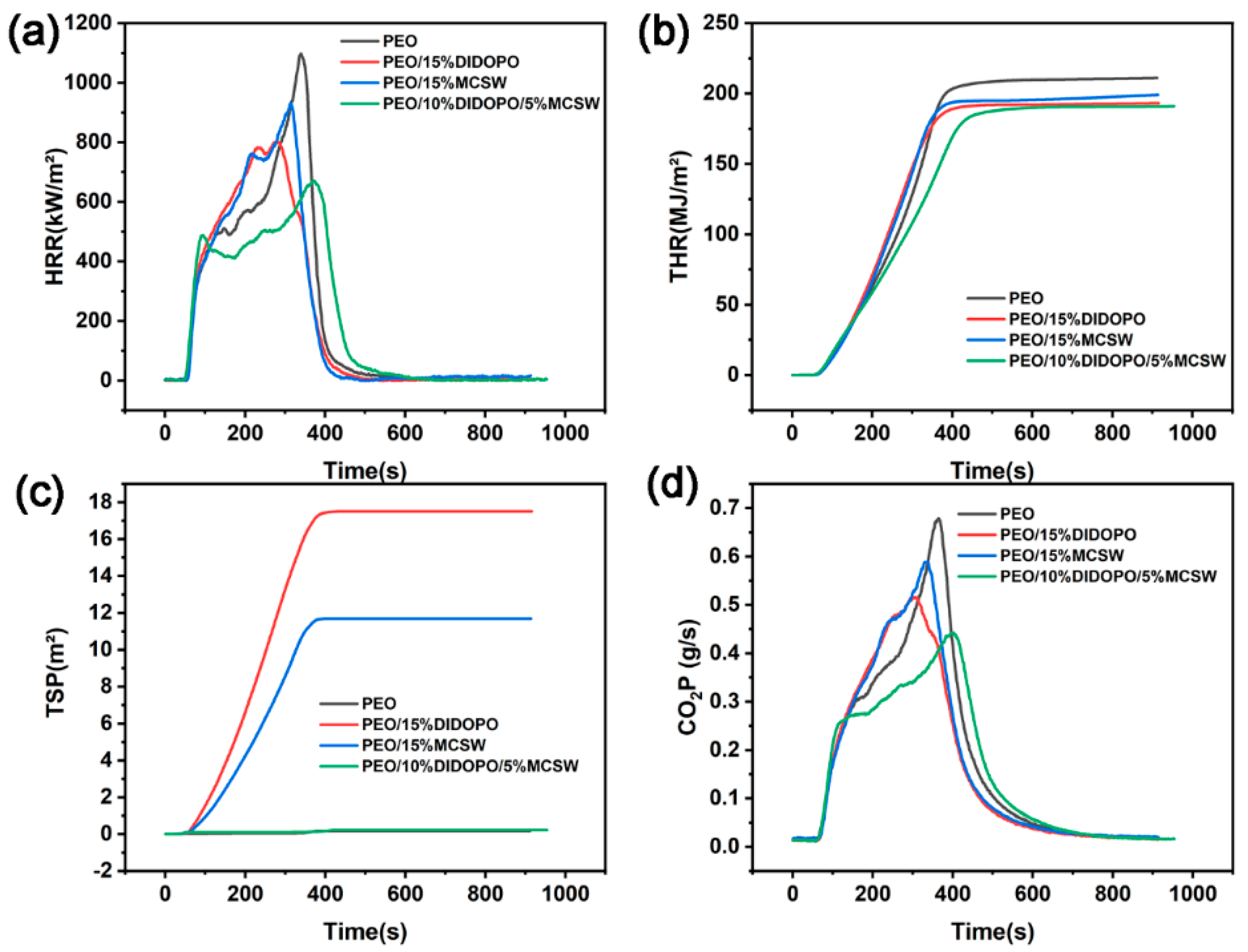
3.3. Cone Calorimetry
3.4. Thermal Stability Analysis
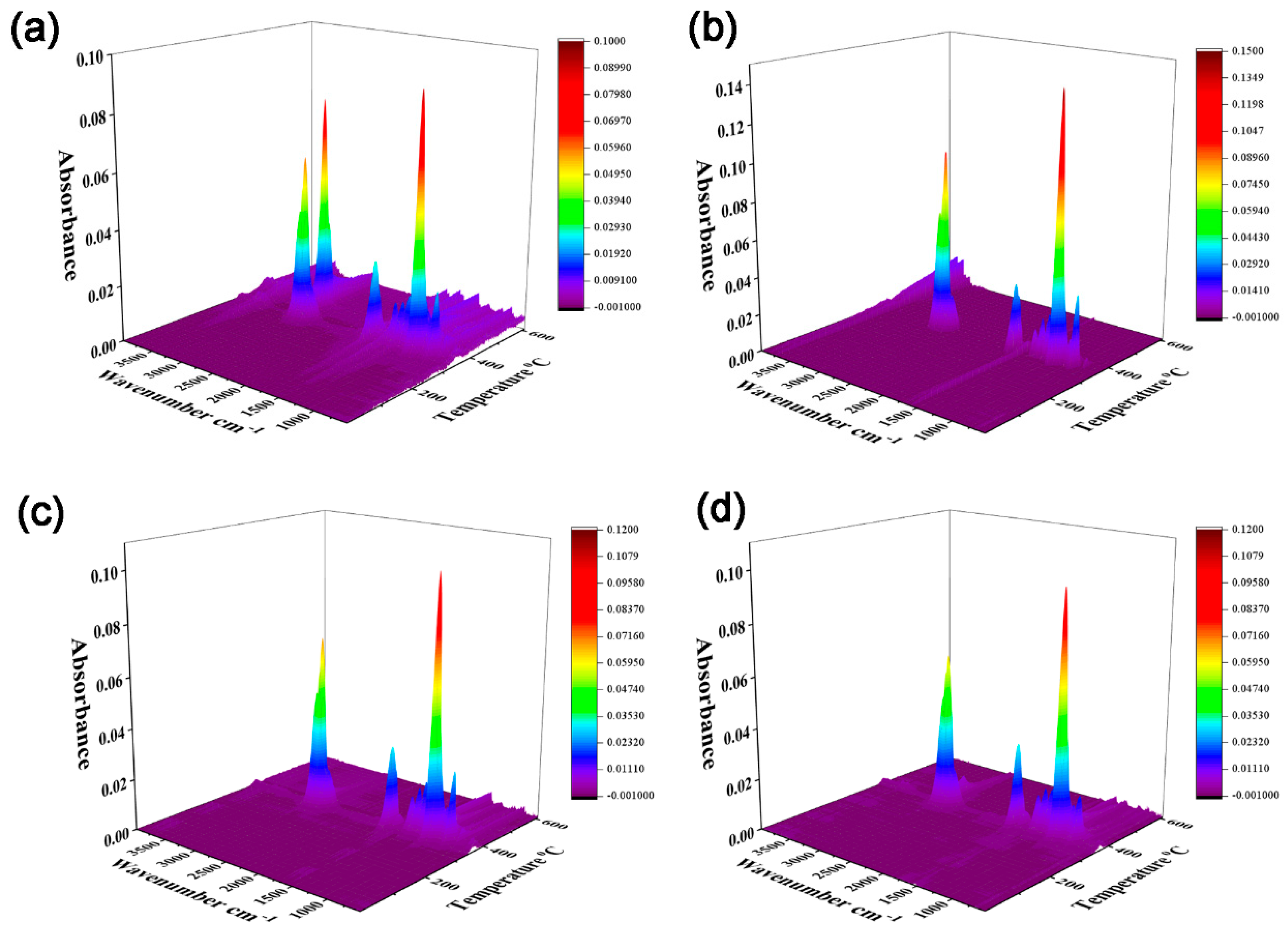
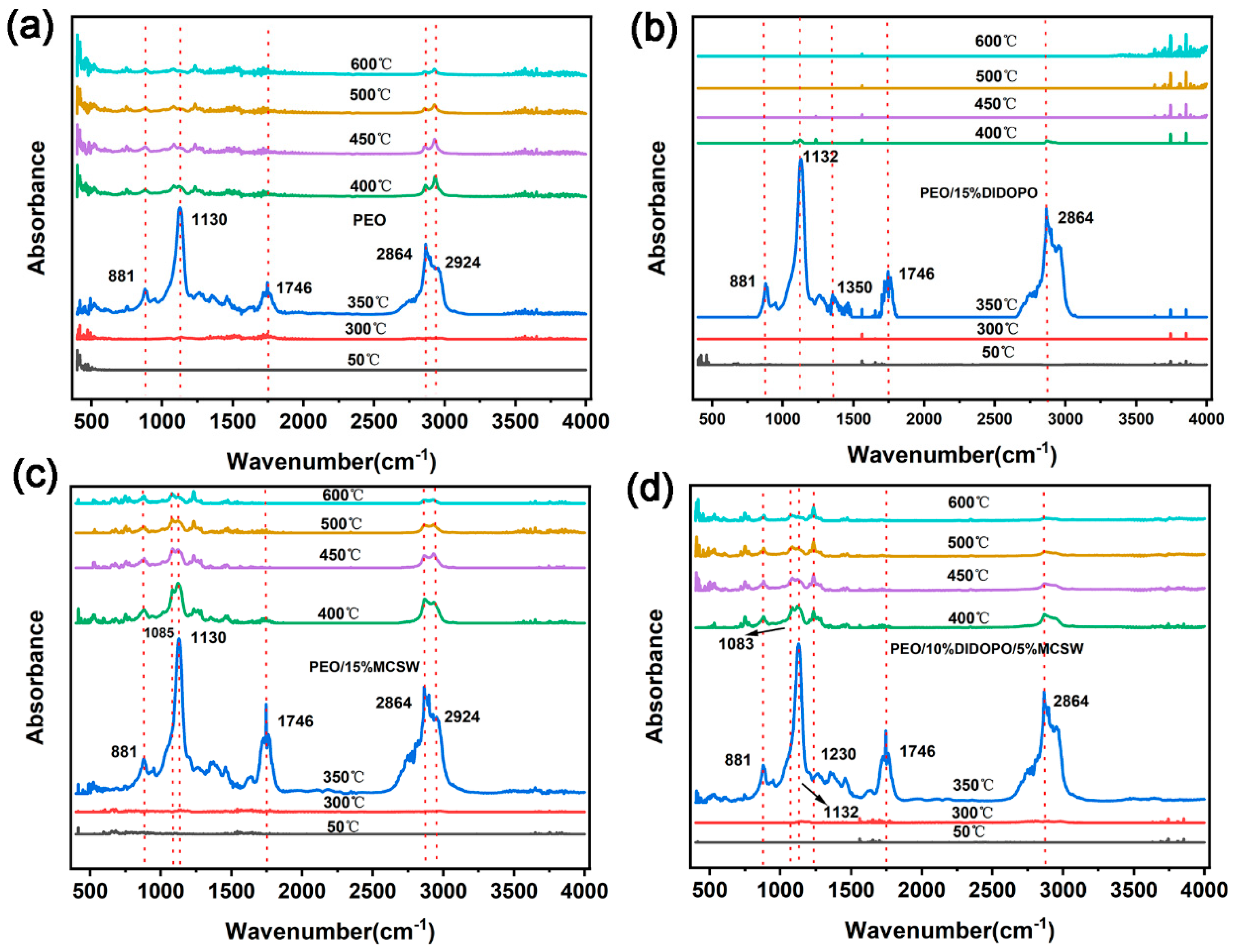
3.5. TGA-FTIR Analysis
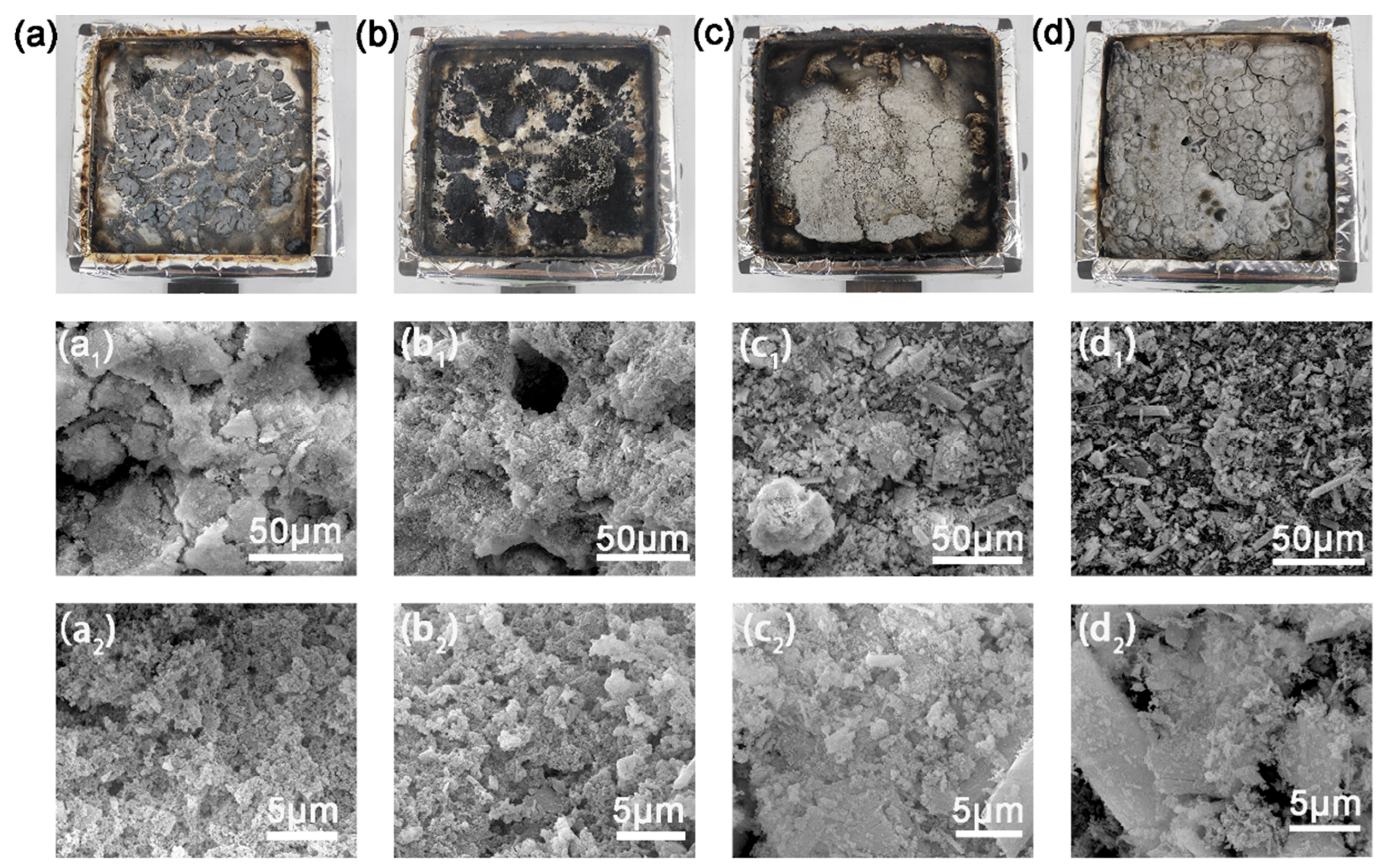
3.6. Analysis of the Char Residues
3.7. Elemental Analysis of the Char Residues
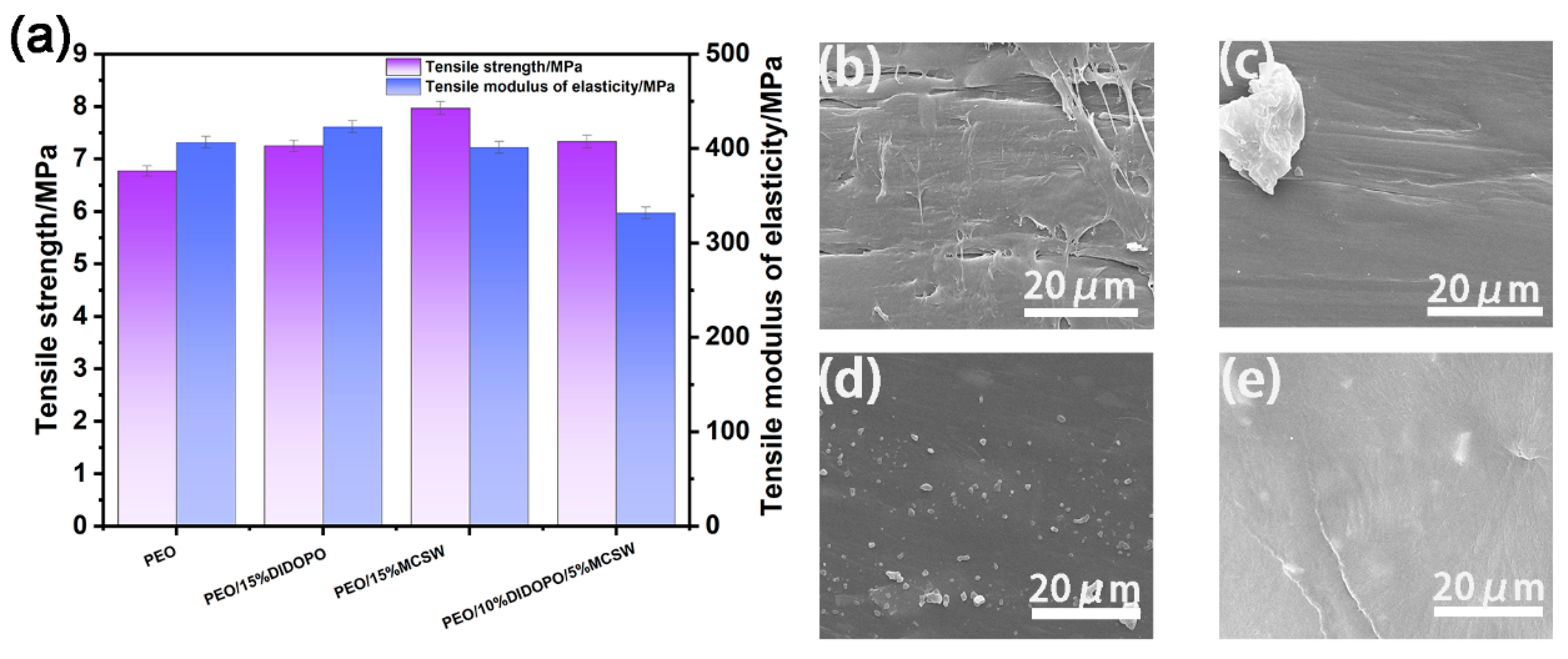
3.8. Mechanical Properties of the PEO Composites
3.9. Analysis of the Rheological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, N.; Zhang, Q.; Xu, Y.; Li, H.; Wei, Z.; Tao, Z.; Wang, Y.; Tang, H. PEO-based composite solid electrolyte for lithium battery with enhanced interface structure. J. Alloys Compd. 2023, 938, 168675. [Google Scholar] [CrossRef]
- Wang, N.; Wei, Y.; Yu, S.; Zhang, W.; Huang, X.; Fan, B.; Yuan, H.; Tan, Y. A flexible PEO-based polymer electrolyte with cross-linked network for high-voltage all solid-state lithium-ion battery. J. Mater. Sci. Technol. 2024, 183, 206–214. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Xia, E.; Wu, Y.; Li, Z. PEO/Li2ZrO3 composite electrolyte for solid-state rechargeable lithium battery. J. Energy Storage 2023, 65, 107283. [Google Scholar] [CrossRef]
- Liu, G.; Yu, H.; Zhu, T.; Chen, J.; Dong, X.; Jia, H.; Sun, J.; Gu, X.; Zhang, S. Constructing flame retardant microspheres for safe and stable poly (ethylene oxide) based All-Solid-State batteries at high voltage. Chem. Eng. J. 2024, 485, 149756. [Google Scholar] [CrossRef]
