Alkaline Extraction and Ethanol Precipitation of High-Molecular-Weight Xylan Compounds from Eucalyptus Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Alkaline Extraction
2.3. Antisolvents Precipitation
3. Results and Discussion
3.1. Alkaline Extraction
Statistical Treatment
| Yield (%) = 22.7 + 5.9 × NaOH + 4.8. × T + 1.8 × t − 1.9 × NaOH2 + 2.4 × NaOH × T | R2 = 93.7% | (1) |
| Xylosaccharides (%) = 1.8 + 0.4 × NaOH + 0.4 × T − 0.2 × NaOH × T | R2 = 88.9% | (2) |
| Absorbance 280 nm = 0.63 + 0.16 × T + 0.18 × NaOH + 0.11 × T × NaOH | R2 = 92.1% | (3) |
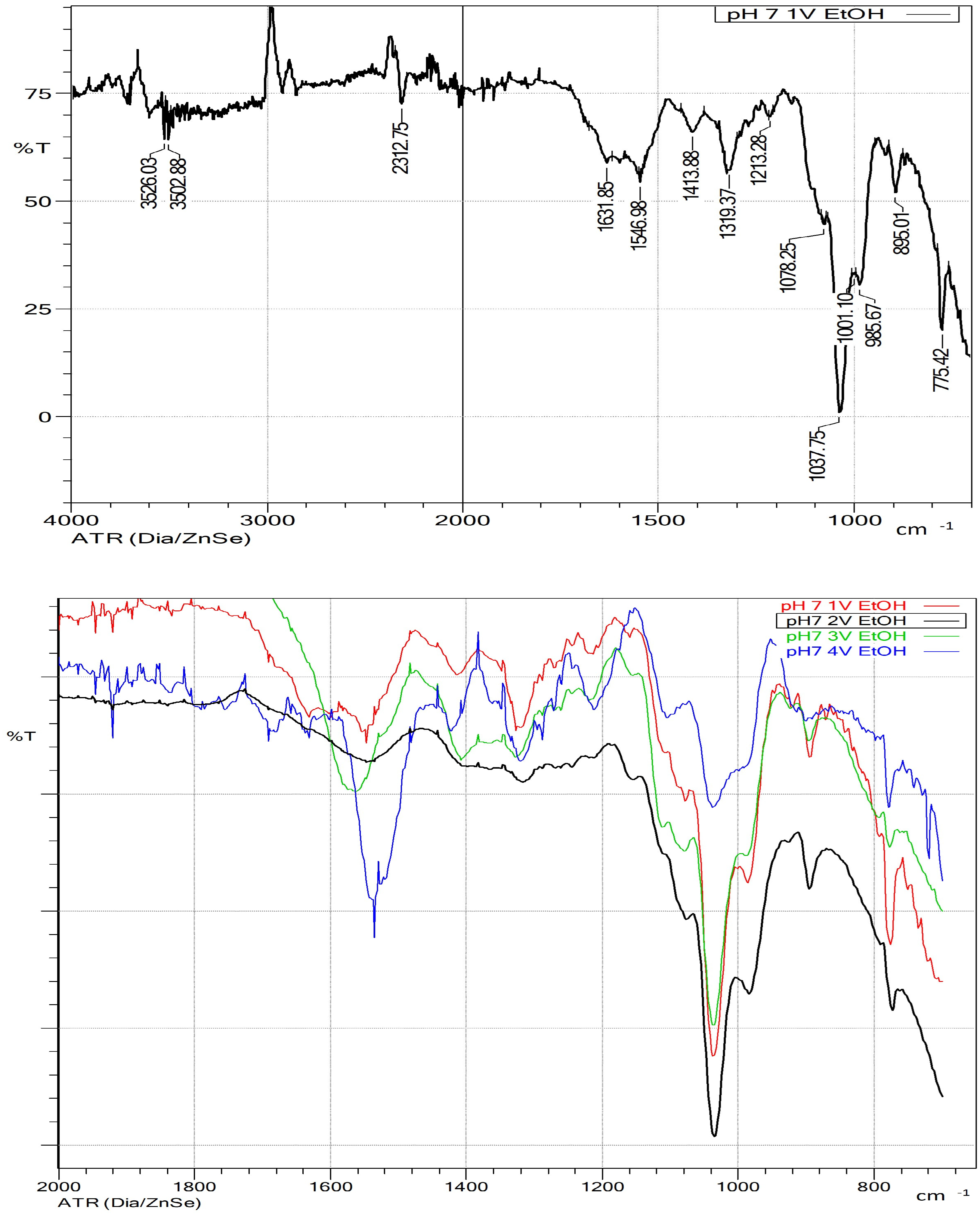
3.2. Ethanol Precipitation
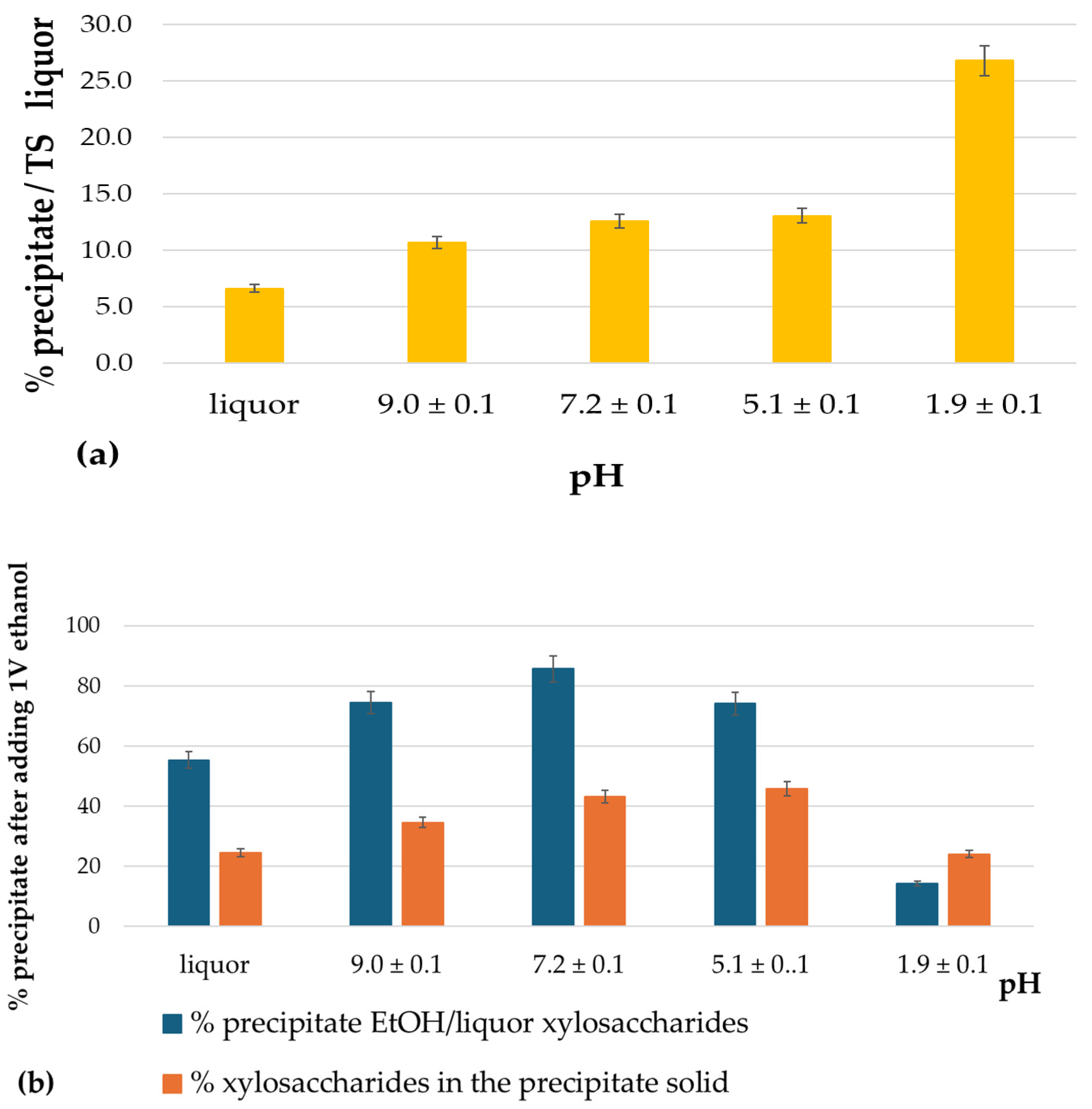
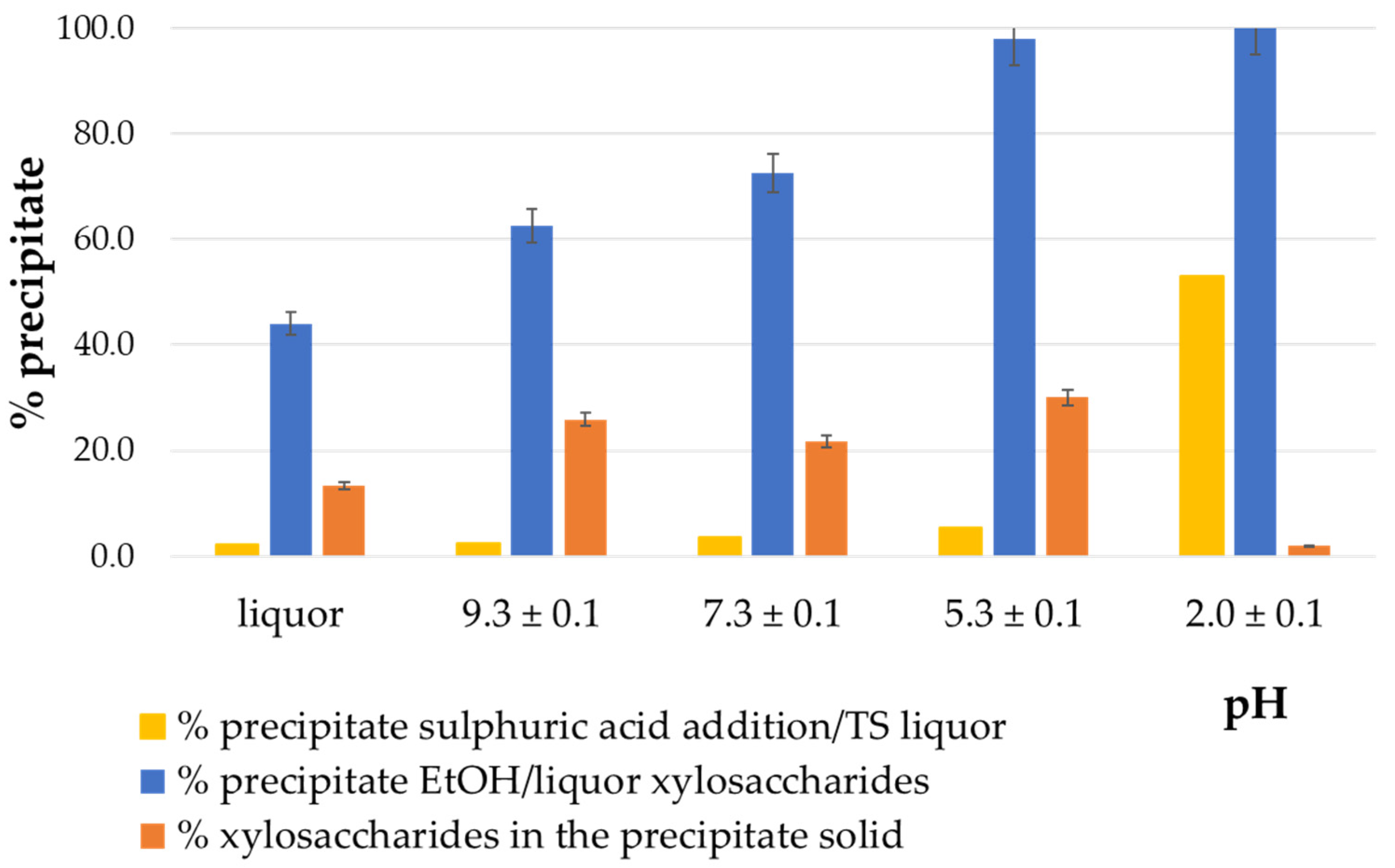
3.2.1. Ethanol Precipitation at Different Liquor pH Levels
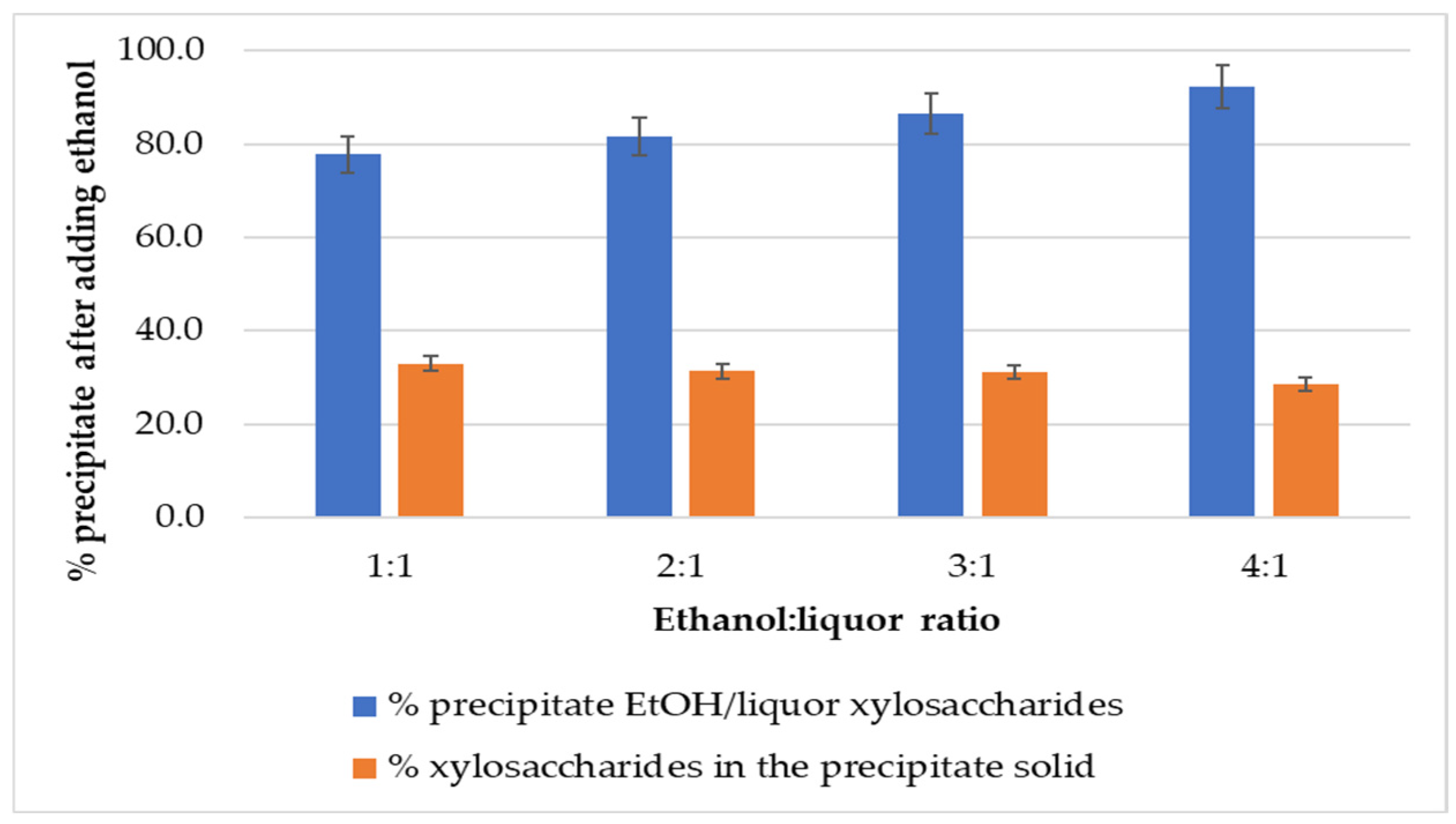
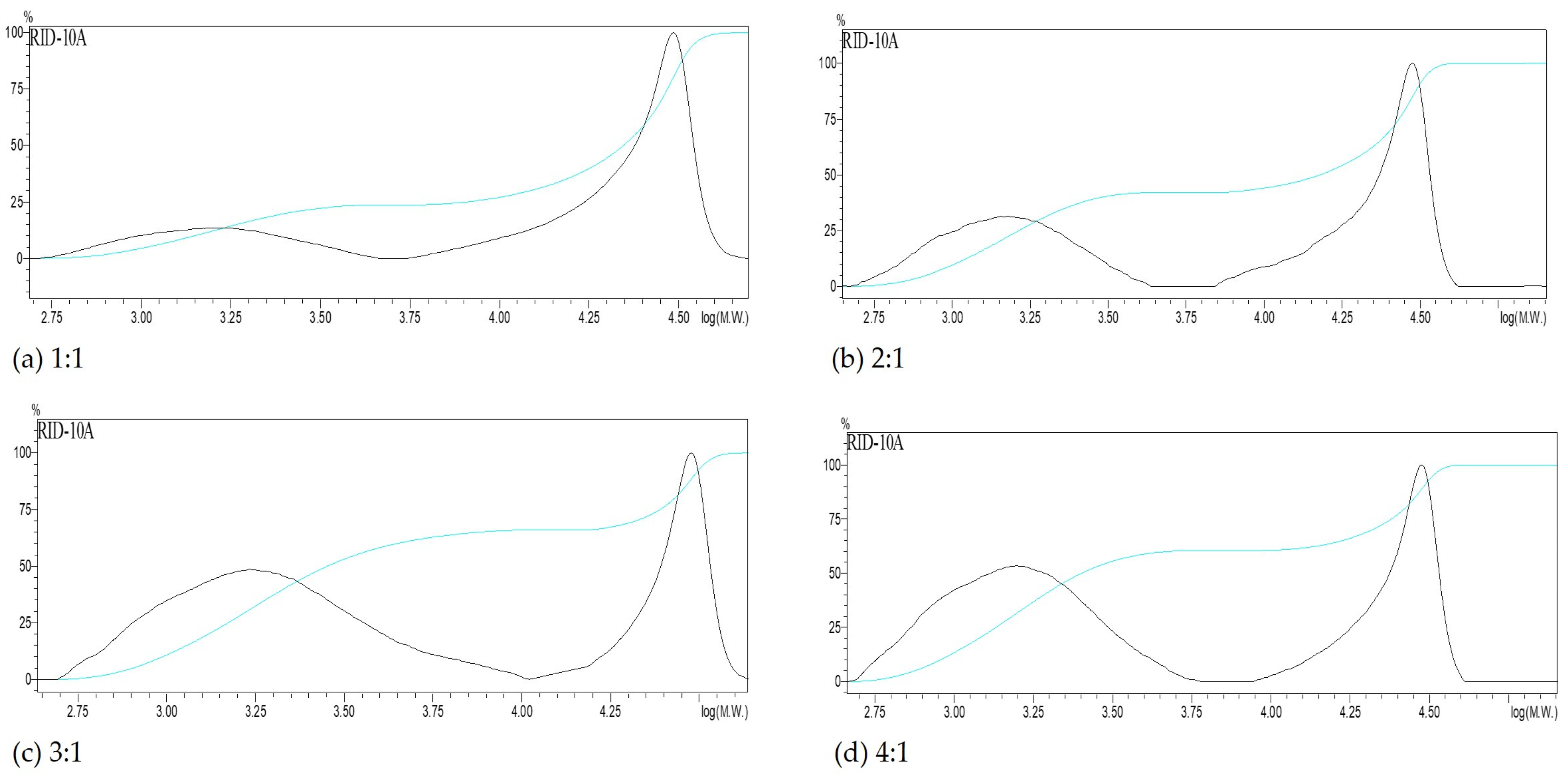
3.2.2. Ethanol-to-Liquor Volume Ratio
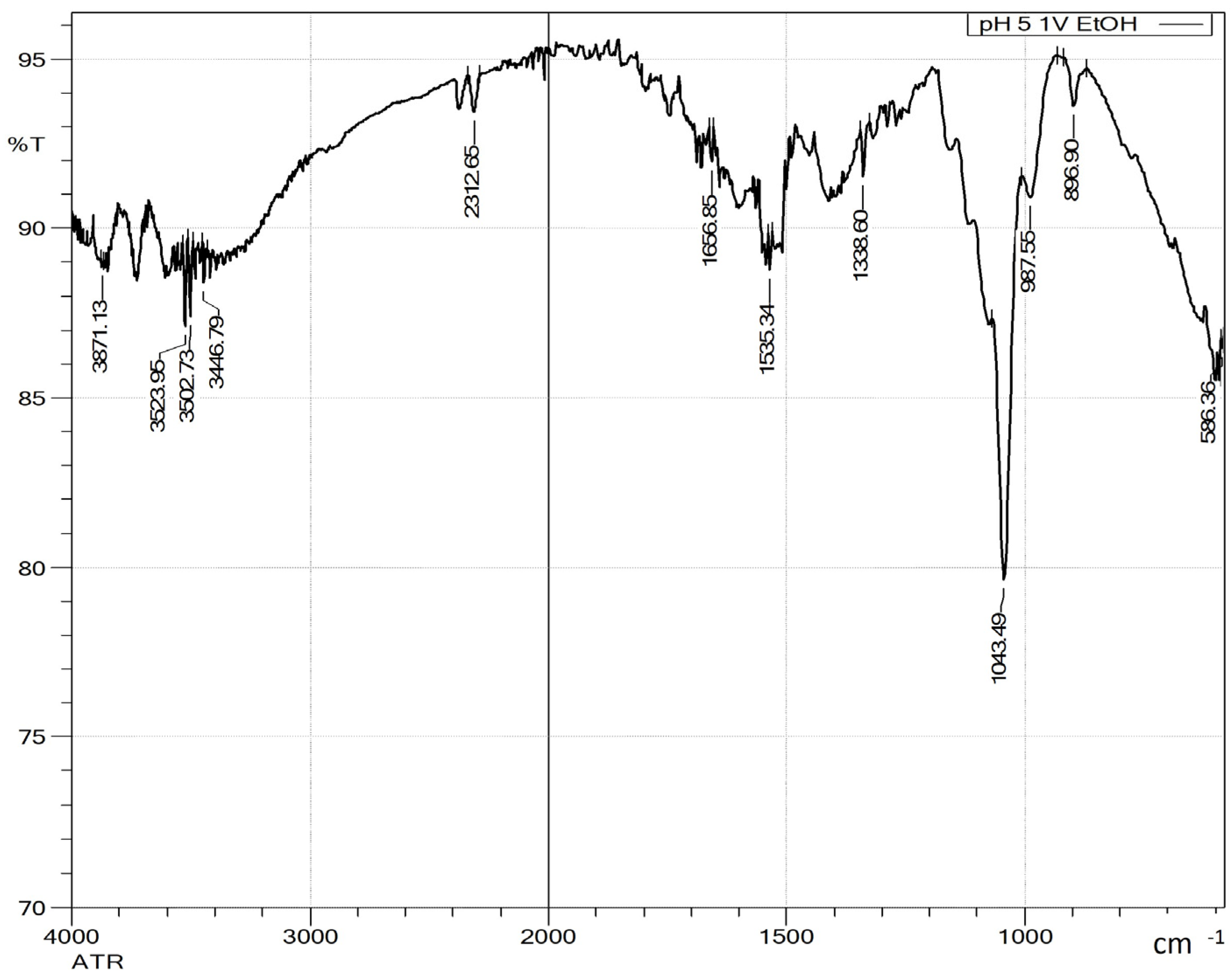
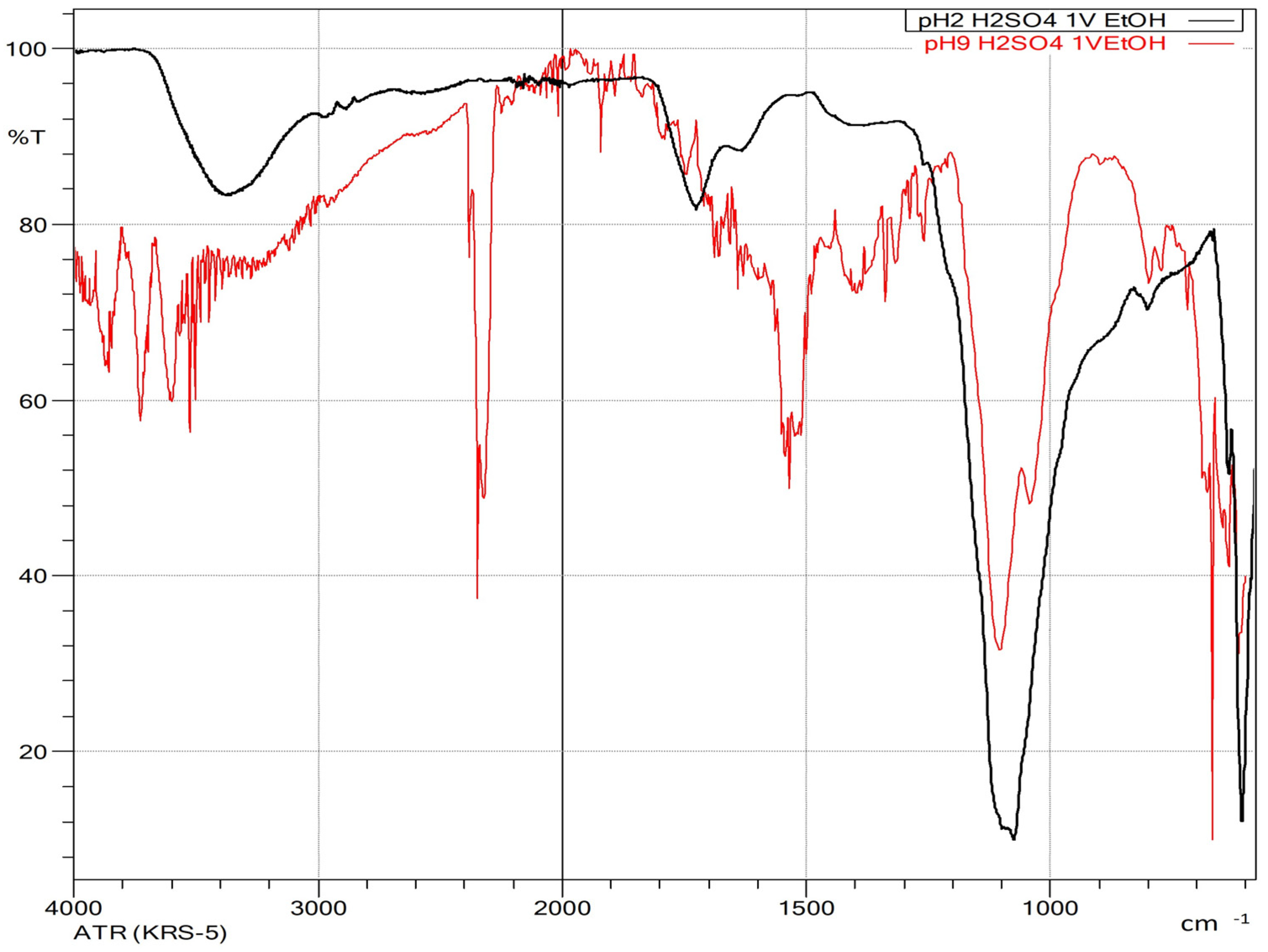
3.2.3. pH Adjustment Using Sulfuric and Acetic Acids Followed by Ethanol-Induced Precipitation
3.3. Precipitation at Different pH Using Dioxane or Isopropanol as Antisolvent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oriez, V.; Peydecastaing, J.; Pontalier, P.Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean. Technol. 2020, 2, 91–115. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.; Moniz, P. Hydrothermal/Liquid Hot Water Pretreatment (Autohydrolysis): A Multipurpose Process for Biomass Upgrading. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier, B.V. Woodhead Publishing: Amsterdam, The Netherlands, 2016; pp. 315–347. ISBN 9780128025611. [Google Scholar]
- Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E. Integrating the New Age of Bioeconomy and Industry 4.0 into Biorefinery Process Design. Bioresources 2022, 17, 5510–5531. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, Q.; Chundawat, S.P.S. Recent Advances on Ammonia-Based Pretreatments of Lignocellulosic Biomass. Bioresour. Technol. 2020, 298, 122446. [Google Scholar] [CrossRef]