- Lim, H.; Chae, M.S.; Jamal, H.; Khan, F.; Jeon, I.; Kim, J.; Kim, J.H. Triple-Layered Noncombustible PEO-Based Solid Electrolyte for Highly Safe Lithium-Metal Batteries. Small 2025, 21, 2406200. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, Y.; Chen, X.; Weng, H.; Wang, C.; Zhu, Y.; Mei, Y. Enhanced safety of polymer solid electrolytes by using black phosphorene as a flame-retardant. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131317. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Chen, H.; Chen, C.; Luan, J.; Dong, C.; Lu, Z. Preparation and thermostability of a Si/P/N synergistic flame retardant containing triazine ring structure for cotton fabrics. Int. J. Biol. Macromol. 2024, 260, 129497. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, J.; Liu, H.; Liu, W.; Zeng, Y.; Liu, R. Low temperature carbonization and synergistic flame retardancy of cotton fabric coated by phosphorus-nitrogen flame retardants. Int. J. Biol. Macromol. 2024, 278, 134873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gomes, M.B.; Yusuf, A.; Yin, G.-Z.; Sun, C.-C.; Wang, D.-Y. Flame-retardant reinforced halloysite nanotubes as multi-functional fillers for PEO-based polymer electrolytes. Eur. Polym. J. 2024, 215, 113246. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Xu, Z.; Li, J.; Yang, H.; Luo, B.; Guo, B.; Wang, K. Design and application of novel multifunctional flame retardants for high-safety solid-state electrolytes in lithium metal batteries. Chem. Eng. J. 2025, 162812. [Google Scholar] [CrossRef]
- Wang, K.; Huang, W.; Tu, C.; Tian, Q.; Fu, Q.; Yan, W. Synthesis of aluminum phosphate–coated sepiolite: Effect on mechanical and rheological properties and flame retardancy of polyethylene oxide. J. Polym. Res. 2024, 31, 131. [Google Scholar] [CrossRef]
- Liao, Y.; Xu, X.; Luo, X.; Ji, S.; Zhao, J.; Liu, J.; Huo, Y. Recent progress in flame-retardant polymer electrolytes for solid-state lithium metal batteries. Batteries 2023, 9, 439. [Google Scholar] [CrossRef]
- Murad, S.; Hamzat, A.K.; Asmatulu, E.; Asmatulu, R. Flame-retardant fiber composites: Synergistic effects of additives on mechanical, thermal, chemical, and structural properties. Adv. Compos. Hybrid Mater. 2025, 8, 31. [Google Scholar] [CrossRef]
- Chai, W.; Su, X.; Xia, Y.; Gao, M.; Li, Y.; Liao, C.; Zheng, Z. An eco-friendly, bio-inspired and synergistic flame retardant system with outstanding water tolerance and ultra-low addition for silicone rubber. Constr. Build. Mater. 2023, 365, 130005. [Google Scholar] [CrossRef]
- Wang, P.; Shi, M.; Ling, J.; Yang, L.; Liu, W.; Zhou, Y.; Xu, J.; Chen, M.; Li, G. The construction of a stable physical–chemical multi-crosslinking structure through a simplified FROMP strategy synergistically enhances the flame retardancy and mechanical properties of PDCPD. Adv. Compos. Hybrid Mater. 2025, 8, 165. [Google Scholar] [CrossRef]