- Wang, H.M.; Chang, G.Q.; Yuan, L.L.; Hou, Q.X.; Sun, R.C. Alkaline Pretreatment toward Sustainable Biorefinery. In Handbook of Biorefinery Research and Technology: Biomass Logistics to Saccharification; Springer: Dordrecht, The Netherlands, 2024; pp. 429–455. ISBN 9789400763081. [Google Scholar]
- Cabrera, M.N.; Felissia, F.; Area, M.C. Concentration and Purification of Xylan-Based Hemicelluloses. In Advances in Chemistry Research; Taylor, J.C., Ed.; Nova, Science and Technology: Caparica, Portugal, 2023; Volume 80, pp. 79–102. ISBN 979-8-89113-008-1. [Google Scholar]
- Lehto, J.; Alén, R. Alkaline Pre-Treatment of Hardwood Chips Prior to Delignification. J. Wood Chem. Technol. 2013, 33, 77–91. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current Perspective on Pretreatment Technologies Using Lignocellulosic Biomass: An Emerging Biorefinery Concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Tenkanen, M. Sustainable Food-Packaging Materials Based on Future Biorefinery Products: Xylans and Mannans. Trends Food Sci. Technol. 2012, 28, 90–102. [Google Scholar] [CrossRef]
- Hansen, N.M.L.; Plackett, D. Sustainable Films and Coatings from Hemicelluloses: A Review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef]
- Arzami, A.N.; Ho, T.M.; Mikkonen, K.S. Valorization of Cereal By-Product Hemicelluloses: Fractionation and Purity Considerations. Food Res. Int. 2022, 151, 110818. [Google Scholar] [CrossRef]
- Jian, H.L.; Lin, X.J.; Zhang, W.A.; Zhang, W.M.; Sun, D.F.; Jiang, J.X. Characterization of Fractional Precipitation Behavior of Galactomannan Gums with Ethanol and Isopropanol. Food Hydrocoll. 2014, 40, 115–121. [Google Scholar] [CrossRef]
- Peng, F.; Peng, P.; Xu, F.; Sun, R. Fractional Purification and Bioconversion of Hemicelluloses. Biotechnol. Adv. 2012, 30, 879–903. [Google Scholar] [CrossRef]
- Hu, X.; Goff, H.D. Fractionation of Polysaccharides by Gradient Non-Solvent Precipitation: A Review. Trends Food Sci. Technol. 2018, 81, 108–115. [Google Scholar] [CrossRef]
- Hu, X.; Liu, C.; Jin, Z.; Tian, Y. Fractionation of Starch Hydrolysate into Dextrin Fractions with Low Dispersity by Gradient Alcohol Precipitation. Sep. Purif. Technol. 2015, 151, 201–210. [Google Scholar] [CrossRef]
- Sun, R.-C.; Tomkinson, J. Essential Guides for Isolation/ Purification of Polysaccharides. Encycl. Sep. Sci. 2000, 4568–4574. [Google Scholar]
- Guo, X.; Meng, H.; Zhu, S.; Tang, Q.; Pan, R.; Yu, S. Stepwise Ethanolic Precipitation of Sugar Beet Pectins from the Acidic Extract. Carbohydr. Polym. 2016, 136, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Alen, R.; Hartus, T. UV Spectrophotometric Determination of Lignin from Alkaline Pulping Liquors. Cellul. Chem. Technol. 1988, 22, 613–618. [Google Scholar]
- Sjöström, E. Wood Chemistry—Fundamentals and Applications, 2nd ed.; Academic Press, Inc.: San Diego, CA, USA, 1993; ISBN 0-12-647481-8. [Google Scholar]
- Alén, R. Basic Chemistry of Wood Delignification. In Forest products Chemistry; Stenius, P., Ed.; Fapet Oy: Helsinki, Finland, 2000; p. 61. ISBN 952-5216-03-9. [Google Scholar]
- van Heiningen, A.; Genco, J.; Yoon, S.; Tunc, M.S.; Zou, H.; Juo, L.; Mao, H.; Pendse, H. Integrated Forest Biorefineries; Near-Neutral Process. In Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass; Zhu, J., Zhang, X., Pan, X., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1067, pp. 443–473. ISBN 9780841226432. [Google Scholar]
- de Carvalho, D.M.; de Queiroz, J.H.; Colodette, J.L. Assessment of Alkaline Pretreatment for the Production of Bioethanol from Eucalyptus, Sugarcane Bagasse and Sugarcane Straw. Ind. Crops Prod. 2016, 94, 932–941. [Google Scholar] [CrossRef]
- Testova, L.; Roselli, A.; Costabel, L.; Kovasin, K.; Tenkanen, M.; Sixta, H. Combined Production of Polymeric Birch Xylan and Paper Pulp by Alkaline Pre-Extraction Followed by Alkaline Cooking. Ind. Eng. Chem. Res. 2014, 53, 8302–8310. [Google Scholar] [CrossRef]
- Vena, P.F.; García-Aparicio, M.P.; Brienzo, M.; Görgens, J.F.; Rypstra, T. Effect of Alkaline Hemicellulose Extraction on Kraft Pulp Fibers from Eucalyptus Grandis. J. Wood Chem. Technol. 2013, 33, 157–173. [Google Scholar] [CrossRef]
- Geng, W.; Narron, R.; Jiang, X.; Pawlak, J.J.; Chang, H.; Park, S.; Jameel, H.; Venditti, R.A. The Influence of Lignin Content and Structure on Hemicellulose Alkaline Extraction for Non-Wood and Hardwood Lignocellulosic Biomass. Cellulose 2019, 26, 3219–3230. [Google Scholar] [CrossRef]