- Xu, B.; Wu, M.; Liu, Y.; Wei, S. Study on flame retardancy behavior of epoxy resin with phosphaphenanthrene triazine compound and organic zinc complexes based on phosphonitrile. Molecules 2023, 28, 3069. [Google Scholar] [CrossRef]
- Wu, H.; Lai, D.; Nan, M.; Cao, W.; Liu, L.; Liu, Y.; Yang, J. Flame retardant polyvinyl alcohol film with self-releasing carbon dioxide and ammonia from phytic acid and urea. J. Appl. Polym. Sci. 2024, 141, e56258. [Google Scholar] [CrossRef]
- Wang, X.; Tu, H.; Xiao, H.; Lu, J.; Xu, J.; Gu, G. A novel halogen-free flame-retardant fabrication for the study of smoke suppression and flame retardancy of polystyrene. Polymer 2023, 283, 126240. [Google Scholar] [CrossRef]
- Tang, E.; Li, Z.; Wu, Z.; Jiang, L.-Y.; Bi, Q.-Q.; Zhang, H.; Zhang, L.; Ran, S.-Y.; de la Vega, J.; Wang, D.-Y. Ultrafast catalytic charring towards anti-flammable thermoplastic polyurethane with superior dripping suppression and fire protection. Polym. Degrad. Stab. 2023, 213, 110369. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Fardosi, A.; Karbasi, M.; Kaseem, M. Advancements in enhancing corrosion protection of Mg alloys: A comprehensive review on the synergistic effects of combining inhibitors with PEO coating. J. Magnes. Alloys 2024, 12, 465–489. [Google Scholar] [CrossRef]
- Tu, J.; Xie, S.; Zhao, Q.; Guan, J.; Wu, C.; Wang, P. Reactive P/S/N-containing synergistic flame retardant towards eco-friendly durable flame-retardant cotton fabric: Flame-retardant property, durability and mechanism. Polym. Test. 2023, 118, 107918. [Google Scholar] [CrossRef]