- Lehuedé, L.; Henríquez, C.; Carú, C.; Córdova, A.; Mendonça, R.T.; Salazar, O. Xylan Extraction from Hardwoods by Alkaline Pretreatment for Xylooligosaccharide Production: A Detailed Fractionation Analysis. Carbohydr. Polym. 2023, 302, 120381. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Jia, N.; Bian, J.; Peng, P.; Sun, R.-C.; Liu, S.J. Isolation and Fractionation of Hemicelluloses From Salix Psammophila. Cellul. Chem. Technol. 2012, 46, 177–184. [Google Scholar]
- Fu, G.-Q.; Hu, Y.-J.; Bian, J.; Li, M.-F.; Peng, F.; Sun, R.-C. Isolation, Purification, and Potential Applications of Xylan. In Production of Materials from Sustainable Biomass Resources; Fang, Z., Smith, R.L.J., Tian, X.-F., Eds.; Springer: Singapore, 2019; Volume 9, pp. 3–35. ISBN 978-981-13-3768-0. [Google Scholar]
- Egüés, I.; Sanchez, C.; Mondragon, I.; Labidi, J. Effect of Alkaline and Autohydrolysis Processes on the Purity of Obtained Hemicelluloses from Corn Stalks. Bioresour. Technol. 2012, 103, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Nie, S.; Li, X.; Huang, X.; Li, Q. Characteristics of the Water- And Alkali-Soluble Hemicelluloses Fractionated by Sequential Acidification and Graded-Ethanol from Sweet Maize Stems. Molecules 2019, 24, 212. [Google Scholar] [CrossRef]
- O’Dwyer, M.H. The Hemicelluloses. Part IV: The Hemicelluloses of Beech Wood. Biochem. J. 1926, 20, 656–664. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Paquot, M.; Wathelet, B. Comparative Study of Alkaline Extraction Process of Hemicelluloses from Pear Pomace. Biomass Bioenergy 2014, 61, 254–264. [Google Scholar] [CrossRef]
- Panthapulakkal, S.; Pakharenko, V.; Sain, M. Microwave Assisted Short-Time Alkaline Extraction of Birch Xylan. J. Polym. Env. 2013, 21, 917–929. [Google Scholar] [CrossRef]
- Kacuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR Study of Plant Cell Wall Model Compounds: Pectic Polysaccharides and Hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Buslov, D.K.; Kaputski, F.N.; Sushko, N.I.; Torgashev, V.I.; Solov’eva, L.V.; Tsarenkov, V.M.; Zubets, O.V.; Larchenko, L.V. Infrared Spectroscopic Analysis of the Structure of Xylans. J. Appl. Spectrosc. 2009, 76, 801–805. [Google Scholar] [CrossRef]
- Kacurakova, M.; Belton, P.S.; Wilson, R.H.; Ebringerova, A. Hydration Properties of Xylan-Type Structures: An FTIR Study of Xylooligosaccharides. J. Sci. Food Agric. 1998, 77, 38–44. [Google Scholar] [CrossRef]
- Balakshin, M.; Capanema, E.; Berlin, A. Isolation and Analysis of Lignin–Carbohydrate Complexes Preparations with Traditional and Advanced Methods, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 42, ISBN 9780444632814. [Google Scholar]
- Puițel, A.C.; Suditu, G.D.; Drăgoi, E.N.; Danu, M.; Ailiesei, G.L.; Balan, C.D.; Chicet, D.L.; Nechita, M.T. Optimization of Alkaline Extraction of Xylan-Based Hemicelluloses from Wheat Straws: Effects of Microwave, Ultrasound, and Freeze–Thaw Cycles. Polymers 2023, 15, 1038. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Qiu, S.; Liu, C.; Zhang, P.; Yin, L.; Chen, F. Rheological Properties and Structural Characteristics of Arabinoxylan Hydrogels Prepared from Three Wheat Bran Sources. J. Cereal Sci. 2019, 88, 79–86. [Google Scholar] [CrossRef]
- Longue, D., Jr.; Ayoub, A.; Venditti, R.A.; Jameel, H.; Colodette, J.L.; Chang, H. min Ethanol Precipitation of Hetero-Polysaccharide Material from Hardwood by Alkaline Extraction Prior to the Kraft Cooking Process. Bioresources 2013, 8, 5319–5332. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Kang, J.; Wang, N.; Xiao, M.; Li, Z.; Wang, C.; Guo, Q.; Hu, X. Arabinoxylan from Wheat Bran: Molecular Degradation and Functional Investigation. Food Hydrocoll. 2020, 107, 105914. [Google Scholar] [CrossRef]
- Umemura, M.; Yuguchi, Y. Solvation of Xyloglucan in Water/Alcohol Systems by Molecular Dynamics Simulation. Cellulose 2009, 16, 361–371. [Google Scholar] [CrossRef]
- Xu, J.; Yue, R.Q.; Liu, J.; Ho, H.M.; Yi, T.; Chen, H.B.; Han, Q. Bin Structural Diversity Requires Individual Optimization of Ethanol Concentration in Polysaccharide Precipitation. Int. J. Biol. Macromol. 2014, 67, 205–209. [Google Scholar] [CrossRef]