- Sun, H.; Wang, W.; Liu, Y.; Wang, Q. A highly efficient, colorless phosphorus–nitrogen synergistic flame retardant for durable flame retardancy in wood pulp paper. Polym. Degrad. Stab. 2023, 215, 110468. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wu, J.; Lu, Q.; Qin, S.C.; Liu, Z.G.; Hu, Y.H.; Zhang, X.W.; Li, J.F.; Yang, D.H. Enhancing mechanical properties of PVC composites through surface composite modified calcium sulfate whiskers. Mater. Sci. Pol. 2024, 42, 55–71. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Ou, L.; Shan, B. Preparation of calcium sulphate whiskers from phosphogypsum for asphalt modification: Rheological properties and microscopic mechanisms. Int. J. Pavement Eng. 2024, 25, 2431607. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Zou, F.; Luo, H.; Cai, J.; Yu, Y. High-Performance Composite Aerogels with CaSO4 Whisker and Nanofiller Synthesized from Lepidolite Lithium Slag. ACS Appl. Polym. Mater. 2024, 6, 15058–15069. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Maiti, S.; Jain, N.; Pathak, A.; Gupta, R.R. Calcium Sulphate whiskers (CSW) an innovative material for civil engineering applications: A critical review of its preparation, characterization, current trends, and prospects. Constr. Build. Mater. 2024, 420, 135624. [Google Scholar]
- Li, D.; Zhao, H.; Jia, Z.; Jia, D.; Rong, J.; Yan, H.; Yu, X.; Zhang, Q. Preparation of phthalonitrile resin/calcium sulfate whisker composites with enhanced wear and heat resistance. High Perform. Polym. 2025, 37, 88–102. [Google Scholar] [CrossRef]