- Bian, J.; Peng, F.; Peng, P.; Xu, F.; Sun, R.C. Isolation and Fractionation of Hemicelluloses by Graded Ethanol Precipitation from Caragana Korshinskii. Carbohydr. Res. 2010, 345, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.Y. Lignocellulosic Biomass Fractionation by Mineral Acids and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Molecules 2019, 24, 4273. [Google Scholar] [CrossRef]
- Kienberger, M.; Maitz, S.; Pichler, T.; Demmelmayer, P. Systematic Review on Isolation Processes for Technical Lignin. Processes 2021, 9, 804. [Google Scholar] [CrossRef]
- da Silva, S.H.F.; Gordobil, O.; Labidi, J. Organic Acids as a Greener Alternative for the Precipitation of Hardwood Kraft Lignins from the Industrial Black Liquor. Int. J. Biol. Macromol. 2020, 142, 583–591. [Google Scholar] [CrossRef]
- Speight, J. Lange’s Handbook of Chemistry, 17th ed.; McGraw Hill: New York, NY, USA, 2016. [Google Scholar]
- Santiago, A.S.; Pascoal Neto, C. Impact of Kraft Process Modifications on Eucalyptus Globulus Pulping Performance and Polysaccharide Retention. Ind. Eng. Chem. Res. 2008, 47, 7433–7440. [Google Scholar] [CrossRef]
- Costabel, L. Alkaline Pre-Extraction of Birch Wood Prior to Alkaline Pulping. Master’s Thesis, Universidad de la República, Montevideo, Uruguay, 2013. [Google Scholar]



| Component | X ± 2σ 1 (%) |
|---|---|
| Glucan | 41.9 ± 0.6 |
| Xylan | 14.5 ± 0.4 |
| Arabinan | 1.6 ± 0.3 |
| Acid soluble lignin | 2.8 ± 0.1 |
| Acid insoluble lignin | 21.9± 0.9 |
| Acetyl groups | 3.1 ± 0.1 |
| Ethanol/water extractives | 3.4 ± 0.2 |
| Ash content | 0.7 ± 0.1 |
| Real Variables | Coded Variables | Extraction Yield (% o.d. Biomass) | Xylosaccharides Content (% o.d.Biomass) | Absorbance at 280 nm (Dilution 1/1500) |
|---|---|---|---|---|
| 105 °C, 6.7 %NaOH, 45 min | −1, −1, −1 | 9.4 ± 0.5 | 0.7 ± 0.1 | 0.247 ± 0.012 |
| 105 °C, 6.7 %NaOH, 90 min | −1, −1, 0 | 12.8 ± 0.5 | 0.9 ± 0.1 | 0.446 ± 0.020 |
| 105 °C, 6.7 %NaOH, 135 min | −1, −1, +1 | 14.2 ± 0.6 | 1.2 ± 0.1 | 0.486 ± 0.019 |
| 105 °C, 11.7 %NaOH, 45 min | −1, 0, −1 | 16.3 ± 0.5 | 1.5 ± 0.1 | 0.284 ± 0.016 |
| 105 °C, 11.7 %NaOH, 90 min | −1, 0, 0 | 19.4 ± 0.7 | 1.7 ± 0.2 | 0.618 ± 0.010 |