- Yan, W.; Yu, J.; Zhang, M.; Qin, S.; Wang, T.; Huang, W.; Long, L. Flame-retardant effect of a phenethyl-bridged DOPO derivative and layered double hydroxides for epoxy resin. RSC Adv. 2017, 7, 46236–46245. [Google Scholar] [CrossRef]
- Cai, M.; Zheng, C.; Li, J.; Shi, C.; Yin, R.; Ren, Z.; Hu, J.; Li, Y.; He, C.; Zhang, Q.; et al. Revealing the role of hydrogen bond coupling structure for enhanced performance of the solid-state electrolyte. J. Colloid Interface Sci. 2023, 652, 529–539. [Google Scholar] [CrossRef]
- Tang, W.; Liao, X.; Qin, Z.; Zeng, Y.; Chen, C.; Zhu, Q.; Mo, Z.; Jin, X. Improving the flame retardancy of epoxy resin by incorporating a bio-based flame retardant and kaolinite. Polym. Degrad. Stab. 2024, 227, 110895. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Yan, Q.; Chen, Q.; Hong, M.; Zhou, Z.-X.; Fu, H. Straightforward synthesis of novel chitosan bio-based flame retardants and their application to epoxy resin flame retardancy. Compos. Commun. 2024, 48, 101949. [Google Scholar] [CrossRef]
- Chen, F.; Qi, P.; Liu, J.; Qian, L.; Sheng, Q.; Chen, J.; Gu, X.; Sun, J.; Zhang, S. Fully bio-based flame retardant and antibacterial coating for polyethylene terephthalate fabric. Prog. Org. Coat. 2023, 182, 107637. [Google Scholar] [CrossRef]
- ASTM D3801; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM International: West Conshohocken, PA, USA, 2020.
- ISO 5660-1; Reaction-to-Fire tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Liu, X.; Zhang, C.; Gao, S.; Cai, S.; Wang, Q.; Liu, J.; Liu, Z. A novel polyphosphonate flame-retardant additive towards safety-reinforced all-solid-state polymer electrolyte. Mater. Chem. Phys. 2020, 239, 122014. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, P.; Li, J.; Sun, J.; Wang, R.; Zhao, H.; Wu, J.; Zheng, Y.; Liu, Q. In-situ formation of macromolecule P/N/Si flame retardant for epoxy resin with low smoke toxicity and excellent flame-retardancy. J. Appl. Polym. Sci. 2023, 140, e53926. [Google Scholar] [CrossRef]
- Cai, H.; Huang, W.; Peng, Y.; Wang, W.; Sun, L. Containing P/N/B/S multi-element flame retardant epoxy resin: Low smoke, low dielectric strength, UV shielding. React. Funct. Polym. 2025, 212, 106226. [Google Scholar] [CrossRef]
- Wang, P.; Chen, X.; Gao, S.; Hu, J.; Yang, L.; Song, J.; Tian, H.; Lu, H.; Shi, M.; Hu, X.; et al. High-performance low-smoke halogen-free flame-retardant composites for Fuxing electric multiple units via synergistic effects of char formation and anti-dripping of clay-based organic sheet silicates. Adv. Compos. Hybrid Mater. 2023, 6, 88. [Google Scholar] [CrossRef]
- Yan, W.; Yu, J.; Zhang, M.; Wang, T.; Wen, C.; Qin, S.; Huang, W. Effect of multiwalled carbon nanotubes and phenethyl-bridged DOPO derivative on flame retardancy of epoxy resin. J. Polym. Res. 2018, 25, 72. [Google Scholar] [CrossRef]
- Bao, H.; Zheng, Z.; Xu, G.; Li, R.; Wang, Q.; Saafi, M.; Ye, J. Performance and mechanism of sand stabilization via microbial-induced CaCO3 precipitation using phosphogypsum. J. Clean. Prod. 2024, 468, 142999. [Google Scholar] [CrossRef]
- Pezzato, L.; Kostelac, L.; Tonelli, L.; Elsayed, H.; Kajánek, D.; Bernardo, E.; Martini, C.; Dabalà, M.; Brunelli, K. Effect of different types of glass powders on the corrosion and wear resistance of Peo coatings produced on 6061 aluminum alloy. Met. Mater. Int. 2025, 31, 636–653. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, Z.; Zou, C.; Feng, Q.; Zhang, J. Study on surface modification of CaSO4 whisker and mechanism of enhancing mechanical properties of oil-well cement. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126408. [Google Scholar] [CrossRef]
- Ahmad, H.; Boutaous, M.; Xin, S.; Pabiou, H.; Siginer, D.A. Rheological Characterization of High-Molecular-Weight Polyethylene Oxide—An Extensive Parametric Experimental Study. J. Fluids Eng. 2023, 145, 021204. [Google Scholar] [CrossRef]
- Chatzaki, T.-M.; Kogchylakis, S.; Vlassopoulos, D.; Anastasiadis, S.H.; Chrissopoulou, K. Towards the understanding of the unusual rheological response of polymer nanocomposites. Eur. Polym. J. 2024, 210, 112903. [Google Scholar] [CrossRef]




| Sample | PEO (wt.%) | DIDOPO (wt.%) | MCSW (wt.%) | Flame Retardancy | |
|---|---|---|---|---|---|
| LOI (%) | UL-94 | ||||
| PEO | 100 | 0 | 0 | 17.9 | No Rating |
| PEO/15% DIDOPO | 85 | 15 | 0 | 23.8 | V-0 |
| PEO/14% DIDOPO/1% MCSW | 85 | 14 | 1 | 24.5 | V-0 |
| PEO/12% DIDOPO/3% MCSW | 85 | 12 | 3 | 25.0 | V-0 |