| 105 °C, 11.7 %NaOH, 135 min | −1, 0, +1 | 21.0 ± 0.6 | 1.7 ± 0.1 | 0.707 ± 0.020 |
| 105 °C, 16.7 %NaOH, 45 min | −1, +1, −1 | 18.4 ± 0.5 | 2.3 ± 0.1 | 0.302 ± 0.014 |
| 105 °C, 16.7 %NaOH, 90 min | −1, +1, 0 | 21.7 ± 0.5 | 1.9 ± 0.1 | 0.729 ± 0.015 |
| 105 °C, 16.7 %NaOH, 135 min | −1, +1, +1 | 21.1 ± 0.7 | 1.9 ± 0.2 | 0.651 ± 0.012 |
| 135 °C, 6.7 %NaOH, 45 min | 0, −1, −1 | 13.3 ± 0.5 | 1.3 ± 0.1 | 0.365 ± 0.016 |
| 135 °C, 6.7 %NaOH, 90 min | 0, −1, 0 | 14.6 ± 0.4 | 1.2 ± 0.2 | 0.473 ± 0.020 |
| 135 °C, 6.7 %NaOH, 135 min | 0, −1, +1 | 15.9 ± 0.6 | 1.1 ± 0.1 | 0.497 ± 0.023 |
| 135 °C, 11.7 %NaOH, 45 min | 0, 0, −1 | 21.7 ± 0.7 | 1.5 ± 0.2 | 0.675 ± 0.016 |
| 135 °C, 11.7 %NaOH, 90 min | 0, 0, 0 | 23.5 ± 0.6 | 1.6 ± 0.2 | 0.347 ± 0.014 |
| 135 °C, 11.7 %NaOH, 135 min | 0, 0, +1 | 25.4 ± 0.7 | 1.8 ± 0.2 | 0.394 ± 0.020 |
| 135 °C, 16.7 %NaOH, 45 min | 0, +1, −1 | 20.9 ± 0.4 | 2.2 ± 0.1 | 0.686 ± 0.016 |
| 135 °C, 16.7 %NaOH, 90 min | 0, +1, 0 | 23.8 ± 0.4 | 2.2 ± 0.2 | 0.776 ± 0.018 |
| 135 °C, 16.7 %NaOH, 135 min | 0, +1, +1 | 25.7 ± 0.6 | 2.2 ± 0.2 | 0.786 ± 0.021 |
| 155 °C, 6.7 %NaOH, 45 min | +1, −1, −1 | 17.6 ± 0.6 | 2.0 ± 0.2 | 0.486 ± 0.013 |
| 155 °C, 6.7 %NaOH, 90 min | +1, −1, 0 | 17.9 ± 0.4 | 2.1 ± 0.1 | 0.507 ± 0.013 |
| 155 °C, 6.7 %NaOH, 135 min | +1, −1, +1 | 19.4 ± 0.5 | 2.2 ± 0.2 | 0.498 ± 0.020 |
| 155 °C, 11.7 %NaOH, 45 min | +1, 0, −1 | 24.0 ± 0.6 | 2.0 ± 0.1 | 0.810 ± 0.021 |
| 155 °C, 11.7 %NaOH, 90 min | +1, 0, 0 | 25.6 ± 0.6 | 2.1 ± 0.2 | 0.803 ± 0.018 |
| 155 °C, 11.7 %NaOH, 135 min | +1, 0, +1 | 27.6 ± 0.7 | 2.2 ± 0.2 | 0.887 ± 0.015 |
| 155 °C, 16.7 %NaOH, 45 min | +1, +1, −1 | 35.1 ± 0.6 | 2.1 ± 0.1 | 1.085 ± 0.018 |
| 155 °C, 16.7 %NaOH, 90 min | +1, +1, 0 | 34.9 ± 0.5 | 2.3 ± 0.1 | 0.995 ± 0.020 |
| 155 °C, 16.7 %NaOH, 135 min | +1, +1, +1 | 38.8 ± 0.6 | 2.5 ± 0.2 | 1.208 ± 0.020 |
| pH | Molecular Weight Distribution | ||||
|---|---|---|---|---|---|
| # | Mn (Da) | Mw (Da) | Mw/Mn | Relative Area (%) | |
| 12.4 | Total | 4080 | 33,243 | 3.6 | |
| 1 | 143,868 | 154,141 | 14.8 | ||
| 2 | 29,864 | 30,428 | 16.0 | ||
| 3 | 11,407 | 11,982 | 42.5 | ||
| 4 | 1325 | 1615 | 1.2 | ||
| 9.1 | Total | 8011 | 16,873 | 1.2 | |
| 1 | 16,062 | 18,433 | 90.7 | ||
| 2 | 1358 | 1633 | 9.3 | ||
| 7.2 | Total | 5255 | 16,147 | 3.1 | |
| 1 | 42,937 | 43,899 | 10.0 | ||
| 2 | 22,815 | 24,378 | 47.8 | ||
| 3 | 1420 | 1930 | 42.2 | ||
| 5.1 | Total | 8310 | 18,658 | 2.2 | |
| 1 | 27,146 | 28,031 | 17.0 | ||
| 2 | 11,569 | 12,574 | 56.5 | ||
| 3 | 1309 | 1699 | 26.5 | ||
| 1.9 | Total | 21,038 | 26,569 | 1.3 | |
| 1 | 44,481 | 46,150 | 17.2 | ||
| 2 | 18,960 | 22,497 | 82.8 | ||
| Ethanol-to-Liquor Volume Ratio | Molecular Weight Distribution | ||||
|---|---|---|---|---|---|
| # | Mn (Da) | Mw (Da) | Mw/Mn | % | |
| 1:1 | Total | 4908 | 19,340 | 3.9 | |
| 1 | 21,401 | 24,798 | 76.3 | ||
| 2 | 1407 | 1718 | 26.7 | ||
| 2:1 | Total | 2885 | 14,838 | 5.1 | |
| 1 | 21,552 | 24,436 | 58.0 | ||
| 2 | 1315 | 1600 | 42.0 | ||
| 3:1 | Total | 2322 | 10,894 | 4.7 | |
| 1 | 26,575 | 27,582 | 34.1 | ||
| 2 | 1577 | 2262 | 65.9 | ||
| 4:1 | Total | 2171 | 11,167 | 5.1 | |
| 1 | 23,606 | 25,559 | 39.6 | ||
| 2 | 1361 | 1740 | 60.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, M.N.; Rossi, A.; Guarino, J.I.; Felissia, F.E.; Area, M.C. Alkaline Extraction and Ethanol Precipitation of High-Molecular-Weight Xylan Compounds from Eucalyptus Residues. Polymers 2025, 17, 1589. https://doi.org/10.3390/polym17121589
Cabrera MN, Rossi A, Guarino JI, Felissia FE, Area MC. Alkaline Extraction and Ethanol Precipitation of High-Molecular-Weight Xylan Compounds from Eucalyptus Residues. Polymers. 2025; 17(12):1589. https://doi.org/10.3390/polym17121589
Chicago/Turabian StyleCabrera, María Noel, Antonella Rossi, Juan Ignacio Guarino, Fernando Esteban Felissia, and María Cristina Area. 2025. "Alkaline Extraction and Ethanol Precipitation of High-Molecular-Weight Xylan Compounds from Eucalyptus Residues" Polymers 17, no. 12: 1589. https://doi.org/10.3390/polym17121589
APA StyleCabrera, M. N., Rossi, A., Guarino, J. I., Felissia, F. E., & Area, M. C. (2025). Alkaline Extraction and Ethanol Precipitation of High-Molecular-Weight Xylan Compounds from Eucalyptus Residues. Polymers, 17(12), 1589. https://doi.org/10.3390/polym17121589