| PEO/10% DIDOPO/5% MCSW | 85 | 10 | 5 | 26.5 | V-0 |
| PEO/8% DIDOPO/7% MCSW | 85 | 8 | 7 | 24.8 | V-1 |
| PEO/15% MCSW | 85 | 0 | 15 | 25.3 | V-0 |
| Sample | T5% | Tmax-1 | Tmax-2 | Char Residue at 700 °C |
|---|---|---|---|---|
| (°C) | (°C) | (°C) | (wt.%) | |
| CSW | 121.8 | 106.8 | 437.6 | 72.44 |
| MCSW | 111.3 | 133.3 | - | 85.71 |
| Sample | TTI | Average Heat Release Rate | p-HRR | THR | TSR | av-EHC | CO2P | TML |
|---|---|---|---|---|---|---|---|---|
| (s) | (kW/m2) | (kW/m2) | (MJ/m2) | (m2/m2) | (MJ/kg) | (g/s) | (wt.%) | |
| PEO | 47 | 507.38 | 1097.8 | 211.01 | 0.174 | 30.446 | 6.02 | 99.4 |
| PEO/15% DIDOPO | 53 | 428.24 | 801.28 | 193.21 | 17.513 | 27.198 | 5.3 | 98.3 |
| PEO/15% MCSW | 53 | 507.69 | 931.31 | 199.07 | 11.687 | 29.388 | 6.01 | 96.2 |
| PEO/10% DIDOPO/5% MCSW | 49 | 394.74 | 670.58 | 190.94 | 0.22 | 30.691 | 4.49 | 89.6 |
| Sample | Flame Inhibition Effect (%) | Barrier Protective Effect (%) | Charring Effect (%) |
|---|---|---|---|
| PEO/15% DIDOPO | 10.7 | 20.3 | 1.1 |
| PEO/15% MCSW | 3.5 | 10.1 | 3.2 |
| PEO/10% DIDOPO/5% MCSW | −0.01 | 32.5 | 9.8 |
| Sample | T5% | Tmax | Char Residue at 700 °C | Tm | Tc |
|---|---|---|---|---|---|
| (°C) | (°C) | (wt.%) | (°C) | (°C) | |
| PEO | 273.96 | 401.5 | - | 58.15 | 42.07 |
| PEO/15% DIDOPO | 321.07 | 403.5 | - | 57.22 | 34.26 |
| PEO/15% MCSW | 295.45 | 400.9 | 2.31 | 57.00 | 37.03 |
| PEO/10% DIDOPO/5% MCSW | 344.51 | 403.3 | 7.82 | 58.88 | 34.17 |
| Sample | Elemental Content (wt.%) | |||||
|---|---|---|---|---|---|---|
| C | N | O | P | S | Ca | |
| PEO | 10.4 | 4.42 | 85.18 | -- | -- | -- |
| PEO/15% DIDOPO | 13.57 | 3.42 | 54.08 | 28.93 | -- | -- |
| PEO/15% MCSW | 3.94 | 2.18 | 43.57 | -- | 21.24 | 29.08 |
| PEO/10% DIDOPO/5% MCSW | 6.54 | 2.23 | 33 | 13.68 | 8.82 | 26.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yan, W.; Huang, W.; Wang, K.; Tian, Q.; Tu, C.; Guan, X.; Wu, S.; Ba, X.; Wei, C.; et al. Construction of Fire-Retardant PEO Composite Based on Calcium Sulfate Whiskers Fabricated from Phosphogypsum and DOPO Derivatives. Polymers 2025, 17, 1588. https://doi.org/10.3390/polym17121588
Zhang J, Yan W, Huang W, Wang K, Tian Q, Tu C, Guan X, Wu S, Ba X, Wei C, et al. Construction of Fire-Retardant PEO Composite Based on Calcium Sulfate Whiskers Fabricated from Phosphogypsum and DOPO Derivatives. Polymers. 2025; 17(12):1588. https://doi.org/10.3390/polym17121588
Chicago/Turabian StyleZhang, Jie, Wei Yan, Weijiang Huang, Kui Wang, Qin Tian, Chunyun Tu, Xingyu Guan, Shaoyuan Wu, Xuan Ba, Chunle Wei, and et al. 2025. "Construction of Fire-Retardant PEO Composite Based on Calcium Sulfate Whiskers Fabricated from Phosphogypsum and DOPO Derivatives" Polymers 17, no. 12: 1588. https://doi.org/10.3390/polym17121588
APA StyleZhang, J., Yan, W., Huang, W., Wang, K., Tian, Q., Tu, C., Guan, X., Wu, S., Ba, X., Wei, C., Ye, T., Chen, J., & Zhang, Y. (2025). Construction of Fire-Retardant PEO Composite Based on Calcium Sulfate Whiskers Fabricated from Phosphogypsum and DOPO Derivatives. Polymers, 17(12), 1588. https://doi.org/10.3390/polym17121588






